Sceptrin–Au nano-aggregates (SANA) for overcoming drug-resistant Gram-negative bacteria†
Jong Min
An‡
a,
Sangrim
Kang‡
b,
Chang Woo
Koh
c,
Sungnam
Park
 c,
Myung Sook
Oh
c,
Myung Sook
Oh
 *de and
Dokyoung
Kim
*de and
Dokyoung
Kim
 *afghi
*afghi
aDepartment of Biomedical Science, Graduate School, Kyung Hee University, Seoul 02447, Republic of Korea. E-mail: dkim@khu.ac.kr
bDivision of Antimicrobial Resistance Research, National Institute of Infectious Diseases (NIID), Korea National Institute of Health, Cheongju, 28459, Republic of Korea
cDepartment of Chemistry, Korea University, Seoul 02841, Republic of Korea
dDepartment of Biomedical and Pharmaceutical Sciences, Graduate School, Kyung Hee University, Seoul 02447, Republic of Korea. E-mail: msohok@khu.ac.kr
eDepartment of Oriental Pharmaceutical Science and Kyung Hee East-West Pharmaceutical Research Institute, College of Pharmacy, Kyung Hee University, Seoul 02447, Republic of Korea
fDepartment of Anatomy and Neurobiology, College of Medicine, Kyung Hee University, Seoul 02447, Republic of Korea
gCenter for Converging Humanities, Kyung Hee University, Seoul 02447, Republic of Korea
hMedical Research Center for Bioreaction to Reactive Oxygen Species and Biomedical Science Institute, School of Medicine, Graduate School, Kyung Hee University, Seoul 02447, Republic of Korea
iKHU-KIST Department of Converging Science and Technology, Kyung Hee University, Seoul 02447, Republic of Korea
First published on 1st July 2022
Abstract
One of the recent advances in medical nanotechnology has been the development of nanoformulations to overcome drug-resistant bacterial infections. Herein, we disclose a new nano-antibiotic formulation based on sceptrin–Au nano-aggregates (SANA), which are drug-metal ion multiple complexes. Sceptrin is a natural compound from a marine organism (sponge) and was reported as a potential compound with drug activities. SANA consists of a sceptrin–Au ion and is a self-assembled nano-formation with electrostatic interaction. Interestingly, SANA showed superior antibiotic/antibiofilm activity toward carbapenem-resistant Gram-negative bacteria with low toxicity to red blood cells and endothelial cells. The working mechanism of SANA was identified with analysis of the extracellular reactive oxygen species level and membrane depolarization of bacteria. The feasibility of SANA as a new nano-antibiotic was demonstrated in CRPA-contaminated medical supplies where SANA inhibited the formation of biofilms as well as the growth of CRPA. This work presents a new concept for the development of next-generation nano-antibiotics and a more feasible clinical translational pathway.
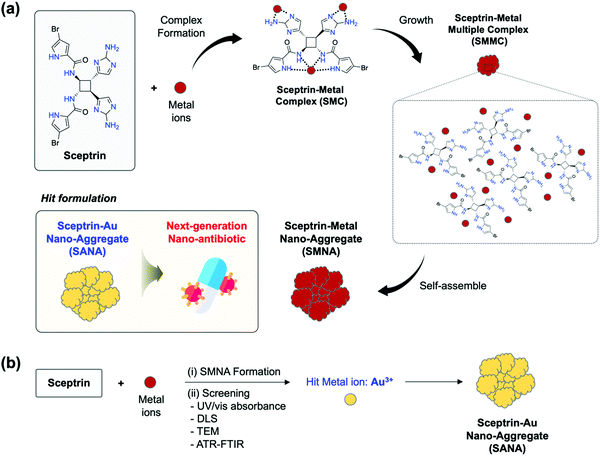
New conceptsGram-negative bacteria (GNB) are responsible for high mortality. Despite significant advances in pharmaceutical therapy and approaches, the misuse and abuse of antibiotics has initiated the advent of drug-resistant bacteria. The development of new antibiotics generally takes 10 years and is costly. Nanomaterial-based antibiotics (nano-antibiotics) have drawn attention from many researchers to shorten the development time for new antibiotics. Therefore, we are reporting a new nano-antibiotic formulation based on a drug–metal complex formation approach, using sceptrin and metal ions. As a hit formulation, sceptrin efficiently forms nano-aggregates with gold ions (Au3+) among various metal ions. The resulting sceptrin–Au nano-aggregates (named SANA) showed superior antibiotic effects against Gram-negative bacteria including drug-resistant Gram-negative bacteria, with high biocompatibility, such as low toxicity to blood cells/endothelial cells. SANA also showed effective antibiotic/biofilm activities in a clinical application (bacteria-contaminated needle). Our work demonstrates that SANA has excellent potential against Gram-negative bacteria, including drug-resistant strains, and provides a new approach based on the complex of a drug and metal ions with wide applicability in clinical fields. |
1. Introduction
Some bacteria are increasingly resistant to existing antibiotics and have become serious threats to human health.1–3 Going through the Covid-19 pandemic, many antibiotic chemicals have been misused, despite having no bacterial coinfections, which led to the generation of drug-resistant bacteria.4To overcome the drug-resistant bacteria, the development of new antibiotics has focused on a new small-molecule or combination of chemicals (natural compounds, drugs, metal ions, etc.) that has a distinct working mechanism to antibiotics to divert the drug resistance pathway.5 However, the development of new antibiotics takes about 10 years with huge costs, which has encouraged many researchers in academia and industry to focus on repositioning existing drugs (drug-repositioning approach) and taking the exploratory approach of mixing substances (cocktail method) to reduce the development period and cost for a new antibiotic.1,6 As another alternative, nanomaterial-based antibiotics (called nano-antibiotic) such as pnictogens complexed with drugs,7 zinc oxide nanocrystals,8 amine-functionalized silica nanoparticles,9,10 and gold/silver nanoparticles/nanoclusters have long been highlighted as the first-generation nano-antibiotics.11–13 However, only a few reports have introduced the anti-bacterial activities of these drug–metal complexes, and it is a very challenging field of research.
Herein, we disclosed a new nano-antibiotic formulation based on the drug–metal complex formation approach using sceptrin (as a drug) and metal ions (Fig. 1). Sceptrin is a natural compound from marine organisms (sponges), and its biological activities, including anti-mobility, anti-muscarin, inhibition of depolarization-induced cellular calcium elevation, somatostatin, and vasoactive intestinal polypeptide, have been reported.14–16 Such features of sceptrin have been reported, but there is no research on its metal complexes and biological applications to date. In this study, we introduced a new approach based on the formation of (i) a sceptrin–metal complex (SMC) via electrostatic interaction of sceptrin and a metal ion, (ii) sceptrin–metal multiple complexes (SMMC), and (iii) sceptrin–metal nano-aggregates (SMNA) via self-assembly of SMMC. As a hit formulation, sceptrin efficiently forms nano-aggregates with gold ions (Au3+) among various metal ions, and the resulting sceptrin–Au nano-aggregates (named SANA) showed superior antibiotic effects. To date, the gold ion and its ligand-complex (auranofin, solganol, etc.) have been widely used for cancer treatment by binding to the protein or DNA in cells, but, to our knowledge, a gold-based complex with a drug for overcoming drug-resistant bacteria has not been reported yet.12,17
The complex formation of sceptrin and gold ions was systemically characterized using density functional theory (DFT) calculation, Job plot analysis, MALDI-TOF/MS analysis, and a Benesi–Hildebrand relation plot. The size of SANA was confirmed by transmission electron microscopy (TEM) imaging analysis. The antibiotic activities of SANA against 10 types of Gram-positive and Gram-negative bacterial strains, including drug-resistance bacteria (Table 1) were evaluated. SANA was confirmed to have several promising properties as a next-generation nano-antibiotic for drug-resistant Gram-negative bacteria (i.e., carbapenem-resistant Pseudomonas aeruginosa; CRPA), which includes (i) enhanced antibiotic activity (minimum inhibitory concentration (MIC): 25 μM), (ii) high antibiofilm activity, (iii) low toxicity for red blood cells (RBCs) and endothelial cells, and (iv) superior antibiotics/antibiofilm activity to medical supplies, confirming that SANA has promising antibiotic/antibiofilm activities with high biocompatibility. We believe that our findings can contribute to overcoming drug-resistant Gram-negative bacteria throughout the clinical fields and translational research.
| Strain | Type of strain | Strain no. |
|---|---|---|
| Gram-negative | ||
| Escherichia coli | E. coli (EC) | ATCC 25922 |
| Klebsiella pneumoniae | K. pneumoniae (KP) | ATCC 13883 |
| Acinetobacter baumannii | Carbapenem-susceptible A. baumannii (AB) | ATCC 19606 |
| Pseudomonas aeruginosa | Carbapenem-susceptible P. aeruginosa (PA) | ATCC 27853 |
| Gram-positive | ||
| Enterococcus faecium | Vancomycin-susceptible E. faecium (EF) | ATCC 29212 |
| Staphylococcus aureus | Methicillin-susceptible S. aureus (SA) | ATCC 29213 |
| MDR-Gram-negative | ||
| Escherichia coli | Carbapenem-resistant E. coli (CREC) | AMC-EC 22365 |
| Klebsiella pneumoniae | Carbapenem-resistant K. pneumoniae (CRKP) | AMC-KP 24272 |
| Acinetobacter baumannii | Carbapenem-resistant A. baumannii (CRAB) | AMC-AB 643 |
| Pseudomonas aeruginosa | Carbapenem-resistant P. aeruginosa (CRPA) | CCARM 2321 |
2. Results and discussion
Rationale
Our research group has focused on developing biologically active tailored molecules and nanomaterials as new antibiotics and anticancer agents.18–20 Recently, we have been interested in identifying new antibiotics based on nano-sized metal–ligand complexes. Among the candidates, we considered sceptrin, which has multiple amine-containing functional groups, such as pyrrole, amide, and alkylamine that can readily coordinate with heavy metal ions.21–23 Sceptrin is a derivative from marine sponges and is not extensively explored as an antibiotic to overcome drug-resistant bacteria. Only one report introduced a binding property of sceptrin toward MreB protein, which is identified as a homolog of actin in bacteria.24 The binding between sceptrin and MreB protein could cause the disorder of the array at dynamic helical filaments around the periphery of the cell, perpendicular to the long axis.24,25 From this paper, we came up with the rationale of this study; multiple binding of amine functionalities within sceptrin could coordinate with the metal ions and have unique bioactivities. As shown in Fig. 1a, we expected that sceptrin could make SMC, SMMC, and SMNA as final products through complex formation, size growth, and self-assembly.We designed a two-step process: (i) SMNA formation of sceptrin in the presence of metal ions. Among the various metal ions, we chose heavy metal ions for the screening considering the possibility of multiple coordination toward sceptrin. (ii) Identification of SMNA using UV/vis absorbance, dynamic light scattering (DLS), transmission electron microscopy (TEM), and attenuated total reflection Fourier-transform infrared (ATR-FTIR) to detect the hit metal ions which form SMNA efficiently (Fig. 1b). Through this process, we found the hit metal ions, Au3+, and prepared the final formulation (SANA). The characterization of SANA was systematically analyzed, and its antibiotic property, mode of action (MoA), and clinical applications were comprehensively demonstrated.
Characterization of SANA
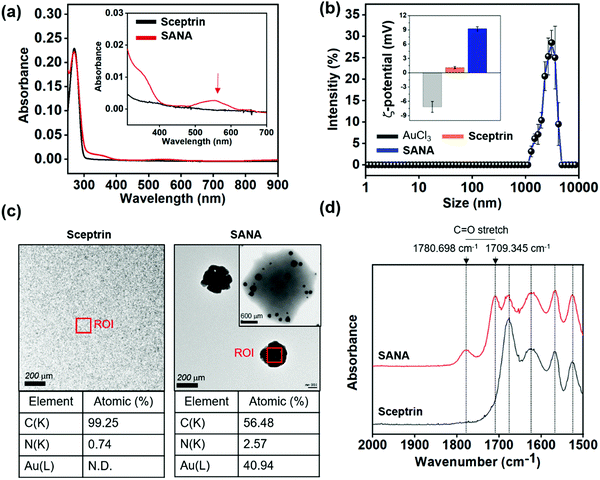
The characterization data of SANA was acquired through SMNA formation and the steps were identified and described in the Rationale section.In the UV/vis absorption spectra analysis of SMNA, significant spectra changes of sceptrin were observed only in the Au3+-treated set (Fig. 2a) and Fe2+-treated set (Fig. S1, ESI†). Generally, metal ions are associated with optical property changes such as in the UV/vis absorption spectra, when they form aggregates (nanoparticles), and those changes are dependent on the size of the ions.26,27 Thus, SANA peaking around 500–600 nm represents the formation of gold nano-aggregates (Fig. 2a), and the peak position corresponds with gold nanoparticles (AuNPs) with the size of 100–200 nm according to existing literature values.28 In addition, a bathochromic shift of the main peak was observed along with the addition of Au3+ (10–100 μM), which clearly showed the aggregate formation of gold ions (Fig. S1, ESI†). In the case of the Fe2+-treated set, the absorption intensity of sceptrin gradually increased depending on the Fe2+ concentration, but the fingerprint peaks of iron aggregates were not observed. The other SMNA formulation showed no significant peak changes.
After the SMNA screening based on the absorption spectra analysis, we focused on SANA. SANA displayed an average hydrodynamic diameter of 2914.5 ± 186.5 nm measured by dynamic light scattering (DLS, Fig. 2b), and its zeta-potential (mV) was measured to be 9.23 ± 0.42 mV (positive), whereas the mV of AuCl3 and sceptrin were −7.17 ± 1.15 mV (negative) and 1.10 ± 0.21 mV (neutral), respectively. In 2006, J.D.S. Newmann et al. reported that the pyrrole functional group could work as a reducing agent for Au3+ to form AuNPs.29 According to this paper, we could assume that the Au3+ coordinates with sceptrin, and the pyrrole group of sceptrin reduces the Au3+ leading to the formation of gold nano-clusters.
At the same time, the less stable gold nano-clusters between sceptrin might undergo self-assembly for stability. To confirm the structure of SANA, we additionally measured the transmission electron microscopy (TEM) image of SANA (Fig. 2c and Fig. S2a, ESI†). The SANA was coated with 1% (w/v) phosphotungstic acid (PTA) solution to visualize the organic region of SANA. As shown in Fig. 2c and Fig. S2a, (ESI†) we confirmed that the size of SANA is around 1–3 μm. This data corresponds to the result of DLS. SMMC was also observed in the primary step (<1 h) that is formed by SANA. In SMMC, a small size (<5 nm) of gold nano-clusters was also observed in the sceptrin–Au complex. Over the incubation time (≥1 h), the gold nano-clusters are expanded to larger gold nano-clusters with a lattice of 2.3 Å. Interestingly, the gold nano-clusters in SANA showed a flower-like formation that recruited the sceptrin–Au complex, and their size was around 150 nm which corresponds to the expected size for the absorption spectrum of gold nano-aggregates (Fig. 2a). In the case of sceptrin itself, we could not observe any aggregates in the TEM image. We thought that the atomic percentage of carbon and nitrogen would be a considerable part of SANA if SANA consists of sceptrin–Au complexes. In element analysis, carbon and nitrogen make up 56.48% and 2.57% of SANA, and the atomic gold (Au) is responsible for 40.94% (Fig. 2c), which indicates that SANA consists of sceptrin–Au complexes. To confirm the degree of aggregation of SANA over time in DI. H2O, the polydispersity index (PDI) was measured by DLS (Fig. S2b, ESI†), and SANA showed no aggregation issues until 4 days.
The attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectrum of SANA was measured to confirm the metal–ligand formation (Fig. 2d). The ATR-FTIR spectrum of SANA displayed a characteristic band corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1780.698 cm−1 and 1709.345 cm−1,30 and the generation of such peaks indicates the restricted vibration freedom of C
O stretching vibration at 1780.698 cm−1 and 1709.345 cm−1,30 and the generation of such peaks indicates the restricted vibration freedom of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O within sceptrin due to the Au3+-coordination.
O within sceptrin due to the Au3+-coordination.
SANA was prepared by a coordinate reaction between sceptrin and Au3+. To understand the coordinate mode, we conducted a quantum chemical DFT simulation and compared the results with experimental data. The DFT calculations were performed using a structurally most stable conformation of sceptrin and its corresponding Au3+ complex-based dispersion-corrected DFT functional. Au3+ has a coordination number of 4 and is square planar, and sceptrin has many functional groups (amine, alkene, bromine, ketone) that can coordinate with Au3+. We expected that the coordination could occur via a square planar coordination manner (ratio of sceptrin and Au; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and the DFT simulation results indicated that all the conformations (Au–amine, Au–alkene, Au–bromine, Au–ketone) were available (Fig. S3 and S4, ESI†). For further verification, we did a Job plot analysis that provided insights into the relative stoichiometries of the organic ligand and metal ions (Fig. S5, ESI†).31 The Job plot analysis between sceptrin and Au3+ was conducted in a mixture solution of dimethyl sulfoxide (DMSO) and DI. H2O (1
2), and the DFT simulation results indicated that all the conformations (Au–amine, Au–alkene, Au–bromine, Au–ketone) were available (Fig. S3 and S4, ESI†). For further verification, we did a Job plot analysis that provided insights into the relative stoichiometries of the organic ligand and metal ions (Fig. S5, ESI†).31 The Job plot analysis between sceptrin and Au3+ was conducted in a mixture solution of dimethyl sulfoxide (DMSO) and DI. H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The results showed a 1
1, v/v). The results showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 stoichiometric ratio between sceptrin and the Au3+ when a difference in maximum absorption intensity was plotted in the mole fraction of sceptrin (Fig. 4a, ESI†). This result was cross-checked by the mass spectrometry analysis using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF, Fig. S5b, ESI†). Interestingly, 1208.4391 M+ was observed as the main mass value of SANA, and it corresponded with the 1
3 stoichiometric ratio between sceptrin and the Au3+ when a difference in maximum absorption intensity was plotted in the mole fraction of sceptrin (Fig. 4a, ESI†). This result was cross-checked by the mass spectrometry analysis using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF, Fig. S5b, ESI†). Interestingly, 1208.4391 M+ was observed as the main mass value of SANA, and it corresponded with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 stoichiometric ratio. Next, we also asserted the binding constant of sceptrin toward the Au3+ (Fig. S5c, ESI†) using the absorption plot and the Benesi–Hildebrand relation.32 The binding constant (Kb) was determined by linear fitting of the absorption titration curve, and it was 1.58 × 102 M−1. This value is low to maintain the stable monomeric complex of Au–sceptrin, so we concluded that the self-assembly of SMC to SMMC/SMNA is inevitable.
3 stoichiometric ratio. Next, we also asserted the binding constant of sceptrin toward the Au3+ (Fig. S5c, ESI†) using the absorption plot and the Benesi–Hildebrand relation.32 The binding constant (Kb) was determined by linear fitting of the absorption titration curve, and it was 1.58 × 102 M−1. This value is low to maintain the stable monomeric complex of Au–sceptrin, so we concluded that the self-assembly of SMC to SMMC/SMNA is inevitable.
Antibiotic properties of SANA
Before the antibiotic property analysis of SANA, we first assayed the antibiotic effect of sceptrin itself against 10 types of Gram-positive and Gram-negative bacterial strains including drug-resistant bacteria (Table 1). Although a report showed the binding property of sceptrin toward MreB protein within Escherichia coli (E. coli; EC),24 there is no report about the MIC values of sceptrin against drug-sensitive and drug-resistant bacterial strains. In our study, MIC assays of sceptrin against bacteria strains (10 types) were conducted using the broth micro broth dilution method in a 96-well plate (Fig. S6, ESI†). The result indicated that sceptrin showed non-inhibition of bacterial growth against EC, KP, EF, SA, CREC, CRKP, CRAB, and CRPA even at high concentration ranges (0–100 μM). Among the tested strains, sceptrin showed a little impact on AB and PA, but it was low to inhibit the bacterial growth (MIC value, AB: 70.8 μM, PA: 75 μM). From the data, we select the hit strains; AB, PA, and drug-resistant strains (CRAB and CRPA), to confirm the antibiotic effect of SANA (Fig. 3a).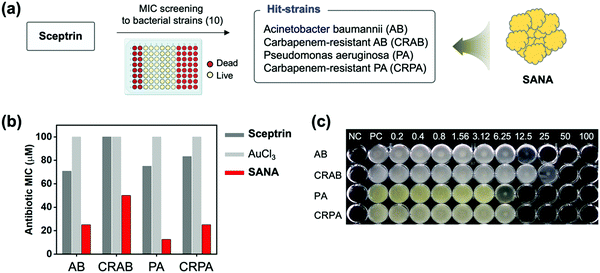 | ||
| Fig. 3 The antibiotic activities of SANA against bacterial strains. (a) Schematic illustration related to the MIC screening of sceptrin against 10 types of bacterial strain (see Table 1 for the strain information). Sceptrin has antibiotic effects on AB and PA strains (Hit-strains) including drug-resistant strains, and SANA was applied for these strains to confirm the enhanced antibiotic effect. (b) MIC values of AuCl3, sceptrin, and SANA toward AB, PA, CRAB, and CRPA strains. These experiments were conducted in quadruplicate. (c) The image of a 96-well plate correlated with the MIC values of SANA against tested strains. Unit: μM. NC: negative control (no bacteria). PC: positive control (non-treated). | ||
First, the MIC assay of SANA against AB, PA, CRAB, and CRPA was evaluated (Fig. 3b and c), and SANA showed a superior antibiotic effect to both strains compared with control sets (sceptrin-treated, AuCl3-treated). In detail, the MIC values of SANA against AB and PA were 25 μM and 12.5 μM, and the MIC values of SANA against CRAB and CRPA were 50 μM and 25 μM. To verify our results, we prepared a mixture of sceptrin and heavy metal ions (Cu2+, Fe2+, Co2+) and confirmed the best results from SANA (Fig. S7, ESI†). The MIC values of SMNA derivatives against AB, PA, CRAB, and CRPA are shown in Table S1 (ESI†). Our data indicate that SANA could be used as a new antibiotic for drug-resistant bacteria. Next, the hemolysis effect of SANA was evaluated to check toxicity for the red blood cells (RBCs), and SANA showed no significant effect even at a high concentration (200 μM) (Fig. S8, ESI†). Such antibiotic and non-hemolysis properties of SANA indicated that it could be used as an antibiotic material in blood without toxicity issues.
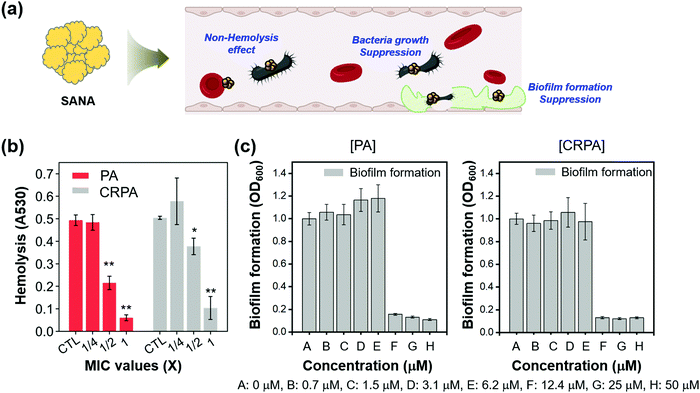
Next, we planned the experiments related to the antibiotic assay of SANA within the blood and the antibiofilm analysis (Fig. 4a). PA and CRPA can often be infected with blood through contaminated medical supplies such as needles in clinical conditions and affect RBCs in the blood mainly disturbing the membrane of RBCs (Fig. S8, ESI,† hemolysis effect of PA and CRPA to RBCs within the blood).33
We hypothesized that the hemolysis effect of PA and CRPA would decrease if SANA has an antibiotic effect against PA and CRPA within the blood, as the biological index of PA and CRPA could decrease by SANA. The effect of SANA on hemolytic suppression activity was investigated after treatment of SANA with the dependent MIC value (0, 1/4 × MIC, 1/2 × MIC, and 1 × MIC) in the presence of PA and CRPA. As seen in Fig. 4b (Fig. S8, ESI†), the hemolysis effect of PA and CRPA significantly decreased within the SANA-treated sets. These data indicate the applicability of SANA to treat blood infections by PA and CRPA. The antibiofilm activity of SANA against PA and CRPA was also examined (Fig. 4c). SANA showed anti-biofilm activity (bar graph) with the MIC value of 12.4 μM against PA and CRPA, which corresponds with the bacterial growth inhibition trend (dot graph).
Additionally, we evaluated the toxicity of SANA toward endothelial cells consisting of blood vessels (Fig. S9, ESI†). SANA showed almost no cellular toxicity (>80% viability) against PA and CRPA at the concentration corresponding to the MIC value (12.5 μM). At the higher concentration (25–50 μM), there was some toxicity (>50% viability), but the value was higher than the Au3+ itself. This result represents the excellent biocompatibility of SANA and its further clinical applicability as an antibiotic/biofilm agent.
Mode of action
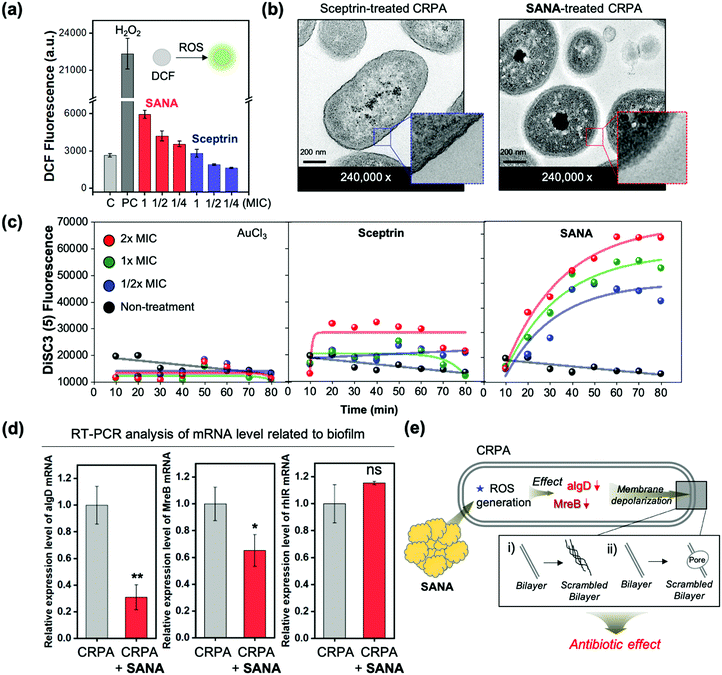
Confirming the antibiotic/antibiofilm activity of SANA against CRPA, we focused on understanding the mode of action (MoA).First, we analyzed the extracellular oxygen species (eROS) level of CRPA after treatment of SANA as such nano-materials generally have MoA based on the membrane damage of bacteria, which has a close correlation with the eROS level. The eROS level was analyzed using the fluorescence method with DCFDA dye. Interestingly, the level of eROS, as primary damage, increased after treatment of SANA (1/4 ×, 1/2 ×, and 1 × MIC; MIC = 25 μM) compared with the PBS-treated set (negative control) and sceptrin-treated set (1/4 ×, 1/2 ×, and 1 × MIC; MIC = 80.3 μM) (Fig. 5a). Like a vicious feedback cycle, the generated eROS could penetrate the bacteria and induce DNA breakage, protein carbonylation, and membrane depolarization, which induces bacterial cell death by amplifying the ROS itself.34
To confirm various aspects of membrane damage (membrane depolarization: scrambled bilayer formation, pore formation), we additionally monitored the morphological changes using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images (Fig. 5b and Fig. S10, S11, ESI†) and fluorescence signal changes of membrane depolarization sensitive dye (DiSC3, dipropylthiadicarbocyanine iodide) after treatment of SANA (1 × MIC, 6 h incubation) to CRPA (Fig. 5c). In the SEM image analysis, the length of the SANA-treated CRPA with a rod shape was markedly reduced (unpaired t-test, vs. non-treatment CRPA; p < 0.001, ***, n = 52) from 1.99 to 1.43 μm, and significant morphology changes was also observed (Fig. S10, ESI†). In the TEM images, the membrane of Gram-negative bacteria had a thick bilayer wall, which was maintained in the sceptrin-treated CRPA (blue box in Fig. 5b), indicating no antibiotic activity of sceptrin itself against CRPA. However, the structure of the scrambled bilayer of the membrane (red box in Fig. 5b) was observed in the SANA-treated CRPA. Generally, a scrambled bilayer could destroy the trans-membrane potential by reducing the viability of the bacteria.35 Thus, the observed scrambling might adversely affect the viability of CRPA. Next, we analyzed the fluorescence signal changes of DiSC3 within CPRA after treatment of AuCl3, sceptrin, and SANA (1/2 ×, 1 ×, and 2 × MIC) to confirm the membrane depolarization (Fig. 5c). DiSC3 could accumulate into the activated bacteria as a fluorescence quenched state, but its fluorescence is recovered when it is released into the extracellular medium via membrane depolarization.36 The AuCl3-treated set did not show significant fluorescence intensity changes of media, indicating that the DiSC3 is accumulated in bacteria. In the case of the sceptrin-treated set, the fluorescence intensity increased (3-fold) after treatment of sceptrin at 2 × MIC. However, the effect was not dependent on the incubation time (0–80 min) or concentration (1/2 ×, 1 ×, and 2 × MIC). However, the SANA-treated set showed increased fluorescence signals as time and concentration increased. Additionally, the disruption of the membrane was confirmed via the protein leakage assay. The bacteria growth curve (OD600) and BCA assay were used to measure the proteins released by disruption of the membrane after treatment of SANA. The measurement of released proteins was performed at the point of lag- and log phase based on the wild-type CRPA (Fig. S12, ESI†). As a result, the protein leakage of the SANA-treatment group was not only higher than in the non-treatment group, but the bacterial growth was also significantly slow in the SANA-treatment group, compared with the non-treatment group.
To explain the effect of SANA, the biotics/biofilm-related mRNA levels of CRPA were analyzed via real-time reverse transcription–polymerase chain reaction (qRT-PCR) (Fig. 5d). As a result, the mRNA level of both MreB and algD was decreased when the CRPA (stationary phase; OD600 ≥ 1.5) was treated with SANA (25 μM). MreB is a main participant in bacterial growth, macromolecular trafficking, and maintenance of cell shape.37,38 In our TEM result (Fig. 5b), a change of morphology was observed, and it is consistent with a decrement in the MreB level. The GDP-mannose 6-dehydrogenase (GDP-D-mannose) encoded by the algD gene is involved in alginate biosynthesis and is one of the key enzymes related to biofilm formation.39 Thus, the decrement of the algD level could be associated with the anti-biofilm effect of SANA. rhlR is a biofilm-related gene, and its mRNA is over-expressed at the top rather than the middle or bottom of the biofilm.40 In our result, the rhlR level tends to increase after treatment of SANA, but it was not significant. As a summary of our results, the SANA could down-regulate the mRNA level related to bacterial morphology, growth, and biofilms as a cascade reaction of ROS, followed by membrane depolarization. We also confirmed the membrane depolarization via observation and experimental measurements such as morphological change, scrambled bilayer, and leakage assays (eROS, proteins, DiSC3). From all experiments related to the antibiotic activities of SANA and MoA analysis, we concluded that SANA could be used as a next-generation nano-antibiotic via membrane depolarization and eROS generation to overcome drug-resistant Gram-negative bacteria.
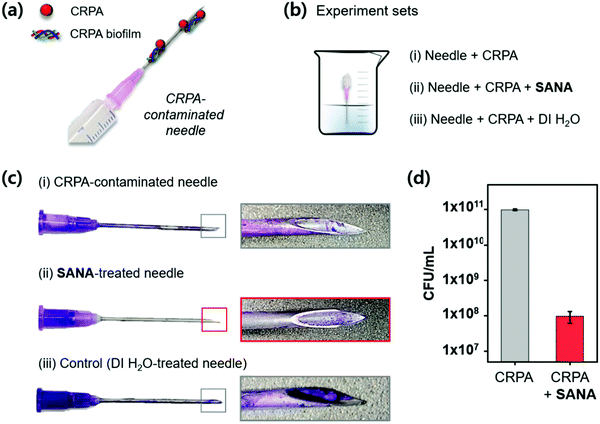
Clinical application
CRPA infection in patients is generally caused by the use of contaminated medical supplies, such as needles (Fig. 6a). To explore the clinical application of SANA, we applied it to the CRPA-contaminated needle expecting the antibiotic and antibiofilm effect. The demonstration was conducted using a needle under three independent conditions (Fig. 6b); (i) needle within CRPA-containing media, (ii) needle within CRPA- and SANA-containing media, and (iii) needle within CRPA- and DI. H2O-containing media. After 12 h incubation, crystal violet (CV) staining was applied, which is commonly used for quantification of biofilm formation.41 As we expected, the CV stained-biofilm of CRPA was not observed in set (ii) even at a low concentration of SANA (1/2 × MIC: 12.5 μM), while a clear CV stained-biofilm of CRPA was observed in set (i) and (iii) (Fig. 6c). Additionally, we asserted the potential for proliferation of CRPA from the contaminated needle after treatment of SANA (1/2 × MIC: 12.5 μM). As shown in Fig. 6d, the colony-forming unit (CFU) assay showed a low activity for proliferation in the group treated with SANA compared with the non-treatment group. From these results, we concluded that SANA has an excellent antibacterial activity to overcome drug-resistant bacteria in the clinical field.3. Conclusions
Herein, we disclosed an approach based on metal–ligand complex formation using the sceptrin drug and a hit formulation of sceptrin–gold (Au) nano-aggregate (SANA) that can kill drug-resistant Gram-negative bacteria as a next-generation nano-antibiotic. Sceptrin has no antibiotic effect on Gram-negative bacteria, but we found that its gold ion complex could have an antibiotic effect for the first time. The preparation method of SANA is simple and effective (mixing sceptrin and AuCl3) and scalable. The systematically acquired characterization data help to understand the macromolecular structure of SANA. SANA showed superior antibiotic/antibiofilm activity toward AB and PA strains, including drug-resistant CRAB and CRPA strains with low toxicity to RBCs and endothelial cells. SANA causes membrane depolarization and eROS generation in the bacteria, which enables it to divert the drug resistance pathway of bacteria. The results in this report reveal the broad applicability of SANA, including its practical application to clinical supplies. This pioneering study presents a new strategy for the development of next-generation nano-antibiotics that holds great potential for bacterial infection therapy.4. Materials and methods
Preparation of SANA
1 mg of sceptrin dihydrochloride (Cat #79703-25-6, SCBT Inc, USA) was dissolved in 144.2 μL of dimethyl sulfoxide (DMSO) with vortex mixing. The AuCl3 solution (10 mM in DI. H2O) was added to a 10 mM sceptrin dihydrochloride solution for final concentrations of 5 mM (DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DI. H2O = 1
DI. H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The mixed solution (bright yellow) was incubated in a shaking incubator (200 rpm) at 37 °C for 1 h. SANA was formed in the given conditions (dark orange). The antibiotic assay was conducted using the as-prepared SANA.
1, v/v). The mixed solution (bright yellow) was incubated in a shaking incubator (200 rpm) at 37 °C for 1 h. SANA was formed in the given conditions (dark orange). The antibiotic assay was conducted using the as-prepared SANA.
MIC Assay
MIC was determined using broth micro broth dilution in cation-adjusted Mueller–Hinton broth (CA-MHB) according to the Clinical and Laboratory Standard Institute (CLSI, 2016) guidelines.42 In this study, we performed the MIC assay for 10 types of strains including MDR bacteria against Au3+, sceptrin, and SANA. Au3+, sceptrin, and SANA were diluted (two-fold) using CA-MHB broth in a round 96-well microplate. The turbidity of all strains was adjusted to a 0.5 McFarland standard (1 × 108 CFU mL−1), 10 μL of bacterial suspension was added to each well of a 96-well microplate, and the final concentration of each strain was approximately 5 × 105 CFU mL−1. The contents of the microplate were mixed well and incubated at 37 °C for 24 h. The lowest concentration of Au3+, sceptrin, and SANA, with no growth, was taken as the MIC value. For the MIC assay, the strain obtained from the microorganism bank was used as the quality control strain. Each experiment was conducted in triplicate or quadruplicate.Biofilm formation assay
Inhibition of biofilm formation was investigated using crystal violet (CV) staining. First, 4 types of bacterial strains (AB, CRAB, PA, CRPA) were cultured in tryptic soy broth (TSB; BD Difco, product no. 211825, Franklin Lakes, NJ, USA) at 37 °C overnight, and then resuspended in fresh TSB to obtain 0.5 McFarland turbidity. After 10-fold dilution in TSB, 200 L TSB with 0.1% glucose and 10 L bacterial suspension (approximately 5 × 105 CFU per well) was seeded into individual wells of a 96-well flat-bottomed polystyrene microwell plate (Corning Costar, product no. 3365, Glendale, ARI. USA). To screen the anti-biofilm activity, SANA was added to the bacterial suspension in concentrations ranging from 0.7–50 μM (2-fold), and the plates were incubated at 37 °C for 24 h. Next, the culture broth and planktonic cells were removed carefully, and the wells were rinsed with phosphate-buffered saline (PBS, pH 7.4) three times and completely dried at 50 °C for 2 h. The dried plates were stained with 1.0% CV at 25 °C for 10 min and gently rinsed with DI. H2O. At this point, the biofilm biomass was observed in a purple ring on the wall of each well. For quantification of the biofilm biomass, 200 μL of 33% glacial acetic acid was added to each well and incubated for 20 min whilst shaking. The optical density of the stained biofilm was measured at 600 nm using a microplate reader (Spark 10M, Tecan, Crailsheim, Germany). Average absorbance for each bacterial strain was determined, and the percentage of the inhibition of biofilm formation was calculated using eqn (1). A biofilm formation assay was performed in three biological replicates, each consisting of two technical replicates.| Inhibition of biofilm formation (%) = 100 × (ODpositive–control – ODexperimental)/ODpositive–control | (1) |
eROS level analysis
The level of extracellular ROS (eROS) was estimated using 2′,7′-dichlorofluorescein diacetate (DCFDA, ab113851, Abcam, UK). CRPA (5 × 105 CFU per well, dark 96-well plate) was initially incubated with different concentrations of Au3+, sceptrin, and SANA (0.5 ×, 1.0 ×, and 2.0 × MIC μg μL−1) at 37 °C for 5 h. The supernatant was obtained at the same volume and then treated with 200 μM of DCFDA in the dark with 30 min incubation. The emission intensity of 2′,7′-dichlorofluorescein (DCF) within the supernatant was recorded at 535 nm using a TECAN microplate reader (Spark 10M, Tecan, Crailsheim, Germany) under the excitation at 503 nm. H2O2 (1 mM) and phosphate buffer (50 mM, pH 7.2) were used as positive and negative controls.Membrane depolarization analysis
The membrane depolarization effect of the Au3+, sceptrin and SANA against CRPA (CCARM 2321) was analyzed using a membrane potential-sensitive fluorescent dye, 3,3′-dipropylthiadicarbocyanine iodide (DiSC3; Sigma-Aldrich, Product No. 53213-94-8, USA), as previously described in our previous work. CRPA was cultured in CA-MHB for 20 h and rinsed three times with PBS (pH 7.4) containing 20 mM glucose (PBS-glu). The rinsed bacteria suspension was adjusted to a 0.5 McFarland turbidity standard (1 × 108 CFU mL−1) in PBS-glu. Aliquots 50 μL of adjusted bacteria suspension were added to a black 96-well flat bottom plate and then incubated with 5 μM DiSC3 at 37 °C for 30 min. After that, CRPA was incubated with Au3+, sceptrin, and SANA at different concentrations (0.5 ×, 1.0 ×, and 2.0 × MIC) for 80 min, and the fluorescence intensity of DiSC3 was monitored every 5 min for 80 min using a TECAN microplate reader (Spark 10M, Tecan, Crailsheim, Germany) at an excitation wavelength of 622 nm and an emission wavelength of 670 nm.Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Kyunghee University and approved by the Animal Ethics Committee of Kyunghee University (Approval number; KHSASP-20-225).Conflicts of interest
The authors declare the following competing financial interest(s): The authors are listed as inventors on a pending patent application related to the technology described in this work.Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education (NRF-2018-R1A6A1A03025124). This research was also supported by the Bio & Medical Technology Development Program of the NRF of Korea funded by the Ministry of Science & ICT (NRF-2022-M3A9H1014157, NRF-2021-M3A9I5030523) and a grant from Korea Health Technology R&D Project of the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI21C0239).References
- M. A. Farha and E. D. Brown, Nat. Microbiol., 2019, 4, 565–577 CrossRef CAS PubMed.
- F. Prestinaci, P. Pezzotti and A. Pantosti, Pathog. Global Health, 2015, 109, 309–318 CrossRef PubMed.
- C. L. Ventola, Pharm. Ther., 2015, 40, 277–283 Search PubMed.
- N. Goel, R. Ahmad, H. Fatima and S. K. Khare, Med. Drug Discovery, 2021, 10, 100089 Search PubMed.
- A. Gupta, S. Mumtaz, C.-H. Li, I. Hussain and V. M. Rotello, Chem. Soc. Rev., 2019, 48, 415–427 RSC.
- I. A. Dutescu and S. A. Hillier, Infect. Drug Resist., 2021, 14, 415–434 CrossRef PubMed.
- C. Liu, J. Shin, S. Son, Y. Choe, N. Farokhzad, Z. Tang, Y. Xiao, N. Kong, T. Xie, J. S. Kim and W. Tao, Chem. Soc. Rev., 2021, 50, 2260–2279 RSC.
- V. Tiwari, N. Mishra, K. Gadani, P. S. Solanki, N. A. Shah and M. Tiwari, Front. Microbiol., 2018, 9, 1218 CrossRef PubMed.
- N. Bouazizi, J. Vieillard, B. Samir and F. Le Derf, Polymers, 2022, 14 DOI:10.3390/polym14030378.
- N. Hao, K. W. Jayawardana, X. Chen and M. Yan, ACS Appl. Mater. Interfaces, 2015, 7, 1040–1045 CrossRef CAS PubMed.
- X. Li, S. M. Robinson, A. Gupta, K. Saha, Z. Jiang, D. F. Moyano, A. Sahar, M. A. Riley and V. M. Rotello, ACS Nano, 2014, 8, 10682–10686 CrossRef CAS PubMed.
- A. Balfourier, J. Kolosnjaj-Tabi, N. Luciani, F. Carn and F. Gazeau, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 22639–22648 CrossRef CAS PubMed.
- S. Eckhardt, P. S. Brunetto, J. Gagnon, M. Priebe, B. Giese and K. M. Fromm, Chem. Rev., 2013, 113, 4708–4754 CrossRef CAS PubMed.
- A. Cipres, D. P. O’Malley, K. Li, D. Finlay, P. S. Baran and K. Vuori, ACS Chem. Biol., 2010, 5, 195–202 CrossRef CAS PubMed.
- R. P. Walker, D. J. Faulkner, D. Van Engen and J. Clardy, J. Am. Chem. Soc., 1981, 103, 6772–6773 CrossRef CAS.
- U. Bickmeyer, C. Drechsler, M. Köck and M. Assmann, Toxicon, 2004, 44, 45–51 CrossRef CAS PubMed.
- L. Freire Boullosa, J. Van Loenhout, T. Flieswasser, J. De Waele, C. Hermans, H. Lambrechts, B. Cuypers, K. Laukens, E. Bartholomeus, V. Siozopoulou, W. H. De Vos, M. Peeters, E. L. J. Smits and C. Deben, Redox Biol., 2021, 42, 101949 CrossRef CAS PubMed.
- S. Kang, K. Sunwoo, Y. Jung, J. K. Hur, K.-H. Park, J. S. Kim and D. Kim, Antibiotics, 2020, 9, 758 CrossRef CAS PubMed.
- J. M. An, Y. Ju, J. H. Kim, H. Lee, Y. Jung, J. Kim, Y. J. Kim, J. Kim and D. Kim, J. Mater. Chem. B, 2021, 9, 4015–4023 RSC.
- R. H. Kang, J. Park, J. Kim, T. Chowdhury, J. H. Oh, J. Kim, J. Shin, M. Kim, C. K. Park, S. Lee, J. Y. Lee and D. Kim, ACS Biomater. Sci. Eng., 2021 DOI:10.1021/acsbiomaterials.1c00653.
- M.-C. Ríos, N.-F. Bravo, C.-C. Sánchez and J. Portilla, RSC Adv., 2021, 11, 34206–34234 RSC.
- P. Padnya, V. Gorbachuk and I. Stoikov, Int. J. Mol. Sci., 2020, 21, 1425 CrossRef CAS PubMed.
- Y. Li, C. Wang, S. Ma, H. Zhang, J. Ou, Y. Wei and M. Ye, ACS Appl. Mater. Interfaces, 2019, 11, 11706–11714 CrossRef CAS PubMed.
- A. D. Rodríguez, M. J. Lear and J. J. La Clair, J. Am. Chem. Soc., 2008, 130, 7256–7258 CrossRef PubMed.
- S. Hussain, C. N. Wivagg, P. Szwedziak, F. Wong, K. Schaefer, T. Izoré, L. D. Renner, M. J. Holmes, Y. Sun, A. W. Bisson-Filho, S. Walker, A. Amir, J. Löwe and E. C. Garner, eLife, 2018, 7, e32471 CrossRef PubMed.
- S. Mourdikoudis, R. M. Pallares and N. T. K. Thanh, Nanoscale, 2018, 10, 12871–12934 RSC.
- N. T. K. Thanh, N. Maclean and S. Mahiddine, Chem. Rev., 2014, 114, 7610–7630 CrossRef CAS PubMed.
- C.-C. Liang, M.-Y. Liao, W.-Y. Chen, T.-C. Cheng, W.-H. Chang and C.-H. Lin, Opt. Express, 2011, 19, 4768–4776 CrossRef CAS PubMed.
- J. D. S. Newman and G. J. Blanchard, Langmuir, 2006, 22, 5882–5887 CrossRef CAS PubMed.
- A. Padermshoke, Y. Katsumoto, H. Sato, S. Ekgasit, I. Noda and Y. Ozaki, Spectrochim. Acta, Part A, 2005, 61, 541–550 CrossRef PubMed.
- J. S. Renny, L. L. Tomasevich, E. H. Tallmadge and D. B. Collum, Angew. Chem., Int. Ed., 2013, 52, 11998–12013 CrossRef CAS PubMed.
- I. D. Kuntz, F. P. Gasparro, M. D. Johnston and R. P. Taylor, J. Am. Chem. Soc., 1968, 90, 4778–4781 CrossRef CAS.
- Y. Y. Hu, J. M. Cao, Q. Yang, S. Chen, H. Y. Lv, H. W. Zhou, Z. Wu and R. Zhang, Emerging Infect. Dis., 2019, 25, 1861–1867 CrossRef CAS PubMed.
- H. Li, X. Zhou, Y. Huang, B. Liao, L. Cheng and B. Ren, Front. Microbiol., 2021, 11, 622534 CrossRef PubMed.
- L. Dietel, L. Kalie and H. Heerklotz, Biophys. J., 2020, 119, 767–779 CrossRef CAS PubMed.
- J. D. te Winkel, D. A. Gray, K. H. Seistrup, L. W. Hamoen and H. Strahl, Front. Cell Dev. Biol., 2016, 4, 29 Search PubMed.
- R. Carballido-López, MMBR, Microbiol. Mol. Biol. Rev., 2006, 70, 888–909 CrossRef PubMed.
- R. M. Figge, A. V. Divakaruni and J. W. Gober, Mol. Microbiol., 2004, 51, 1321–1332 CrossRef CAS PubMed.
- P. J. Tatnell, N. J. Russell and P. Gacesa, Microbiology, 1994, 140, 1745–1754 CrossRef CAS PubMed.
- A. C. Pérez-Osorio, K. S. Williamson and M. J. Franklin, J. Bacteriol., 2010, 192, 2991–3000 CrossRef PubMed.
- G. A. O’Toole, J. Visualized Exp., 2011, 47, 2437–2438 Search PubMed.
- F. R. Cockerill, M. Wikler, J. Alder, M. Dudley, G. Eliopoulos, M. Ferraro, D. Hardy, D. Hecht, J. Hindler and J. Patel, Clin. Lab. Stand. Inst., 2012, 32, M07–A09 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nh00279e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |