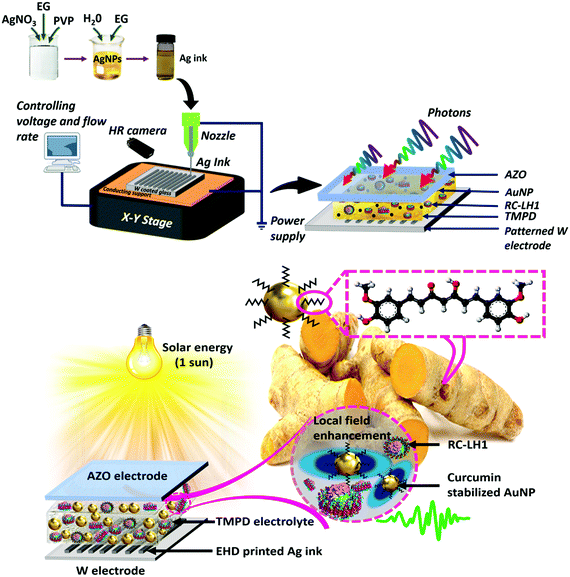
Plasmonic protein electricity generator†
Nikita
Paul‡
a,
Lakshmi
Suresh‡
 a,
Yixin
Chen
b,
Yaoxin
Zhang
a,
Fuad Indra
Alzakia
a,
Victor
Vogt
c,
Michael R.
Jones
*d,
Zi Jing
Wong
*bc and
Swee Ching
Tan
a,
Yixin
Chen
b,
Yaoxin
Zhang
a,
Fuad Indra
Alzakia
a,
Victor
Vogt
c,
Michael R.
Jones
*d,
Zi Jing
Wong
*bc and
Swee Ching
Tan
 *a
*a
aDepartment of Materials Science and Engineering, National University of Singapore, 9 Engineering Drive 1, Singapore 117575, Singapore. E-mail: msetansc@nus.edu.sg
bDepartment of Aerospace Engineering, Texas A&M University, 701 H.R. Bright Building, College Station, TX 77843, USA. E-mail: zijing@tamu.edu
cDepartment of Materials Science and Engineering, Texas A&M University, 207 Reed McDonald Building, College Station, TX 77843, USA
dSchool of Biochemistry, Biomedical Sciences Building, University of Bristol, University Walk, Bristol, BS8 1TD, UK. E-mail: m.r.jones@bristol.ac.uk
First published on 6th January 2022
Abstract
Interest in acquiring green energy from sunlight is driving research into the incorporation of biological photosynthetic materials into biohybrid devices. A potential way to enhance solar energy conversion by photosynthetic proteins is to couple them to plasmonic nanomaterials to enhance absorption of incident radiation. In this work, a variety of plasmonic nanoparticles were used to boost the photocurrent output of a Protein Electricity Generator (PEG). Mixing gold nanoparticles (NPs) of five different architectures into the photoprotein/electrolyte contents of the cell was found to increase device performance, the most effective being ∼120 nm diameter star-shaped clusters that caused a ∼six-fold increase in photocurrent at the optimum dopant level. In addition, high-resolution electrohydrodynamic printing was used to create parallel line and square lattice patterns of silver nanoparticle ink on the tungsten rear electrode of the cells. Patterns with a 700 nm spacing between lines boosted photocurrents by up to three-fold and the effects of the gold and silver nanoparticles were additive, such that the ideal combination produced a ∼19-fold increase in photocurrent and device efficiency. We attribute the elevated performance to plasmonic enhancement of absorbance and scattering effects that increase the path length for photons in the device. Use of rear electrodes with silver nanoparticle lines and grids at 1100 nm spacing did not increase photocurrents, highlighting the importance of precision printing of nanostructures for the enhancement of device performance.
New conceptsRC-LH1 from purple photosynthetic bacteria such as Rhodobacter (Rba.) Sphaeroides was used as the photovoltaic material for this study as it can accomplish the transduction of absorbed light energy through a highly effective photochemical charge separation process which produces an electron for every photon absorbed. Turmeric, one of the most commonly used South-East Asian spices, was utilised as an alternative for conventional chemical reducing agents for producing the plasmonic gold nanoparticles used in this study. Unlike in conventional routes, using turmeric eliminates the need for additional capping agents as it provides an inherent capping feature preventing the nanoparticles from agglomerating and thus, improving the stability of nanoparticles from a few days to years! In order to improve the light harvesting ability of RC-LH1 in the useful range of the solar spectrum, an embedded matrix of metal nanoparticles and periodic plasmonic patterns was created using the green synthesis route and electrohydrodynamic printing. Electrohydrodynamic printing (EHD) provides high resolution sub-micron-nano- scale printing compared to other standard printing techniques. In combination, the EHD printed silver and green synthesised gold plasmonic nanostructures were capable of producing up to a ∼1900% improvement in the performance of RC-LH1 Protein Electricity Generator cells. |
Introduction
In recent years, there has been considerable interest in the use of molecules, membranes and cells from photosynthetic organisms as components in bio-photoelectrochemical cells for a variety of energy and sensing applications.1–12 Such components are environmentally benign and recyclable,13 and there is scope for their precise manipulation using the tools of molecular biology to engineer their interactions with each other14 and with man-made materials.15,16 One of the preferred photosynthetic proteins for photovoltaic applications is the Reaction Centre-Light Harvesting 1 (RC-LH1) complex from the purple bacterium Rhodobacter sphaeroides (Fig. S1, ESI†).3,17–20 The bacteriochlorophyll (BChl) and carotenoid pigments of the cylindrical LH1 domain gather the energy of incident photons and pass it to a central RC domain where a series of photochemical reactions take place. Transfer of excited state energy to the BChl special pair (P) within the RC produces a singlet excited electronic state (P*) capable of reducing a quinone (QB) at the opposite side of the protein, producing a photo-oxidized special pair (P+). This creates a metastable charge separated state (P+QB−), which, in a device setting, can be harnessed for photocurrent generation through a variety of mechanisms. In one such, electrons are carried from the protein to an anode by an electrolyte whilst new electrons enter the protein from the opposing cathode to reduce P+.19,21 A primary focus of research into bio-photovoltaics is to uncover ways to utilise such green photosynthetic materials as efficiently as possible. As individual photosynthetic pigment molecules harvest only certain regions of the available solar spectrum, one area of interest is improvement of overall light absorption across the useful solar spectral range (300 nm to 1200 nm). Possible ways to achieve this include the conjugation of photosynthetic materials with additional absorbing components, either natural,14,22 synthetic15,23–27 or both,16 and manipulation of cell design to enhance absorption by the protein through reflection and scattering.28,39 Some of these strategies have commonalities with the use of plasmonics to enhance the performance of thin film solar cells.29One way to enhance light harvesting is through the use of gold nanoparticles (NPs). Since the typical size of a gold NP is much smaller than the wavelength of visible light, a strong interaction takes place between the free conduction electrons of the metal and the incident light wave. This causes the surface conduction band electrons to coherently oscillate with the generation of a dipole in the nanoparticle, termed a surface plasmon (Fig. S2, ESI†).30–32 When the frequency of the electromagnetic radiation matches the frequency of the vibrating electrons a localized surface plasmon resonance (LSPR) condition is met. As the LSPR induces strong absorption of visible light, gold NPs can act as “solar concentrator antennas” to harvest incident sunlight and store the photon energy in the LSPR modes.32–36 This is associated with a strong local electric field enhancement due to resonance.37,38 Several groups have presented organic solar cells containing noble metal NPs-that display superior performance due to enhanced absorption.35,39,40 Through LSPR enhanced scattering, noble metal NPs can also improve the performance of a solar cell by increasing the path length for light within the active layer.29,33,41 By careful tuning of the size, shape and concentration of NPs, one can tune the performance of a device by optimising scattering.42 Metal NPs also bring the possibility of enhancement of performance through improved electrical conductivity.27
Gold NPs have emerged as important engineering materials across a variety of disciplines due to their stability, tuneable surface features, biocompatibility and low cytotoxicity. Physical, chemical, and biological synthesis approaches can be used to synthesize Au nanoparticles of various shapes and sizes. The use of physical or chemical routes, however, can involve high energy consumption, produce low yields, increase synthesis costs, and cause environmental damage. Another drawback in their production is that conventional synthesis routes can require the use of toxic reducing and capping reagents such as sodium borohydride, hydrazine and polyvinyl pyrrolidone (PVP).43,44 Biological processes usually involve the employment of organisms such as bacteria, fungi, yeast, algae or plants. However, the use of microorganisms can pose risk due to pathogenicity concerns, as well as requiring the maintenance of large-scale cultures. As a result, greener methods of gold nanoparticle synthesis using plant components (e.g. apple, green tea, tulsi) have been explored.45 One such employs extracts of turmeric (Curcuma longa), a well-known spice plant that is enriched with curcumin, a bio-active polyphenol that contributes to turmeric's distinctive colour. Curcumin is used widely for its anti-inflammatory, anti-oxidant, anti-carcinogenic and anti-viral properties.46–48 It has been applied to the green synthesis of gold NPs, either as a capping reagent49 or as a combined reducing/capping reagent.50–52
In addition to simple mixing of NPs into the volume of a photoactive material, where precision in spacing is not possible, there has also been great interest in the patterning of both front and back electrodes with nanostructures comprising noble metal layers or NPs to support enhanced absorption and light scattering by surface plasmon resonances (SPR).53,54 Periodic plasmonic patterns with dimensions comparable to, or less than, the wavelength of incident light can produce a longer optical path within a solar cell.55 The interaction of light waves with a nanostructured surface is the basis for the development of SPR-based sensing devices which find applications in biotechnology, security, environmental protection, quality inspection.56–59 Also, silver offers good light trapping efficiency in the visible spectrum, which is the range of interest for most photovoltaic cells.60,61 It is suitable for use both in the form of discrete NPs and for the fabrication of larger scale nanopatterned coatings and periodic structures through NP deposition.61
The creation of micro- and nano-scale surface textures for printed electronics has gained much attention and is a focus for much industrial and academic research. A number of printing techniques have been developed including photolithography, electron beam lithography, inject printing and focused ion beam milling. Despite successes, these methods suffer from disadvantages that can result in high production costs including complex hardware, excessive use of printing materials, limitations in the area that can be patterned and slow printing rates.62 Electrohydrodynamic (EHD) printing is a technique that can address such issues, providing a means to print high resolution patterns on scales ranging from tens of nanometres to hundreds of micrometres, and with a more efficient use of resources. In an EHD printer an electrified jet is obtained by applying a potential difference between the ink nozzle and the substrate. EHD printing of electrodes is mainly performed using metal nanoparticle inks due to their inertness, high conductivity, and non-oxidizing properties.62 Direct printing using EHD technology has been use to fabricate a wide variety of components for thin field transistors,63,64 sensors,65 photovoltaic cells,66 optoelectronic devices67,68 and micro-electromechanical systems.69
The present work explores enhancement of the performance of a bio-photovoltaic cell through the incorporation of two types of plasmonic metal NP (Fig. 1). Within the photoactive layer, the protein/electrolyte solution was blended with one of five types of gold NP synthesised by a green route using curcumin extracted from turmeric root as a reducing/capping agent. The relative merits of incorporating different amounts of spherical, triangular plate and star-shaped spherical clusters were examined to identify the optimal NP architecture and doping level. In addition, after optimising flow rate, ink viscosity and applied voltage, silver NPs were EHD printed in micron-width parallel lines and grids on the tungsten surface of the back electrode of the bio-photovoltaic cell (Fig. 1). It was found that, in combination, these silver and gold plasmonic nanostructures were capable of producing up to a ∼19-fold improvement in the external quantum efficiency of these RC-LH1 Plasmonic Protein Electricity Generator (PPEG). This is a stepping stone in the development of bio-solar cells in which LSPR, SPR and light scattering enhance performance.
Experimental
Materials
Aluminium-doped ZnO (AZO) conductive glass with a sheet resistance of less than 10 Ω sq−1 was purchased from LaTech Scientific Supply, Singapore. N,N,N′,N′-Tetramethyl-p-phenylenediamine (TMPD, 99%), silver(I) nitrate, PVP, ethylene glycol (EG, ≥99%) and gold(III) chloride (HAuCl4) were purchased from Sigma Aldrich. Distilled water was used for all experimental and cleaning purposes. All chemicals were used without any further purification. RC-LH1 complexes devoid of the PufX protein70 were isolated from photosynthetic membranes using n-dodecyl β-D-maltopyranoside (DDM), and were purified as described previously.71,72 They were stored at −80 °C as a concentrated solution in 20 mM Tris (pH 8.0)/0.04% (w/v) DDM prior to use. Structural models of PufX-deficient RC–LH1 complexes are based on the X-ray crystal structure of the similar complex from Thermochromatium tepidum.73 The concentration of RC-LH1 protein was such that the absorbance at 874 nm was equivalent to ∼690 absorbance units cm−1.Synthesis of gold NPs
Turmeric root used for the extraction of curcumin was purchased from a local market in Singapore. A 10 g sample of roots was soaked in 50 mL of water overnight and then used to extract curcumin the following day. The mixture was heated to 95 °C and stirred continuously until it reduced to a 25 mL concentrate. The resulting extract was filtered to remove impurities before being used for the synthesis of NPs. Curcumin–NaOH solution (pH 8) was formed by mixing 5 mL of curcumin solution with 100 mM NaOH until a pH of 8 was achieved.To synthesize gold NPs, curcumin extract was mixed with gold(III) chloride (HAuCl4)50 and the resulting colour change from yellow to blue/purple, depending on the size and shape of obtained nanoparticles, was monitored visually. To form spherical gold NPs, a volume of curcumin-NaOH solution was slowly added dropwise to an equal volume of HAuCl4 solution (10 mM) under continuous stirring for 30 min, 90 min or overnight to obtain NPs of 90 nm diameter (NPs-90), 50 nm diameter (NPs-50) or 10 nm diameter (NPs-10), respectively. Triangular plate gold NPs (NPs-T) were synthesised by maintaining a mixture of curcumin and HAuCl4 solutions at pH 4 and stirring overnight. Synthesis of star-shaped gold NPs (NPs-S) was achieved as reported earlier74 by adding 1 mL of 100 mM hydroxylamine solution (in water) to 5 mL of curcumin solution at pH 12 and stirring for 120 seconds. A 100 μL aliquot of 10 mM HAuCl4 in distilled water was then rapidly mixed in and fast stirring was maintained until a deep blue coloration developed.74 NP formation was followed by six cycles of sonication (5 min) and centrifugation (1 h) to remove the supernatant mixture (water plus curcumin), and the NP residue was then dispersed in distilled water for subsequent use. Approximate concentrations for the 10 nm diameter, 50 nm diameter, 90 nm diameter, triangular plate and star shaped NP solutions in water were 15.7, 3.91, 2.33, 0.07 and 0.02 mg mL−1 respectively.
A low concentration of each gold NP dispersed in ethanol was used for transmission electron microscopy (TEM). One drop of a gold suspension was placed on a Cu mesh grid and allowed to dry overnight. An EM208S (Philips) transmission electron microscope operating at an accelerating voltage of 100 kV was used to visualise structure and record electron diffraction patterns.
Patterning of electrodes with silver NP ink
Silver NPs were prepared by heating 50 mL of ethylene glycol (reducing agent) with 0.5 g PVP (dispersing agent) at 80 °C with continuous stirring. A 50 mL volume of 0.1 M silver nitrate solution was then added whilst maintaining a pH of 10 through dropwise addition of ammonia. Resulting silver NPs (average particle diameter of 12 ± 2 nm) were dispersed evenly in a water–ethylene glycol mixture (at a weight percentage of silver NPs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG of 10
EG of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35) and directly used as an ink for EHD printing. The detailed procedure can be found elsewhere.75
35) and directly used as an ink for EHD printing. The detailed procedure can be found elsewhere.75
Electrode substrates were prepared by sputtering tungsten metal (200 nm thickness) onto a precleaned AZO glass substrate using an RF sputter (110 W and 15 minutes). To form patterned electrodes, the formulated silver NP ink was applied to a substrate from a glass capillary nozzle of inner diameter 1 μm mounted on the EHD printing head (Enjet, Korea) by a digitally controlled pressure pump. During printing on the tungsten electrode substrate, a high-speed pattern camera attached to the system captured magnified images of the ink jet. The distance between the nozzle and the substrate was adjusted to 70 μm in 0.1 μm steps using a X–Y movable stage to hold a pre-cleaned tungsten-coated electrode. The print speed was fixed at 40 mm s−1. After the printing process, the substrate was annealed at 150 °C for 30 min in a vacuum furnace (HF Kejing) to evaporate the residual solvent. Four patterns of silver NP lines were printed, denoted DG-1 to DG-4 (see text). Surface images of the patterned electrodes were recorded using a Zeiss Sigma 300 scanning electron microscope (SEM).
For optimisation of the silver NP ink, an Anton Paar modular compact rheometer (MCR302) was used to perform rheological measurements with a parallel plate probe (PP25-SN 17002). Tests were carried out using a 1.5 mL sample in rotation mode.
Device fabrication
Prior to device fabrication, top AZO electrodes were thoroughly cleaned by sonicating in acetone and ethanol, followed by rising twice with deionized water. An electrolyte solution comprising TMPD redox mediator in 0.01 mM Tris–HCl (pH 7.5) with a final concentration adjusted to 100 mM was used for all the devices. Cyclic voltammetry of the electrolyte was recorded using an electrochemical workstation (CHI 660D) at a scan rate of 50 mV s−1. A tungsten electrode was employed as the working electrode, Ag/AgCl (3 M KCl) as the reference electrode and a platinum sheet as the counter electrode.To form a cell, a piece of parafilm with a section removed to form a central well was adhered to the conductive side of the AZO electrode and 6 μL of protein/electrolyte mixture in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was pipetted out into the cavity. The back electrode was brought in contact and the device was sealed using binder clips to prevent leakage of the working solution.
1 was pipetted out into the cavity. The back electrode was brought in contact and the device was sealed using binder clips to prevent leakage of the working solution.
To compensate for varying stock solution concentrations, each type of gold NP was mixed with the protein/TMPD solution in a volume ratio that gave a uniform ratio of mg gold to mg protein. This enabled comparison of different NP morphologies. Cells were then fabricated as above. As mentioned above, synthesis of spherical nanoparticles was easier and had a higher yield than triangular and star shaped NPs. So, considering this concentration difference, we tried to approximately balance out the amount of gold NPs added to the RC–LH1 devices. For e.g. 0.15 μL of 10 nm, 0.6 μL of 50 nm, 1 μL of 90 nm, 33 μL of triangular plate and ∼100 μL of star shaped NP accounted for the same amount of gold NPs irrespective of the yield difference. After normalizing the concentration difference, each of these quantified volume of gold NPs solution had approximately 2.33 μg of gold NPs. To examine different levels of doping, the amount of gold NPs was also doubled, tripled and quadrupled based on the above normalised values; maintaining a constant protein concentration for all the cases. In the text, this is denoted by 1 vol% to 4 vol%.
Cells fabricated using an optimized vol% of one of five types of gold NP and one of four types of patterned back electrode were named in accord with the combination used – e.g. DG-1 + NP-S for a cells with star-shaped gold NPs and the DG-1 patterned back electrode. In addition to these 20 cells, reference cells without any gold NPs and an unpatterned tungsten back electrode were fabricated and labelled as “Control”. An Active area of 0.3 cm2 was used for all measurements.
Device characterization
Photoresponses of assembled cells were measured under illumination from a tungsten halogen light (100 mW cm−2) on an active area of 0.2 cm2 using Keithley K2450 source meter. For EQE, measurements were performed using Zolix (SCS10-X150-DSSC) IPCE instrument with a quartz tungsten halogen lamp as the light source. To study the electrochemical behaviour of the device, electrochemical impedance spectroscopy (EIS) was performed using an electrochemical workstation (CHI 660D) over a frequency range from 1 to 105 Hz with a 10 mV amplitude. Conductivity, electrophoretic mobility and zeta potentials of the different NPs were measured using a zeta potential analyzer. Absorbance spectra were recorded using a Shimadzu UV-Vis spectrophotometer.Results
Synthesis of gold NPs
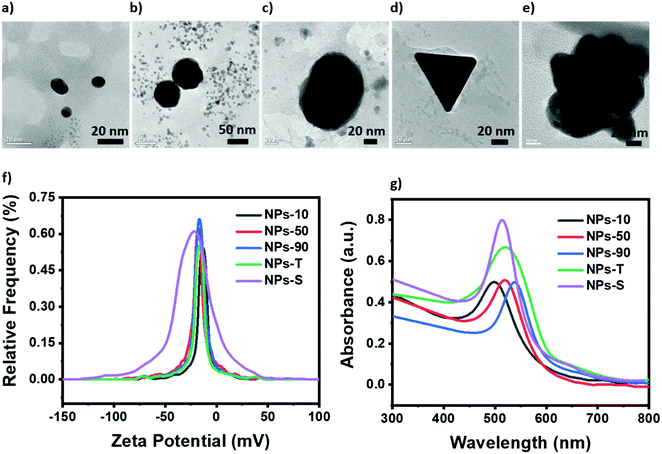
In green synthesis of gold NPs,50 the carboxyl group of curcumin (enolic) was used to reduce HAuCl4 and stabilise the NPs (Fig. S3, ESI†). The enolate and two phenolate OH groups of curcumin were first deprotonated to generate Cur3−, and then electrons from the O− groups were used to reduce Au3+ to Au0, producing a transparent solution. These Au0 atoms form initial nucleates which can then grow further in size either through diffusion or a surface process-controlled mechanism.38,50 Spherical curcumin-capped gold NPs were grown by incubating an alkaline solution of curcumin with HAuCl4 for different times (see Materials and methods). Triangular plate gold NPs were formed by mixing curcumin and HAuCl4 solutions at pH 4, whilst star-shaped gold NPs were formed in the presence of hydroxylamine.74Transmission electron microscopy (TEM) was used to document successful synthesis of curcumin-stabilized gold NPs. Spherical NPs (Fig. 2a–c) were well-dispersed, with a nearly uniform shape and diameter, and with no significant agglomeration. Particle diameter distributions are shown in Fig. S4 (ESI†). NP size was determined by incubation time, NPs with the smallest diameter (dubbed “NPs-10”) being obtained by stirring overnight whilst those with the largest diameter (“NPs-90”) being obtained after only 30 mins stirring. Triangular plate NPs (“NPs-T”, Fig. 2d) were distributed around an edge length of 100 nm while star-shaped NPs (“NPs-S”, Fig. 2e) were on the order of 120 nm in diameter (Fig. S4, ESI†). Small angle electron diffraction (SAED) confirmed that the NPs were crystalline, and further showed that the triangular plate NP was a single crystal (Fig. S5, ESI†).
The NPs showed a negative surface charge (Fig. 2f), with average zeta (ζ) potentials ranging from −22 mV to −12 mV indicating that the NPs are extremely stable in their environment. It was also observed, for the spherical NPs, that the peak height increased as the diameter of the NP increased, an effect attributable to an increasing surface charge density. The conductivity, electrophoretic mobility and zeta potentials of the different NPs are summarized in Table S1 (ESI†).
All NPs possessed a localized surface plasmon resonance absorption band (Fig. 2g) with a maximum at 495 nm (NPs-10), 515 nm (NPs-50), 537 nm (NPs-90), 520 nm (NPs-T) or 513 nm (NPs-S). For the spherical NPs this maximum wavelength correlated with diameter, being most blue-shifted for the smallest NP in accordance with Mie theory.76
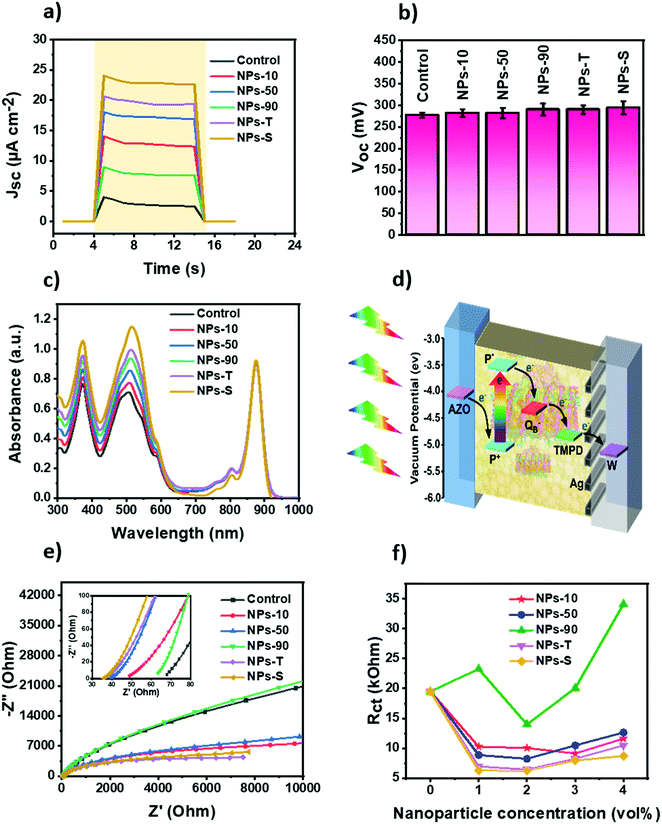
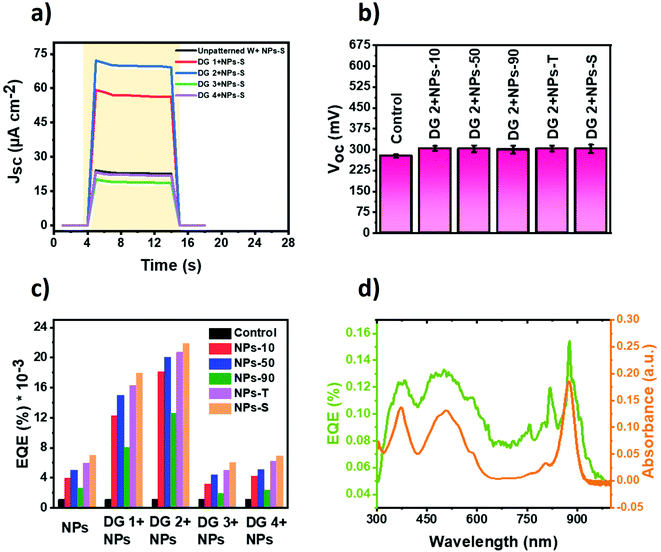
In addition, a series of cells was constructed in which the protein/electrolyte mixture was doped with one of the five types of gold NPs (see Materials and methods). Amounts of each NP stock solution added were adjusted such that the absolute amount of gold added to each cell was constant (denoted “1 vol%”). In addition, the amount was doubled, tripled and quadrupled (denoted 2, 3 or 4 vol%) to identify an optimal level. In all cases, doping the protein/electrolyte mixture with a gold NP enhanced the short circuit photocurrent density (Jsc) of the Protein Electricity Generator (Fig. S7, ESI†). The optimum effect was seen at 2 vol% with the exception of NPs-10 where the optimum was at 3 vol%. The magnitude of the enhancement of current density proceeded in the order NPs-S > NPs-T > NPs-50 > NPs-10 > NPs-90 (Fig. 3a). The strongest effect was seen with star-shaped NPs, where the Jsc was improved by approximately 6-fold, from 4 μA cm−2 to 24 μA cm−2, by simply adding 2 vol% of NPs. The decrease in Jsc at NP levels above 2 vol%, (or 3 vol% for the smallest NPs) is attributed to scattering by the NPs dominating over enhanced absorption due to the NPs.
To check the possibility of the device producing a photocurrent in the absence of the photoprotein, five cells lacking protein but with each of the five different types of NP were fabricated (Fig. S8, ESI†). A weak photocurrent was generated in these devices due to photo-excitation of the NPs, charge separation being accomplished by the transfer of photoexcited electrons from the gold particle to the tungsten electrode. The relative magnitudes of the weak current densities were in accordance with the trend in the protein-containing devices, as discussed above. The transient nature of the weak photocurrents seen in these control devices was in marked contrast to the strong and stable photocurrents that could be sustained in the presence of the photoprotein (Fig. 3a).
The open circuit voltage (Voc) obtained for each of the five protein cells with different types of NP was not significantly different from the control cell with just RC-LH1 protein and TMPD, with a value of around 0.28–0.29 V (Fig. 3b). However, there was a prolongation of the discharge time for all of the cells with NPs, suggesting the trapping of charges on the surfaces of the gold NPs (data not shown).
Absorption spectra of the RC-LH1 cells are shown in Fig. 3c. Compared to a cell with just RC-LH1 proteins, the cells with an optimal 2 vol% gold NPs (or 3 vol% for the NPs-10 cell) showed a general increase in absorbance below 600 nm. This is attributed to a combination of light scattering by the gold NPs throughout the visible region and overlap of their absorbance band between 450 and 600 nm (Fig. 2g) with the absorbance bands of the carotenoid light harvesting cofactors of the RC-LH1 complex between 420 and 600 nm. Absorbance in the 300 to 600 nm range was enhanced by a factor of 1.1 using NPs-10, 1.22 using NPs-50, 1.34 using NPs-90, 1.42 using NPs-T and 1.64-fold using NPs-S. It is of note that this absorbance enhancement was much lower than the photocurrent enhancement seen with each NP (e.g. a 6-fold enhancement in Jsc for a 1.64-fold enhancement of absorbance in NPs-S cells).
The proposed mechanism for photocurrent generation based on the vacuum potentials of the components and the direction of current flow is represented schematically in Fig. 3d. Illumination causes charge separation across the RC protein to oxidise the primary electron donor BChls (P+) and reduce a ubiquinone-10 at the QB binding site, forming a radical pair P+QB−. The water-soluble TMPD mediator shuttles electrons to the back electrode from the QB site of the protein, followed by re-reduction of the oxidized P BChls at the front AZO electrode.
Electrochemical impedance spectra (EIS) were recorded for the RC-LH1/AuNPs cells. The equivalent Randles circuit is shown in Fig. S9a (ESI†). Adding gold NPs to the devices tended to decrease both the sheet resistance (Rs) and charge transfer resistance at the electrode–electrolyte surface (Rct). In accord with the observed enhancement of Jsc, values of Rs were lowest for 3 vol% of the NPs–10 gold NPs and 2 vol% for the remainder; EIS for these optimal cells are collated in Fig. 3e. A similar trend was seen for Rct (Fig. 3f). Further additions of NPs above this optimal level increased impedance values (Fig. S9b–f, ESI†) indicating hindrance of electron flow that can likely be attributed to the accumulation of NPs at the tungsten electrode grain boundaries.77 Of the five types of gold NP used, the star shaped particles provided the best enhancement of charge transport, possibly due to their higher specific surface area, followed by the triangular plates (Fig. 3e and f).
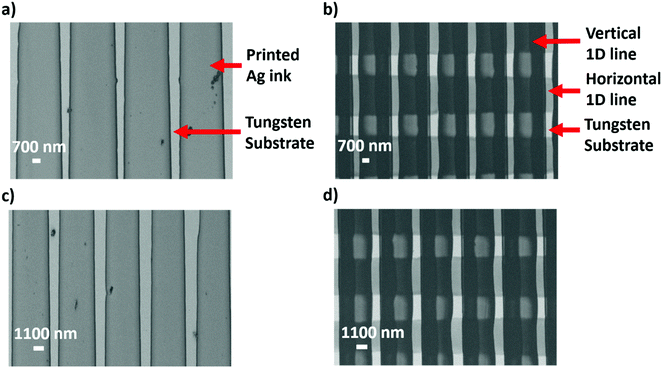
The applied voltage and flow rate were experimentally optimized for effective EHD printing. Initially, with a voltage of 0.8 kV, the ink was unable to flow out continuously through the capillary nozzle. However, on increasing the applied voltage to 2 kV, micro dripping and an intermittent jet were converted to cone jet that was able to form a high-resolution pattern. At a low flow rate of 0.4 μL min−1 it was difficult for the ink to come out completely from the nozzle and a thin jet was formed that was incapable of uniform printing. In contrast at a high flow rate of 2.4 μL min−1 the diameter of the jet became too large and overly thick patterns were printed. After series of trials, an optimum voltage of 2 kV and a flow rate of 1.6 μL min−1 were identified that could print features of size down to 700 nm using nozzle diameters as small as 1 μm. Evaporation caused particle aggregation the formation of continuous compact lines. Four different patterns were printed on bare tungsten electrode substrates (Fig. 4a–d). These were a 1-D continuous line pattern with the distance between lines maintained at 700 nm (denoted DG-1), a 2-D square lattice pattern with the distance between the vertical lines maintained at 700 nm (denoted DG-2), a 1-D continuous line pattern with the distance between lines maintained at 1100 nm (denoted DG-3) and a 2-D square lattice with a distance between the vertical lines maintained at 1100 nm (denoted DG-4). The width and height for each line was ∼2.8–3 μm and ∼200 nm, respectively (Fig. S11a and b, ESI†). For DG-2 and DG-4, the spacing of the horizontal lines was ∼2200 nm. Energy-dispersive X-ray spectroscopy (EDS) was performed to examine the elemental mapping of the Ag printed ink. Fig. S12a–d (ESI†) shows the elemental maps, highlighting silver as the dominant element. The printed patterns formed a non-uniformly distributed aggregate, conductive network structure that maintained a good contact with the bare W electrode below.
For control devices fabricated with patterned electrodes but no gold NPs in the protein/electrolyte contents, the highest Jsc was obtained for the DG-2 pattern followed by DG-1, DG-3 and DG-4 (Fig. S13, ESI†). Sets of cells were fabricated in which an optimised amount of each of the five gold NPs was combined with one of four types of nanopatterned back electrode. Sample photocurrent transients for the set with 2 vol% NPs–S gold NPs are shown in Fig. 5a. The DG-3 and DG-4 patterns had no significant effect on Jsc, but the DG-1 and DG-2 patterns brought about a strong boost in photocurrent. Equivalent results were obtained for sets of cells containing the four remaining types of gold NP, the DG-3/4 patterns proving ineffective but the DG-1/2 patterns producing a large increase in photocurrent (Table 1). The DG-1 and DG-2 increased Jsc in a manner that retained the relative efficacy of the different types of gold NP (Fig. S14a and b, ESI†) and these relative efficacies were not changed by the ineffective DG-3 and DG-4 patterns (Fig. S15a and b, ESI†).
| Device | W Jsc (μA cm−2) | DG-1 Jsc (μA cm−2) | DG-2 Jsc (μA cm−2) | DG-3 Jsc (μA cm−2) | DG-4 Jsc (μA cm−2) |
|---|---|---|---|---|---|
| a Control cell with protein/electrolyte but no gold NPs. b Percentages in parentheses are current as a percentage of that in a cell with a plain tungsten back electrode and the same gold NPs. | |||||
| Controla | 4 | 23.6 | 30.43 | 9.27 | 5.68 |
| +NPs-90 | 8.8 | 27 (307%)b | 42 (477%) | 6.5 (−26%) | 8 (−9%) |
| +NPs-10 | 14 | 41 (293%) | 60 (429%) | 10.5 (−25%) | 14.45 (3%) |
| +NPs-50 | 18 | 50 (278%) | 67 (372%) | 14.5 (−19%) | 17.5 (−3%) |
| +NPs-T | 20.6 | 55 (267%) | 69 (335%) | 17 (−17%) | 20.75 (1%) |
| +NPs-S | 23.9 | 60 (251%) | 73 (305%) | 20 (−16%) | 23 (−4%) |
To again check the possibility of such a device producing a photocurrent in the absence of the RC-LH1 protein, an optimised amount of each of the five gold NPs was combined with DG-2 nanopatterned back electrode in five control cells. Photocurrent densities in the range of 1 μA cm−2 to 2.7 μA cm−2 were observed (Fig. S16, ESI†), higher than those obtained for equivalent control cells with a plain tungsten electrode (Fig. S8, ESI†) but similarly transient in nature, decaying over several seconds. The higher output of the control cells with a nanopatterned back electrode is attributed to scattering of the incident radiation that increased the path for absorption of light within the device.
The longer-term stability of photocurrent output was examined in the RC-LH1 cell fabricated using star-shaped AuNPs and a DG-2 back electrode (Fig. S17 in ESI†). These cells exhibited photocurrent output over a period of a couple of hours under continuous illumination with visible light.
The Voc for all cells with nanopatterned back electrodes was uniformly in the region of 0.3 V, only marginally higher (∼0.02 V) than that seen with an unpatterned back electrode (see Fig. 5b for the DG-2 electrode). This was consistent with the similar work functions of silver and tungsten. In addition, a slightly higher voltage response than seen in the control cell can be attributed to the formation of nano-traps which serves as nano-capacitors.78
Changes in Jsc and Voc translated into increases in external quantum efficiency (EQE) which was estimated by dividing the product of Jsc and Voc by the 100 mW cm−2 total input power.79 Fill factors were not taken into account due to transient nature of photocurrent. Values of EQE for 25 cells with different gold NP content and different plain or patterned back electrodes are compiled in Fig. 5c, and compared to that of a cell with no gold NPs and a plain back electrode. The comparison highlights (1) consistency in the relative effects of the different types of gold NP, (2) the contrast in effectiveness of the DG-1/2 silver patterns versus the DG-3/4 patterns and (3) the very large (>18-fold) boost in EQE achieved by the optimal combination of NP-S gold NPs and the DG-2 pattern of silver NP lines.
In all cells, including the DG-2 + NPs-S variant with the strongest Jsc, comparison of the EQE action spectrum with the absorbance spectrum of the protein (Fig. 5d) further validated that RC-LH1 complexes were responsible for photoresponse. The EQE spectra exhibited a broad band between 420 and 640 nm attributable mainly to the carotenoids of the LH1 component and peaks above 750 nm attributable to the RC and LH1 BChls, with an EQE maximum of 0.15%.
Sheet and charge transfer resistances were determined for cells fabricated with a nanopatterned back electrode and an optimized gold NP content. Compared to an unpatterned back electrode, the diameter of the semi-circular arc (Rct) shrank and was lowest for the cell with the DG-2 lattice, which offers a higher surface area of deposited silver nanoparticles than the remainder, including the parallel line DG-1 pattern (Fig. S18a and b, ESI†). An increase in the interfacial surface area of the electrode would be expected to improve electrical performance by enabling better charge collection and by lowering the electrical sheet resistance. In accord, in all cases values of RS were uniformly lower than for an equivalent cell with an unpatterned back electrode. A lower interfacial resistance would allow efficient electron transfer to the electrodes via the TMPD electrolyte. This can be understood in terms of the high ionic conductivity due to presence of many interconnected nano-networks of silver particles. The same trend was observed for Rct, the lowest being for the DG-2 pattern with the remainder in the order DG-1 < DG-4 < DG-3 (Fig. S18e, ESI†). Data on the photovoltaic and electrical performance results of cells fabricated with the nanopatterned electrodes are collated in Tables S2–S5 (ESI†).
Optical simulations
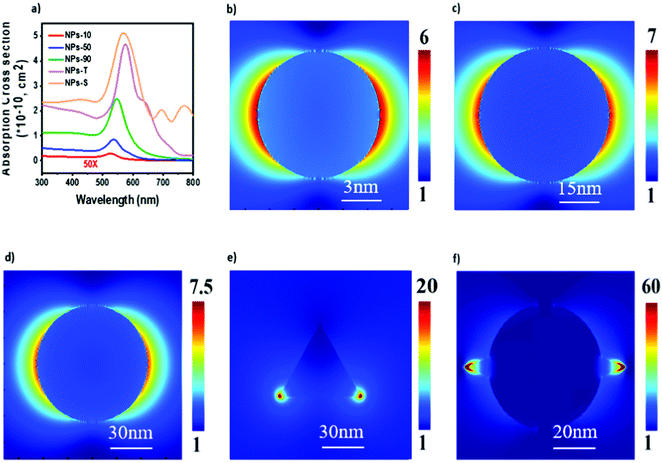
In order to understand optical effects due to the incorporation of gold NPs into the cells, numerical simulations were carried out using Lumerical FDTD Solutions (ANSYS Inc.). Each NP was embedded in water environment (refractive index n = 1.333) and exposed to plane-wave incident light. Simulated absorption cross section spectra are shown in Fig. 6a. For single spherical NPs, a larger diameter produced a stronger absorption cross section, and the peak wavelength red-shifted, matching the experimental absorbance spectra. Absorption from a single triangular plate or star-shaped cluster was stronger than for the spherical NPs, owing to the existence of edges and tips that induce the near-field enhancement from LSPR modes. Fig. 6b–f shows the electric field enhancement |E|/|E0| distribution of the different NPs. The spherical NPs induce a light trapping effect owing to LSPR, while the enhancement factor |E|/|E0| was less than 10. In comparison, the triangular plates and nanostars induce higher near-field enhancement, attributing to the highly confined electrical field at the edge regions. A more than 10-fold electric field enhancement was found for the triangular plates and star-shaped clusters, leading to stronger light absorption and agreeing well with the observed device performance increase.Discussion
A general challenge in the development of thin film solar cells is to increase the proportion of incident solar energy that is harvested. The same consideration applies to Protein Electricity Generator based on photosynthetic materials, where a notable feature is selectivity in the wavelengths of solar energy absorbed due to the particular biological pigments responsible for light harvesting. The data above demonstrate that the efficiency of solar energy harvesting by such cells can be boosted by incorporation of two types of plasmonic nanoparticle. Mixing gold NPs with the protein solution in the bulk of the device produced, optimally, a ∼six-fold increase in photocurrent. The strongest effect was seen for star-shaped NP clusters which had the most complex surface architecture and were associated with the strongest electric field intensity enhancements. Furthermore, the printing of parallel lines or grids of silver NPs on the rear tungsten electrode produced, optimally, a further nearly three-fold increase in photocurrent. Overall, through incorporation of both gold and silver NPs into the device in different ways, an up to ∼18-fold increase in photocurrent was achievable.Previous studies of the interactions of gold or silver NPs with photosynthetic proteins have focussed in the main on the photophysical consequences, including enhanced absorption and energy transfer.80–92 A smaller number of studies have reported the impact of plasmonic interactions on photocurrents from photosynthetic materials on electrodes or in cells. Enhancements have been seen after placing Photosystem I complexes on a silver island film93 and decorating thylakoid membranes with gold nanorods.94 In solar cells using LHCII as a sensitiser, output was improved by including silver nanoprisms95 or gold or silver nanospheres and nanoplates.96 In the study most directly relevant to the present one, a 2.4-fold enhancement was seen when Rba. sphaeroides RC-LH1 complexes were deposited on a nanostructured silver electrode rather than a planar silver electrode, an effect attributed to plasmon-enhanced light harvesting.39
For gold NPs in solution, the enhancement in photocurrent is likely to be attributable to multiple effects. The free electrons of gold NPs oscillate and resonate with the electric field of incident light giving rise to a localised optical near-field effect (oscillating electric field). This can excite the RC-LH1 protein complexes more efficiently than incident far-field light, thus providing an absorption improvement. The gold NPs also function as light capturing antenna for adjacent protein complexes. In addition, the presence of the gold NPs in the protein matrix causes enhanced scattering which can increase the optical path length in the cell, enabling enhanced absorption by bacteriochlorophyll and carotenoid pigments.
The green synthesis of five different types of gold NP allowed the importance of morphology to be explored. For the spherical morphology, the strongest photocurrent enhancement was seen for 50 nm-diameter NPs. The absorption cross section of the spherical NPs increased with increased diameter, consistent with 50 nm NPs producing a stronger current enhancement than 10 nm NPs. However this correlation did not hold and there was a marked drop in photocurrent enhancement seen with the larger 90 nm NPs, an effect attributed to significant scattering losses.97 The intermediate sized 50 nm NPs thus likely provided a compromise between a desired strong absorption cross section and an undesired strong scattering loss, producing an optimal photocurrent enhancement for this morphology of NP. Somewhat stronger enhancements were seen with the triangular gold nanoplates and in particular, the star-shaped gold nanoclusters. The degree of light absorption enhancement was the highest for these star-shaped NPs (Fig. 6) due to the availability of a larger surface area which can lead to significant changes in the magnitude of the electric field around the NP. Evidently the benefits of the high absorption cross section of these ∼120 nm diameter clusters were less offset by the deficits of strong scattering than was the case for the 90 nm diameter spherical NPs.
As well as testing different morphologies, different levels of doping with gold NPs were also examined. For each type of NP an optimal level of doping was seen beyond which the photocurrent enhancement dropped off. This can be ascribed to a saturation effect based on enhanced parasitic absorption.38 Higher concentrations of gold NPs could also possibly cause a short circuit between the two electrodes due to aggregation. Although there was a drop at higher doping levels for all five types of gold NP, the photocurrent was always higher than seen in their absence, suggesting that benefits of their addition always outweighed such drawbacks.
Silver NPs with a ∼12 nm average diameter were used to print nanostructured patterns on the rear tungsten electrode, comprising lines of ∼2.8–3 μm diameter and ∼200 nm thickness. As the two metals have a similar work function this had only a marginal effect on Voc, but two of the four patterns tested produced substantive increases in Jsc that were proportionate to the current achievable by a particular protein/gold NP combination (Fig. 7a). In part this may have been attributable to an increased surface area for the back electrode, and also local plasmonic enhancement of absorption by both surface and localised resonances. Nanostructuring should also cause scatter of incident radiation, again increasing the path for absorption of light within the device (Fig. S19 in ESI†).
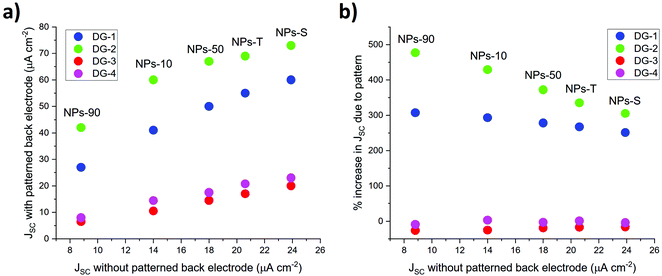 | ||
| Fig. 7 Effect on photocurrent density of patterning of rear electrode with silver NP lines. (a) Comparison of Jsc with and without patterning. (b) Percentage increase in Jsc due to patterning. | ||
An indication that the latter effect may have been dominant came for the interesting observation that the degree of enhancement of the photocurrent depended strongly on the geometry of the pattern of silver NPs. Parallel lines with a 700 nm spacing produced a 250% to 500% enhancement of the photocurrent obtained from a reference cell with the same protein/gold NP composition (Fig. 7b). The effect scaled approximately linearly with the current density from the reference cell, being strongest at the lowest reference current density. The greatest enhancement was seen with the DG-2 grid pattern. In marked contrast to this, both parallel line and grid patterns of ∼3 μm diameter lines with a 1100 nm spacing had either only a marginal positive or small negative effect on the photocurrent density. This showed that patterning of rear electrode did not have a positive effect on output, but rather it was the details of the patterning that was important. One possibility is that visible light is scattered more effectively by lines or grids with 700 nm spacing than with a wider 1100 nm spacing. Although the DG-2 grid supported higher photocurrents than the DG-1 parallel lines, and covered a greater proportion of the surface of the tungsten electrode, there was no correlation of photocurrent enhancement with surface coverage; the effective DG-1 lines involved a much lower surface coverage than ineffective DG-4 grid. Rather it was the spacing of the lines that was the crucial factor.
Conclusions
Instead of increasing the amount of RC-LH1 protein complexes in a bio solar cell, increasing absorption by the protein complexes provides an alternative solution to enhancing the device photo-performance. To explore this, a more efficient light trapping scheme has been devised by incorporating into a bio-photovoltaic device gold NPs synthesised through a green route and a nanostructured rear electrode printed using silver NP ink. Creation of the latter required experimental optimisation of flow rate, applied voltage and ink to achieve a jetting performance suitable for high resolution EHD printing. Photocurrent output could be optimised by manipulating the type and amount of gold NPs doped into the bulk of the device, and the spacing and pattern of lines of silver NP ink on the back electrode. The optimal device with 120 nm diameter star-shaped gold NPs and a grid of silver NP lines with 700 nm spacing exhibited an ∼19-fold increase in photocurrent and EQE relative to a cell devoid of gold or silver NPs. We conclude that by careful implementation of multiple scattering strategies and a reduction in reflective losses in bio-solar modules, our approach can lead to enhanced photochemical reactions, with significant photoconversion efficiency and photocurrent improvements.Author contributions
Nikita Paul: conceptualization, methodology, investigation, writing – original draft, writing – review and editing. Lakshmi Suresh: conceptualization, methodology, green synthesis of nanoparticles, investigation, writing-original draft, writing-review and editing. Zhang Yaoxin: writing-review and editing. Fuad Indra Alzakia: conducted preliminary studies on EHD printing. Yixin Chen: numerical simulation and analysis. Victor Vogt: numerical simulation and analysis. Zi Jing Wong: simulation and guidance. Michael R. Jones: protein synthesis and purification, writing-review and editing. Swee Ching Tan: conceptualization, supervision, funding acquisition, project administration.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
S. C. T. acknowledges the financial support from MOE AcRF 1 (R-284-000-161-114 and R-284-000-174-114). Z. J. W. acknowledges financial support from President's Excellence Fund (X-Grant). M. R. J. acknowledges support from the Biotechnology and Biological Sciences Research Council of the UK (project BB/I022570/1).References
- A. Badura, T. Kothe, W. Schuhmann and M. Rögner, Wiring photosynthetic enzymes to electrodes, Energy Environ. Sci., 2011, 4, 3263–3274 RSC.
- F. Wang, X. Liu and I. Willner, Integration of photoswitchable proteins, photosynthetic reaction centers and semiconductor/biomolecule hybrids with electrode supports for optobioelectronic applications, Adv. Mater., 2013, 25, 349–377 CrossRef CAS PubMed.
- S. K. Ravi, et al., Bio-photocapacitive tactile sensors as a touch-to-audio braille reader and solar capacitor, Mater. Horiz., 2020, 7, 866–876 RSC.
- S. K. Ravi, et al., Photosynthetic bioelectronic sensors for touch perception, UV-detection, and nanopower generation: Toward self-powered E-skins, Adv. Mater., 2018, 30, 1802290 CrossRef.
- S. K. Ravi and S. C. Tan, Progress and perspectives in exploiting photosynthetic biomolecules for solar energy harnessing, Energy Environ. Sci., 2015, 8, 2551–2573 RSC.
- K. Nguyen and B. D. Bruce, Growing green electricity: Progress and strategies for use of Photosystem I for sustainable photovoltaic energy conversion, Biochim. Biophys. Acta – Bioenerg., 2014, 1837, 1553–1566 CrossRef CAS PubMed.
- S. K. Ravi, et al., Photosynthetic apparatus of Rhodobacter sphaeroides exhibits prolonged charge storage, Nat. Commun., 2019, 10, 902 CrossRef PubMed.
- V. M. Friebe and R. N. Frese, Photosynthetic reaction center-based biophotovoltaics, Curr. Opin. Electrochem., 2017, 5, 126–134 CrossRef CAS.
- S. K. Ravi, V. S. Udayagiri, L. Suresh and S. C. Tan, Emerging role of the band-structure approach in biohybrid photovoltaics: A path beyond bioelectrochemistry, Adv. Funct. Mater., 2018, 28, 1705305 CrossRef.
- F. Milano, A. Punzi, R. Ragni, M. Trotta and G. M. Farinola, Photosynthetic bacteria: Photonics and optoelectronics with bacteria: Making materials from photosynthetic microorganisms (Adv. Funct. Mater. 21/2019), Adv. Funct. Mater., 2019, 29, 1970141 CrossRef.
- S. K. Ravi, et al., A mechanoresponsive phase-changing electrolyte enables fabrication of high-output solid-state photobioelectrochemical devices from pigment–protein multilayers, Adv. Mater., 2018, 30, 1704073 CrossRef PubMed.
- V. K. Singh, et al., Biohybrid photoprotein-semiconductor cells with deep-lying redox shuttles achieve a 0.7 V photovoltage, Adv. Funct. Mater., 2018, 1703689 CrossRef.
- L. Suresh, J. V. Vaghasiya, M. R. Jones and S. C. Tan, Biodegradable protein-based photoelectrochemical cells with biopolymer composite electrodes that enable recovery of valuable metals, ACS Sustainable Chem. Eng., 2019, 7, 8834–8841 CrossRef CAS.
- J. Liu, V. M. Friebe, R. N. Frese and M. R. Jones, Polychromatic solar energy conversion in pigment–protein chimeras that unite the two kingdoms of (bacterio)chlorophyll-based photosynthesis, Nat. Commun., 2020, 11, 1542 CrossRef CAS PubMed.
- J. Liu, J. Mantell, N. Di Bartolo and M. R. Jones, Mechanisms of self-assembly and energy harvesting in tuneable conjugates of quantum dots and engineered photovoltaic proteins, Small, 2019, 15, 1804267 CrossRef PubMed.
- J. Liu, J. Mantell and M. R. Jones, Minding the gap between plant and bacterial photosynthesis within a self-assembling biohybrid photosystem, ACS Nano, 2020, 14, 4536–4549 CrossRef CAS PubMed.
- P. Qian, et al., Three-dimensional structure of the Rhodobacter sphaeroides RC-LH1-PufX complex: Dimerization and quinone channels promoted by PufX, Biochemistry, 2013, 52, 7575–7585 CrossRef CAS.
- S. C. Tan, L. I. Crouch, S. Mahajan, M. R. Jones and M. E. Welland, Increasing the open-circuit voltage of photoprotein-based photoelectrochemical cells by manipulation of the vacuum potential of the electrolytes, ACS Nano, 2012, 6, 9103–9109 CrossRef CAS PubMed.
- S. C. Tan, L. I. Crouch, M. R. Jones and M. Welland, Generation of alternating current in response to discontinuous illumination by photoelectrochemical cells based on photosynthetic proteins, Angew. Chem., Int. Ed., 2012, 51, 6667–6671 CrossRef CAS PubMed.
- S. K. Ravi and S. C. Tan, Solar Energy Harvesting with Photosynthetic Pigment–Protein Complexes, Springer, 2020 DOI:10.1007/978-981-15-6333-1.
- N. Paul, et al., Self-powered all weather sensory systems powered by Rhodobacter sphaeroides protein solar cells, Biosens. Bioelectron., 2020, 165, 112423 CrossRef CAS.
- K. J. Grayson, et al., Augmenting light coverage for photosynthesis through YFP-enhanced charge separation at the Rhodobacter sphaeroides reaction centre, Nat. Commun., 2017, 8, 13972 CrossRef CAS.
- A. Operamolla, et al., “Garnishing” the photosynthetic bacterial reaction center for bioelectronics, J. Mater. Chem. C, 2015, 3, 6471–6478 RSC.
- P. K. Dutta, et al., A DNA-directed light-harvesting/reaction center system, J. Am. Chem. Soc., 2014, 136, 16618–16625 CrossRef CAS.
- R. R. Tangorra, et al., Bio-hybrid photoconverter by covalent functionalization of the photosynthetic reaction center of Rhodobacter sphaeroides with fluorescein isothiocyanate, MRS Online Proc. Libr., 2014, 1722, 39–46 Search PubMed.
- O. Hassan Omar, et al., Synthetic antenna functioning as light harvester in the whole visible region for enhanced hybrid photosynthetic reaction centers, Bioconjugate Chem., 2016, 27, 1614–1623 CrossRef CAS PubMed.
- R. Ragni, et al., Bursting photosynthesis: designing ad hoc fluorophores to complement the light harvesting capability of the photosynthetic reaction center, MRS Online Proc. Libr., 2014, 1689, 13–19 Search PubMed.
- L. Suresh, et al., 1200% enhancement of solar energy conversion by engineering three dimensional arrays of flexible biophotoelectrochemical cells in a fixed footprint encompassed by Johnson solid shaped optical well, Nano Energy, 2021, 79, 105424 CrossRef CAS.
- H. A. Atwater and A. Polman, Plasmonics for improved photovoltaic devices, Nat. Mater., 2010, 9, 205–213 CrossRef CAS PubMed.
- X. Huang and M. A. El-Sayed, Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy, J. Adv. Res., 2010, 1, 13–28 CrossRef.
- W. Shi, J. Casas, M. Venkataramasubramani and L. Tang, Synthesis and characterization of gold nanoparticles with plasmon absorbance wavelength tunable from visible to near infrared region, ISRN Nanomater., 2012, 2012, 659043 Search PubMed.
- V. Amendola, R. Pilot, M. Frasconi, O. M. Maragò and M. A. Iatì, Surface plasmon resonance in gold nanoparticles: a review, J. Phys.: Condens. Matter, 2017, 29, 203002 CrossRef PubMed.
- H. Shen, P. Bienstman and B. Maes, Plasmonic absorption enhancement in organic solar cells with thin active layers, J. Appl. Phys., 2009, 106, 73109 CrossRef.
- D. Duche, et al., Improving light absorption in organic solar cells by plasmonic contribution, Sol. Energy Mater. Sol. Cells, 2009, 93, 1377–1382 CrossRef CAS.
- B. P. Rand, P. Peumans and S. R. Forrest, Long-range absorption enhancement in organic tandem thin-film solar cells containing silver nanoclusters, J. Appl. Phys., 2004, 96, 7519–7526 CrossRef CAS.
- J. A. Schuller, et al., Plasmonics for extreme light concentration and manipulation, Nat. Mater., 2010, 9, 193–204 CrossRef CAS PubMed.
- M. I. Stockman, Plasmonics: Theory and Applications, 1957 Search PubMed.
- S. A. Maier, Plasmonics: Fundamentals and Applications, 2007 Search PubMed.
- K. Kim and D. L. Carroll, Roles of Au and Ag nanoparticles in efficiency enhancement of poly(3-octylthiophene)/C60 bulk heterojunction photovoltaic devices, Appl. Phys. Lett., 2005, 87, 203113 CrossRef.
- A. J. Morfa, K. L. Rowlen, T. H. Reilly, M. J. Romero and J. van de Lagemaat, Plasmon-enhanced solar energy conversion in organic bulk heterojunction photovoltaics, Appl. Phys. Lett., 2008, 92, 13504 CrossRef.
- D. Derkacs, S. H. Lim, P. Matheu, W. Mar and E. T. Yu, Improved performance of amorphous silicon solar cells via scattering from surface plasmon polaritons in nearby metallic nanoparticles, Appl. Phys. Lett., 2006, 89, 93103 CrossRef.
- Y. A. Akimov and W. S. Koh, Design of plasmonic nanoparticles for efficient subwavelength light trapping in thin-film solar cells, Plasmonics, 2011, 6, 155–161 CrossRef CAS.
- T. Nakagawa and Y. Takagai, Simple synthesis of gold nanoparticles by sodium borohydride reduction method and their ligand exchange reaction, Bunseki Kagaku, 2019, 68, 751–755 CrossRef CAS.
- K. M. Koczkur, S. Mourdikoudis, L. Polavarapu and S. E. Skrabalak, Polyvinylpyrrolidone (PVP) in nanoparticle synthesis, Dalton Trans., 2015, 44, 17883–17905 RSC.
- J. Singh, et al., ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation, J. Nanobiotechnology, 2018, 16, 84 CrossRef CAS PubMed.
- M.-H. Teiten, S. Eifes, M. Dicato and M. Diederich, Curcumin-the paradigm of a multi-target natural compound with applications in cancer prevention and treatment, Toxins, 2010, 2, 128–162 CrossRef CAS PubMed.
- H. Hatcher, R. Planalp, J. Cho, F. M. Torti and S. V. Torti, Curcumin: From ancient medicine to current clinical trials, Cell. Mol. Life Sci., 2008, 65, 1631–1652 CrossRef CAS PubMed.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Bioavailability of curcumin: Problems and promises, Mol. Pharm., 2007, 4, 807–818 CrossRef CAS PubMed.
- S. Manju and K. Sreenivasan, Gold nanoparticles generated and stabilized by water soluble curcumin-polymer conjugate: blood compatibility evaluation and targeted drug delivery onto cancer cells, J. Colloid Interface Sci., 2012, 368, 144–151 CrossRef CAS PubMed.
- K. Sindhu, A. Rajaram, K. J. Sreeram and R. Rajaram, Curcumin conjugated gold nanoparticle synthesis and its biocompatibility, RSC Adv., 2014, 4, 1808–1818 RSC.
- D. K. Singh, R. Jagannathan, P. Khandelwal, P. M. Abraham and P. Poddar, In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: an experimental and density functional theory investigation, Nanoscale, 2013, 5, 1882–1893 RSC.
- C. Sreelakshmi, et al., Green synthesis of curcumin capped gold nanoparticles and evaluation of their cytotoxicity, Nanosci. Nanotechnol. Lett., 2013, 5, 1258–1265 CrossRef CAS.
- J.-Y. Lee and P. Peumans, The origin of enhanced optical absorption in solar cells with metal nanoparticles embedded in the active layer, Opt. Express, 2010, 18, 10078–10087 CrossRef CAS PubMed.
- R. A. Pala, J. White, E. Barnard, J. Liu and M. L. Brongersma, Design of Plasmonic Thin-Film Solar Cells With Broadband Absorption Enhancements, Adv. Mater., 2009, 21, 3504–3509 CrossRef CAS.
- C. Min, et al., Enhancement of optical absorption in thin-film organic solar cells through the excitation of plasmonic modes in metallic gratings, Appl. Phys. Lett., 2010, 96, 133302 CrossRef.
- J. Homola, S. S. Yee and G. Gauglitz, Surface plasmon resonance sensors: Review, Sens. Actuators, B, 1999, 54, 3–15 CrossRef CAS.
- K. V. Gobi, H. Tanaka, Y. Shoyama and N. Miura, Continuous flow immunosensor for highly selective and real-time detection of sub-ppb levels of 2-hydroxybiphenyl by using surface plasmon resonance imaging, Biosens. Bioelectron., 2004, 20, 350–357 CrossRef CAS PubMed.
- D. Habauzit, J. Chopineau and B. Roig, SPR-based biosensors: A tool for biodetection of hormonal compounds, Anal. Bioanal. Chem., 2007, 387, 1215–1223 CrossRef CAS PubMed.
- D. R. Shankaran, K. V. Gobi and N. Miura, Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest, Sens. Actuators, B, 2007, 121, 158–177 CrossRef CAS.
- P. M. Voroshilov, V. Ovchinnikov, A. Papadimitratos, A. A. Zakhidov and C. R. Simovski, Light trapping enhancement by silver nanoantennas in organic solar cells, ACS Photonics, 2018, 5, 1767–1772 CrossRef CAS.
- M. Rycenga, et al., Controlling the synthesis and assembly of silver nanostructures for plasmonic applications, Chem. Rev., 2011, 111, 3669–3712 CrossRef CAS PubMed.
- Y. J. Yang, et al., Drop-on-demand electrohydrodynamic printing of high resolution conductive micro patterns for MEMS repairing, Int. J. Precis. Eng. Manuf., 2018, 19, 811–819 CrossRef.
- S. Khan, et al., Direct patterning and electrospray deposition through EHD for fabrication of printed thin film transistors, Curr. Appl. Phys., 2011, 11, S271–S279 CrossRef.
- Y. G. Lee and W.-S. Choi, Electrohydrodynamic jet-printed zinc–tin oxide TFTs and their bias stability, ACS Appl. Mater. Interfaces, 2014, 6, 11167–11172 CrossRef CAS PubMed.
- K. Kang, et al., Micropatterning of metal oxide nanofibers by electrohydrodynamic (EHD) printing towards highly integrated and multiplexed gas sensor applications, Sens. Actuators, B, 2017, 250, 574–583 CrossRef CAS.
- S.-E. Park, S. Kim, D.-Y. Lee, E. Kim and J. Hwang, Fabrication of silver nanowire transparent electrodes using electrohydrodynamic spray deposition for flexible organic solar cells, J. Mater. Chem. A, 2013, 1, 14286–14293 RSC.
- E. Sutanto, Y. Tan, M. S. Onses, B. T. Cunningham and A. Alleyne, Electrohydrodynamic jet printing of micro-optical devices, Manuf. Lett., 2014, 2, 4–7 CrossRef CAS.
- V. Wood, et al., Inkjet-printed quantum dot–polymer composites for full-color AC-driven displays, Adv. Mater., 2009, 21, 2151–2155 CrossRef CAS.
- J. Lessing, et al., Inkjet printing of conductive inks with high lateral resolution on omniphobic “RF Paper” for paper-based electronics and MEMS, Adv. Mater., 2014, 26, 4677–4682 CrossRef CAS PubMed.
- J. Liu, et al., Engineered photoproteins that give rise to photosynthetically-incompetent bacteria are effective as photovoltaic materials for biohybrid photoelectrochemical cells, Faraday Discuss., 2018, 207, 307–327 RSC.
- S. K. Ravi, et al., Enhanced output from biohybrid photoelectrochemical transparent tandem cells integrating photosynthetic proteins genetically modified for expanded solar energy harvesting, Adv. Energy Mater., 2017, 7, 1601821 CrossRef.
- V. M. Friebe, et al., Plasmon-enhanced photocurrent of photosynthetic pigment proteins on nanoporous silver, Adv. Funct. Mater., 2016, 26, 285–292 CrossRef CAS.
- S. Niwa,
et al., Structure of the LH1–RC complex from Thermochromatium tepidum at 3.0
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Å, Nature, 2014, 508, 228 CrossRef CAS PubMed.
Å, Nature, 2014, 508, 228 CrossRef CAS PubMed. - L. Minati, F. Benetti, A. Chiappini and G. Speranza, One-step synthesis of star-shaped gold nanoparticles, Colloids Surf., A, 2014, 441, 623–628 CrossRef CAS.
- M. Vaseem, K. M. Lee, A. Hong and Y. Hahn, Inkjet printed fractal-connected electrodes with silver nanoparticle ink, ACS Appl. Mater. Interfaces, 2012, 3300–3307 CrossRef CAS PubMed.
- S. Link and M. A. El-Sayed, Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals, Int. Rev. Phys. Chem., 2000, 19, 409–453 Search PubMed.
- W.-H. Tseng, et al., Shape-dependent light harvesting of 3D gold nanocrystals on bulk heterojunction solar cells: Plasmonic or optical scattering effect?, J. Phys. Chem. C, 2015, 119, 7554–7564 CrossRef CAS.
- M. Pusty and P. M. Shirage, Gold nanoparticle–cellulose/PDMS nanocomposite: a flexible dielectric material for harvesting mechanical energy, RSC Adv., 2020, 10, 10097–10112 RSC.
- V. K. Singh, et al., Biohybrid photoprotein–semiconductor cells with deep-lying redox shuttles achieve a 0.7 V photovoltage, Adv. Funct. Mater., 2018, 28, 1703689 CrossRef.
- I. Kim, et al., Metal nanoparticle plasmon-enhanced light-harvesting in a photosystem I thin film, Nano Lett., 2011, 11, 3091–3098 CrossRef CAS PubMed.
- J. B. Nieder, R. Bittl and M. Brecht, Fluorescence studies into the effect of plasmonic interactions on protein function, Angew. Chem., Int. Ed., 2010, 49, 10217–10220 CrossRef CAS PubMed.
- R. Pamu, V. P. Sandireddy, R. Kalyanaraman, B. Khomami and D. Mukherjee, Plasmon-enhanced photocurrent from photosystem I assembled on Ag nanopyramids, J. Phys. Chem. Lett., 2018, 9, 970–977 CrossRef CAS PubMed.
- F. Kyeyune, et al., Strong plasmonic fluorescence enhancement of individual plant light-harvesting complexes, Nanoscale, 2019, 11, 15139–15146 RSC.
- R. Pamu, B. J. Lawrie, B. Khomami and D. Mukherjee, Broadband plasmonic photocurrent enhancement from photosystem I assembled with tailored arrays of Au and Ag nanodisks, ACS Appl. Nano Mater., 2021, 4, 1209–1219 CrossRef CAS.
- S. R. Beyer, et al., Hybrid nanostructures for enhanced light-harvesting: Plasmon induced increase in fluorescence from individual photosynthetic pigment–protein complexes, Nano Lett., 2011, 11, 4897–4901 CrossRef CAS PubMed.
- M. Brecht, M. Hussels, J. B. Nieder, H. Fang and C. Elsässer, Plasmonic interactions of photosystem I with Fischer patterns made of gold and silver, Chem. Phys., 2012, 406, 15–20 CrossRef CAS.
- Ł. Bujak, et al., Polarization control of metal-enhanced fluorescence in hybrid assemblies of photosynthetic complexes and gold nanorods, Phys. Chem. Chem. Phys., 2014, 16, 9015–9022 RSC.
- N. Czechowski, et al., Large plasmonic fluorescence enhancement of cyanobacterial photosystem I coupled to silver island films, Appl. Phys. Lett., 2014, 105, 43701 CrossRef.
- E. Wientjes, J. Renger, A. G. Curto, R. Cogdell and N. F. van Hulst, Strong antenna-enhanced fluorescence of a single light-harvesting complex shows photon antibunching, Nat. Commun., 2014, 5, 4236 CrossRef CAS PubMed.
- I. Ashraf, et al., Effects of irregular bimetallic nanostructures on the optical properties of photosystem I from Thermosynechococcus elongatus, Photonics, 2015, 2, 838–854 CrossRef CAS.
- A. Tsargorodska, et al., Strong coupling of localized surface plasmons to excitons in light-harvesting complexes, Nano Lett., 2016, 16, 6850–6856 CrossRef CAS PubMed.
- I. Ashraf, et al., Temperature dependence of metal-enhanced fluorescence of photosystem I from Thermosynechococcus elongatus, Nanoscale, 2017, 9, 4196–4204 RSC.
- M. Szalkowski, et al., Plasmonic enhancement of photocurrent generation in a photosystem I-based hybrid electrode, J. Mater. Chem. C, 2020, 8, 5807–5814 RSC.
- Y. J. Kim, et al., Plasmon-stimulated biophotovoltaic cells based on thylakoid–AuNR conjugates, J. Mater. Chem. A, 2020, 8, 24192–24203 RSC.
- K. Yao, et al., Nano-bio hybrids of plasmonic metals/photosynthetic proteins for broad-band light absorption enhancement in organic solar cells, J. Mater. Chem. A, 2016, 4, 13400–13406 RSC.
- Y. Yang, H. B. Gobeze, F. D’Souza, R. Jankowiak and J. Li, Plasmonic enhancement of biosolar cells employing light harvesting complex II incorporated with core–shell metal@TiO2 nanoparticles, Adv. Mater. Interfaces, 2016, 3, 1600371 CrossRef.
- N. Chander, et al., Size and concentration effects of gold nanoparticles on optical and electrical properties of plasmonic dye sensitized solar cells, Sol. Energy, 2014, 109, 11–23 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: All data needed to evaluate the conclusions in the paper are present in the paper and/or the supplementary materials. Additional data related to this paper may be requested from the authors. See DOI: 10.1039/d1nh00569c |
| ‡ Both the authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |