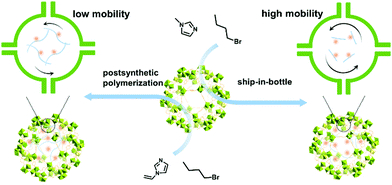
Optimizing the mobility of active species in ionic liquid/MIL-101 composites for boosting carbon dioxide conversion†
Xi
Liu
,
Meili
Ding
,
Pan
Ma
and
Jianfeng
Yao
 *
*
College of Chemical Engineering, Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Key Lab for the Chemistry & Utilization of Agricultural and Forest Biomass, Nanjing Forestry University, Nanjing, Jiangsu 210037, China. E-mail: jfyao@njfu.edu.cn
First published on 30th November 2021
Abstract
Imidazole ionic liquids were encapsulated in the cavities of a metal–organic framework (MOF), via ship-in-bottle and post-synthetic polymerization strategies to afford IL@MIL-101 and polyILs@MIL-101, respectively. It is found that the catalyst with high mobility has better performance of carbon dioxide conversion.
With the increase of human activities, the concentration of greenhouse gases (GHGs) in the atmosphere is increasing, which not only causes global warming but also threatens the environment.1,2 Carbon dioxide (CO2), as the major GHG, has accumulated rapidly in the atmosphere over the past several decades and is suspected to cause global climate change.3,4 Moreover, as a free and renewable C1 resource, CO2 can be used to make many value-added chemicals and materials.5–7 This is not only a reliable way to reduce man-made greenhouse gas emissions, but also a very attractive strategy to realize the carbon cycle and further reduce our dependence on petrochemical products.8 Among the strategies towards CO2 fixation, cycloaddition reaction of epoxy compounds and CO2 is an ideal reaction on account of its 100% atom economy and product versatility. In the past two decades, a wide range of catalyst systems have been explored for this strategy, including metal complexes, ionic liquids (ILs), metal–organic frameworks (MOFs), and porous organic polymers (POPs).9–15 Despite the remarkable progress achieved in this field, most catalysts usually suffer from some inevitable defects. For example, several homogeneous catalysts are widely used in CO2 conversion in industry. Nevertheless, this process typically requires harsh reaction conditions and/or complicated separation and purification of products. In addition, co-catalysts cannot be avoided for use in most heterogeneous catalyst systems to achieve desired yields. Therefore, there is still an urgent demand for designing heterogeneous, robust, and highly active catalysts for the chemical transformation of CO2 under mild conditions.
MOFs, as a new class of crystalline solid material with porous structures, have extensive applications in many fields.16–22 By virtue of their high surface areas, exposed active sites, designable structures and tunable nanochannels, these porous materials have become promising catalysts for effective CO2 adsorption and chemical transformation.9–13,23 In spite of the fact that numerous MOFs possess excellent catalytic performances in CO2 chemical fixation under mild reaction conditions, the use of pure MOFs to achieve high catalytic efficiency is still a daunting challenge without adding any halide catalyst. To tackle this issue, a valid approach is combining MOFs with other highly active catalysts such as ILs.24–35 However, this approach commonly involves prerequisites and defects: particular sites (e.g. coordinatively unsaturated metal sites) for modification and the loss of original features.28–30 Therefore, it would be worth exploring a general strategy to prepare IL-MOF composites without compromising any characteristics of the components. Although, some IL-MOF composites have been developed for CO2 chemical fixation,24–32 the role of active species’ mobility in the catalytic performance of MOF catalysts has been rarely studied.
Bearing this in mind, we introduced imidazole ILs into the channels of MIL-101 through ship-in-bottle and post-synthetic polymerization strategies, respectively, to afford IL@MIL-101 and polyILs@MIL-101 (Scheme 1). MIL-101 possesses high surface area and appropriate pore structure, ensuring that ILs can be encapsulated in the MOF channels. Additionally, the combination of MIL-101 and ILs is independent due to the weak interactions between the two components, which is beneficial for the preservation of individual characteristics of the participating materials. Based on the hierarchical porous structure, efficient CO2 enrichment, and the effective cooperation between different active sites, IL@MIL-101 shows excellent catalytic performance toward the coupling of epoxides and CO2 under 1 bar and 70 °C without the aid of any co-catalyst, surpassing the homogeneous IL and MIL-101. Astonishingly, even with a similar IL loading, surface area, pore structure and CO2 adsorption ability, the yield of polyILs@MIL-101 cyclic carbonate decreased significantly because of the decreased mobility of active species caused by the polymerization of the ILs in the MIL-101 pores.
MIL-101 with a hierarchical pore structure is one of the extensively studied MOFs.36 By removing the coordinated water molecules, the exposed Cr3+ centers are capable of acting as Lewis acid sites. In addition, owing to its decent chemical and thermal stability, high surface area and hierarchical pore sizes, MIL-101 provides an ideal catalyst support for encapsulating active species. Typically, a small monomer, 1-methylimidazole, was “pre-seeded” into the pores of MIL-101, and then an excess amount of another monomer (1-bromobutane) was added to afford IL@MIL-101, like ship-in-a-bottle. In parallel, polyILs@MIL-101 was facilely synthesized based on post-synthetic polymerization initiated by a free radical initiator. Both IL and polyILs can be effectively trapped within the cavities of MIL-101 due to the size confinement. The structural stability of IL@MIL-101 was confirmed by a leaching test. To this end, silver nitrate (AgNO3) was added to 3-butyl-1-methyl-1H-imidazol-3-ium bromide ([Bmim]Br) aqueous solution and the filtrate obtained by ultrasonic treatment of IL@MIL-101 with water (Fig. S1, ESI†). As expected, a pale yellow precipitate was formed immediately at the bottom of the bottle containing [Bmim]Br aqueous solution, whereas no particularly significant changes were found in the filtrate of the composite catalyst (Fig. S1, ESI†). This result indicates the desirable stability of IL@MIL-101, signifying its potential for long-term use. The parallel experiments show that the contents of IL and polyILs within the two composites were ∼9.3 and 9.1 wt%, respectively (Tables S1 and S2, ESI†).
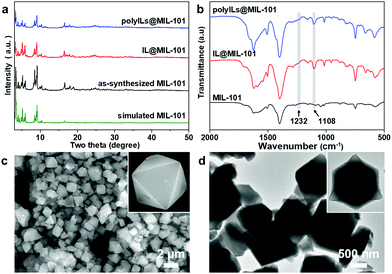
The crystal phases of several materials were verified by powder X-ray diffraction (XRD) (Fig. 1a). The XRD patterns of the two composites showed obvious characteristic peaks related to pristine MIL-101, indicating the good preservation of the crystalline structure of MIL-101 upon IL and polyILs encapsulation. The introduction of IL was successfully confirmed by Fourier transform infrared (FT-IR) spectroscopy. Compared with MIL-101, both composites show additional peaks at 1232 and 1108 cm−1, which belong to the characteristic absorption bands of the imidazole ring (Fig. 1b).37 The thermogravimetric analysis (TGA) profile of MIL-101 displays a significant weight loss at 380 °C (Fig. S2, ESI†). In comparison, IL@MIL-101 and polyILs@MIL-101 started to collapse from 360 and 350 °C, respectively. The lower degradation temperature of the two composites was due to the decomposition of loaded IL.32
The X-ray photoelectron spectroscopy (XPS) survey spectrum revealed the state of nitrogen and bromine elements in the two composites (Fig. S3, ESI†). The nitrogen 1s spectrum shows a clear peak at 399.2 and 400.7/400.9 eV (Fig. S3a, ESI†), confirming the presence of non-ionic nitrogen atoms and a positively charged nitrogen atom on the imidazole ring.38 In addition, the spectrum of bromine 3d has weak peaks at 68.1/69.1 eV and 66.7/67.7 eV (Fig. S3b, ESI†), corresponding to the binding energy of bromide ions.39 Altogether, these results suggest that the [Bmim]Br molecule has been successfully synthesized and integrated with the framework of MIL-101. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were adopted to study the morphology of these materials. Both SEM and TEM images of the two composites show a similar octahedral morphology of pristine MIL-101 with smooth and clean surfaces (Fig. 1c and d, Fig. S4 and S5, ESI†), manifesting that the formation processes of both composites are mild synthetic routes and can easily be extended to other MOF systems.
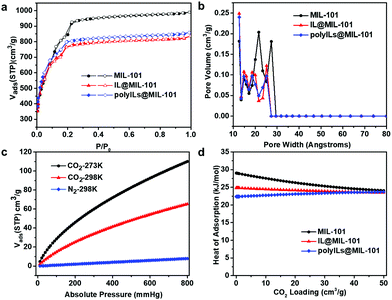
For evaluating the pore characteristics of all catalysts, N2 adsorption/desorption isotherms were obtained at 77 K (Fig. 2a). MIL-101 displays a high Brunauer–Emmett–Teller (BET) surface area of 3150 m2 g−1 with a decent pore volume of 1.32 cm3 g−1. By comparison, the BET surface area of IL@MIL-101 and polyILs@MIL-101 fell to 2457 and 2529 m2 g−1, respectively. The pore size distribution, obtained by density functional theory (DFT) analysis, shows that the hierarchical pores of the pristine MOF are well inherited in both composite materials (Fig. 2b). Compared with the micropores of most MOFs, graded pores are more conducive to mass migration. The pore size shrinkage of these two composites is similar, indicating that the cavity of MIL-101 is partially occupied by IL. Additionally, the relatively small pores in the two IL@MOF composites might effectively improve their CO2 capture ability.
To verify this hypothesis, the CO2 uptake capacities of three materials were tested at different temperatures. The adsorption amounts of the original MIL-101 under 1 bar are 84 and 45 cm3 g−1 at 273 and 298 K, respectively (Fig. S6, ESI†). To our delight, IL@MIL-101 displays extraordinary CO2 sorption performance (110 cm3 g−1 under 273 K and 1bar, 65 cm3 g−1 under 298 K and 1 bar) (Fig. 2c), despite the decline in BET surface area and pore volume. This result can be attributed to the presence of extra-small pores after loading ILs. Under 1 bar, the CO2 adsorptive amounts of polyILs@MIL-101 at 273 and 298 K are 110 and 63 cm3 g−1, respectively (Fig. S6, ESI†). In comparison the high N2 uptake capacities at 77 K, negligible N2 was adsorbed by IL@MIL-101 at 298 K (Fig. 2c), implying its outstanding CO2/N2 selectivity. Interestingly, the zero-coverage Qst values of these two composites calculated on the basis of the virial method are slightly lower than that of MIL-101 (Fig. 2d). This indicates that the two composites would demand lower energy input for regeneration compared with MIL-101. Since the Qst value at zero-surface coverage is often strongly affected by the polarity and basicity of adsorption sites and their steric availability,40 the slightly higher Qst of MIL-101 can be attributed to the abundant available adsorption sites resulting from MIL-101's large specific surface area.
Encouraged by the high specific surface area, hierarchical porous structure, desirable CO2 adsorption ability, and synergistic effects of Cr3+ centers and halide anions, we investigate the activities of the above-mentioned catalysts toward the coupling reaction of epichlorohydrin (ECH) and CO2 in the absence of co-catalyst (Fig. 3a). Remarkably, IL@MIL-101 shows the highest catalytic performance (94% yield) for turning ECH to (chloromethyl)ethylene carbonate upon 24 h of reaction at 70 °C and 1 bar of CO2. To investigate the cooperation between two types of active sites from MIL-101 and IL, control experiments were conducted. In contrast to IL@MIL-101, MIL-101 and the homogeneous [Bmim]Br catalyst only achieved a target product yield of 25 and 65%, respectively. With respect to the physical mixture of MIL-101 and IL, 90% of ECH can be converted to the target product. However, the homogeneous nature of the IL makes this catalytic system difficult to be recycled. Altogether, these results demonstrated that the remarkable performance of the optimal catalyst was attributable to the synergistic effects between exposed Cr3+ centers and halide anions from MIL-101 and IL. Beyond these, the influence of CO2 enrichment of MIL-101 on its catalytic conversion efficiency should not be ignored. It should be noted that MIL-101 attains a negative charge (−23.1 mV) in acetonitrile solution. This signifies that the imidazolium cation of IL is inclined to interact with negatively charged MOF particles through electrostatic interaction during the catalytic process.
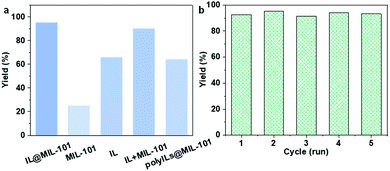 | ||
| Fig. 3 (a) The yield of cycloaddition reactions catalyzed by different catalysts. (b) The yield of cyclic carbonates for five consecutive catalytic reactions. | ||
To further understand the role of mobility of the active species in catalysis, polyILs@MIL-101 was also utilized as a catalyst. The yield of product dropped significantly to 64% under the same conditions. In view of the similar pore structure, BET surface area, active species, and CO2 enrichment ability at 70 °C (Fig. S7, ESI†), the huge difference between the activities of two catalysts was mainly due to the relatively high mobility of IL within the MOF. In the case of polyILs@MIL-101, polyILs, obtained from the self-assembly of 1-vinylimidazole and 1-bromobutane, were combined with MIL-101. The active species’ mobility is restricted to the flexibility of the polymer chains in the nanochannels of MIL-101 to a large extent. Unlike polyILs@MIL-101, IL encapsulated in IL@MIL-101 tends to be more mobile and accessible, thus significantly improving the catalytic efficiency of the composite.
The recyclability and catalytic stability of the IL@MIL101 catalytic system was assessed under mild conditions. Taking advantage of the heterogeneity, IL@MIL-101 can be readily separated using centrifugation. Notably, IL@MIL101 maintains good catalytic activity in five independent CO2 cycloaddition reactions (Fig. 3b). In addition, after five repeated reactions, the crystallinity and morphology of IL@MIL-101 are almost the same as before the reaction. (Fig. S8 and S9, ESI†). The decrease of BET surface area was not obvious (Fig. S10, ESI†), indicating its good recoverability and structural stability.
Inspired by the excellent catalytic activity of IL@MOF for the coupling reaction of CO2 and ECH, different epoxy compounds containing different functional groups are used to investigate their general applicability (Table S3, ESI†). Impressively, most of the substituted epoxy compounds are smoothly converted into the corresponding five-membered ring carbonates with high yields, suggesting the great substrate tolerance of IL@MIL-101. It should be noted that 1,2-epoxyhexane and cyclohexene oxide underwent low catalytic conversion even upon increasing the reaction time and temperature or changing the reaction solvent (entries 5 and 6). The relatively low efficiency may be ascribable to the large steric hindrance arising from long alkyl side chains and bulky cyclohexane groups.
According to the existing catalytic results and previous literature,9,11 a plausible reaction mechanism was deduced (Scheme 2). At the beginning, adsorbed epoxide is activated by the interaction between Lewis acidic Cr3+ sites and an O atom from the epoxide. In the second step, the Br− anion of IL conducts a nucleophilic attack on the C atom with relatively low steric hindrance in the epoxide, causing the breaking of the C–O bond in the epoxide. Subsequently, the O atom of the intermediate carries out a nucleophilic attack on the C atom of CO2 to form a carbonic acid complex. Finally, a cyclic carbonate is achieved by an intramolecular cyclization in the above complex with the regeneration of the composite catalyst.
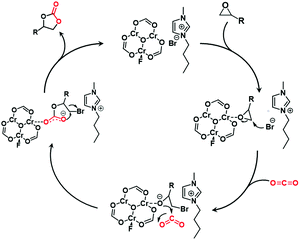 | ||
| Scheme 2 Proposed reaction mechanism for cycloaddition of epoxides with CO2 catalyzed by IL@MIL-101. | ||
Conclusions
To sum up, an imidazole-based IL has been encapsulated into a hierarchically porous MOF (MIL-101) to afford ionic liquid@MOF composites via two facile methods. It is found that active species’ mobility plays a distinct role in the CO2 chemical fixation. Even in the absence of any co-catalysts, IL in situ incorporated in MIL-101 (IL@MIL-101) displays superior catalytic properties for the catalytic CO2 fixation reaction in comparison with polyILs@MIL-101. The fantastic catalytic performance over IL@MIL-101 is ascribed to high surface area, hierarchically porous structure, the coexistence of two types of active sites, high mobility of active species and heterogeneous nature. These factors synergistically promote the CO2 enhancement, the conversion of substrates and mass transfer. Given the structural diversity of ionic liquids and MOFs, this work could open up a novel way to achieve MOF-based composites with tailor-made functionality in catalysis.Author contributions
Xi Liu: methodology, investigation, formal analysis, and writing – original draft. Meili Ding: conceptualization, writing – reviewing, editing and funding acquisition. Pan Ma: writing – reviewing. Jianfeng Yao: validation, supervision, and writing – reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support by the National Natural Science Foundation of China (22001125) and Natural Science Foundation of Jiangsu Province Youth Fund (BK20200767).References
- Z. Zhang, S.-Y. Pan, H. Li, J. Cai, A. G. Olabi, E. J. Anthony and V. Manovic, Renewable Sustainable Energy Rev., 2020, 125, 109799 CrossRef CAS.
- J. Alcalde, S. Flude, M. Wilkinson, G. Johnson, K. Edlmann, C. E. Bond, V. Scott, S. M. V. Gilfillan, X. Ogaya and R. S. Haszeldine, Nat. Commun., 2018, 9, 2201 CrossRef.
- M. D. Aminu, S. A. Nabavi, C. A. Rochelle and V. Manovic, Appl. Energy, 2017, 208, 1389–1419 CrossRef CAS.
- J. Li, Y. Zhang and N. Kornienko, New J. Chem., 2020, 44, 4246–4252 RSC.
- G. Singh, J. Lee, A. Karakoti, R. Bahadur, J. B. Yi, D. Y. Zhao, K. AlBahily and A. Vinu, Chem. Soc. Rev., 2020, 49, 4360–4404 RSC.
- Z. Xie, X. Zhang, Z. Zhang and Z. Zhou, Adv. Mater., 2017, 29, 1605891 CrossRef.
- G. Y. Zang, P. P. Sun, E. Yoo, A. Elgowainy, A. Bafana, U. Lee, M. Wang and S. Supekar, Environ. Sci. Technol., 2021, 55, 7595–7604 CrossRef CAS PubMed.
- O. S. Bushuyev, P. De Luna, C. T. Dinh, L. Tao, G. Saur, J. van de Lagemaat, S. O. Kelley and E. H. Sargent, Joule, 2018, 2, 825–832 CrossRef CAS.
- M. Ding, R. W. Flaig, H.-L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC.
- A. Schoedel, Z. Ji and O. M. Yaghi, Nat. Energy, 2016, 1, 16034 CrossRef CAS.
- H. He, J. A. Perman, G. Zhu and S. Ma, Small, 2016, 12, 6309–6324 CrossRef CAS.
- T. K. Pal, D. De and P. K. Bharadwaj, Coord. Chem. Rev., 2020, 408, 213173 CrossRef CAS.
- C. A. Trickett, A. Helal, B. A. Al-Maythalony, Z. H. Yamani, K. E. Cordova and O. M. Yaghi, Nat. Rev. Mater., 2017, 2, 17045 CrossRef CAS.
- R. R. Shaikh, S. Pornpraprom and V. D’Elia, ACS Catal., 2018, 8, 419–450 CrossRef CAS.
- K. Huang, C.-L. Sun and Z.-J. Shi, Chem. Soc. Rev., 2011, 40, 2435–2452 RSC.
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle III, M. Bosch and H.-C. Zhou, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- L. Jiao, J. Wang and H.-L. Jiang, Acc. Mater. Res., 2021, 2, 327–339 CrossRef CAS.
- X. Ma, H. Liu, W. Yang, G. Mao, L. Zheng and H.-L. Jiang, J. Am. Chem. Soc., 2021, 143, 12220–12229 CrossRef CAS.
- G. Cai, P. Yan, L. Zhang, H.-C. Zhou and H.-L. Jiang, Chem. Rev., 2021, 121, 12278–12326 CrossRef CAS.
- Y. Sun, Q. Du, F. Wang, P. Dramou and H. He, New J. Chem., 2021, 45, 1137–1162 RSC.
- R.-G. Lin, G.-J. Cao, J.-D. Lin and Y.-L. Wang, New J. Chem., 2015, 39, 9075–9078 RSC.
- J. Long, W. Dai, M. Zou, B. Li, S. Zhang, L. Yang, J. Mao, P. Mao, S. Luo and X. Luo, Microporous Mesoporous Mater., 2021, 318, 111027 CrossRef CAS.
- R. Babu, J. F. Kurisingal, J. S. Chang and D. W. Park, ChemSusChem, 2018, 11, 924–932 CrossRef CAS PubMed.
- Y. Jiang, Z. Wang, P. Xu and J. Sun, Cryst. Growth Des., 2021, 21, 3689–3698 CrossRef CAS.
- E. S. Larrea, R. Fernández de Luis, A. Fidalgo-Marijuan, E. M. Maya, M. Iglesias and M. I. Arriortua, CrystEngComm, 2020, 22, 2904–2913 RSC.
- H. Ji, K. Naveen, W. Lee, T. S. Kim, D. Kim and D.-H. Cho, ACS Appl. Mater. Interfaces, 2020, 12, 24868–24876 CrossRef CAS.
- B. J. Yao, L. G. Ding, F. Li, J. T. Li, Q. J. Fu, Y. J. Ban, A. Guo and Y. B. Dong, ACS Appl. Mater. Interfaces, 2017, 9, 38919–38930 CrossRef CAS.
- D. Ma, B. Li, K. Liu, X. Zhang, W. Zou, Y. Yang, G. Li, Z. Shi and S. Feng, J. Mater. Chem. A, 2015, 3, 23136–23142 RSC.
- N. A. Khan, Z. Hasan and S. H. Jhung, Chem. Commun., 2016, 52, 2561–2564 RSC.
- M. Ding and H.-L. Jiang, ACS Catal., 2018, 8, 3194–3201 CrossRef CAS.
- M. Taherimehr, B. Van de Voorde, L. H. Wee, J. A. Martens, D. E. De Vos and P. P. Pescarmona, ChemSusChem, 2017, 10, 1283–1291 CrossRef CAS.
- J. Liang, R. P. Chen, X. Y. Wang, T. T. Liu, X. S. Wang, Y. B. Huang and R. Cao, Chem. Sci., 2017, 8, 1570–1575 RSC.
- B. Aguila, Q. Sun, X. Wang, E. O’Rourke, A. M. Al-Enizi, A. Nafady and S. Ma, Angew. Chem., Int. Ed., 2018, 57, 10107–10111 CrossRef CAS.
- G. Ferey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef CAS.
- A. Mehrdad and E. Parvini, J. Mol. Liq., 2017, 243, 324–332 CrossRef CAS.
- X. Y. Li, Y. Z. Li, Y. Yang, L. Hou, Y. Y. Wang and Z. Zhu, Chem. Commun., 2017, 53, 12970–12973 RSC.
- G. Chen, Y. Zhang, J. Xu, X. Liu, K. Liu, M. Tong and Z. Long, Chem. Eng. J., 2020, 381, 122765 CrossRef CAS.
- V. Zeleňák, M. Skřínska, A. Zukal and J. Čejka, Chem. Eng. J., 2018, 348, 327–337 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and figures/tables referred to in the text. See DOI: 10.1039/d1nj04914c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |