Open Access Article
Open Access ArticleOxygen-evolving hollow polydopamine alleviates tumour hypoxia for enhancing photodynamic therapy in cancer treatment†
Qichen
Zhan‡
 *a,
Xuan
Han‡
b,
Jiankang
Mu
a,
Xianqing
Shi
c,
Yuhan
Zheng
a,
Ting
Wang
d,
Tao
Cao
*a,
Xuan
Han‡
b,
Jiankang
Mu
a,
Xianqing
Shi
c,
Yuhan
Zheng
a,
Ting
Wang
d,
Tao
Cao
 a,
Yulu
Xi
a,
Zhongpei
Weng
g,
Xiaoqing
Wang
g and
Peng
Cao
*aef
a,
Yulu
Xi
a,
Zhongpei
Weng
g,
Xiaoqing
Wang
g and
Peng
Cao
*aef
aSchool of Pharmacy, Nanjing University of Chinese Medicine, Nanjing, Jiangsu 210023, China. E-mail: zhanqichen@njucm.edu.cn; cao_peng@njucm.edu.cn
bSchool of Chinese Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu 210023, China
cDepartment of Public Experimental Teaching, Nanjing University of Aeronautics and Astronautics, Nanjing, Jiangsu 211106, China
dInstitute of Chemical Industry of Forest Products, Chinese Academy of Forestry, Nanjing, Jiangsu 210042, China
eAffiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu 210028, China
fZhenjiang Hospital of Chinese Traditional and Western Medicine, Zhenjiang, Jiangsu 212002, China
gGaoyou Hospital of Traditional Chinese Medicine, Yangzhou, Jiangsu 225600, China
First published on 19th October 2022
Abstract
Hypoxia, a characteristic hallmark of solid tumours, restricts the therapeutic effect of photodynamic therapy (PDT) for cancer treatment. To address this issue, a facile and nanosized oxygen (O2) bubble template is established by mixing oxygenated water and water-soluble solvents for guiding hollow polydopamine (HPDA) synthesis, and O2 is encapsulated in the cavity of HPDA. HPDA with abundant catechol is designed as a carrier for zinc phthalocyanine (ZnPc, a boronic acid modified photosensitizer) via borate ester bonds to fabricate nanomedicine (denoted as HZNPs). The in vitro and in vivo results indicate that O2-evolving HZNPs could alleviate tumour hypoxia and enhance PDT-anticancer efficiency. Melanin-like HPDA with a photothermal conversion rate (η) of 38.2% shows excellent synergistic photothermal therapy (PTT) efficiency in cancer treatment.
Introduction
Malignant cellular proliferation and vascular malformation lead to hypoxia formation in solid tumours.1,2 Insufficient oxygen (O2) supply and consumption induce the invasion and metastasis of tumour cells.3,4 Hypoxia severely limits the treatment outcome of O2-medicated cancer therapies, especially photodynamic therapy (PDT).5Great efforts have been made to alleviate tumour hypoxia for enhancing drug therapy efficiency, and these types can be divided into two categories, supplying exogenous O2 to tumours (carrier or carrier-free) and catalysing endogenous hydrogen peroxide (H2O2) or H2O to generate O2.6–8 Delivering exogenous O2 to alleviate tumour hypoxia is the most direct and effective method, and hyperbaric O2 therapy increasing the pO2 in the plasma and tumour tissues has been used in clinical PDT for cancer treatment.9 In recent years, haemoglobin (Hb), liposomes and macrovesicle materials were designed as carriers for O2 delivery. Hb, an iron-rich metalloprotein in red blood cells composed of globin and heme, could highly bind and deliver O2 in intravenous transmission.10 However, free Hb is susceptible to auto-oxidation during circulation and results in renal toxicity and cardiovascular complications.11 Liposomes embedded with microbubbles have been approved by the FDA as an ultrasound contrast agent for echocardiography.12 Its disadvantage is that the O2 loading efficiency is approximately 10% by volume, indicating that it is sufficient for delivering potent bioactive gases, such as NO and Xe, but not suitable for O2.13 Compared with liposomes, hollow materials have a core and a high loading efficiency for O2. How to fabricate a satisfactory O2 carrier to alleviate tumour hypoxia is still a big challenge.
Polydopamine (PDA) is an ideal carrier for drug delivery because of its good biocompatibility.14 PDA formation shares many common characteristics with the melanin synthetic pathway, and its photothermal conversion rate (η) could reach up to 40%.15 Under alkaline conditions, dopamine (DA) monomers were oxidized to DA quinone and a five-membered ring, then rearranged to 5,6-dihydroxyindoles and aggregated with other polymers.16 More importantly, the polymerization rate of DA at the gas–liquid interface is higher than that in the solutions,17,18 indicating that the O2 bubble template may guide PDA polymerization. Chen's group reported a method for O2-rich PDA microcapsule preparation in 2021, but the micron-sized aggregates limited its further application in cancer treatment.19
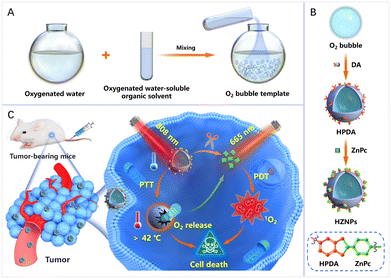
As shown in Scheme 1, a facile and nanosized O2 bubble template is reported by mixing oxygenated water and oxygenated water-soluble organic solvents. The O2 bubble template guided DA polymerization to form hollow PDA (HPDA) and encapsulate O2 simultaneously. Furthermore, HPDA is designed as a carrier for zinc phthalocyanine (ZnPc) to fabricate pH-responsive nanomedicine (denoted HZNPs). The ZnPc release rate of HZNPs reached 64.9% in a simulated tumour microenvironment. The in vitro and in vivo results indicated that HZNPs could deliver O2 to alleviate tumour hypoxia, inhibit the expression of hypoxia-induced factor-1 (HIF-1α), and enhance the anticancer efficiency of PDT. Upon the combination of light irradiation (808 nm + 665 nm), HZNP treated groups achieved satisfactory outcomes by PTT and PDT.
Results and discussion
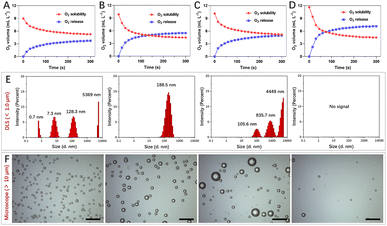
Gas solubility in solvents is closely related to polarity, and mixing different solvents could change gas solubility, such as water and water-soluble organic solvents.20–22 Polarity difference changes resulted in gas accumulation which escapes in the form of bubbles.23 To obtain nanosized O2 bubbles, the air (mainly N2 and O2) dissolved in water and water-soluble organic solvents was removed using a vacuum pump. These solvents were replenished with O2 to form O2-rich solutions respectively.After mixing oxygenated water and four water-soluble alcohols respectively, O2 solubility in these mixtures was monitored using an O2 dissolving meter. The results revealed that O2 solubility decreased and the biggest change was the mixture of n-propanol and water (Fig. 1A–D). The excess O2 escaped from methanol/water, ethanol/water, isopropanol/water, and n-propanol/water was 3.71 mL L−1, 5.46 mL L−1, 4.97 mL L−1 and 7.14 mL L−1, respectively. We further studied the size and morphology of O2 bubbles by taking n-propanol/water as an instance at different time points. As shown in Fig. 1E, the dynamic light scattering (DLS) distribution showed that the sizes of O2 bubbles were uneven, ranging from 0.7 nm to 5369 nm. These small O2 bubbles were fragile and fused with other bubbles to form larger bubbles. Interestingly, these O2 bubbles with an average size of 188.5 nm remained in existence stably for 10 min. Their generation, fusion and escape processes were also confirmed using a microscope (Fig. 1F).
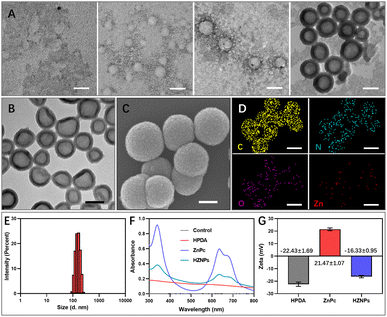
The polymerization rate of DA monomers at the gas–liquid interface is two times faster than that in solution.17 Therefore, we speculated that O2 bubbles are promising as a template for guiding DA polymerization to fabricate HPDA. To prove this hypothesis, after mixing oxygenated water and oxygenated n-propanol, DA monomers were added to the mixing solution. The PDA formation process was monitored using a transmission electron microscope (TEM). As shown in Fig. 2A, DA cyclization and covalent cross-linking to form composite films at the gas–liquid interface occur. The Raman spectra confirmed that HPDA was similar to eumelanin (Fig. S1†). Compared with PDA nanobowls, HPDA would not break with the increasing pressure inside the O2 bubbles.23 However, after removing the O2 bubble template, DA polymerized to form PDA nanospheres (Fig. S2†). We further utilized the O2 bubble template generated by mixing other oxygenated water-soluble organic solvents (methanol, alcohol, and isopropanol) with oxygenated water to prepare HPDA successfully (Fig. S3†), indicating the general applicability of the O2 bubble template for guiding PDA polymerization. The shell thickness of HPDA could be easily controlled by adjusting the reaction time (Fig. S4†).
Furthermore, HPDA was designed as a carrier for ZnPc via boric acid ester to fabricate HZNPs. As shown in Fig. 2B and C, HZNPs were observed by using TEM and a scanning electron microscope (SEM), and HZNPs presented regular hollow structures with an average size of 156.1 nm (Fig. 2E). The elemental mapping patterns of HZNPs showed that C, N and O were derived from HPDA and ZnPc while Zn was derived from ZnPc, confirming the successful preparation of HZNPs (Fig. 2D). In addition, HZNPs showed UV-vis absorption in the B-band (200–300 nm) and Q-band (600–800 nm), which was attributed to the characteristic π–π* electron transition of ZnPc.24 The broad-spectrum absorption ability of HPDA resulted in the increasing absorbance of HZNPs (Fig. 2F). As shown in Fig. 2G, ZnPc loading in HZNPs also increased the zeta potential of HZNPs. The inductively coupled plasma spectrometer (ICP) data indicated that the average loading efficiency of ZnPc in HZNPs was 0.2891 mg mg−1 (Table S1†). To further study the stability of HZNPs, the DLS distribution of HZNPs was detected in PBS and the cell culture medium. As shown in the DLS results, no obvious HZNP aggregation was detected in PBS or DMEM+10% bovine serum, indicating its good stability in these bio-surroundings (Fig. S5†).
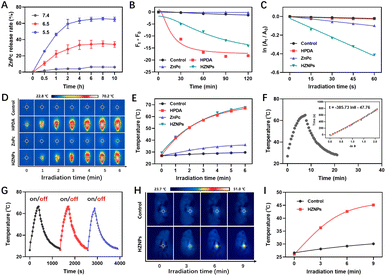
HPDA with abundant catechol could form borate ester bonds with ZnPc, and the pH-responsive borate ester bond was broken under acidic conditions.25 The vigorous metabolism and profound hypoxia in tumour tissue led to cell glycolysis and superfluous acid substance generation.26,27 Then, pH-triggered ZnPc release behaviours of HZNPs were studied under acidic conditions. As shown in Fig. 3A and S6,† the release rate of ZnPc from HZNPs increased obviously under acidic conditions while a slight change was observed under normal conditions, and the ZnPc release rate reached 64.9% when the pH is 5.5, which will recover PDT-mediated antitumor activity (Fig. 3A, S6 and S7†).
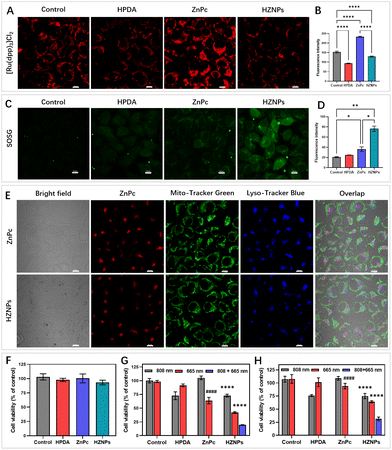
Even though HPDA has good stability due to its extensive covalent and noncovalent interactions, HPDA could be degraded in H2O2 aqueous solution because the –OH group in HPDA was oxidized to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –COOH groups (Fig. S8†). To study the encapsulation ability of HZNPs for O2, the Ru(dpp)3Cl2, a commercial O2-quenching fluorescence probe, was used to detect the O2 levels of different samples. As shown in Fig. 3B and S9,† the fluorescence intensity of the HPDA and HZNP treated groups decreased obviously while the ZnPc treated group showed a slight change, indicating that HPDA and HZNPs could increase the O2 level under hypoxic conditions. We further investigated the effect of HPDA-mediated PTT on the stability of HZNPs. After being dispersed in H2O2 aqueous solution for 30 min, HZNPs decomposed slightly at room temperature (Fig. S10†). In contrast, after laser irradiation for 30 min, HZNPs decomposed rapidly, revealing that PTT may promote the O2 release and alleviate tumour hypoxia. Singlet oxygen (1O2), a pivotal evaluation indicator for the photosensitizer, is positively related to the O2 level.28 After light irradiation, the 1O2 generation ability of different drugs was evaluated using 3,3′-(anthracene-9,10-diyl)dipropanoic acid (ADPA). As can be seen from Fig. 3C and S11,† the ADPA absorbance bleaching at 378 nm showed that the order of 1O2 generation efficiency of different drugs was kHZNPs (6.71 × 10−3) > kZnPc (1.67 × 10−3) > kHPDA (0.39 × 10−3) > kControl (0.34 × 10−3), indicating that the high 1O2 generation efficiency of HZNPs was attributed to ZnPc and the increasing O2 level.
O and –COOH groups (Fig. S8†). To study the encapsulation ability of HZNPs for O2, the Ru(dpp)3Cl2, a commercial O2-quenching fluorescence probe, was used to detect the O2 levels of different samples. As shown in Fig. 3B and S9,† the fluorescence intensity of the HPDA and HZNP treated groups decreased obviously while the ZnPc treated group showed a slight change, indicating that HPDA and HZNPs could increase the O2 level under hypoxic conditions. We further investigated the effect of HPDA-mediated PTT on the stability of HZNPs. After being dispersed in H2O2 aqueous solution for 30 min, HZNPs decomposed slightly at room temperature (Fig. S10†). In contrast, after laser irradiation for 30 min, HZNPs decomposed rapidly, revealing that PTT may promote the O2 release and alleviate tumour hypoxia. Singlet oxygen (1O2), a pivotal evaluation indicator for the photosensitizer, is positively related to the O2 level.28 After light irradiation, the 1O2 generation ability of different drugs was evaluated using 3,3′-(anthracene-9,10-diyl)dipropanoic acid (ADPA). As can be seen from Fig. 3C and S11,† the ADPA absorbance bleaching at 378 nm showed that the order of 1O2 generation efficiency of different drugs was kHZNPs (6.71 × 10−3) > kZnPc (1.67 × 10−3) > kHPDA (0.39 × 10−3) > kControl (0.34 × 10−3), indicating that the high 1O2 generation efficiency of HZNPs was attributed to ZnPc and the increasing O2 level.
Melanin-like PDA has strong photothermal properties,29 and the photothermal conversion rate (η) of HZNPs was measured with a photothermal imager. After laser irradiation for 5 min, the temperature of HPDA and HZNP solutions reached up to 67.1 °C and 67.6 °C respectively, while that of ZnPc was 36.2 °C, revealing that the η of HZNPs was mainly derived from HPDA (Fig. 3D and E). To calculate the η of HPDA, the absorbance of HPDA was detected to be 0.3073 at 808 nm (Fig. S12†). The temperature of HPDA solution raised to the highest upon laser irradiation, then, the laser was turned off, and the natural cooling temperature of HPDA solution was recorded at different points of time. The cooling time (t) curve versus the negative natural logarithm of temperature driving force (−ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) is shown in Fig. 3F. The time constant (τ) for heat transfer in the system was calculated to be 385.73 and the η was determined to be 38.2%. Three photothermal cycles showed that photothermal conversion capacity decreased slightly, indicating that HPDA has good stability (Fig. 3G). Furthermore, the photothermal properties of HZNPs were measured in tumour-bearing mice. After laser irradiation for 9 min, the temperature of the right solid tumour reached up to 45.1 °C, suggesting that HZNPs could kill tumour cells by PTT (Fig. 3H and I).
θ) is shown in Fig. 3F. The time constant (τ) for heat transfer in the system was calculated to be 385.73 and the η was determined to be 38.2%. Three photothermal cycles showed that photothermal conversion capacity decreased slightly, indicating that HPDA has good stability (Fig. 3G). Furthermore, the photothermal properties of HZNPs were measured in tumour-bearing mice. After laser irradiation for 9 min, the temperature of the right solid tumour reached up to 45.1 °C, suggesting that HZNPs could kill tumour cells by PTT (Fig. 3H and I).
HZNP alleviating hypoxia behaviour in vitro was detected using Ru(dpp)3Cl2 under hypoxic conditions.30 Compared with others, the fluorescence intensity of the ZnPc treated group increased obviously upon light irradiation because of PDT-depletion of O2. However, even though PDT consumed O2, the HZNP treated group still showed no fluorescence change, which indicated that O2 released from HZNPs alleviated intracellular hypoxia (Fig. 4A and B). O2 is an important component in PDT since the light initiated 1O2 generated by photosensitizers needs O2 motivation.30 Therefore, we speculated that O2-evolving HZNPs could enhance the anticancer activity of PDT. To verify this speculation, Singlet Oxygen Sensor Green (SOSG) was used to measure the intracellular 1O2 generation ability of different drugs. Under hypoxic conditions, the HPDA and ZnPc treated groups all showed weak fluorescence while the HZNP treated group showed fluorescence enhancement upon light irradiation (Fig. 4C and D). All these results above indicated that HZNPs released O2 and improved the generation ability of ZnPc in tumour cells.
To further elucidate drugs' anticancer mechanisms, the intracellular localization of ZnPc and HZNPs was measured using a confocal laser scanning microscope. As shown in Fig. 4E, the colocalization overlap of drugs and probes (Mito-Tracker Green or Lyso-Tracker Blue) suggested that the action site of HZNPs was the lysosome. Cell Counting Kit-8 (CCK8) assay was used to detect drugs' dark toxicity and phototoxicity under normoxic and hypoxic conditions. First of all, we tested the IC50 value of each formulation. The IC50 values of light treated groups were remarkably lower than those of non-light treated groups, indicating that light was one crucial factor in damaging tumour cells (Table S2†). Furthermore, all drugs showed no cytotoxicity without light irradiation (Fig. 4F). After different light irradiations, the HPDA treated group and ZnPc treated group showed PTT-induced (808 nm) and PDT-induced (665 nm) 4T1 cell growth inhibition effects, respectively. The HZNPs have combined and more efficient PTT and PDT anticancer effects under normoxic conditions (Fig. 4G). In contrast, the PDT-induced cell death rate by ZnPc decreased obviously under hypoxic conditions, and the anticancer efficiency of HZNPs was superior to that of ZnPc, indicating that HZNPs could alleviate tumour hypoxia (Fig. 4H).
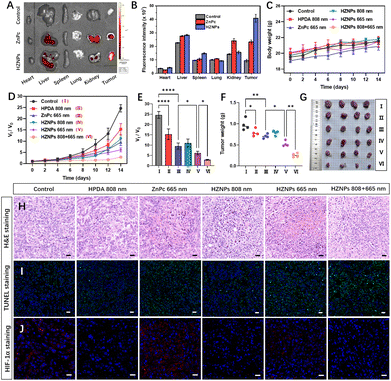
The phototoxicity of different drugs in vivo was studied in tumour-bearing mice by monitoring the tumour volume change during 14 days of treatment. The metabolism distribution of ZnPc and HZNPs in mice was monitored using an IVIS Lumina LT system. Compared with the ZnPc treated group, the stronger fluorescence intensity of the HZNP treated group suggested that HZNPs have a better retention effect in solid tumours (Fig. 5A, B and S13†). In addition, the relative amount of the Zn element in the main organs and tumours of tumour-bearing mice was measured by inductively coupled plasma mass spectrometry (ICP-MS). The relative amount of the Zn element in tumours treated with HZNPs was 8.16 mg g−1, 2.6 times that of the ZnPc treated group (3.15 mg g−1). Compared with the ZnPc treated group, HZNPs accumulated in the kidney decreased, which was beneficial to reduce the side effects of ZnPc (Table S3†). Many literature studies reported that nanomedicine accumulated in tumour and inflamed tissue by the enhanced EPR effect.31,32 Recent scientific studies indicated the passive accumulation of nanomedicines at the tumour site is not as important as the proposed classical EPR effect because of few gaps along tumour blood vessels.33 Therefore, the trans endothelial pathway and EPR effect may both contribute to the high accumulation of HZNPs at tumour sites.
There was no body weight decrease in mice in our study (Fig. 5C). The haematoxylin and eosin (H&E) stained tissue slices of main organs dissected from sacrificial mice showed no pathological features (Fig. S14†). The tumour growth inhibition of different drugs was studied by tumour volume tracking and H&E staining. HPDA treated with 808 nm light and ZnPc treated with 665 nm light showed tumour growth suppression to some extent via PTT (19.18%) and PDT (27.88%), respectively. The anticancer efficiency (46.29%) of HZNPs + 665 nm was superior to that (27.88%) of ZnPc + 665 nm, which could be ascribed to the hypoxia alleviating ability of HZNPs at tumour sites. The synergetic PTT and PDT of HZNPs achieved the best anticancer efficiency (73.91%, Fig. 5D–G). H&E staining of pathological tumour slices showed the most serious nuclei damage (purple blue, stained with haematoxylin) occurred in the group of HZNPs treated with 808 nm plus 665 nm light irradiation (Fig. 5H). After treatment with different formulations, the cell apoptosis in the excised tumour tissues was studied by means of TUNEL. As shown in Fig. 5I, the cell apoptosis of the HZNPs + 665 nm treated group was higher than that of the ZnPc + 665 nm treated group, and HZNPs treated with combination light (808 nm + 665 nm) had the best anticancer efficiency, which was consistent with the results of H&E staining. Furthermore, the hypoxia-inducible factor (HIF)-1α staining assay was used to evaluate the hypoxic condition in the tumours of different groups. The tumour immunofluorescence of ZnPc treated with 665 nm light was stronger than that of control, implying that the O2-depletion of PDT resulted in HIF-1α overexpression. Even if the PDT process consumed O2 in tumours, the immunofluorescence intensity was still weak in the HZNP treated group, indicating that the HIF-1α expression was inhibited by the O2-evolving HZNPs (Fig. 5J).
Conclusions
In conclusion, a facile and nanosized O2 bubble template was reported by mixing oxygenated water and water-soluble organic solvents. O2-evolving HPDA was prepared based on the nano-sized O2 bubble template, which would inspire the gas bubble template for guiding materials synthesis. The in vitro and in vivo results revealed that HZNPs could release O2 and alleviate hypoxia to enhance the PDT efficiency. Under a combination of light (808 nm + 665 nm) irradiation, HZNPs achieved synergetic and satisfactory anticancer efficiency, and the tumour growth rate was suppressed up to 73.91% when compared to that of control mice. Our study provides a novel strategy for O2 delivery by nanomaterials in cancer treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (grant no. 82104686; 82125037) and Nanjing University of Chinese Medicine (XPT82104686).Notes and references
- C. Zhang, W. J. Qin, X. F. Bai and X. Zhang, Nano Today, 2020, 35, 100960 CrossRef CAS.
- X. Li, K. Kwon, T. Guo, Z. Liu and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 11522 CrossRef CAS PubMed.
- W. Meng, Y. Hao, C. He and G. Zhu, Mol. Cancer, 2019, 18, 1–14 CrossRef PubMed.
- E. L. LaGory and A. J. Giaccia, Nat. Cell Biol., 2016, 18, 356 CrossRef CAS PubMed.
- X. Liang, M. Chen, P. Bhattarai, S. Hameed and Z. Dai, ACS Nano, 2020, 14, 13569 CrossRef PubMed.
- Y. Wan, L. H. Fu, C. Li, J. Lin and P. J. Huang, Adv. Mater., 2021, 33, 2103978 CrossRef CAS PubMed.
- Y. Cheng, H. Cheng, C. Jiang, X. Qiu, K. Wang, W. Huan, A. Yuan, J. Wu and Y. Q. Hu, Nat. Commun., 2015, 6, 8785 CrossRef CAS PubMed.
- R. Li, C. Zhang, B. Xie, W. Yu, W. Qiu, H. Cheng and X. Zhang, Biomaterials, 2019, 194, 84 CrossRef CAS PubMed.
- I. Moen and L. E. Stuhr, Target. Oncol., 2012, 7, 233 CrossRef PubMed.
- H. Wang, J. Li, Y. Wang, X. Gong, X. Xu, J. Wang, Y. Li, X. Sha and Z. Zhang, J. Control. Release, 2020, 319, 25 CrossRef CAS PubMed.
- T. Li, X. Jing and Y. Huang, Macromol. Biosci., 2011, 11, 865 CrossRef CAS PubMed.
- S. M. Fix, M. A. Borden and P. A. Dayton, J. Control. Release, 2015, 209, 139 CrossRef CAS PubMed.
- S. Huang, D. D. McPherson and R. C. MacDonald, Ultrasound Med. Biol., 2008, 34, 1272 CrossRef PubMed.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057 CrossRef CAS PubMed.
- Q. Zhan, X. Shi, J. Zhou, L. Zhou and S. Wei, Small, 2019, 15, 1803926 CrossRef PubMed.
- N. F. Della Vecchia, R. Avolio, M. Alfè, M. E. Errico, A. Napolitano and M. d'Ischia, Adv. Funct. Mater., 2013, 23, 1331 CrossRef CAS.
- F. Ponzio, P. Payamyar, A. Schneider, M. Winterhalter, J. Bour, F. Addiego, M. P. Krafft, J. Hemmerle and V. Ball, J. Phys. Chem. Lett., 2014, 5, 3436 CrossRef CAS PubMed.
- H. Yang, Q. Wu, L. Wan and Z. Xu, Chem. Commun., 2013, 49, 10522 RSC.
- B. Wu, Z. Sun, J. Wu, J. Ruan, P. Zhao, K. Liu, C. Zhao, J. Sheng, T. Liang and D. Chen, Angew. Chem., Int. Ed., 2021, 60, 9284 CrossRef CAS PubMed.
- S. Yang, S. M. Dammer, N. Bremond, H. J. Zandvliet, E. S. Kooij and D. J. Lohse, Langmuir, 2007, 23, 7072 CrossRef CAS PubMed.
- S. Karpitschka, E. Dietrich, J. R. Seddon, H. J. Zandvliet, D. Lohse and H. J. Riegler, Phys. Rev. Lett., 2012, 109, 066102 CrossRef PubMed.
- L. A. Palmer, D. Cookson and R. N. Lamb, Langmuir, 2011, 27, 144 CrossRef CAS PubMed.
- Q. Zhan, X. Shi, D. Fan, L. Zhou and S. Wei, Chem. Eng. J., 2021, 404, 126443 CrossRef CAS.
- A. Ogunsipe, D. Maree and T. J. Nyokong, J. Mol. Struct., 2003, 650, 131 CrossRef CAS.
- R. Liu, Y. Guo, G. Odusote, F. Qu and R. D. Priestley, ACS Appl. Mater. Interfaces, 2013, 5, 9167 CrossRef CAS PubMed.
- V. Estrella, T. Chen, M. Lloyd, J. Wojtkowiak, H. H. Cornnell, A. Ibrahim-Hashim, K. Bailey, Y. Balagurunathan, J. M. Rothberg, B. F. Sloane, J. Johnson, R. A. Gatenby and R. J. Gillies, Cancer Res., 2013, 73, 1524 CrossRef CAS PubMed.
- R. A. Cardone, V. Casavola and S. J. Reshkin, Nat. Rev. Cancer, 2005, 5, 786 CrossRef CAS PubMed.
- H. Zhao, J. Xu, Y. Wang, C. Sun, L. Bao, Y. Zhao, X. Yang and Y. Zhao, ACS Nano, 2022, 16, 3070 CrossRef CAS PubMed.
- X. Zeng, M. Luo, G. Liu, X. Wang, W. Tao, Y. Lin, X. Ji, L. Nie and L. Mei, Adv. Sci., 2018, 5, 1800510 CrossRef PubMed.
- H. Chen, J. Tian, W. He and Z. Guo, J. Am. Chem. Soc., 2015, 137, 1539 CrossRef CAS PubMed.
- H. Maeda, H. Nakamura and J. Fang, Adv. Drug Delivery Rev., 2013, 65, 71 CrossRef CAS PubMed.
- H. Kang, S. Rho, W. R. Stiles, S. Hu, Y. Baek, D. W. Hwang, S. Kashiwagi, M. S. Kim and H. S. Choi, Adv. Healthcare Mater., 2020, 9, 1901223 CrossRef CAS PubMed.
- S. Sindhwani, A. M. Syed, J. Ngai, B. R. Kingston, L. Maiorino, J. Rothschild, P. MacMillan, Y. Zhang, N. U. Rajesh, T. Hoang, J. Wu, S. Wilhelm, A. Zilman, S. Gadde, A. Sulaiman, B. Ouyang, Z. Lin, L. Wang, M. Egeblad and W. Chan, Nat. Mater., 2020, 19, 566 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2na00549b |
| ‡ Q. C. Zhan and X. Han contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |