Open Access Article
Open Access ArticleHigh-efficiency and simple synthesis of Ti3C2Tx nanoscrolls by surface energy modulation and cryogenic freezing†
Ke
Tan‡
,
Sen
Liu‡
,
Yang
Liu
*,
Linrui
Hou
 * and
Changzhou
Yuan
* and
Changzhou
Yuan
 *
*
School of Material Science & Engineering, University of Jinan, Jinan, 250022, P. R. China. E-mail: mse_liuy@ujn.edu.cn; mse_houlr@ujn.edu.cn; mse_yuancz@ujn.edu.cn
First published on 27th October 2022
Abstract
To alleviate the restacking issue of 2D Ti3C2Tx itself, we purposefully explore a simple but efficient method for controllable construction of 1D Ti3C2Tx nanoscrolls with a high efficiency of ∼90.5%, with detailed regulation of the feed concentrations and surface energy of Ti3C2Tx. The involved transformation mechanism from 2D nanosheets to 1D nanoscrolls is reasonably proposed.
In recent years, as a new member of two-dimensional (2D) materials, transition metal carbides or carbon/nitride MXenes, with a structure similar to that of graphene, were first reported by Gogotsi in 2011, and afterwards immediately attracted enormous attention worldwide.1 MXenes are usually prepared by a selective etching-assisted method by removing the A-layer elements (group IIIA or IVA elements, such as Al, Ga, Si or Ge) in the Mn+1AXn (n = 1–3) phase. And the original Mn+1XnTx (M refers to transition metals and X represents carbon and/or nitrogen) structure can remain unchanged, with –F, –OH and other anion surface functional groups on the surface of nanosheets (NSs) shown as Tx, exhibiting excellent electrical, optical and mechanical properties.2,3 Compared to traditional 2D materials, MXenes are also endowed with great chemical reactivity and hydrophilicity with functional groups, which is expected to be an ideal matrix material for constructing nanocomposites.4 Furthermore, the obtained MXene NSs can be exfoliated to monolayer-enriched MXene colloidal solutions towards promising electromagnetic interference shielding and energy storage materials.5–7 However, mono-layered MXene NSs are always restacked and brought together because of van der Waals interaction and hydrogen bonds, hindering their practical applications because of the poor ion conductivity and electromagnetic wave consumption, which can be solved well by morphology transformation.8
The specific morphologies of MXenes play a great role in improving or expanding their properties.9–12 It has been shown that Ti2C MXene NSs can be transformed into crumpled sheets, spheres and scrolls by selective intercalation of p-phosphonic calix[n]arenes, showing new physico-chemical properties.9 Alkalized MXene nanoribbons with extended interlayer spacing were prepared with excellent Na+/K+-storage properties.10 Ti3C2Tx MXene NSs could be scrolled by spray-drying or freeze-drying with Si nanoparticles for electrode materials for Li-ion batteries.11 Besides, the Ti3C2 aerogel synthesized by a simple self-assembly method displays exceptional electrochemical performance for supercapacitors.12
Herein, we proposed a highly efficient method to synthesize one-dimensional (1D) Ti3C2Tx nanoscrolls through surfactant-assisted lyophilization. Competitively, the formation efficiency of 1D Ti3C2Tx nanoscrolls by this way can reach as high as ∼90.5% just by optimizing the concentrations of Ti3C2Tx NSs dispersion and surfactants. More importantly, with detailed investigations, the essential roles of colloidal solution concentration and surfactants in the scrolling behaviour of Ti3C2Tx NSs were rationally put forward.
The Ti3C2Tx nanoscrolls were prepared as follows. First, 2.0 g of LiF powder was dissolved in 40 mL of 9 M HCl solution, and stirred for 30 min to prepare etch solution. 2.0 g of Ti3AlC2 MAX (11 Technology Co. Ltd.) was added into the solution slowly. After being stirred at 30 °C for 24 h, the suspension was centrifuged at 3500 rpm and washed with deionized (DI) water until the pH value of the solution went up to 6. Second, the obtained precipitate was dispersed into 40 mL of ethyl alcohol and delaminated by sonication under Ar for 1 h. DI water was added after the precipitate was retained in the container by certification. The delaminated Ti3C2Tx NSs dispersion can be obtained after being sonicated under Ar for 1 h and centrifuged at 3500 rpm for 5 min, for the formation of a supernatant. The concentration was tested by vacuum drying a certain volume of dispersion at 60 °C for 8 h. Third, 5.0 wt% of surfactants including sodium dodecyl sulfate (SDS), polyvinyl pyrrolidone (PVP) or hexadecyl trimethyl ammonium bromide (CTAB) was dispersed in the diluted Ti3C2Tx NSs dispersion of 0.1 g mL−1 by stirring at room temperature for 1 h, respectively. Finally, the solutions with SDS, PVP or CTAB were transferred to tubes in liquid N2 for freezing and drying in freeze-drying equipment for 48 h, and then denoted as Ti3C2Tx-S, Ti3C2Tx-P and Ti3C2Tx-C, respectively. Other Ti3C2Tx samples were prepared by the same procedure just with the exception of different concentrations of the Ti3C2Tx NSs dispersion and the absence of the surfactant, and named Ti3C2Tx-n (n = dispersion concentration). Ti3C2Tx dispersion (0.1 g mL−1) is directly frozen in a refrigerator (−18 °C), along with the other parameters the same as Ti3C2Tx-S, and the product was named Ti3C2Tx-r.
The structures and morphologies of samples were investigated by field-emission scanning electron microscopy (FESEM, JEOL-6300F, 15 kV), transmission electron microscopy (TEM), scanning TEM (STEM), and high-resolution TEM (HRTEM) (JEOL JEM 2100 system) with an energy dispersive X-ray spectroscopy (EDS) system. Crystalline phases were examined using a Rigaku Ultima IV X-ray diffractometer with a Cu Kα radiation source.
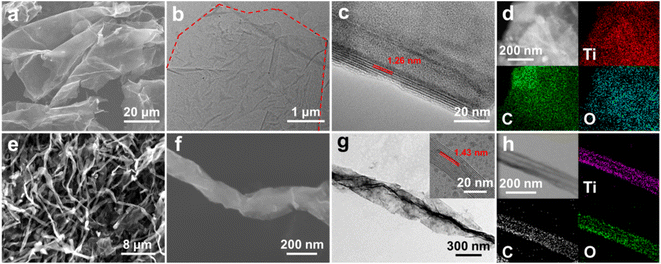
The morphologies of Ti3C2Tx NSs and Ti3C2Tx-S were characterized by FESEM and (HR)TEM techniques. Typically, micro-sized Ti3C2Tx NSs with transparent features (Fig. 1a and b) and a spacing between layers of ∼1.18 nm (Fig. S1†) are evidenced, confirming the successful fabrication of high-quality few-layered Ti3C2Tx NSs. It can be visually authenticated by the HRTEM observation (Fig. 1c). One should especially note that some cracked NSs still exist (Fig. 1a) in the freeze-fried Ti3C2Tx NSs of original concentration after being frozen by liquid nitrogen. With such a phenomenon in mind, more experiments on feed concentrations are designed later. STEM and corresponding EDX mapping images (Fig. 1d) reveal that there are rich oxygen-containing functional groups on Ti3C2Tx NSs. After modification with SDS, rapid freezing by liquid nitrogen and freeze-drying, most Ti3C2Tx NSs are rolled up to Ti3C2Tx-S (Fig. 1e). The diameter of Ti3C2Tx nanoscrolls is usually ∼200–500 nm, as derived from FESEM and TEM images (Fig. 1f and g). Strikingly, the topological transformation efficiency of Ti3C2Tx-S is as high as ∼90.5%, assessed by counting the number of NSs and nanoscrolls quantitatively in the SEM image (Fig. S2†). The HRTEM image (Fig. 1g) shows that the layer spacing is expanded to ∼1.43 nm after rolling-up, corresponding to the low-angle shift tendency of the (002) crystal plane (Fig. S1†), indicating that the addition of SDS and the freezing process further expand the layer spacing. Fig. 1h evidently proves that the nanoscrolls are still Ti3C2Tx with oxygen-containing functional groups.
From the discovery of broken Ti3C2Tx NSs mentioned before, the sheets dispersion is diluted to successively decreasing concentrations and freeze-dried directly after freezing with liquid N2 without SDS. The morphologies of these samples with different concentrations ranging from 2.0 to 0.1 g mL−1 (Fig. 2a–f) visualize that more Ti3C2Tx NSs can be scrolled up and more broken NSs appear as the concentration decreases. Specifically, Ti3C2Tx-2.0 (Fig. 2a) still presents a sheet-shape structure, and Ti3C2Tx-1.0 (Fig. 2b) is partly broken. As the concentration decreases, most NSs of Ti3C2Tx-0.8 (Fig. 2c) are broken and nanoscrolls start to appear in Ti3C2Tx-0.5 (Fig. 2d). More and more nanoscrolls are discerned with further decreasing the concentration of Ti3C2Tx dispersion, particularly for the case of Ti3C2Tx-0.1 (Fig. 2f). Note that the NSs start to roll up inward from the edge closer to freshly formed ice in Ti3C2Tx-0.8, while the NSs in Ti3C2Tx-0.2 and Ti3C2Tx-0.1 are half/full rolling up with a lower rolling degree than Ti3C2Tx-S. Their digital pictures (the insets in Fig. 2) show that the volume of Ti3C2Tx in the form of aerogels gets smaller and smaller as the dispersion concentration decreases. It can be rationally attributed to the larger size of Ti3C2Tx NSs and the too fast growth rate of ice crystals by liquid N2 freezing. When the concentration is reduced to a certain extent, the rapidly growing ice crystals tear the NSs apart into smaller sizes as they cannot hinder them, as revealed by the schematic illustration (Fig. S3†).
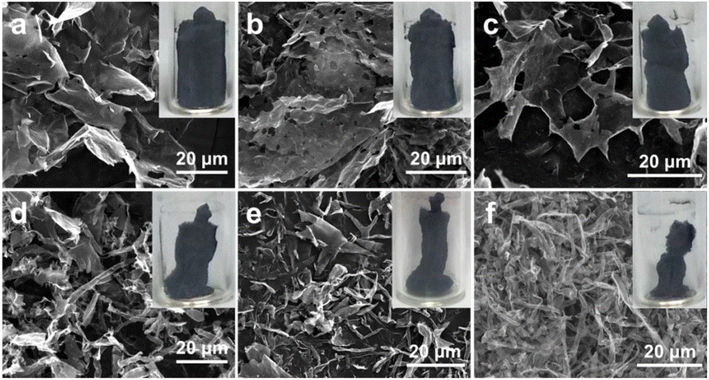 | ||
| Fig. 2 FESEM images and digital pictures (the insets) of (a) Ti3C2Tx-2.0, (b) Ti3C2Tx-1.0, (c) Ti3C2Tx-0.8, (d) Ti3C2Tx-0.5, (e) Ti3C2Tx-0.2 and (f) Ti3C2Tx-0.1 samples. | ||
As mentioned above, Ti3C2Tx NSs modified with the anion surfactant of SDS can be well rolled-up after freezing by liquid N2 and freezing-drying. To better understand the phenomenon, two other surfactants (i.e., cationic CTAB and non-ionic PVP) are evaluated as well like SDS with Ti3C2Tx NSs, and their morphological transformation results are shown in Fig. 3a–d. Interestingly, for the case of Ti3C2Tx-C, most of the Ti3C2Tx NSs are still flat and half-rolled up sheets, along with merely a small number of nanoscrolls (Fig. 3a and b). In contrast, Ti3C2Tx-P (Fig. 3c and d) consists of half-rolled up NSs and nanoscrolls, about half of each. The distinct difference among the three cases should be related to the different natures of the three surfactants. Generally, the anionic surfactant of SDS further can enhance the electronegativity of the NSs with original anion surface functional groups (–F and –OH), while a cationic surfactant or non-ionic surfactant could reduce the electronegativity and enhance the stability of Ti3C2Tx NSs. When the temperature drops rapidly, Ti3C2Tx NSs tend to roll up and shrink to reduce surface energy. And more pronounced the tendency to roll up becomes, the greater the electronegativity.
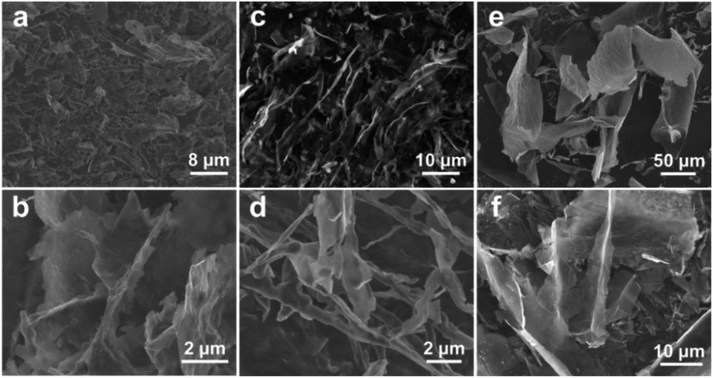 | ||
| Fig. 3 FESEM images of (a and b) Ti3C2Tx-C and (c and d) Ti3C2Tx-P frozen with liquid N2, and (e and f) Ti3C2Tx-r. | ||
For figuring out the influence of the dropping rate of freezing temperature, Ti3C2Tx NSs are frozen under ∼−18 °C in a refrigerator for comparison. Clearly, the integral NSs even larger than ∼50 μm in size are obvious for Ti3C2Tx-r (Fig. 3e and f), which is not broken by ice crystals. This can be attributed to ice crystals that just grow along the NSs when the temperature is dropped slowly and the stress work on the NSs is too small to break and/or squeeze them much (Fig. S4†). This can be used for the delamination of MXenes, as reported in detail before.13
Combining the above phenomenon of different concentrations and surfactant modifications, the morphological transformations of Ti3C2Tx NSs themselves manifest in different forms, as schematically summarized in Fig. 4. When the concentration is relatively high, the stress generated by the growth of ice crystals can only break the Ti3C2Tx NSs and rolling-up of them cannot be achieved because of their mutual obstacles. As regards the relatively low concentration, some of the broken Ti3C2Tx NSs roll up for lower surface energy to accommodate the super low environment, so the porous NSs and nanoscrolls co-exist in the case. After being modified with SDS, nearly all Ti3C2Tx NSs roll up when frozen with liquid nitrogen as they are more unstable. As for Ti3C2Tx-S, some structures in the intermediate state show how broken Ti3C2Tx NSs rolled up (Fig. S5a and b†). When the ice crystal punctures Ti3C2Tx NSs, a temperature difference in a localized area containing ice and broken NSs is created temporarily. The ambient temperature at the edge of the NSs near the ice is much higher than that at the other part farther away from the ice, so the NSs are rolled up from this edge for a more stable state. The interconnected types of nanoscrolls show a typical scrolling morphology containing trunk-to-trunk and head-to-trunk structures (Fig. S5c–f†). Commonly, the trunk-to-trunk structure is formed by crossing mutually independent NSs, and the head-to-trunk structure is formed from the same NSs.
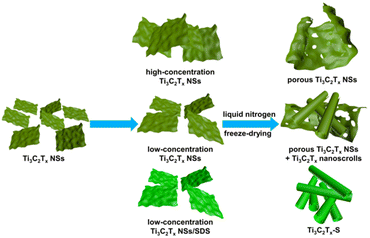 | ||
| Fig. 4 Schematic illustration for synthesizing different Ti3C2Tx structures through regulation of concentrations and electronegativity. | ||
Conclusions
In summary, a highly efficient and simple method was devised to controllably synthesize 1D Ti3C2Tx nanoscrolls by surface energy modulation and cryogenic freezing. The formation of 1D Ti3C2Tx nanoscrolls was rationally proposed with detailed morphological and structural characteristics. Benefitting from the enhanced electronegativity by an anionic surfactant and the fast freezing rate of liquid nitrogen, Ti3C2Tx NSs were spontaneously rolled up to keep stable as they are in the area of temporary temperature imbalance. More meaningfully, our contribution here will provide a functional building block to construct 1D versatile Ti3C2Tx nanoscroll based composites, hugely promoting comprehensive research and applications of Ti3C2Tx MXenes in energy storage, electromagnetic interference shielding, catalytic conversion, and beyond.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (No. 52072151, 52171211, 5210021292, and 52271218) and Taishan Scholars (No. ts201712050).References
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- C. J. Lu, L. Yang, B. Z. Yan, L. B. Sun, P. G. Zhang, W. Zhang and Z. M. Sun, Adv. Funct. Mater., 2020, 30, 2000852 CrossRef CAS.
- H. C. Huang, H. Su, H. T. Zhang, L. D. Xu, X. Chu, C. F. Hu, H. Liu, N. J. Chen, F. Y. Liu, W. Deng, B. N. Gu, H. P. Zhang and W. Q. Yang, Adv. Electron. Mater., 2018, 4, 1800179 CrossRef.
- Q. W. Wang, H. B. Zhang, J. Liu, S. Zhao, X. Xie, L. X. Liu, R. Yang, N. Koratkar and Z. Z. Yu, Adv. Funct. Mater., 2018, 29, 1806819 CrossRef.
- W. Z. Bao, L. Liu, C. Y. Wang, S. Choi, D. Wang and G. X. Wang, Adv. Energy Mater., 2018, 8, 1702485 CrossRef.
- M. Z. Yu, Z. Y. Wang, J. S. Liu, F. Sun, P. J. Yang and J. S. Qiu, Nano Energy, 2019, 63, 103880 CrossRef CAS.
- K. Li, M. Y. Liang, H. Wang, X. H. Wang, Y. S. Huang, J. Coelho, S. Pinilla, Y. L. Zhang, F. W. Qi, V. Nicolosi and Y. X. Xu, Adv. Funct. Mater., 2020, 30, 2000842 CrossRef CAS.
- A. Vaughn, J. Ball, T. Heil, D. J. Morgan, G. I. Lampronti, G. Maršalkaitė, C. L. Raston, N. P. Power and S. Kellici, Chem.–Eur. J., 2017, 23, 8128–8133 CrossRef CAS PubMed.
- P. C. Lian, Y. F. Dong, Z. S. Wu, S. H. Zheng, X. H. Wang, S. Wang, C. L. Sun, J. Q. Qin, X. Y. Shi and X. H. Bao, Nano Energy, 2017, 40, 1–8 CrossRef CAS.
- J. N. Meng, F. F. Zhang, L. Zhang, L. Y. Liu, J. T. Chen, B. J. Yang and X. B. Yan, J. Energy Chem., 2020, 46, 256–263 CrossRef.
- L. Li, M. Y. Zhang, X. T. Zhang and Z. G. Zhang, J. Power Sources, 2017, 364, 234–241 CrossRef CAS.
- X. W. Huang and P. Y. Wu, Adv. Funct. Mater., 2020, 30, 1910048 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2na00520d |
| ‡ Ke Tan and Sen Liu equally contributed to the work. |
| This journal is © The Royal Society of Chemistry 2022 |