A visible light driven 3D hierarchical CoTiO3/BiOBr direct Z-scheme heterostructure with enhanced photocatalytic degradation performance†
Lijie
Wang
ab,
Qiang
Li
 *ab,
Xiaoxiao
Lu
ab,
Zhenfei
Tian
ab,
Shiwu
He
ab and
Jinfeng
Zhang
ab
*ab,
Xiaoxiao
Lu
ab,
Zhenfei
Tian
ab,
Shiwu
He
ab and
Jinfeng
Zhang
ab
aCollege of Physics and Electronic Information, Huaibei Normal University, Huaibei, 235000, P. R. China. E-mail: qiangli@chnu.edu.cn
bAnhui Province Key Laboratory of Pollutant Sensitive Materials and Environmental Remediation, Huaibei Normal University, Huaibei, 235000, P. R. China
First published on 17th November 2021
Abstract
A series of novel three-dimensional (3D) CoTiO3/BiOBr (CTBB) hierarchical heterostructures were prepared via a simple hydrothermal method. In comparison with pure CoTiO3 and BiOBr, all the CTBB nanocomposites display enhanced photocatalytic performance toward dye decomposition. Particularly, CTBB-5 reveals the best photocatalytic efficiency for RhB removal with a K of 0.2030 min−1, which is about 50.75 and 3.02-fold higher than that of pure CoTiO3 and BiOBr, respectively. The outstanding activity is further demonstrated by MO photodegradation. The dramatically enhanced activity can be attributed to the increased surface area and reduced recombination probability of charge carriers. Furthermore, CTBB-5 exhibits excellent stability after repetitively running four times. The h+ and ˙O2− are identified as the dominating reactive species contributing to the oxidation reaction. Finally, a possible Z-scheme charge transfer mechanism is presented and analyzed in detail.
1. Introduction
With the rapid development of the printing and dyeing industry, dye wastewater has become one of the sources of water pollution. Printing and dyeing wastewater has the characteristics of high toxicity, high alkalinity, and large total amount.1 Moreover, it is very difficult to purify.2–4 Chemical coagulation, physical adsorption and biological treatment methods have been used to solve the problem of wastewater pollution. However, the cost of these technologies is very high, and their practical use is difficult. Due to its potentially energy-efficient, low-cost and environment friendly nature, semiconductor photocatalysis technology has been widely used in environmental remediation.5–7A variety of photocatalysts have been developed, such as TiO2,8 Bi2MoO6,9 CeO2,10 BiOI,11 WO3,12 and metal chalcogenide semiconductor.13–17 In particular, more and more research studies are focusing on BiOBr photocatalytic materials,18–20 due to their excellent stability and high activity.21 BiOBr is a medium band gap (∼2.7 eV) semiconductor,22 which can respond to visible light. In addition, the [Bi2O2]2+ slabs and Br− slabs in BiOBr make it a unique layered structure, which could suppress the recombination of photo-generated carriers and increase the photocatalytic performance.23 However, the performance of single BiOBr photocatalyst was unsatisfactory due to the rapid carrier recombination rate and relatively small photoresponse range to visible light.24 Constructing a heterojunction has been acknowledged as an effective means to increase the activity of a single-phase catalyst because it can broaden the light absorption range and strengthen the separation of photogenerated charge carriers.25–27 For instance, Zhang et al. fabricated flower-like Ag3VO4/BiOBr via a simple chemical deposition method and Ag3VO4/BiOBr exhibited improved photocatalytic activity compared to single BiOBr and Ag3VO4 toward the removal of dye.28 Recently, CoTiO3 has drawn extensive attention in constructing heterojunctions because of its narrow band gap (∼2.25 eV) and suitable band structure.29–31 Hence, CoTiO3 was selected to build heterojunction with BiOBr to improve the photocatalytic efficiency. For instance, Mao et al. synthesized the CoTiO3/BiOBr heterojunction by the precipitation–deposition method, which enhanced the performance of the photocatalyst to degrade RhB.32
The photocatalyst with a hierarchical structure has aroused the attention of many researchers since the unique hierarchical structure can not only enlarge the light absorption range, but also increase the specific surface area.33 In addition, the photocatalyst with a hierarchical structure is very stable and can ensure a high quantum yield.34 High-efficiency photocatalysts with a hierarchical structure have been widely reported, like AgIO3/BiVO4,35 N-TiO2/Bi2MoO6,36 AgBr/Bi2WO6,37etc. Therefore, in-depth research on hierarchical photocatalysts will promote the development of photocatalytic technology.
Herein, the 3D hierarchical CoTiO3/BiOBr (CTBB) heterostructures were prepared by loading 2D BiOBr nanosheets on the surface of CoTiO3 microrods via a simple solvothermal method. The CTBB composites broaden optical absorption and promote the fast separation of charge. Therefore, CTBB-5 composite displays superior photocatalytic performance toward degradation of RhB and MO compared to pure CoTiO3 and BiOBr under visible light illumination. In addition, the effects of some key operating parameters are examined. Finally, the mechanism of charge carrier transfer and photocatalytic reaction are revealed.
2. Experimental
2.1 Synthesis of CoTiO3 powder
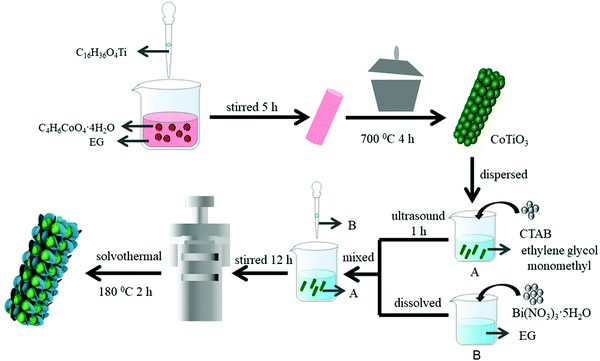
CoTiO3 was synthesized by a coprecipitation method based on a previous report.38 Firstly, 2.49 g of Co(CH3COO)2·4H2O was added to 60 ml of ethylene glycol (EG), and then 3.4 ml of titanium butoxide was added to the above solution under magnetic stirring. The reaction was continued under vigorous stirring for 5 h. The precursors were gathered by alternative centrifugation and cleaned with ethyl alcohol three times. The wet products were dried at 60 °C for a whole night under vacuum. Finally, CoTiO3 microrods were gained by calcining the precipitates at 700 °C in air.2.2 Preparation of composite materials
The CTBB composites were prepared by a simple solvothermal process, as shown in Scheme 1. Typically, 4 mmol CTAB was added to 50 mL of ethylene glycol monomethyl ether. Then, a certain amount of CoTiO3 was dispersed in the above solution by ultrasonic treatment for one hour to generate a homogeneous solution (solution A). Meanwhile, 4 mmol Bi(NO3)3·5H2O was added to glycol to form solution B. Then, the solutions A and B were thoroughly mixed and stirred for 12 hours. The homogeneous solution was shifted to a 100 ml Teflon-lined vessel, followed by heat-treatment at 180 °C for 2 h. Eventually, the CTBB precipitate was gathered by centrifugation and washed with ethyl alcohol and distilled water. Then, the sample was dried in air at 343 K for a whole night. Since the theoretical weight percentages of CoTiO3 were 2%, 5% and 15% in CTBB composites, the as-synthesized samples were labeled as CTBB-2, CTBB-5 and CTBB-15, respectively. Pure BiOBr was prepared by the same method without CoTiO3.2.3 Characterization
X-ray diffraction (XRD) patterns were recorded using an X-ray diffractometer (Empyrean, PANalytical) with Cu Kα radiation. The morphologies of the samples were probed by scanning electron microscopy (FESEM, SU8220, Japan). Transmission electron microscopy (TEM) images of the prepared catalyst were recorded using a JEM 2100 microscope. An X-ray photoelectron spectrometer (XPS, Axis Supra+) was employed to explore the chemical composition and chemical states of CTBB composites. The UV-vis diffuse reflectance spectroscopy (DRS) was performed using a UV-vis spectrophotometer (PerkinElmer Lambda 950). Using a PLS920 fluorescence spectrometer, the photoluminescence (PL) emission spectrum was obtained. The BET surface areas of the samples were analyzed using Micrometrics ASAP 2460.2.4 Photocatalytic activity test
The photocatalytic performance of the catalyst was explored by removing RhB (15 mg L−1) and MO (10 mg L−1). A 500 W xenon lamp equipped with a 400 nm filter was used as the light source. The typical photodegradation process was as follows: before irradiation, 100 ml of RhB solution containing 20 mg catalyst was vigorously stirred for one hour in the dark to achieve adsorption–desorption equilibrium. Then, the above suspension was continuously irradiated with visible light under stirring. At specified time intervals, 4 mL of the suspension was taken out and centrifuged to get rid of the photocatalyst. Finally, the concentration variation of RhB and MO was detected using a spectrophotometer at wavelengths of 554 and 465 nm, respectively.2.5 Photoelectrochemical test
The photoelectrochemical performance of the photocatalysts was measured on an electrochemical workstation (CHI 760E, Shanghai Chenhua, China). A standard three-electrode system was used with Ag/AgCl as the reference electrode, Pt wire as the counter electrode and catalyst-modified FTO as the working electrode, which was prepared by smearing the catalyst suspension on an FTO conducting glass, followed by drying under natural conditions. A sodium sulfate solution was employed as the electrolyte.3. Results and discussion
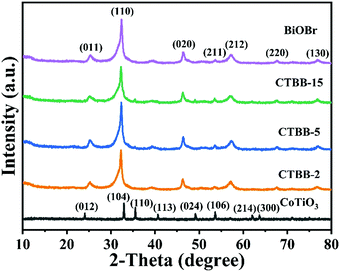
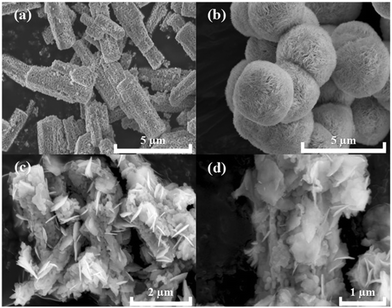
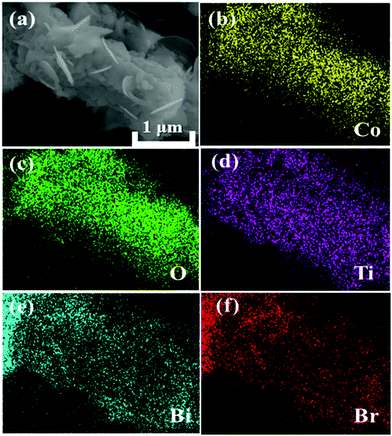
The crystallographic structures of CoTiO3 microrods, BiOBr nanosheets and CTBB composites are exhibited in Fig. 1. The diffraction peaks of BiOBr are ascribed to the tetragonal BiOBr crystalline phase (JCPDS no. 73-2061).39 The 2θ peaks at 25.3°, 32.3°, 46.3°, 53.6°, 57.3°, 67.6° and 76.8°can be well indexed to the crystal planes of (011), (110), (020), (211), (212), (220) and (130), respectively. The diffraction peaks of CoTiO3 can be referenced to the (012), (104), (110), (113), (024), (106), (214) and (300) planes of rhombohedral CoTiO3 (JCPDS no. 15-0866),38 respectively. The characteristic peaks of CTBB composites exhibited the coexistence of both CoTiO3 and BiOBr. Moreover, the peak intensity of the (110) crystal plane of CoTiO3 increased with increase of the CoTiO3 content in CTBB composites. Furthermore, no other diffraction peak was detected in the CTBB samples, suggesting that the purity of CTBB composites was high.The morphology of the as-fabricated materials was characterized by SEM. As displayed in Fig. 2a, pure CoTiO3 appears as microrods with an average length of ∼5 µm and a diameter of ∼2 µm. The CoTiO3 microrods are composed of irregular nanoparticles. The morphology of pristine BiOBr (Fig. 2b) is microspheres with size in the range of 2.0–3.0 µm, which are made up of abundant ultrathin nanosheets. Fig. 2c and d demonstrate the typical SEM images of CTBB-5, many BiOBr nanosheets are anchored on the surface of CoTiO3 microrods. The outcomes of EDX analysis (see Fig. S1, ESI†) show the presence of O, Ti, Co, Br and Bi elements in the CTBB-5 composite. The obtained mass percentages of CoTiO3 (5.25%) are very close to the theoretical value. Fig. 3b–f show the EDS mapping of Co, O, Ti, Bi and Br elements, indicating that BiOBr nanoflakes are uniformly deposited on the surface of CoTiO3 microrods.
In order to study the detailed structure of the CTBB composite, TEM and HRTEM characterization studies were performed. As displayed in Fig. 4a, it is clear that 2D BiOBr nanosheets are tightly attached to the CoTiO3 microrods, which is in line with the outcomes of the SEM observation. The HRTEM image (Fig. 4b) displays that the lattice fringe spacings are 2.72 and 1.95 nm, corresponding to the (104) plane of CoTiO3 and the (020) plane of BiOBr (Fig. 4b), respectively.
XPS measurements were utilized to investigate the surface elemental composition and chemical state of CTBB-5. The peaks of Bi, Br, Ti, Co, and O can be observed in the survey spectrum (Fig. 5a). Fig. 5b displays that the Bi 4f spectrum splits into two peaks at 163.8 and 158.5 eV, corresponding to Bi 4f5/2 and Bi 4f7/2, respectively.40 The Br 3d spectrum in Fig. 5c can be fitted into two peaks at around 67.8 and 68.8 eV, which belong to Br 3d5/2 and Br 3d7/2, respectively.20 In Fig. 5d, the asymmetrical Ti 2p1/2 and 2p3/2 signals are fitted into two peaks at 465.1 and 457.7 eV.30Fig. 5e shows two peaks at 796.0 and 779.3 eV, which can be assigned to Co 2p1/2 and Co 2p3/2, respectively.41,42 In O 1s, it can be viewed that there are two peaks at the binding energies of 529.3 and 531.3 eV, which is due to lattice oxygen and the surface adsorbed oxygen species, respectively.43 The results of XPS characterization indicate that the samples are composed of CoTiO3 and BiOBr.
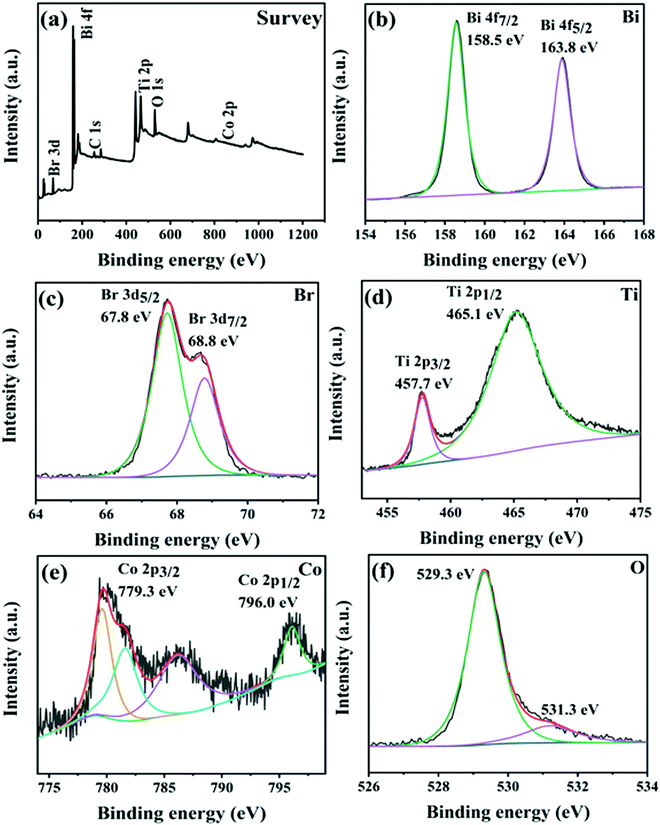 | ||
| Fig. 5 (a) XPS survey spectra of CTBB-5. High-resolution XPS spectra of (b) Bi 4f, (c) Br 3d, (d) Ti 2p, (e) Co 2p and (f) O 1s for CTBB-5. | ||
It is well known that the photoabsorption properties are a key factor related to the photocatalytic performance of the catalyst. Therefore, the UV-vis DRS of all composites were studied. As exhibited in Fig. 6a, BiOBr exhibits an absorption edge at approximately 430 nm, which is similar to the value reported earlier.44 Pure CoTiO3 displays forceful absorption in the visible light range. The O2−–Ti4+ charge transfer interaction would produce sharp areas below 483 nm.38,45 In addition, two absorption bands appear at 537 and 610 nm, which are attributed to the crystal field splitting of CoTiO3.46,47 For CTBB composite, the intensity of light absorption becomes stronger with the increase of CoTiO3, indicating the interaction between CoTiO3 and BiOBr. The band gap energies are calculated according to the following equation:
| αhν = B(hν − Eg)n/2 |
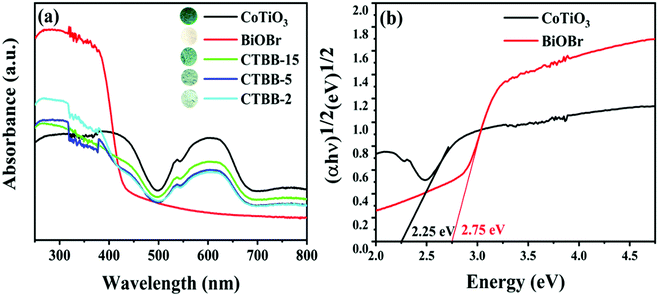 | ||
| Fig. 6 (a) UV-vis diffuse reflectance spectra of all as-prepared photocatalysts and (b) plots of (αhν)1/2versus hν for CoTiO3 and BiOBr. | ||
To probe the BET surface area of the catalyst, the N2 adsorption–desorption isotherm of the samples was measured. As displayed in Fig. 7, the N2 adsorption–desorption isotherm is in accord with the type-IV isotherm, suggesting that the material has a mesoporous structure. The specific surface areas of pure CoTiO3, BiOBr and CTBB-5 are 8.32, 30.98 and 43.51 m2 g−1, respectively. The specific surface area of CTBB-5 is 5.2 and 1.4 times higher than that of CoTiO3 and BiOBr, respectively. Therefore, the CTBB-5 composite can supply more active sites for photocatalytic reaction, which is conducive to mineralizing of pollutants.
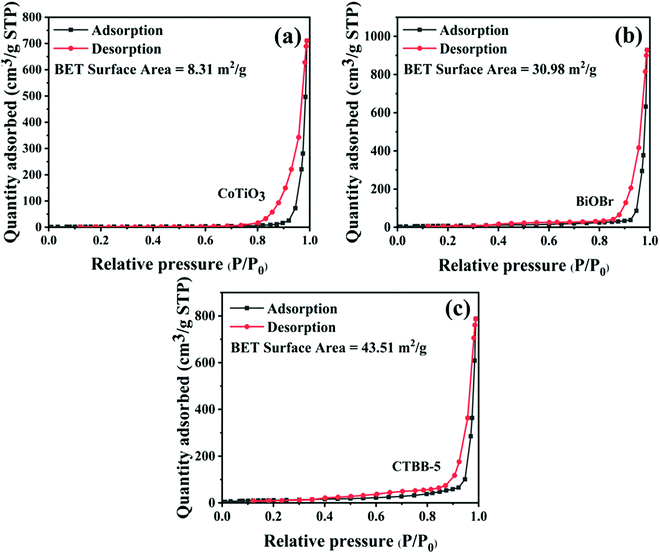 | ||
| Fig. 7 Nitrogen adsorption–desorption isotherm curves of (a) CoTiO3, (b) BiOBr and (c) CTBB-5 composites. | ||
The activities of the samples were assessed by photocatalytic degradation of RhB and MO. Before photodegradation tests, adsorption–desorption equilibrium is achieved by stirring for 60 min in the dark (see Fig. S2, ESI†). Among them, CTBB-5 shows the highest adsorption efficiency, which could be due to the larger specific surface areas. Fig. 8a shows that the degradation of RhB is negligible without catalyst, suggesting RhB is stable. When BiOBr and CoTiO3 are introduced, the degradation efficiency reaches 86.8% and 12.0% in 25 minutes, respectively. Compared to pure BiOBr and CoTiO3, CTBB composites show superior photocatalytic performance. The degradation rate of RhB increases to 98.7%, 99.8% and 97.6% with CTBB-2, CTBB-5 and CTBB-15, respectively. Among them, CTBB-5 exhibits the optimal photocatalytic activity. In addition, the degradation rate over physical mixture is about 71.7%, which is lower than CTBB-5 composite. To study the reaction kinetics of degradation of pollutants by all samples, a pseudo-first-order kinetic model was used.
| ln(C0/Ct) = kt |
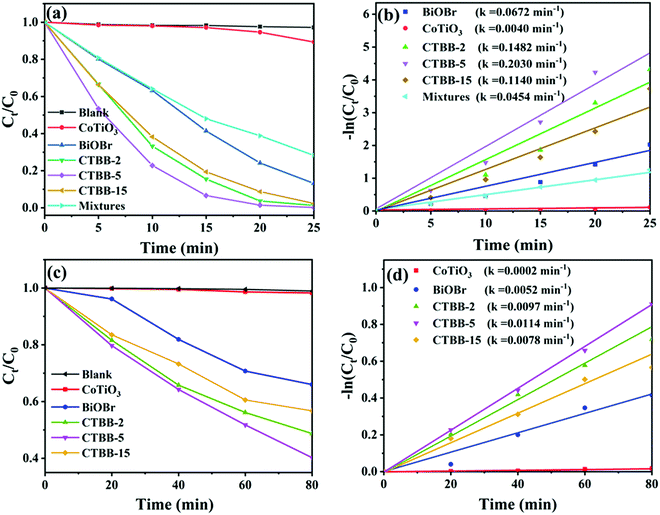 | ||
| Fig. 8 The degradation rates of RhB (a) and MO (c) by photocatalysts under visible light irradiation. The reaction rate constants of RhB (b) and MO (d) over photocatalysts. | ||
In addition, the removal efficiency of MO by all photocatalysts was also studied. As demonstrated in Fig. 8c, the degradation rate of MO by all samples follows the order: CTBB-5 (59.8%) > CTBB-2 (51.3%) > CTBB-15 (42.3%) > BiOBr (34.0%) > CoTiO3 (1.7%). Moreover, the rate constant of CTBB-5 towards MO is 0.0114 min−1, which is 56.75, 2.17, 1.17 and 1.45 times higher than those of CoTiO3 (0.0002 min−1), BiOBr (0.0052 min−1), CTBB-2 (0.0097 min−1) and CTBB-15 (0.0078 min−1), respectively. The result is consistent with the upshot of RhB degradation.
The effect of concentration and initial pH value of RhB solutions on the activity of CTBB-5 was investigated. Fig. 9a exhibits that when the concentration of RhB increases from 15 to 30 mg L−1, the decomposition efficiency of RhB by CTBB-5 decreases from 99.8% to 83.0%. This could be due to the reason that a high concentration of RhB could block visible light absorption by CTBB-5, which reduces the number of photogenerated charge carriers, causing the decline of photoactivity. To study the effect of initial pH on the photocatalytic efficiency, HCl and NaOH were employed to adjust the pH of the RhB solution to 3, 7, 9 and 11; the pH of the original RhB solution is approximately 5. As displayed in Fig. 9b, the degradation efficiency of RhB decreases with the increase of pH values. This phenomenon could be explained as follows: at a higher pH, abundant OH− strongly combines with cationic RhB molecules, inhibiting them from adsorbing onto the catalyst surface.50 While at a lower pH, the degradation of RhB is realized predominately by h+; therefore, at pH = 3, CTBB-5 achieves the highest degradation efficiency.
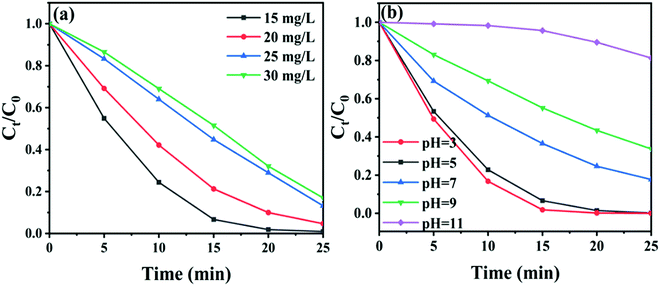 | ||
| Fig. 9 (a) The influence of different RhB concentrations on the photodegradation process, (b) the influence of different pH values on the photodegradation process. | ||
Photoluminescence (PL) spectra were obtained to study charge separation. Generally, a lower PL intensity implies better separation efficiency of charge and superior photocatalytic performance.51Fig. 10a demonstrates the PL peaks of CoTiO3, BiOBr and CTBB are concentrated at around 460 nm and the PL intensity of CTBB-5 is much lower than those of CoTiO3 and BiOBr, indicating that the charge recombination is effectually restrained in the CTBB-5 composite. Transient photocurrent can be utilized to evaluate the separation and transfer efficiency of photoinduced charge carriers. A higher photocurrent reveals the greater separation probability of photogenerated carriers.52,53Fig. 10b displays the photocurrent response of pure CoTiO3, BiOBr and CTBB-5. Among them, CTBB-5 shows the highest photocurrent, suggesting more efficient separation of electron–hole pairs in CTBB-5, which coincides with the results of PL. These results imply that the combination of CoTiO3 and BiOBr could greatly enhance the separation of photogenerated carriers.
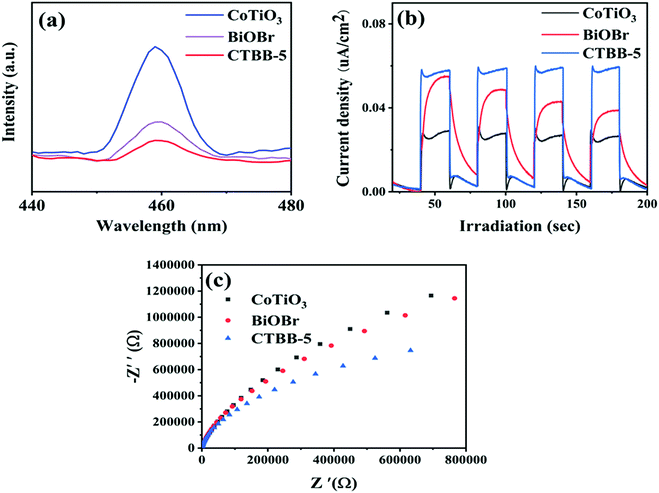 | ||
| Fig. 10 (a) PL spectra, (b) photocurrent response and (c) EIS Nyquist plots of the as-fabricated photocatalysts. | ||
To further study the transfer of charge in the photocatalyst, the EIS spectra of pure CoTiO3, BiOBr and CTBB-5 were obtained (Fig. 10c). The smaller the arc radius on the EIS Nyquist curve, the faster the transfer of photogenerated charges.54 It is clear that the arc radius of CTBB-5 composite is significantly smaller than that of CoTiO3 and BiOBr, indicating that the assembly of BiOBr and CoTiO3 is conducive to accelerating the transfer of photogenerated charge.
To explore the reusability and stability of the CTBB composite, a four-cycle test was carried out by degrading RhB over CTBB-5 composite. As shown in Fig. 11a, CTBB-5 still retains a high photocatalytic efficiency (93.6%) after four photocatalytic cycles. Furthermore, the used CTBB-5 shows negligible changes in the phase (Fig. 11b) and chemical state (see Fig. S3, ESI†) compared with the fresh one. These results manifest that the CTBB-5 composite possesses high stability.
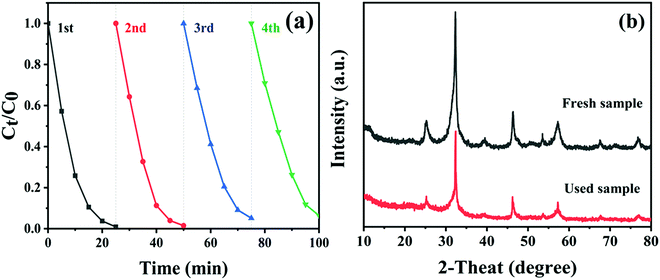 | ||
| Fig. 11 (a) The photostability of CTBB-5 over 4 runs. (b) XRD patterns of CTBB-5 composite before and after 4 runs. | ||
To detect the major active substances involved in the photocatalytic removal of RhB dyes by CTBB-5, isopropanol (IPA), triethanolamine (TEOA) and p-benzoquinone (BQ) were used as the capture agents for hydroxyl radical (˙OH), hole (h+) and superoxide radicals (˙O2−), respectively.55 As shown in Fig. 12a, the degradation rate of RhB hardly changes after the addition of IPA, indicating that the effect of ˙OH on RhB decomposition is negligible. Nevertheless, when TEOA and BQ are introduced into the RhB solution, the degradation rate declines to 76.5% and 14.4% (Fig. 12b), respectively. These results imply that both h+ and ˙O2− are the dominating active substances in the process of RhB degradation.
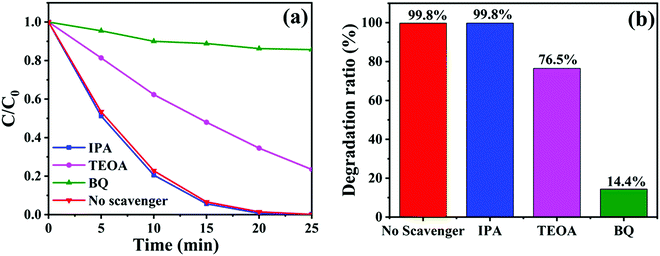 | ||
| Fig. 12 (a) Degradation curves and (b) degradation ratios of RhB by CTBB-5 in the presence of different scavengers. | ||
The intermediates generated (see Fig. S4, ESI†) during the photocatalytic reaction for RhB degradation were analyzed by LC-MS. Based on the identified intermediates and previous studies,56–58 a possible degradation pathway (see Fig. S5, ESI†) was formulated. Firstly, the RhB molecules were oxidized by active radicals and released ethyl groups; a series of N-deethylated intermediates R1, R2, R3 and R4 could be successively generated. Subsequently, product R4 could be converted to intermediate R5 by releasing benzene ring and functional groups. Eventually, product R5 would be continuously attacked and mineralized into CO2 and H2O molecules.
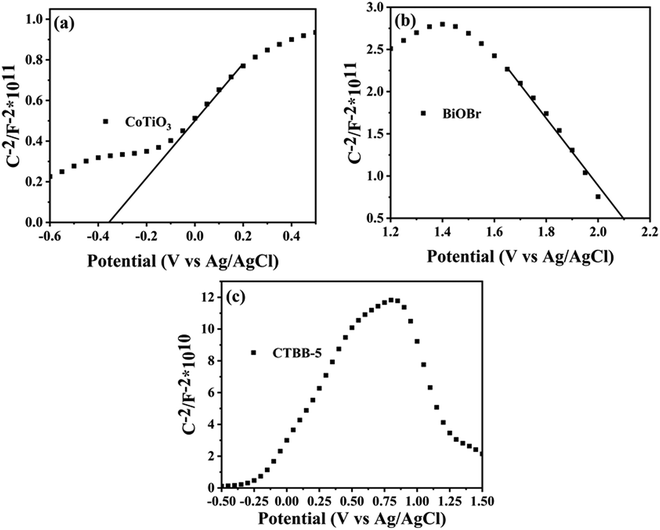
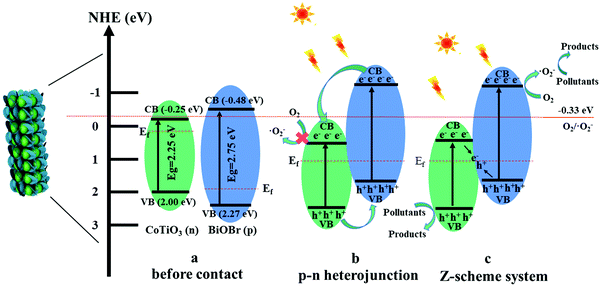
To study the conduction type of samples, the Mott–Schottky experiments were carried out. The positive slope of the plot suggests that CoTiO3 is an n-type semiconductor (Fig. 13a), while the negative slope indicates that BiOBr (Fig. 13b) is a p-type semiconductor, which coincide with the previous reports.44,59 It is worth noting that a reversed V shape curve is obtained for CTBB-5 (Fig. 13c), implying the generation of p–n junction between BiOBr and CoTiO3.60 The Fermi levels (Ef) of CoTiO3 and BiOBr are −0.35 and 2.17 eV (vs. Ag/AgCl), respectively. The electrode potential vs. Ag/AgCl can be converted to normal hydrogen electrode (NHE) potential by using Nernst equation: ENHE = EAg/AgCl + 0.197 eV.61 According to the reports, the valence band (VB) is 0.1 eV lower than Ef for p-type semiconductors, while the conduction band (CB) is 0.1 eV higher than Ef for n-type semiconductors.61 Therefore, the ECB of CoTiO3 and EVB of BiOBr are calculated to be −0.25 and 2.27 eV vs. NHE. Accordingly, the ECB of BiOBr and EVB of CoTiO3 are −0.48 and 2.0 eV, respectively, based on the formula: Eg = EVB − ECB.
From the above results, a possible photocatalytic mechanism for the degradation of pollutants over the CTBB heterojunction is proposed in Fig. 14. Before connection, the nested band structures of single BiOBr and CoTiO3 are shown in Fig. 14a, which seem unfavorable for the separation of photogenerated charge carriers. However, when BiOBr combines with CoTiO3 to form an effective heterojunction, the electrons migrate from BiOBr to CoTiO3 until an equalization of Ef takes place. The energy bands of BiOBr and CoTiO3 shift upward and downward, respectively. Under irradiation with visible light, both BiOBr and CoTiO3 can be motivated to produce charge. The transfer of the photoexcited charge is schematically described in Fig. 14b and c. If the carriers migrate in the CTBB according to Fig. 14b, the accumulated e− in the CB of CoTiO3 cannot react with oxygen to generate ˙O2− since the redox potential for ˙O2− generation (O2/˙O2− = −0.33 eV)62 is more negative than the ECB of CoTiO3 (−0.25 eV).62 This result is not in keeping with the free radical capture experiment which implies that ˙O2− is a major active substance in the photodegradation reaction. Hence, we deem that the charge carriers in the hybrid catalyst followed a Z-scheme mechanism (Fig. 14c). The photoinduced e− from CoTiO3 directly recombines with h+ in the VB of BiOBr through the tight contact interface, promoting the efficient charge separation. The electrons on the CB of BiOBr could reduce O2 to produce ˙O2− since its CB potential is more negative than E (O2/˙O2−). The EVB of CoTiO3 was more negative than E (OH−/˙OH), so the h+ from CoTiO3 could not oxidize OH− to generate ˙OH. Consequently, both ˙O2− and h+ play a significant role in RhB decomposition, which is in accord with the results of quenching experiments.
4. Conclusions
In summary, 3D hierarchical CTBB hybrid photocatalysts were successfully fabricated through a facile solvothermal method for the first time. A series of characterization results suggest that BiOBr nanoplates are uniformly anchored on the CoTiO3 microrods. The as-obtained CTBB heterostructures present significantly improved photocatalytic performance toward the removal of MO and RhB compared to single CoTiO3 and BiOBr. The amount of CoTiO3 exhibits an apparent effect on the photocatalytic activity of CTBB, where the optimum mass ratio of CoTiO3 is 5.0%. The mineralization ability of RhB and MO by CTBB-5 is 3.02 and 2.17 times higher than that of BiOBr. This significantly improved activity could be mainly due to the increased surface area, enhanced light absorption and efficient charge separation in the CTBB heterostructures. More importantly, the CTBB-5 composite also shows excellent photostability after four runs.Author contributions
Lijie Wang: conceptualization, methodology, formal analysis, validation and writing – original draft. Xiaoxiao Lu: methodology, data curation and formal analysis. Shiwu He: methodology, formal analysis and validation. Zhenfei Tian: methodology and formal analysis. Qiang Li: resources, supervision, funding acquisition, project administration and writing – review & editing. Jinfeng Zhang: funding acquisition and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully appreciate the generosity of the financial support of National College Students Innovation and Entrepreneurship Training Program of China (202010373017), the National Natural Science Foundation of China (51572103) and Natural Science Foundation of Anhui Province (1908085QF293).References
- S. L. Chiam, S. Y. Pung and F. Y. Yeoh, Environ. Sci. Pollut. Res. Int., 2020, 27, 5759–5778 CrossRef CAS.
- R. Xie, K. Fang, Y. Liu, W. Chen, J. Fan, X. Wang, Y. Ren and Y. Song, J. Mater. Sci., 2020, 55, 11919–11937 CrossRef CAS.
- Y. Huang, Y. Zhou and K. Wang, Environ. Sci. Pollut. Res. Int., 2020, 27, 41503–41514 CrossRef CAS.
- M. Hasanpour and M. Hatami, J. Mol. Liq., 2020, 309, 113094 CrossRef CAS.
- Z. Fu, X. Zhao, S. Zhang and Z. Fu, Mater. Chem. Phys., 2021, 259, 124004 CrossRef CAS.
- H. Sun, F. Guo, J. Pan, W. Huang, K. Wang and W. Shi, Chem. Eng. J., 2021, 406, 126844 CrossRef CAS.
- L. Luo, X. Shen, L. Song, Y. Zhang, B. Zhu, J. Liu, Q. Chen, Z. Chen and L. Zhang, J. Alloys Compd., 2019, 779, 599–608 CrossRef CAS.
- M. Miodyńska, A. Mikolajczyk, B. Bajorowicz, J. Zwara, T. Klimczuk, W. Lisowski, G. Trykowski, H. P. Pinto and A. Zaleska-Medynska, Appl. Catal., B, 2020, 272, 118962 CrossRef.
- G. Yang, Y.-A. Zhu, Y. Liang, J. Yang, K. Wang, Z. Zeng, R. Xu and X. Xie, Appl. Surf. Sci., 2021, 539, 148038 CrossRef CAS.
- X. Du, Z. Zhang, H. Chen and P. Liang, Appl. Surf. Sci., 2020, 499, 143939 CrossRef CAS.
- C. Zhang, W. Fei, H. Wang, N. Li, D. Chen, Q. Xu, H. Li, J. He and J. Lu, J. Hazard. Mater., 2020, 399, 123109 CrossRef CAS.
- L. Zhang, X. Hao, Y. Li and Z. Jin, Appl. Surf. Sci., 2020, 499, 143862 CrossRef CAS.
- Z. Zhuge, X. Liu, T. Chen, Y. Gong, C. Li, L. Niu, S. Xu, X. Xu, Z. A. Alothman, C. Q. Sun, J. G. Shapter and Y. Yamauchi, Chem. Eng. J., 2021, 421, 127838 CrossRef CAS.
- X. Liu, B. Liu, L. Li, Z. Zhuge, P. Chen, C. Li, Y. Gong, L. Niu, J. Liu, L. Lei and C. Q. Sun, Appl. Catal., B, 2019, 249, 82–90 CrossRef CAS.
- B. Liu, X. Liu, J. Liu, C. Feng, Z. Li, C. Li, Y. Gong, L. Pan, S. Xu and C. Q. Sun, Appl. Catal., B, 2018, 226, 234–241 CrossRef CAS.
- Z. Shao, X. Meng, H. Lai, D. Zhang, X. Pu, C. Su, H. Li, X. Ren and Y. Geng, Chin. J. Catal., 2021, 42, 439–449 CrossRef CAS.
- D. Kong, X. Ruan, J. Geng, Y. Zhao, D. Zhang, X. Pu, S. Yao and C. Su, Int. J. Hydrogen Energy, 2021, 46, 28043–28052 CrossRef CAS.
- Z. Gao, B. Yao, F. Yang, T. Xu and Y. He, Mater. Sci. Semicond. Process., 2020, 108, 104882 CrossRef CAS.
- Y. Ren, Y. Yang, X. Jing, X. Wang and H. Song, Mater. Lett., 2019, 257, 126681 CrossRef CAS.
- C. Hua, X. Dong, Y. Wang, N. Zheng, H. Ma and X. Zhang, J. Mater. Sci., 2019, 54, 9397–9413 CrossRef CAS.
- M. Yadav, S. Garg, A. Chandra and K. Hernadi, J. Colloid Interface Sci., 2019, 555, 304–314 CrossRef CAS PubMed.
- W. Huang, X. Hua, Y. Zhao, K. Li, L. Tang, M. Zhou and Z. Cai, J. Mater. Sci.: Mater. Electron., 2019, 30, 14967–14976 CrossRef CAS.
- M. Yadav, S. Garg, A. Chandra and K. Hernadi, Ceram. Int., 2019, 45, 17715–17722 CrossRef CAS.
- J. Di, J. Xia, Y. Ge, L. Xu, H. Xu, J. Chen, M. He and H. Li, Dalton Trans., 2014, 43, 15429–15438 RSC.
- J. Xiong, H.-Y. Zeng, C.-R. Chen, G.-F. Xiao and D.-S. An, J. Alloys Compd., 2020, 833, 154898 CrossRef CAS.
- C. Su, D. Zhang, X. Pu, Z. He, X. Hu, L. Li and G. Hu, J. Chem. Technol. Biotechnol., 2021, 96, 2976–2985 CrossRef CAS.
- D. Zhang, X. Liu, S. Wang, B. Fan, Z. Shao, C. Su and X. Pu, J. Alloys Compd., 2021, 869, 159390 CrossRef CAS.
- J. Zhang and Z. Ma, Mol. Catal., 2017, 436, 190–198 CrossRef CAS.
- S. Aynehband, E. Nouri, M. R. Mohammadi and Y. Li, New J. Chem., 2019, 43, 3760–3768 RSC.
- B. Lin, S. Li, Y. Peng, Z. Chen and X. Wang, J. Hazard. Mater., 2021, 406, 124675 CrossRef CAS PubMed.
- Y. Xu, J. Mo, Q. Liu, X. Wang and S. Ding, Catal. Sci. Technol., 2020, 10, 2040–2046 RSC.
- W. Mao, K. Bao, F. Cao, B. Chen, G. Liu, W. Wang and B. Li, Ceram. Int., 2017, 43, 3363–3368 CrossRef CAS.
- L. Zhi, S. Zhang, Y. Xu, J. Tu, M. Li, D. Hu and J. Liu, J. Colloid Interface Sci., 2020, 579, 754–765 CrossRef CAS.
- Z. Chai, X. Pan, F. Cui, Q. Ma, J. Zhang, M. Liu, Y. Chen, L. Li and T. Cui, Mater. Lett., 2020, 275, 128168 CrossRef CAS.
- Y. Si, Y. Chen, Y. Fu, X. Zhang, F. Zuo, T. Zhang and Q. Yan, J. Alloys Compd., 2020, 831, 154820 CrossRef CAS.
- L. Zhang, Q. Shen, L. Yu, F. Huang, C. Zhang, J. Sheng, F. Zhang, D. Cheng and H. Yang, CrystEngComm, 2020, 22, 5481–5490 RSC.
- D. Huang, J. Li, G. Zeng, W. Xue, S. Chen, Z. Li, R. Deng, Y. Yang and M. Cheng, Chem. Eng. J., 2019, 375, 121991 CrossRef CAS.
- R. Ye, H. Fang, Y.-Z. Zheng, N. Li, Y. Wang and X. Tao, ACS Appl. Mater. Interfaces, 2016, 8, 13879–13889 CrossRef CAS.
- H. Cheng, B. Huang, Z. Wang, X. Qin, X. Zhang and Y. Dai, Chem, 2011, 17, 8039–8043 CrossRef CAS.
- S. Li, Z. Wang, X. Xie, G. Liang, X. Cai, X. Zhang and Z. Wang, J. Hazard. Mater., 2020, 391, 121407 CrossRef CAS PubMed.
- N. Kitchamsetti, R. J. Choudhary, D. M. Phase and R. S. Devan, RSC Adv., 2020, 10, 23446–23456 RSC.
- H. Li, Q. Gao, G. Wang, B. Han, K. Xia and C. Zhou, Chem. Eng. J., 2020, 392, 123819 CrossRef CAS.
- L. Zhang, X. Yue, J. Liu, J. Feng, X. Zhang, C. Zhang, R. Li and C. Fan, Sep. Purif. Technol., 2020, 231, 115917 CrossRef CAS.
- Y. L. Ling and Y. Z. Dai, Appl. Surf. Sci., 2020, 509, 145201 CrossRef CAS.
- K. Wangkawong, S. Phanichphant, D. Tantraviwat and B. Inceesungvorn, J. Colloid Interface Sci., 2015, 454, 210–215 CrossRef CAS PubMed.
- J. Zou and W. Zheng, Ceram. Int., 2016, 42, 8198–8205 CrossRef CAS.
- S. Rasalingam and R. T. Koodali, CrystEngComm, 2016, 6, 868–871 RSC.
- J. C. Medina, N. S. Portillo-Vélez, M. Bizarro, A. Hernández-Gordillo and S. E. Rodil, Dyes Pigm., 2018, 153, 106–116 CrossRef CAS.
- L. Ma, G. Wang, C. Jiang, H. Bao and Q. Xu, Appl. Surf. Sci., 2018, 430, 263–272 CrossRef CAS.
- Y. Qiao, X. Meng and Z. Zhang, Appl. Surf. Sci., 2019, 470, 645–657 CrossRef CAS.
- T. Wang, S. Liu, W. Mao, Y. Bai, K. Chiang, K. Shah and J. Paz-Ferreiro, J. Hazard. Mater., 2020, 389, 121827 CrossRef CAS PubMed.
- J. Yu, Z. Chen, L. Zeng, Y. Ma, Z. Feng, Y. Wu, H. Lin, L. Zhao and Y. He, Sol. Energy Mater. Sol. Cells, 2018, 179, 45–56 CrossRef CAS.
- Z. Feng, L. Zeng, Q. Zhang, S. Ge, X. Zhao, H. Lin and Y. He, J. Environ. Sci., 2020, 87, 149–162 CrossRef.
- S. Fu, X. Liu, Y. Yan, L. Li, H. Liu, F. Zhao and J. Zhou, Sci. Total Environ., 2019, 694, 133756 CrossRef CAS.
- Q. Zhang, M. Wang, M. Ao, Y. Luo, A. Zhang, L. Zhao, L. Yan, F. Deng and X. Luo, J. Alloys Compd., 2019, 805, 41–49 CrossRef CAS.
- H. Li, Q. Gao, G. Wang, B. Han, K. Xia and C. Zhou, Appl. Surf. Sci., 2021, 536, 147787 CrossRef CAS.
- J. Chen, X. Xiao, Y. Wang and Z. Ye, Appl. Surf. Sci., 2019, 467-468, 1000–1010 CrossRef CAS.
- Y. Liu, H. Guo, Y. Zhang, X. Cheng, P. Zhou, G. Zhang, J. Wang, P. Tang, T. Ke and W. Li, Sep. Purif. Technol., 2018, 192, 88–98 CrossRef CAS.
- H. Ramezanalizadeh and E. Rafiee, Mater. Sci. Semicond. Process., 2020, 113, 105055 CrossRef CAS.
- D. Zhang, Y. Tang, X. Qiu, J. Yin, C. Su and X. Pu, J. Alloys Compd., 2020, 845, 155569 CrossRef CAS.
- J. Xu, B. Feng, Y. Wang, Y. Qi, J. Niu and M. Chen, Front. Chem., 2018, 6, 393 CrossRef CAS PubMed.
- X. Li, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 2603–2636 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nj04252a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |