Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ambient pressure synthesis of unstable bulk phases of strongly correlated rare-earth nickelates†
Nicholas
Smieszek‡
 a,
Xinran
Li‡
a and
Vidhya
Chakrapani
a,
Xinran
Li‡
a and
Vidhya
Chakrapani
 *ab
*ab
aHoward P. Isermann Department of Chemical and Biological Engineering, Rensselaer Polytechnic Institute, Troy, New York-12180, USA
bDepartment of Physics, Applied Physics, and Astronomy, Rensselaer Polytechnic Institute, Troy, New York-12180, USA. E-mail: chakrv@rpi.edu
First published on 26th July 2022
Abstract
Despite the outstanding electrical, electrochemical, and optical properties of rare-earth (R)-doped nickelates (RNiO3), a bottleneck in its device applications is the need for high-pressure, typically in excess of 100 bars, to stabilize this phase during synthesis. To date, no known near-ambient pressure synthesis process exists for the synthesis of bulk RNiO3 with ionic radii of R lower than that of Nd (such as Sm, Eu, and Gd) in the lanthanide series due to the increasing thermodynamic instability of Ni3+ cations at ambient pressures. In the present study, we report a set of conditions for the successful synthesis of bulk SmNiO3 and NdNiO3 through a sol–gel synthesis procedure followed by annealing at ambient pressure to stabilize Ni3+. Rietveld refinement analysis shows the composition of crystalline SmNiO3 and NdNiO3 phases to be as high as 50 wt% and 96 wt%, respectively. Consequently, sharp, well-defined insulator–metal transitions (IMTs) involving resistance changes of 2–3 orders of magnitude could be achieved, which is comparable to that seen in high-pressure synthesized samples reported in the literature.
1. Introduction
Rare-earth nickelates are an important class of functional materials that exhibit a diverse range of remarkable properties and functionalities, such as the observation of size-tunable insulator–metal transitions (IMTs),1,2 low-temperature superconductivity,3,4 unusual antiferromagnetic ordering,5 and strong bifunctional electrocatalytic activity,6 which can be exploited for advanced optoelectronics,7 photodetection,8 neuromorphic and synaptic devices,9 smart windows, sensors,10 regenerative fuel cells11 and other energy storage devices.12 The flexibility of the perovskite structure of RNiO3 allows for efficient doping of various rare-earth ions, which affects the rotation, tilt, and distortion of the NiO6 octahedra, which gives rise to tunable structural, electrical, optical and magnetic properties.13 For instance, the IMT temperature of RNiO3 can be systematically tuned from low to high with the rare-earth ions of decreasing ionic radius, such as 130 K with Pr, 200 K with Nd, 400 K with Sm, and 460 K with Eu in RNiO3.14Unfortunately, the facile bulk synthesis of RNiO3 is currently a bottleneck to both the fundamental studies and exploit its unique properties in a wider range of device applications. The underlying cause for the synthetic challenge is the difficulty in stabilizing higher valent Ni3+ cations in the RNiO3 lattice (except LaNiO3), which is thermodynamically unstable under typical oxide growth conditions at low or ambient pressures.15 Most reported bulk RNiO3 synthesis methods use high pressure and high temperature conditions of 150 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bar of O2 and 800 to 1000 °C for several days.1,16 Such extreme conditions require the use of specialized equipment that can neither be developed in a common laboratory nor is readily available commercially. Therefore, developing strategies for low pressure bulk synthesis or stabilization is highly attractive. Thin films can be obtained without extreme annealing conditions through epitaxial stabilization on lattice-matched single crystal substrates.17–19 However, this condition restricts the thickness of the oxide layer that can be used without the onset of instability as well as the choice of substrates and the type of device that can be employed for experiments.20
000 bar of O2 and 800 to 1000 °C for several days.1,16 Such extreme conditions require the use of specialized equipment that can neither be developed in a common laboratory nor is readily available commercially. Therefore, developing strategies for low pressure bulk synthesis or stabilization is highly attractive. Thin films can be obtained without extreme annealing conditions through epitaxial stabilization on lattice-matched single crystal substrates.17–19 However, this condition restricts the thickness of the oxide layer that can be used without the onset of instability as well as the choice of substrates and the type of device that can be employed for experiments.20
The difficulty in low-pressure Ni3+ stabilization exacerbates as the ionic radius of the rare earth dopant decreases. Thermodynamic analysis by Jaramillo et al.15 shows that the Gibbs free energy of the formation of the RNiO3 phase increases with the decreasing ionic radius of the rare earth dopant. This may be related to the increased bending of the Ni–O–Ni bond angle, defined in terms of the tolerance factor (t), occurring in RNiO3 phases with smaller size R atoms, which may affect the cohesive energy of the crystal lattice. Table 1 summarizes the reported experimental conditions commonly employed for the synthesis of several bulk nickelate phases. The near-ambient pressure syntheses of bulk and nanostructured La-,21 Pr-22 and Nd23–25-doped RNiO3 have been reported through several techniques, such as the solid-state flux method,21 solid-state nitrate annealing,22 sol–gel precipitation and annealing,23 electrospinning,26 and hydrothermal synthesis.25 However, no report exists for the low-pressure synthesis of nickelates with rare earth elements past Nd in the lanthanide series (such as Sm, Eu, etc.). This is consistent with the phase diagrams reported by Jaramillo et al.15 for Pr-, Nd-, and SmNiO3, which indicate that the lowest O2 partial pressures required for the thermodynamic stabilization of the RNiO3 phase at a typical synthesis temperature of 700 °C are 10−2 bar, 1.2 bar, and 180 bar, respectively. Vibhu et al.27 reported that annealing citrate sol–gel of precursors at 850 °C for 48 h under oxygen flow synthesis can produce oxygen-deficient PrNiO3−δ. The phase was reported to be stable up to 950 °C in ambient air. Similarly, Tiwari and Rajeev28 reported the successful synthesis of the nearly stoichiometric composition of NdNiO2.92 with continuous annealing of sol–gel synthesized pellets at 800 °C in O2 at ambient pressure for 7 days. However, no synthesis condition exists for the ambient-pressure preparation of bulk SmNiO3. In the present study, we report two aging protocols to stabilize Ni3+ without the need for high-pressure annealing conditions. Our results show that both SmNiO3 and NdNiO3 phases can be crystallized/stabilized through either a high temperature aging process in the temperature range of 650–700 °C for 1–3 weeks or via slow aging of the as-synthesized sub-stoichiometric phase in ambient air for 6–8 months. Both conditions lead to an increase in the crystalline RNiO3 content of the lattice and give rise to sharp insulator-to-metal transitions involving a resistance change of at least 2–3 orders of magnitude.
| Bulk phase | Ambient pressure synthesis? | Synthesis method and conditions | Ref. |
|---|---|---|---|
| LaNiO3 | Yes | Solid-state flux method at 800 °C for 72 h or 1300 °C for 48 h in air | 21 |
| Electrospinning + annealing at 950 °C for 1 h | 12 | ||
| PrNiO3−δ | Yes | Solid-state nitrate annealing at 850 °C for 48 h under O2 flow | 22,27 |
| NdNiO3 | Yes | Sol–gel precipitation and annealing at 650 °C in O2 for 5 days | 23 |
| Hydrothermal synthesis + annealing at 400 °C for 3 h. | 25 | ||
| Electrospinning + annealing at 800 °C under O2 flow for 4 h | 26 | ||
| High pressure synthesis | |||
| NdNiO3−δ: solid state precursor/sol gel preparation + annealing at 50 bar O2 for 25 h | 33 | ||
| SmNiO3 | No | High pressure synthesis Non-stoichiometric oxygen deficient: Solid state precursor/sol gel preparation + 900 °C annealing at 50 bar O2 for 25 h | 33 |
| Stoichiometric: solid state precursor/sol gel preparation + 1000 °C annealing at 150–200 bar O2 for several days | 16 | ||
| EuNiO3 and other RNiO3 | No | EuNiO3: Solid state precursor/sol gel preparation + 1000 °C annealing at 200 bar O2 for 3 days | 14 |
Others: Solid state precursor/sol gel preparation + 950 °C annealing at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bar O2 for 12 minutes 000 bar O2 for 12 minutes |
1 |
2. Experimental
Bulk nickelates of Sm and Nd doping were synthesized using a modified sol–gel method of Vassiliou et al.,23 which involved dissolving Sm(NO3)3·6H2O or Nd(NO3)3·6H2O along with Ni(NO3)2·6H2O and citric acid in 100 mL of deionized water in a 1:1:1.67 molar ratio. Next, 1.5 mL of 0.5 M ethylenediaminetetraacetic acid (EDTA) was added, and the pH was adjusted to 7 using NH4OH. The solution was heated to 80 °C with constant stirring until most of the water evaporated and a gel was formed. The resulting gel was dried at 100 °C for 12 hours, ground into a powder, and further annealed at 400 °C for 6 hrs. The resulting powder was pelletized under 4 metric tons of force for 10 minutes and subsequently annealed again at 700 °C for 12 hours.The composition of the nickelate pellets was determined by X-ray diffraction (XRD), which was recorded using a PANalytic X’Pert Pro diffractometer using Cu Kα radiation. We applied the Rietveld refinement to the diffraction patterns using the FULLPROF program and the theoretically-calculated diffraction patterns of possible phases from the Materials Project Database. Metal–insulator transitions were recorded by measuring the two-probe resistance on the sintered pellets using a home-built liquid nitrogen-cooled temperature stage.
3. Results and discussion
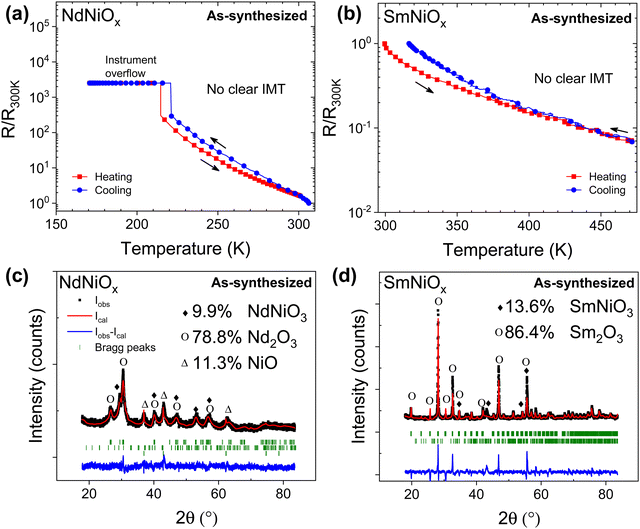
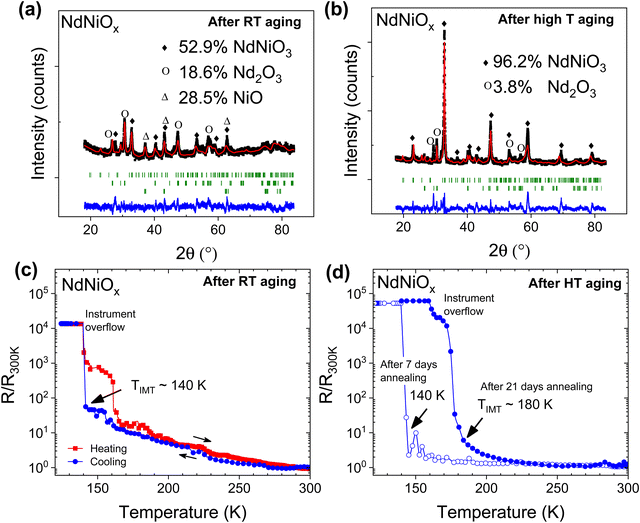
Fig. 1a and b show the resistance profiles of as-synthesized Nd-doped (NdNiOx) and Sm-doped (SmNiOx) nickelate pellets as a function of temperature, respectively. Neither samples exhibit a sharp temperature-induced IMT at expected temperatures of ∼200 K and 400 K, respectively. However, such a profile is similar to that reported in the literature for these nickelates synthesized under low pressure conditions.6,15 The composition of the crystalline phases of the as-synthesized pellets was determined by the Rietveld refinements of the measured XRD patterns and is shown in Fig. 1c and d. The fitting parameters and results are summarized in Table S1 in the ESI.† The points in the spectra represent the experimental data while the solid lines correspond to the fitted pattern. The green bars under the coordinate system are the calculated Bragg positions of different phases. The ‘noise-like’ curve below the green bars represents the difference between the experimental and calculated data. The refinement results show that the predominant crystalline phase in the as-synthesized pellet is the cubic Sm2O3 and trigonal Nd2O3 phases in Sm-doped and Nd-doped nickelate pellets, respectively, with only 10–15% of the RNiO3 phase. It is likely that at the low synthesis temperature, either the RNiO3 phase formation was not completed within the synthesis time due to the difficulty in stabilizing Ni3+ or the as-formed RNiO3 phase is amorphous and, therefore, not detectable by XRD. The latter is very likely to have occurred because the composition of crystalline phases does not account for the total Ni content of the sample.To promote the crystallization of the RNiO3 phase, we tested the effect of aging on the as-synthesized pellets using two methods, which involved: (i) high temperature (HT) aging from 650 °C to 700 °C for 1–3 weeks under ambient air and (ii) aging of the pellets at room temperature (RT) in a laboratory air environment for 6–8 months, followed by annealing at 700 °C for 12 hours. The compositional and electrical characteristics of both sets of samples were determined, which are summarized in Fig. 2 and 3 and Table 2. According to the Rietveld refinement of the XRD patterns, the crystalline RNiO3 content in both Sm- and Nd-doped samples increased with aging under both sets of conditions. In Nd-doped pellet (NdNiOx), the weight percent of the crystalline NdNiO3 phase increased from ∼10% in the as-synthesized sample to 53% with aging at RT (Fig. 2a). However, a higher improvement was seen in samples that underwent HT aging, wherein the crystalline NdNiO3 concentration increased from 10% to 61.7% after 7 days of continuous annealing and further increased to 96.2% upon annealing for two additional weeks (Fig. 2b). Thus, nearly phase-pure, fully crystallized NdNiO3 can be achieved through accelerated aging without the need for high-pressure annealing conditions. The obtained RNiO3 phase is likely oxygen-deficient. This result confirms the prior work of Tiwari and Rajeev,28 who reported the successful synthesis of the nearly stoichiometric composition of NdNiO2.92 with continuous annealing of sol–gel synthesized pellets at 800 °C in an O2 environment at ambient pressure for 7 days. The diffractogram indicates the sample to consist of an orthorhombically distorted perovskite structure with a Pnma space group symmetry. The refined lattice parameters were a = 5.41635 Å, b = 5.55054 Å, and c = 7.68114 Å.3 These values are very close to but slightly greater than values observed in high-pressure synthesized bulk samples whose values are a = 5.3891 Å, b = 5.3816 Å and c = 7.6101 Å.1
| Annealing conditions | % of RNiO3 (R = Nd or Sm) | % of R2O3 | % of NiO |
|---|---|---|---|
| NdNiOx | |||
| Pristine | 9.9 | 78.8 | 11.3 |
| RT aging | 52.9 | 28.5 | 28.52 |
| 650 °C for 7 days | 64.4 | 35.6 | |
| 650 °C for 7 + 14 days | 96.2 | 3.8 | |
| SmNiOx | |||
| Pristine | 13.64 | 86.36 | |
| RT aging | 42.9 | 57.1 | |
| 650 °C for 7 days | 34.13 | 65.87 | |
| 650 °C for 7 + 14 days | 30.60 | 69.40 | |
| 650 °C for 28 days | 39.71 | 60.29 | |
| 700 °C for 4 days | 43.18 | 56.82 | |
| 700 °C for 4 + 5 days | 49.31 | 50.69 | |
With the increase in the NdNiO3 content, the aged Nd-doped sample showed a well-defined and sharp IMT at temperatures between 140 K and 180 K with more than 4 orders of magnitude change in the electrical resistance (Fig. 2c and d). In both the RT-aged sample and the HT aged sample that were annealed for 7 days, the TIMT occurred at 140 K. However, in the HT-aged sample with the highest NdNiO3 content of 96%, the TIMT occurred at 180 K, which is close to the 180–200 K reported in earlier studies.14,28
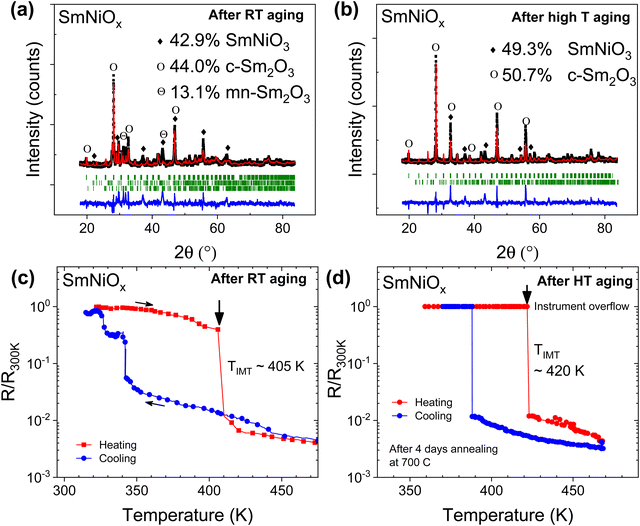
Similar to NdNiOx, both aging methods led to a substantial increase in the SmNiO3 concentration in Sm-doped pellets (Fig. 3a and b). The weight percent of SmNiO3 increased from ∼14% in the as-synthesized sample to 43% with aging at RT and to 34% upon HT-annealing at 650 °C for 1 week. The Rietveld fitting analysis indicates that a monoclinic (mn) Sm2O3 phase at a concentration of 13.1% may be present along with cubic (c) Sm2O3 (44%) in RT-aged Sm-doped pellets. Increasing the annealing time to 4 weeks did not significantly increase the composition of SmNiO3 (39.7%). Instead, it was found that annealing at 700 °C for 9 days could increase the RNiO3 composition close to 50%. The XRD refinement results indicate that this SmNiO3 phase consists of an orthorhombically distorted perovskite structure with a Pnma space group symmetry (Fig. 3a and b). The refined lattice parameters were a = 5.8256 Å, b = 4.9550 Å, and c = 7.7341 Å, which are somewhat higher than the values of a = 5.3283 Å, b = 5.4374 Å and c = 7.5675 Å reported for high-pressure synthesized bulk SmNiO3.16 The lower wt% of RNiO3 in Sm-doped pellets compared to Nd-doped pellets after aging points to the increasing difficulty in stabilizing Ni3+ as the ionic radius of the rare-earth dopant decreases. Furthermore, SmNiO3 also required a longer aging time of 6 to 8 months for stabilization, while NdNiO3 required a RT aging time of only 1 to 4 weeks. Attempts at decreasing the aging time by either increasing or decreasing the ambient humidity had no effect on the aging process (data not shown). Despite the mixed phase with a lower RNiO3 content compared to that seen in Nd-doped pellets, aged Sm-doped pellets showed a very well-defined and sharp IMT at temperatures between 400 K and 420 K (Fig. 3c and d) with a resistance change of 102–103, which is comparable to the 102 resistance change seen for high-pressure synthesized bulk16 and thin films.15
While the XRD results indicate the formation of the crystalline RNiO3 phase with aging, X-ray photoemission spectroscopy (XPS) was performed to confirm the increase in the Ni3+ content. Unlike XRD that only probes the crystalline phases, composition changes probed through XPS reflect changes in both amorphous and crystalline phases. Fig. 4 compares the Ni 2p3/2 core-level XPS spectrum of as-synthesized and aged Nd- and Sm-doped pellets. The extrinsic loss structure of each spectrum was subtracted using a Shirley-type correction. The Ni 2p3/2 peak was fit using multiplet envelope peaks of theoretically predicted29,30 free Ni2+ and Ni3+ ions using the previously reported procedure of Biesinger et al.31 and Qi et al.32 Fitting was performed by constraining both the binding energy (BE) positions and the full-width-at-the-half maximum (FWHM) of the peaks to the same values between different spectra, and is summarized in Table S2 in the ESI.† In mixed-valent nickel oxides, the predominant Ni2+ peaks appear at a lower BE (853.7) while the peaks of Ni3+ occur at higher BEs (854.6, 855.3, and 856.5 eV).31 The multiplet-fitting analysis shows that the Ni3+ contents of Nd- and Sm-doped pellets increase with both RT- and HT-aging. In RT-aged Nd-doped pellet, the Ni3+/Ni2+ ratio increased modestly from 2.2 to 2.6. In this sample, XRD analysis indicated an increase in the NdNiO3 content from 9% to 52.9%. However, a larger increase in the Ni3+/Ni2+ concentration ratio from 1.2 to 3.5 was observed in the Sm-doped sample, in which XRD analysis showed an increase in the SmNiO3 content from 13.6% to 49.3%. In line with this, the O/Ni ratio in Sm-doped pellet increased by 160% after HT-aging. We note that the particulate nature of the samples in the present study prevented the evaluation of relative changes in the Ni and O stoichiometry from the integrated peak areas. However, the comparative analysis of the O/Ni ratio between pristine and aged samples can still be performed. These results suggest that aging promotes both the formation of RNiO3 and crystallization of amorphous RNiO3 present in the as-synthesized sample, and thus lead to an increase in the Ni3+ content relative to the Ni2+ content through oxygen incorporation into the lattice. The valence band (VB) spectra of both as-synthesized and aged samples show them to be p-type semiconductors (Fig. S1 in the ESI†).
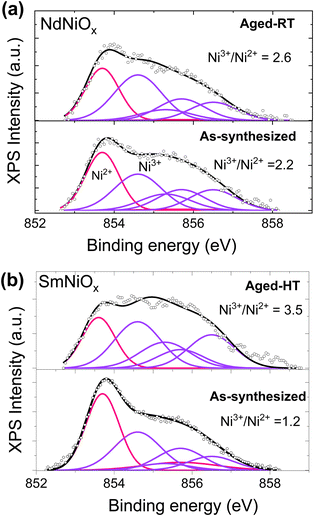 | ||
| Fig. 4 XPS spectra of the Ni 2p3/2 core-level spectra of Nd-doped (a) and Sm-doped nickelate pellets (b) before and after aging. Each spectrum was fitted with Ni2+ and Ni3+ multiplet envelopes using the procedure of Biesinger et al.31 The summary of the fitting analysis of the fitted peaks and their peak energy positions, areas, and FWHMs is given in Table S2 in the ESI.† | ||
Based on the XRD and XPS results, we briefly consider the mechanism of RNiO3 stabilization. The formation/decomposition reaction of RNiO3 can be written as
| 0.5R2O3 + Ni2+O + 0.25O2 ↔ RNi3+O3 | (R1) |
4. Conclusion
In summary, ambient-pressure aging, involving either a slow ambient-air oxidation for 6–8 months or an accelerated aging at a higher temperature of 650 °C for 1–3 weeks, of bulk Sm-doped and Nd-doped nickelate pellets prepared by the conventional solid-state preparation technique can stabilize Ni3+ and form the RNiO3 phase. The Rietveld refinement analysis of crystalline phases shows a composition as high as 96 wt% of NdNiO3 and 49.3 wt% of SmNiO3 can be obtained through aging. Consequently, sharp well-defined insulator-to-metal transitions (IMTs) involving resistance changes of 102–103 could be achieved in mixed-phase samples, which is comparable to that seen in high-pressure synthesized samples, and may be of practical importance in many device applications. Further work is needed to explore how aging helps in stabilizing nickelates doped with R cations of even lower ionic radii than Sm, such as Eu and Y, and are currently underway. The preliminary results indicate that they require even longer annealing time, spanning several weeks, than SmNiO3 for stabilization.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
N. S. and X. L. performed the experiments. V. C. wrote the manuscript. All the authors contributed towards data analysis and manuscript preparation.Conflicts of interest
All the authors declare that they have no conflicts of interest.Acknowledgements
The authors would like to thank the National Science Foundation, DMR award (No: 1709649), and Rensselaer Polytechnic Institute (RPI) for financial support. N. S. and X. L. also gratefully acknowledge the partial support of the Howard P. Isermann fellowship provided by the Department of Chemical and Biological Engineering at RPI.References
- G. Demazeau, A. Marbeuf, M. Pouchard and P. Hagenmuller, J. Solid State Chem., 1971, 3, 582–589 CrossRef CAS.
- M. L. Medarde, J. Phys.: Condens. Matter, 1997, 9, 1679 CrossRef CAS.
- J. Chaloupka and G. Khaliullin, Phys. Rev. Lett., 2008, 100, 016404 CrossRef PubMed.
- D. Li, K. Lee, B. Y. Wang, M. Osada, S. Crossley, H. R. Lee, Y. Cui, Y. Hikita and H. Y. Hwang, Nature, 2019, 572, 624–627 CrossRef CAS PubMed.
- V. Scagnoli, U. Staub, A. Mulders, M. Janousch, G. Meijer, G. Hammerl, J. Tonnerre and N. Stojic, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 100409 CrossRef.
- L. Wang, K. A. Stoerzinger, L. Chang, J. Zhao, Y. Li, C. S. Tang, X. Yin, M. E. Bowden, Z. Yang and H. Guo, Adv. Funct. Mater., 2018, 28, 1803712 CrossRef.
- Z. Li, Y. Zhou, H. Qi, Q. Pan, Z. Zhang, N. N. Shi, M. Lu, A. Stein, C. Y. Li, S. Ramanathan and N. Yu, Adv. Mater., 2016, 28, 9117–9125 CrossRef CAS PubMed.
- L. Wang, L. Chang, X. Yin, L. You, J.-L. Zhao, H. Guo, K. Jin, K. Ibrahim, J. Wang, A. Rusydi and J. Wang, Appl. Phys. Lett., 2017, 110, 043504 CrossRef.
- J. Shi, S. D. Ha, Y. Zhou, F. Schoofs and S. Ramanathan, Nat. Commun., 2013, 4, 2676 CrossRef PubMed.
- L. Xuchen, X. Tingxian and D. Xianghong, Sens. Actuators, B, 2000, 67, 24–28 CrossRef CAS.
- R. N. Singh, A. N. Jain, S. K. Tiwari, G. Poillerat and P. Chartier, J. Appl. Electrochem., 1995, 25, 1133–1138 CAS.
- D. K. Hwang, S. Kim, J.-H. Lee, I.-S. Hwang and I.-D. Kim, J. Mater. Chem., 2011, 21, 1959–1965 RSC.
- J. B. Goodenough, Rep. Prog. Phys., 2004, 67, 1915–1993 CrossRef CAS.
- J. B. Torrance, P. Lacorre, A. I. Nazzal, E. J. Ansaldo and C. Niedermayer, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 8209–8212 CrossRef CAS PubMed.
- R. Jaramillo, F. Schoofs, S. D. Ha and S. Ramanathan, J. Mater. Chem. C, 2013, 1, 2455–2462 RSC.
- P. Lacorre, J. B. Torrance, J. Pannetier, A. I. Nazzal, P. W. Wang and T. C. Huang, J. Solid State Chem., 1991, 91, 225–237 CrossRef CAS.
- M. A. Novojilov, O. Y. Gorbenko, I. E. Graboy, A. R. Kaul, H. W. Zandbergen, N. A. Babushkina and L. M. Belova, Appl. Phys. Lett., 2000, 76, 2041–2043 CrossRef CAS.
- A. Kaul, O. Gorbenko, I. Graboy, M. Novojilov, A. Bosak, A. Kamenev, S. Antonov, I. Nikulin, A. Mikhaylov and M. Kartavtzeva, MRS Proc., 2011, 755, DD7.1 CrossRef.
- A. A. Bosak, A. A. Kamenev, I. E. Graboy, S. V. Antonov, O. Y. Gorbenko, A. R. Kaul, C. Dubourdieu, J. P. Senateur, V. L. Svechnikov and H. W. Zandbergen, Thin Solid Films, 2001, 400, 149–153 CrossRef CAS.
- C. Girardot, S. Pignard, F. Weiss and J. Kreisel, Appl. Phys. Lett., 2011, 98, 241903 CrossRef.
- A. Wold, B. Post and E. Banks, J. Am. Chem. Soc., 1957, 79, 4911–4913 CrossRef CAS.
- V. Vibhu, A. Flura, A. Rougier, C. Nicollet, S. Fourcade, T. Hungria, J.-C. Grenier and J.-M. Bassat, J. Energy Chem., 2020, 46, 62–70 CrossRef.
- J. K. Vassiliou, M. Hornbostel, R. Ziebarth and F. J. Disalvo, J. Solid State Chem., 1989, 81, 208–216 CrossRef CAS.
- S. Kim, D. H. Truyen, T. H. Kim and C. W. Bark, J. Nanosci. Nanotechnol., 2020, 20, 4239–4243 CrossRef CAS PubMed.
- M. Sivakumar, K. Pandi, S.-M. Chen, Y.-H. Cheng and M. Sakthivel, New J. Chem., 2017, 41, 11201–11207 RSC.
- M. S. Medina, B. N. Ramirez, P. M. G. L. Ferreira, H. P. Huang, A. Zenatti, A. J. C. Lanfredi and M. T. Escote, Nano Express, 2020, 1, 010028 CrossRef.
- V. Vibhu, A. Flura, C. Nicollet, S. Fourcade, N. Penin, J.-M. Bassat, J.-C. Grenier, A. Rougier and M. Pouchard, Solid State Sci., 2018, 81, 26–31 CrossRef CAS.
- A. Tiwari and K. P. Rajeev, Solid State Commun., 1998, 109, 119–124 CrossRef.
- R. P. Gupta and S. K. Sen, Phys. Rev. B: Condens. Matter Mater. Phys., 1974, 10, 71–77 CrossRef CAS.
- R. P. Gupta and S. K. Sen, Phys. Rev. B: Condens. Matter Mater. Phys., 1975, 12, 15–19 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, L. W. M. Lau, A. Gerson and R. S. C. Smart, Surf. Interface Anal., 2009, 41, 324–332 CrossRef CAS.
- Q. Wang, A. Puntambekar and V. Chakrapani, Nano Lett., 2016, 16, 7067–7077 CrossRef CAS PubMed.
- I. V. Nikulin, M. A. Novojilov, A. R. Kaul, S. N. Mudretsova and S. V. Kondrashov, Mater. Res. Bull., 2004, 39, 775–791 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00415a |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2022 |