A true color palette: binary metastable photonic pigments†
Likang
Zhou
a,
Junhao
Fei
a,
Wei
Fang
b,
Luqing
Shao
b,
Qianjiang
Liu
a,
Huiwen
He
a,
Meng
Ma
 a,
Yanqin
Shi
a,
Si
Chen
a,
Yanqin
Shi
a,
Si
Chen
 *a and
Xu
Wang
*a
*a and
Xu
Wang
*a
aCollege of Materials Science and Engineering, Zhejiang University of Technology, Hangzhou 310014, China. E-mail: wangxu@zjut.edu.cn; chensi@zjut.edu.cn
bInterdisciplinary Center for Quantum Information, State Key Laboratory of Modern Optical Instrumentation, College of Optical Science and Engineering, College of Optical Science and Engineering, Zhejiang University, Hangzhou 310027, China
First published on 22nd June 2022
Abstract
Different from the traditional concept that binary photonic crystals can only reproduce mixed colors due to the simple superposition of the photonic band gaps, precisely addressable “true colors” obtained from volume fraction deviation of binary photonic crystals with metastable structures are reported here. Inspired by the mussels’ adhesion and longhorn beetles’ photonic scales, a binary metastable amorphous photonic crystal was obtained by enhancing the driving forces and customizing the surface roughness of building blocks to regulate the thermodynamic and dynamic factors simultaneously. By controlling the volume fraction of two building blocks, the tunable photonic bandgap varies linearly in the visible region. Furthermore, the “true violet” that cannot be obtained by conventional color mixing is reproduced with the particular ultraviolet characteristics of red photonic pigment's metastable structures, which complement the palette effect of “true colors”. Meanwhile, due to the self-adhesion and post-modification of building blocks, the stability of photonic pigments is further improved. The binary photonic pigments not only solve the dilemma of mixed colors, but also realize the tunability and multiplicity of “true colors”, offering a new choice for the color palette of the world.
New conceptsBy virtue of the physical coloration mechanism, the structural colors of photonic pigments have the characteristics of never-fading and environmental friendliness, which have become the ideal option for future coloration materials. However, despite the attractive “true color” properties corresponding to the individual photonic bandgaps, such as high color resolution and stability, conventional photonic crystals cannot achieve the color multiplicity as traditional pigments through mixing, which greatly restricts the development of photonic pigments, even though the color manipulation can be realized by superposition of photonic bandgaps in the binary steady-state photonic system, at the cost of losing the essential merits of “true colors”. Inspired by the natural phenomena, the binary metastable photonic structures were constructed and tuned to obtain the precisely addressable “true colors”. A quantitative correlation between the volume fraction and photonic bandgap has been established. Moreover, the unique ultraviolet properties of metastable photonic structures are dexterously exploited to reproduce “true violet”, realizing the palette effect of “true colors”. These photonic pigments not only solve the essential dilemma of the mixed colors from the existing colorants, but also realize the tunability and versatility of “true colors”, which facilitate the further development of the binary photonic crystals and provide a new approach to photonic displays. |
Introduction
As you might recall, a photograph of “the dress” became a viral phenomenon on the Internet in 2015. This single photograph set the worldwide Internet alight with a burning question: is this dress blue and black or white and gold?1–4 This phenomenon that had caused billions of public discussions originated from the same object will display different colors under diverse light sources. This is because the vast majority of colors seen by people are mixed colors composed of a variety of coloration units, which selectively absorb or reflect the light of different wavelength ranges to realize the manipulation of colors. Therefore, mixed colors are inevitably distorted when the light sources change significantly.5,6 In contrast, “true colors”, which are controlled by a dominant reflection peak, have greater color constancy.7–10 More importantly, true colors also have the essential advantage in resolution. Nowadays, the almost fanatical pursuit of high resolution on electronic screens and prints is also derived from the coloration mechanism of mixed colors: the close arrangement and combination of sub-pixels.11–14 Whether additive color mixing or subtractive color mixing needs a variety of colors as primary colors for mixing, so the resolution of color is mainly limited by the size and the array of coloration units.15,16 In contrast to mixed colors, true colors consist of only one coloration unit and theoretically have nearly infinite resolution. However, true colors cannot be obtained by mixing colors, which significantly limits the diversity of true colors. Therefore, despite the apparent drawbacks of mixed colors, the mixture of coloration units is currently the only solution to expand the palette of the world.Although photonic crystals immediately come to our mind as a novel way to obtain “true colors” based on spatially ordered micro-nano structures, it is much more complicated than mixed colors to achieve the diversity of colors due to the physical coloration mechanism.17,18 Unlike conventional chemical pigments, the structural coloration of single-component photonic crystals is mainly attributed to interference and reflection.19–21 As a result, changing the diffracting plane by adjusting the particle sizes,22–25 spacing26–33 and molecular interactions34 of building blocks, changing the refractive indices by controlling the properties of the medium,35–37 and tuning the spectrum composition by introducing other components of photoluminescence38,39 are basically the limited options to change structural colors. However, with these complicated and imprecise manipulating methods, the photonic crystals are still challenging to achieve the multicolored palette like “mixed colors”.
Since the true colors generated by single-component photonic crystals lack the color diversity, can the mixture and combination of two photonic crystals reproduce colors like pigments to expand the true color palette? When the building blocks of two kinds of photonic crystals are mixed, in order to obtain long-range ordered binary photonic crystals, the self-assembly process of the binary colloidal particle system is usually dominated by thermodynamics. For example, under the action of weak driving forces such as electrostatic repulsion and capillary force, the two components slowly co-assemble in the state of the lowest energy of the binary system to obtain a long-range ordered steady structure.40–44 Nevertheless, this steady-state binary photonic crystal obtained by the thermodynamics-dominated mixing mode only retains the coloration properties of the dominant articles, and it is difficult to achieve mixing and reproducing true color.
So how about the combination of two kinds of photonic structures? Through 4.5 billion years of evolution and selection, Nature provides a combinatorial coloring mode dominated by dynamics for binary photonic crystals reproducing colors. Longhorn beetle divided coarse chitin nanoparticles of different sizes into amorphous structures of different colors. These amorphous primary coloration units were further combined in a pointillistic way to reproduce different colors macroscopically.45 Takeoka et al. simulated this dynamics-dominated combinatorial strategy. By introducing external forces and limiting the assembly space and time, the different color photonic crystal spheres were constructed as primary coloration units and further combined to reproduce multicolors.15,16 Although this combinatorial mode can realize macroscopic color manipulation, it was achieved by the two kinds of photonic crystals selectively reflecting different wavelengths of light independently. Therefore, the obtained color was essentially the mixed colors of multiple coloration units rather than true colors, which cannot change the existing dilemma of mixed colors: low color resolution and constancy. Therefore, it is also difficult for this dynamics-dominated combinatorial coloring mode to obtain true colors.
Different from common sense that only the steady-state structures can display colors in binary photonic systems,40–44 a binary metastable amorphous photonic crystal was obtained by enhancing the driving forces and customizing the surface roughness of building blocks to regulate the thermodynamic and dynamic factors simultaneously. Based on the binary metastable photonic crystals, precisely addressable “true colors” with the palette effect are constructed, which solved the dilemma that the existing photonic crystals cannot mix and regulate the optical properties. Inspired by the super adhesion of mussels, a form of building block encapsulated by polydopamine (PDA) was synthesized, which endowed the building blocks with “anchors” of self-adhesion and post-modification.46–49 In terms of thermodynamics, the interaction forces between the PDA moieties replaced the original electrostatic repulsion and capillary force to enhance the aggregation trend of the building blocks and broke the thermodynamic steady state dominated by weak driving forces. From the perspective of dynamics, under the guidance of the coloration mechanism of longhorn beetles,45 the roughness of the PDA layer on the surface of building blocks was customized further to control the overall crystallization tendency of the system. Benefiting from these superior properties of PDA@SiO2 NPs, the delicate metastable structure can even be constructed in the rapid preparation process of spraying. This binary metastable amorphous photonic structure realized the mixing and reproducing of colors of photonic pigments, which had the high resolution of true colors and the diversity of mixed colors, and further developed a “true color” palette conforming to the additive color mixing. In addition, benefiting from the unique properties of the photonic pigments, the stability and applications have been further expanded. We believe that this novel photonic pigment and its unique true color mixing mode can paint our world in glorious multicolors.
Results and discussion
In order to realize mixing and reproducing of colors of photonic pigments, it is necessary to break the steady-state photonic structures dominated by thermodynamics. Therefore, the metastable equilibrium between thermodynamics and dynamics of the assembly system was achieved by adjusting the building blocks’ interaction forces and surface roughness and the metastable photonic structure was obtained. As the representative of super adhesion, the interaction forces of mussels originate from the adhesive protein produced by themselves. Dopamine (DA), as a representative substance of adhesion, was used to provide the “anchors” between building blocks and control the surface morphology of building blocks. As shown in Fig. 1a, by virtue of the oxidative self-polymerization of dopamine, the inner core SiO2 NPs were encapsulated by the PDA layer and the self-adhesive and rough building blocks, PDA@SiO2 nanoparticles (PDA@SiO2 NPs), were obtained to mimic the rough building blocks in the photonic scales of longhorn beetles (Fig. 1b).45 According to the scanning electron microscopy (SEM) images of the building blocks, the surface of the unmodified SiO2 NPs was smooth and flat (Fig. S1, ESI†), while Fig. 1c–f clearly shows that under different DA concentrations, the SiO2 NPs were encapsulated by PDA layers with different roughnesses. In addition, in Fig. S2 (ESI†), the stretching vibration peak (1614 cm−1) and the deformation vibration peak (1497 cm−1) of the benzene skeleton characteristic of PDA appeared in the infrared spectrum of PDA@SiO2 NPs, indicating that PDA had been successfully encapsulated on SiO2 NPs to provide “anchors” that can interact with each other. Furthermore, the roughness of the building blocks will affect the crystallization trend of the assembly system, so the dynamic behaviors were regulated by changing the roughness of the PDA@SiO2 NPs. As shown in Fig. 1c–f, with the increase of DA concentration, the surface morphology of building blocks first changed from smooth to a non-dense “raspberry-like” morphology, then to the dense wrinkle accumulation, and finally to relatively flat. The specific surface areas of PDA@SiO2 NPs with different roughnesses are shown in Fig. 1g. With the DA concentration increasing, the BET surface area of PDA@SiO2 NPs reached the maximum when the DA concentration was 2.6 mM. After that, the BET surface area gradually decreased, but it was still higher than that of unmodified SiO2 NPs. These results indicated that when DA concentration was increased to 2.6 mM, the non-dense “raspberry-like” morphology had the highest roughness. After that, the surface of PDA@SiO2 NPs tended to be dense and the roughness gradually decreased. This controllable rough surface was caused by the fact that DA tended to form oligomers at the initial stage of reaction under the appropriate pH environment and then oligomers aggregated on the surface of SiO2 NPs to form the non-dense and uneven surfaces. With the increase of DA concentration, folds and grooves were gradually filled by aggregates of PDA and the surface morphology tended to be relatively flat, which meant that the surface roughness of PDA@SiO2 NPs was effectively controlled. So far, PDA@SiO2 NPs can not only enhance the interaction forces between nanoparticles by virtue of the self-adhesion of PDA to break the thermodynamic equilibrium, but also customize their surface roughness to regulate the dynamic trend, which were the ideal building blocks for metastable photonic crystals.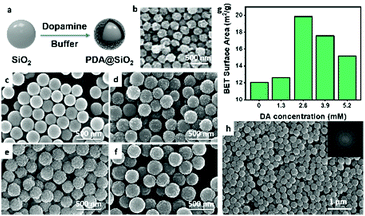 | ||
| Fig. 1 (a) Schematic illustration of the preparation of PDA@SiO2 NPs; (b) SEM image of chitin NPs of longhorn beetles Anoplophora graafi;42 (© https://doi.org/10.1364/OE.18.014430 [2010] Optica Publishing Group). (c–f) SEM images of PDA@SiO2 NPs prepared with different DA concentrations: (c) 1.3 mM, (d) 2.6 mM, (e) 3.9 mM, and (f) 5.2 mM; (g) BET surface areas of PDA@SiO2 NPs prepared with different DA concentrations; (h) SEM image of the photonic pigments prepared by PDA@SiO2 NPs (DA 2.6 mM). Inset: The corresponding 2D FT pattern of the SEM image. | ||
After obtaining the ideal building blocks of PDA@SiO2 NPs, the metastable photonic pigments can be constructed by a facile spraying method. Different from the lengthy and delicate preparation process of conventional photonic crystals, PDA@SiO2 NPs can form short-range ordered metastable photonic structures in the limited assembly space and time of the spraying process due to the self-adhesion. According to the SEM image of single-component photonic pigments prepared by the spraying method (Fig. 1h), it can be seen that similar to the arrangement of chitin nanoparticles of longhorn beetle (Fig. 1b), PDA@SiO2 NPs in the photonic pigments exhibited the amorphous structure, which was confirmed by two-dimensional Fourier transform (2D FT) of the SEM image. Unlike the patterns of 2D FT corresponding to the thermodynamically dominated assembly of unmodified SiO2 NPs, which were hexagonal peaks (Fig. S1, ESI†), which originated from the presence of long-range ordered crystalline structures in the steady-state, the 2D FT result of the photonic pigments (Fig. 1h) displayed a symmetrical circular pattern centered around the origin, indicating that the spatial periodicity of this arrangement was consistent in all directions with these planes,50–53 which was completely different from the long-range ordered steady-state structures formed by the assembly system under the domination of thermodynamics. The reasons for obtaining this metastable amorphous structure were comprehensive: one was the thermodynamic factors, the interaction forces between PDA@SiO2 NPs were strengthened, which replaced the driving forces only composed of electrostatic repulsion and capillary forces, making building blocks more inclined to form short-range order; the other was the dynamic behavior; the rough surface and the limited assembly mode also significantly reduced the crystallization tendency of this system and the metastable amorphous photonic structures with short-range ordered and spatial isotropy were obtained under the joint influence of thermodynamics and dynamics.
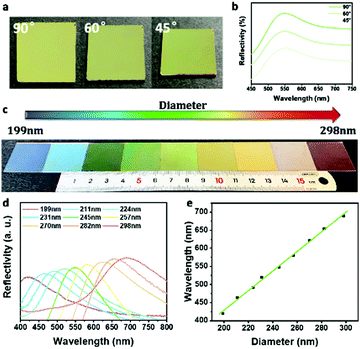
The optical properties of the single-component photonic pigments based on the metastable structures were further studied. As shown in Fig. 2a, the bright green of the photonic pigment prepared by 245 nm PDA@SiO2 NPs remained constant when the viewing angle changed. The corresponding reflection spectrums (Fig. 2b) showed that the dominant reflection peaks had no obvious displacement under different test angles, which proved the low angle dependence of the non-iridescent color. This was because there was no direction discrimination in the metastable amorphous structures. When the photons entered this micro-nano structure, the light scattered coherently under the action of interference and scattered evenly in all directions, forming the isotropic photonic band gap to realize the low angle dependence. This non-iridescent feature endows the photonic pigments with angle independence and wide-angle display, which is more in line with the logic of practical applications than the traditional crystalline photonic pigments. In addition, as shown in Fig. 2c, the single-component photonic pigments with different primary hues ranging from blue to red were obtained by changing the diameter of building blocks from 199 nm to 298 nm. According to Fig. 2d and e, the hue of photonic pigments was consistent with the dominant reflection wavelength in the corresponding reflection spectrum, which linearly increased with the particle size of building blocks. Although the single-component photonic pigments cannot generate more precise and colorful true colors, which were limited by the accuracy and robustness of the preparation process of building blocks, these photonic pigments with different primary hues can be used as primary colors to provide a foundation for the binary photonic pigments.
On the basis of the obtained single-component metastable photonic pigments, the mixing and reproducing colors that occurred at the level of building blocks were realized, which were more advanced than the mixed coloration of longhorn beetle Anoplophora graafi (Fig. 3a). A binary metastable amorphous photonic pigment was obtained, developing a brand-new “true color” palette conforming to the additive color mixing. As shown in Fig. 3b, since the photonic pigments were structurally colored, the optical trichromatic colors red, green, and blue, which conformed to the additive color mixing were selected as the primary colors to study the rules of mixing and reproducing colors. The binary photonic pigments were prepared by the mixing of building block dispersions corresponding to the trichromatic colors in pairs with equal volume fractions. Different from the subtractive mixing of pigments, the colors of binary photonic pigments mixed in pairs conformed to the mixing rule of additive colors (red + green = yellow, green + blue = cyan, red + blue = purple), which proved that the photonic pigments followed the palette effect of additive color mixing. As shown in Fig. 3c, when the building blocks with the diameter of 298 nm and 231 nm were mixed in equal volume, the reflection peak previously attributed to the two components disappeared, replaced by a new single reflection peak located between the original two peaks. By contrast, the simulated reflection spectrum superimposed by two kinds of photonic structures (dotted line in Fig. 3c) showed that the double reflection peak was composed of two peaks at 673 nm and 523 nm, which, respectively, belong to the photonic structures constructed by the diameter of 298 nm and 231 nm PDA@SiO2 NPs. It can be inferred that with the change of the proportion of two kinds of photonic structures, the reflection intensity of the two peaks will be affected, but the position of the double peak will not significantly shift and form a single dominant peak similar to that of binary photonic pigments. The mixing of green and blue photonic pigments (Fig. S3, ESI†) also showed a similar result, which meant that the coloration mode of the binary photonic pigments was not the self-sorting assembly of two kinds of building blocks or the simple superposition of two coloration units.11–14 The color obtained from binary photonic pigments corresponded to the newly generated single peak in the reflection spectrum, which was a more microscopic and subtle “true color”. In order to verify this theory, the coloration mechanism of binary photonic pigments was further studied. The in situ SEM image of the binary photonic pigment and corresponding 2D-FT (Fig. 3d) showed that in the binary system, the two building blocks still maintained the close-packing mode of long-range disorder and short-range order instead of self-sorting assembly. It should be noted that when the surface roughness of building blocks was low, the binary system still had a strong tendency for crystallization and it could not achieve mixing and reproducing colors by the same preparation method. As shown in Fig. S4 (ESI†), the building blocks with lower roughness tended to self-sorting assemble, in which the same kind of building blocks was gathered and the corresponding reflection spectrum also showed that the optical properties of the binary system were still similar to those of the original single-component system, which were determined by the dominant building blocks, without the new monochromatic peak corresponding to the binary metastable structures. Therefore, controlling the dynamic behaviors through the surface roughness of building blocks was crucial for constructing the binary metastable structures. Based on these metastable binary structures, the three-dimensional (3D) model of the binary photonic structure was simulated. As shown in Fig. 3e, the random close-packing structure composed of two kinds of nanoparticles was generated. The refractive index of nanoparticles in the model was set to be the same as that of PDA@SiO2 NPs (nPDA@SiO2 = 1.45) and the reflection spectrum of the simulated structure was calculated using the 3D finite difference time domain (3D-FDTD) method. By comparing the reflection spectrums of simulated structure and photonic structure (Fig. 3f), it can be found that the results were in good agreement, further proving that this unique color mixing mode can obtain “true color” in spectroscopy. This novel color mixing mechanism was derived from the deliberately designed building blocks and the limited assembly mode. The introduction of PDA broke the steady-state dominated by thermodynamics and enhanced the interaction forces between binary building blocks. Moreover, the controllable roughness and the limited assembly space and time further introduced the dynamic behaviors to reduce the crystallization trend between the same building blocks. The assembly trend between all building blocks tended to be homogeneous and a binary metastable structure was obtained, which preliminarily realized the true color mixing.
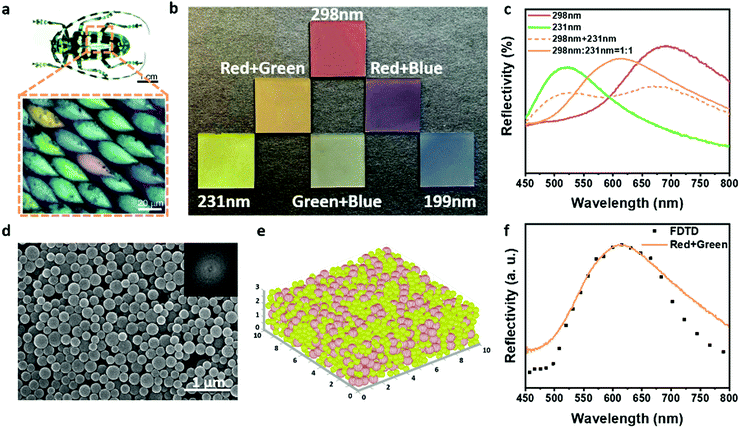 | ||
| Fig. 3 (a) Optical image of longhorn beetles A. Graafi. Inset: Optical microscopic image of the mixed array of coloration units;42 (© https://doi.org/10.1364/OE.18.014430 [2010] Optica Publishing Group); (b) optical images of trichromatic single-component photonic pigments (red: 298 nm, green: 231 nm, and blue: 199 nm) and the binary photonic pigments; (c) reflection spectrums of binary photonic pigments prepared by 298 nm and 231 nm PDA@SiO2 NPs (dotted line: the simulated reflection spectrum); (d) SEM image of binary photonic pigments. Inset: The corresponding 2D FT pattern of the SEM image; (e) the simulated model of binary photonic pigments; (red spheres: 298 nm, green spheres: 231 nm); (f) measured (solid line) and calculated by the 3D-FDTD method (dotted line) reflection spectrums of binary photonic pigments. | ||
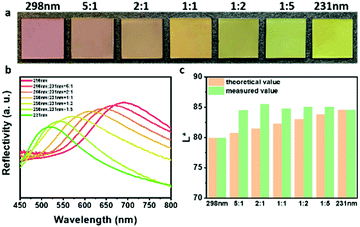
In order to further explore this color palette effect, the influence of the volume ratio of the two components on the optical properties of the binary photonic pigments was studied. As shown in Fig. 4a, it was confirmed with the naked eye that with the increment of the proportion of green building blocks (231 nm), the hue of the photonic pigments changed from red to orange, to yellow, and then to green, which was exactly the continuous change process from red to green in the visible spectrum. The corresponding reflection spectrums of these photonic pigments (Fig. 4b) showed that the hue of binary photonic pigments was consistent with the dominant reflection wavelength. The wavelength at the dominant reflection linearly increased with the volume fraction of 298 nm PDA@SiO2 NPs (Fig. S5, ESI†). This meant that using two photonic pigments with the known dominant reflection peaks as primary colors, the binary photonic pigment's peak position can be adjusted by the volume fraction ratio to achieve controlling hues of true colors of photonic pigments. This result can save people from learning abstract and tedious color matching training and make color mixing efficient and repeatable. It was also worth noting that, as shown in Fig. 4c, through colorimetry calculation, in the L*a*b* color space with the D50 light source and 2° field of view, the photonic pigments prepared by mixing with different proportions showed higher brightness than that of primary photonic pigments, which was attributed to coloration mechanism of structural colors. Therefore, the binary photonic pigments can not only obtain precise addressable “true colors” by controlling the volume fraction of two building blocks, but also have the brightness enhancement property of additive colors.
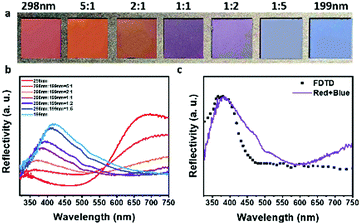
Dexterously, it was the special ultraviolet optical properties of the metastable amorphous photonic structures that had been used to reproduce the violet that could not be obtained by mixing in a conventional way, so that the mechanism of the true color palette was further improved. As shown in Fig. 3c and Fig. S3 (ESI†), the wavelength of yellow obtained by mixing red and green and cyan obtained by mixing green and blue was between the two original peaks before mixing. Contrastingly, in Fig. 5a, through naked-eye observation, with the variation of the volume fraction of 298 nm and 231 nm PDA@SiO2 NPs, the hue of binary photonic crystals gradually transformed from red or blue to variant violet, which was not the intermediate hue between red and blue light. The corresponding reflection spectrums of these binary photonic pigments (Fig. 5b) showed that for the violet photonic pigment, the main reflectance peak characteristic of “true colors” was also located on the shorter wavelength side of the blue photonic pigments as shown by the hue, which meant that “true violet” had been successfully obtained by tuning the construction of the binary photonic crystals. This was attributed to the specificity of optical properties of the amorphous photonic pigments. Similar to the stimulation curve of red-sensitive cone cells of the human eye and the resulting CIE 1931 red matching functions (Fig. S6, ESI†), the red amorphous photonic pigments also showed two distinct reflection peaks, one at a red wavelength and the other one at an ultraviolet wavelength. Furthermore, the secondary peak in the ultraviolet region was generated by the high-frequency photonic pseudogap caused by the short-range ordered array.45,51,54 As shown in Fig. 5b, due to the existence of the secondary reflection peak, when red and blue photonic pigments are mixed in different proportions, the new single peak shifted continuously and regularly between the blue peak and the secondary red peak, realizing the additive color mixing of violet. Similarly, the reflection spectrum obtained by 3D-FDTD calculation of the simulated structure of violet binary photonic pigment was compared with the experimental reflection spectrum (Fig. 5c) and the overall consistency between theory and experiment was acceptable. In a nutshell, by virtue of special ultraviolet properties, binary photonic pigments subtly reproduced the violet that did not exist in the mixed colors, which complemented the missing part of the traditional color palette effect.
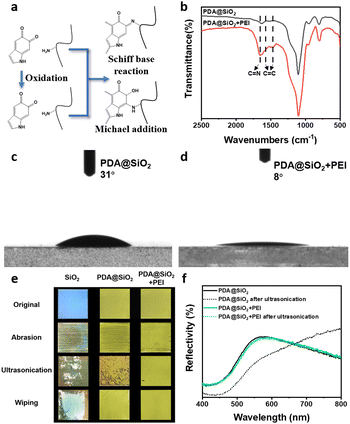
Different from the steady-state photonic structure dominated by thermodynamics or dynamics, the stability of metastable photonic structures is particularly critical for practical applications and the feature of never-fading. In contrast to encapsulating photonic crystals by introducing other components to improve the stability,55–59 inspired by the super-adhesion of mussels, a two-step strategy for enhancing the stability of photonic pigments without changing the optical properties was developed. Firstly, PDA endowed the building blocks with self-adhesion. Abundant catechol groups on the surface of PDA@SiO2 NPs provide “anchors” that can interact with various interfaces,46–49 enhancing the stability between the building blocks and substrate. Then, benefiting from the strong post-modification of PDA, polyethyleneimine (PEI) was introduced into the metastable photonic structures as a cross-linking agent. The interaction forces between the building blocks were strengthened through Michael addition and Schiff base reaction with PDA to achieve the stabilization of metastable structures. (Fig. 6a).60,61 As shown in Fig. 6b, the FT-IR spectrum of PEI-modified photonic pigments showed a new peak at 1654 cm−1, which was derived from the formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond between PDA and PEI. Due to the generation of covalent bonds, the conjugate benzene caused the vibration peaks of the C
N bond between PDA and PEI. Due to the generation of covalent bonds, the conjugate benzene caused the vibration peaks of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C skeleton to redshift from 1629 cm−1 and 1519 cm−1 to 1567 cm−1 and 1469 cm−1. In addition, the reaction between PDA and PEI can be further proved by the surface wettability of photonic pigments before and after modification. In Fig. 6c and d, the water contact angle of unmodified photonic pigment was approximately 31°, while that of PEI-modified photonic pigment was 8°. With the introduction of PEI, the inherent abundance of amino groups of PEI significantly improved the hydrophilicity of photonic pigments, which proved the occurrence of reaction between PDA and PEI. Furthermore, the stability of photonic pigments was studied by abrasion, ultrasonication, and finger wiping. As shown in Fig. 6e, after a series of damaging operations, the arrays of SiO2 NPs were almost completely destroyed, while the photonic pigments constructed by PDA@SiO2 NPs partially detached under abrasion and ultrasonication. The photonic pigments modified by PEI had no noticeable damage under the destructive operations and retained the initial structure colors. The corresponding reflection spectrum (Fig. 6f) showed that, in the case of the photonic pigment prepared by PDA@SiO2 NPs, the reflection peak originally located at 568 nm was redshifted and its peak width increased. This was because the metastable state of the photonic structures was destroyed, which led to the increase in the spacing of the building blocks and the destruction of the order degree of the phonic structure. In contrast, the photonic pigments modified by PEI still maintained the original reflection peak after ultrasonication. These results clearly demonstrate that the two-step method was used to enhance the interaction force both between the building block and the substrate and each building block significantly improved the stability of metastable amorphous photonic pigments, which is expected to establish the advantages for future applications of photonic pigments.
C skeleton to redshift from 1629 cm−1 and 1519 cm−1 to 1567 cm−1 and 1469 cm−1. In addition, the reaction between PDA and PEI can be further proved by the surface wettability of photonic pigments before and after modification. In Fig. 6c and d, the water contact angle of unmodified photonic pigment was approximately 31°, while that of PEI-modified photonic pigment was 8°. With the introduction of PEI, the inherent abundance of amino groups of PEI significantly improved the hydrophilicity of photonic pigments, which proved the occurrence of reaction between PDA and PEI. Furthermore, the stability of photonic pigments was studied by abrasion, ultrasonication, and finger wiping. As shown in Fig. 6e, after a series of damaging operations, the arrays of SiO2 NPs were almost completely destroyed, while the photonic pigments constructed by PDA@SiO2 NPs partially detached under abrasion and ultrasonication. The photonic pigments modified by PEI had no noticeable damage under the destructive operations and retained the initial structure colors. The corresponding reflection spectrum (Fig. 6f) showed that, in the case of the photonic pigment prepared by PDA@SiO2 NPs, the reflection peak originally located at 568 nm was redshifted and its peak width increased. This was because the metastable state of the photonic structures was destroyed, which led to the increase in the spacing of the building blocks and the destruction of the order degree of the phonic structure. In contrast, the photonic pigments modified by PEI still maintained the original reflection peak after ultrasonication. These results clearly demonstrate that the two-step method was used to enhance the interaction force both between the building block and the substrate and each building block significantly improved the stability of metastable amorphous photonic pigments, which is expected to establish the advantages for future applications of photonic pigments.
Due to the fact that the photonic pigments have the “true color” mixing mode, which follows the palette rule of additive color mixing, it has incomparable advantages in practical applications compared with conventional pigments. In the 19th century, French painter Seurat tried to draw color dots at close distances with the technique of pointillism, so that the different colors of light were reflected from the color dots and finally mixed in the human eye to improve the brightness of prints. However, this tremendous scientific experiment failed to achieve the desired results due to the limited sizes and distances of color dots and the inherent disadvantages of subtractive colors. As shown in Fig. 7a and b, the intensely colored and bright “Vase with Irises” was prepared by spraying with suspensions of PDA@SiO2 NPs with different diameters, different DA contents (Fig. S7, ESI†), and different mixing ratios. Compared with the original work,62 photonic painting not only achieved multicolors like mixed colors but also had the unique advantages of true colors. The mixing of the photonic pigments followed the principle of additive color mixing and the brightness of the mixed colors increased, which can easily achieve the effect of increased brightness presented by pointillists. More importantly, different from the color resolution in the normal color mixing mode, which was limited by the size or arrangement of color dots, the true colors displayed by the mixed photonic pigments had the almost infinite resolution in theory. Therefore, the photonic pigments realized the diversity of true colors and overcame the shortcomings of the existing mixed colors, which were expected to repaint the color palette of the world.
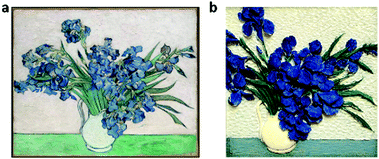 | ||
Fig. 7 (a) The original work of “Vase with Irises”, created by van Gogh in 189062 (the red arrow in the original image was removed in the version, © https://doi.org/10.1186/s40494-017-0131-8 [2017] Springer International Publishing). (b) photonic picture of “Vase with Irises” drawn by spraying methods (iris petals: 199 nm, 2.6 mM DA; leaves 224 nm, 1.3 mM DA; table: 245 nm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 199 nm = 1 199 nm = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.3 mM DA; vase: 298 nm 1, 1.3 mM DA; vase: 298 nm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 nm = 1 231 nm = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.7 mM). 1, 0.7 mM). | ||
Conclusions
In summary, inspired by natural creatures, we developed the “true color” photonic pigments based on the binary metastable photonic structures, which were capable of mixing and reproducing colors. The building blocks of this pigment were PDA@SiO2 NPs with self-adhesion and adjustable roughness. The single-component photonic pigments had the short-range ordered and spatial isotropic metastable structures and its non-iridescent structural colors can be further mixed as primary colors to obtain the binary photonic pigments. Different from the majority of binary photonic crystals, which only displayed colors in a steady-state, the obtained binary metastable photonic structures achieved precise addressable “true colors” with volume fraction correlation. More importantly, in this palette system, the binary photonic structures provided the particular ultraviolet characteristic to reproduce the violet, which dexterously complemented the color palette effect. In addition, benefiting from the self-adhesion and post-modification of building blocks, the stability of metastable structures was significantly improved, ensuring the practical applications of these materials. Furthermore, the photonic pigments had the characteristics of brightness enhancement, which conventional pigments cannot achieve through the facile mixed spraying and realized the tunability and multiplicity of true colors. Therefore, we believe that these novel photonic pigments and their unique true color palette mode can repaint our world in glorious multicolors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51773180, 52003237, and 21875009) and the Fundamental Research Funds for the Provincial Universities of Zhejiang (RF-B2019002, 2021C01087, and 2021C01125).References
- D. H. Brainard and A. C. Hurlbert, Curr. Biol., 2015, 25, R551–R554 CrossRef CAS PubMed.
- K. R. Gegenfurtner, M. Bloj and M. Toscani, Curr. Biol., 2015, 25, R543–R544 CrossRef CAS PubMed.
- R. Lafer-Sousa, K. L. Hermann and B. R. Conway, Curr. Biol., 2015, 25, R545–R546 CrossRef CAS PubMed.
- A. D. Winkler, L. Spillmann, J. S. Werner and M. A. Webster, Curr. Biol., 2015, 25, R547–R548 CrossRef CAS PubMed.
- A. R. Robertson, Rep. Prog. Phys., 1978, 41, 469–510 CrossRef CAS.
- P. Lipton, Science, 2007, 316, 834 CrossRef CAS.
- V. Walsh, Curr. Biol., 1995, 5, 703–705 CrossRef CAS PubMed.
- A. Hurlbert, Curr. Biol., 1999, 9, R558–R561 CrossRef CAS PubMed.
- D. H. Foster, Trends Cognit. Sci., 2003, 7, 439–443 CrossRef PubMed.
- C. Witzel and K. R. Gegenfurtner, Annu. Rev. Vis. Sci., 2018, 4, 475–499 CrossRef PubMed.
- C. Wang, X. Lin, C. G. Schäfer, S. Hirsemann and J. Ge, Adv. Funct. Mater., 2020, 31, 2008601 CrossRef.
- H. Eoh, Y. Jung, C. Park, C. E. Lee, T. H. Park, H. S. Kang, S. Jeon, D. Y. Ryu, J. Huh and C. Park, Adv. Funct. Mater., 2021, 32, 2103697 CrossRef.
- K. Li, T. Li, T. Zhang, H. Li, A. Li, Z. Li, X. Lai, X. Hou, Y. Wang, L. Shi, M. Li and Y. Song, Sci. Adv., 2021, 7, eabh1992 CrossRef CAS PubMed.
- S. U. Kim, Y. J. Lee, J. Liu, D. S. Kim, H. Wang and S. Yang, Nat. Mater., 2022, 21, 41–46 CrossRef CAS PubMed.
- Y. Ohtsuka, M. Sakai, T. Seki, R. Ohnuki, S. Yoshioka and Y. Takeoka, ACS Appl. Mater. Interfaces, 2020, 12, 54127–54137 CrossRef CAS PubMed.
- M. Sakai, H. Kim, Y. Arai, T. Teratani, Y. Kawai, Y. Kuwahara, K. Abe, Y. Kuwana, K. Ikeda, K. Yamada and Y. Takeoka, ACS Appl. Nano Mater., 2020, 3, 7047–7056 CrossRef CAS.
- S. John, Phys. Rev. Lett., 1987, 58, 2486–2489 CrossRef CAS PubMed.
- E. Yablonovitch, Phys. Rev. Lett., 1987, 58, 2059–2062 CrossRef CAS PubMed.
- S. Kinoshita, S. Yoshioka and J. Miyazaki, Rep. Prog. Phys., 2008, 71, 076401 CrossRef.
- A. G. Dumanli and T. Savin, Chem. Soc. Rev., 2016, 45, 6698–6724 RSC.
- Z. Cai, Z. Li, S. Ravaine, M. He, Y. Song, Y. Yin, H. Zheng, J. Teng and A. Zhang, Chem. Soc. Rev., 2021, 50, 5898–5951 RSC.
- Y. Ohtsuka, T. Seki and Y. Takeoka, Angew. Chem., Int. Ed., 2015, 54, 15368–15373 CrossRef CAS PubMed.
- Y. Li, Q. Fan, X. Wang, G. Liu, L. Chai, L. Zhou, J. Shao and Y. Yin, Adv. Funct. Mater., 2021, 31, 2010746 CrossRef CAS.
- X. Li, D. Zhao, K. J. Shea, X. Li and X. Lu, Mater. Horiz., 2021, 8, 932–938 RSC.
- A.-Q. Xie, J. Guo, L. Zhu and S. Chen, Chem. Eng. J., 2021, 415, 128950 CrossRef CAS.
- Z. Chen, F. Fu, Y. Yu, H. Wang, Y. Shang and Y. Zhao, Adv. Mater., 2019, 31, e1805431 CrossRef PubMed.
- M. Li, H. Tan, L. Jia, R. Zhong, B. Peng, J. Zhou, J. Xu, B. Xiong, L. Zhang and J. Zhu, Adv. Funct. Mater., 2020, 30, 2000008 CrossRef CAS.
- Y. Wang, L. Shang, G. Chen, L. Sun, X. Zhang and Y. Zhao, Sci. Adv., 2020, 6, eaax8258 CrossRef CAS PubMed.
- X. Lai, J. Peng, Q. Cheng, A. P. Tomsia, G. Zhao, L. Liu, G. Zou, Y. Song, L. Jiang and M. Li, Angew. Chem., Int. Ed., 2021, 60, 14307–14312 CrossRef CAS PubMed.
- W. Niu, X. Cao, Y. Wang, B. Yao, Y. Zhao, J. Cheng, S. Wu, S. Zhang and X. He, Adv. Funct. Mater., 2021, 31, 2009017 CrossRef CAS.
- Y. Wu, Y. Wang, S. Zhang and S. Wu, ACS Nano, 2021, 15, 15720–15729 CrossRef CAS PubMed.
- Y. Dong, Z. Ma, D. P. Song, G. Ma and Y. Li, ACS Nano, 2021, 15, 8770–8779 CrossRef CAS PubMed.
- Y. Liu, Q. Fan, G. Zhu, G. Shi, H. Ma, W. Li, T. Wu, J. Chen, Y. Yin and J. Guan, Mater. Horiz., 2021, 8, 2032–2040 RSC.
- Z. Zhang, Z. Chen, Y. Wang, Y. Zhao and L. Shang, Adv. Funct. Mater., 2021, 32, 2107242 CrossRef.
- R. Chen, D. Feng, G. Chen, X. Chen and W. Hong, Adv. Funct. Mater., 2021, 31, 2009916 CrossRef CAS.
- Y. Wang, X. Cao, J. Cheng, B. Yao, Y. Zhao, S. Wu, B. Ju, S. Zhang, X. He and W. Niu, ACS Nano, 2021, 15, 3509–3521 CrossRef CAS PubMed.
- Y. Qi, H. Yang and S. Zhang, Chem. Eng. J., 2022, 428, 130859 CrossRef CAS.
- M. Sakai, T. Seki and Y. Takeoka, Small, 2018, 14, e1800817 CrossRef PubMed.
- F. Bian, L. Sun, H. Chen, Y. Wang, L. Wang, L. Shang and Y. Zhao, Adv. Sci., 2022, 9, e2105278 CrossRef PubMed.
- T. Biben and J. P. Hansen, Phys. Rev. Lett., 1991, 66, 2215–2218 CrossRef CAS PubMed.
- S. Auer and D. Frenkel, Nature, 2001, 409, 1020–1023 CrossRef CAS PubMed.
- P. Velikov Krassimir, G. Christova Christina, P. A. Dullens Roel and A. van Blaaderen, Science, 2002, 296, 106–109 CrossRef CAS PubMed.
- D. Wang and H. Möhwald, Adv. Mater., 2004, 16, 244–247 CrossRef CAS.
- J. Yu, Q. Yan and D. Shen, ACS Appl. Mater. Interfaces, 2010, 2, 1922–1926 CrossRef CAS PubMed.
- B. Q. Dong, X. H. Liu, T. R. Zhan, L. P. Jiang, H. W. Yin, F. Liu and J. Zi, Opt. Express, 2010, 18, 14430–14438 CrossRef CAS PubMed.
- H. Lee, M. Dellatore Shara, M. Miller William and B. Messersmith Phillip, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- H. Lee, B. P. Lee and P. B. Messersmith, Nature, 2007, 448, 338–341 CrossRef CAS PubMed.
- F. Bian, J. Wu, H. Wang, L. Sun, C. Shao, Y. Wang, Z. Li, X. Wang and Y. Zhao, Small, 2018, 14, e1803551 CrossRef PubMed.
- C. Zhang, L. Xiang, J. Zhang, C. Liu, Z. Wang, H. Zeng and Z.-K. Xu, Chem. Sci., 2022, 13, 1698–1705 RSC.
- R. O. Prum, R. H. Torres, S. Williamson and J. Dyck, Nature, 1998, 396, 28–29 CrossRef CAS.
- Y. Takeoka, S. Yoshioka, A. Takano, S. Arai, K. Nueangnoraj, H. Nishihara, M. Teshima, Y. Ohtsuka and T. Seki, Angew. Chem., Int. Ed., 2013, 52, 7261–7265 CrossRef CAS PubMed.
- L. Shi, Y. Zhang, B. Dong, T. Zhan, X. Liu and J. Zi, Adv. Mater., 2013, 25, 5314–5320 CrossRef CAS PubMed.
- Y. Takeoka, Polym. J., 2014, 47, 106–113 CrossRef.
- Y. Naoi, T. Seki, S. Yoshioka and Y. Takeoka, Mol. Cryst. Liq. Cryst., 2019, 688, 105–113 CrossRef CAS.
- H. Zhang, W. Niu and S. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 24639–24647 CrossRef CAS PubMed.
- Y. Wang, W. Niu, C. Y. Lo, Y. Zhao, X. He, G. Zhang, S. Wu, B. Ju and S. Zhang, Adv. Funct. Mater., 2020, 30, 2000356 CrossRef CAS.
- W. Niu, X. Wang, Y. Zheng, S. Wu, M. Hua, Y. Wang, X. Zhang, A. I. Y. Tok, X. He and S. Zhang, Small, 2020, 16, e2003638 CrossRef PubMed.
- J. Choi, M. Hua, S. Y. Lee, W. Jo, C.-Y. Lo, S.-H. Kim, H.-T. Kim and X. He, Adv. Opt. Mater., 2020, 8, 1901259 CrossRef CAS.
- K. Zhao, X. Cao, Y. Alsaid, J. Cheng, Y. Wang, Y. Zhao, X. He, S. Zhang and W. Niu, Chem. Eng. J., 2021, 426, 130870 CrossRef CAS.
- H.-C. Yang, K.-J. Liao, H. Huang, Q.-Y. Wu, L.-S. Wan and Z.-K. Xu, J. Mater. Chem. A, 2014, 2, 10225–10230 RSC.
- H.-C. Yang, W. Zhong, J. Hou, V. Chen and Z.-K. Xu, J. Membr. Sci., 2017, 523, 1–7 CrossRef CAS.
- S. A. Centeno, C. Hale, F. Carò, A. Cesaratto, N. Shibayama, J. Delaney, K. Dooley, G. van der Snickt, K. Janssens and S. A. Stein, Herit. Sci., 2017, 5, 18 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nh00232a |
| This journal is © The Royal Society of Chemistry 2022 |