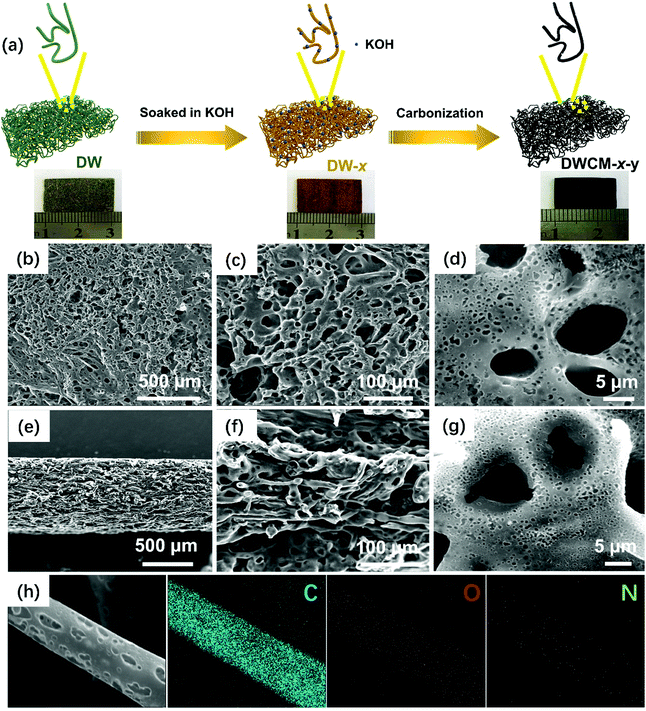
Recycling decoration wastes toward a high-performance porous carbon membrane electrode for supercapacitive energy storage devices†
Mengxia
Cui
abc,
Fang
Wang
abc,
Zhengguo
Zhang
abc and
Shixiong
Min
 *abc
*abc
aSchool of Chemistry and Chemical Engineering, North Minzu University, Yinchuan, 750021, P. R. China. E-mail: sxmin@nun.edu.cn
bKey Laboratory of Chemical Engineering and Technology, State Ethnic Affairs Commission, North Minzu University, Yinchuan, 750021, P. R. China
cNingxia Key Laboratory of Solar Chemical Conversion Technology, North Minzu University, Yinchuan, 750021, P. R. China
First published on 15th November 2021
Abstract
Using discarded wastes as carbon precursors to prepare high value-added carbon electrode materials for supercapacitors (SCs) can not only enrich the selection of precursors to reduce the cost but also alleviate the environmental pressure on recovering these wastes. Herein, a porous carbon membrane (DWCM) was fabricated by direct carbonization of decoration waste using KOH as an activator and employed as a binder-free self-supported electrode for aqueous SC. The DWCM electrode features a large specific surface area (917 m2 g−1) and high porosity (0.5 cm3 g−1) and thus exhibits superior electrolyte wettability. Moreover, the DWCM electrode possesses excellent structural integrity and mechanical strength, providing continuous 3D networks for rapid electron transfer. Attributing to the above structural merits, the best DWCM-1-1000 electrode even with a weight of ∼30 mg exhibits the highest specific capacitance (Cs) (areal capacitance, Ca) of 338.2 F g−1 (10.6 F cm−2) at 0.5 A g−1 in a three-electrode system in 6 M KOH. An assembled symmetric SC using two DWCM-1-1000 electrodes can stably operate at a potential window of 0–1.4 V showing a high Cs (Ca) of 138.3 F g−1 (8.9 F cm−2) at 0.2 A g−1. Notably, this SC shows excellent cycling stability (98% capacitance retention and 100% coulombic efficiency after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) and presents a maximum energy density of 9.7 Wh kg−1 at a power density of 139.6 W kg−1. Also, it is found that reducing the thickness of the electrode will further improve the capacitive performance of the DWCM-based SC device. This work opens a new route of using decoration waste to develop high-performance self-supported porous carbon membranes for practical energy storage.
000 cycles) and presents a maximum energy density of 9.7 Wh kg−1 at a power density of 139.6 W kg−1. Also, it is found that reducing the thickness of the electrode will further improve the capacitive performance of the DWCM-based SC device. This work opens a new route of using decoration waste to develop high-performance self-supported porous carbon membranes for practical energy storage.
Introduction
The development of high-performance and cost-efficient energy storage devices is in great demand to sustainably utilize electricity produced from renewable solar, wind, and hydroenergies.1 Carbon-based supercapacitors (SCs) based on electrochemical double-layer mechanism have attracted increasing attention as a kind of promising electricity storage devices as they possess the merits of high power density, excellent long cycling life, and fast charging/discharging kinetics.2,3 However, limited by the low energy density (<5 Wh kg−1) compared to those of Li-batteries and fuel cells (>200 Wh kg−1), the practical application of carbon-based SCs has been severely suppressed.4 According to the general rule (E = 0.5 × C × (ΔV)),5 there are mainly two ways to enhance the energy density of carbon-based SCs: (1) extending the working potential window using organic or ionic liquid electrolytes and (2) increasing the specific capacitance (C) by developing advanced carbon electrode materials with large specific surface area (SSA) and an abundant hierarchically porous structure. For electrolyte solutions used in SCs so far, compared with aqueous electrolytes, although ionic liquids (ILs)6–8 and organic electrolytes9 typically have wider operating potential windows (>3.0 V),10 they typically display high viscosity and low conductivity especially at lower temperatures and have disadvantages of high toxicity, flammability, and high cost, thus largely limiting their practical applications in commercial SCs.11 Therefore, extensive efforts have been devoted to the development of advanced carbon-based electrode materials with superior capacitive performances. Over the past decades, a number of carbon nanomaterials such as carbon black, carbon nanofibers (CNFs), carbon nanotubes (CNTs), graphene, and graphdiyne have been extensively employed as electrode materials for carbon-based SCs with improved energy density.12–17 However, the complex preparation and thus high cost of the above carbon nanomaterials largely restrict the large-scale commercialization of carbon-based SCs. Therefore, it is still essential to develop efficient carbon electrode materials at lower costs for further improving the practical applicability of SCs.In recent years, porous carbon materials derived from natural biomass, biomass wastes, and other carbon-containing wastes have stood out as promising electrode materials to replace carbons prepared from expensive chemicals for application in SCs due to their reduced cost and high performance. In this context, the natural biomass-derived carbon materials with inherited unique microstructures have shown impressive capacitive performances in SCs.18–22 However, the biological heterogeneity of biomass precursors may result in a large performance uncertainty. In addition, the added value of the biomass-derived carbons is relatively low because the biomass can be effectively transformed into high-value-added chemicals and fuels. In contrast to natural biomass, the massively produced carbon-containing industrial and domestic wastes during the construction of buildings, housing decoration, and food production/processing are a class of more economic carbon sources for the preparation of value-added porous carbons as this is a truly “waste-to-treasure” conversion process.23–25 However, the practical use of waste-derived carbons as electrode materials in SCs has been largely hampered by their powdered status. The powdered carbons have to be cast/coated on conductive current collectors by using polymer binders and additives. The presence of insulated binders in the electrode would not only increase the series resistance for the electron transfer but also limit the electrolyte diffusion by blocking pores as well as exposed surfaces achievable by the porous structure, thus leading to inferior capacitive and rate performance. In addition, the weak interface adhesion of active material/binder/collector would largely reduce the cycling stability of SC devices during long-term use.26–30 Therefore, the practical use of waste-based carbon in SCs still needs a rational design of electrode structure by integrating the microscopic merits of powdered porous carbons with advantages of the macroscopic architecture.
In this work, a robust and hierarchically porous carbon membrane electrode, denoted as DWCM, was fabricated by direct two-step carbonization of housing decoration wastes in the presence of KOH as the activator. The effects of the concentration of KOH and carbonization temperature on the textural structure (specific surface area, pore size and distribution, graphitic degree) of the obtained DWCM electrodes were systematically investigated. The as-fabricated DWCM was directly used as a binder- and additive-free self-supported electrode for assembling an aqueous symmetric SC. Benefiting from the large surface area, high porosity, excellent electrolyte wettability, as well as structural integration, the DWCM electrode exhibits excellent capacitive performance with high specific capacitance (areal capacitance), cycling stability, and energy density superior to most of the reported powdered carbons.
Experimental section
Chemicals and materials
All the reagents were of analytical grade and used as received without further purification. The deposited wooden plastic plates (Fig. S1, ESI†), which are the composite of wood scraps and thermoplastic polymers, were collected during the housing decoration. All solutions were prepared with ultrapure water (18 MΩ cm).Fabrication of decoration waste-based carbon membrane (DWCM)
The decoration waste (DW) was first cut into thin slices (2 cm ×1.5 cm × 1 mm), which were then soaked into KOH solutions of different concentrations (0.1, 0.5, and 1 M) at room temperature for 12 h and then dried in a vacuum oven at 60 °C for 12 h. The as-obtained samples were denoted as DW-x, where x represents the concentration of KOH solution. After drying, the DW-x was pre-carbonized in air at 260 °C for 6 h and then carbonized at different carbonization temperatures (800, 900, 1000, 1100, and 1200 °C) for 2 h with a ramping rate of 5 °C min−1 under a flow Ar gas (40 mL min−1). The resulting carbon membrane electrodes were denoted as DWCM-x-y, where y represents the carbonization temperature. After cooling to room temperature under the protection of Ar atmosphere, the as-obtained black carbon membrane DWCM-x-y was carefully polished with 2000 grit sandpaper to obtain a slice of ∼800 μm thickness, washed with ethanol and water three times to remove the residual carbon, and dried in a vacuum oven at 60 °C for 12 h.Characterizations
X-ray diffraction (XRD) was used to analyze the phase structure of the membrane electrodes using a Rigaku Smartlab diffractometer with a Ni filtrated Cu Kα radiation in the 2θ range of 5–80° with a scanning rate of 5° min−1. Scanning electron microscopy (SEM) images to study the morphologies and microstructures of the membrane electrodes were taken using a ZEISS EVO 10 microscope. The compositions of the membrane electrodes were analyzed using energy-dispersive X-ray spectroscopy (EDX) attached to the SEM instrument. Transmission electron microscopy (TEM) images were collected on an FEI Talos F200X transmission electron microscope. X-ray photoelectron spectroscopy (XPS) was carried out using a Thermo Scientific Escalab-250Xi XPS spectrometer equipped with a monochromatic Al Kα X-ray source to analyze the chemical compositions and valence states of the membrane electrodes. Raman spectra of the membrane electrodes were collected using a Horiba Evolution Raman spectrometer with a 532 nm laser as an excitation source. Micromeritics ASAP 2460 at 77 K was used to calculate the specific surface area of the electrodes using the Brunauer–Emmett–Teller (BET) equation. The compression tests were carried out using a single-column system (HZ-1003) at a constant loading speed of 3 mm min−1. Thermogravimetric (TG) measurements were conducted using an STA 449 F5 Jupiter simultaneous thermal analyzer at a heating rate of 5 °C min−1 from 30 to 1200 °C under a nitrogen atmosphere.Electrochemical measurements
The electrochemical tests were performed on a CorrTest3103 (CorrTest, Wuhan, China) electrochemical workstation in a three-electrode system with Pt sheet and Ag/AgCl as the counter and reference electrodes, respectively. The as-fabricated DWCM-x-y electrodes were directly used as the working electrode. The capacitance performances of the DWCM-x-y electrodes were evaluated using cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) tests in 6 M KOH aqueous solution at a potential range of −1 to 0 V vs. Ag/AgCl. Electrochemical impedance spectroscopy (EIS) measurements were recorded in the frequency range from 0.01 Hz to 100 kHz with an amplitude of 10 mV. The specific capacitance (Cs in F g−1) and areal capacitance (Ca in F cm−2) of DWCM-x-y electrodes were calculated from the discharge curves using the following eqn (1) and (2):31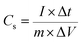 | (1) |
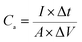 | (2) |
Moreover, a symmetric SC was assembled in a CR2025 stainless steel coin cell with two nearly identical DWCM-x-y electrodes using a cellulose membrane as the separator and 6 M KOH aqueous solution as the electrolyte. The cycling stability of the assembled SCs was tested using a LANHE CT3001A (Wuhan Land Co., China). The specific capacitance (Cm in F g−1) and areal capacitance (CA in F cm−2) were calculated from the GCD curves according to the following eqn (3)–(6):32
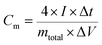 | (3) |
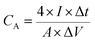 | (4) |
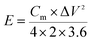 | (5) |
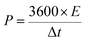 | (6) |
Results and discussion
The overall synthesis process of porous DWCM electrodes is illustrated in Fig. 1a. First, the decoration waste (DW) was cut into the thin slices with a thickness of ∼1 mm and then soaked in an aqueous solution containing KOH to obtain the KOH-treated DW slice (DW-x). The color change from green to brown for the DW slice after the KOH treatment indicates the partial dissolution of the lignin content of the wood scraps. Afterward, the KOH-treated DW slice was facilely converted into hierarchically porous DWCM via a direct two-step carbonization process, during which both the wood scraps and thermoplastic polymers in DW were decomposed, cross-linked, and finally carbonized to form a continuous porous carbon framework with KOH acting as an etching agent to create numerous micropores on the carbon skeleton. In order to understand the carbonization behavior of DW and further determine the proper carbonation temperature range for preparing DWCM electrodes, the thermogravimetric (TG) analysis of DW was first performed. As shown in Fig. S2 (ESI†), the major weight loss for DW occurs at a temperature range from 200 to 400 °C due to the dehydration and degradation processes of binders and thermoplastic polymers. As the carbonization temperature is increased from 400 to 800 °C, the weight loss gradually increases, which can be attributed to the release of gases during complex cross-linked reactions. In the temperature range of 800 to 1000 °C, the weight loss becomes negligible due to the formation of a graphitic carbon matrix. Further increasing the carbonization temperatures will lead to structural degradation, as evidenced by noticeable weight loss in the temperature range of 1000–1200 °C. Therefore, the carbonization temperature range was selected to be 800–1200 °C. The as-obtained DWCM electrodes largely retain the macrostructures of the original DW slice but exhibit a significant volume shrinkage after carbonization. This implies that DWCM electrodes may have a porous structure.33,34 In addition, all the obtained DWCM electrodes exhibit good mechanical strength during the polishing and washing processes. In fact, the mechanical properties of the best DWCM-1-1000 electrode were measured. As shown in Fig. S3a–c (ESI†), the DWCM-1-1000 electrode exhibits high stress of 14.8 MPa at 50% compression rate and Young's modulus in the elastic region is 1.4 MPa in the compressive strain range of 5 to 15%. In addition, it is also disclosed by the stress–strain curve (Fig. S3d–f, ESI†) that the DWCM-1-1000 electrode can also endure a tensile strength of ∼750 kPa at a tensile strain of 10.7%, ensuring its direct application as a binder- and additive-free self-supported membrane electrode.Morphology and microstructures of DWCM electrodes were investigated using scanning electron microscopy (SEM). The effect of the concentration of KOH activator on the microstructures of the DWCM electrodes was first studied. As shown in Fig. S4 and S5 (ESI†), when concentrations of KOH were 0–0.1 M, a large number of tubular carbon fibers can be observed on the surfaces of DWCM-0-1000 and DWCM-0.1-1000. An enlarged side-view SEM image of DWCM-0-1000 shows that the surface of tubular carbon fibers is quite smooth without any obvious porous structure (Fig. S4d, ESI†). Increasing the concentration of KOH solution to 0.5 M (Fig. S6, ESI†), only a few random pores can be seen on the surface of DWCM-0.5-1000. When the concentration of KOH reaches 1 M, the porous structure becomes more obvious and the pores are distributed uniformly. The top-view SEM images of the DWCM-1-1000 are shown in Fig. 1b–d. A large number of pores can be clearly observed and form relatively ordered highly porous carbon frameworks, which are composed of interconnected carbon nanosheets. Fig. 1e–g display the side-view SEM images of DWCM-1-1000 with a thickness of ∼800 μm. It can be confirmed that the pores not only exist on the surface of DWCM-1-1000 but also uniformly distribute throughout the microchannels, which would be expected to provide a large SSA for charge storage, and the interconnected carbon tubulars can provide a continuous pathway for rapid electron transfer. Furthermore, the energy-dispersed X-ray (EDX) elemental maps of DWCM-1-1000 (Fig. 1h) show that C, N, and O elements are major elements and uniformly distributed on the entire electrode, indicating the homogenous N and O doping, which will be beneficial to enhancing the electrochemical performance. Meanwhile, the influence of the carbonization temperature on the microstructures of DWCM electrodes was also studied. At a low carbonization temperature of 800 °C, the surface of the resulting DWCM-1-800 becomes loose and rough with a few pores formed on the microchannels (Fig. S7, ESI†). As the carbonization temperature is increased to 900 °C, the surface and microchannels of DWCM-1-900 get relatively rougher, but the distribution of pores in the microchannel is not uniform (Fig. S8, ESI†). With further increase of the carbonization temperature to optimal 1000 °C, the formed pores become abundant and uniform in the microchannel of the resulting DWCM-1-1000, which is conducive to the adsorption and rapid transport of electrolyte ions, thereby enhancing the electrochemical performance. As the carbonization temperatures are increased to 1100 (Fig. S9, ESI†) and 1200 °C (Fig. S10, ESI†), most of the pores in the as-fabricated DWCM-1-1100 and DWCM-1-1200 electrodes collapse, which would limit their capability to provide a large surface area for efficient charge storage. These results suggest that the pore structure of the DWCM electrodes can be effectively modulated by synergistically tuning the concentration of the KOH activator and the carbonization temperature, which establishes a facile yet effective activation-carbonization strategy for the development of high-performance carbon membrane based on the wastes.
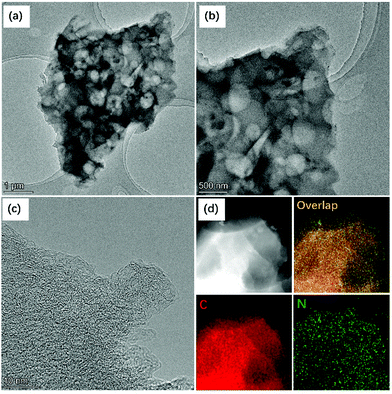
To further study the microstructures of the DWCM-1-1000 electrode, transmission electron microscopy (TEM) imaging was performed. As shown in the low-magnification TEM images in Fig. 2a and b, the DWCM-1-1000 electrode has a 3D interconnected porous structure with numerous cross-linked macropores and mesopores in the carbon matrix, consistent with the results of SEM analysis. No evident lattice fringe can be observed in the high-resolution TEM (HRTEM) (Fig. 2c), suggesting a low graphitization degree of the carbon matrix. In addition, the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image and the corresponding energy-dispersive X-ray (EDX) elemental maps (Fig. 2d) reveal that the DWCM-1-1000 electrode mainly consists of C element and N heteroatoms are uniformly doped in the carbon matrix.
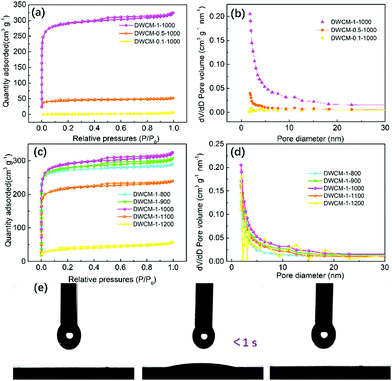
Surface areas and porous structures of the DWCM electrodes were then investigated by N2 adsorption/desorption measurements. Fig. 3a shows the isotherms of DWCM-x-1000 electrodes prepared with different concentrations of KOH activator. It is observed that the concentration of the KOH activator has a significant influence on the textural structures of DWCM electrodes. At lower concentrations of KOH, the resulting DWCM-0.1-1000 and DWCM-0.5-1000 electrodes show featureless isotherms, indicating that they only have a small number of macropores and mesopores with random sizes due to aggregation of the carbon nanosheets. The measured Brunauer–Emmett–Teller (BET) specific surface areas are only 3.51 and 141.7 m2 g−1 for DWCM-0.1-1000 and DWCM-0.5-1000, respectively. In addition, it can be seen from Fig. 3b and Table 1 that DWCM-0.1-1000 and DWCM-0.5-1000 show limited pore volumes, further rationalizing that the low BET surface areas for both electrodes are mainly due to the lack of pores. As 1.0 M KOH was used, the isotherm of the resulting DWCM-1-1000 is a combination of type I and type IV isotherms, suggesting the existence of abundant micro- and mesopores. Notably, the calculated BET surface area of DWCM-1-1000 is as high as 917.0 m2 g−1, which would be crucial for providing large area for the adsorption of electrolyte ions, in turn enhancing the capacitive performance of the electrode. On the other hand, compared to the effect of concentration of the KOH activator, the carbonization temperature shows a less pronounced effect on the surface areas and pore structures of the resulting DWCM electrodes. As shown in Fig. 3c and d, all DWCM electrodes prepared at different carbonization temperatures show a similar adsorption behaviour at a fixed concentration of KOH, except for the DWCM-1-1200 electrode. As also shown in Table 1, the specific surface area of DWCM electrodes only slightly increases from 851.1 to 917.0 m2 g−1 as the carbonization temperature is increased from 800 to 1000 °C. In addition, variations in the pore diameter and volume with carbonization temperature are also not obvious. However, as the carbonization temperature is increased to 1100 and 1200 °C, both specific surface areas and pore volumes of the resulting DWCM-1-1100 and DWCM-1-1200 electrodes greatly decrease, which can be attributed to the microstructure degradation (pore collapse) at higher carbonization temperatures, consistent with the results of SEM analysis. The above results clearly indicate that the concentration of KOH and carbonization temperature are the dominant factors that determine the surface area and porosity of the resulting DWCM electrodes. At lower concentrations of KOH (0.1–0.5 M), the etching capability of KOH toward DW is limited, thus the formation of pores is suppressed, resulting in a relatively small surface area. Meanwhile, at high carbonization temperatures (1100 and 1200 °C), the pores in DWCM electrodes tend to collapse, resulting in a lower specific surface area and pore volume. Therefore, at the optimal concentration of KOH (1 M) and appropriate carbonization temperature (1000 °C), the reaction of KOH and DW becomes more sufficient, leading to the formation of a large number of pores over broad length scales from micro- to meso- to macropores in the resulting DWCM-1-1000. The existence of macropores would provide a fast pathway for electrolyte transport, while the micro-and mesopores supply a large specific surface area for enhancing the capacitance performance. In addition, the existence of multi-level pores would render DWCM-1-1000 excellent electrolyte wettability. As shown in Fig. 3e and Video S1 (ESI†), the electrolyte droplet can completely permeate into the DWCM-1-1000 microchannels with a contact angle approaching approximately 0° in a short time of <1 s. In combination with the hierarchically porous structure, such a good electrolyte wettability ensures fast transport of electrolyte at the electrode/electrolyte interface and hence rendering DWCM with improved electrochemical capacitive performance.
| Sample | Specific surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|---|
| DWCM-0.1-1000 | 3.5 | 0.009 | 10.1 |
| DWCM-0.5-1000 | 141.7 | 0.081 | 2.3 |
| DWCM-1-800 | 851.1 | 0.449 | 2.1 |
| DWCM-1-900 | 876.3 | 0.475 | 2.2 |
| DWCM-1-1000 | 917.0 | 0.501 | 2.2 |
| DWCM-1-1100 | 698.6 | 0.37 | 2.1 |
| DWCM-1-1200 | 120.6 | 0.08 | 3.0 |
The elemental compositions and valence states of the DW slice and DWCM-1-1000 electrode were investigated using X-ray photoelectron spectroscopy (XPS). The XPS survey spectrum of the DW slice (Fig. S11, ESI†) reveals that it mainly consists of C (85.3 at%), O (10.4 at%), and N (4.3 at%) elements. N atoms in DW might come from the wood scraps as well as the wood binders and additives.35 As shown in Fig. 4a, the XPS survey spectrum indicates that DWCM-1-1000 mainly consists of C (89.58 at%) and O (8.97 at%) elements, with a small amount of N (1.45 at%) dopants, which is consistent with EDX results. Deconvolution of the high-resolution C 1s spectrum (Fig. 4b) gives three peaks located at 284.4, 285.5, and 288.7 eV, which can be ascribed to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, and C
C, C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.36 The N 1s spectrum (Fig. 4c) is well-fitted by three peaks at 398.4, 400.2 and 402.1 eV, which are attributed to pyridinic N, pyrrolic N, and graphitic N species, respectively.37 It has been reported that graphitic N can improve the electrical conductivity of carbon materials and thus facilitate the activity of electron transfer reactions.38 The fitting of O 1s spectrum (Fig. 4d) yields two peaks at 530.1 eV for C–O and 532.5 eV for C
O, respectively.36 The N 1s spectrum (Fig. 4c) is well-fitted by three peaks at 398.4, 400.2 and 402.1 eV, which are attributed to pyridinic N, pyrrolic N, and graphitic N species, respectively.37 It has been reported that graphitic N can improve the electrical conductivity of carbon materials and thus facilitate the activity of electron transfer reactions.38 The fitting of O 1s spectrum (Fig. 4d) yields two peaks at 530.1 eV for C–O and 532.5 eV for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, which would be beneficial for improving the electrolyte wettability of the DWCM electrodes, as evidenced by the contact angle measurements.
O groups, which would be beneficial for improving the electrolyte wettability of the DWCM electrodes, as evidenced by the contact angle measurements.
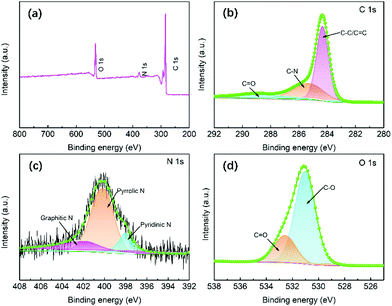 | ||
| Fig. 4 (a) XPS survey spectra and high-resolution (b) C 1s, (c) N 1s, and (d) O 1s XPS spectra of the DWCM-1-1000 electrode. | ||
The phase structures of DWCM-x-y electrodes were investigated using X-ray diffraction (XRD). In XRD patterns of DWCM-x-y electrodes (Fig. 5a and b), two broad diffraction peaks at approximately 23 and 44° can be observed, which can be attributed to (002) and (100) crystal planes of carbon with a low degree of graphitization, respectively.39 Notably, the peak intensity of DWCM-x-y first increases and then decreases with increasing concentration of the KOH activator and carbonization temperature. Specifically, as shown in Fig. 5a, with increasing concentration of KOH, the (002) peak of DWCM-x-1000 reveals a slight shift toward the lower angle, which may be due to the expansion of the space between the atomic carbon layers, which results in an increase in the pore and defect density.40 Meanwhile, the intensity of the (100) peak gradually enhances with increasing carbonization temperature to 1000 °C, attributing to the enhanced degree of crystallization at higher carbonization temperatures. Beyond 1000 °C, however, the intensity of the (100) peak becomes weaker with increased carbonation temperature due to the reduction of graphitization degree, which also hinders the electron transfer (Fig. 5b). On the other hand, it can be observed from the XRD pattern of DWCM-1-1000 that there is an obvious upward at low diffraction angle region (5–10°), meaning the formation of abundant micropores, which is in good coherence with N2 adsorption/desorption results.41 Raman spectra were collected to further study the structural information of DWCM electrodes. As shown in Fig. 5c, all the DWCM electrodes display two characteristic peaks located at 1338 cm−1 for D-band and 1597 cm−1 for G-band.42 The intensity ratios of the D-band to G-band (ID/IG) are found to be 0.970, 0.980, 1.002, 1.008, and 1.038 for DWCM-0.1-1000, DWCM-0.5-1000, DWCM-1-800, DWCM-1-900, and DWCM-1-1000 electrodes, respectively. It can be noticed that the value of ID/IG is increased with the increase of KOH concentration, which indicates the formation of more defects, most likely due to the formation of micropores originating from the sufficient reaction of KOH and carbon, as evidenced by XRD results. On the other hand, it can be noted that the ID/IG ratio of DWCM electrodes increases with increasing carbonization temperature, also implying the evolution of structural defects due to the formation of reorganization of carbon framework at a higher temperature. As a result, the DWCM-1-1000 electrode features abundant multilevel pores, numerous defects, and high bulk crystallization degree, all of which are beneficial for providing fast pathways for electrolyte transport, supplying large contact surface area for the adsorption of electrolyte ions, and offering low-resistance pathways for electron transfer, and in turn potentially enhancing the capacitive performance.
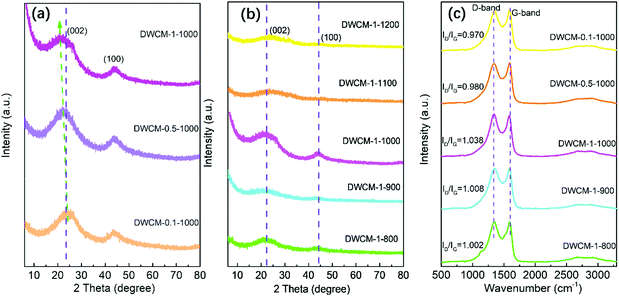 | ||
| Fig. 5 XRD patterns of (a) DWCM-x-1000 electrodes, (b) DWCM-1-y electrodes, and (c) Raman spectra of DWCM-x-y electrodes. | ||
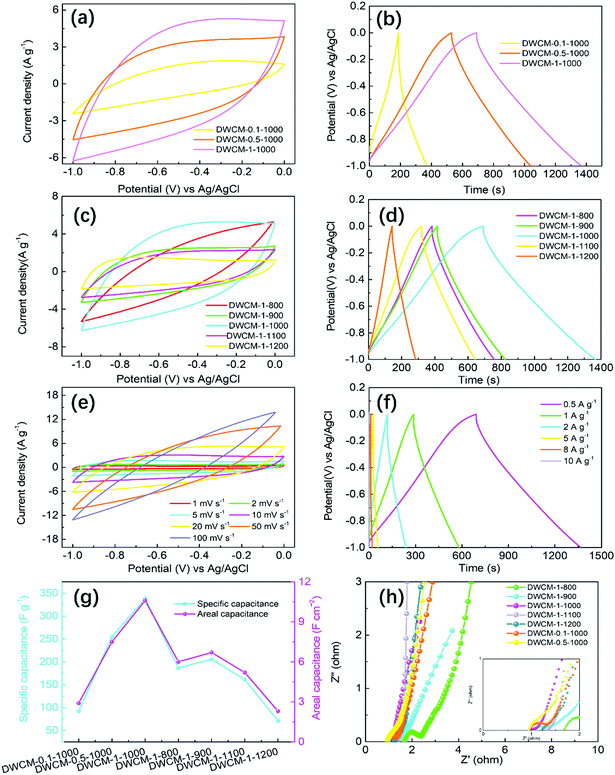
The electrochemical performance of DWCM-x-1000 electrodes prepared using different concentrations of KOH activator were first tested in a three-electrode system in 6 M KOH electrolyte at a voltage window of −1 to 0 V. Noted that the average weight of the prepared DWCM electrode varies from 30 to 40 mg depending on the preparation conditions. Fig. 6a shows the cyclic voltammetry (CV) curves of DWCM-x-1000 electrodes recorded at a scan rate of 20 mV s−1. All the DWCM-x-1000 electrodes display a quasi-rectangular shape and no obvious redox peaks can be observed. This indicates that DWCM-x-1000 electrodes store electricity following an electrical double-layer (EDL) mechanism without obvious pseudocapacitance contribution.43 Among the electrodes tested, the DWCM-1-1000 electrode shows the larger CV curve area compared to DWCM-0.1-1000 and DWCM-0.5-1000 electrodes, demonstrating that it has the highest capacitive performance, which depends on the increased pore volume and the increased SSA by KOH activation. Fig. 6b shows the galvanostatic charge–discharge (GCD) curves of DWCM-x-1000 electrodes at a current density of 0.5 A g−1. Notably, shape distortion can be observed in the GCD curve of the DWCM-0.1-1000 electrode, which may be due to its high internal series resistance caused by low conductivity and irregular pore size distribution. In contrast, the GCD curves of DWCM-0.5-1000 and DWCM-1-1000 electrodes exhibit a typical symmetrical triangular shape with a negligible IR drop, suggesting an outstanding capacitive performance. In addition, the DWCM-1-1000 electrode exhibits a longer discharge time than DWCM-0.1-1000 and DWCM-0.5-1000 electrodes, demonstrating good capacitive characteristics.
In addition, the effect of carbonization temperature on the electrochemical performance of DWCM electrodes was also studied. The CV curves of DWCM-1-y electrodes at a scan rate of 20 mV s−1 are shown in Fig. 6c. The CV curves of all DWCM-1-y electrodes show quasi-rectangular shapes with no distortion, demonstrating an excellent EDL capacitive behavior, and the DWCM-1-1000 electrode possesses the largest integral area of the CV curve, revealing the largest charge storage capability with the best capacitive behavior. The GCD curves of DWCM-1-y electrodes are shown in Fig. 6d. All the GCD curves exhibit a typical symmetrical triangular shape, and the charge/discharge curves have a good linear shape, again indicating nearly ideal EDL capacitive behavior of DWCM-1-y electrodes. Again, the DWCM-1-1000 electrode exhibits the longest discharge time than other DWCM-1-y electrodes, which evidences its highest capacitance. These results are well in accordance with the results of CV tests. Overall, the capacitive performance of the DWCM-1-y electrodes follows an order of DWCM-1-1000 > DWCM-1-900 > DWCM-1-800 > DWCM-1-1100 ≫ DWCM-1-1200. The outstanding capacitance performance of the DWCM-1-1000 electrode may be attributed to its larger specific surface area, optimal pore size distribution, and superior electrolyte wettability, while the DWCM-1-y electrodes obtained at much lower (800 °C) or higher (1200 °C) temperatures typically have insufficient surface area and pores and thus lead to inferior capacitive performance. Fig. 6e shows CV curves of the DWCM-1-1000 electrode at different scan rates ranging from 1 to 100 mV s−1. As the scan rate increases, CV curves for the DWCM-1-1000 electrode still maintain a nearly rectangular shape, even at a high scan rate of 50 mV s−1, indicating that this electrode has excellent rate capability and the best reversibility. However, as the scan rate is increased to 100 mV s−1, the CV curve of the DWCM-1-1000 electrode slightly deviates from a quasi-rectangular shape. Given that the thickness of the electrode is ∼800 μm, this might be due to insufficient diffusion of electrolyte ions from the solid/liquid interface to the interior of the electrode, which will result in slow kinetics for the electrochemical adsorption of electrolyte ions. Additionally, CV curves of DWCM-x-1000 (Fig. S12a and b, ESI†) and DWCM-1-y (Fig. S13, ESI†) electrodes only maintain the quasi- rectangular shape even at lower scan rates of less than 20 mV s−1 because of the lower SSA and pore volume of DWCM-x-1000 electrodes and the low degree of graphitization and thicker walls of cavities in DWCM-1-y electrodes, which are not favorable for the effective transportation of the electrolyte ions.
Fig. 6f shows GCD curves of DWCM-1-1000 electrode at current densities from 0.5 to 10 A g−1. The GCD curves of the DWCM-1-1000 electrode still exhibit symmetric triangular shape curves with a negligible IR drop, even at 10 A g−1. This result suggests excellent reversibility of the DWCM-1-1000 electrode, which may be attributed to its large SSA and high porosity that can facilitate rapid ion diffusion and transportation. Specifically, the specific capacitance (Cs) (areal capacitance (Ca)) values of the DWCM-1-1000 electrode calculated are 338.2 F g−1 (10.6 F cm−2), 294.6 F g−1 (9.2 F cm−2), 240.5 F g−1 (7.5 F cm−2), 143.2 F g−1 (4.5 F cm−2), 82.1 F g−1 (2.6 F cm−2), and 63.3 F g−1 (2.0 F cm−2) at the current densities of 0.5, 1, 2, 5, 8 and 10 A g−1, respectively, which are higher than the obtained values of other DWCM-x-1000 (Fig. S12c and d, ESI†) and DWCM-1-y electrodes (Fig. S14, ESI†). Consequently, the excellent capacitive performance of DWCM-1-1000 can be attributed to synergistic effects of the use of proper concentration of KOH and selection of carbonization temperature, which result in large SSA, hierarchically porous structure, and appropriate size distribution as these structural characteristics are favorable for the electrolyte penetration, ion diffusion, and electron transfer in the electrode. Fig. 6g depicts plots of Cs and Ca of DWCM electrodes at a current density of 0.5 A g−1. The calculated Cs and Ca values from GCD curves are 92.1 F g−1 (3.2 F cm−2), 254.6 F g−1 (8.5 F cm−2), 338.2 F g−1 (10.6 F cm−2), 186.6 F g−1 (6.0 F cm−2), 205.5 F g−1 (6.7 F cm−2), 161.3 F g−1 (5.2 F cm−2), and 71.1 F g−1 (2.3 F cm−2) for DWCM-0.1-1000, DWCM-0.5-1000, DWCM-1-1000, DWCM-1-800, DWCM-1-900, DWCM-1-1100 and DWCM-1-1200 electrodes, respectively. The capacitance performance of the optimal DWCM-1-1000 electrode is comparable to even much higher than those of recently reported powdery and self-supported carbon materials (Table 2). The electrochemical impedance spectroscopy (EIS) measurements were carried out in the frequency ranging from 0.01 Hz to 100 kHz, and the results are shown in Fig. 6h. The Nyquist plots of the DWCM electrodes can be divided into two parts, including a semicircle in the high-frequency region and a steep line in the low-frequency region. In the low-frequency region, the DWCM-1-1000 curve exhibits a larger slope than other DWCM electrodes, which is indicative of faster ion diffusion and migration, further revealing a great capacitive performance of the DWCM-1-1000 electrode. In the high-frequency region, the intercept between the EIS curve and the x-axis represents the equivalent series resistance (Rs) and the semicircle diameter corresponds to Rct (interfacial charge transfer resistance) of the electrode material. Notably, at low carbonization temperatures, Rs of the obtained DWCM-1-800 (1.68 Ω), DWCM-1-900 (1.07 Ω), DWCM-1-1100 (1.34 Ω) and DWCM-1-1200 (1.27 Ω) are obviously higher than that of DWCM-1-1000 electrode (0.98 Ω) (inset of Fig. 6h). The lower Rs of the DWCM-1-1000 electrode is mainly attributed to the fast ion response within the mesopore-dominant hierarchically porous structure and the low series resistance of the DWCM-1-1000 electrode. In addition, Rct of the DWCM-1-1000 electrode is also obviously lower than those of other DWCM-1-y electrodes, again indicating a higher ion-diffusion efficiency of DWCM-1-1000. All above results indicate that the DWCM-1-1000 electrode possesses favorable charge transfer and ion diffusion kinetics, which can be a suitable electrode material for high-performance SC.
| Material | Form | C s (F g−1) | S BET (m2 g−1) | Cycling stability | Ref. |
|---|---|---|---|---|---|
| WHAC | Powders | 271.5 (0.5 A g−1) | 1200 | 82% (5000 cycles) | 44 |
| Rice husk | Powders | 147 (0.1 A g−1) | 2696 | 85% (5000 cycles) | 45 |
| HPTC | Powders | 225 (2 mV s−1) | 1094 | 90% (5000 cycles) | 46 |
| CLR | Powders | 250 (0.5 A g−1) | 1787.2 | 99.7% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
47 |
| CS-CuCl2 | Powders | 273.9 (0.5 A g−1) | 2957.8 | 96.5% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
48 |
| NS-HPC | Powders | 285 (0.5 A g−1) | 1431 | 88.8% (5000 cycles) | 49 |
| PTS | Powders | 220 (0.5 A g−1) | 2201 | 100% (5000 cycles) | 50 |
| SSAC | Powders | 257.2 (0.5 A g−1) | 1674 | 111% (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
51 |
| ANPC | Powders | 243.2 (0.5 A g−1) | 1749 | 96.5% (5000 cycles) | 52 |
| AC-650 | Powders | 162 (0.5 A g−1) | 1016.4 | 94.6% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
53 |
| CDCM | Membrane | 196 (0.2 A g−1) | 682 | 90% (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
24 |
| LCM | Membrane | 213.4 (0.2 A g−1) | 973 | 96.9% (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
54 |
| DWCM-1-1000 | Membrane | 338.2 (0.5 A g−1) | 917.05 | 98% (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
This work |
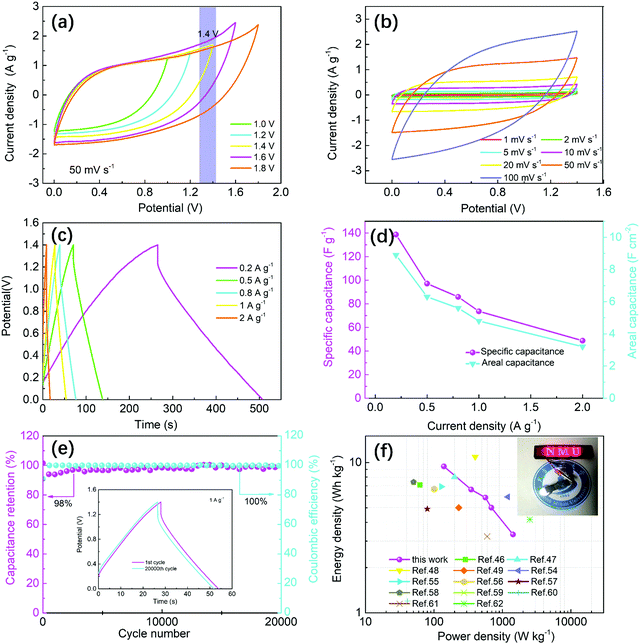
Benefiting from the excellent mechanical strength, superior wettability and outstanding capacitance in three-electrode system, two identical DWCM-1-1000 electrodes were directly used to assembly a symmetric SC device without using any binder and additive. The CV curves of DWCM-1-1000-based SC at different operating potential windows are shown in Fig. 7a. It can be observed that a rapid current increase occurs at 1.6 V due to electrolyte splitting. Therefore, for the sake of safety, the following tests were performed in a rational potential window of 0–1.4 V. The CV curves of DWCM-1-1000-based SC maintain the quasi-rectangular shape without significant distortion even at high scan rates of 100 mV s−1, indicating an excellent rate capability (Fig. 7b). The GCD curves display a quasi-symmetric triangle shapes at current densities from 0.2 to 2 A g−1 (Fig. 7c), demonstrating an ideal reversible double-layer capacitive behaviour. Additionally, as shown in Fig. 7d, the calculated specific capacitances (areal capacitances) of the DWCM-1-1000-based SC are 138.3 F g−1 (8.9 F cm−2), 97.1 F g−1 (6.3 F cm−2), 85.9 F g−1 (5.6 F cm−2), 73.6 F g−1 (4.8 F cm−2), and 48.8 F g−1 (3.2 F cm−2) at current densities of 0.2, 0.5, 0.8, 1, and 2 A g−1, respectively. The value of the specific capacitance for the DWCM-1-1000 based SC at 1 A g−1 is higher than other powdered carbon-based SCs but slightly inferior to the recently reported carbon membrane-based SCs (Table S1, ESI†). In addition, the DWCM-1-1000-based SC demonstrates excellent cycling stability in the potential window of 0–1.4 V, maintaining over 98% of its original capacitance and 100% coulombic efficiency after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a charging/discharging current density of 1 A g−1 (Fig. 7e). Almost similar GCD curves of the 1st cycle and the 20
000 cycles at a charging/discharging current density of 1 A g−1 (Fig. 7e). Almost similar GCD curves of the 1st cycle and the 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000th cycle also indicate a good cycling stability of DWCM-1-1000-based SC (inset of Fig. 7e). This may be a benefit from the hierarchically porous structure, large specific surface area, appropriate size distribution, and lower impedance of the DWCM-1-1000 electrode. Fig. 7f shows the relationship between power density and energy density calculated from GCD curves of DWCM-1-1000-based SC. At the power density of 139.6 W kg−1, the energy density is as high as 9.7 Wh kg−1, which is higher than that observed in aqueous SC devices based on recently developed powdery and self-supported carbon materials.46–49,54–62 In addition, to further demonstrate the practical application of the DWCM-based SC in practical energy storage, a 124 red LED-moulded “NMU” logo (minimum operating potential is 2.0 V) was successfully lighted and it remained on at least 10 s using two symmetric DWCM-1-1000-based SC devices, as shown in the inset of Fig. 7f and Video S2 (ESI†). These results prove that the obtained DWCM-1-1000 carbon membrane can be used as an excellent electrode material for supercapacitors.
000th cycle also indicate a good cycling stability of DWCM-1-1000-based SC (inset of Fig. 7e). This may be a benefit from the hierarchically porous structure, large specific surface area, appropriate size distribution, and lower impedance of the DWCM-1-1000 electrode. Fig. 7f shows the relationship between power density and energy density calculated from GCD curves of DWCM-1-1000-based SC. At the power density of 139.6 W kg−1, the energy density is as high as 9.7 Wh kg−1, which is higher than that observed in aqueous SC devices based on recently developed powdery and self-supported carbon materials.46–49,54–62 In addition, to further demonstrate the practical application of the DWCM-based SC in practical energy storage, a 124 red LED-moulded “NMU” logo (minimum operating potential is 2.0 V) was successfully lighted and it remained on at least 10 s using two symmetric DWCM-1-1000-based SC devices, as shown in the inset of Fig. 7f and Video S2 (ESI†). These results prove that the obtained DWCM-1-1000 carbon membrane can be used as an excellent electrode material for supercapacitors.
It should also be noted that the IR drop of assembled SC is as high as 0.16 V even at 0.2 A g−1, which is most likely a result of thick DWCM electrode (∼800 μm) that is unfavourable for the rapid electrolyte transportation and electron transfer, thereby resulting in a high resistance to limit the capacitive performance of the SC device.63 In order to address this issue, we also prepared a thinner DWCM-1-1000 electrode (∼400 μm) and tested its electrochemical performance. As shown in Fig. S15 (ESI†), reducing the thickness of the DWCM-1-1000 electrode is beneficial for reducing the IR drop (from 0.07 V for 400 μm electrode to 0.16 V for 800 μm electrode, Fig. S15a, ESI†) and thus enhancing the specific capacitance (161.8 and 138.3 F g−1 for 400 and 800 μm electrodes at 0.2 A g−1, respectively, Fig. S15b, ESI†), rate capability (35.3% and 45.3% retentions for 400 and 800 μm electrodes, respectively, Fig. S15c, ESI†), and energy density (11.0 Wh kg−1 at a power density of 139.83 W kg−1 for 400 μm and 9.7 Wh kg−1 at a power density of 139.6 W kg−1 for 800 μm, Fig. S15d, ESI†). The results demonstrate that adjusting the thickness of electrode materials can efficiently optimize electrochemical performance.
Conclusions
In summary, we have facilely fabricated a hierarchically porous carbon membrane (DWCM) based on the direct two-step carbonation of decoration waste using KOH as an activator, which can be directly used as the binder- and additive-free self-supported electrode for an aqueous SC. By adjusting the concentration of KOH solution and carbonization temperature, the pore structure and surface area of the DWCM can be well regulated, and the obtained DWCM-1-1000 electrode displays a high SSA of 917.04 m2 g−1 and superior electrolyte wettability, which can be conducive to the adsorption of electrolyte ions and the rapid transport of electrolyte. Meanwhile, the DWCM-1-1000 electrode possesses excellent mechanical strength and can be directly used as the electrode material to assemble supercapacitor devices. These structural synergies render the best DWCM-1-1000-based SC a high operating potential window up to 1.4 V and a maximum energy density of 9.7 Wh kg−1. Transforming decoration wastes into self-supported high-performance carbon electrode materials provides new insight into the development of high-performance energy storage devices at lower costs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Natural Science Foundation of Ningxia Province (2021AAC02016 and 2021AAC03201), the Leading Talents Program of Science and Technology Innovation in Ningxia Province (2020GKLRLX14), the Foundation of Academic Top-notch Talent Support Program of the North Minzu University (2019BGBZ08), the Innovation and Entrepreneurship Projects for Returnees of Ningxia Province, and the Cooperative Scientific Research Project of Chunhui Plan of Ministry of Education of China (201900081).References
- A. Gopalakrishnan and S. Badhulika, J. Ind. Eng. Chem., 2018, 68, 257–266 CrossRef CAS.
- C. Merlet, B. Rotenberg, P. Madden, P. Taberna, P. Simon, Y. Gogotsi and M. Salanne, Nat. Mater., 2012, 11, 306–310 CrossRef CAS PubMed.
- M. Salanne, B. Rotenberg, K. Naoi, K. Kaneko, P. Taberna, C. Grey, B. Dunn and P. Simon, Nat. Energy, 2016, 1, 16070 CrossRef CAS.
- A. Shah, A. Zahid, H. Subhan, A. Munir, F. J. Iftikhar and M. Akbar, Sustainable Energy Fuels, 2018, 2, 1398–1429 RSC.
- X. M. Wu, B. Huang, Q. G. Wang and Y. Wang, J. Mater. Chem. A, 2019, 7, 19017–19025 RSC.
- K. L. V. Aken, M. Beidaghi and Y. Gogotsi, Angew. Chem., 2015, 1272, 4888–4891 CrossRef.
- S. Sahoo, K. Krishnamoorthy, P. Pazhamalai, V. K. Mariappan, S. Manoharan and S. J. Kim, J. Mater. Chem. A, 2019, 7, 21693–21703 RSC.
- N. Yadav, N. Yadav and S. A. Hashmi, J. Power Sources, 2020, 451, 227771 CrossRef CAS.
- P. Pazhamalai, K. Krishnamoorthy, S. Sahoo, V. Kumar Mariappan and S. J. Kim, Chem. Eng. J., 2020, 387, 123886 CrossRef CAS.
- C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang and J. Zhang, Chem. Soc. Rev., 2015, 44, 7484 RSC.
- Q. Dou, L. Liu, B. Yang, J. Lang and X. Yan, Nat. Commun., 2017, 8, 2188 CrossRef.
- M. B. Arvas, M. Gencten and Y. Sahin, Ionics, 2021, 27, 2241–2256 CrossRef CAS.
- L. Miao, H. Duan, Z. W. Wang, Y. W. Lv, W. Xiong, D. Z. Zhu, L. H. Gan, L. C. Li and M. X. Liu, Chem. Eng. J., 2020, 382, 122945 CrossRef CAS.
- M. B. Arvas, H. Gürsu, M. Gencten and Y. Sahin, J. Energy Storage, 2021, 35, 102328 CrossRef.
- M. B. Arvas, M. Gençten and Y. Sahin, Int. J. Energy Res., 2020, 44, 1624–1635 CrossRef CAS.
- M. B. Arvas, N. Karatepe, M. Gencten and Y. Sahin, New J. Chem., 2021, 45, 6928–6939 RSC.
- O. Gorduk, M. Gencten, S. Gorduk, M. Sahin and Y. Sahin, J. Energy Storage, 2021, 33, 102049 CrossRef.
- L. L. Ji, B. Wang, Y. L. Yu, N. X. Wang and J. B. Zhao, Electrochim. Acta, 2020, 331, 135348 CrossRef CAS.
- T. K. Zhao, X. R. Peng, X. Zhao, J. T. Hu, T. Jiang, X. F. Lu, H. Zhang, T. H. Li and I. Ahmad, J. Alloys Compd., 2019, 817, 153057 CrossRef.
- Z. H. Bi, Q. Q. Kong, Y. F. Cao, G. H. Sun, F. Y. Su, X. X. Wei, X. M. Li, A. Ahmad, L. J. Xie and C. M. Chen, J. Mater. Chem. A, 2019, 7, 16028–16045 RSC.
- J. Du, L. Liu, Y. F. Yu, Z. P. Hu, B. B. Liu and A. B. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 40062–40069 CrossRef CAS.
- G. A. Ferrero, M. Sevilla and A. B. Fuertes, Sustainable Energy Fuels, 2017, 1, 127–137 RSC.
- J. Niu, R. Shao, J. J. Liang, M. L. Dou, Z. L. Li, Y. Q. Huang and F. Wang, Nano Energy, 2017, 36, 322–330 CrossRef CAS.
- J. Cheng, Q. Xu, X. Wang, Z. Li, F. Wu, J. Shao and H. Xie, Sustainable Energy Fuels, 2019, 3, 1215–1224 RSC.
- J. W. Liu, S. X. Min, F. Wang and Z. G. Zhang, J. Power Sources, 2020, 466, 228347 CrossRef CAS.
- A. Gopalakrishnan, T. D. Raju and S. Badhulika, Carbon, 2020, 168, 209–219 CrossRef CAS.
- Y. Cui, H. Wang, X. Xu, Y. Lv, J. Shi, W. Liu, S. Chen and X. Wang, Sustainable Energy Fuels, 2018, 2, 381–391 RSC.
- O. E. Eleri, K. U. Azuatalam, M. W. Minde, A. M. Trindade, N. Muthuswamy, F. L. Lou and Z. X. Yu, Electrochim. Acta, 2020, 362, 137152 CrossRef CAS.
- S. Dutta, A. Bhaumik and K. Wu, Energy Environ. Sci., 2014, 7, 3574–3592 RSC.
- Q. Chen, J. Chen, Y. Zhou, C. Song, Q. Tian, J. Xu and C. Wong, Appl. Surf. Sci., 2018, 169, 30241 Search PubMed.
- S. Yang, S. Wang, X. Liu and L. Li, Carbon, 2019, 147, 540–549 CrossRef CAS.
- T. Qin, Z. Wan, Z. Wang, Y. Wen, M. Liu, S. Peng, D. He, J. Hou, F. Huang and G. Cao, J. Power Sources, 2016, 336, 455–464 CrossRef CAS.
- S. Kulandaivalu and Y. Sulaiman, J. Power Sources, 2019, 419, 181–191 CrossRef CAS.
- J. Huang, J. Wu, F. Dai and C. M. Li, Chem. Commun., 2019, 55, 9168–9171 RSC.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250–1280 RSC.
- S. A. Shah, X. Shen, M. Xie, G. Zhu, Z. Ji, H. Zhou, K. Xu, X. Yue, A. Yuan, J. Zhu and Y. Chen, Small, 2019, 15, 1804545 CrossRef.
- L. Hao, J. Ning, B. Luo, B. Wang, Y. Zhang, Z. Tang, J. Yang, A. Thomas and L. Zhi, J. Am. Chem. Soc., 2015, 137, 219–225 CrossRef CAS PubMed.
- C. Largeot, C. Portet, J. Chmiola, P. L. Taberna and Y. Gogotsi, P. Simon, J. Am. Chem. Soc., 2018, 130, 2730–2731 CrossRef.
- G. Zhang, T. Guan, M. Cheng, Y. Wang, N. Xu, J. Qiao, F. Xu, Y. Wang, J. Wang and K. Li, J. Power Sources, 2019, 448, 227446 CrossRef.
- K. Zhang, M. Liu, T. Zhang, X. Min, Z. Wang, L. Chai and Y. Shi, J. Mater. Chem. A, 2019, 7, 26838–26848 RSC.
- Q. Niu, K. Gao, Q. Tang, L. Wang, L. Han, H. Fang, Y. Zhang, S. Wang and L. Wang, Carbon, 2017, 123, 290–298 CrossRef CAS.
- J. Liu, Y. Deng, X. Li and L. Wang, ACS Sustainable Chem. Eng., 2015, 4, 177–187 CrossRef.
- H. Peng, Y. P. Xu, Y. P. Jiang, X. Wang, R. Zhao, F. Q. Wang, L. Li and G. F. Ma, Sustainable Energy Fuels, 2021, 5, 4965–4972 RSC.
- M. M. Baig and I. H. Gul, Biomass Bioenergy, 2021, 144, 105909 CrossRef CAS.
- E. Y. Teo, L. Muniandy, E. P. Ng, F. Adam, A. R. Mohamed, R. Jose and K. F. Chong, Electrochim. Acta, 2016, 192, 110–119 CrossRef CAS.
- L. Chen, T. Ji, L. Brisbin and J. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 12230–12237 CrossRef CAS.
- X. L. Wang, Y. Z. Li, C. Yang, Y. L. Cao, X. T. Su and M. U. Tahir, Int. J. Energy Res., 2020, 45, 1–11 Search PubMed.
- L. P. Zheng, X. C. Dai, Y. H. Ouyang, Y. L. Chen and X. Y. Wang, J. Energy Storage, 2021, 33, 102152 CrossRef.
- M. G. Shang, J. Zhang, X. C. Liu, Y. Liu, S. P. Guo, S. M. Yu, S. Filatov and X. B. Yi, Appl. Surf. Sci., 2021, 542, 148697 CrossRef CAS.
- A. Wang, K. Sun, R. T. Xu, Y. J. Sun and J. C. Jiang, J. Cleaner Prod., 2021, 283, 125385 CrossRef CAS.
- M. J. Kim, H. Lim, X. T. Xu, M. S. A. Hossain, J. Na and N. N. Awaludin, Microporous Mesoporous Mater., 2021, 312, 110757 CrossRef CAS.
- G. X. Lin, R. G. Ma, Y. Zhou, Q. Liu, X. P. Dong and J. C. Wang, Electrochim. Acta, 2018, 261, 49–57 CrossRef CAS.
- D. W. Lan, M. Y. Chen, Y. C. Liu, Q. L. Liang, W. W. Tu, Y. Y. Chen, J. J. Liang and F. Qiu, J. Mater. Sci.: Mater. Electron., 2020, 31, 18541–18553 CrossRef.
- J. W. Liu, S. X. Min, F. Wang and Z. G. Zhang, Energy Technol., 2020, 8, 2000391 CrossRef CAS.
- Y. Cheng, L. Huang, X. Xiao, B. Yao, L. Yuan, T. Li, Z. Hu, B. Wang, J. Wan and J. Zhou, Nano Energy, 2015, 15, 66–74 CrossRef CAS.
- X. Hao, J. Wang, B. Ding, Y. Wang, Z. Chang, H. Dou and X. Zhang, J. Power Sources, 2017, 352, 34–41 CrossRef CAS.
- Y. Gong, D. Li, C. Luo, Q. Fu and C. Pan, Green Chem., 2017, 19, 4132–4140 RSC.
- P. Li, H. Xie, Y. Liu, J. Wang, Y. Xie, W. Hu, T. Xie, Y. Wang and Y. Zhang, J. Power Sources, 2019, 439, 227096 CrossRef CAS.
- A. Gopalakrishnam and S. Badhulika, J. Ind. Eng. Chem., 2018, 68, 25 Search PubMed.
- P. Schlee, O. Hosseinaei, D. Baker, A. Landmér, P. Tomani, M. J. Mostazo-López, D. Cazorla-Amorós, S. Herou and M.-M. Titirici, Carbon, 2019, 145, 470 CrossRef CAS.
- Y. Liu, J. Zhou, L. Chen, P. Zhang, W. Fu, H. Zhao, Y. Ma, X. Pan, Z. Zhang, W. Han and E. Xie, ACS Appl. Mater. Interfaces, 2015, 7, 23515 CrossRef CAS.
- Q. Li, W. Xie, D. Liu, Q. Wang and D. He, Electrochim. Acta, 2016, 222, 1445 CrossRef CAS.
- Z. Y. Zhang, F. Xiao, L. H. Qian, J. W. Xiao, S. Wang and Y. Q. Liu, Adv. Energy Mater., 2014, 4, 1400064 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures. See DOI: 10.1039/d1nj04738h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |