Open Access Article
Open Access ArticleOne-pot synthesis of robust dendritic sulfur quantum dots for two-photon fluorescence imaging and “off–on” detection of hydroxyl radicals and ascorbic acid†
Jisuan
Tan
a,
Yiheng
Song
a,
Xuanjun
Dai
a,
Guan
Wang
b and
Li
Zhou
 *a
*a
aKey Laboratory of New Processing Technology for Nonferrous Metal and Materials (Ministry of Education), Guangxi Key Laboratory of Optical and Electronic Materials and Devices, and College of Materials Science and Engineering, Guilin University of Technology, Guilin 541004, China. E-mail: zhouli@glut.edu.cn
bInstitute of Sustainability for Chemicals, Energy and Environment, A*STAR, Singapore, 138634, Singapore
First published on 23rd August 2022
Abstract
The straightforward preparation of fluorescent sulfur quantum dots (SQDs) with good photostability and biocompatibility and multifunction remains a challenge. Herein, a simple method to improve the performance of SQDs is reported, that is, using hyperbranched polyglycerol (HPG) as a ligand to direct the synthesis of dendritic HPG-SQD nanocomposites from cheap elemental sulfur. Thanks to the protection of HPG, the HPG-SQDs show much better biocompatibility and photostability as compared with the widely reported polyethylene glycol (PEG) ligand-capped SQDs (PEG-SQDs). In addition, the HPG-SQDs also present excellent aqueous solubility, stable fluorescence against environmental variation, good cell uptake capability, and strong single- and two-photon fluorescence. Moreover, the HPG-SQDs display sensitive and selective fluorescence “off–on” behavior to hydroxyl radicals (˙OH) and ascorbic acid (AA), respectively, and thereby hold potential as a fluorescent switch to detect ˙OH and AA. For the first time, the utilization of two-photon fluorescence of HPG-SQDs to monitor ˙OH and AA in cells is demonstrated in this study.
Introduction
Sulfur quantum dots (QDs), novel metal-free luminescent nanodots, have attracted more and more attention because of their unique composition and attractive attributes, as well as numerous potential applications.1 Additionally, SQDs can now be directly synthesized from cheap elemental sulfur.2–5 However, most of the surface ligands reported so far cannot completely stabilize SQDs, resulting in poor photostability of SQDs and difficulty of tolerating long time UV-light illumination, which seriously limits the practical applications of SQDs.4,6,7 For instance, the fluorescence intensity of polyethylene glycol (PEG)-capped SQDs declined by 48% after exposure to a UV lamp for 10 min.4 In addition, although SQDs do not contain heavy metal ions, they are generally slightly more toxic than other metal-free fluorescent nanodots, such as carbon dots and graphene quantum dots.8,9 Therefore, there is still an urgent need to explore an effective strategy to produce SQDs with high photostability and good biocompatibility.As one of the most crucial reactive oxygen species in vivo, hydroxyl radicals (˙OH) can cause oxidative damage to various biomolecules including proteins, nucleic acids, carbohydrates and lipids, and thus their production process and content are related to various diseases (e.g., tumors, cardiovascular diseases, and aging).10 Ascorbic acid (AA), also known as vitamin C, has good antioxidant effects and is an essential nutrient for the treatment and prevention of a variety of diseases. Nevertheless, an excessive intake or lack of AA may lead to diarrhea, acidosis, cancer and kidney stones.11 So far, a variety of sensing technologies have been developed for the detection of ˙OH and AA, among which fluorescence detection technology has attracted much attention due to its high sensitivity, simple operation and low cost.12 Nevertheless, most reported fluorescent probes suffer from the disadvantages of high toxicity, low sensitivity, poor water solubility and high cost. As such, the development of an applicable fluorescent probe for sensitive detection of ˙OH and AA remains a challenge. On the other hand, although SQDs have been studied as a fluorescent probe for bioimaging,13 the reported SQDs generally present short wavelength emission (e.g., blue fluorescence) and are excited using UV light, which is prone to photobleaching and is susceptible to the organism's own fluorescence. In this regard, two-photon fluorescence imaging technology can effectively overcome the above drawbacks due to its advantages of low spontaneous fluorescence, large penetration depth and less photobleaching.14 However, to date, the employment of SQDs as a two-photon fluorescence probe has rarely been studied.
In this contribution, we report a simple one-pot strategy to prepare multifunctional dendritic SQDs from cheap elemental sulfur by employing hyperbranched polyglycerol (HPG) as a ligand (Fig. 1a). Different from previously reported ligands, the biocompatible HGP with a highly branched molecular structure and numerous hydroxyl groups can simultaneously endow SQDs with superior photostability and good biocompatibility.15 Moreover, the obtained HPG-SQDs can be utilized not only as a single-photon fluorescence switch for sensing of ˙OH and AA in water, but also as a two-photon fluorescence probe for imaging and monitoring of ˙OH and AA in cells.
Results and discussion
The synthesis process of the HPG-SQDs is depicted in Scheme 1. Fluorescent HPG-SQDs can be easily prepared by agitating an aqueous mixture of sublimed sulfur, NaOH, and HPG in an oxygen atmosphere at 95 °C for 24 h. The formation of HPG-SQDs could be achieved by the following reactions.4,16| Elemental sulfur + NaOH → Na2S + Na2SO3 + H2O | (1) |
| (x−1)S + S2− → Sx2− | (2) |
| Sx2− + O2 + HPG → HPG-SQDs + SO32− + SO42− | (3) |
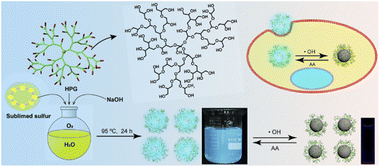 | ||
| Scheme 1 Schematic illustration of the synthesis of HPG-SQDs for fluorescence “off–on” sensing of ˙OH and AA. | ||
Firstly, the elemental sulfur can react with NaOH to produce sodium polysulfide (Na2Sx). Subsequently, the pure oxygen atmosphere can accelerate the oxidation of polysulfide ions (Sx2−) to zero-valent sulfur atoms and facilitate the production of SQDs in the presence of HPG.4
Notably, no fluorescent product was formed without addition of sublimed sulfur or HPG. After purification by dialysis against deionized water, a transparent aqueous solution of HPG-SQDs was obtained and it could emit bright cyan fluorescence under a 365 nm UV lamp (Scheme 1 and Fig. S1†).
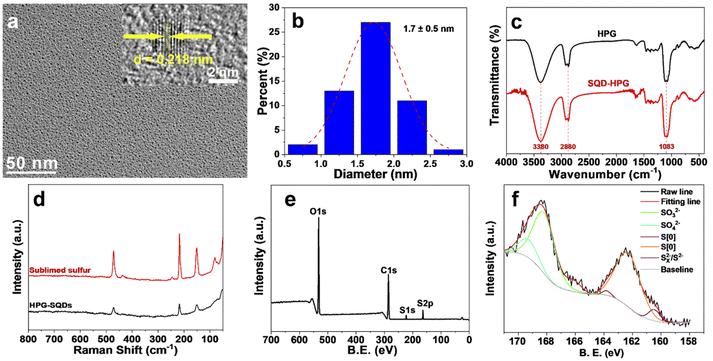
Transmission electron microscopy (TEM) images (Fig. 1a) show that HPG-SQDs have a small and uniform size, with a mean diameter of about 1.7 nm (Fig. 1b). The corresponding high-resolution TEM (HRTEM) image (Fig. 1a) indicates that HPG-SQDs possess obvious lattice fringes with a crystal plane spacing of 0.218 nm, which is similar to other reported SQDs.4,17,18 The Fourier transformed infrared spectroscopy (FTIR) results (Fig. 1c) reveal that all absorption peaks of HPG-SQDs are consistent with those of HPG. Hence, no new chemical bonds were formed between HPG and SQDs. Furthermore, the Raman spectrum of HPG-SQDs (Fig. 1d) is very similar to that of sublimed sulfur, which demonstrated that the resultant fluorescent nanodots are SQDs. The X-ray photoelectron spectroscopy (XPS) result (Fig. 1e) shows that the HPG-SQDs sample consists of carbon, oxygen, and sulfur elements. The corresponding high-resolution XPS S 2p spectrum (Fig. 1f) indicates the presence of four kinds of sulfur including atomic sulfur (S[0]), SO32−, SO42− and Sx2−/S2− in HPG-SQDs,1e,2,4 which is consistent with the reaction equations. The sulfur content in HPG-SQDs was determined by elemental analysis (EA). The EA result (Table S1†) showed that the mass fractions of sulfur, carbon, hydrogen and oxygen were 32.48 wt%, 30.37 wt%, 6.23 wt% and 30.92 wt% (calculated result), respectively.
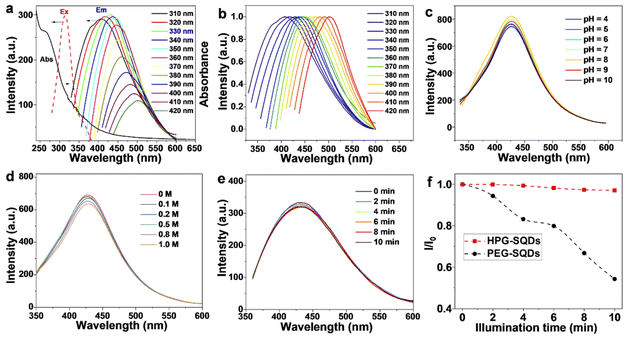
Owing to the capping of HPG, the HPG-SQDs present superior aqueous solubility and stability, and there was no precipitate in its aqueous solution even after being stored for 3 months. In the UV-vis absorption spectrum of HPG-SQDs (Fig. 2a), an absorption peak at about 264 nm can be observed, which is attributed to the n → π* transition of sulfur atoms.16,17 Like other reported SQDs,4,18,19 the emission peaks of HPG-SQDs can be tuned to some extent by manipulating the excitation wavelength (λex) (Fig. 2a and b). With the λex of 330 nm, the strongest emission peak appeared at around 427 nm (Fig. 2a), which agrees well with the excitation spectrum. Meanwhile, the absolute fluorescence quantum yield (QY) of HPG-SQDs was measured to be 6.8%, which compares favorably to that of many reported SQDs.1 The fluorescence decay curve of HPG-SQDs (Fig. S2†) can be fitted by a triple-exponential function as follows:
Y(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) + A3 exp(−t/τ2) + A3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ3) exp(−t/τ3) | (4) |
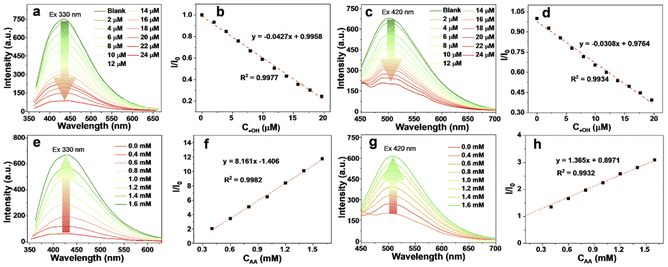
Interestingly, the HPG-SQDs display remarkable fluorescence quenching behavior towards ˙OH. As shown in Fig. 3a and c, regardless of λex at 330 nm or 420 nm, the fluorescence intensity of HPG-SQDs declined continuously with increasing concentration of ˙OH. After mixing with 20 μM of ˙OH, the fluorescence intensity of HPG-SQDs decreased by 76.2% and 60.6% with a λex of 330 nm or 420 nm, respectively. Additionally, this fluorescence quenching process could reach equilibrium within 2 min (Fig. S4†). All these results indicate that the ˙OH can quench the fluorescence of HPG-SQDs. Furthermore, the quenching efficiency (I/I0, where I and I0 represent the fluorescence intensity of HPG-SQDs in the presence and absence of ˙OH, respectively) was highly dependent on the concentration of ˙OH with a good linear correlation in the range of 0.5–20 μM, offering an opportunity for the quantitative detection of ˙OH (Fig. 3b and d). The limit of detection at the excitation wavelength of 330 nm (signal-to-noise is 3) was calculated to be 0.23 ± 0.03 μM, which is superior to those of numerous reported nanoprobes (see Table S2†).10a,12 To probe the quenching mechanism, the UV-vis absorption spectra and fluorescence–decay curves of HPG-SQDs before and after mixing ˙OH were compared. Clearly, the addition of ˙OH leads to the red shift of the absorption peak (from 264 nm to 290 nm) (Fig. S5†) and the shortening of fluorescence lifetime (from 2.35 ns to 2.06 ns) (Fig. S2†). Therefore, the fluorescence quenching process may be a dynamic process. The oxidation of the surface of HPG-SQDs by ˙OH would affect the non-radiative path of HPG-SQDs.
In addition to high sensitivity, the HPG-SQDs also showed excellent selectivity. In comparison with ˙OH, the fluorescence quenching effect of other common metal ions (e.g., Fe2+, Fe3+, Ni2+, K+, Zn2+, Mn2+, Cu2+, Mg2+, Ag+, Cd2+ and Pd2+) (Fig. S6†) and molecules (e.g., H2O2, AA, histidine, glycine, arginine, cysteine, glucose and lysine) (Fig. S7†) on HPG-SQDs was negligible or very slight even when their concentration was as high as 50 μM. It is worth noting that the quenched fluorescence can be effectively and selectively recovered by adding AA (Fig. S8†). No matter λex was 330 nm (Fig. 3e) or 420 nm (Fig. 3g), the fluorescence intensity of the HPG-SQDs/˙OH system enhanced with increasing the addition amount of AA and presented a good linear relationship between 0.4 mM and 1.6 mM (Fig. 3f and h). In addition, the fluorescence recovery process can be completed within 3 min (Fig. S9†). It is noteworthy that the fluorescence lifetime of the HPG-SQDs/˙OH system recovered from 2.06 ns to 2.23 ns after AA addition (Fig. S2†), and meanwhile the absorption peak shifted from 290 nm to 264 nm (Fig. S5†), which was consistent with HPG-SQDs. Hence, this fluorescence recovery phenomenon induced by AA may be attributed to the fact that AA is a typical reducing agent, which can eliminate the oxidation effect of ˙OH to HPG-SQDs. Moreover, this fluorescence quenching/recovery behavior of HPG-SQDs is repeatable although the extent of switching back and forth gradually weakened (Fig. S10†). Therefore, the HPG-SQDs hold potential as a fluorescent nanoprobe for the sensitive detection of ˙OH and AA.
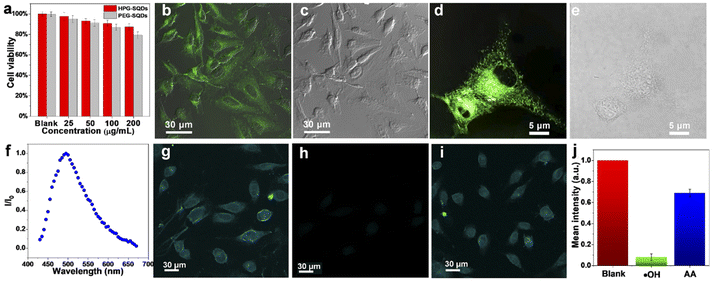
The toxicity of HPG-SQDs was evaluated with HeLa cells using thiazolyl blue tetrazolium bromide (MTT) assay. As shown in Fig. 4a, even after incubation with a high concentration of HPG-SQDs (e.g., 100 μg mL−1) for 24 h, the HeLa cells still displayed over 90% of cell viability. Moreover, the HPG-SQDs exhibited much lower cytotoxicity than that of widely reported PEG-SQDs with the same concentration, suggesting that the biocompatible HPG can endow the SQDs with good biocompatibility. In addition, utilizing fluorescent HPG-SQDs to stain HeLa cells was also investigated. Confocal laser-scanning microscopy (CLSM) images (Fig. 4b–e) show that the cell cytoplasm of HeLa cells could emit obvious green fluorescence after staining with HPG-SQDs (50 μg mL−1), demonstrating the successful uptake of HPG-SQDs by the cells. However, in practical bioimaging applications, single-photon short-wavelength fluorescence imaging is susceptible to photobleaching and spontaneous fluorescence of organisms. Considering that two-photon fluorescence imaging technology can effectively overcome the above shortcomings, the two-photon fluorescence characteristics of HPG-SQDs were studied. Fortunately, the HPG-SQDs showed distinct two-photon fluorescence with an emission peak at about 495 nm under the excitation of an 800 nm femtosecond pulse laser (Fig. 4f). As such, using HPG-SQDs in two-photon fluorescence imaging and in vitro detection of ˙OH and AA was further studied using a multiphoton fluorescence microscope (Fig. 4g–j and S11†). As depicted in Fig. 4g, the HeLa cells after incubation with HPG-SQDs (50 μg mL−1) present bright two-photon fluorescence. In accordance with the single-photon emission spectra, the two-photon fluorescence of HeLa cells also weakened after addition of ˙OH (Fig. 4h), and then recovered to a large extent after mixing in AA (Fig. 4i). The corresponding statistical analysis results showed that the two-photon fluorescence intensity of HPG-SQD stained HeLa cells decreased by nearly 90% after mixing in ˙OH (30 μM), and then recovered to 70% after adding AA (1.5 mM) (Fig. 4j). To our knowledge, this is the first example of two-photon fluorescence detection in cells based on SQDs to date.
Conclusions
In conclusion, this study reports the first example of effective enhancement of photostability, biocompatibility and versatility of SQDs using a hyperbranched polymer ligand. Thanks to the introduction of multihydroxy biocompatible HPG, the dendritic HPG-SQD nanocomposites simultaneously showed superior aqueous solubility, stable fluorescence, good photostability, low cytotoxicity, good cell uptake capability, and strong single- and two-photon fluorescence. In addition, HPG-SQDs also exhibited sensitive and selective fluorescence quenching/recovery behavior to ˙OH and AA, respectively, and thus could be employed as a fluorescent switch to detect ˙OH and AA. Moreover, this study also demonstrated for the first time that the two-photon fluorescence of HPG-SQDs can be exploited to perform cell imaging and monitoring of ˙OH and AA in cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U21A2097), Natural Science Foundation of Guangxi Province (No. 2017GXNSFFA198002), and Bagui Scholar Program of Guangxi Province.Notes and references
- (a) P. Gao, G. Wang and L. Zhou, ChemPhotoChem, 2020, 4, 5235–5244 CrossRef CAS; (b) Y. Shi, P. Zhang, D. Yang and Z. Wang, Chem. Commun., 2020, 56, 10982–10988 RSC; (c) A. Pal, F. Arshad and M. P. Sk, Adv. Colloid Interface Sci., 2020, 285, 102274 CrossRef CAS; (d) L. Liu, Y. Zhang, R. Yuan and H. Wang, Anal. Chem., 2020, 92, 15112–15119 CrossRef CAS PubMed; (e) S. Li, D. Chen, F. Zheng, H. Zhou, S. Jiang and Y. Wu, Adv. Funct. Mater., 2014, 24, 7133–7138 CrossRef CAS.
- L. Shen, H. Wang, S. Liu, Z. Bai, S. Zhang, X. Zhang and C. Zhang, J. Am. Chem. Soc., 2018, 140, 7878–7884 CrossRef CAS PubMed.
- H. Wang, Z. Wang, Y. Xiong, S. Kershaw, T. Li, Y. Wang, Y. Zhai and A. Rogach, Angew. Chem., Int. Ed., 2019, 58, 7040–7044 CrossRef CAS PubMed.
- Y. Song, J. Tan, G. Wang, P. Gao, J. Lei and L. Zhou, Chem. Sci., 2020, 11, 772–777 RSC.
- P. Gao, Z. Huang, J. Tan, G. Lv and L. Zhou, ACS Sustainable Chem. Eng., 2022, 10, 4634–4641 CrossRef CAS.
- Z. Huang, J. Lei, H. Ruan, Y. Gong, G. Wang and L. Zhou, Microchem. J., 2021, 164, 106031 CrossRef CAS.
- S. Rong, Q. Chen, G. Xu, F. Wei, J. Yang, D. Ren, X. Cheng, X. Xia, J. Li, M. Gao, Q. Hu and Y. Cen, Sens. Actuators, B, 2022, 353, 131146 CrossRef CAS.
- (a) S. T. Yang, L. Cao, P. G. Luo, F. Lu, X. Wang, H. Wang, M. J. Meziani, Y. Liu, G. Qi and Y. P. Sun, J. Am. Chem. Soc., 2009, 131, 11308–11309 CrossRef CAS PubMed; (b) S. Bayda, E. Amadio, S. Cailotto, Y. Frión-Herrera, A. Perosa and F. Rizzolio, Nanoscale Adv., 2021, 3, 5183–5221 RSC; (c) S. Zeng, J. Long, J. Sun, G. Wang and L. Zhou, Carbohydr. Polym., 2022, 279, 119015 CrossRef CAS PubMed.
- (a) Q. Liu, B. Guo, Z. Rao, B. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436–2441 CrossRef CAS PubMed; (b) Y. Chong, Y. Ma, H. Shen, X. Tu, X. Zhou, J. Xu, J. Dai, S. Fan and Z. Zhang, Biomaterials, 2014, 35, 5041–5048 CrossRef CAS PubMed.
- (a) S. Liu, J. Zhao, K. Zhang, L. Yang, M. Sun, H. Yu, Y. Yan, Y. Zhang, L. Wu and S. Wang, Analyst, 2016, 141, 2296–2302 RSC; (b) Z. Li, T. Liang, S. Lv, Q. Zhuang and Z. Liu, J. Am. Chem. Soc., 2015, 137, 11179–11185 CrossRef CAS PubMed.
- (a) X. Lv, H. Man, L. Dong, J. Huang and X. Wang, Food Chem., 2020, 326, 126935 CrossRef CAS PubMed; (b) Y. Duan, J. Tan, Z. Huang, Q. Deng, S. Liu, G. Wang, L. Li and L. Zhou, Carbohydr. Polym., 2020, 249, 116882 CrossRef CAS PubMed; (c) Z. Wei, W. Lu, C. Pan, J. Ni, H. Zhao, G. Huang and C. Wang, Dalton Trans., 2022, 51, 10290–10297 RSC.
- (a) Q. Zhao, R. Zhang, D. Ye, S. Zhang, H. Chen and J. Kong, ACS Appl. Mater. Interfaces, 2017, 9, 2052–2058 CrossRef CAS PubMed; (b) Y. Sun, L. Pang, X. Guo and H. Wang, Microchim. Acta, 2022, 189, 60 CrossRef CAS PubMed; (c) F. Shi, Y. Zhang, W. Na, X. Zhang, Y. Li and X. Su, J. Mater. Chem. B, 2016, 4, 3278–3285 RSC.
- (a) H. Ruan and L. Zhou, Front. Bioeng. Biotechnol., 2022, 10, 909727 CrossRef PubMed; (b) C. Zhang, P. Zhang, X. Ji, H. Wang, H. Kuang, W. Cao, M. Pan, Y. Shi and Z. Wang, Chem. Commun., 2019, 55, 13004–13007 RSC; (c) J. Lei, Z. Huang, P. Gao, J. Sun and L. Zhou, Part. Part. Syst. Charact., 2021, 38, 2000332 CrossRef CAS; (d) F. Arshad, M. P. Sk, S. K. Maurya and H. R. Siddique, ACS Appl. Nano Mater., 2021, 4, 3339–3344 CrossRef CAS.
- (a) C. Yang, K. Chen, M. Chen, X. Hu, S. Y. Huan, L. Chen, G. Song and X. B. Zhang, Anal. Chem., 2019, 91(4), 2727–2733 CrossRef CAS PubMed; (b) A. Zhu, Z. Luo, C. Ding, B. Li, S. Zhou, R. Wang and Y. Tian, Analyst, 2014, 139, 1945–1952 RSC.
- (a) D. Wilms, S. E. Stiriba and H. Frey, Acc. Chem. Res., 2010, 43, 129–141 CrossRef CAS PubMed; (b) S. Abbina, S. Vappala, P. Kumar, E. M. Siren, C. C. La, U. Abbasi, D. E. Brooks and J. N. Kizhakkedathu, J. Mater. Chem. B, 2017, 5, 9249–9277 RSC; (c) H. Frey and R. Haag, Rev. Mol. Biotechnol., 2002, 90, 257–267 CrossRef CAS; (d) L. Zhou, J. Geng, G. Wang, J. Liu and B. Liu, Polym. Chem., 2013, 4, 5243–5251 RSC; (e) B. Huang, D. Wang, G. Wang, F. Zhang and L. Zhou, J. Colloid Interface Sci., 2017, 508, 214–221 CrossRef CAS PubMed.
- (a) S. Liu, H. Wang, A. Feng, J. Chang, C. Zhang, Y. E. Shi, Y. Zhai, V. Biju and Z. Wang, Nanoscale Adv., 2021, 3, 4271–4275 RSC; (b) Z. Huang, Y. Gao, Z. Huang, D. Chen, J. Sun and L. Zhou, Microchem. J., 2021, 170, 106656 CrossRef CAS.
- Y. Sheng, Z. Huang, Q. Zhong, H. Deng, M. Lai, Y. Yang, W. Chen, X. Xia and H. Peng, Nanoscale, 2021, 13, 2519–2526 RSC.
- G. Qiao, L. Liu, X. Hao, J. Zheng, W. Liu, J. Gao, C. Zhang and Q. Wang, Chem. Eng. J., 2020, 382, 122907 CrossRef CAS.
- L. Xiao, Q. Du, Y. Huang, L. Wang, S. Cheng, Z. Wang, T. N. Wong, E. K. L. Yeow and H. Sun, ACS Appl. Nano Mater., 2019, 2, 6622–6628 CrossRef CAS.
- F. Arshad and M. P. Sk, ACS Appl. Nano Mater., 2020, 3, 3044–3049 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2na00498d |
| This journal is © The Royal Society of Chemistry 2022 |