Open Access Article
Open Access ArticleTetra-coordinated boron-appended zinc(II)-salen: a highly selective fluorescence-based sensor for Sm3+ ions via sensitization†
Prakash
Nayak
a,
Anna Chandrasekar
Murali
a,
Vadapalli
Chandrasekhar
 *bc and
Krishnan
Venkatasubbaiah
*bc and
Krishnan
Venkatasubbaiah
 *a
*a
aSchool of Chemical Sciences, National Institute of Science Education and Research (NISER), an OCC of HOMI Bhabha National Institute, Bhubaneswar-752050, Odisha, India. E-mail: krishv@niser.ac.in
bTata Institute of Fundamental Research Hyderabad, Gopanpally, Hyderabad-500 046, India
cDepartment of Chemistry, Indian Institute of Technology Kanpur, Kanpur-208016, India
First published on 7th June 2022
Abstract
Selective detection of metal ions is very important from the environmental and biological points of view. In this regard, selective discrimination between lanthanide ions is very difficult and challenging owing to their similar atomic radii. Herein, we report a tetra-coordinated boron-based salen ligand that shows selective fluorescence enhancement with Zn2+ ions. More interestingly, isolated zinc complex 6a was able to detect Sm3+ ions selectively. The luminescence color of complex 6a changed from green to red upon addition of Sm3+ ions, which makes this design highly selective in discriminating Sm3+ ions in the presence of other lanthanide ions at a very low concentration of 1 × 10−5 M.
Introduction
In recent years, the design and synthesis of tri- and tetra-coordinated boron compounds have received considerable attention due to their applications in diverse fields such as light-emitting devices, photovoltaics, sensors and organic field-effect transistors.1 The ability to attain a planar conformation, stability due to saturation of Lewis acidity and rigidity of the basic structure makes tetra-coordinated boron-based fluorophores more favorable alternatives over three-coordinated boron compounds. Among the different tetra-coordinated boron compounds, B–N coordinated compounds have secured considerable attention because of their widespread applications.2 We and others have recently shown that B–N coordinated compounds can show oxygen sensing behavior, photochromism, non-linear optical properties etc.2a,g,i,l,m,3Selective detection of metal ions has emerged as an important area of research due to its implications in the area of environmental, biological and clinical studies. Several probes or sensors have been reported for the detection of different metal ions employing colorimetric and/or fluorometric techniques.4 Fluorescence-based probes or sensors have received considerable attention owing to their simplicity, selectivity and sensitivity towards specific metal ions. Among the different metal ions, lanthanide ions constitute a family with a very similar and a non-discriminative coordination behavior. On the other hand, the use of lanthanides in industrial applications is ever burgeoning, including in magnetism, displays, components of nuclear control rods and even as catalysts to name a few.5 As some or many of the lanthanide ions are toxic in nature their detection is an important task. Although there are reports on the detection of different lanthanide ions,6 selective discrimination and detection of lanthanide ions is a major challenge in view of their similarity in terms of size and chemical behavior.
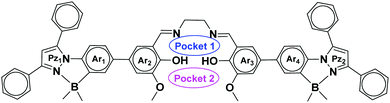
Taking advantage of the emissive and electron-transporting properties of the B–N coordinated fluorophores with the chelating ability of salen ligands, which can find use in catalysis and materials chemistry, we designed a tetra-coordinated boron-functionalized pyrazole-based salen ligand (Fig. 1). We envisioned that this molecular architecture will enable the selective detection of Zn2+ using pocket 1 and also further expected that pocket 2 will discriminate lanthanide ions.
Results and discussion
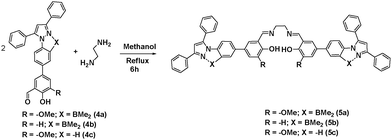
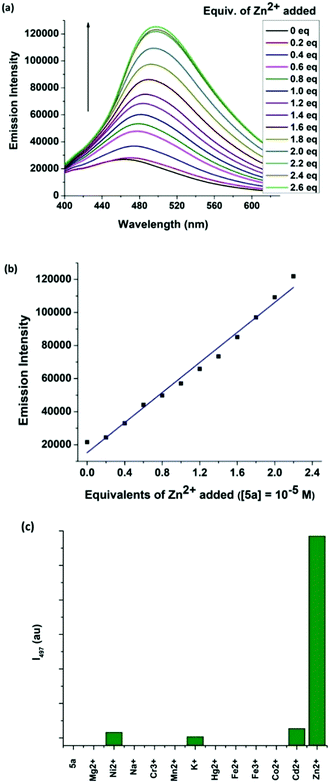
Selective detection of zinc ion plays a vital role in the chemical and biological sciences owing to its important role in gene expression, apoptosis, cellular metabolism and neurotransmission.7 A number of analytical techniques such as UV/Vis, fluorescence, atomic absorption spectroscopy, and electrochemical methods are used for the detection of Zn2+ ions.8 Among the different methods, fluorescent sensors have shown superior sensitivity. This motivated us to investigate the coordinating ability of boron functionalized compound 5a as a luminescent probe for Zn2+. The synthetic route to pyrazole-based boron functionalized imine ligand 5a is outlined in Scheme 1. Compounds 1–3 were prepared according to the literature reported methods.3g Treatment of 2 and 3a (Scheme S1, ESI†) with Na2CO3 and a catalytic amount of Pd(PPh3)4 under Suzuki coupling conditions resulted in a moderate yield (61%) of 4a. Synthesis of the required ligand 5a (Scheme 1) was achieved in an excellent yield (92%) by a simple condensation reaction of 2 equivalents of 4a with ethylene diamine in dry methanol as a solvent. Compound 5a was characterized using multi-nuclear NMR and HRMS. Furthermore, compound 5a was also analysed using 1H–1H COSY (Fig. S8, ESI†).The UV/Vis absorption and fluorescence spectra of 5a (10 μM) reveal an absorption maximum at 330 nm and an emission maximum at 464 nm. To understand the sensing properties of 5a, the absorption and fluorescence spectra of 5a were recorded with different amounts of Zn2+ ions. Sequential addition of Zn2+ ions (0–2.4 eq.) to 5a resulted in a new absorption peak at 353 nm with an isobestic point at 339 nm (Fig. S2, ESI†). As shown in Fig. 2, the fluorescence intensity gradually increased with sequential addition of Zn2+ ions (0–2.4 eq.). Furthermore, a selectivity test was performed using various cations such as Cd2+, Co2+, Fe3+, Fe2+, Hg2+, K+, Mn2+, Cr3+, Na+, Ni2+ and Mg2+ to investigate the binding interaction of 5a (10 μM). As shown in Fig. 2(c), only Zn2+ caused an enhanced emission, while other cations were silent. This result indicates that 5a discriminates Zn2+ from other cations, which can be detected by green emission.
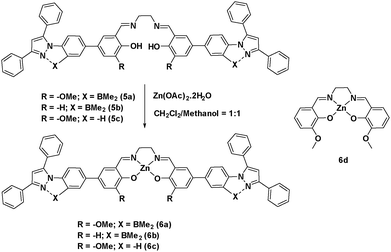
To determine the binding mode of the zinc ion with salen, we independently synthesized (Scheme 2) the Zn-salen and characterized it using different techniques like 1H, 13C, and 11B. The disappearance of the OH peak at 13.61 ppm of ligand 5a and the up-field shifting of four ethylene protons from 3.94 ppm in 5a to 3.74 ppm in 6a confirms the formation of Zn2+ complex, 6a. To our delight X-ray quality crystals of complex 6a could be grown in the DMF/CH3CN solvent system. The crystal structure confirms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of 5a with Zn2+ (Fig. S1, ESI†). The detection limit for Zn2+ was calculated and found to be 72 nM. These results reveal the applicability of 5a in determining Zn2+ ions with high selectivity.
1 binding of 5a with Zn2+ (Fig. S1, ESI†). The detection limit for Zn2+ was calculated and found to be 72 nM. These results reveal the applicability of 5a in determining Zn2+ ions with high selectivity.
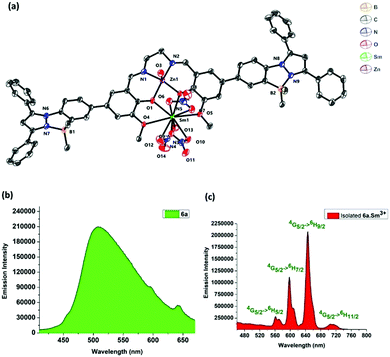
Complex 6a crystallizes in a triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The molecular structure reveals that the zinc atom adopts a five-coordinate distorted square pyramidal geometry. The zinc atom is 0.447 Å above the plane formed by N2O2 coordination from the salen motif. The axial position is occupied by a water molecule with the bond length of 2.066 Å. The Zn–O/N bond distances and angles observed for compound 6a are consistent with previously reported Zn2+-salen complexes.9 The pyrazoles (Fig. 1, Pz1 and Pz2) and the N-phenyls (Fig. 1, Ar1 and Ar4) form a three cycle co-planarized system via B–N coordination. The torsional angles observed for Ar3–Ar4 and Ar2–Ar1 are 18.5° and 32.3°, respectively, which indicates that Zn-salen and the B–N coordinated pyrazole moieties have slight deviation from planarity.
space group. The molecular structure reveals that the zinc atom adopts a five-coordinate distorted square pyramidal geometry. The zinc atom is 0.447 Å above the plane formed by N2O2 coordination from the salen motif. The axial position is occupied by a water molecule with the bond length of 2.066 Å. The Zn–O/N bond distances and angles observed for compound 6a are consistent with previously reported Zn2+-salen complexes.9 The pyrazoles (Fig. 1, Pz1 and Pz2) and the N-phenyls (Fig. 1, Ar1 and Ar4) form a three cycle co-planarized system via B–N coordination. The torsional angles observed for Ar3–Ar4 and Ar2–Ar1 are 18.5° and 32.3°, respectively, which indicates that Zn-salen and the B–N coordinated pyrazole moieties have slight deviation from planarity.
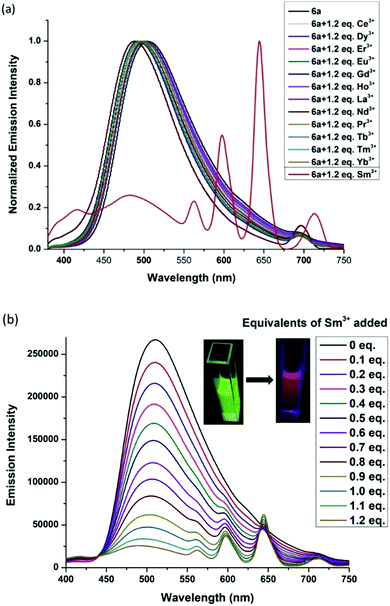
With this success, we further investigated the coordination ability of pocket 2 in 6a towards lanthanide ions. To our surprise complex 6a discriminated lanthanide ions and was able to detect Sm3+ ions selectively using pocket 2. We examined the emission spectra of complex 6a upon addition of 1.2 equiv. of various lanthanide ions such as Sm3+, Ce3+, Dy3+, Er3+, Eu3+, Gd3+, Ho3+, La3+, Pr3+, Tb3+, Tm3+ and Yb3. Except for Sm3+ ions, all other ions showed a weak fluorescence quenching at 500 nm (Fig. 3). However, a strong fluorescence quenching at 500 nm and enhanced emission peaks at 560 nm, 602 nm and 645 nm were observed on addition of 1.2 equivalents of Sm3+ ions to complex 6a. This could be due to an efficient energy transfer from 6a to the Sm3+ center upon complexation. These are characteristic peaks of Sm3+ emission resulting from the deactivation of the 4G5/2 excited state to the 6HJ ground state (J = 9/2, 7/2, and 5/2).10 To determine the capability of 6a to detect Sm3+ in an aqueous medium, 1.5 equivalents of Sm3+ ions in water were added to 6a in THF solution, which showed an emission reminiscent of the methanol/THF system. This result suggests that 6a can also be used to discriminate lanthanide ions in the THF/H2O medium.
In general, the lanthanide ion sensitization process takes place from the triplet state of the sensitizer.11 To understand the electronic properties of complex 6a, and the sensitization phenomenon, TD-DFT calculations were performed using the optimized ground-state geometry of 6a. The T1 triplet energy of complex 6a was predicted using the corresponding TD-DFT calculation results of 6a, which gives an energy of 1053 nm/9497 cm−1 (Table S2, ESI†). The triplet state energy of 6a is lower than the emissive state of Sm3+ ions (558 nm/17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 cm−1), which proves that the sensitization of Sm3+ by 6a is not taking place from the triplet state. Previous reports have also shown that the sensitization of Ln3+ centers can occur from the singlet state of the sensitizers.12 From TD-DFT calculations, it was calculated that the S1 singlet state energy of 6a (388 nm/25
900 cm−1), which proves that the sensitization of Sm3+ by 6a is not taking place from the triplet state. Previous reports have also shown that the sensitization of Ln3+ centers can occur from the singlet state of the sensitizers.12 From TD-DFT calculations, it was calculated that the S1 singlet state energy of 6a (388 nm/25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773 cm−1) is above the emissive state 4G5/2 of Sm3+. Thus, complex 6a is expected to sensitize Sm3+ emission from its singlet S1 state.
773 cm−1) is above the emissive state 4G5/2 of Sm3+. Thus, complex 6a is expected to sensitize Sm3+ emission from its singlet S1 state.
In order to investigate the interactions between 6a and Sm3+, we reacted complex 6a with Sm(NO3)3·6H2O in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 ratio in the CHCl3/EtOH solvent system at room temperature and isolated pure 6a·Sm3+ complex as a yellow precipitate. Single crystals of 6a·Sm3+ were grown by slow evaporation of DMF and methanol. To our delight, single-crystal X-ray diffraction analysis revealed the formation of a 1
1.5 ratio in the CHCl3/EtOH solvent system at room temperature and isolated pure 6a·Sm3+ complex as a yellow precipitate. Single crystals of 6a·Sm3+ were grown by slow evaporation of DMF and methanol. To our delight, single-crystal X-ray diffraction analysis revealed the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct, in which Sm3+ forms a nine-coordinated complex with 6a (Fig. 4). Complex 6a·Sm3+ crystallizes in the monoclinic system with a space group of P21/c. The complex 6a is bound to the samarium ion through four oxygen atoms, in which the Sm1–O4 (2.667(4) Å) and Sm1–O5 (2.770(4) Å) bond distances are significantly longer than the Sm1–O1 (2.372(4) Å) and Sm1–O2 (2.357(3) Å) bond distances. The samarium ion is further coordinated to three nitrate ions adopting a distorted square pyramidal structure achieving a coordination number of nine. The zinc ion adopts a distorted square pyramidal coordination environment in the heterometallic complex. The Sm⋯Zn distance is 3.454 Å, suggesting that there is no direct Sm⋯Zn bond.
1 adduct, in which Sm3+ forms a nine-coordinated complex with 6a (Fig. 4). Complex 6a·Sm3+ crystallizes in the monoclinic system with a space group of P21/c. The complex 6a is bound to the samarium ion through four oxygen atoms, in which the Sm1–O4 (2.667(4) Å) and Sm1–O5 (2.770(4) Å) bond distances are significantly longer than the Sm1–O1 (2.372(4) Å) and Sm1–O2 (2.357(3) Å) bond distances. The samarium ion is further coordinated to three nitrate ions adopting a distorted square pyramidal structure achieving a coordination number of nine. The zinc ion adopts a distorted square pyramidal coordination environment in the heterometallic complex. The Sm⋯Zn distance is 3.454 Å, suggesting that there is no direct Sm⋯Zn bond.
To determine the solid state emission properties of the 6a·Sm3+ complex, we excited the complex at 350 nm. The solid state emission of 6a·Sm3+ shows well-resolved peaks at 562 nm, 598 nm, 646 nm and 712 nm, which correspond to the transitions, 4G5/2 → 6H5/2, 4G5/2 → 6H7/2, 4G5/2 → 6H9/2 and 4G5/2 → 6H11/2, respectively. Among the emission peaks, the most intense emission at 645 nm corresponds to the 4G5/2 → 6H9/2 transition (Fig. 4). The complex 6a showed green emission whereas 6a·Sm3+ shows red emission in the solid state (Fig. 4).
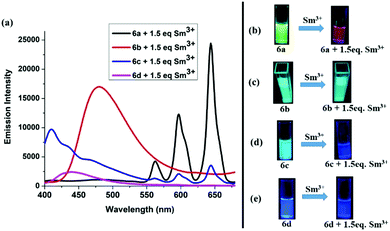
The –OMe functionality plays an important role in forming the complex with Sm3+ ions. To test our hypothesis, we made compound 6b (Scheme 2) which does not have any –OMe functionality. As expected, the emission of compound 6b did not alter upon addition of Sm3+ ions (Fig. S5, ESI†). To investigate the importance of boron coordination, we synthesized complex 6c (Scheme 2) that does not have boron in the framework and characterized it using 1H and 13C spectroscopy. Complex 6c showed a blue-shifted absorption and emission with respect to 6a (Fig. S7, ESI†). This signifies that boron coordination increases the rigidity of the pyrazole moiety, which resulted in slightly higher quantum yield and higher emission intensity for 6a with respect to 6c (Table S2, ESI†). When 1.5 equivalent of Sm3+ was added to 6c (1 × 10−5 M), we observed very less intense Sm3+ characteristic peaks in the emission spectrum and some residual emission from 6c (Fig. 5(a)). When the solution was kept under a hand held UV-lamp, no red emission was observed unlike for complex 6a (Fig. 5(d)). When 1.5 equiv. of Sm3+ was added to 6a, we observed intense Sm3+ characteristic peaks in the emission spectrum with no residual emission form 6a and intense red emission under a hand held UV-lamp (Fig. 5(a and b)). We believe that non-radiative decay due to lack of rigidity in complex 6c resulted in less intense red emission with respect to 6a. The lack of rigidity in 6c also resulted in inefficient energy transfer from the tri-phenyl pyrazole moiety to the Sm3+ center and thus caused relatively less intense red emission.
Dong and co-workers6h reported a bimetallic Zn–Sm complex derived from a Salamo-type ligand and also observed selective sensitization of the Sm3+ ions. Although structural characterization and emission for the Zn(II)–Sm(III) complex was presented, there is no discussion about the concentration of the solution used for this study. We synthesized compound 6d, which is analogues to the complex of Dong and co-workers, and analysed its potential to test Sm3+ under identical conditions that we used in our study. Use of 6d as a probe did not show any emission color change (conc. 1 × 10−5 M), when 1.5 equiv. of Sm3+ was added to the THF/methanol solution of 6d (Fig. 5(a and e)). At the same time, 6a made it possible to detect Sm3+ at 1 × 10−5 M concentration.
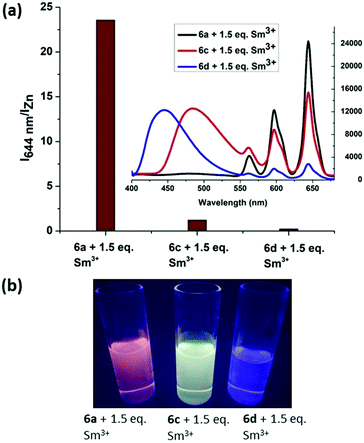
In order to compare the energy transfer in 6a, 6c and 6d, we increased the concentration from 1 × 10−5 M to 5 × 10−5 M and plotted I644nm/IZn (Izn = emission maximum of the respective zinc complex) for all complexes with the addition of 1.5 equiv. Sm3+ ions. It was observed that the I644nm/IZn ratio for 6a + 1.5 equiv. Sm3+ is almost 100 times higher than that of 6d and 20 times higher than that of 6c at the same concentration. The inset emission spectra, shown in Fig. 6(a), reveal the presence of residual emission from 6c at 484 nm and 6d at 444 nm. This residual emission from 6c and 6d masks the red emission from Sm3+, which can be observed under a hand held UV-Lamp (Fig. 6(b)). From these studies we conclude that our design has the advantage to discriminate Sm3+ ions at a very low concentration.
Conclusions
To conclude, we have successfully synthesized a tetra-coordinated boron-based salen ligand that exhibits excellent selectivity for the detection of Zn2+ ions using pocket 1. Furthermore, the zinc complex 6a, showed remarkable luminescence response at a very low concentration upon the addition of Sm3+ ions and discriminates them from other lanthanide ions. X-Ray crystallography and other studies reveal that pocket 2 is responsible for the selective binding and sensitization of Sm3+ ions via a cooperative use of the B–N coordinated pyrazole motif and the –OMe functionality. The sensitivity of 6a was found to be 100 fold that of 6d. The design described here will be an excellent lead for the further development of ligands for the selective discrimination of metal ions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Department of Atomic Energy (DAE) for financial support. We thank Dr U. Louderaj for helpful discussion about DFT studies. We also thank Principal Scientist Dr R. Natarajan from IICB-Kolkata for his help with X-ray studies and Dr Mriganka Sadhukan for elemental analysis.Notes and references
- (a) F. Jäkle, Chem. Rev., 2010, 110, 3965–4022 CrossRef PubMed; (b) Y.-L. Rao, H. Amarne and S. Wang, Coord. Chem. Rev., 2012, 256, 759–770 CrossRef CAS; (c) D. Li, H. Zhang and Y. Wang, Chem. Soc. Rev., 2013, 42, 8416–8433 RSC; (d) Z. M. Hudson and S. Wang, Acc. Chem. Res., 2009, 42, 1584–1596 CrossRef CAS PubMed; (e) D. Frath, J. Massue, G. Ulrich and R. Ziessel, Angew. Chem., Int. Ed., 2014, 53, 2290–2310 CrossRef CAS PubMed; (f) K. Tanaka and Y. Chujo, NPG Asia Mater., 2015, 7, e223 CrossRef CAS; (g) P.-Z. Chen, L.-Y. Niu, Y.-Z. Chen and Q.-Z. Yang, Coord. Chem. Rev., 2017, 350, 196–216 CrossRef CAS; (h) L. Ji, S. Griesbeck and T. B. Marder, Chem. Sci., 2017, 8, 846–863 RSC; (i) E. von Grotthuss, A. John, T. Kaese and M. Wagner, Asian. J. Org. Chem., 2018, 7, 37–53 CrossRef CAS; (j) T. W. Hudnall, C.-W. Chiu and F. P. Gabbaï, Acc. Chem. Res., 2009, 42, 388–397 CrossRef CAS PubMed; (k) J. F. Araneda, W. E. Piers, B. Heyne, M. Parvez and R. McDonald, Angew. Chem., Int. Ed., 2011, 50, 12214–12217 CrossRef CAS PubMed; (l) X. Su, T. A. Bartholome, J. R. Tidwell, A. Pujol, S. Yruegas, J. J. Martinez and C. D. Martin, Chem. Rev., 2021, 121, 4147–4192 CrossRef CAS PubMed; (m) R. R. Maar, R. Zhang, D. G. Stephens, Z. Ding and J. B. Gilroy, Angew. Chem., Int. Ed., 2019, 58, 1052–1056 CrossRef CAS PubMed; (n) R. Zhao, C. Dou, Z. Xie, J. Liu and L. Wang, Angew. Chem., Int. Ed., 2016, 55, 5313–5317 CrossRef CAS PubMed; (o) S. Sa, A. C. Murali, P. Nayak and K. Venkatasubbaiah, Chem. Commun., 2021, 57, 10170–10173 RSC.
- (a) A. Wakamiya, T. Taniguchi and S. Yamaguchi, Angew. Chem., Int. Ed., 2006, 45, 3170–3173 CrossRef CAS PubMed; (b) D. L. Crossley, I. A. Cade, E. R. Clark, A. Escande, M. J. Humphries, S. M. King, I. Vitorica-Yrezabal, M. J. Ingleson and M. L. Turner, Chem. Sci., 2015, 6, 5144–5151 RSC; (c) M. Grandl, T. Kaese, A. Krautsieder, Y. Sun and F. Pammer, Chem. – Eur. J., 2016, 22, 14373–14382 CrossRef CAS PubMed; (d) S. K. Mellerup, K. Yuan, C. Nguyen, Z. H. Lu and S. Wang, Chem. – Eur. J., 2016, 22, 12464–12472 CrossRef CAS PubMed; (e) M. Yusuf, K. Liu, F. Guo, R. A. Lalancette and F. Jäkle, Dalton. Trans., 2016, 45, 4580–4587 RSC; (f) D. L. Crossley, R. Goh, J. Cid, I. Vitorica-Yrezabal, M. L. Turner and M. J. Ingleson, Organometallics, 2017, 36, 2597–2604 CrossRef CAS; (g) K. Liu, R. A. Lalancette and F. Jäkle, J. Am. Chem. Soc., 2017, 139, 18170–18173 CrossRef CAS PubMed; (h) S. Schraff, Y. Sun and F. Pammer, J. Mater. Chem. C, 2017, 5, 1730–1741 RSC; (i) J. Wang, B. Jin, N. Wang, T. Peng, X. Li, Y. Luo and S. Wang, Macromolecules, 2017, 50, 4629–4638 CrossRef CAS; (j) M. Mamada, G. Tian, H. Nakanotani, J. Su and C. Adachi, Angew. Chem., Int. Ed., 2018, 57, 12380–12384 CrossRef CAS PubMed; (k) S. Wang, K. Yuan, M. F. Hu, X. Wang, T. Peng, N. Wang and Q. S. Li, Angew. Chem., Int. Ed., 2018, 57, 1073–1077 CrossRef CAS PubMed; (l) B. P. Dash, I. Hamilton, D. J. Tate, D. L. Crossley, J.-S. Kim, M. J. Ingleson and M. L. Turner, J. Mater. Chem. C, 2019, 7, 718–724 RSC; (m) Y. Li, H. Meng, T. Liu, Y. Xiao, Z. Tang, B. Pang, Y. Li, Y. Xiang, G. Zhang, X. Lu, G. Yu, H. Yan, C. Zhan, J. Huang and J. Yao, Adv. Mater., 2019, 31, e1904585 CrossRef PubMed; (n) K. Liu, R. A. Lalancette and F. Jäkle, J. Am. Chem. Soc., 2019, 141, 7453–7462 CrossRef CAS PubMed; (o) J. Full, S. P. Panchal, J. Gotz, A. M. Krause and A. Nowak-Krol, Angew. Chem., Int. Ed., 2021, 60, 4350–4357 CrossRef CAS PubMed; (p) A. C. Murali, P. Nayak and K. Venkatasubbaiah, Dalton. Trans., 2022, 51, 5751–5771 RSC; (q) Z. Dominguez, R. Lopez-Rodriguez, E. Alvarez, S. Abbate, G. Longhi, U. Pischel and A. Ros, Chem. – Eur. J., 2018, 24, 12660–12668 CrossRef CAS PubMed.
- (a) C. Li, S. K. Mellerup, X. Wang and S. Wang, Organometallics, 2018, 37, 3360–3367 CrossRef CAS; (b) S. K. Mellerup, C. Li, X. Wang and S. Wang, J. Org. Chem., 2018, 83, 11970–11977 CrossRef CAS PubMed; (c) X. Li, Y. Shi, N. Wang, T. Peng and S. Wang, Chem. – Eur. J., 2019, 25, 5757–5767 CrossRef CAS PubMed; (d) Z. He, L. Liu, Z. Zhao, S. K. Mellerup, Y. Ge, X. Wang, N. Wang and S. Wang, Chem. – Eur. J., 2020, 26, 12403–12410 CrossRef CAS PubMed; (e) M. Vanga, S. Sa, A. Kumari, A. C. Murali, P. Nayak, R. Das and K. Venkatasubbaiah, Dalton Trans., 2020, 49, 7737–7746 RSC; (f) D. Kunchala, S. Sa, P. Nayak, J. Ponniah and S. K. Venkatasubbaiah, Organometallics, 2019, 38, 870–878 CrossRef CAS; (g) V. Mukundam, S. Sa, A. Kumari, R. Das and K. Venkatasubbaiah, J. Mater. Chem. C, 2019, 7, 12725–12737 RSC.
- (a) K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS PubMed; (b) L. Wang, X. Gong, Q. Bing and G. Wang, Microchem. J., 2018, 142, 279–287 CrossRef CAS; (c) P. Ravichandiran, A. Boguszewska-Czubara, M. Masłyk, A. P. Bella, S. A. Subramaniyan, P. M. Johnson, K. S. Shim, H. G. Kim and D. J. Yoo, ACS Sustainable Chem. Eng., 2019, 7, 17210–17219 CrossRef CAS; (d) N. Yadav and A. K. Singh, J. Electrochem. Soc., 2019, 166, B644–B653 CrossRef CAS; (e) A. Gul, M. Oguz, A. N. Kursunlu and M. Yilmaz, Dyes Pigm., 2020, 176, 108221 CrossRef CAS; (f) M. Sahu, A. Kumar Manna, K. Rout, J. Mondal and G. K. Patra, Inorg. Chim. Acta, 2020, 508, 119633 CrossRef CAS; (g) Y. Zhang, W. Wang, R. Li, E. Zhang, Z. Li, L. Tang, B. Han, X. Hou and J. J. Wang, Spectrochim. Acta, Part A, 2020, 230, 118050 CrossRef CAS PubMed; (h) T. Samanta and R. Shunmugam, Mater. Adv., 2021, 2, 64–95 RSC.
- (a) F. T. Edelmann, Chem. Soc. Rev., 2012, 41, 7649–7964 RSC; (b) J. Kido and Y. Okamoto, Chem. Rev., 2002, 102, 2357–2368 CrossRef CAS PubMed; (c) S. Faulkner, S. J. A. Pope and B. P. Burton-Pye, Appl. Spectrosc. Rev., 2005, 40, 1–31 CrossRef CAS; (d) C. Andraud and O. Maury, Eur. J. Inorg. Chem., 2009, 4357–4371 CrossRef CAS; (e) C. J. Weiss and T. J. Marks, Dalton Trans., 2010, 39, 6576–6588 RSC; (f) S. D. Bennett, B. A. Core, M. P. Blake, S. J. Pope, P. Mountford and B. D. Ward, Dalton Trans., 2014, 43, 5871–5885 RSC; (g) Z. Ahmed and K. Iftikhar, Inorg. Chem., 2015, 54, 11209–11225 CrossRef CAS PubMed; (h) V. V. Utochnikova, N. N. Solodukhin, A. N. Aslandukov, L. Marciniak, I. S. Bushmarinov, A. A. Vashchenko and N. P. Kuzmina, Org. Electron., 2017, 44, 85–93 CrossRef CAS; (i) F. Zinna, M. Pasini, F. Galeotti, C. Botta, L. Di Bari and U. Giovanella, Adv. Funct. Mater., 2017, 27, 1603719 CrossRef; (j) Y. Qiao and E. J. Schelter, Acc. Chem. Res., 2018, 51, 2926–2936 CrossRef CAS PubMed; (k) Y. Zhang, S. Liu, Z.-S. Zhao, Z. Wang, R. Zhang, L. Liu and Z.-B. Han, Inorg. Chem. Front., 2021, 8, 590–619 RSC; (l) P. Kalita, J. Goura, J. M. Herrera, E. Colacio and V. Chandrasekhar, ACS Omega, 2018, 3, 5202–5211 CrossRef CAS PubMed; (m) A. K. Bar, P. Kalita, M. K. Singh, G. Rajaraman and V. Chandraselkar, Coord. Chem. Rev., 2018, 367, 163–216 CrossRef CAS; (n) P. Kalita, N. Ahmed, A. K. Bar, S. Dey, A. Jana, G. Rajaraman, J.-P. Sutter and V. Chandrasekhar, Inorg. Chem., 2020, 59, 6603–6612 CrossRef CAS PubMed; (o) V. V. Utochnikova, E. V. Latipov, A. I. Dalinger, Y. V. Nelyubina, A. A. Vashchenko, M. Hoffmann, A. S. Kalyakina, S. Z. Vatsadze, U. Schepers, S. Bräse and N. P. Kuzmina, J. Lumin., 2018, 202, 38–46 CrossRef CAS; (p) L. Wang, Z. Zhao, C. Wei, H. Wei, Z. Liu, Z. Bian and C. Huang, Adv. Opt. Mater., 2019, 7, 1801256 CrossRef.
- (a) D. A. Chowdhury, T. Ogata and S. Kamata, Anal. Chem., 1996, 68, 366–370 CrossRef CAS; (b) C. E. Lisowski and J. E. Hutchison, Anal. Chem., 2009, 81, 10246–10253 CrossRef CAS PubMed; (c) C. Han, L. Zhang and H. Li, Chem. Commun., 2009, 3545–3547 RSC; (d) P. Das, A. Ghosh and A. Das, Inorg. Chem., 2010, 49, 6909–6916 CrossRef CAS PubMed; (e) T. Gorai and U. Maitra, Angew. Chem., Int. Ed., 2017, 56, 10730–10734 CrossRef CAS PubMed; (f) P. Harvey, A. Nonat, C. Platas-Iglesias, L. S. Natrajan and L. J. Charbonniere, Angew. Chem., Int. Ed., 2018, 57, 9921–9924 CrossRef CAS PubMed; (g) G. I. Vargas-Zuniga and J. L. Sessler, Sensors for Lanthanides and Actinides, in The Rare Earth Elements: Fundamentals and Applications, ed. Atwood, D. A., Wiley, 2012, pp. 561–573 Search PubMed; (h) S.-S. Zheng, W.-K. Dong, Y. Zhang, L. Chen and Y.-J. Ding, New J. Chem., 2017, 41, 4966–4973 RSC.
- (a) M. P. Cuajungco and G. J. Lees, Neurobiol. Dis., 1997, 4, 137–169 CrossRef CAS PubMed; (b) D. Beyersmann and H. Haase, Biometals, 2001, 14, 331–341 CrossRef CAS PubMed; (c) D. Beyersmann, Materialwiss. Werkstofftech., 2002, 33, 764–769 CrossRef CAS; (d) S. G. Bell and B. L. Vallee, ChemBioChem, 2009, 10, 55–62 CrossRef CAS PubMed; (e) W. Maret, Biometals, 2011, 24, 411–418 CrossRef CAS PubMed; (f) W. Maret, Adv. Nutr., 2013, 4, 82–91 CrossRef CAS PubMed; (g) S. Triboulet, C. Aude-Garcia, L. Armand, A. Gerdil, H. Diemer, F. Proamer, V. Collin-Faure, A. Habert, J. M. Strub, D. Hanau, N. Herlin, M. Carriere, A. Van Dorsselaer and T. Rabilloud, Nanoscale, 2014, 6, 6102–6114 RSC; (h) T. Kambe, T. Tsuji, A. Hashimoto and N. Itsumura, Physiol. Rev., 2015, 95, 749–784 CrossRef CAS PubMed; (i) W. Maret, Metallomics, 2015, 7, 202–211 CrossRef CAS PubMed; (j) L. C. Costello and R. B. Franklin, Arch. Biochem. Biophys., 2016, 611, 100–112 CrossRef CAS PubMed; (k) K. Grüngreiff, D. Reinhold and H. Wedemeyer, Ann. Hepatol., 2016, 15, 7–16 CrossRef PubMed; (l) W. Maret, Int. J. Mol. Sci., 2017, 18, 2285 CrossRef PubMed; (m) X. Yang, H. Wang, C. Huang, X. He, W. Xu, Y. Luo and K. Huang, Sci. Rep., 2017, 7, 14669 CrossRef PubMed; (n) W. Maret, Free Radical Biol. Med., 2019, 134, 311–326 CrossRef CAS PubMed.
- (a) K. Hanaoka, K. Kikuchi, H. Kojima, Y. Urano and T. Nagano, Angew. Chem., Int. Ed., 2003, 42, 2996–2999 CrossRef CAS PubMed; (b) Y. Xu, J. Meng, L. Meng, Y. Dong, Y. Cheng and C. Zhu, Chem. – Eur. J., 2010, 16, 12898–12903 CrossRef CAS PubMed; (c) Z. Xu, K.-H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601–610 CrossRef CAS PubMed.
- (a) M. E. Germain, T. R. Vargo, P. G. Khalifah and M. J. Knapp, Inorg. Chem., 2007, 46, 4422–4429 CrossRef CAS PubMed; (b) X.-Q. Song, Y.-Q. Peng, G.-Q. Cheng, X.-R. Wang, P.-P. Liu and W.-Y. Xu, Inorg. Chim. Acta, 2015, 427, 13–21 CrossRef CAS.
- Y. Hasegawa, Y. Kitagawa and T. Nakanishi, NPG Asia Mater., 2018, 10, 52–70 CrossRef CAS.
- (a) E. A. Varaksina, I. V. Taydakov, S. A. Ambrozevich, A. S. Selyukov, K. A. Lyssenko, L. T. Jesus and R. O. Freire, J. Lumin., 2018, 196, 161–168 CrossRef CAS; (b) Z. Ahmed and K. Iftikhar, Dalton Trans., 2019, 48, 4973–4986 RSC; (c) F. P. Aguiar, I. F. Costa, J. G. P. Espínola, W. M. Faustino, J. L. Moura, H. F. Brito, T. B. Paolini, M. C. F. C. Felinto and E. E. S. Teotonio, J. Lumin., 2016, 170, 538–546 CrossRef CAS; (d) Z. Li, P. Li, Q. Xu and H. Li, Chem. Commun., 2015, 51, 10644–10647 RSC; (e) J.-C. G. Bünzli, Coord. Chem. Rev., 2015, 293–294, 19–47 CrossRef; (f) L. Sun, Y. Qiu, T. Liu, J. Feng, W. Deng and L. Shi, Luminescence, 2015, 30, 1071–1076 CrossRef CAS PubMed; (g) P. Kalita, P. Nayak, N. Ahmed, J. M. Herrera, K. Venkatasubbaiah, E. Colacio and V. Chandrasekhar, Dalton Trans., 2020, 49, 15404–15416 RSC.
- (a) A. Guenet, F. Eckes, V. Bulach, C. A. Strassert, L. De Cola and M. W. Hosseini, ChemPhysChem, 2012, 13, 3163–3171 CrossRef CAS PubMed; (b) E. Kasprzycka, V. A. Trush, V. M. Amirkhanov, L. Jerzykiewicz, O. L. Malta, J. Legendziewicz and P. Gawryszewska, Chem. – Eur. J., 2017, 23, 1318–1330 CrossRef CAS PubMed; (c) J. R. G. Thorne, J. M. Rey, R. G. Denning, S. E. Watkins, M. Etchells, M. Green and V. Christon, J. Phys. Chem. A, 2002, 106, 4014–4021 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data, and copies of the 1H and 13C NMR spectra. CCDC 2152949 and 2152950. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ma00516f |
| This journal is © The Royal Society of Chemistry 2022 |