Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Robust and high barrier thermoplastic starch – PLA blend films using starch-graft-poly(lactic acid) as a compatibilizer†
Binh M.
Trinh
,
Debela T.
Tadele
 and
Tizazu H.
Mekonnen
and
Tizazu H.
Mekonnen
 *
*
Department of Chemical Engineering, Institute of Polymer Research, Waterloo Institute of Nanotechnology, University of Waterloo, Waterloo, ON, Canada. E-mail: tmekonnen@uwaterloo.ca
First published on 24th June 2022
Abstract
This work reports the design of a single-layer robust and good barrier sustainable films by using graft polymer compatibilizers. A starch-graft-poly(lactic acid) (St-PLA) copolymer was synthesized and its efficacy as a compatibilizer of thermoplastic starch (TPS)/PLA blends was studied in detail. The St-PLA copolymer is synthesized using a tin(II) 2-ethylhexanoate initiator, employing ring-opening surface graft polymerization. The microstructure and morphology development of the blends are studied using a polarized optical microscope (POM), scanning electron microscope (SEM), differential scanning calorimetry (DSC), and rheology investigations. Results indicate that the addition of the St-PLA at 2.5 wt% and 5 wt% in the TPS/PLA blend via a melt extrusion process has tremendously improved the compatibility of TPS/PLA blends. Moreover, the PLA crystal spherulite size demonstrates considerable reduction with the addition of the St-PLA compatibilizer despite maintaining similar melt processing parameters. As a result of the improvement in microstructure and morphology changes, the compatibilized blends exhibit outstanding oxygen barrier and thermomechanical properties. Thus, the compatibilization effects provided by St-PLA offer a non-reactive approach to improve the overall morphology and physical properties of the immiscible TPS–PLA blends. Such blend can be used as a single layer flexible packaging material and allows repeated recycling.
1. Introduction
The growing movement to impose strict environmental regulations on single-use plastics has accelerated innovation interest in sustainable materials as replacement for polyolefins and other traditional plastics.1 The use of natural macromolecules to design biodegradable or compostable plastics offers potential solutions to concerns associated with the production and end-of life of synthetic plastics.2–4 Among the various sustainable polymers, poly(lactic acid) (PLA), poly(butylene succinate) (PBS), and poly(hydroxyalkanoate) (PHA), thermoplastic starch (TPS) attracted considerable interest. Other synthetic biodegradable polymers, such as poly(butylene adipate-co-terephthalate) (PBAT), poly(e-caprolactone) (PCL), and poly(glycolic acid) (PGA), have also been extensively investigated as sustainable polymer alternatives.4,5PLA is perhaps the most investigated and utilized bio-based and compostable bioplastic produced from renewable resources, such as corn, sugarcane, bagasse, etc. It is appealing due to its ease of processability using conventional processing techniques, thermal stability, good mechanical properties, excellent moisture barrier properties, and acceptable cost structure.6 However, the extensive utilization of PLA to displace polyolefins in packaging and engineering applications is still limited due to its high brittleness, poor oxygen barrier properties, and high cost compared to conventional polyolefin-based plastics.7,8 To enhance the gas barrier, conductivity, thermal, and physic-mechanical properties of immiscible blends, the incorporation of bio-based fillers such as modified nanoclays and carbon black particles have been employed as alternate solutions to improve thermoplastic composites as packaging materials.9,10 Another commonly used method is to combine PLA with other biopolymers that can complement PLA's weakness. For instance, thermoplastic starch (TPS) is an excellent candidate to complement the limitations of PLA in packaging and other applications of commercial interest owing to its inherent attributes of biodegradability, abundancy, high oxygen barrier properties and most importantly, cost-structure.11–13 Furthermore, many studies have found that a blend of TPS and PLA has the potential to bring a balance in the desirable complementary properties between both materials. Polymer blends can either be miscible or immiscible, in which the miscibility of polymer blends refers to a homogenous morphology with a negative Gibb's free energy of mixing.14 Binary blends of TPS and PLA are immiscible due to the poor interfacial tension and phase adhesion and rheology factors, which results in poor microstructure development. Thus, producing a stable immiscible blend and controlling the microstructure of TPS and PLA remained a remarkably challenging task. In addition to process optimization, the use of additives, such as compatibilization agents can mitigate such challenges.
Compatibilizers for immiscible blends can be classified into two main categories: (1) in situ: reactive compatibilizers containing reactive functional groups (maleic anhydride, epoxy, etc.) that can interconnect different polymer phases by generating chemical bonds between functional groups (hydroxyl, carboxyl, etc.) of the polymer chains; (2) ex situ: non-reactive compatibilizers (typically premade copolymer) that are miscible to all constituents in the blends.15,16
Several studies have reported the improvement in the mechanical properties of PLA/TPS blends by increasing the interfacial adhesion via utilizing various in situ compatibilizers, with various degrees of success.7,17–20 Huneault et al. successfully prepared PLA/TPS blends using maleic anhydride compatibilizer, resulting in a considerably ductile blend over the uncompatibilized alternative.17 Another study by Turco et al., demonstrated biodegradable films of PLA thermoplastic corn starch blend using epoxidized oil as an in situ compatibilizer.19 While the interfacial adhesion between the hydrophobic PLA and hydrophilic TPS improved as a result of the epoxidized oil, phase separation between the PLA and TPS resumed with secondary processes. As a result, the compatibilization did not enhance mechanical properties. Shi et al. utilized a different in situ approach of synthesizing glycidyl methacrylate grafted poly(ethylene octane) (GPOE) to be used as the third component of a PLA/TPS/GPOE ternary blend, which showed significant improvement in mechanical morphology, water absorption, and degradation properties.20
On the other hand, the utilization of ex situ starch-graft-copolymer in immiscible TPS/PLA blend has been previously proposed, the information obtained is still limited. Recently, there have been numerous attempts to attach PLA chains onto anhydroglucose units (AGU) (e.g., starch, nanocellulose, carboxymethyl cellulose) by utilizing “grafting from” technique to grow the oligomer chains from lactic acid (LA) or L-lactide and generate graft copolymers.21–24 It is believed that, in theory, the PLA block from the graft copolymer can be miscible to the PLA phase, while the starch block can be miscible to the TPS phase in the blend. Thus, making the graft copolymer Starch-graft-PLA (St-PLA) an interesting and viable option to improve the miscibility of TPS/PLA blend. To the best of our knowledge, investigation on the effects of ex situ St-PLA compatibilizer on the overall morphology, crystallinity, and barrier properties of TPS/PLA blend has not been explored in previous research. Thus, this work offers an in-depth investigation on the chemical characterization of the ex situ compatibilizer St-PLA synthesized through the ring open polymerization (ROP) reaction and carries out a comprehensive study on the interaction between TPS and PLA polymer chains bridged together by the ex situ compatibilizer St-PLA. The compatibilized blend's morphology, rheology, crystallinity, and physical properties as the results of incorporating the compatibilizer were also reported.
2. Methods and materials
2.1. Materials
Corn starch (containing approximately 73% amylopectin and 27% amylose) was supplied by Sigma Aldrich, USA. Polylactide (PLA) resin (ρ = 1.24 g cm−3, MW = 168![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) in pellet form (Ingeo™ biopolymer 4043D general grade) was obtained from Nature Works LLC, Minnetonka, MN, USA. L-Lactide monomer was purchased from TCI America. Other chemicals such as tin(II) 2-ethylhexanoate (Sn (Oct)2), potassium bromide (KBr) in powder form, sodium hydroxide (NaOH) pellets, 10.2 N hydrochloric acid (HCl), dimethyl sulfoxide (DMSO) (99.9%), deuterated DMSO (DMSO-d6), methanol (MeOH), ethanol (EtOH) (99.0%), and rubbing alcohol (2-propanol) (99.0%), were all purchased from Sigma Aldrich, USA. All chemicals were used as received.
000 g mol−1) in pellet form (Ingeo™ biopolymer 4043D general grade) was obtained from Nature Works LLC, Minnetonka, MN, USA. L-Lactide monomer was purchased from TCI America. Other chemicals such as tin(II) 2-ethylhexanoate (Sn (Oct)2), potassium bromide (KBr) in powder form, sodium hydroxide (NaOH) pellets, 10.2 N hydrochloric acid (HCl), dimethyl sulfoxide (DMSO) (99.9%), deuterated DMSO (DMSO-d6), methanol (MeOH), ethanol (EtOH) (99.0%), and rubbing alcohol (2-propanol) (99.0%), were all purchased from Sigma Aldrich, USA. All chemicals were used as received.
2.2. Methods
| Copolymer (compatibilizer) | Starch (g) | Lactide (g) |
|---|---|---|
| St-PLA 1 | 10 | 2.5 |
| St-PLA 2 | 10 | 5 |
| St-PLA 3 | 10 | 10 |
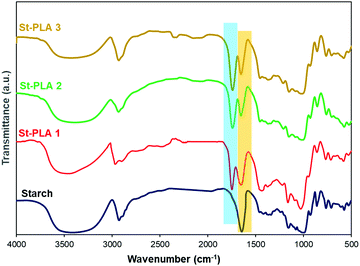
Fourier transform infrared spectroscopy (FTIR). The grafting of PLA chains onto corn starch particles was characterized at ambient temperature using FTIR (Nicolett 6700, Thermo Scientific Inc.). Native and modified starch samples for the IR were prepared by mixing the powder samples with KBr salt (2 mg sample and 200 mg KBr) and pressing them into pellets. On the other hand, PLA was prepared by adding the PLA in chloroform solvent directly to a potassium bromide (KBr) salt pellet and dried under vacuum overnight. FTIR scans, in transmittance mode, were recorded in the range of 4000 to 500 cm−1 under the same conditions as the background.
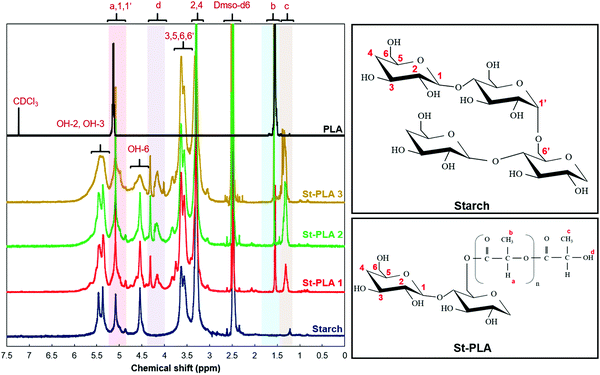
Proton nuclear magnetic resonance (H-NMR). Proton nuclear magnetic resonance (H-NMR) spectra of the baseline and grafted starch (St-PLA) at different modification levels were recorded to analyze the chemical changes resulting from the reaction. For this, the samples were dissolved in deuterated dimethyl sulfoxide (DMSO-d6) at 50 °C and sealed in 5 mm NMR analysis tubes with a ratio of 10 mg solid sample to 0.7 mL solvent. The H-NMR spectra were collected from 0 to 7 ppm using Bruker 500 MHz high-resolution NMR (Bruker-SpectroSpin 500 MHz Ultra shield, Bruker Corporation, MA).
Thermogravimetric analysis (TGA). The impact of PLA grafting on the thermal degradation behaviour of starch was evaluated by conducting TGA on pristine and modified samples. The tests were carried out on dried samples using TA instrument TGA Q500. The test was carried out from 30 to 500 °C at 10 °C min−1 heating rate and a nitrogen flow of 20 mL min−1.
Differential scanning calorimetry (DSC). The thermal transitions profiles of starch, TPS, PLA, and TPS/PLA (non-compatibilized and compatibilized) blends were analyzed using DSC (Q2000, TA instrument, USA). For the test, accurately weighted samples of 5 mg were placed in a sealed aluminum pan, and the test was run in a nitrogen atmosphere of 50 mL min−1. An empty aluminum pan was used as a reference for all scanning measurements. The thermal profiles were obtained from 10 to 190 °C with the ramping rate of 10 °C min−1. For the blend film samples, specimens were first scanned from 20 to 200 °C at a ramping rate of 10 °C min−1 to remove the thermal profile. Next, the samples were kept at 200 °C for 5 minutes, cooled down to −75 °C at cooling rate of 10 °C min−1, and heating up again from −80 to 200 °C at the ramping rate of 10 °C min−1. The percentage crystallinity of PLA phases within the blend was determined using eqn (1).
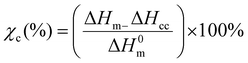 | (1) |
Water contact angle (WCA). Using a custom-built optical sessile drop system, the water contact angle (WCA) measurement of starch and St-PLAs was carried out. About 5 μL of the water droplet was dropped onto the prepared pellets, and images were taken after 10 seconds. A built-in Matlab tool was used to measure the contact angles of the water droplets.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, respectively.
000 g mol−1, respectively.
| Sample | Blend compositions | Compatibilizer | |
|---|---|---|---|
| TPS (%) | PLA (%) | St-PLA2 (%) | |
| TPS | 100 | 0 | — |
| TPS/PLA | 70 | 30 | — |
| TPS/PLA/1%St-PLA | 70 | 30 | 1 |
| TPS/PLA/2.5%St-PLA | 70 | 30 | 2.5 |
| TPS/PLA/5%St-PLA | 70 | 30 | 5 |
| PLA | 0 | 100 | — |
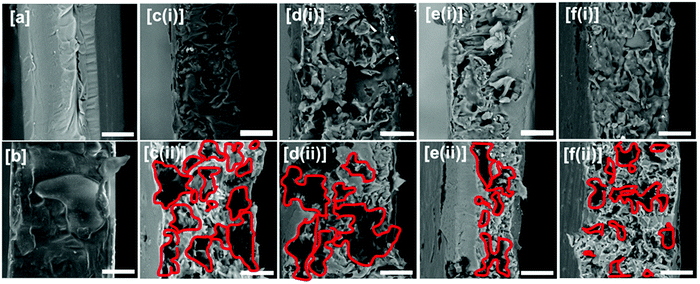
Scanning electron microscopy. The fractured surface morphologies of neat and compatibilized blend films were investigated using FEI SEM (Quanta FEG-SEM 250, Oxford Instrument) at an accelerating voltage of 20 kV. The fractured surfaces were coated with gold sputtering and mounted onto stubs with double-sided carbon adhesive tape prior to scanning.
To further determine the phase morphologies of the immiscible blend, phase-extracted samples were prepared. The cryofractured samples of each blended formulation was immersed in 10.2 N HCl solution at 25 ± 2 °C for 8 h to remove the TPS phase. The TPS leached specimens were then rinsed with water and ethanol and dried at 70 °C for 48 h.
Polarizing optical microscope (POM). A polarizing optical microscope (Olympus BX53M polarizing optical microscope, USA) was employed to determine the crystal morphologies of TPS–PLA blends with and without the co-grafted-compatibilizer St-PLA. Pictures were collected at 20× objective magnifications.
Rheometer. Rheology measurements, such as the complex viscosity, elastic modulus (G′), and viscous modulus (G′′) of the various emulsion formulations were studied at 180 °C using a stress-controlled rotational rheometer (HAAKE Mars 3, Thermo Scientific) with a parallel plate geometry and a gap of 1 mm between the plates. Rheology samples with diameters and thicknesses of 15 and 3 mm, respectively, were used for the measurement.
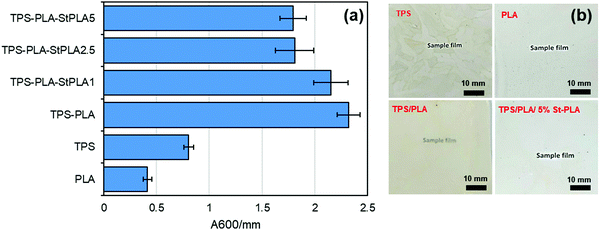
UV-Vis. The light transmittance of the samples was recorded using Cary 100 Bio UV-Vis's spectrophotometer at the visible wavelength of 600 nm to determine the transparency of the films. Opacity values were calculated by converting the transmittance at wavelength 600 nm to absorbance and dividing by the films’ thickness.
Barrier properties. The water vapor permeability of the film formulations was evaluated in accordance with ASTM E96/E96M-16. The water vapor transmission rates (WVT) were measured using barrier cups with an exposed area of 10 cm2. The barrier cups were filled with 7 mL distilled water (RH = 100%) with the film placed over the opening of the cups and tightly clamped with screw lids. The cups were then placed inside a sealed chamber at 23 °C and 30% relative humidity. The weight loss resulting from water permeation through the film was recorded at different time intervals up to seven days. WVT values (g m−2 h−1) were then determined using eqn (2):
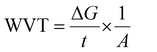 | (2) |
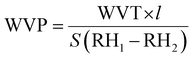 | (3) |
The oxygen permeability (OP) tests were performed using a customized bubble flow rate setup. Films (18.10 cm2) were used to seal a two-chamber cartridge attached to an oxygen gas source on one end and a bubble flow meter on the other end. The pressure difference between the two chambers was set at 5 psi, allowing the oxygen to permeate through the membrane. The flux of bubbles was determined by counting the time the bubbles took to travel 50 mL volumetric unit. The OP values (cm3 m m−2 day−1 Pa−1) was then calculated using eqn (4):
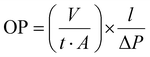 | (4) |
Tensile property testing. Tensile property testing was carried out using a Universal tensile testing unit (AGS-X series, Shimadzu, Japan) with a 500 N load cell according to ASTM D882-18. Films were cut into 70 × 10 mm test pieces with a gauge length of 50 mm. The sample's thickness was collected by taking the average values of 3 measurement points across each sample. Testing was conducted at room temperature and 5 mm min−1 strain rate. At least five samples were tested for each formulation, and the average was reported.
3. Results and discussions
3.1. Characterization of starch-graft-PLA copolymer
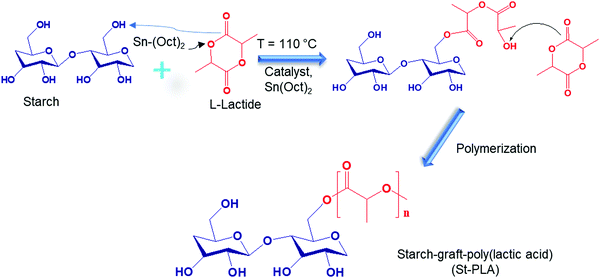
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) attributed to the grafting of PLA oligomers onto starch, as illustrated in Scheme 1. Other studies on the synthesis of PLA grafts reported the emergence of a similar peak.24,28 Another evidence for the grafting of lactic acid oligomer on starch was the progressive reduction of the broad hydroxyl vibration at 3600–3000 cm−1, indicating that (–OH) group was substituted. The stretching of PLA's –CH could overlap with starch's strong –CH stretching at 2930 cm−1. Peaks at 1650 cm−1, assigned to absorbed water molecules on starch's structure, reduced with the increase in the grafting of PLA, which could be associated with an increase in hydrophobicity. Overall, the results obtained from IR spectra indicate that the lactide was successfully grafted and polymerized into PLA oligomers on the anhydroglucose units of starch through ROP reaction with Sn (Oct)2 as the catalyst initiator.
O) attributed to the grafting of PLA oligomers onto starch, as illustrated in Scheme 1. Other studies on the synthesis of PLA grafts reported the emergence of a similar peak.24,28 Another evidence for the grafting of lactic acid oligomer on starch was the progressive reduction of the broad hydroxyl vibration at 3600–3000 cm−1, indicating that (–OH) group was substituted. The stretching of PLA's –CH could overlap with starch's strong –CH stretching at 2930 cm−1. Peaks at 1650 cm−1, assigned to absorbed water molecules on starch's structure, reduced with the increase in the grafting of PLA, which could be associated with an increase in hydrophobicity. Overall, the results obtained from IR spectra indicate that the lactide was successfully grafted and polymerized into PLA oligomers on the anhydroglucose units of starch through ROP reaction with Sn (Oct)2 as the catalyst initiator.
The NMR signals of commercial-grade PLA was also examined for comparison purpose. The PLA sample was dissolved in deuterated chloroform (CDCl3), which correspond to the NMR peak at 7.2 ppm, as shown on the PLA spectrum in Fig. 2. Furthermore, the PLA displayed two distinctive peaks at approximately 5.2 and 1.5 ppm, which correspond to the hydrogen atoms attached at the carbon backbone (position a) and the methyl group –CH3 (position b), respectively.33,34
The spectra of the synthesized St-PLA revealed additional signals that correspond to the grafted PLA oligomer, as well as the lactic acid monomer, on the AGUs. The emergence of an additional signal at approximately 1.5 ppm that was not observed in starch, and the overlapping signal intensity at about 5.2 ppm, were emanating from the grafted PLA oligomer on the AGU of starch. Similar peaks were also observed in the PLA spectra, implying that the PLA chains were successfully synthesized and grafted on the starch structure. Also, it is noteworthy to mention the gradual increase in the intensity and eventually overlapping of peaks at 5.20 ppm with the increase in L-lactide concentration, indicating the ring opening polymerization of PLA oligomers. The degree of substitutions (DS) of OH with the modifying agent can usually be determined via integrating anomeric proton peaks at 5.20 ppm and the hydroxyl protons from PLA oligomer chain end at 4.40–4.00 ppm. However, the DS for St-PLA sample was not feasible due to the interference of the overlapping peaks at 5.2 ppm. The presence of new peaks at around 4.4–4.2 ppm, as well as a peak at 1.3 ppm attributed to the protons at position c, were detected for only the St-PLA samples. It is suspected that since the synthesized PLA on the AGU unit has much shorter chain lengths compared to commercial PLA, the peaks at 1.3 ppm were potent in St-PLA samples while completely missing in the commercial PLA spectra. A similar observation was reported by Noivoil and Yoksan,35 in which they reported a similar peak at 1.3 ppm position corresponding to the methyl group proton at the PLA chains end. Thus, it was determined that the grafted PLA chains were oligomers instead of fully grown polymer chains. On the other hand, the emergence of a peak at 4.4–4.2 ppm, could plausibly correspond to the proton signal of O–H at position d. Thus, the NMR results confirmed the lactic acid grafting and subsequent polymerization onto the starch chain.
 | ||
| Fig. 3 (a) TGA spectra, (b) DTGA spectra, (c) DSC spectra, and (d) WCA of starch and St-PLA at different grafting level. | ||
The thermal profiles of St-PLA samples were further evaluated via DSC, and results are shown in Fig. 3(c). The DSC of the pristine starch presented a smooth line with no peaks, indicating the absence of transition phase (cold crystallization, melting point) in the thermal run. On the contrary, all the St-PLA samples exhibited an endothermic peak at around 150 °C, attributed to the melting of the PLA oligomers grafted onto starch. One notable observation was the lack of cold crystallization peaks in the St-PLA profiles. Based on their work on the grafting of PLLA onto starch Chen et al., suggested that the crystallization behaviours of the starch-PLLA depend on the PLLA length.21 Thus, in line with the NMR observation, the grafted PLA chains in this study did not produce many long-chain PLA, which explains the missing cold crystallization peaks expected in the St-PLA samples. Overall, the results from DSC indicated that PLA oligomers were successfully synthesized on the surface of the starch. However, the chains are short, which was demonstrated by the thermogram's lack of cold crystallization peaks.
3.2. Compatibilized blends of TPS–PLA
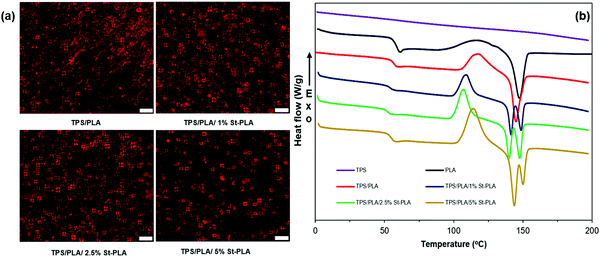
The crystalline structure of the TPS/PLA blends was further evaluated by monitoring the change in the thermal transitions provided by DSC. A summary of the various transition temperatures during the second heating, such as glass transition temperature (Tg), cold crystallization temperature (Tcc), melting temperature (Tm), cold crystallization enthalpy, melting enthalpy, and crystallinity are presented in Fig. 5(b) and Table S1 (ESI†). It was noted that the TPS behaved more like an amorphous material and did not exhibit any measurable thermal transition from the DSC. On the other hand, PLA displayed clear Tg, Tcc, and Tm, with a low crystallinity level (10.3%), indicating its semicrystalline behavior. It was interesting to observe that the TPS/PLA blend without compatibilizer exhibit only one melting peak (at 145 °C) similar to PLA's thermogram, while all the blends containing St-PLA exhibit two distinctive peaks. This was attributed to the two melting peaks of TPS/PLA corresponding to the two distinguished behaviours of PLA chains in the blend. The first peak around 140–143 °C was attributed to PLA entangled with TPS chains due to the enhanced interaction caused by the St-PLA. This crystalline structure would melt faster due to the ease of the chain segments to move to a lower energy level. A similar observation was reported by Zhang et al. on the reactive blend of PLA with natural rubber, in which the authors have observed two distinctive peaks in their compatible blend.36 Since the uncompatiblized TPS/PLA blend lacks compatibility, the lower temperature DSC thermogram was not observed. On the contrary, the second peak at 149–151 °C corresponds to the crystalline melting of PLA chains, similar to the existing peaks in PLA and TPS/PLA samples.
As shown in Table S1 (ESI†), the crystallinity has gradually reduced with the increasing concentration of St-PLA incorporated into the formulations. At 5 wt% St-PLA, the crystallinity of the blend has declined to only 1.86%. The observed reduction in crystallinity was in line with the POM imaging observations. Moreover, the reduction in crystallinity content within the polymers indicate a greater increase in the intensity of Tg (step height).37 Similarly, the blends containing the St-PLA compatibilizer exhibited gradual enhancement in the region of Tcc transition. Most likely, these peaks intensification are attributed to the additional enthalpy required for the nuclei to form crystalline structures due to the addition of the St-PLA copolymer compatibilizers. The enhancement in step height and cold crystallization peaks are most evident in the TPS/PLA/5% St-PLA sample, showing that the crystallinity of the compatibilized blend has diminished. Thus, it was determined that the improved dispersion of PLA in the TPS continuous phase could reduce the PLA crystallization due to the St-PLA compatibilizer interference.
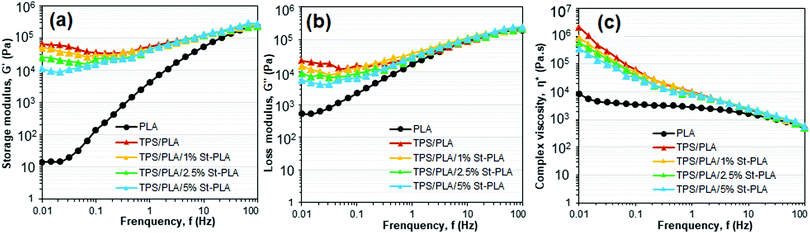 | ||
| Fig. 6 Rheology showing (a) storage modulus, (b) loss modulus, (c) and complex viscosity as function of frequency of PLA and TPS/PLA blends. | ||
Moreover, since the shearing deformation of TPS chains was easier to recover, it would inherently dissipate less energy and reduce the loss modulus. This is because TPS chains are not as mobile as PLA with glycerol as a plasticizer. On the other hand, the overall moduli of all blends did not change regardless of the compatibilizer content at higher frequency.
Fig. 6(c) displays the complex viscosity of neat PLA and various TPS/PLA blends at 180 °C. PLA exhibited characteristic Newtonian behaviour with the viscoelastic plateau at a lower frequency, while TPS/PLA blends displayed non-Newtonian shear thinning behaviour in the entire frequency region. The observed shear-thinning behaviour of the TPS/PLA blends was attributed to the dominant viscosity of the continuous TPS matrices due to forming a rigid three-dimensional starch network. Similar viscosity behaviour was noted in a recent investigation on the morphology–rheology relationship in co-plasticized TPS/PLA blend.38 The gradual decrease in the complex viscosity at lower frequency was observed with increasing incorporation of the St-PLA. As anticipated, the increased miscibility between the polymer chains of TPS and PLA due to interfacial bridging by St-PLA allows the chains to exhibit better movements at low frequency and reduce the overall intermolecular cohesive energy, which ultimately resulted in the complex viscosity reduction of the compatibilized samples. This reduction in complex viscosity trend can be correlated to diminishing in the crystalline structure of the blend. The drops in crystallites of TPS/PLA blend may break the ordered structures and facilitates mobility freedom for the branched PLA chains, which would lower the overall moduli and complex viscosity at lower shear rates. Overall, the dynamic rheology result gave crucial information on the compatibilization effect and processability of the TPS/PLA blend, which further supported the morphology observations.
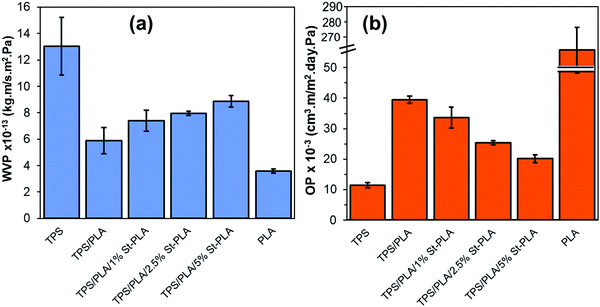 | ||
| Fig. 8 Comparison of (a) WVP values, and (b) OP values of neat TPS, PLA, non-compatibilized, and compatibilized TPS/PLA blends. | ||
It was also anticipated that the addition of St-PLA compatibilizer to the TPS/PLA blend would improve the overall moisture barrier properties of the films due to finer dispersion of PLA phase in the TPS matrices, as indicated from the results obtained from SEM and AFM analysis (Fig. S2, ESI†). However, contrary to expectation, the incremental addition of compatibilizer St-PLA in the blend formulation gradually increases the WVP of the TPS/PLA blend film, indicating that St-PLA diminished the water barrier properties of the blend films. The reason for this counterintuitive observation could be related to the reduction in the crystalline structures. A study conducted by Duan and Thomas investigated the correlation between the rate of crystallization of PLA and the solubility factor that contributes to the water vapour transmission rate (WVTR).42 The authors have found out that the crystalline spherulite can be treated as “impermeable” by water vapor, and that the solubility coefficient of the semi-crystalline PLA is the same as the solubility coefficient of the amorphous phase of PLA. Based on their investigations, it was concluded that the WVP is directly related to the crystalline spherulite structures. In this study, the results from POM and DSC suggested that the crystallinity of the TPS/PLA blend was reduced with the addition of St-PLA compatibilizer, which explained the increasing WVP as shown in Fig. 8(a).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ration PLA/TPS (blend compatibilized by oligo lactic acid–PLA.35 The researchers reported a reduction in the OP values by up to 64% compared to neat PLA and up to 20% compared to the same blend without compatibilizers.
1 ration PLA/TPS (blend compatibilized by oligo lactic acid–PLA.35 The researchers reported a reduction in the OP values by up to 64% compared to neat PLA and up to 20% compared to the same blend without compatibilizers.
Furthermore, the oxygen permeability experimental results were compared with the values predicted by the Maxwell and Bruggerman equations, which are among the most commonly used predictive models to determine the effective permeability of particulate filled continuous phase system.43–45 Maxwell's model assumes that the dispersed polymer phase consists of spherical solids that are homogeneous in the continuous phase, while Bruggeman equation utilized the concept of effective medium theory and is more applicable in randomly distributed dispersed phase systems. Therefore, the theoretical permeability (P) of TPS/PLA blend can be computed using either of Maxwell (eqn (5)) and Bruggeman (eqn (6)):
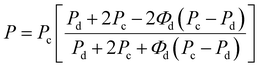 | (5) |
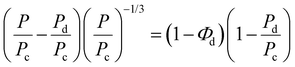 | (6) |
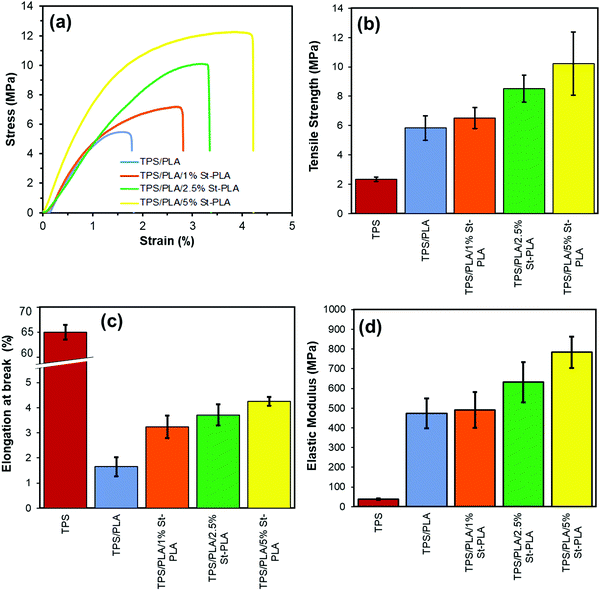
It was evident that the addition of the St-PLA compatibilizer had both reinforcing and plasticization effect on the TPS/PLA blend. At a loading level of only 1 wt%, the compatibilization effect was not observed as the amount was not significant enough to make a difference in mechanical properties. With the use of 5 wt% St-PLA compatibilizer, the tensile strength of the uncompatibilized TPS/PLA blend increased from 5.83 to 10.21 MPa, which was a 75% improvement. Moreover, the ductility of the blend has also considerably improved with the gradual increase in the St-PLA concentration, which indicate an enhancement in the toughness of the compatibilized blend. At the highest loading of St-PLA (5 wt%), the elongation at break of the binary blends increased from 1.65 to 4.25% (∼160% increase), showing significant improvement in the film's flexibility. This augmentation in the film flexibility showed a great potential to mitigate the brittleness caused by poor interfacial compatibility of the immiscible TPS and PLA materials. The elastic modulus, similar to tensile strength and flexibility, showed a tremendous improvement using the St-PLA compatibilizer. For instance, the use of 5 wt% St-PLA led to a 66% increase in the modulus compared to the uncompatibilized samples. Overall, extensive tensile property improvements were noted using the St-PLA ex situ compatibilizer, owing to the blends enhanced interfacial adhesion and phase dispersion.
4. Conclusion
Starch-graft-PLA (St-PLA) copolymer was successfully synthesized via the ROP reaction of lactide to deposit PLA oligomer on the starch backbone as confirmed via FTIR, NMR, DSC, and WCA evaluations. The use of optimal concentration of the St-PLA as a compatibilizer of TPS/PLA blend displayed outstanding compatibility and miscibility of the phases through interfacial bridging between the TPS and PLA chains. Consequently, the fabricated TPS/PLA films exhibited enhanced tensile properties in conjunction with superior oxygen barrier properties owing to the excellent distribution of PLA phases in the TPS matrices. Nevertheless, it was also noted that the bridging between TPS and PLA phases through the ex situ compatibilization reduced the crystalline morphology of PLA structures. While such crystal spherulite reduction of the PLA promoted increased flexibility and transparency of the fabricated films, it also caused an undesirable moisture barrier reduction. Overall, this research conclusively demonstrated the great potential of using St-PLA co-polymers as a sustainable compatibilizing agent of TPS/PLA blends. The studied compatibilized blend is expected to be compostable with substantial application potential for food, pharmaceutical, and biomedical packaging, owing to its outstanding oxygen barrier and other physicomechanical properties.Author contributions
Binh M. Trinh: methodology, writing – original draft, writing – review & editing, results analysis, and conceptualization. Debela T. Tadele: writing – original draft, writing – review & editing, and results analysis. Tizazu H. Mekonnen: conceptualization, writing – review & editing, resources, and supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The financial support of NSERC and Canada Foundation for Innovation is greatly appreciated. We would also like to thank Dr Arvind Gupta for the technical support he offered on this work.References
- R. Muthuraj and T. Mekonnen, Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends, Polymer, 2018, 145, 348–373, DOI:10.1016/j.polymer.2018.04.078.
- O. H. P. Gunawardene, C. Gunathilake, S. M. Amaraweera, N. M. L. Fernando, D. B. Wanninayaka, A. Manamperi, A. K. Kulatunga, S. M. Rajapaksha, R. S. Dassanayake, C. A. N. Fernando and A. Manipura, Compatibilization of starch/synthetic biodegradable polymer blends for packaging applications: A review, J. Compos. Sci., 2021, 5, 300, DOI:10.3390/JCS5110300.
- A. K. Mohanty, M. Misra and G. Hinrichsen, Biofibres, biodegradable polymers and biocomposites: An overview, Macromol. Mater. Eng., 2000, 276–277, 1–24, DOI:10.1002/(SICI)1439-2054(20000301)276:1<1::AID-MAME1>3.0.CO;2-W.
- T. Mekonnen, P. Mussone, H. Khalil and D. Bressler, Progress in bio-based plastics and plasticizing modifications, J. Mater. Chem. A, 2013, 1, 13379–13398, 10.1039/c3ta12555f.
- T. Gurunathan, S. Mohanty and S. K. Nayak, A review of the recent developments in biocomposites based on natural fibres and their application perspectives, Composites, Part A, 2015, 77, 1–25, DOI:10.1016/j.compositesa.2015.06.007.
- B. M. Trinh, C. C. Chang and T. H. Mekonnen, Facile fabrication of thermoplastic starch/poly(lactic acid) multilayer films with superior gas and moisture barrier properties, Polymer, 2021, 223, 123679, DOI:10.1016/J.POLYMER.2021.123679.
- B. Palai, M. Biswal, S. Mohanty and S. K. Nayak, In situ reactive compatibilization of polylactic acid (PLA) and thermoplastic starch (TPS) blends; synthesis and evaluation of extrusion blown films thereof, Ind. Crops Prod., 2019, 141, 111748, DOI:10.1016/J.INDCROP.2019.111748.
- S. Detyothin, A. Kathuria, W. Jaruwattanayon, S. E. M. Selke and R. Auras, Poly(Lactic Acid) Blends, Poly(Lactic Acid), John Wiley & Sons, Inc., Hoboken, NJ, USA, 2010, pp.227–271 DOI:10.1002/9780470649848.ch16.
- S. K. Ghosh, T. K. Das, S. Ghosh, S. Remanan, K. Nath, P. Das and N. C. Das, Selective distribution of conductive carbonaceous inclusion in thermoplastic elastomer: A wet chemical approach of promoting dual percolation and inhibiting radiation pollution in X-band, Compos. Sci. Technol., 2021, 210, 108800, DOI:10.1016/J.COMPSCITECH.2021.108800.
- S. K. Ghosh, T. K. Das, S. Ganguly, K. Nath, S. Paul, D. Ganguly and N. C. Das, Silane functionalization of sodium montmorillonite and halloysite (HNT) nanoclays by ‘grafting to’ method to improve physico-mechanical and barrier properties of LLDPE/clay nanocomposites, Polym. Bull., 2022, 2022, 1–29, DOI:10.1007/S00289-022-04281-4.
- C. C. Chang, B. M. Trinh and T. H. Mekonnen, Robust multiphase and multilayer starch/polymer (TPS/PBAT) film with simultaneous oxygen/moisture barrier properties, J. Colloid Interface Sci., 2021, 593, 290–303, DOI:10.1016/J.JCIS.2021.03.010.
- E. Ojogbo, E. O. Ogunsona and T. H. Mekonnen, Chemical and physical modifications of starch for renewable polymeric materials, Mater. Today Sustain., 2020, 7–8, 100028, DOI:10.1016/j.mtsust.2019.100028.
- T. Mekonnen, P. Mussone, H. Khalil and D. Bressler, Progress in bio-based plastics and plasticizing modifications, J. Mater. Chem. A, 2013, 1, 13379–13398, 10.1039/C3TA12555F.
- D. Jubinville, B. P. Chang, J. M. Pin, A. K. Mohanty and M. Misra, Synergistic thermo-oxidative maleation of PA11 as compatibilization strategy for PA6 and PBT blend, Polymer, 2019, 179, 121594, DOI:10.1016/J.POLYMER.2019.121594.
- R. Muthuraj, M. Misra and A. K. Mohanty, Biodegradable compatibilized polymer blends for packaging applications: A literature review, J. Appl. Polym. Sci., 2018, 135, 45726, DOI:10.1002/APP.45726.
- D. Jubinville, E. Esmizadeh, S. Saikrishnan, C. Tzoganakis and T. Mekonnen, A comprehensive review of global production and recycling methods of polyolefin (PO) based products and their post-recycling applications, Sustainable Mater. Technol., 2020, 25, e00188, DOI:10.1016/J.SUSMAT.2020.E00188.
- M. A. Huneault and H. Li, Morphology and properties of compatibilized polylactide/thermoplastic starch blends, Polymer, 2007, 48, 270–280, DOI:10.1016/J.POLYMER.2006.11.023.
- J. Wootthikanokkhan, P. Kasemwananimit, N. Sombatsompop, A. Kositchaiyong and S. Isarankura, na Ayutthaya, N. Kaabbuathong, Preparation of modified starch-grafted poly(lactic acid) and a study on compatibilizing efficacy of the copolymers in poly(lactic acid)/thermoplastic starch blends, J. Appl. Polym. Sci., 2012, 126, E389–E396, DOI:10.1002/app.36896.
- R. Turco, R. Ortega-Toro, R. Tesser, S. Mallardo, S. Collazo-Bigliardi, A. C. Boix, M. Malinconico, M. Rippa, M. Di Serio and G. Santagata, Poly(lactic acid)/thermoplastic starch films: Effect of cardoon seed epoxidized oil on their chemicophysical, mechanical, and barrier properties, Coatings, 2019, 9, 1–20, DOI:10.3390/coatings9090574.
- Q. Shi, C. Chen, L. Gao, L. Jiao, H. Xu and W. Guo, Physical and degradation properties of binary or ternary blends composed of poly(lactic acid), thermoplastic starch and GMA grafted POE, Polym. Degrad. Stab., 2011, 96, 175–182, DOI:10.1016/J.POLYMDEGRADSTAB.2010.10.002.
- L. Chen, X. Qiu, M. Deng, Z. Hong, R. Luo, X. Chen and X. Jing, The starch grafted poly(l-lactide) and the physical properties of its blending composites, Polymer, 2005, 46, 5723–5729, DOI:10.1016/j.polymer.2005.05.053.
- H. Eslami, C. Tzoganakis and T. H. Mekonnen, Constructing pristine and modified cellulose nanocrystals based cured polychloroprene nanocomposite films for dipped goods application, Composites Part C: Open Access., 2020, 1, 100009, DOI:10.1016/J.JCOMC.2020.100009.
- H. Eslami, T. Costas and T. H. Mekonnen, Surface graft polymerization of lactic acid from the surface of cellulose nanocrystals and applications in chloroprene rubber film composites, Cellulose, 2020, 27, 5267–5284, DOI:10.1007/s10570-020-03167-w.
- R. Lipsa, N. Tudorachi, C. Vasile, A. Chiriac and A. Grigoras, Novel environmentally friendly copolymers carboxymethyl starch grafted poly(lactic acid), J. Polym. Environ., 2013, 21, 461–471, DOI:10.1007/S10924-012-0470-1/FIGURES/10.
- S. Jia, D. Yu, Y. Zhu, Z. Wang, L. Chen and L. Fu, Morphology, crystallization and thermal behaviors of PLA-based composites: Wonderful effects of hybrid GO/PEG via dynamic impregnating, Polymers, 2017, 9(10), 528, DOI:10.3390/polym9100528.
- E. Ojogbo, R. Blanchard and T. Mekonnen, Hydrophobic and melt processable starch-laurate esters: Synthesis, structure-property correlations, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 2611–2622, DOI:10.1002/pola.29237.
- B. M. Trinh and T. Mekonnen, Hydrophobic esterification of cellulose nanocrystals for epoxy reinforcement, Polymer, 2018, 155, 64–74, DOI:10.1016/j.polymer.2018.08.076.
- S. S. Zamir, M. R. Frouzanmehr, M. Nagalakshmaiah, A. Ajji, M. Robert and S. Elkoun, Chemical compatibility of lactic acid-grafted starch nanocrystals (SNCs) with polylactic acid (PLA), Polym. Bull., 2019, 76, 3481–3499, DOI:10.1007/S00289-018-2548-Y.
- J. Liu, X. Wang, R. Bai, N. Zhang, J. Kan and C. Jin, Synthesis, characterization, and antioxidant activity of caffeic-acid-grafted corn starch, Starch – Stärke., 2018, 70, 1700141, DOI:10.1002/star.201700141.
- E. Ojogbo, R. Blanchard and T. Mekonnen, Hydrophobic and melt processable starch-laurate graft polymers: Synthesis, structure – property correlations, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 2611–2622, DOI:10.1002/pola.29237.
- E. Ojogbo, V. Ward and T. H. Mekonnen, Functionalized starch microparticles for contact-active antimicrobial polymer surfaces, Carbohydr. Polym., 2020, 229, 115422, DOI:10.1016/j.carbpol.2019.115422.
- Y. Wen, F. Ye, J. Zhu and G. Zhao, Corn starch ferulates with antioxidant properties prepared by N,N′-carbonyldiimidazole-mediated grafting procedure, Food Chem., 2016, 208, 1–9, DOI:10.1016/j.foodchem.2016.03.094.
- K. Suganuma, T. Asakura, M. Oshimura, T. Hirano, K. Ute and H. N. Cheng, NMR analysis of poly(lactic acid) via statistical models, Polymers, 2019, 11, 1–8, DOI:10.3390/polym11040725.
- B. M. Trinh, E. O. Ogunsona and T. H. Mekonnen, Thin-structured and compostable wood fiber-polymer biocomposites: Fabrication and performance evaluation, Composites, Part A, 2021, 140, 106150, DOI:10.1016/j.compositesa.2020.106150.
- N. Noivoil and R. Yoksan, Oligo(lactic acid)-grafted starch: A compatibilizer for poly(lactic acid)/thermoplastic starch blend, Int. J. Biol. Macromol., 2020, 160, 506–517, DOI:10.1016/j.ijbiomac.2020.05.178.
- C. Zhang, Y. Huang, C. Luo, L. Jiang and Y. Dan, Enhanced ductility of polylactide materials: Reactive blending with pre-hot sheared natural rubber, J. Polym. Res., 2013, 20(121), 1–9, DOI:10.1007/S10965-013-0121-9.
- J. Shawe, R. Riesen, J. Widmann and M. Schubnell, Interpreting DSC curves Part 1: Dynamic measurements, 2000.
- M. Esmaeili, G. Pircheraghi, R. Bagheri and V. Altstädt, Poly(lactic acid)/coplasticized thermoplastic starch blend: Effect of plasticizer migration on rheological and mechanical properties, Polym. Adv. Technol., 2019, 30, 839–851, DOI:10.1002/PAT.4517.
- Y. Lin, E. Bilotti, C. W. M. Bastiaansen and T. Peijs, Transparent semi-crystalline polymeric materials and their nanocomposites: A review, Polym. Eng. Sci., 2020, 60, 2351–2376, DOI:10.1002/PEN.25489.
- E. Ogunsona, E. Ojogbo and T. Mekonnen, Advanced material applications of starch and its derivatives, Eur. Polym. J., 2018, 108, 570–581, DOI:10.1016/j.eurpolymj.2018.09.039.
- E. Hablot, S. Dewasthale, Y. Zhao, Y. Zhiguan, X. Shi, D. Graiver and R. Narayan, Reactive extrusion of glycerylated starch and starch-polyester graft copolymers, Eur. Polym. J., 2013, 49, 873–881, DOI:10.1016/j.eurpolymj.2012.12.005.
- Z. Duan and N. L. Thomas, Water vapour permeability of poly(lactic acid): Crystallinity and the tortuous path model, J. Appl. Phys., 2014, 115, 064903, DOI:10.1063/1.4865168.
- E. E. Gonzo, M. L. Parentis and J. C. Gottifredi, Estimating models for predicting effective permeability of mixed matrix membranes, J. Membr. Sci., 2006, 277, 46–54, DOI:10.1016/J.MEMSCI.2005.10.007.
- A. Rybak, A. Rybak and P. Sysel, Modeling of gas permeation through mixed-matrix membranes using novel computer application MOT, Appl. Sci., 2018, 8, 1–20, DOI:10.3390/app8071166.
- J. H. Petropoulos, A comparative study of approaches applied to the permeability of binary composite polymeric materials, J. Polym. Sci., Polym. Phys. Ed., 1985, 23, 1309–1324, DOI:10.1002/POL.1985.180230703.
- E. de, M. Teixeira, A. A. S. Curvelo, A. C. Corrêa, J. M. Marconcini, G. M. Glenn and L. H. C. Mattoso, Properties of thermoplastic starch from cassava bagasse and cassava starch and their blends with poly(lactic acid), Ind. Crops Prod., 2012, 37, 61–68, DOI:10.1016/J.INDCROP.2011.11.036.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00501h |
| This journal is © The Royal Society of Chemistry 2022 |