Open Access Article
Open Access ArticleNanostructured Ni(OH)2–ZnO mixed crystals as recyclable catalysts for the synthesis of N-unsubstituted 1,2,3-triazoles†
Priyanuj Krishnann
Hazarika
,
Priyanka
Gogoi
,
Roktopol
Hazarika
,
Kalyanjyoti
Deori
 * and
Diganta
Sarma
* and
Diganta
Sarma
 *
*
Department of Chemistry, Dibrugarh University, Dibrugarh, Assam 786004, India. E-mail: kalchemdu@gmail.com; dsarma22@gmail.com
First published on 21st September 2022
Abstract
A novel and sustainable way of constructing medicinally active compounds, 4-aryl-1,2,3-(NH)-triazoles, has been developed by employing Ni(OH)2–ZnO nanostructured mixed crystals. The one-pot multicomponent synthesis giving nanosheet-like reusable catalysts in PEG-400 solvent is quite efficient, economical and can tolerate a wide range of substrates with excellent yields.
Nanostructured materials have been harnessed in an array of revolutionary applications1 as an alternative to traditional catalysts in a greener and more sustainable way, mainly due to their incredible surface-areas2 and tremendous mechanical3 and catalytic4 properties. In addition, good selectivity, high stability and recyclability have manifested these materials to be involved as a potent applicant in various organic reactions such as chemo-selective oxidation,5 oxidative amination,6ipso hydroxylation7 and many more.8–10
The enormous applicability of nanomaterials has encouraged us to incorporate them into the synthesis of a biologically active heterocyclic scaffold, NH-1,2,3-triazole, using a greener approach. This five-membered aromatic nucleus belongs to the kingdom of 1,2,3-triazoles. Due to its good resemblance to the amide bonds, this structural motif has been exploited for the synthesis of a large number of medicinal scaffolds11 to treat deadly diseases like AIDS, cancer and tuberculosis. In particular, 4-(2-chlorophenyl)-1H-1,2,3-triazole possesses some intriguing features to act as an IDO (indoleamine 2,3-dioxygenase) inhibitor for cancer immunotherapy12 and 4-(3,4-dibromophenyl)-1H-1,2,3-triazole serves as a methionine aminopeptidase enzyme13 that produces anticancer and antibacterial agents.
Various methodologies have been put forward to access these compounds that mainly follow the general procedures such as the condensation reaction between olefin and azides14 or the direct reaction between sodium azide and nitroolefin15 or the multicomponent synthesis from a nitroalkane, aldehyde and NaN3.16 However, a large number of these protocols mainly use copper17 or palladium18-based catalysts and other catalytic systems such as Amberlyst,19p-TsOH,20 Bi2WO6/water,21etc., which limits their practical use at industrial levels including low yield or substrate polymerization.22 Moreover, copper is cytotoxic to mammalian cells, which hinders the use of these protocols in biological systems.23
Thus, owing to an increasing demand for the development of a sustainable strategy for the synthesis of NH-triazole, we have pioneered a greener and an efficient catalytic system for the aforementioned purposes. As such, ZnO nanoparticles stabilized/supported on CTAB (cetyl trimethyl ammonium bromide) as a highly stable catalyst have been selected for our study. This biodegradable and non-toxic white powder material has a large energy band gap (3.37 eV) and 60 meV bonding energy, which endows it with tremendous chemical and thermal stabilities.24 Moreover, ZnO NPs have a large surface area and their morphologies such as nanowires25 or nanorods26 can be modified by controlling the reaction time, temperature or surfactants27 and this makes the nanoparticles highly effective as a heterogenous catalyst.28 CTAB here acts as a stabilizer or capping agent that prevents the particles from agglomerating by obstructing overgrowth of these particles.29
However, when we explored the catalytic activity of ZnO supported on CTAB nanomaterials in the multicomponent synthesis of N-unsubstituted triazoles, the product obtained was not very satisfactory. In recent years, nickel-based catalysts have been in the spotlight for the development of N-substituted 1,2,3-triazoles.30–34 Getting fortified from these works, we thought of incorporating nickel into our catalytic system with a view that it may introduce some synergistic effect on ZnO nanoparticles, which will enhance the formation of N-unsubstituted triazoles with a gratifying yield. Hence, by using the co-precipitation method, we prepared Ni(OH)2–ZnO nanostructured mixed crystals, which when added to the reaction mixture produced the desired triazole with an excellent yield (∼96%)(Fig. S1, ESI†).
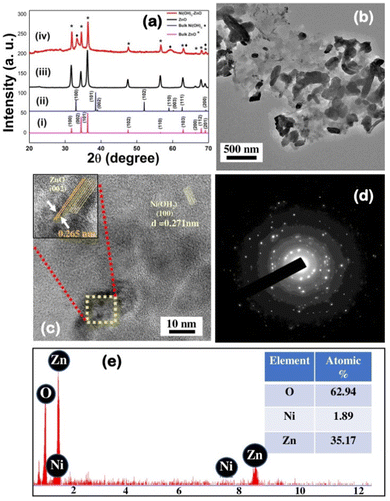
The as-synthesized ZnO and Ni(OH)2–ZnO nanocrystals were probed for structural and morphology study with the help of powder XRD and HRTEM analysis. As can be seen from Fig. 1a(iii), all the distinct XRD peaks are completely matching with the wurtzite structure of ZnO (JCPDS card no. 005-0064, space group P63mc). After addition of nickel precursor to the zinc acetate solution, the PXRD of the final crystalline precipitate exhibited two additional characteristic peaks at 33° and 60° corresponding to the (100) and (003) planes of Ni(OH)2 (JCPDS card no. 14-117, space group P3m1) [Fig. 1a(iv)]. As suggested by XRD, the crystal growth of the as-synthesized nanocrystal is along the highest intensity peak, i.e. along the (101) plane, which justifies the sheet-like morphology of the above-mentioned nanocrystal.35 The low magnification TEM image shows that the as-prepared Ni(OH)2–ZnO nanocrystals consist of sheet-like architectures of irregular shapes (Fig. 1b). Focusing on individual sheets under HRTEM clearly visualizes the lattice fringes of the (002) planes of ZnO and (100) planes of Ni(OH)2, which clearly indicates the formation of mixed crystal type nanostructured particles (Fig. 1c). The interplanar distance between the (002) planes of ZnO is measured to be 2.65 nm, which matches well with the standard data.36 The selected area electron diffraction (SAED) pattern depicts the pure crystalline nature of the nanocrystal (Fig. 1d). The surface topography has been studied with the help of field emission scanning electron microscopy (FESEM) showing hazy cloud-like morphology of the nanocrystal (Fig. S2, ESI†). EDX justifies the presence of all the elements with stoichiometric ratios in the as-synthesized nanocrystal (Fig. 1e).
To further confirm the oxidation state of the metals present in the Ni(OH)2–ZnO nanocrystal, high resolution XPS was performed. The Zn 2p spectrum in Fig. 2a depicts the presence of two doublets of binding energies of 1021.3 eV and 1044.5 eV, which can be assigned to Zn 2p3/2 and Zn 2p1/2, respectively. The difference in binding energies between the two peaks is 23.2 eV which reveals the presence of ZnO in the crystal.37–39 The O 1s spectrum in Ni(OH)2–ZnO (Fig. 2b) shows its standard binding energy peak at 531.3 eV. Fig. 2c shows Ni 2p3/2 and Ni 2p1/2 core peaks appearing at 856.2 eV and 873.8 eV with energy spacing of 17 eV. The corresponding satellite peaks are observed at 862.4 eV and 880.4 eV and have the same energy spacing of 17 eV, which indicates the presence of Ni(OH)2 with Ni in 2+ oxidation state.40 The survey spectrum (Fig. 2d) of the sample confirms the presence of 2p states of both Ni and Zn along with 1s states of oxygen and carbon.
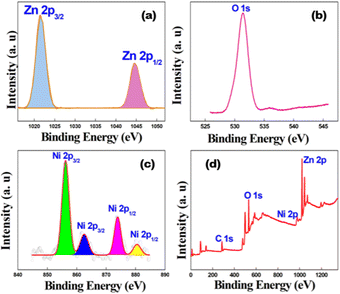 | ||
| Fig. 2 The high resolution XPS spectra of (a) Zn 2p, (b) O 1s, and (c) Ni 2p and (d) the wide survey scan of the as-synthesized Ni(OH)2–ZnO nanocrystal. | ||
The catalytic activity of the newly developed nanomaterial was studied for the synthesis of 4-aryl-NH-1,2,3-triazoles for which 4-chlorobenzaldehyde (1 mmol), nitromethane (2 mmol) and sodium azide (3 mmol) were chosen as the model substrates. The reaction was performed in PEG-400 at 100 °C and the results obtained are depicted in Table S1 (ESI†). Different quantities of the catalyst were used for investigation and it was found that with 25 mg of the catalyst, the desired product was obtained in a superior yield (96%). However, with a decrease in catalyst loading from 25 mg to 15 mg and 10 mg, the progress of the reaction was hampered and none of them exhibited better yields than the former amount (Table S1, ESI†).
In order to find the most appropriate solvent, different polar and non-polar solvents were screened and PEG-400 was found to be the best solvent. Remarkably, water and ethanol failed to promote the reaction with better yields probably due to the low solubility of the substrates in these polar solvents. When the feasibility of this catalytic system was evaluated with different solvents such as dimethylsulphoxide (DMSO), dimethylformamide (DMF), dichloromethane (DCM), ethylene glycol and toluene, the cycloadduct was produced with relatively inferior yields (Table S2, ESI†).
When the reaction was performed at room temperature, only a trace amount of the product was obtained even after increasing the amount of the catalyst and the time duration for more than 4 hours. However, on elevating the temperature, the reaction began to show much improved yield. At 100 °C, the reaction afforded the 4-aryl-NH-1,2,3-triazoles with the best yield signifying it to be the optimum temperature (Table S2, ESI†).
Furthermore, reaction with zinc acetate and nickel acetate as the catalysts could produce the product in 45% and 50% yields, respectively. Moreover, in the absence of the catalyst, the result acquired is quite unsatisfactory, which clearly articulated the need for the developed catalyst (Table S3, ESI†).
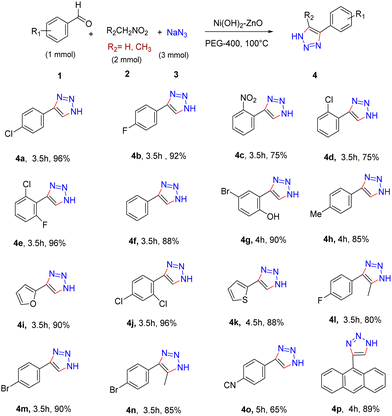
Next, we explored various aromatic aldehydes and nitro alkanes in order to evaluate the applicability of the catalyst (Scheme 1). The reaction of benzaldehyde with nitromethane under the optimized catalytic conditions provided the corresponding triazole in 88% yield (4f). On exploring other aromatic aldehydes bearing various functional groups, it was found that substrates bearing halo substituents such as fluoro, bromo and chloro (4a, 4b, 4d, 4e, 4j, and 4m) and nitro groups (4c) afforded the respective triazoles with 75–96% yield. However, 4-cyanobenzaldehyde yielded the triazole product with a lower yield (4o).
Aldehydes containing electron-donating groups such as hydroxyl (4g) or methyl (4h) also participated smoothly under the reaction conditions. In addition to this, heterocyclic aldehydes also get transformed into the desired products with excellent yields (4i, 4k, and 4p). Furthermore, the reactions of nitroethane with different aldehydes were investigated and they reacted with ease to form NH-triazoles with good yields (4n, 4l). From Scheme 1, it could be summarized that the newly developed catalyst is quite amicable to a wide range of aldehydes and nitroalkanes regardless of the electronic nature of the substituents present on the aldehydes and nitroalkanes.
Moreover, the catalyst can be reused successfully for up to three times without any significant drop in its activity (Fig. S3, ESI†).
In order to demonstrate the synthetic effectiveness of this protocol on an industrial level, we performed two gram-scale reactions using 4-fluorobenzaldehyde and 4-bromobenzaldehyde and both of them yielded the respective triazoles in appreciable yields (Table S4, ESI†).
The enhanced catalytic activity of the mixed nanostructured Ni(OH)2–ZnO crystal is believed to be due to the synergistic effect of both Ni and Zn. Here, zinc oxide acts as the active site, which is attacked by the oxygen atom of the nitroolefin because zinc oxide is Lewis acidic in nature.41 Ni(OH)2, on the other hand, may impose some synergistic effect on ZnO nanomaterials, which enhances the yield of the N-unsubstituted triazoles. The synergistic effect of Ni(OH)2 and ZnO can be demonstrated from our experimental works as shown in Table S3 (ESI†). When ZnO nanomaterial was used alone for carrying out the reaction, a yield of about 80% of the NH-triazole was obtained. Similarly, Ni(OAc)2 and Ni(OH)2, used alone gave only 50% and 45% yields of the desired product, respectively. But, when the catalyst Ni(OH)2–ZnO was employed for the multicomponent reaction, 96% of the product was formed, which indicates that Ni and Zn may have some cooperative effect on each other.42
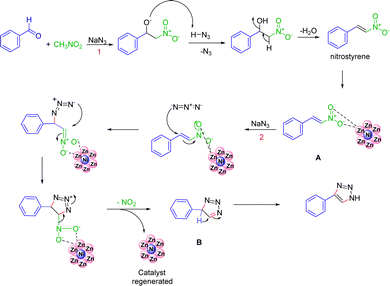
Based on the literature a plausible mechanism has been proposed. The mechanism first involves the reaction between nitroalkane and aldehyde to form nitrostyrene, which coordinates with the surface of the catalyst (A). Then, the nucleophilic azide adds to the electrophilic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the intermediate followed by cyclization and loss of a nitro group to form B. Further aromatisation results in the formation of the triazole with regeneration of the catalyst41 (Fig. 3).
C double bond of the intermediate followed by cyclization and loss of a nitro group to form B. Further aromatisation results in the formation of the triazole with regeneration of the catalyst41 (Fig. 3).
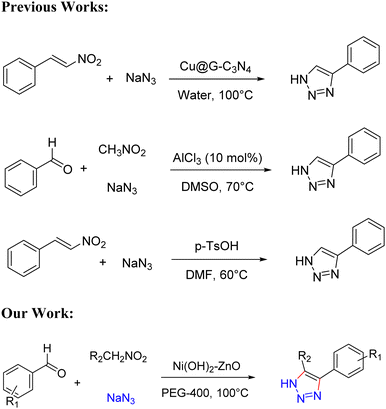
Our catalytic system, Ni(OH)2–ZnO nanostructured mixed crystals, is believed to be more environmental friendly and efficient than the previous works for the synthesis of the NH-triazoles. We have compared our protocol with the literature reports (Scheme 2).15,20,43
In a nutshell, we have prepared a highly stable heterogeneous catalyst that showed appreciable catalytic activity in the multicomponent synthesis of 4-aryl-NH-1,2,3-triazoles. The Ni(OH)2–ZnO mixed nanocrystals supported on CTAB are simple to prepare, sturdy, recoverable and easily reusable for up to three times without any significant loss in their catalytic performance. Apart from this, the catalyst is also able to tolerate a wide range of aldehydes and nitro alkanes, which makes this methodology suitable for the sustainable synthesis of various unsubstituted NH-triazoles.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. S. and K. D. are thankful to CSIR, New Delhi, India for a research grant [Grant No. 02(0399)/21/EMR-II]. K. D. is grateful to SERB-DST, India (Grant EEQ/2018/000326) for financial assistance. P. K. H. thanks CSIR, New Delhi for a research fellowship. The authors acknowledge IISER-Mohali for TEM analysis, CSIR-NEIST Jorhat for XPS, STIC Cochin for XRD, CSIC Dibrugarh University for NMR measurements and Dibrugarh University for providing all infrastructural facilities.Notes and references
- (a) H. B. Dias, M. I. B. Bernardi, V. S. Marangoni, A. C. de, A. Bernardi, A. N. S. Rastelli and A. C. Hernandes, Mater. Sci. Eng., C, 2019, 96, 391 CrossRef CAS PubMed; (b) M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371 RSC; (c) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS PubMed; (d) R. S. Varma, Green Chem., 2014, 16, 2027 RSC; (e) J. Damodharan, Mater. Today, 2021, 37, 383 CAS.
- J. Govan and Y. K. Gun'ko, Nanomaterials, 2014, 4, 222 CrossRef.
- D. Guo, G. Xie and J. Luo, J. Phys. D: Appl. Phys., 2014, 47, 013001 CrossRef CAS.
- M. C. Daniel and D. Astru, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed.
- A. Saxena, A. Kumar and S. Mozumdar, J. Mol. Catal. A: Chem., 2007, 269, 35 CrossRef CAS.
- M. Behzadi, M. M. Hashemi, M. Roknizadeh, S. Nasiri and A. R. Saadatabadi, New J. Chem., 2021, 45, 3242 RSC.
- V. Sadhasivam, M. Harikrishnan, G. Elamathi, R. Balasaravanan, S. Murugesan and A. Siva, New J. Chem., 2020, 44, 6222 RSC.
- L. L. Chng, N. Erathodiyil and J. Y. Ying, Acc. Chem. Res., 2013, 46, 1825 CrossRef CAS PubMed.
- K. Hong, M. Sajjadi, J. M. Suh, K. Zhang, M. Nasrollahzadeh, H. W. Jang, R. S. Varma and M. Shokouhimehr, ACS Appl. Nano Mater., 2020, 3, 2070 CrossRef CAS.
- A. M. Ferretti, A. Ponti and G. Molteni, Tetrahedron Lett., 2015, 56, 5727 CrossRef CAS.
- (a) L. S. Kallander, Q. Lu, W. Chen, T. Tomaszek, G. Yang, D. Tew, T. D. Meek, G. a Hofmann, C. K. Schulz-Pritchard, W. W. Smith, C. a Janson, M. D. Ryan, G. F. Zhang, K. O. Johanson, R. B. Kirkpatrick, T. F. Ho, P. W. Fisher, M. R. Mattern, R. K. Johnson, M. J. Hansbury, J. D. Winkler, K. W. Ward, D. F. Veber and S. K. Thompson, J. Med. Chem., 2005, 48, 5644 CrossRef CAS PubMed; (b) T. Weide, S. A. Saldanha, D. Minond, T. P. Spicer, J. R. Fotsing, M. Spaargaren, J.-M. Frère, C. Bebrone, K. B. Sharpless, P. S. Hodder and V. V. Fokin, ACS Med. Chem. Lett., 2010, 1, 150 CrossRef CAS PubMed; (c) A. R. Gakovic, J. J. Csanádi, E. A. Djurendic, O. Klisuric, G. Bogdanovic and K. M. P. Gaši, Tetrahedron Lett., 2009, 50, 4107 CrossRef; (d) Q. Huang, M. Zheng, S. Yang, C. Kuang, C. Yu and Q. Yang, Eur. J. Med. Chem., 2011, 46, 5680 CrossRef CAS; (e) U. F. Röhrig, S. R. Majjigapu, A. Grosdidier, S. Bron, V. Stroobant, L. Pilotte, D. Colau, P. Vogel, B. J. Van den Eynde, V. Zoete and O. Michielin, J. Med. Chem., 2012, 55, 5270 CrossRef PubMed.
- U. F. RoHrig, L. Awad, A. Grosdidier, P. Larrieu, V. Stroobant, D. Colau, V. Cerundolo, A. J. G. Simpson, P. Vogel and B. Van den Eynde, J. Med. Chem., 2010, 53, 1172 CrossRef CAS PubMed.
- L. S. Kallander, Q. Lu, W. Chen, T. Tomaszek, G. Yang, D. Tew, T. D. Meek, G. A. Hofmann, C. K. Schulz-Pritchard and W. W. Smith, J. Med. Chem., 2005, 48, 5644 CrossRef CAS.
- (a) J. Li, D. Wang, Y. Zhang, J. Li and B. Chen, Org. Lett., 2009, 11, 3024 CrossRef CAS; (b) T. Jin, S. Kamijo and Y. Yamamoto, Tetrahedron Lett., 2004, 45, 9435 CrossRef CAS; (c) L. H. Lu, J. H. Wu and C. H. Yang, J. Chin. Chem. Soc., 2008, 55, 414 CrossRef CAS.
- S. Payra, A. Saha and S. Banerjee, ChemCatChem, 2018, 10, 5468 CrossRef CAS.
- A. Garg, R. Hazarika, N. Dutta, B. Dutta and D. Sarma, ChemistrySelect, 2021, 6, 7266 CrossRef CAS.
- Y. Chen, G. Nie, Q. Zhang, S. Ma, H. Li and Q. Hu, Org. Lett., 2015, 17, 1118 CrossRef CAS.
- J. John, J. Thomas, N. Parekh and W. Dehaen, Eur. J. Org. Chem., 2015, 4922 CrossRef CAS.
- H. Zhang, D. Z. Dong and Z. L. Wang, Synthesis, 2016, 131 Search PubMed.
- X. J. Quan, Z. H. Ren, Y. Y. Wang and Z. H. Guan, Org. Lett., 2014, 16, 5728 CrossRef CAS PubMed.
- B. Paplal, S. Nagaraju, V. Palakollu, S. Kanvah, B. V. Kumar and D. Kashinath, RSC Adv., 2015, 5, 57842 RSC.
- A. Saha, C. M. Wu, R. Peng, R. Koodali and S. Banerjee, Eur. J. Org. Chem., 2019, 104 CrossRef CAS.
- (a) A. J. Link, M. K. S. Vink, N. J. Agard, J. A. Prescher, C. R. Bertozzi and D. A. Tirrell, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10180 CrossRef CAS PubMed; (b) G. J. Brewer, Chem. Res. Toxicol., 2010, 23, 319 Search PubMed.
- (a) D. C. Kennedy, C. S. McKay, M. C. B. Legault, D. C. Danielson, J. A. Blake, A. F. Pegoraro, A. Stolow, Z. Mester and J. P. Pezacki, J. Am. Chem. Soc., 2011, 133, 17993 CrossRef CAS PubMed; (b) M. Aminuzzaman, L. P. Ying, W.-S. Goh and A. Watanabe, Bull. Mater. Sci., 2018, 41, 1 CrossRef CAS.
- V. Errico, G. Arrabito, E. Fornetti, C. Fuoco, S. Testa, G. Saggio, S. Rufini, S. Cannata, A. Desideri and C. Falconi, ACS Appl. Mater. Interfaces, 2018, 10, 14097 CrossRef CAS PubMed.
- P. Ravirajan, A. M. Peiró, M. K. Nazeeruddin, M. Graetzel, D. D. C. Bradley, J. R. Durrant and J. Nelson, J. Phys. Chem. B, 2006, 110, 7635 CrossRef CAS PubMed.
- A. Sirelkhatim, S. Mahmud, A. Seeni, N. H. M. Kaus, L. C. Ann, S. K. M. Bakhori, H. Hasan and D. Mohamad, Nano-Micro Lett., 2015, 7, 219 CrossRef CAS.
- J. Strunk, K. Kahler, X. Xia and M. Muhler, Surf. Sci., 2009, 603, 1776 CrossRef CAS.
- (a) J. Tang, J. Huang and S. Q. Man, Spectrochim. Acta, Part A, 2013, 103, 349 CrossRef CAS PubMed; (b) P. Phukan, S. Agarwal, K. Deori and D. Sarma, Catal. Lett., 2020, 150, 2208 CrossRef CAS.
- S. Boggala, V. Perupogu, S. Varimalla, K. Manda and V. Akula, Mol. Catal., 2022, 521, 112191 CrossRef CAS.
- V. Camberlein, N. Kraupner, N. B. Karroum, E. Lipka, R. D. Poulain, B. Deprez and D. Bosc, Tetrahedron Lett., 2021, 73, 153131 CrossRef CAS.
- W. G. Kim, M. E. Kang, J. B. Lee, M. H. Jeon, S. Lee, J. Lee, B. Choi, P. M. S. D. Cal, S. Kang, J. M. Kee, G. J. L. Bernardes, J. U. Rohde, W. Choe and S. Y. Hong, J. Am. Chem. Soc., 2017, 139, 12121 CrossRef CAS PubMed.
- Z. Hashemi, J. Albadi and M. Jalal, Res. Chem. Intermed., 2021, 47, 5291 CrossRef CAS.
- P. Choudhury, S. Chattopadhyay, G. De and B. Basu, Mater. Adv., 2021, 2, 3042 RSC.
- Q. Wang, D. Yang, Y. Qiu, X. Zhang, W. Song and Lizhong Hu, Appl. Phys. Lett., 2018, 112, 063906 CrossRef.
- X. Li, Y. Li, G. Sun, N. Luo, B. Zhang and Z. Zhang, Nanomaterials, 2019, 9, 723 CrossRef PubMed.
- S. S. Patil, M. G. Mali, M. S. Tamboli, D. R. Patil, M. V. Kulkarni, H. Yoon, H. Kim, S. S. Al-Deyab, S. S. Yoon, S. S. Kolekar and B. B. Kale, Catal. Today, 2016, 260, 126 CrossRef CAS.
- J. Das, S. K. Pradhan, D. R. Sahu, D. K. Mishra, S. N. Sarangi, B. B. Nayak, S. Verma and B. K. Roul, J. Appl. Phys., 2011, 109, 103508 CrossRef.
- R. Gaashani, S. Radiman, A. R. Daud, N. Tabet and Y. Al-Douri, Ceram. Int., 2013, 39, 2283 CrossRef.
- M. Kumar and S. Deka, ACS Appl. Mater. Interfaces, 2014, 6, 16071 CrossRef CAS PubMed.
- R. Hazarika, A. Garg, S. Chetia, P. Phukan, A. Kulshrestha, A. Kumar, A. Bordoloi, A. J. Kalita, A. K. Guha and D. Sarma, Appl. Catal., A, 2021, 625, 118338 CrossRef CAS.
- H. Inagaki, A. Nishikawa, Y. Sugita and T. Tsuji, J. Nucl. Sci. Technol., 2003, 3, 143 CrossRef.
- Q. Hu, Y. Liu, X. Deng, Y. Li and Y. Chena, Adv. Synth. Catal., 2016, 358 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR, SEM and optimisation tables. See DOI: https://doi.org/10.1039/d2ma00824f |
| This journal is © The Royal Society of Chemistry 2022 |