Open Access Article
Open Access Articleg-C3N4/dendritic fibrous nanosilica doped with potassium for photocatalytic CO2 reduction†
Sushma A.
Rawool
a,
Yusuf
Kar
b and
Vivek
Polshettiwar
 *a
*a
aDepartment of Chemical Sciences, Tata Institute of Fundamental Research (TIFR), Mumbai, India
bShell Technology Center, Bangalore, India. E-mail: vivekpol@tifr.res.in; Tel: (+91) 8452886556
First published on 30th September 2022
Abstract
Photocatalytic CO2 conversion is a promising process for the reduction of CO2 into useful chemicals and fuels using solar energy. In this work, we have synthesized potassium-doped g-C3N4 coated over dendritic fibrous nanosilica (K-CN/DFNS) and studied the photocatalytic CO2 conversion under visible light illumination. K-CN/DFNS was synthesized by heating KOH-treated melamine in a vacuum-sealed quartz tube by varying the amounts of potassium salt. The scanning electron microscopy images confirmed the coating of potassium-doped g-C3N4 over DFNS, while the absorption spectra show extended absorption in the visible region and redshift in the bandgap as compared to the undoped CN/DFNS. XPS analysis further confirms the interaction of potassium ions with g-C3N4. During the photocatalytic CO2 conversion, the selectivity towards the formation of hydrogen (from water splitting) was the highest for the g-C3N4 and CN/DFNS catalysts, whereas no hydrogen generation was observed using the potassium-doped catalysts. The highest methane yield observed was 1.7 μmol g−1 for g-C3N4 in 4 h using K-CN/DFNS-2 (with 6 wt% of potassium ions). The high activity and improved selectivity of K-CN/DFNS were due to the extended absorption in the visible region, better separation of charge carriers, shifting of the conduction band and valence band positions towards positive potentials, and moderate adsorption of CO2 molecules on its surface.
Introduction
The generation of greenhouse gases is a major concern in order to protect the climate from global warming. Fossil fuels produce a large amount of CO2 during their burning and an increase in CO2 level of up to 590 ppm can result in a rise in the global temperature by 1.9 °C.1 Thus, different methodologies have been developed to tackle the issue of excessive anthropogenic CO2 in the environment.2–4 One of the best methods is photocatalytic CO2 conversion using solar energy, where a photocatalyst harvests solar energy to convert CO2 into valuable fuels like methane, methanol, formic acid etc., using water as a proton source.3,4 Different metal oxides, sulfides, nitrides, and oxynitrides have been studied as photocatalysts.3–6 Among them, metal-free organic polymeric graphitic carbon nitride (g-C3N4) has attracted tremendous attention because of its low bandgap of 2.7 eV, its inexpensive, environmentally friendly, and chemically inert nature and also the fact that the reduction potential of CO2 conversion falls within its bandgap.7,8 It can be synthesized using nitrogen-rich molecules like urea, melamine, cyanamide, dicyandiamide etc.7–11Scheme S1 (ESI†) shows the presence of a different type of nitrogen in g-C3N4, which helps in CO2 adsorption and activation because of the lone pair of electrons present on it. The pyridinic nitrogen group shows Lewis basicity because of the presence of a lone pair of electrons and thus helps in the adsorption and activation of CO2 molecules.11 This nitrogen has a higher negative charge as it can retain the lone pair of electrons in a planar structure and thus can bind CO2 more effectively than other types of nitrogen present in g-C3N4. Quaternary graphitic nitrogen shows Lewis acidity as the electrons from its π* antibonding orbital are less accessible for CO2 binding. Also, the other types of nitrogen species formed during incomplete polymerization, such as terminal -NH2, -NH groups and N-oxides, exhibit some basicity and thus, g-C3N4 is suitable for adsorption and activation of CO2 molecules.11 In spite of having these advantages, the photocatalytic activity of g-C3N4 is low because of limited light absorption until 450 nm in the visible region of the solar spectrum, high charge recombination rate, and low surface area.8
For efficient g-C3N4 photocatalyst design, adsorption of CO2, visible light absorption, and charge carrier separation and transfer to carry out redox reactions are the key factors that need to be considered. Different strategies such as doping of cations or anions, composite formation with different semiconductors, tuning of the morphology, and loading of metal nanoparticles have been adopted to modify the structural, electronic and optical properties of g-C3N4.8 Among these, the doping of cations into the g-C3N4 matrix offers several advantages, such as tuning the bandgap by creating impurity states and the generation of active sites for trapping of photoexcited electrons and holes.12 Potassium-doped g-C3N4 has been studied for photocatalytic hydrogen generation and dye degradation.13–17 To the best of our knowledge, CO2 conversion was barely reported using potassium-doped g-C3N4.18,19 Doping of potassium ions into the matrix of g-C3N4 was found to be beneficial in extending absorption in the visible region and efficient separation of charge carriers.13–17 It was also anticipated that the presence of an alkali metal ion like potassium would help in improving the CO2 adsorption capacity of g-C3N4.18,19 Sun et al.19 introduced an amino group and intercalated the potassium ion in g-C3N4 by post-treatment of g-C3N4 and KOH. They reported that the generation of the excess amino group improves CO2 adsorption and activation and the presence of intercalated potassium ions enhanced the separation and transfer of charge carriers. The methane and CO generation was found to be 5.6 and 5.4 times higher than that of g-C3N4, respectively. Recently, Wang et al. reported CO generation at the rate of 8 μmol g−1 h−1 over (2.08 at%) potassium-doped g-C3N4.18 Previously, we reported hydrogen generation using g-C3N4 decorated over dendritic fibrous nanosilica (DFNS) and it was found to be seven times higher as compared to that of the pristine g-C3N4.20 The improvement in activity was attributed to the visible light enhancement, efficient separation of charge carriers and high surface area.
In continuation of our work on DFNS-based catalytic systems,20–24 in this work, we doped potassium ions within the framework of g-C3N4 coated over DFNS to further improve the photocatalytic CO2 conversion. Melamine treated with KOH was used as a precursor for the synthesis of potassium-doped g-C3N4. This is the first time that we have reported the synthesis of potassium-doped g-C3N4 coated over DFNS using a vacuum-sealed quartz tube. The amount of potassium ions was varied and the modifications in the optical, electronic and structural properties of g-C3N4 were assessed by different characterization techniques. The catalysts were investigated for photocatalytic CO2 conversion under visible light illumination. The changes in the structure of g-C3N4 brought by doping with potassium ion and the role of DFNS as the support were investigated and the impact of structural changes was correlated with the photocatalytic property of the sample. Preferential evolution of methane over hydrogen generation was observed using potassium-doped samples.
Experimental
Synthesis
Potassium hydroxide-treated melamine (K-melamine) was synthesized by dispersing potassium hydroxide (KOH) and melamine in distilled water. The solution was stirred for 2 h. The solvent was evaporated to dryness at 80 °C in an oven. The amount of KOH varied from 3.2, 25.5 and 45.5 wt% of melamine. K-melamine (250 mg) and DFNS (500 mg) were mixed well by grinding for 1 h. During grinding, ethanol was added after every 20 min to ensure homogeneous mixing. After grinding, the mixture was kept in an oven at 80 °C for 12 h. The dried sample (86 mg) was transferred to a quartz tube and the tube was evacuated at 0.3–0.6 mbar overnight and then sealed under vacuum. The sealed tube was then heated in two steps, first at 400 °C for 2 h and then at 550 °C for 2 h at a heating rate of 10 °C min−1. After cooling down to room temperature, the tube was broken to obtain a powder catalyst. The detailed synthesis procedure is given in Fig. S1 (ESI†). The sample was washed with water four times to remove the impurities. The samples were referred to as K-CN/DFNS-1, K-CN/DFNS-2 and K-CN/DFNS-3 for 3.2, 25.5 and 45.5 wt% of KOH used during synthesis. Pristine g-C3N4 without DFNS and CN/DFNS without K doping were also synthesized as controls using the same protocol.Characterization
Scanning electron microscopy (SEM) was performed on a Zeiss ULTRA instrument. For sample preparation, powders were dispersed in ethanol with the assistance of sonication and a drop of solution was placed on an aluminum SEM grid. The X-ray diffraction (XRD) patterns were recorded using a Panalytical X’Pert Pro powder X-ray diffractometer using Cu Kα radiation. A fixed amount of sample and KBr were mixed to record the FTIR spectra of the samples using a JASCO FT/IR-4700 instrument. The thermal stability of the samples was evaluated by using thermogravimetric analysis (TGA, Mettler Toledo). TGA was carried out by heating the samples in an alumina pan at a heating rate of 10 °C min−1 from 30 °C to 800 °C under an air atmosphere. UV-visible DRS spectra were recorded using a Jasco spectrophotometer (Jasco V-770). Lifetime measurements were carried out by using a femto/picosecond laser as the excitation source and employing the time-correlated single photon counting (TCSPC) technique. For fluorescence lifetime measurements, 1 ps pulses of 732 nm radiation from a Ti-sapphire femto/picosecond laser (Spectra-Physics, Mountain View, CA), pumped by a Nd:YLF laser (Millenia X, Spectra-Physics), were frequency-doubled to 396 nm by using a frequency doubler/triplet (GWU, Spectra-Physics). The fluorescence decay curves were obtained at a laser repetition rate of 4 MHz using a microchannel plate photomultiplier (model R2809u, Hamamatsu Corp.) coupled to a TCSPC setup. The instrument response function (IRF) at 396 nm was obtained using a dilute colloidal suspension of a dried non-dairy creamer. The full width at half-maximum (FWHM) of the IRF was 90 ps and the number of channels used was 1024. All the data were fitted with triexponential fitting. It gives three-lifetime values of τ1, τ2 and τ3 corresponding to amplitudes, A1, A2 and A3, respectively. Average lifetime (τavg) was determined by using the following equation:26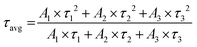 | (1) |
The surface area was calculated by applying Brunauer–Emmett–Teller (BET) theory to N2 physisorption data, which was recorded using a Micromeritics Flex3 analyzer. Approximately 100 mg of each sample was degassed at 120 °C for 12 h prior to N2 sorption analysis. CO2 adsorption of the catalyst was studied using a Micromeritics Flex3 analyzer at 25 °C.
The XPS analysis of the sample was done using monochromated Al-Kα radiation (1486.6 eV) as an X-ray source. The carbon signal at 284.6 eV was used as an internal reference. Peak fitting of the spectra was performed using XPSPEAK 4.1 software. The relative positions of the valence band (EVB) and conduction band (ECB) against NHE were calculated from the XPS valence band spectra:27
E VB against NHE is estimated using the following equation:
| EVB = ΔE − EVac + Ws | (2) |
E CB is estimated using the following equation:
| ECB = EVB − EG | (3) |
Photocatalytic CO2 conversion
Photocatalytic CO2 conversion was carried out in a Pyrex photoreactor (volume 50 mL). 1 wt% Pt was deposited over the K-CN/DFNS sample by a chemical reduction method using NaBH4 as a reducing agent. (1 wt%) Pt/K-CN/DFNS sample (15 mg) was mixed with a small amount of water to form a paste and spread over the flat bottom of the reactor, having an area of 2.5 cm2. Then the reactor was dried at 80 °C in an oven for 30 min. After this, CO2 was passed through a water bubbler to bring a mixture of CO2 and H2O vapor into the photoreactor for 1 h at a flow rate of 200 mL min−1. The reactor was irradiated with visible light (wavelengths 385–740 nm) using a 300 W xenon lamp (MAX 303 Asahi Spectra Co. Ltd). The output power of the lamp was adjusted to deliver 318 mW cm−2 at the reactor. The photograph of the experimental setup is given in Fig. S2 (ESI†). Hydrogen and methane yields were monitored by gas chromatography (Agilent 7890B, Agilent Technologies) equipped with a thermal conductivity detector (TCD) and a flame ionization detector (FID). To compare the photocatalytic activity of the photocatalyst, the total consumed electron number (TCEN) was calculated. The methane generation requires 8 electrons. The formula used for the calculation of TCEN is as follows: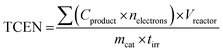 | (4) |
To confirm that the hydrocarbon products are generated by the reduction of CO2, isotopically labeled experiments were carried out. For this experiment, 13CO2 was used as a reactant. The generated gaseous products after the reaction were analyzed by using GC coupled with mass spectrometry (Agilent 7890B, Agilent Technologies). For GC-MS analysis, helium was used as the carrier gas and an Agilent hybrid column CP7430 as the stationary phase.
Results and discussion
g-C3N4 is a potential candidate for photocatalytic CO2 conversion. However, low surface area, the high recombination rate of charge carriers and limited light absorption in the visible region affect its photocatalytic properties. In our previous publication, to overcome these issues, we coated g-C3N4 over a high surface area support DFNS (CN/DFNS).20 It showed seven times better hydrogen generation as compared to the pristine g-C3N4. The enhancement in photocatalytic activity was attributed to the high surface area, extended visible light absorption in the 450–650 nm range and improved charge separation due to the formation of an interface between DFNS and g-C3N4. The samples were further tested for CO2 conversion. Unfortunately, it showed hydrogen as a major product and a low yield of methane. To improve its CO2 conversion process, increase the selectivity towards CO2 reduction products and suppress hydrogen generation, in this work, potassium ions were doped into the matrix of g-C3N4 loaded over DFNS (K-CN/DFNS).For the synthesis of K-CN/DFNS, KOH-treated melamine was mixed with DFNS and placed in a vacuum-sealed quartz tube and then heated at 400 °C for 2 h and 550 °C for 2 h. The amount of potassium was varied by changing the KOH amount from 3.2 to 45.5 wt% of melamine. The morphology of the samples was analyzed by SEM (Fig. 1). The SEM images of DFNS showed the presence of a fiber-like morphology with particle size in the range of 300–500 nm, whereas that of the CN/DFNS and K-CN/DFNS samples revealed that the layers of g-C3N4 were grafted over the DFNS nanosheets, giving it a complete sphere-shape appearance (Fig. 1). The amount of potassium and g-C3N4 content in the sample was evaluated using EDX analysis (Table 1). The amount of potassium varied from 0.74, 6.06 and 18.9 wt% with respect to g-C3N4 for the K-CN/DFNS-1, K-CN/DFNS-2 and K-CN/DFNS-3 samples, respectively. The content of g-C3N4 was found to be lower in the case of K-CN/DFNS-3 as compared to the K-CN/DFNS-1 and K-CN/DFNS-2 samples.
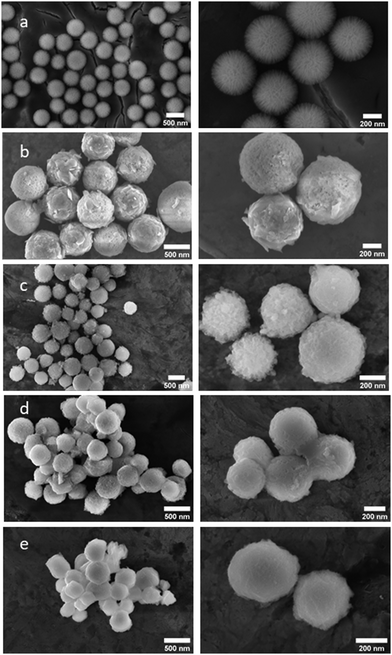 | ||
| Fig. 1 The SEM images of the (a) DFNS, (b) CN/DFNS, (c) K-CN/DFNS-1, (d) K-CN/DFNS-2 and (e) K-CN/DFNS-3 samples (images from left to right indicate low resolution and high-resolution images). | ||
| Sample | Amount of potassium with respect to g-C3N4 by EDX (wt%) | Amount of g-C3N4 from TGAa (wt%) | Bandgap measurement by UV-Visible DRS (eV) | Surface area (m2 g−1) | Pore volume (cm3 g−1) |
|---|---|---|---|---|---|
| a Amount of g-C3N4 calculated from the weight loss in the region of 220–670 °C in the thermogram. | |||||
| DFNS | — | — | — | 622 | 0.719 |
| g-C3N4 | — | — | 2.70 | 15 | 0.05 |
| CN/DFNS | — | 21.9 | 2.76 | 150 | 0.163 |
| K-CN/DFNS-1 | 0.74 | 19.8 | 2.74 | 88 | 0.158 |
| K-CN/DFNS-2 | 6.06 | 13.3 | 2.69 | 55 | 0.062 |
| K-CN/DFNS-3 | 18.9 | 2.7 | 2.80 | 79 | 0.086 |
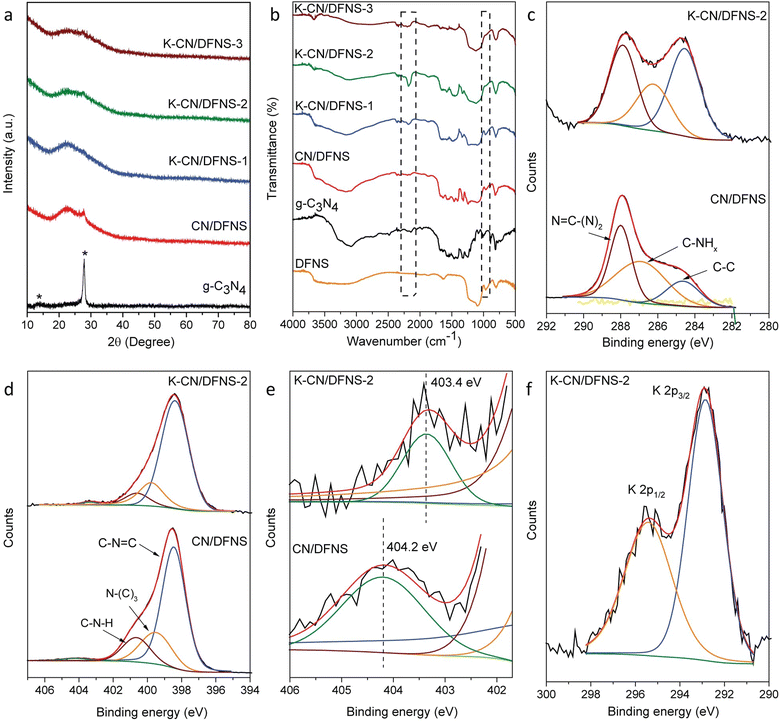
To determine the structural modification after doping with potassium ions, the PXRD, FTIR and XPS analyses of the K-CN/DFNS samples were recorded. The PXRD pattern of g-C3N4 (Fig. 2a) shows peaks at 13.2° and 27.9° corresponding to the 100 and 002 planes, which were ascribed to in-planar repeating units with a period of 0.672 nm and layered staking with the inter-planar spacing of 0.319 nm of aromatic systems, respectively.25,28 K-CN/DFNS samples show a very small peak at 27.7° corresponding to the 002 plane of g-C3N4. The absence of other peaks at 13.2° corresponding to the 100 plane could be because of its lower content and the presence of overlapping broad peaks of amorphous SiO2 at 22.7°.
To investigate the structural modification after doping with potassium ions, the FTIR spectra of the samples were recorded (Fig. 2b). The FTIR spectrum of g-C3N4 shows absorption bands at 810 and 890 cm−1 corresponding to the breathing mode of heptazine rings and the deforming mode of NH groups, respectively.29 The absorption bands observed in the range of 1200–1700 cm−1 correspond to stretching modes of the CN heterocycle.29 The absorption band at 1641 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration mode. Also, the absorption bands characteristic of aromatic C–N stretching were observed at 1247, 1326, 1432 and 1565 cm−1. The broad band in the range of 3000–4000 cm−1 attributed to stretching modes of the terminal –NH2 or –NH groups arises due to the presence of uncondensed amine groups and the adsorbed water molecules.18,29 Also, a small absorption band is observed at 2145 cm−1, which might correspond to the C–N stretching mode of the C
N stretching vibration mode. Also, the absorption bands characteristic of aromatic C–N stretching were observed at 1247, 1326, 1432 and 1565 cm−1. The broad band in the range of 3000–4000 cm−1 attributed to stretching modes of the terminal –NH2 or –NH groups arises due to the presence of uncondensed amine groups and the adsorbed water molecules.18,29 Also, a small absorption band is observed at 2145 cm−1, which might correspond to the C–N stretching mode of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (cyano) group. The FTIR spectrum of DFNS shows absorption bands at 1097 and 1192 cm−1 corresponding to transverse optical (TO) and longitudinal optical (LO) modes of the asymmetric stretching of the Si–O–Si bond, respectively.30 The weak absorption band at 966 cm−1 is attributed to Si–OH or Si–O-stretching and the absorption band at 807 cm−1 is due to Si–O–Si symmetric stretching.30,31 The band around 1638 cm−1 corresponds to bending vibrations of -OH and the broadband centered around 3400 cm−1 belongs to -OH and the adsorbed H2O molecules.20,30 The K-doped samples and undoped sample show the characteristic FTIR absorption bands of both DFNS and g-C3N4.
N (cyano) group. The FTIR spectrum of DFNS shows absorption bands at 1097 and 1192 cm−1 corresponding to transverse optical (TO) and longitudinal optical (LO) modes of the asymmetric stretching of the Si–O–Si bond, respectively.30 The weak absorption band at 966 cm−1 is attributed to Si–OH or Si–O-stretching and the absorption band at 807 cm−1 is due to Si–O–Si symmetric stretching.30,31 The band around 1638 cm−1 corresponds to bending vibrations of -OH and the broadband centered around 3400 cm−1 belongs to -OH and the adsorbed H2O molecules.20,30 The K-doped samples and undoped sample show the characteristic FTIR absorption bands of both DFNS and g-C3N4.
It is found that the peak at 2180 cm−1 corresponds to the asymmetric stretching mode of the cyano group and increases with an increase in the potassium content from 1 to 6 wt%. The cyano group appears as a result of incomplete polymerization.32 The excess KOH may prevent the polymerization of melamine, leading to defects in the K-doped samples. Also, the peak at 966 cm−1 corresponding to the Si–OH stretching disappeared in the case of the K-CN/DFNS-2 and K-CN/DFNS-3 samples, which might be due to the C–N–Si linkages that formed between Si–OH and –NH2 groups of g-C3N4, which was verified by the solid-state 13C and 1H NMR study in our previous publication.20 In the case of K-CN/DFNS-3, the weak absorption band in the region of 1200–1700 cm−1 does not appear prominently because of the lower loading of g-C3N4.
Furthermore, to learn more about the oxidation state of different elements and the interaction between potassium ions and g-C3N4, the XPS spectra of CN/DFNS, K-CN/DFNS-2 and K-CN/DFNS-2-used samples were analyzed. A survey scan of CN/DFNS shows the presence of C, N, O and Si on the surface, while K-CN/DFNS shows the existence of C, N, O, Si and K elements.
Fig. 2c shows a high-resolution spectrum of C 1s, which was deconvoluted into three peaks, namely 284.6, 286.9 and 287.9 eV, which could be assigned to the adventitious carbon, C–NHx species, and sp2 hybridized carbon N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–(N)2, respectively.19 An increase in the area of the peak at 287.9 eV indicates the higher amount sp2 hybridized carbon N
C–(N)2, respectively.19 An increase in the area of the peak at 287.9 eV indicates the higher amount sp2 hybridized carbon N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–(N)2 of g-C3N4 in CN/DFNS as compared to the K-CN/DFNS-2 sample. This can be correlated with the higher amount of g-C3N4 in CN/DFNS than in the K-CN/DFNS-2 sample.
C–(N)2 of g-C3N4 in CN/DFNS as compared to the K-CN/DFNS-2 sample. This can be correlated with the higher amount of g-C3N4 in CN/DFNS than in the K-CN/DFNS-2 sample.
The high-resolution N 1s spectra of the samples were deconvoluted into four peaks (Fig. 2d). The peaks appeared at 398.5, 399.5, 400.6 and 404.2 eV. The peak at 398.5 eV corresponds to the sp2 N present in triazine rings, i.e. C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds,19 while the peaks at 399.5 and 400.6 eV correspond to the tertiary nitrogen group N–(C)3 and amino group present due to incomplete polymerization, respectively.19 The peak appearing at 404.2 eV is ascribed to the charging effect of π excitations of the C–N conjugated system.33 The peak at 404.2 eV in CN/DFNS shifted to 403.4 eV in the potassium-doped g-C3N4 sample (Fig. 2e). This indicates that the damage of the π electrons might be due to the disorder in the structure of g-C3N4.19 Not much considerable shift was observed with other types of nitrogen species present in the K-CN/DFNS sample.
C bonds,19 while the peaks at 399.5 and 400.6 eV correspond to the tertiary nitrogen group N–(C)3 and amino group present due to incomplete polymerization, respectively.19 The peak appearing at 404.2 eV is ascribed to the charging effect of π excitations of the C–N conjugated system.33 The peak at 404.2 eV in CN/DFNS shifted to 403.4 eV in the potassium-doped g-C3N4 sample (Fig. 2e). This indicates that the damage of the π electrons might be due to the disorder in the structure of g-C3N4.19 Not much considerable shift was observed with other types of nitrogen species present in the K-CN/DFNS sample.
Peaks at binding energy 292.8 and 295.4 eV in the K-CN/DFNS sample were assigned to K 2p3/2 and K 2p1/2, respectively (Fig. 2f). These peaks could be due to the existence of K–N bonds.16 Also, the presence of the K 2s peak at 377.8 eV confirms the existence of K–N bonds (Fig. S3a, ESI†).16,34 The high-resolution O 1s spectrum shows peaks at positions of 532.6 and 533.7 eV originating from Si–O–Si bonds of silica and water molecules adsorbed on the surface, respectively (Fig. S3b, ESI†).23,35 The peak at binding energy of 103.4 eV corresponds to the Si 2p of the siloxane framework of DFNS (Fig. S3c, ESI†).23
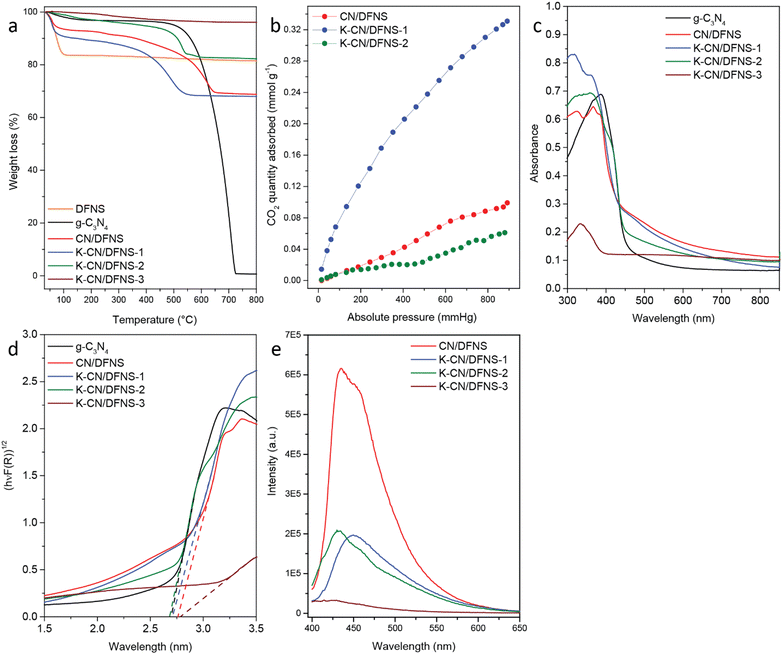
Thermogravimetric analysis of the sample was carried out to study the thermal stability of the material. TGA analysis from 30–800 °C in the presence of air was carried out (Fig. 3a). The thermogram of DFNS shows a weight loss in the region of 43–104 °C, corresponding to the loss of adsorbed water molecules. Pristine g-C3N4 showed weight losses at 220–346 °C and 448–711 °C corresponding to the weight losses due to the decomposition of unreacted melamine or melam and decomposition of g-C3N4.36,37 The thermogram of CN/DFNS showed weight losses in three steps. Step I: 34–132 °C corresponded to the weight loss due to physically adsorbed water. Step II: 132–283 °C was attributed to the weight loss due to the decomposition of residual intermediate products formed during the reaction. Step III: 310–670 °C was attributed to the thermal decomposition of g-C3N4.20 The thermograms of the K-CN/DFNS-1, K-CN/DFNS-2 and K-CN/DFNS-3 samples also show significant weight losses in two different regions. The weight loss occurred in two steps. Step I: 40–115 °C was attributed to the adsorbed water on the surface. Step II: 220–600 °C corresponded to the decomposition of g-C3N4. The offset temperature was found to increase from 593 °C to 749 °C with the increase in the potassium content.
N2 sorption analysis of K-CN/DFNS was performed to understand the textural properties and it showed a Type IV isotherm with hysteresis, indicating the mesoporous nature of the samples (Fig. S4 and S5, ESI†). The pores in the DFNS arise due to the gap between the fibres. CN/DFNS coated with 21.9 wt% of g-C3N4 shows a surface area of 150 m2 g−1 lower than that of DFNS (622 m2 g−1) but higher than g-C3N4 (15 m2 g−1). On the increase in the potassium content, the surface area varied from 88, 55 and 79 m2 g−1 for K-CN/DFNS-1, K-CN/DFNS-2 and K-CN/DFNS-3, respectively (Table 1). The pore volumes of the samples change from 0.158, to 0.062 and 0.086 cm3 g−1 for K-CN/DFNS-1, K-CN/DFNS-2 and K-CN/DFNS-3, respectively. The decrease in pore volume and surface area indicates the growth of g-C3N4 on the DFNS fibers and also within the pores (channels) of the DFNS. The presence of potassium resulted in the distortion of the structure of g-C3N4, which could have resulted in a further decrease in the pore size and surface area as compared to the undoped sample. Due to the lower loading of g-C3N4 in K-CN/DFNS-3, a slight increase in surface area was observed.
CO2 adsorption over different samples is given in Fig. 3b and Fig. S6 (ESI†). Among all the samples, K-CN/DFNS-1 has shown the highest CO2 adsorption of 0.31 mmol g−1 at 780 mm Hg of CO2 pressure and 298 K temperature. The improved adsorption could be due to increased basic sites in g-C3N4. These basic sites could be the increased –NH2 groups in the samples. K-CN/DFNS-2 is supposed to show higher CO2 adsorption as it has a higher content of potassium ions, around 6 wt%. However, it exhibits lower adsorption of CO2. This could be due to the incomplete polymerization of g-C3N4, as evident from the presence of the cyano group, which might result in a decrease in the amino groups in the sample. The adsorption of CO2 in the cases of CN/DFNS and K-CN/DFNS-2 could be due to the presence of pyridinic nitrogen and terminal amino groups.
The optical properties of the samples were evaluated by recording the UV-Visible DRS absorption spectra of the samples (Fig. 3c). The bandgap of the samples was calculated using Tauc plots (Fig. 3d) and tabulated in Table 1. The bandgap of pristine g-C3N4 was found to be 2.70 eV, while that of CN/DFNS was found to be 2.76 eV (Fig. 3c). CN/DFNS shows extended absorption in the range of 450–650 nm due to n to π* transition resulting from the distortion in the structure of g-C3N4.38 This distortion could be due to the formation of C–N–Si linkages between DFNS and g-C3N4 or the confined growth of g-C3N4 within the pores of DFNS.20 With the increase in the potassium content, the bandgap increases. The bandgap was found to be 2.71, 2.68 and 2.78 eV for K-CN/DFNS-1, K-CN/DFNS-2 and K-CN/DFNS-3, respectively. The bandgap value is found to be slightly lower for K-CN/DFNS-2. The lower content of g-C3N4 in the case of K-CN/DFNS-3 resulted in the generation of thin sheets of g-C3N4 grafted over DFNS. This might have resulted in the blueshift of the bandgap and less absorption of light. Along with the decrease in the bandgap on increasing the loading of potassium ions until 6 wt%, the absorption in the region of 450–650 nm increases. This absorption in the region of 450–650 nm occurs due to n → π* transition.38 These n → π* transitions are forbidden in perfectly symmetric and planar units.17 This transition becomes prominent with the increasing disorder in the structure of g-C3N4. In the case of doped samples, the presence of potassium ions inhibits the polymerization of g-C3N4, as evident from the presence of the cyano group in the FTIR spectra. This indicates that the presence of potassium ions not only affects the structural and thermal properties but also affects the electronic and optical properties of g-C3N4.
To gain insight into the separation of charge carriers, the steady-state and time-resolved photoluminescence spectra were recorded. The samples were excited at 380 nm and the emission spectra were recorded. Fig. 3e shows the steady-state photoluminescence spectra of the CN/DFNS and K-CN/DFNS samples. It was observed that the potassium-doped samples showed low emission intensity as compared to the CN/DFNS. This infers that the rate of radiative recombination of charges is low in the K-CN/DFNS samples as compared to CN/DFNS. K-CN/DFNS-3 (18.9 wt% potassium) showed the lowest emission intensity, while its photocatalytic activity is low. CN/DFNS showed a broad emission peak centered at 434 nm, while in the case of the K-CN/DFNS samples, the emission peak was blue shifted. These results are in concurrence with the UV-Visible DRS spectra, where the bandgaps of the potassium-doped (6 wt% and 18.9 wt%) samples were found to be higher than that of CN/DFNS.
To further understand the lifetimes of charge carriers, time-resolved decay profiles were recorded. The samples were excited at 395 nm and decay profiles were recorded. The lifetime decay profile was fitted with the tri-exponential fitting decay eqn 1 (Table 2). In the case of doped samples, the average lifetimes were found to increase with an increase in the potassium amount. The contribution from the shortest lifetime (τ1) increases with an increase in the potassium content. This indicates the effective transfer of charges to the impurity states or defect centers created after doping with potassium ions. This lowering in average lifetime as compared to g-C3N4 might be due to the increased charge recombination by the non-radiative pathway. This was also evident from the lowering of the PL intensity in the steady-state experiments.
| Sample name | τ 1 (ns) | A 1 | τ 2 (ns) | A 2 | τ 3 (ns) | A 3 | χ 2 | τ avg (ns) |
|---|---|---|---|---|---|---|---|---|
| Note: For all the samples excitation wavelength was 380 nm and emission wavelength was around 434 nm. | ||||||||
| g-C3N4 | 0.841 | 0.406 | 2.509 | 0.484 | 7.445 | 0.11 | 1.08 | 3.97 |
| CN/DFNS | 0.525 | 0.581 | 1.909 | 0.354 | 6.927 | 0.064 | 1.02 | 3.17 |
| K-CN/DFNS-1 | 0.122 | 0.791 | 1.271 | 0.166 | 5.27 | 0.043 | 1.694 | 1.45 |
| K-CN/DFNS-2 | 0.048 | 0.895 | 0.821 | 0.08 | 4.236 | 0.025 | 2.926 | 2.35 |
| K-CN/DFNS-3 | 0.018 | 0.92 | 0.988 | 0.061 | 4.929 | 0.02 | 1.004 | 3.11 |
Photocatalytic CO2 conversion using K-CN/DFNS
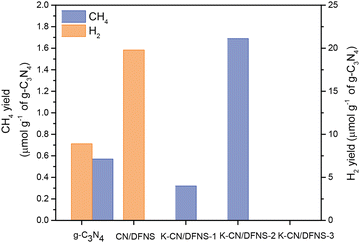
Photocatalytic CO2 conversion of the samples was evaluated under visible light illumination (Fig. 4). Moist CO2 was passed over 15 mg of K-CN/DFNS/Pt (1 wt% Pt was pre-deposited by chemical reduction method) in a batch reactor for 1 h and then irradiated under a 300 W Xe lamp (384–750 nm, 318 mW cm−2). Quantification of H2 and CH4 generated after 4 h of reaction was monitored by GC-TCD-FID. CN/DFNS showed only hydrogen generation, whereas in the case of potassium-doped samples, no H2 generation was observed. The optimum amount of potassium content is needed to get the maximum photocatalytic activity. In this case, 6 wt% potassium-doped g-C3N4 coated over DFNS shows the highest activity and preferential CO2 conversion over hydrogen generation. The highest methane yield observed was 1.7 μmol g−1 of g-C3N4 in 4 h using K-CN/DFNS-2 (6 wt% of potassium ion). The methane generation observed over the K-CN/DFNS-2 sample was found to be nearly three times higher than that of the pristine g-C3N4. The overall activity for methane generation was estimated from total consumed electrons using eqn (4) (TCEN). The TCEN for different samples were found to be 1.14, 0.64, and 3.4 μmol g−1 h−1 for the g-C3N4, K-CN/DFNS-1 and K-CN/DFNS-2, respectively. This enhancement in activity could be attributed to the improved light absorption, enhanced separation of charge carriers and suitable structural changes brought by the presence of the optimum amount of potassium.On the contrary, despite having lower CO2 adsorption, K-CN/DFNS-2 has shown better photocatalytic activity. CO2 adsorption is not the only criterion that defines the better yield in photocatalytic CO2 conversion. Other factors such as light absorption and the separation of charge carriers also play important roles. Therefore, in the case of K-CN/DFNS-2 the redshift in the absorption edge and the improved separation of charge carriers are the factors that influence the photocatalytic activity. In spite of having a greater average lifetime and extended absorption in the visible region, K-CN/DFNS-3 has not shown any conversion. This could be attributed to the lower amount of g-C3N4 loading over DFNS and increased bandgap, which further obstruct the light absorption properties and thus result in the lower amount of charge carrier generation.
To confirm the source of the carbon, isotope-labeled experiments were performed using 13CO2 and the products were analyzed by GC-MS. The mass fragmentation is given in Fig. S7 (ESI†). A signal at m/z = 17 corresponds to 13CH4. This confirms that methane is generated from the reduction of CO2. In addition to this, the sample recovered after the CO2 conversion reaction, K-CN/DFNS-2-used, was also studied by XPS (Fig. S8, ESI†). Not much change in the spectrum was observed. This implies that the catalyst remains stable after the photocatalytic reaction.
It is difficult to compare the yields of the products because of various experimental conditions such as intensity of the light source, amount of catalyst and water, reactor design, and distance between the reactor and light source. Very few reports are found on potassium-doped g-C3N4 systems.18,19 The methane yield reported in the current manuscript is lower than that in the reported literature on potassium-doped g-C3N4 (Table S1, ESI†). However, the activity for CO2 conversion using K-CN/DFNS was found to be higher than those of some of the doped g-C3N4 systems reported in the literature.39–42
This is the first time that the role of DFNS as a support and the effects of various amount contents of potassium ion on the photocatalytic property of g-C3N4 are studied. In this case, CN/DFNS, which showed a generation of primary hydrogen, was structurally modified by doping with potassium to tune the selectivity for CO2 reduction. The structural changes brought by doping of potassium ions, such as generation of a cyano group, formation of K–N bonds and the interface between DFNS and g-C3N4 through C–N–Si bonds, modification in the absorption spectrum and enhancement in charge carrier dynamics, have positively contributed to the improvement in activity and selectivity of the potassium-doped samples.
Band alignment and mechanism of photocatalytic CO2 conversion
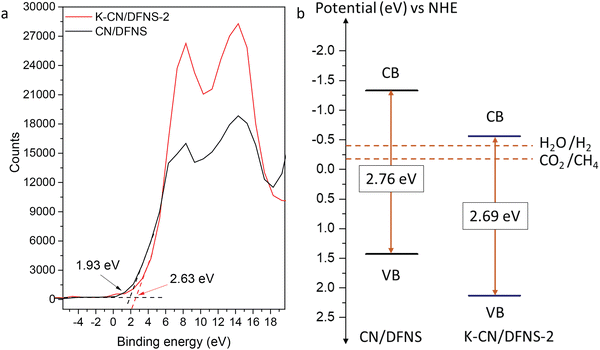
To understand the band structure and relative position of CB and VB, the valence band XPS spectra were recorded. The valence band XPS spectra of K-CN/DFNS-2 sample shows a slight shift in the valence band edge as shown in Fig. 5a. The structural changes brought by the presence of potassium ions, such as the existence of a cyano group and the disordered structure of g-C3N4 might have resulted in a shift in the position of the conduction and valence band edge.From the valence band XPS spectra, the differences between the Fermi level and valence band maximum (ΔE) of CN/DFNS and K-CN/DFNS-2 were found to be 1.93 and 2.63 eV, respectively. EVB was calculated using eqn (2). The valence band (EVB) of CN/DFNS and K-CN/DFNS-2 were found to be 1.43 and 2.13 eV, respectively. The conduction band (ECB) was calculated using the bandgap value estimated from EVB and the Tauc plot (Table 1 and Fig. 3d). The ECB values of CN/DFNS and K-CN/DFNS-2 were found to be −1.33 and −0.56 eV, respectively. Fig. 5b shows the positions of the conduction and valence bands in the CN/DFNS and K-CN/DFNS-2 samples.
The thermodynamic potential for the generation of methane and hydrogen are as follows:43
| CO2 + 8H+ + 8e− → CH4 + 2H2O E0 = −0.24 V vs. NHE |
| 2H+ + 2e− → H2 E0 = −0.41 V vs. NHE |
Due to the presence of potassium ions, both ECB and EVB of the K-CN/DFNS-2 samples have altered. The K-CN/DFNS-2 sample has a suitable band position for the generation of methane from CO2 and reduction of water. The position of the CB is more negative than the standard reduction potential of CO2, thus making its photocatalytic reduction feasible.
Lowering the conduction band potential to vary the potential of photogenerated electrons is one of the strategies to tune the product selectivity.44 In this case, the lowering of ECB of the K-CN/DFNS-2 sample by −0.77 eV as compared to that of CN/DFNS might be the reason for the improved selectivity for CO2 conversion.
The following mechanism was predicted from the observed data: when light falls on K-CN/DFNS, an electron gets excited to the conduction band by leaving behind a hole. The photogenerated hole can oxidize water and release protons, while the electron can migrate to Pt, where it can reduce CO2 in the presence of a proton to give methane. As compared to pristine g-C3N4, K-CN/DFNS-2 has shown better activity for methane generation and prohibited hydrogen generation due to the enhanced absorption of light, better separation of charge carriers, the shift of the conduction band and valence band towards positive potentials and moderate adsorption of CO2.
Conclusions
In this work, we were able to tune the selectivity of K-CN/DFNS and preferentially reduce CO2 over water reduction to generate hydrogen by simple potassium ion doping into the matrix of g-C3N4. It was observed that the potassium ion doping affected the structural, thermal, optical and electronic properties of g-C3N4. The SEM images confirm the grafting of the layers of potassium-doped g-C3N4 over DFNS. The presence of potassium ions hampers the polymerization of g-C3N4, which was evident from the presence of the cyano group in the FTIR spectra of the potassium-doped samples. The XPS analysis further confirms the interaction of potassium ions with the nitrogen of g-C3N4 and the disordered structure of g-C3N4. The lowering of the bandgap and the extended absorption in visible light was observed until 6 wt% of potassium ion loading. Also, the lifetime of the charge carriers increases with an increase in the potassium content. Improved productivity for CO2 conversion and suppression of hydrogen generation reaction was found over the potassium-doped samples. Among all the samples, K-CN/DFNS-2 (with 6 wt% of potassium ion doped in g-C3N4) has shown the highest CO2 conversion, with a 1.7 μmol g−1 of g-C3N4 yield of methane, in 4 h under visible light irradiation. The UV-DRS, PL, and lifetime measurement studies indicated that the improved activity and preferential CO2 reduction over hydrogen generation were due to the extended absorption in the visible region, better separation of charge carriers, shifting of the conduction band and valence band towards positive potentials and moderate adsorption of CO2. This study will be useful for the designing of catalysts with improved selectivity for CO2 conversion over hydrogen generation.Conflicts of interest
There are no conflicts to declare.References
- M. Khalil, J. Gunlazuardi, T. A. Ivandini and A. Umar, Photocatalytic conversion of CO2 using earth-abundant catalysts: A review on mechanism and catalytic performance, Renewable Sustainable Energy Rev., 2019, 113, 109246 CrossRef CAS.
- L. Xu, Y. Xiu, F. Liu, Y. Liang and S. Wang, Research progress in conversion of CO2 to valuable fuels, Molecules, 2020, 25, 3653 CrossRef CAS PubMed.
- T. Kong, Y. Jiang and Y. Xiong, Photocatalytic CO2 conversion: What can we learn from conventional COx hydrogenation?, Chem. Soc. Rev., 2020, 49, 6579–6591 RSC.
- U. Ulmer, T. Dingle, P. N. Duchesne, R. H. Morris, A. Tavasoli, T. Wood and G. A. Ozin, Fundamentals and applications of photocatalytic CO2 methanation, Nat. Commun., 2019, 10, 3169 CrossRef PubMed.
- Z. Bi, R. Guo, X. Hu, J. Wang, X. Chen and W. Pan, Research progress on photocatalytic reduction of CO2 based on LDH materials, Nanoscale, 2022, 14, 3367–3386 RSC.
- Y. Miao, R. Guo, J. Gu, Y. Liu, G. Wu, C. Duan and W. Pan, Oxygen vacancy-rich BiO2−x: Super-active co-catalyst on g-C3N4 for efficient visible-light photocatalytic CO2 reduction, J. CO2 Util., 2021, 44, 101377 CrossRef CAS.
- G. Dong, Y. Zhang, Q. Pan and J. Qiu, A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties, J. Photochem. Photobiol., C, 2014, 20, 33–50 CrossRef CAS.
- G. Mamba and A. K. Mishra, Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation, Appl. Catal., B, 2016, 198, 347–377 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, A metal-free polymeric photocatalyst for hydrogen production from water under visible light, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- D. J. Martin, K. Qiu, S. A. Shevlin, A. D. Handoko, X. Chen, Z. Guo and J. Tang, Highly efficient photocatalytic H2 evolution from water using visible light and structure-controlled graphitic carbon nitride, Angew. Chem., Int. Ed., 2014, 53, 9240–9245 CrossRef CAS.
- S. Samanta and R. Srivastava, Catalytic conversion of CO2 to chemicals and fuels: the collective thermocatalytic/photocatalytic/electrocatalytic approach with graphitic carbon nitride, Mater. Adv., 2020, 1, 1506–1545 RSC.
- L. Jiang, X. Yuan, Y. Pan, J. Liang, G. Zeng, Z. Wu and H. Wang, Doping of graphitic carbon nitride for photocatalysis: A review, Appl. Catal., B, 2017, 217, 388–406 CrossRef CAS.
- Y. Wang, S. Zhao, Y. Zhang, J. Fang, Y. Zhou, S. Yuan, C. Zhang and W. Chen, One-pot synthesis of K-doped g-C3N4 nanosheets with enhanced photocatalytic hydrogen production under visible-light irradiation, Appl. Surf. Sci., 2018, 440, 258–265 CrossRef CAS.
- J. Ma, W. Zhou, X. Tan and T. Yu, Potassium ions intercalated into g-C3N4-modified TiO2 nanobelts for the enhancement of photocatalytic hydrogen evolution activity under visible-light irradiation, Nanotechnology, 2018, 29, 21 Search PubMed.
- J. Jiang, S. Cao, C. Hu and C. Chen, A comparison study of alkali metal-doped g-C3N4 for visible-light photocatalytic hydrogen evolution, Chin. J. Catal., 2017, 38, 1981–1989 CrossRef CAS.
- S. Hu, F. Li, Z. Fan, F. Wang, Y. Zhao and Z. Lv, Band gap-tunable potassium doped graphitic carbon nitride with enhanced mineralization ability, Dalton Trans., 2015, 44, 1084–1092 RSC.
- H. Katsumata, K. Sakakibara, I. Tateishi, M. Furukawa and S. Kaneco, Structurally modified graphitic carbon nitride with highly photocatalytic activity in the presence of visible light, Catal. Today, 2020, 352, 47–53 CrossRef CAS.
- S. Wang, J. Zhan, K. Chen, A. Ali, L. Zeng, H. Zhao, W. Hu, L. Zhu and X. Xu, Potassium-doped g-C3N4 achieving efficient visible-light-driven CO2 reduction, ACS Sustainable Chem. Eng., 2020, 8(22), 8214–8222 CrossRef CAS.
- Z. Sun, S. Wang, Q. Li, M. Lyu, T. Butburee, B. Luo, H. Wang, J. M. T. A. Fischer, C. Zhang, Z. Wu and L. Wang, Enriching CO2 activation sites on graphitic carbon nitride with simultaneous introduction of electron-transfer promoters for superior photocatalytic CO2-to-fuel conversion, Adv. Sustainable Syst., 2017, 1, 1700003 CrossRef.
- S. A. Rawool, A. Samanta, T. G. Ajithkumar, Y. Kar and V. Polshettiwar, Photocatalytic hydrogen generation and CO2 conversion using g-C3N4 decorated dendritic fibrous nanosilica: role of interfaces between silica and g-C3N4, ACS Appl. Energy Mater., 2020, 3, 8150–8158 CrossRef CAS.
- A. Maity, R. Belgamwar and V. Polshettiwar, Facile synthesis protocol to tune size, textural properties & fiber density of dendritic fibrous nanosilica (DFNS): applications in catalysis and CO2 capture, Nat. Protoc., 2019, 14, 2177–2204 CrossRef CAS.
- V. Polshettiwar, Dendritic Fibrous Nano-Silica (DFNS): Discovery, Synthesis, Formation Mechanism, Catalysis, and CO2 Capture-Conversion, Acc. Chem. Res., 2022, 55, 1395–1410 CrossRef CAS PubMed.
- A. K. Mishra, R. Belgamwar, R. Jana, A. Datta and V. Polshettiwar, Defects in nanosilica catalytically convert CO2 to methane without any metal and ligand, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 6383–6390 CrossRef CAS.
- A. Maity, S. Chaudhari, J. J. Titman and V. Polshettiwar, Nanosponges of acidic amorphous aluminosilicate for catalysis, plastic degradation and CO2 to fuel conversion, Nat. Commun., 2020, 11, 3828 CrossRef PubMed.
- K. Li, F.-Y. Su and W.-D. Zhang, Modification of g-C3N4 nanosheets by carbon quantum dots for highly efficient photocatalytic generation of hydrogen, Appl. Surf. Sci., 2016, 375, 110–117 CrossRef CAS.
- Principles of Fluorescence Spectroscopy, ed., Lakowicz J. R., Springer, USA, 2006 Search PubMed.
- J. Huang, G. Nie and Y. Ding, Metal-free enhanced photocatalytic activation of dioxygen by g-C3N4 doped with abundant oxygen-containing functional groups for selective N-deethylation of Rhodamine B, Catalysts, 2020, 10, 6 CrossRef CAS.
- Y. Yuan, L. Zhang, J. Xing, M. I. B. Utama, X. Lu, K. Du, Y. Li, X. Hu, S. Wang, A. Genç, R. Dunin-borkowski, J. Arbiol and Q. Xiong, High-yield synthesis and optical properties of g-C3N4, Nanoscale, 2015, 7, 12343–12350 RSC.
- L. Ge, F. Zuo, J. Liu, Q. Ma, C. Wang, D. Sun, L. Bartels and P. Feng, Synthesis and efficient visible light photocatalytic hydrogen evolution of polymeric g-C3N4 Coupled with CdS quantum dots, J. Phys. Chem. C, 2012, 116, 13708–13714 CrossRef CAS.
- R. Singh, N. Bayal, A. Maity, D. J. Pradeep, J. Trébosc, P. K. Madhu, P. Lafon and V. Polshettiwar, Probing the interfaces in nanosilica-supported TiO2 photocatalysts by solid-state NMR and in situ FTIR, ChemNanoMat, 2018, 4, 1231–1239 CrossRef CAS.
- B. Lin, C. Xue, X. Yan, G. Yang, G. Yang and B. Yan, Facile fabrication of novel SiO2/g-C3N4 core–shell nanosphere photocatalysts with enhanced visible light activity, Appl. Surf. Sci., 2015, 357, 346–355 CrossRef CAS.
- M. Zhang, X. Bai, D. Liu, J. Wang and Y. Zhu, Enhanced catalytic activity of potassium-doped graphitic carbonnitride induced by lower valence position, Appl. Catal., B, 2015, 164, 77–81 CrossRef CAS.
- J. Yu, K. Wang, W. Xiao and B. Cheng, Photocatalytic reduction of CO2 into hydrocarbon solar fuels over g-C3N4–Pt nanocomposite photocatalysts, Phys. Chem. Chem. Phys., 2014, 16, 11492–11501 RSC.
- J. Sharma, T. Gora, J. D. Rimstidt and R. Staley, X-Ray photoelectron spectra of the alkali azides, Chem. Phys. Lett., 1972, 15, 232 CrossRef CAS.
- A. Thøgersen, J. H. Selj and E. S. Marstein, Oxidation effects on graded porous silicon anti-reflection coatings, J. Electrochem. Soc., 2012, 159(5), D276–D281 CrossRef.
- Y. Zhao, Z. Liu, W. Chu, L. Song, Z. Zhang, D. Yu, Y. Tian, S. Xie and L. Sun, Large-Scale synthesis of nitrogen-rich carbon nitride microfibers by using graphitic carbon nitride as precursor, Adv. Mater., 2008, 20, 1777–1781 CrossRef CAS.
- S. C. Yan, Z. S. Li and Z. G. Zou, Photodegradation performance of g-C3N4 fabricated by directly heating melamine, Langmuir, 2009, 25, 10397–10401 CrossRef CAS PubMed.
- M. K. Bhunia, S. Melissen, M. R. Parida, P. Sarawade, J. Basset, D. H. Anjum, O. F. Mohammed, P. Sautet, T. L. Bahers and K. Takanabe, Dendritic tip-on polytriazine-based carbon nitride photocatalyst with high hydrogen evolution activity, Chem. Mater., 2015, 27, 8237–8247 CrossRef CAS.
- Q. Huang, J. Yuab, S. Cao, C. Cui and B. Cheng, Efficient photocatalytic reduction of CO2 by amine-functionalized g-C3N4, Appl. Surf. Sci., 2015, 358, 350–355 CrossRef CAS.
- K. Wang, Q. Li, B. Liu, B. Cheng, W. Ho and J. Yu, Sulfur-doped g-C3N4 with enhanced photocatalytic CO2- reduction performance, Appl. Catal., B, 2015, 176–177, 44–52 CAS.
- X. Liu, P. Wang, H. Zhai, Q. Zhang, B. Huang, Z. Wang, Y. Liu, Y. Dai, X. Qin and X. Zhang, Synthesis of synergetic phosphorus and cyano groups (–C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) modified g-C3N4 for enhanced photocatalytic H2 production and CO2 reduction under visible light irradiation, Appl. Catal., B, 2018, 232, 521–530 CrossRef CAS.
N) modified g-C3N4 for enhanced photocatalytic H2 production and CO2 reduction under visible light irradiation, Appl. Catal., B, 2018, 232, 521–530 CrossRef CAS. - M. Arumugam, M. Tahir and P. Praserthdam, Effect of nonmetals (B, O, P, and S) doped with porous g-C3N4 for improved electron transfer towards photocatalytic CO2 reduction with water into CH4, Chemosphere, 2022, 286(2), 131765 CrossRef CAS PubMed.
- J. Pan, X. Wu, L. Wang, G. Liu, G. Q. Lu and H.-M. Cheng, Synthesis of anatase TiO2 rods with dominant reactive {010} facets for the photoreduction of CO2 to CH4 and use in dye-sensitized solar cells, Chem. Commun., 2011, 47, 8361–8363 RSC.
- J. Fu, K. Jiang, X. Qiu, J. Yu and M. Liu, Product selectivity of photocatalytic CO2 reduction reactions, Mater. Today, 2020, 32, 222–243 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00809b |
| This journal is © The Royal Society of Chemistry 2022 |