Fungal bioactive macrolides†
Antonio
Evidente
 *
*
Department of Chemical Sciense, University of Naples Federico II, Complesso Universitario Monte S. Angelo, Via Cintia 4, 80126, Naples, Italy. E-mail: evidente@unina.it
First published on 20th June 2022
Abstract
Covering: 2000 to 2022
Natural products are a vital source of compounds for use in agriculture, medicine, cosmetics, and other fields. Macrolides are a wide group of natural products found in plants and microorganisms. They are a group of polyketides constituted of different-sized rings and characterized by the presence of a lactone group. These compounds show different biological activities, such as antiviral, antiparasitic, antifungal, antibacterial, immunosuppressive, herbicidal, and cytotoxic activities. This review is focused on macrolides isolated from fungal sources, examining their biological activities, stereochemistry, and structure–activity relationships. The review reports the chemical and biological characterization of fungal macrolides isolated in the last four decades, with assistance from SciFinder searches. A critical evaluation of the most recent reviews covering this area is also provided. The content provided in this review is of interest to chemists focusing on natural substances, plant pathologists and physiologists, botanists, mycologists, biologists, and pharmacologists. Furthermore, it is of interest to farmers and agri-food specialists and those working in the medicinal and cosmetic industries due to the potential practical application of macrolides. Politicians could also be interested in this class of natural compound, as the practical application of these macrolides in the above-cited fields could reduce environmental pollution and increase consumer satisfaction with respect to food, providing reduced or zero risk to human and animal health along with increased nutraceutical value.
1 Introduction
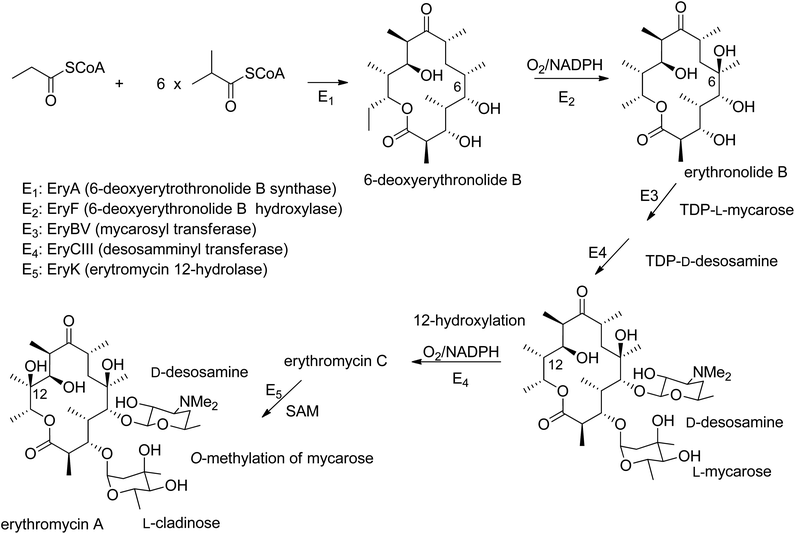
Natural products are a vital source of components for use in agriculture, medicine, cosmetics, and other fields. Despite advances in the isolation and structural characterization of organic compounds, only a fraction of existing natural products has been reported to date.1 Among living organisms, fungi are able to produce secondary metabolites belonging to numerous classes of naturally occurring compounds, and these frequently consist of original and complex carbon skeletons. Macrolides are often reported in the form of secondary metabolites produced by phytopathogenic, marine, and endophytic fungi. These compounds show various biological activities, such as antiviral, antiparasitic, antifungal, antibacterial, immunosuppressive, herbicidal, and cytotoxic activities.2 They are a group of polyketides constituted of different-sized rings and characterized by the presence of a lactone group.3 The term “macrolide” was introduced for the first time by Woodward in 1957,4 and it has been extensively used to describe antibiotics with 14-, 15-, and 16-membered rings.2 Research carried out in the last few decades has extended this term to smaller-sized macrocyclic lactones, such as 10-, 11-, 12-, and 13-membered rings, and to compounds with rings constituted of more than 16 members. The largest discovered natural macrolide is a lactone constituted of a 66-membered ring. The biosynthetic pathways of macrolides start, as expected, from acetate units, but they also involve propionate units. Macrolides with antibiotic activities have been extensively studied in terms of practical application and as biosynthesis models. Erythromycin A, which is produced from the soil-dwelling bacterium Saccharopolyspora erythraea, represents a biosynthesis model (Fig. 1). In fact, common metabolic intermediates are employed in the formation of erythromycins, and the nature of the enzymatic reactions occurring in the formation of these precursors is of interest. Similar processes are involved in the synthesis of other complex natural products.5,6In the present manuscript, the sources, isolation, and biological characterization of fungal macrolides obtained from 1980 to 2021 are reported. Furthermore, a critical evaluation of recent reviews concentrating on macrolides is also provided. The content of this review is of interest to chemists of natural substances, plant pathologists and physiologists, botanists, mycologists, biologists, and pharmacologists. Furthermore, it should also be of interest to farmers and agri-food specialists and those working in medicinal and cosmetic industries due to the potential practical application of macrolides.
2 Critical discussion of recent reviews covering macrolides
A review published in 2021 selectively reported on macrolides isolated from different marine organisms, including sponges, marine microorganisms and zooplankton, cnidarians, mollusks, red algae, bryozoans, and tunicates.7 The same topic, but restricted to marine macrolides with antibacterial and antifungal activities, was covered in Karpiński's review in 2019.8 Another review, also published in 2021, highlighted structures, mechanisms of action, pharmacology, and human cellular interactions in relation to some macrolides isolated from different organisms that may have potential therapeutic applications.2 Some reviews have also focused only on macrolides belonging to one class of lactones, for example the review by Drager et al. in 19969 that provided an overview of 10-membered lactones of natural origin; the review by Janas and Przybylski in 201910 that reported 14- and 15-membered lactone macrolides; and the review by Arsic et al. in 201811 that focused on 16-membered antibiotic macrolides. However, an advanced review on bioactive fungal macrolides, not restricted to marine organisms or focusing only on specifically sized macrolides, is yet to be reported.3 Sources, isolation, and biological activities of fungal macrolides
This discussion below describes fungal sources and the isolation and structures of macrolides, divided into subsections on the basis of the ring-size, starting from 10-membered rings and moving up to 20-or-higher-membered macrolides. This discussion also describes biological activities. A table (Table 1) reporting compound names, ring sizes, fungus producers, biological activities, and related references is provided for all macrolides.| Number | Number of ring members | Compound | Fungal producer | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | 10 | Diplodialide A | Diplodia pinea | Not tested | 12 |
| 2 | “ | Diplodialide B | “ | “ | “ |
| 3 | “ | Diplodialide C | “ | “ | “ |
| 4 | “ | Diplodialide D | “ | “ | “ |
| 5 | “ | Pyrenolide A | Pyrnophora teres | Fungicidal | 13 and 14 |
| 6 | “ | Pyrenolide B | “ | “ | “ |
| 7 | Pyrenolide C | “ | “ | “ | |
| 8 | “ | Thiobiscephalosporide A | Caphalosporium aphidicola | Antibiotic | 16 and 17 |
| 9 | “ | Thiobiscephalosporide B | “ | Not tested | 19 |
| 10 | “ | Thiobiscephalosporide C | “ | “ | |
| 11 | “ | Pinolidoxin | Dimydella pinodes | Phytotoxic | 20 and 23–26 |
| 12 | “ | 7-epi-pinolidoxin | “ | “ | 21 |
| 13 | “ | 5,6-Dihydropinolidoxin | “ | “ | 21 |
| 14 | “ | 5,6-Epoxypinolidoxin | “ | Non-toxic | 21 |
| 15 | “ | Pinolide | “ | Phytotoxic | 28 |
| 16 | “ | Microcarpalide | Endophytic fungus of Ficus microcarpa L. | Anti-microfilament | 29–33 |
| 17 | “ | Cephalosporide G | C. aphidicola | Not tested | 38 |
| 18 | “ | Putaminoxin | Phoma putaminum | Phytotoxic | 25 and 39 |
| 19 | “ | Putaminoxin B | “ | Non-toxic | 40 |
| 20 | Putaminoxin C (cyclononendione) | “ | Phytotoxic | 40 | |
| 21 | “ | Putaminoxin D | “ | Non-toxic | 41 |
| 22 | “ | Putaminoxin E | “ | “ | 41 |
| 23 | “ | 5-O-Formylputaminoxin B | “ | “ | 41 |
| 24 | “ | Herbarumin I | Phoma erbarum | Inhibition of radicle growth; inhibition of calmodulin-dependent enzymes | 43, 46 and 47 |
| 25 | “ | Herbarumin II | “ | “ | “ |
| 26 | “ | Herbarumin III | “ | 48 | |
| 27 | “ | Multiplolides A | Xylaria multiplex | Antiprotozoal | 50 |
| 28 | “ | Multiplolides B | “ | “ | “ |
| 29 | “ | Modiolide A | Paraphaeosphaeria sp. (N-119) | Antimicrobial; phytotoxic | 52 and 61 |
| 30 | Modiolide B | “ | Antimicrobial | “ | |
| 31 | “ | Stagonolide | Stagonospora cirsii | Phytotoxic; weakly antibiotic | 59 |
| 32 | “ | Stagonolide B | “ | Non-toxic | 60 |
| 33 | “ | Stagonolide C | “ | Phytotoxic | “ |
| 34 | “ | Stagonolide D | “ | Non-toxic | “ |
| 35 | “ | Stagonolide E | “ | “ | “ |
| 36 | “ | Stagonolide F | “ | “ | “ |
| 37 | “ | Stagonolide G | “ | “ | 61 |
| 38 | “ | Stagonolide H | “ | Phytotoxic | “ |
| 39 | “ | Stagonolide I | “ | “ | “ |
| 40 | “ | Stagonolide J | “ | Phytotoxic, cytotoxic, and antiprotozoal | 62 |
| 41 | “ | Stagonolide K | “ | “ | “ |
| 42 | “ | Stagochromene (chromene-4,5-dione) | “ | “ | “ |
| 43 | “ | Hypocreolide A | Hypocrea lacteal | Moderately antimicrobial | 63 |
| 44 | 11 | Aspercyclides A | Aspergillu sp. | Inhibition of IgE binding | 66 and 67 |
| 45 | “ | Aspercyclides B | “ | Not active | “ |
| 46 | “ | Aspercyclides C | “ | “ | “ |
| 47 | 10 | Sumalactone A | Penicillium sumatrense | “ | 68 |
| 48 | “ | Sumalactone B | “ | “ | “ |
| 49 | 11 | Sumalactone C | “ | “ | “ |
| 50 | “ | Sumalactone D | “ | “ | “ |
| 51 | 12 | Curvularin | Curvularia spp. | Not tested | 69–71 |
| 52 | “ | α,β-deydrocurvularin | “ | Inhibition of IgE binding | “ |
| 53 | “ | cis-Deydrocurvularin | Penicillium citrinoviride | “ | 72 |
| 54 | “ | Citreofuran | “ | “ | “ |
| 55 | “ | 12-Oxocurvularin | “ | “ | “ |
| 56 | “ | 11α-Hydroxycurvularin | “ | “ | “ |
| 57 | “ | 11β-Hydroxycurvularin | “ | Sporulation suppressing factor | 72–74 |
| 58 | “ | 11α-Methoxycurvularin | “ | Not tested | 73 |
| 59 | “ | 11-b-Methoxycyrvularin | “ | “ | “ |
| 60 | “ | 11,12-Dihydroxycurvularin | “ | “ | “ |
| 61 | “ | 12-Hydroxy-10,11-trans-dehydrocurvularin | “ | “ | “ |
| 62 | “ | Cladospolide A | Cladosporium cladosporioides | Root growth inhibitor | 75 |
| 63 | “ | Cladospolide B | “ | Root growth promoter | “ |
| 64 | “ | Cladospolide C | Cladosporium tenuissimum | Not tested | 76 |
| 65 | “ | Cladospolide D | Cladosporium sp. | Fungicidal | 77 |
| 66 | “ | Patulolide B | Penicillium urticae | Specific antimicrobial | 79 |
| 67 | “ | Patulolide C | “ | “ | “ |
| 68 | “ | Patulolide A | Mutant of P. urticae | Specific antimicrobial | 79 and 82 |
| 69 | “ | (5S)-5-hydroxylasiodiplodin | Lasiodiplodia theobromae | Weak potato microtuber inducer | 83 |
| 70 | “ | (5R)-5-hydroxylasiodiplodin | “ | “ | “ |
| 71 | “ | 5-Oxalasodiplodin | “ | “ | “ |
| 72 | “ | (3R),(4S)-4-hydroxylasiodiplodin | “ | “ | 85 |
| 73 | “ | (3R),(6R)-6-hydroxy-de-O-methyllasiodiplodin | “ | “ | “ |
| 74 | “ | (3R),(5R)-5-hydroxy-de-O-methyllasiodiplodin | “ | “ | “ |
| 75 | “ | Pandangolide 1 | Fungal strain I96S215 | No activity | 86 |
| 76 | “ | Pandagolide 2 | “ | “ | “ |
| 77 | “ | Pandagolide 3 | Cladosporium herbarum | “ | 87 |
| 78 | “ | Pandagolide 4 | “ | “ | “ |
| 79 | “ | Sporiolides A | Cladosporium sp. | Cytotoxic and antifungal | 88 |
| 80 | “ | Sporiolides B | “ | Cytotoxic and antibacterial | “ |
| 81 | “ | 6-Oxo-de-O-methyllasiodiplodin | Endophytic fungus ZZF36 | Antibacterial | 89 |
| 82 | “ | (E)-9-etheno-lasiodiplodin | “ | “ | “ |
| 83 | “ | Curvulone A | Curvularia sp. | Not tested | 90 |
| 84 | “ | Dendrodolide A | Dendrodochium sp. | Cytotoxic | 91 |
| 85 | “ | Dendrodolide B | “ | “ | “ |
| 86 | “ | Dendrodolide C | “ | “ | “ |
| 87 | “ | Dendrodolide D | “ | “ | “ |
| 88 | “ | Dendrodolide E | “ | “ | “ |
| 89 | “ | Dendrodolide F | “ | “ | “ |
| 90 | “ | Dendrodolide G | “ | “ | “ |
| 91 | “ | Dendrodolide H | “ | “ | “ |
| 92 | “ | Dendrodolide I | “ | “ | “ |
| 93 | “ | Dendrodolide J | “ | “ | “ |
| 94 | “ | Dendrodolide K | “ | “ | “ |
| 95 | “ | Dendrodolide L | “ | “ | “ |
| 96 | “ | Dendrodolide M | “ | “ | “ |
| 97 | “ | Dendrodolide N | Plenodomus influorescence co-cultured with Pyrenochaeta nobilis | No activity | 92 |
| 98 | “ | Sumalarin A | Penicillium sumatrense | Cytotoxic activity | 93 |
| 99 | “ | Sumalarin B | “ | “ | “ |
| 100 | “ | Sumalarin C | “ | “ | “ |
| 101 | “ | (3R,7R)-7-Hydroxy-de-O-methyllasiodiplodin | Trichoderma sp. (strain 307) | Inhibition of glucosidase | 94 |
| 102 | “ | (3R)-5-oxo-de-O-methyllasiodiplodin | “ | “ | “ |
| 103 | “ | (3R)-7-Oxo-de-O-methyllasiodiplodin | “ | No activity | “ |
| 104 | “ | Thiocladospolide F | Cladosporium oxysporum | No activity | 95 |
| 105 | “ | Thiocladospolide G | “ | Cytotoxic | “ |
| 106 | “ | Thiocladospolide H | “ | No activity | “ |
| 107 | “ | Thiocladospolide I | “ | “ | “ |
| 108 | “ | Thiocladospolide J | “ | “ | |
| 109 | 13 | Brefeldin | Several fungi sp. | Phytotoxic; cytotoxic | 96–98 |
| 110 | “ | Thermolide A | Talaromyces thermophiles | Immunosuppressant; anticancer; antibiotic; antitubercular; nematocidal | 99–102 |
| 111 | “ | Thermolide B | “ | “ | “ |
| 112 | “ | Thermolide C | “ | Immunosuppressant; anticancer; antibiotic, antitubercular | “ |
| 113 | “ | Thermolide D | “ | “ | “ |
| 114 | “ | Thermolide E | “ | “ | “ |
| 115 | “ | Thermolide F | “ | “ | “ |
| 116 | “ | Maleaoride A | Penicillium meleagrinum var. viridiflavum | Antifungal activity | 103 |
| 117 | “ | Maleaoride B | “ | “ | “ |
| 118 | “ | PF1163 A | “ | “ | |
| 119 | “ | PF1163B | “ | “ | |
| 120 | “ | PF1163 D | “ | “ | |
| 121 | “ | PF1163 F | “ | “ | |
| 122 | “ | PF1163H | “ | “ | |
| 123 | 14 | Zearalenone | Fusarium sp. | Mycotoxic; growth promoting | 104–106 |
| 124 | “ | α-Zearalenol | Streptomyces griseu | Binding affinity to rat uterine estrogen receptor | 106 |
| 125 | “ | β-Zearalenol | Mucor bainier | “ | “ |
| 126 | “ | α-Zearalanol | Aspergillus ochraceous Aspergillus niger | “ | “ |
| 127 | “ | β-Zearalanol | “ | “ | “ |
| 128 | “ | 8′(S)-Hydroxyzearalenone | Saccharomyces cerevisiae or Streptomyces rimous or Cunninghamells bainieri | “ | “ |
| 129 | “ | 2,4-Dimethoxy zearalenone | “ | “ | “ |
| 130 | “ | Zearalenone 2-methyl ether | “ | No activity | “ |
| 131 | “ | Zearalenone-4-O-β-glucoside | “ | Binding affinity to rat uterine estrogen | “ |
| 132 | “ | 8′-Hydroxyzearalanol | Penicicillium sp. | No activity | 107 |
| 133 | “ | 2′-Hydroxyzearalanol | “ | “ | “ |
| 134 | “ | Hypothemycin | Coriolus versicolor, Hypomyces trichothecoides, Aigialu parvus | Antimalarial; cytotoxic; antimicrobial | 108, 109, 110, 111 and 113 |
| 135 | “ | Bartanol | Cytospora sp. | No activity | 111 |
| 136 | “ | Bartallol | “ | “ | “ |
| 137 | “ | 14-Macrolide | Varicosporina ramulosa | No activity | 112 |
| 138 | “ | “ | “ | Antifungal | “ |
| 139 | “ | “ | “ | No activity | “ |
| 140 | “ | “ | “ | Antifungal | “ |
| 141 | “ | “ | “ | “ | “ |
| 142 | “ | Aigialomycins A | Aigialus parvus | No activity | 115 |
| 143 | “ | Aigialomycins B | “ | “ | “ |
| 144 | “ | Aigialomycins C | “ | “ | “ |
| 145 | “ | Aigialomycins D | “ | Antimalarial | “ |
| 146 | “ | Aigialomycins E | “ | No activity | |
| 147 | “ | Cochliomycin A | Cochliobolus lanatus | Antilarval; antibiotic | 115 and 116 |
| 148 | “ | Cochliomycin B | “ | No activity | “ |
| 149 | “ | Cochliomycin C | “ | “ | “ |
| 150 | “ | Zeanol | Drechslera portulacae Paecilomyces sp. | Antiprotozoal; antifouling; cytotoxic | 115 and 117 |
| 151 | “ | LL-Z1640-1 | Cochliobolus lanatus, Lederle colture Z1640 | “ | 115 and 117 |
| 152 | “ | LL-Z1640-2 | “ | No activity | 117 |
| 153 | “ | LL-Z1640-3 | “ | “ | “ |
| 154 | “ | LL-Z1640-4 | “ | “ | “ |
| 155 | “ | Paecilomycin A | Paecilomyces sp. | Antifungal | 119 |
| 156 | “ | Paecilomycin B | “ | “ | “ |
| 157 | “ | Paecilomycin E | “ | “ | “ |
| 158 | “ | Paecilomycin F | “ | Antiplasmodial; cytotoxic | 115 and 119 |
| 159 | “ | Benquoine | Phomopsis sp., Mycosphaerella rubella | Antimicrobial; cytotoxic | 120 |
| 160 | “ | Seiricuprolide | Phomopsis sp., Seiridium cupressi (syn. Diplodia cupressi) | Phytotoxic | 121, 123 and 127 |
| 161 | “ | Pestalotioprolides A | Pestalotiopsis spp. | Not tested | 124 |
| 162 | “ | Pestalotioprolides B | “ | “ | “ |
| 163 | “ | Mutolide | Lepidosphaeria sp, Aplosporella javeedii | Anti-inflammatory; cytotoxic; antibacterial | 125 and 126 |
| 164 | “ | 6,7,8,9-Tetrahydromutolide | A. javeedii | Not tested | 126 |
| 165 | “ | Pestalotioprolides C | Pestalotiopsis microspora | Cytotoxic | 127 |
| 166 | “ | Pestalotioprolides D | “ | No activity | “ |
| 167 | “ | Pestalotioprolides E | “ | Cytotoxic | “ |
| 168 | “ | Pestalotioprolides F | “ | “ | “ |
| 169 | “ | Pestalotioprolides G | “ | No activity | “ |
| 170 | “ | Pestalotioprolides H | “ | “ | “ |
| 171 | “ | 7-O-Methylnigrosporolide | “ | Cytotoxic | “ |
| 172 | “ | Nigrosporolide | “ | No activity | “ |
| 173 | “ | 4,7-Dihydroxy-13-tetradeca-2,5,8-trienolide | “ | “ | “ |
| 174 | 16 | A26771B | Penicillium turbatum Penicillium fuscum and P. camembertii/clavigerum | Antimicrobial activity | 128 and 129 |
| 175 | “ | Berkeleylactone A | Penicillium fuscum and P. camembertii/clavigerum | Antimicrobial activity | 130 |
| 176 | “ | Berkeleylactone B | “ | “ | “ |
| 177 | “ | Berkeleylactone C | “ | “ | “ |
| 178 | “ | Berkeleylactone D | “ | “ | “ |
| 179 | “ | Berkeleylactone E | “ | “ | “ |
| 180 | “ | Berkeleylactone F | “ | “ | “ |
| 181 | “ | Berkeleylactone G | “ | “ | “ |
| 182 | “ | Berkeleylactone H | “ | “ | “ |
| 183 | “ | Rizoxhin | Rizhopus chinensis | Cytotoxic; phytotoxic; antifungal | 131 and 132 |
| 184 | “ | WF-1360B | Rizhopus sp. | Cytotoxic | 132 |
| 185 | “ | WF-1360C | “ | “ | “ |
| 186 | “ | WF-1360E | “ | “ | “ |
| 187 | “ | WF-1360F | “ | “ | “ |
| 188 | “ | Macrosphelides E | Periconia byssoide | Cytotoxic | 133 |
| 189 | “ | Macrosphelides F | “ | “ | “ |
| 190 | “ | Macrosphelides G | “ | “ | “ |
| 191 | “ | Macrosphelides H | “ | “ | “ |
| 192 | “ | Macrosphelides I | “ | “ | “ |
| 193 | “ | Macrosphelides A | Macrosphaeropsis sp. FO 5050 | Anti-cell adherence | 133–135 |
| 194 | “ | Macrosphelides B | “ | “ | “ |
| 196 | “ | Macrosphelides C | “ | “ | “ |
| 196 | “ | Macrosphelides D | “ | “ | “ |
| 197 | “ | Myrothecines A | Myrothecium roridum | Cytotoxic | 136 |
| 198 | Myrothecines B | “ | “ | “ | |
| 199 | Myrothecines C | “ | “ | “ | |
| 200 | 18 | Myrothecines H | “ | “ | 137 |
| 201 | “ | Myrothecines I | “ | “ | “ |
| 202 | “ | 12,13-Deoxyroridin E | “ | “ | “ |
| 203 | “ | 12′-Hydroxyroridin E | “ | “ | 137 and 138 |
| 204 | “ | Roridin E | “ | “ | 138 |
| 205 | “ | Verrucarin A | “ | “ | “ |
| 206 | “ | Verrucarin J | “ | “ | “ |
| 207 | “ | Roridin Q | “ | “ | 138 |
| 208 | “ | 2′,3′-deoxyroridin D | “ | Cytotoxic; antimicrobial | “ |
| 209 | “ | Roridin R | Myrotecium sp. | Cytotoxic | “ |
| 210 | 16 | Trichobotryside A | Trichobotrys effuse | Antifouling; cytotoxic; antimicrobial | 140 |
| 211 | 18 | Trichobotryside B | “ | “ | “ |
| 212 | “ | Trichobotryside C | “ | “ | “ |
| 213 | “ | Borrelidin | Streptomyces rochei co-culture with Rhinocladiella similis | Cytotoxic; antibacterial | 141 and 142 |
| 214 | “ | Borrelidin F | “ | “ | “ |
| 215 | 20 | Rickiol A | Hypoxylon rickii | Cytotoxic | 143 |
| 216 | “ | Rickiol B | “ | “ | “ |
| 217 | “ | Rickiol C | “ | Cytotoxic, antibiotic | “ |
| 218 | “ | Rickiol D | “ | Cytotoxic | “ |
| 219 | “ | Rickiol E | “ | “ | “ |
| 220 | 22 | Rickiol A2 | “ | Cytotoxic, antibiotic | “ |
| 221 | “ | Rickiol E2 | “ | Cytotoxic | “ |
| 222 | 24 | Rickiol A3 | “ | “ | “ |
| 223 | “ | Rickiol E3 | “ | Cytotoxic, antibiotic | |
| 224 | 22 | Wortmannilactone A | Talaromyces wortmannii | Cytotoxic | 144 |
| 225 | “ | Wortmannilactone B | “ | “ | “ |
| 226 | “ | Wortmannilactone C | “ | “ | “ |
| 227 | “ | Wortmannilactone D | “ | “ | “ |
| 228 | 24 | Eushearilide | Eupenicillium shearii | Antifungal | 145 |
| 229 | “ | JBIR-19 | Metarhizium sp. fE61 | “ | 146 |
| 230 | “ | JBIR-20 | “ | “ | “ |
| 231 | “ | Preussolide A | Preussia typharum | “ | 147 |
| 232 | “ | Preussolide B | “ | “ | “ |
| 233 | “ | 15G256α | Hypoxylon oceanicum | Antimicrobial | 148 and 149 |
| 234 | 25 | 15G256α-1 | “ | No activity | 148 |
| 235 | 24 | 15G256β | “ | “ | 148 and 149 |
| 236 | 25 | 15G256τ | “ | Antimicrobial | “ |
| 237 | 31 | 15G256ω | “ | “ | “ |
| 238 | 24 | Calcaride A | Calcarisporium sp. KF525 | Antimicrobial | 149 |
| 239 | “ | Calcaride B | “ | No activity | “ |
| 240 | 25 | Calcaride C | “ | “ | “ |
3.1. 10-membered macrolides
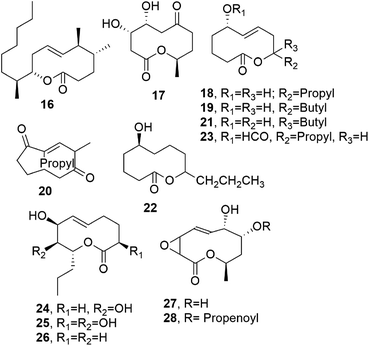
When considering the last four decades, the first four nonenolides, named diplodialides A–D (1–4, Fig. 2), were isolated from Diplodia pinea, which is a worldwide causal agent of the yellowing and necrosis of different species of pine, including exotic ones.12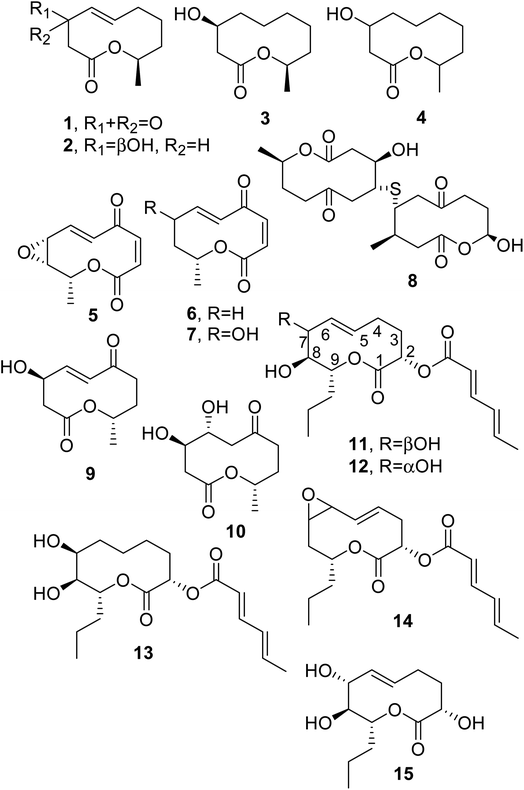 | ||
| Fig. 2 The structures of 10-membered macrolides isolated from Diplodia pinea (1–4), Pyrenophora teres (5–7), Cephalosporium aphidicola (8–10), and Didymella pinodes (11–15). | ||
Pyrenolide A (5, Fig. 2) was isolated from Pyrenophora teres, which is a worldwide pathogen of barley, barley grass, and wheat. Pyrenolide A showed growth inhibition and morphogenic activities toward fungi.13 Successively, two new nonenolides, named pyrenolides B and C (6 and 7, Fig. 2), were isolated from organic extracts of the same fungal culture filtrates. When compounds 5 and 6 were tested at a concentration of 50 μg per disc toward growing hyphae of the phytopathogenic fungus Cochliobolous lunata, the growth of the hyphae was inhibited, and they degenerated into many irregularly swollen hyphae.14 Some years later, pyrenolide D was also isolated from the same fungus, but it appeared not be a macrolide and instead was a tricyclic γ-spirolactone with cytotoxic activity.15
Thiobiscephalosporolide A (8, Fig. 2), is a thiobisnonenolide isolated from Cephalosporium aphidicola, a fungus very well known for the production of aphidicolin. This possesses strong antibiotic activity and showed a marked inhibitory effect toward the growth of deoxyribonucleic acid (DNA) viruses.16 The structure of compound 8 was determined via chemical spectroscopic and X-ray analysis.17 Some of its chemical transformations were subsequently reported.18 The industrial fermentation of C. aphidicola allowed for the isolation of other nonenolides, named cephalosporolides B and C (9 and 10, Fig. 2), together with other lactones.19
Ascochyta pinodes (subsequently reclassified as Didymella pinodes), when grown on wheat kernels, produced a tetrasubstituted nonenolide as a main phytotoxin, and this was named pinolidoxin (11, Fig. 2). A. pinodes causes peas (Pisum satioum L.) to develop anthracnose, resulting in severe lesions and the necrosis of plant leaves and pods. Pinolidoxin, when tested at 3 × 10−2 M via puncture assays on bean and pea leaves and on pods, caused a marked decrease in turgidity, followed by the appearance of severe necrotic lesions. On brine shrimp (Artemia salina L.) assayed at a concentration of 5.9 × 10−4 M, it induced weak mycotoxicity.20 Toxin 11 was isolated from culture filtrates of D. pinodes together with three analogues, which were characterized as 7-epi-, 5,6-dihydro-, and 5,6-epoxy-pinolidoxin, respectively (12–14, Fig. 2). Assayed on pea and bean leaves at 15 μg of toxin/droplet of solution, epi-pinolidoxin and dihydro-pinolidoxin caused necrotic lesions, whereas epoxy-pinolidoxin was inactive. In brine shrimp assays using a concentration of 5 μg mL−1, only epi-pinolidoxin and epoxy-pinolidoxin were active.21
At the time of the isolation of pinolidoxin (11), its absolute configuration was not established. An attempt made by some of the authors allowed for the determination of the absolute configuration at the C-7, C-8, and C-9 chiral centers of 11 using chemical and spectroscopic methods. In particular, four stereoisomeric fully benzoylated 2,3-erythro-1,2,3,4-heptanetetrols, corresponding to the C6–C18 portion of 11, were synthesized starting from meso-tartaric acid. Among them, the tetrabenzoate having “natural” stereochemistry and suitable for absolute configuration assignment was selected and compared with the natural analogue obtained from the degradation of 11. The determination of the absolute configuration at C-7, C-8, and C-9 was carried out via the application of Mosher's method22 to both intermediates and to pinolidoxin itself. In the same work, starting from commercially available D-erythronolactone, the stereoselective synthesis of a protected form of the C6–C18 portion of pinolidoxin was also described.23 The absolute configuration of compound 11 was subsequently assigned by Fustner et al. in 2002,24 who realized its total enantioselective synthesis and that of herbarumin I and II produced from Phoma herbarum (see below). Pinolidoxin and the herbarumins not only share the same lactone ring but they also have potential to act as bioherbicides. In particular, pinolidoxin (11) is a potent inhibitor of phenylalanine ammonia lyase (PAL) activity without having any effects on cell growth and viability.25,26 The ability of 11 to negatively affect plant self-defense renders nonenolides as highly promising lead structures in the search for novel herbicidal agents. This was also supported by the significant phytotoxic activity reported to be shown by lethaloxin (see below) isolated from Mycosphaerella lethalis, which resulted as being identical to 7-epi-pinolidoxin.27 The scant availability of natural pinolidoxin and the other cited nonenolides is a serious limitation when it comes to further investigating their biological activities and structure–activity relationships. Thus, the efforts of Fustner and collaborators was focused on overcoming this handicap, realizing the total enantioselective synthesis of 10-membered lactones based on a concise approach. The key step in this approach is ring-closure metathesis (RCM) to synthesize pinolidoxin and the herbarumin series, with their absolute configurations assigned starting from commercially available D-ribose.24
Subsequently, a new nonenolide, named pinolide (15, Fig. 2) was produced from a more aggressive strain of Didymella pinodes, isolated from an infected pea plant. Pinolide was isolated together with pinolidoxin, and herbarumin II and 2-epi-herbarumin II (see below), being pinolidoxin the main metabolite. Pinolidoxin showed phytotoxic activity towards the leaves of the host plant and other legumes and weeds, while the other nonenolides appeared to be non-toxic.28
Microcarpalide (16, Fig. 3), another nonenolide close to pinolidoxin, was isolated from the endophytic fungus of Ficus microcarpa L., a plant present on the Hawaiian Islands. A fungal organic extract showed strong anti-microfilament activity.29 Macrocarpalide showed anti-microfilament activity at a concentration of 5 μg mL−1, resulting in the loss of 50–75% of the regular actin cytoskeleton in A-10 rat smooth muscle cells. The previously reported cytotoxicity of 16 is significantly low, with IC50 values towards the KB and LoVo cancer cell lines of 50 and 90 μg mL−1, respectively.29 This noteworthy difference in bioactivity thresholds allows microcarpalide to be proposed as a potential lead structure in the preparation of non-toxic anti-actin agents30–32 to be used in chemical biology, medicinal chemistry, and crop protection.33
Microcarpalide and pinolidoxin were used as hydroxylated 10-membered macrolide models in comparison with latrunculin A, the marine nonenolide isolated from the Red Sea sponge Negombata magnifica and used as a standard in the field.35 Both compounds 11 and 16 showed less-potent selective actin-binding with respect to the standard and markedly reduced activity. This result prompted the re-evaluation of the biological activities of other fungal nonenolides, using microcarpalide as a model. Thus, its total enantioselective synthesis, similar to those of pinolidoxin and its analogues, was realized to have ring-closing metathesis as a key step.24 This work obviously permitted the assignment of the absolute configuration of 11, which was not previously determined during its first isolation from D. pinodes.20 Microcarpalide, pinolidoxin, and a series of synthesised 10-membered macrolides were assayed to estimate the effects of small molecules on the actin cytoskeleton. Most of the 10-membered macrolides tested at 5 μM showed a similar ability to induce detectable actin microfilament disruption. Pinolidoxin exhibited stronger activity than microcarpalide.33
Lethaloxin, as reported above, was isolated from M. lethalis, a pathogen of sweet clover Melilotus sp., causing so-called black stem.34 Lethaloxin showed antibacterial and antifungal activities, but its phytotoxicity was not assayed.27 This metabolite, whose stereochemistry was assigned, was also reviewed together with other metabolites produced by different Mycosphaerella strains.36 Its similarity with pinolidoxin was finally established by García-Fortanet et al. in 2005,37 who realized its stereoselective synthesis and that of microcarpalide, using the commercially available chiral reagents (R)-glycidol, (S,S)-tartaric acid, and D-ribose as starting materials and using a synthetic strategy different from that reported by Furstner et al. in 2002.24
Cephalosporolide G (17, Fig. 3) was isolated together with diplodialide B and Z-3-methylpent-2-en-l,5-dioic acid from Cephalosporium aphidicola. Compound 17 was shown to be an isomer of thiobiscephalosporoside C (10), produced, as reported above, by the same fungus.38
Another group of phytotoxic nonenolides, named putaminoxin and putaminoxin B, D, and E (18, and 19, 21, and 22, Fig. 3), was isolated together with a cyclononendione named putaminoxin C and 5-O-formylputaminoxin (20 and 23, Fig. 3) from culture filtrates of Phoma putaminum.39–41 This fungus was proposed as a mycoherbicide to biologically control Erigeron annuus L. Pers, which is also commonly known as annual fleabane. This is a weedy and indigenous plant from North America, and it is widely found in fields and pastures all over Europe. In particular, the S absolute configuration was assigned to the chiral carbinol C-5 using Horeau's GC method.42 When tested on leaves from host and non-host plants (several weeds and agrarian plants), putaminoxin (18) showed a wide toxicity range, performing strongly towards the leaves of E. annuus.39 As already cited for pinolidoxin, compound 18 was also a potent inhibitor of phenylalanine ammonia lyase (PAL) activity without having any effects on the cell growth and viability.25 Putaminoxin C (20), when assayed toward host and non-host plants, showed similar phytotoxicity to compound 18, while putaminoxin B (19) was non-toxic.40 Putaminoxin D and E (21 and 22) and the artefact 5-O-formylputaminoxin (23), obtained due to the acidification with formic acid of the fungal culture filtrate before extraction with EtOAc, did not show phytotoxicity.41 When assayed against the bacteria Pseudomonas sp. and Bacillus megaterium, the fungus Geotrichum candidum, and brine shrimp (Artemia salina L.), neither the putaminoxins nor compound 20 showed antibiotic, fungicide, or zootoxic activities.39–41
Two nonenolides, named herbarumins I and II (24 and 25, Fig. 3) were isolated from Phoma herbarum,43 which is a worldwide plant pathogen that can be isolated from herbaceous and weedy plants, soil, and water. This fungus also showed other biological activity, such as the in vitro inhibition of the growth of Chlorella pyrenoidosa and phytotoxicity upon the artificial infection of seedlings of Avena fatua44 and dandelion (Taraxacum officinale).45 When the compounds were applied to Amaranthus hypochondriacus L., which is a very dangerous weed, both nonenolides 24 and 25 induced the significant inhibition of seedling radicle growth.43 Their total synthesis was realized as mentioned above by Furstner et al. in 2001 and 2002.46,47
From the culture filtrates of the same fungus, a further nonenolide, named herbarumin III (26, Fig. 3), was isolated. Compounds 24–26 interacted with bovine-brain calmodulin, inhibiting the activation of the calmodulin-dependent enzyme AMP phosphodiesterase.48
Recently P. herbarum was isolated as the pathogen responsible for leaf spot disease suffered by cherry palm (Pseudophoenix sargentii) in Chiang Mai Province, Thailand.49
Multiplolides A and B (27 and 28, Fig. 3) are two nonenolides isolated from Xylaria multiplex BCC 1111, and they belong to a genus species group showing interesting antimycobacterial, antimalarial, and antifungal activities. However, the two nonenolides 27 and 28 when tested at 20 μg mL−1 did not show toxicity against the malarial parasite Plasmodium falciparum and were not cytotoxic towards the BC-1 and KB cell lines.50
Xestodecalactones A-C (Fig. S1 in the ESI†) were isolated from culture filtrates of Penicillium cf. montanense obtained from the marine sponge Xestospongia exigua, which was collected from the Bali Sea, Indonesia. They are similar to 10-membered macrolides, as they are constituted of fused nonenolide and resorcinol rings. In particular, xextodecalactones B and C, having different chirality at C-9 and C-11, are diastereomers. All nonenolides were tested for their antifungal activities, and only xestodecalactone B was active against the yeast Candida albicans.51 These nonenolides are close to several fungal metabolites, such as sporostatin (Fig. S1 in the ESI†), which was isolated from the terrestrial fungus Sporomiella sp. M5032. The latter compound is a potent and specific inhibitor of EGF (epidermal growth factor) receptor kinase.51
Modiolides A and B (29 and 30, Fig. 4) were isolated together with a new linear pentaketide, named modiolin, from the fungus Paraphaeosphaeria sp. (strain N-119). This fungus was obtained from the horse mussel Modiolus auriculatus, collected at Hedo Cape, Okinawa Island. Both nonenolides 29 and 30 showed antibacterial activity against Micrococcus luteus and antifungal activity against Neurospora crassa (MIC values of 16.7 and 33.3 μg mL−1, respectively).52
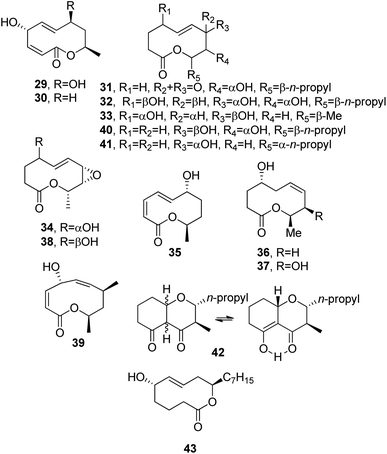 | ||
| Fig. 4 The structures of 10-membered macrolides isolated from Paraphaeosphaeria sp. (29 and 30), Stagonospora cirsii (31–42), and Hypocrea lacteal (43). | ||
Cirsium arvense L. is a perennial weed causing serious problems through arable land in North America, New Zealand, and Europe, including the European part of the Russian Federation. This weed has developed resistance to mechanical treatment methods and pesticides.53,54 Some phytopathogenic fungi, which all produce phytotoxins and could act as potential bioherbicides, were isolated from infected plant tissue in the form of Septoria cirsii and Phyllostitica cirsii. These two pathogens produced phytotoxic metabolites, such as β-nitropropionic acid,55 phyllostictines A–D,56,57 phyllostoxin, and phyllostin.58
Stagonospora cirsii, a foliar pathogen of C. arvense, was also proposed as a mycoherbicide for the biocontrol of this weed. Preliminary studies carried out on its liquid culture filtrates demonstrated that S. cirscii was also able to produce a plethora of phytotoxins. The main one was a nonenolide, named stagonolide (31, Fig. 4). Compound 31 was a non-host specific but selective phytotoxin. In fact, when assayed at 5 × 10−3 M, it was more toxic toward C. arvense leaves than tomato and pepper (both Solanaceae). When it was assayed at 5 × 10−6 M, it was a strong root growth inhibitor of host plant seedlings and some other Asteraceae species, such as lettuce, sow-thistle, and sunflower (Lactuca sativa, Sonchus arvensis and Helianthus annuus, respectively). It appeared less toxic toward wheat (Triticum aestivum) and radish (Raphanus sativus) seedling growth. Compound 31 was not toxic toward the protozoa Colpoda steinii and it exhibited only weak antimicrobial activity toward Candida tropicalis, Escherichia coli, and Bacillus subtillis.59 When grown on millet, S. cirsii produced eight other nonenolides, named stagonolides B-I (32–39, Fig. 4), while another resulting example was identified as modiolide A (29), the fungal nonenolide reported above. All the nonenolides (29 and 32–39) were assayed via a leaf-puncture method toward C. arvensis leaves and compared to stagonolide (31); only stagonolide H and I (38 and 39) and modiolide A showed phytotoxicity. Furthermore, only stagonolide H (39) inhibited chicory seedling root growth. As reported above for stagonolide (31), stagonolide C (33) exhibited weak toxicity toward C steinii, while the other stagonolides appeared to be non-toxic.60,61
Later, from the same fungus S. cirsii, a further nonenolide and nonanolide, named stagonolides J and K (40 and 41, Fig. 4), were isolated together with stagonolide (31), a close chromene-4,5-dione, named stagochromene A (42, Fig. 4), and herbarumin I (24). Stagochromene A (42) was isolated as a mixture of keto and enol tautomeric forms in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. All compounds isolated were tested on Sonchus arvensis via leaf puncture, and they showed different ranges of phytotoxicity, while antimicrobial, cytotoxic, and antiprotozoal activities were modest or absent.62
1 ratio. All compounds isolated were tested on Sonchus arvensis via leaf puncture, and they showed different ranges of phytotoxicity, while antimicrobial, cytotoxic, and antiprotozoal activities were modest or absent.62
Hypocreolide A (43, Fig. 4) is a nonenolide purified from culture filtrates of Hypocrea lacteal IBWF 02002, which is a strain obtained from fruiting bodies collected near Kaiserslautern, Germany. Its absolute configuration was initially not assigned due to the flexibility of the macrocyclic ring, but it was subsequently attributed upon its total enantioselective synthesis using transition-metal-catalyzed asymmetric reactions. Nonenolide 43 showed moderate antimicrobial activities against various tested fungi and bacteria.63
Xestodecalactones D–F (Fig. S2, ESI†) are three compounds close to nonenolides that were isolated together with corynesidone D and four other known compounds from the culture of an organic extract of Corynespora cassiicola.64 The fungus was obtained from the leaves of the Chinese mangrove Laguncularia racemosa which is spread along the Pacific and Atlantic coasts and tropical southern Asia and used as a medicinal plant.65 Xestodecalactone D–F are constituted of a nonenolide ring fused to a 1,2,3-trisubstituted benzene. All compounds were tested against a panel of human pro-21-tein kinases and only corynesidone B and 6-(3-hydroxybutyl)-7-O-methylspinochrome B inhibited some kinases, such as IGF1-R and VEGF-R2, with IC50 values in the low micromolar range.66
3.2. 11-membered macrolides
The family of 11-membered macrolides is small with respect to the other-sized groups. Natural compounds belong to this group and those described up to 2008 were reviewed by Ferraz et al.,66 who reported their structures and illustrated different total synthesis methods.Here, briefly, data reported by Ferraz et al. in 200866 on the sources and biological activities of 11-membered macrolides are integrated with the related literature to date.
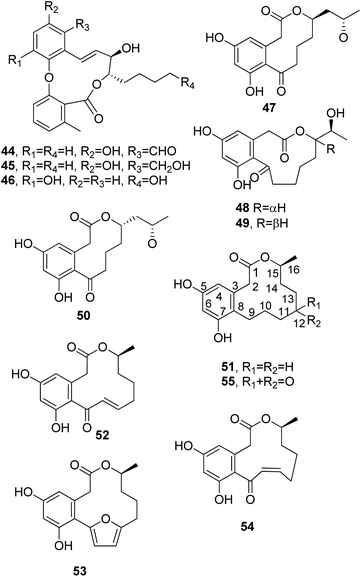
Aspercyclides A–C (44–46, Fig. 5), which are biphenylether-lactones, were isolated from an extract of Aspergillus sp. obtained from a soil sample collected in Olduvai Gorge, Upper Strata, Tanzania.67 The fungal strain resulted from screening carried out to find compounds to treat allergic diseases; immunoglobin E (IgE) binds to the high-affinity IgE receptor on mast cells and basophils and causes release of inflammatory compounds. Thus, natural substances that are able to inhibit ligand binding and avoid the production of these toxins causing inflammation can consequently reduce or block asthma and other allergic diseases. An organic extract of a culture of Aspergillus sp. showed this type of inhibitory activity. Among three 11-membered macrolides isolated, aspercyclide A (44) inhibited IgE binding, with an IC50 value of 200 μM.66,67
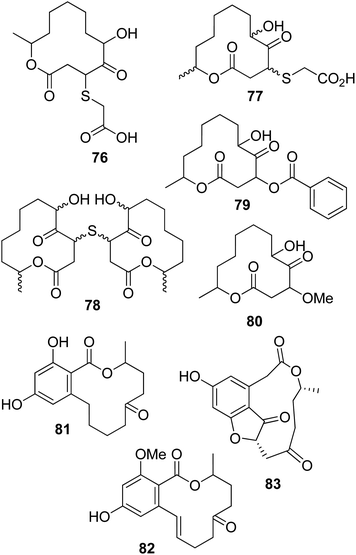
Sumalactones A–D (47–50, Fig. 5), four differently sized benzomacrolides, were isolated together curvularin and α,β-dehydrocurvularin, which are 12-membered macrolides (51 and 52, Fig. 5, see below), from Penicillium sumatrense, which is a marine fungus obtained from deep-sea sediment from the Indian Ocean. Sumalactones A and D (47 and 50) contain 10-membered macrocyclic rings, while sumalactones B and C (48 and 49) are constituted of 11-membered rings and thus belong to this rare group of macrolides reported above. All compounds were assayed for their inhibitory activities toward LPS-induced NO production in RAW 264.7 macrophages. Only α,β-dehydrocurvularin (52) showed strong inhibition of NO production, with an IC50 value range of 0.91–0.03 μM; in comparison, the positive control L-NMMA (NG-monomethyl-L-arginine) had an IC50 value range of 41.91–1.27 mM.68
3.3. 12-membered macrolides
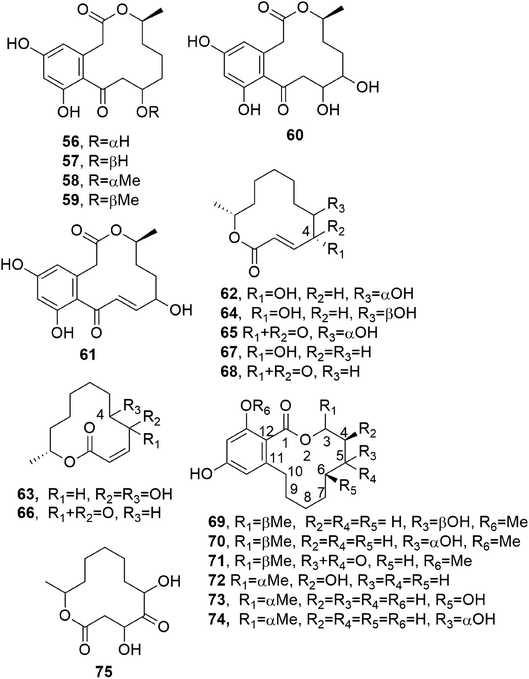
Curvularin-type macrolides are 12-membered-ring keto lactones fused to 1,3-diydroxybenzene (resorcinol). The first two members of this group isolated as naturally occurring compounds were curvularin and α,β-dehydrocurvularin (51 and 52), and these were obtained from Curvularia species.69,70 At that time, some studies of the biosynthesis of curvularin showed that the head-to-tail linkage of eight acetic acid units is involved in its pathway.71Cis-dehydrocurvularin, citreofuran, 12-oxocurvularin (53–55, Fig. 5), 11-α-hydroxy-curvularin and 11-β-hydroxy-curvularin (56 and 57, Fig. 6) were isolated together with curvularin and α,β-dehydrocurvularin from the mycelium of the hybrid strain ME 0005 derived from Penicillium citreo-viride B. IFO 4692 and 6200.72 Subsequently, 11-α-methoxy-, 11-β-methoxy-, 11,12-dihydroxy-, and 12-hydroxy-10,11-trans-dehydro-curvularin (58–61, Fig. 6) were also isolated from the same fungus.73 Furthermore, previously reported β-hydroxycurvularin, isolated together with β-deydrocurvularin from Alternaria tomato as sporulation-suppressing factors,74 was identified as 11-β-hydroxycurvularin (57) upon a comparison of its spectroscopic data with those of 11-α-hydroxy- and 11-β-hydroxy-curvularin (56 and 67).73
Cladospolides A and B (62 and 63, Fig. 6), two diastereomeric 12-macrolides were isolated as two plant growth promotion agents from culture filtrates of Cladosporium cladosporioides FI-113.75 When assayed on lettuce (Lactuca sativa L., cv. Great Lakes 366) and rice (Oryza sativa L., v. Tosan 38) seedlings at 100 ppm, compound 62 inhibited root elongation by about 60%. In contrast, macrolactone 63, tested at 100 and 300 ppm, promoted the root elongation of seedlings by about 20% and 30%, respectively. Thus, these results showed the close relationship between biological activity and stereochemistry, as the inverted configuration of the hydroxyl group at the C-4 position strongly affected the activity.75 Subsequently, a third diastereomer, named cladospolide C (64, Fig. 6), was isolated together with compounds 62 and 63 from Cladosporium tenuissimum, whose culture filtrates showed retardant activity toward rice seedlings.76 Further investigations allowed the isolation of cladospolide D (65, Fig. 6) from Cladosporium sp.77 Compound 65, when assayed at a concentration of 1 mg mL−1 (10 μg per disk), inhibited Pyricularia oryzae KB180 and Mucor racemosus KF223 (IFO 4581), with IC50 values of 29 and 0.15 μg mL−1, respectively. No antimicrobial activity was observed towards several other tested bacteria and fungi.77 The total enantioselective synthesis of cladospolide C (64) was realized in eight steps with 5% total yield, using methyl acrylate, (3R,4R)-1,5-hexadiene-3,4-diol, and (6R)-6-hepten-2-ol as the starting materials. Two cross-metathesis reactions and Yamaguchi esterification were used to obtain 64.78
Patulolides B and C (66 and 67, Fig. 6) were isolated from culture filtrate of Penicillium urticae S11R59,79 which is one of the mutants used to performed studies of the biosynthesis of patulin.80 Patulin, a disubstituted dihydrofuropyran-2-one, is a well-known mycotoxin which usually contaminates apples, causing serious food safety issues around the world.81 Previously, from a culture of the mutant P. urticae S11 (ATCC 48165), a similar macrolide, named patulolide A (68, Fig. 6), was isolated.82 Patulolides A, B, and C were tested against several fungi, yeasts, and bacteria. Compounds 66 and 68 inhibited some of the microorganisms tested, and their activities were stronger than that of 67, which showed the weakest inhibition. Furthermore, the yeasts were more sensitive than the fungi. Macrolide 67 showed no antibacterial activity. Patulolide A (68) showed higher antibacterial activity against gram-positive bacteria than patulolide B (66), which, in turn, exhibited higher toxicity against enteric bacteria. Patulolide A and B (68 and 66) did not inhibit gram-negative bacteria.79
(5R)- and (5S)-5-hydroxylasiodiplodin and 5-oxalasiodiplodin (69–71, Fig. 6) were isolated from culture filtrate of Lasiodiplodia theobromae IFO. Compounds 69 and 70 had, only at very high concentrations, weak potato micro-tuber inducing activities.83 Theobroxide, jasmonic acid, and mellein were previously isolated from the same fungus.84 Subsequently, three hydroxylasiodiplodins were isolated from the fungal mycelium and characterized as (3R,4S)-4-hydroxylasiodiplodin, (3R,6R)-6-hydroxy-de-O-methyllasiodiplodin, and (3R,5R)-5-hydroxy-de-O-methyllasiodiplodin (72–74, Fig. 6). Their bioactivities were tested at a concentration of 10−4 M, with all compounds being active in terms of potato micro-tuber induction, and compound 72 shows the strongest activity. Jasmonic acid and theobroxide, tested under the same conditions, showed activity at concentrations of 10−6 and 10−5 M, respectively.85
Pandangolide 1 and pandangolide 2 (75, Fig. 6 and 76, Fig. 7) were isolated from culture filtrates of the fungal strain I96S215, which was obtained from a tissue sample of a marine sponge collected in Indonesia in October 1996. Iso-cladospolide B, seco-patulolide C, and the already known cladospolide B (63) were isolated from the same organic extract. When all the isolated compounds were tested against a panel of gram-positive and gram-negative bacteria and yeast at a concentration of 250 μg per well, activity was observed.86 Subsequently, pandangolide 3 and 4 (77 and 78, Fig. 7) were isolated together with the known pandangolide 2, cladospolide B, and iso-cladospolide B from organic extracts of Cladosporium herbarum. This fungus was obtained from the marine sponge Callyspongia aerizusa collected in Indonesia. 5-Hydroxymethyl-2-furancarboxylic acid, also known as Sumiki's acid, and its acetyl derivative were also isolated from the same organic extract. The latter two compounds were active against B. subtilis and Staphylococcus aureus, while they were inactive against E. coli and C. albicans. The other macrolides did not exhibit any inhibition of the growth of gram-positive and gram-negative bacteria.87
Sporiolides A and B (79 and 80, Fig. 7) were isolated from cultured filtrates of Cladosporium sp., a fungus which was obtained from the brown alga Actinotrichia fragilis collected from an Okinawan island in Japan. Macrolides 79 and 80 showed cytotoxicity against murine lymphoma L1210 cells with IC50 values of 0.13 and 0.81 μg mL−1, respectively. Sporiolide A (79) also showed antimicrobial activity against Candida albicans, Cryptococcus neoformans, Aspergillus niger, Neurospora crassa, and Micrococcus luteus, while sporiolide B (80) exhibited toxicity only against this last type of bacteria.88
6-Oxo-de-O-methyllasiodiplodin and (E)-9-etheno-lasiodiplodin (81 and 82, Fig. 7) were isolated together with lasiodiplodin, de-O-methyllasiodiplodin, and 5-hydroxy-de-O-methyllasiodiplodin from mycelium extracts of a brown alga endophytic fungus (no. ZZF36) obtained from the South China Sea. All the metabolites, except 82, which was isolated in very low amounts, were tested against six aerobic reference strains. De-O-methyllasiodiplodin and 5-hydroxy-de-O-methyllasiodiplodin showed the inhibition of S. aureus, with MICs of 6.25 and 100 μg mL−1, respectively, while lasiodiplodin inhibited the growth of S. aureus, B. subtilis, and Fusarium oxysporum, with MICs of 25, 50, and 100 μg mL−1, respectively.89
Curvulone A (83, Fig. 7) was isolated together with a di-substituted resorcine, named curvulone B, and a close to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of (11R,15R)-11-hydroxycurvularin and the R enantiomer of α,β-dehydrocurvularin from a strain of Curvularia sp. This fungus was obtained from the marine alga Gracilaria folifera.90
1 mixture of (11R,15R)-11-hydroxycurvularin and the R enantiomer of α,β-dehydrocurvularin from a strain of Curvularia sp. This fungus was obtained from the marine alga Gracilaria folifera.90
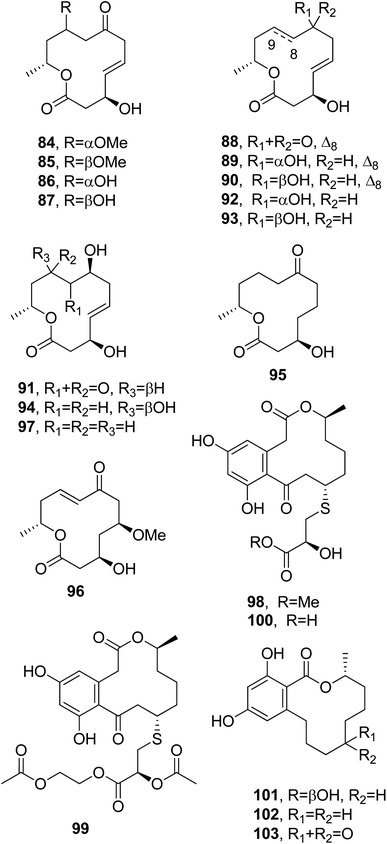
Dendrodolides A–M (84–96, Fig. 8) were isolated from Dendrodochium sp., a fungus which was obtained from the sea cucumber Holothuria nobilis Selenka, collected from the South China Sea. The cytotoxic activities of compounds 84–96 were tested in vitro against SMMC-7721, HCT116, and A549 tumor cell lines. Compounds 84–88, 90–92, 94, and 95 exhibited different degrees of inhibition of the growth SMMC-7721 and HCT116 cells, while no toxicity was observed toward A549 cells.91 Subsequently, dendrodolide N (97, Fig. 8) was isolated together with dendrodolide E (88), two rare azaphilones, namely spiciferinone and the new analog 8a-hydroxy-spiciferinone, and cephalochromin from a co-culture of Plenodomus influorescens and Pyrenochaeta nobilis, which were obtained from marine sediment isolated from Windebyer Noor (Baltic Sea).92
Among all the compounds tested, only cephalochromin showed anti-phytopathogenic activity against Xanthomonas campestris and Phytophthora infestans, with IC50 values of 0.9 and 1.7 μg mL−1, respectively.92
Sumalarins A–C (98–100, Fig. 8), which are sulphur-containing 12-membered macrolides, were isolated together with already known curvularin, dehydrocurvularin, and curvularin-7-O-β-D-glucopyranoside from the extract of Penicillium sumatrense MA-92. The fungus was obtained from the rhizospheres of the mangrove Lumnitzera racemose, which was collected at WenChang on Hainan island, China. All the compounds were assayed for their cytotoxicity against Du145, HeLa, Huh 7, MCF-7, NCI-H460, SGC-7901, and SW1990 tumor cell lines. Sumalarins A–C (98–100) and dehydrocurvularin showed cytotoxic activity against each of the tested cell lines, with IC50 values ranging from 3.8 to 10 μM, while curvularin was non-toxic.93
(3R,7R)-7-Hydroxy-de-O-methyllasiodiplodin, (3R)-5-oxo-de-O-methyllasiodiplodin, and (3R)-7-oxo-de-O-methyllasiodiplodin (101–103, Fig. 8) were isolated together with two new sesquiterpenes, named microsphaeropsisin B and C, and twelve already known compounds from a co-culture of Trichoderma sp. 307 and the aquatic pathogenic bacterium Acinetobacter johnsonii B2. All compounds were assayed for their glucosidase inhibitory and cytotoxic activities. Compounds 101 and 102 exhibited strong glucosidase inhibitory activity, with IC50 values of 25.8 and 54.6 μM, respectively.94
Thiocladospolides F–J (104–108, Fig. 9) were isolated together with known biogenetic-related analogues from the mangrove-derived endophytic fungus Cladosporium oxysporum, which was obtained from the surface-sterilized roots of Avicennia marina (Forssk.) Vierh (Acanthaceae). When tested for cytotoxic activity, only thiocladospolide G (105) showed activity against the aquatic pathogen Edwardsiella tarda, with a MIC value of 4 μg mL−1. The other tested compounds were non-toxic.95
3.4. 13-membered macrolides
Brefeldin A (109, Fig. 9) also named decumbin, cyanein, ascotoxin, and sinergisidin,96 is a 13-membered macrolide antibiotic isolated from a number of fungal genera: Alternaria, Ascochyta, Penicillium, Curvularia, Cercospora, and Phyllosticta.97 In fact, brefeldin A was also isolated with α,β-dehydrocurvularin (52) from Alternaria zinniae, a fungus proposed for the biocontrol of Xanthium occidentale that causes leaf necrosis on the weedy host plant. This plant is a widespread noxious weed in Australian summer crops and pastures.97 Subsequently, brefeldin A (109) was isolated as a cytotoxic metabolite from Paecilomyces sp. and Aspergillus clavatus, which were obtained from Taxus mairei and Torreya grandis collected in southeast China.98Thermolides A–F (110–115, Fig. 9) were isolated from Talaromyces thermophilus.99 They belong to a class of PKS-NRPS hybrid metabolites possessing a 13-membered lactam-bearing macrolactone; these are mainly reported as bacteria metabolites but rarely as fungi ones.100 This 13-membered macrolide subgroup shows a wide range of noteworthy biological activities, including immunosuppressant, anticancer, antibiotic, antitubercular, Hsp90 inhibiting, and antitumor activities.101,102 Thermolides A and B (110 and 111) exhibited strong inhibitory activity against three well-known nematodes, with LC50 values of 0.5–1 μg mL−1, comparable with avermectins, commercial nematocidal agents.99
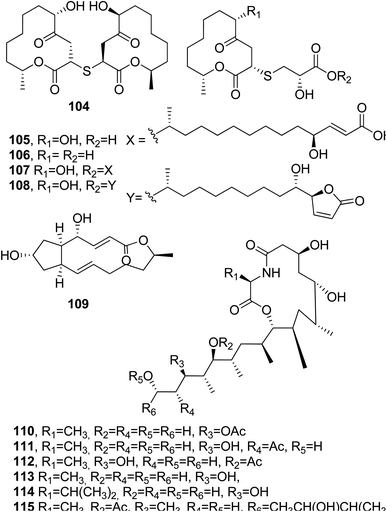
Melearorides A and B (116 and 117, Fig. 10) were isolated together with the closely related known 13-membered macrolides PF1163A, B, D, F, and H (118–122, Fig. 11) from Penicillium meleagrinum var. viridiflavum, which is a strain of a marine-derived fungus. The 13-membered macrolides 116–122 were tested via a checkerboard assay, and they showed synergistic activity with fluconazole against azole-resistant Candida albicans.103
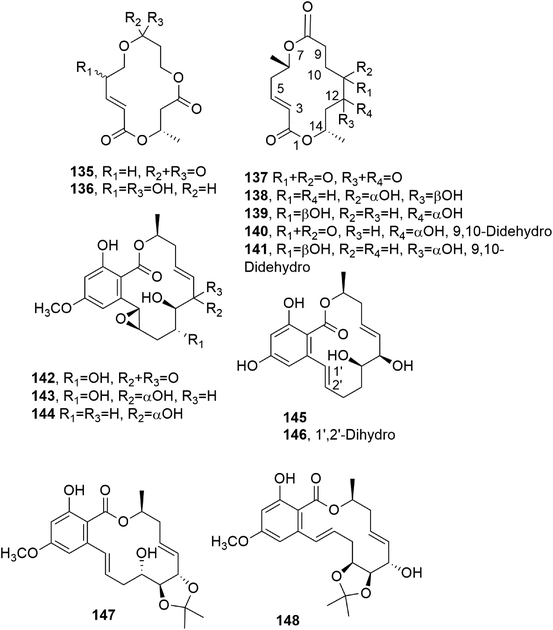 | ||
| Fig. 11 The structures of 14-membered macrolides isolated from Cytospora sp. (135 and 136),Varicosporina ramulosa (137–141), and Aigialus parvus (142–146) and Cochliobolus lanatus (147 and 148). | ||
3.5. 14-membered macrolides
Zearalenone (123, Fig. 10) is a 14-membered macrolide produced by several Fusarium species which possesses estrogenic and growth-promoting activities; it can impair fertility in cows and cause hyperestrogenism in swine with heavy consequent economic losses.104,105 Some studies were also carried out on its microbial transformation using different microorganisms. α- and β-zearalenol (124 and 125, Fig. 10) were obtained using Streptomyces griseus (ATCC 13273) and Mucor bainieri, respectively, while α-zearalanol and β-zearalanol (126 and 127, Fig. 10) were obtained from Aspergillus ochraceous (NRRL 405) and Aspergillus niger (X-170). Furthermore, when the fermentation process was induced by Saccharomyces cereuisiae (NRRL Y2034), Streptomyces rimosus (NRRL 2988), or Cunninghamella bainieri (ATCC 9244B), zearalenone, 8′(S)-hydroxyzearalenone, 2,4-dimethoxyzearalenone, zearalenone 2-methyl ether, and zearalenone-4-O-β-glucoside (123, 128–131, Fig. 10) were obtained. Among all the compounds tested (123, its 2-methoxy derivative, and 124–129), only the metabolites having a free 4-phenolic group had binding affinities toward rat uterine estrogen receptors.106Subsequently, 8′-hydroxyzearalanone and 2′-hydroxyzearalanol (132 and 133) were isolated together with zearalanone, β-zearalanol, zearalenone, and β-zearalenonol from the marine-derived fungus Penicilliumx sp. All of the six macrolides showed activity when tested for radical-scavenging and antibacterial activities against methicillin-resistant S. aureus and multi-drug-resistant S. aureus, for the ability to protect against ultraviolet A, and for the ability to inhibit the enzyme tyrosinase.107
Hypothecim (134, Fig. 10) is a resorcinol macrolide isolated from Colorius versicolor, and it showed strong anticancer activity against P38 leukemia cells. The cytotoxic activity was essentially due to 134. The structure previously reported when it was isolated from Hypomyces trichothecoides was corrected,108,109 and its relative configuration was assigned based on X-ray analysis.110
Bartanol and bartallol (135 and 136, Fig. 11) were isolated from Cytospora sp., and they showed antibiotic activity.111
Five 14-membered macrolides (135–139, Fig. 11) were isolated together with ergosterol from the fungus Varicosporina ramulosa obtained from Cytoseira sp., which is an alga collected from Tenerife, Spain. The macrolides 140 and 141 inhibited the growth of Eurotium repens, while compound 138 showed antifungal activity against both E. repens and Ustiloga violacea.112
Aigialomycins A–E (142–146, Fig. 11), other 14-membered resorcylic macrolides, were isolated together with the known compound hypothemycin (134) from the mangrove fungus, Aigialus parvus BCC 5311, whose extract showed antimalarial activity (IC50: 8 μg mL−1). Among all the compounds tested (142–146 and 134), only hypothemycin (134) and aigialomycin D (145) showed antimalarial activity, with IC50 values of 2.2 and 6.6 μg mL−1, respectively. They also exhibited cytotoxic activity against human epidermoid carcinoma (KB cells), human breast cancer cells (BC-1 cells), and African green monkey kidney fibroblasts (Vero cells).113 Hypothemycin (134) and dihydrohypothemycin, which were previously isolated from H. trichothecoides, showed antibiotic activity against protozoan Tetrahymena furgasoni and the plant pathogenic fungi Ustilago maydis and Botrytis allii.110,111 When other studies were conducted on metabolites produced from the fungus Aigialus parvus, six new nonaketides, namely aigialomycin F and G, 7,8-dihydroaigialospirol, 4-deoxy-7,8-dihydroaigialospirol, and two rearranged macrolides, were isolated together with aigialospirol, 4-O-demethylhypothemycin and aigialone.114
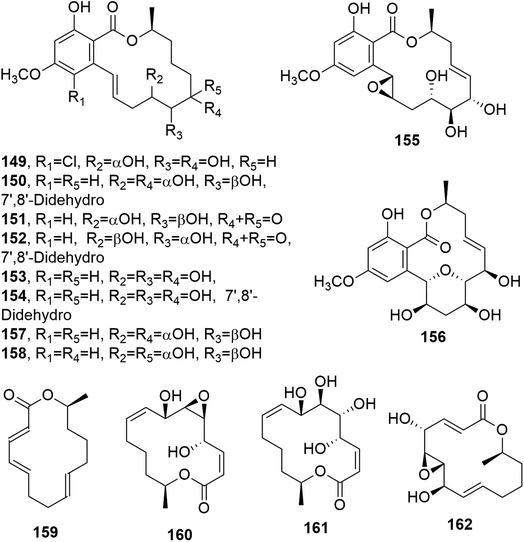
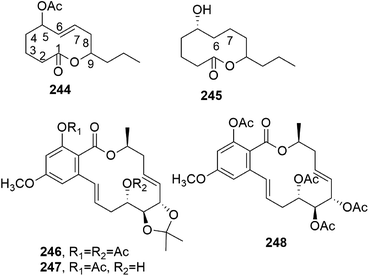
Cochliomycins A–C (147 and 148, Fig. 11, and 149, Fig. 12) are three 14-membered resorcylic lactones which were isolated together with the four analogues zeaenol, LL-Z1640-1, LL-Z1640-2 (150–152, Fig. 12), and paecilomycin F (158, Fig. 12) from the culture filtrate of Cochliobolus lunatus. This fungus was obtained from the gorgonian coral Dichotella gemmacea, collected from the Weizhou coral reef in the South China Sea.115 The absolute configurations of cochliomycin C (149) and paecilomycin F (158, Fig. 12) were subsequently corrected by the same authors.116 The macrolides 147 and 148 showed a rare natural acetonide group, while 149 is a 5-chloro-substituted lactone. Zeaenol (150) was previously isolated together with zearalenone (123) and two other closely related 14-membered macrolides from Drechslera portulacae, which is the causal agent of a leaf spotting disease affecting purslane (Portulaca oleracea). The latter is a noxious worldwide weed affecting many crops, such as corn, vegetables, cotton, and rice.117 The macrolides LL-Z1640-1 and LL-Z1640-2 (151 and 152, Fig. 12) were isolated together with the zearalenone analogues LL-Z1640-3 and LL-Z1640-4 (153 and 154, Fig. 12) from an unidentified fungus, Lederle culture Z1640. This fungus was investigated for the production of secondary metabolites, as its culture filtrate inhibited the growth and motility of ciliated protozoan Tetrahymena pyriformis.118 Paecilomycin F (158, Fig. 12) together with three other β-resorcylic lactones, named paecilomycins A, B, and E (155–157, Fig. 12), and aigilomycin zeaenol (148), aigialomycin D (143), aigialomycin F, and aigialospirol were isolated from a mycelial solid culture of Paecilomyces sp. SC0924. This fungus showed antifungal activity against Peronophythora litchii, which causes litchi (Litchi chinensis Sonn.) fruit rot. Paecilomycin C and D are two trisubstituted benzofuranones. When all the metabolites were assayed against Plasmodium falciparum line 3D7, compounds 158 and aigialomycin F showed antiplasmodial activity, with IC50 values of 20.0 and 10.9 nM, respectively, while aigialomycin B and paecilomycin E and F (157 and 158) exhibited moderate activity against P. falciparum line Dd2.119
All the metabolites of Cochliobolus lunatus were tested against a larval settlement of the barnacle Balanus amphitrite, inducing its total inhibition. Cochliomycin A (147) exhibited inhibitory activity against a larval settlement up to 5.0 μg mL−1 but it showed associated toxicity. The same compound (147), its 2,4-O,O′-diacetyl and 2-O-acetyl derivatives, and the macrolides 150, 151, and 158 showed strong antifouling activity at nontoxic concentrations, with EC50 values of 1.2, 15.4, 12.5, 5.0, 5.3, and 17.9 μg mL−1, respectively. Among the metabolites tested, only cochliomycin A (147) showed antibacterial activity against S. aureus, E. coli, and Micrococcus tetragenus. Furthermore, compound 151 showed moderate cytotoxicity against A549 and HepG2 tumor cell lines, with IC50 values of 44.5 and 98.6 μM, respectively.115
Phomopsis sp., isolated from healthy galanga (Alpinia malaccensis), produced eight polyketides in its liquid culture, including two 14-membered macrolides: benquoine and the δ-lactone 2,3-dihydroxytetradecan-5-olide (DHTO) (159 and 160, Fig. 12). Benquoine (159) is the ethyl ester derivative of 13-dihydroxytetradeca-2,4,8-trienoic acid, which is produced by Valsa ambiens. This compound (159) is concomitantly produced with 6,13-dihydroxytetradeca-2,4,8-trienoic acid (DHTTA) previously isolated from Mycosphaerella rubella.120 DHTO was previously isolated from Seiridium cupressi and named seiricuprolide.121 Seiricuprolide (160) was produced by S. cupressi (now reclassified as Diplodia cupressi) together with other phytotoxic metabolites, such as seridins, seiricardins, and cyclopaldic acid. This fungus, together with Seiridium cardinale and Seiridium unicorne, is the causal agent of canker suffered by Italian cypress (Cupressus sempervirens L.), which is a very destructive disease.122 The absolute configuration of 160 was subsequently determined via X-ray diffractometry analysis.123 Benquoine exhibits antimicrobial activity against gram-positive bacteria and cytotoxicity against the HCT-116 cancer cell line.120
Two macrolides closely related to seiricuprolide and named pestalotioprolides A and B (161 and 162, Fig. 12) were isolated together with three α-pyrones, named pestalotiopyrones A–C, from Pestalotiopsis spp. PSU-MA92 and PSU-MA119, which are two endophytic fungi obtained from the mangrove plants Rhizophora apiculata and Rhizophora mucronata, respectively.124
Mutolide (163, Fig. 13) was isolated from the coprophilous fungus Lepidosphaeria sp. (PM0651419), which was collected from Rajkot, India. Compound 163 showed anti-inflammatory activity, mitigating the LPS-induced secretion of pro-inflammatory cytokines TNF-α and IL-6 from THP-1 cells and from human peripheral blood mononuclear cells (hPBMCs). It also inhibited the secretion of another pro-inflammatory cytokine, IL-17, from anti-hCD3/anti-hCD28-stimulated hPBMCs. As NF-κB is a major transcription factor involved in the secretion of pro-inflammatory cytokines, including IL-17, an advanced study of the mode of action of mutolide (163) suggested that the compound inhibited NF-κB activation and translocation from the cytoplasm into the nucleus. Mutolide (163), administered orally at 100 mg kg−1 in mice, exhibited significant inhibition of the LPS-induced release of TNF-α from Balb/c in an acute model of inflammation. These results suggested that mutolide can be used to treat inflammatory diseases.125 6,7,9,10-Tetrahydromutolide (164, Fig. 13) was isolated together with mutolide (163) and five sesterterpenes from the endophytic fungus Aplosporella javeedii obtained from stem tissue of Orychophragmus violaceus (L.) (Brassicaceae) collected in Beijing. Mutolide (163) also showed significant cytotoxicity against the L5178Y cell line and against human Jurkat J16 and Ramos cells, with IC50 values of 0.4, 5.8, and 4.4 μM, respectively. Studies of its mode of action suggested that 163 also induces apoptotic cell death. In addition, it also showed low antibacterial activity against Mycobacterium tuberculosis H37Rv, and its close metabolite 162 showed activity against S. aureus, with a MIC of 100 μM.126
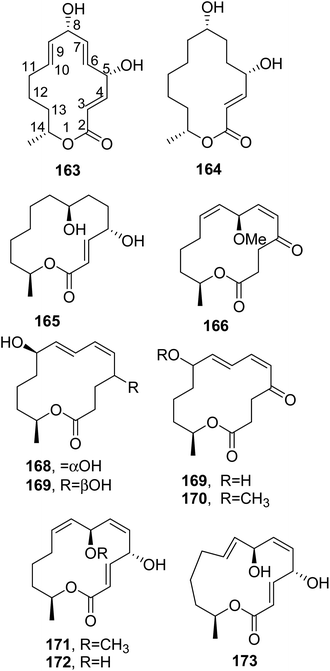 | ||
| Fig. 13 The structures of 14-membered macroldes isolated from Lepidosphaeria sp. and Aplosporella javeedii (163 and 164) and Pestalotiopsis microspore (165–173). | ||
Another fungus that produced seiricuprolide (160) is Pestalotiopsis microspora, and this also produced pestalotioprolide B-H (162, Fig. 12 and 165–170, Fig. 13), 7-O-methylnigrosporolide, nigrosporolide, and 4,7-dihydroxy-13-tetradeca-2,5,8-trienolide (171–173, Fig. 13). P. microspora is an endophyte obtained from fresh fruits of mangrove plants Drepanocarpus lunatus (Fabaceae) collected in Cameroon. Compounds 166, 167, 170 and 171 showed strong cytotoxic activity against the murine lymphoma cell line L5178Y, with IC50 values of 0.7, 5.6, 3.4, and 3.9 μM, respectively, while compound 168 also showed similar activity against the human ovarian cancer cell line A2780 with an IC50 value of 1.2 μM.127
3.6. 16-membered macrolides
The antibiotic A26671B (174, Fig. 14) was isolated together with a group of epipolythiopiperazinedione antibiotics from Penicillium turbatum, which was obtained from soil from Mt Ararat in Eastern Turkey.128 Compound 174, when tested at 100 μg mL−1, showed moderate activity against gram-positive bacteria, mycoplasma, and fungi, while it had no activity against Proteus sp., Salmonella typhosa, Salmonella typhimurium, the Klebsiella enterobacter group, E. coli, and Pseudomonas aeruginosa. In addition, it was inactive when administered subcutaneously against S. aureus, Streptococcus pyogenes, and Streptococcus pneumoniae infections in mice, and the toxicity resulted in an LD50 value of 62 mg kg−1.129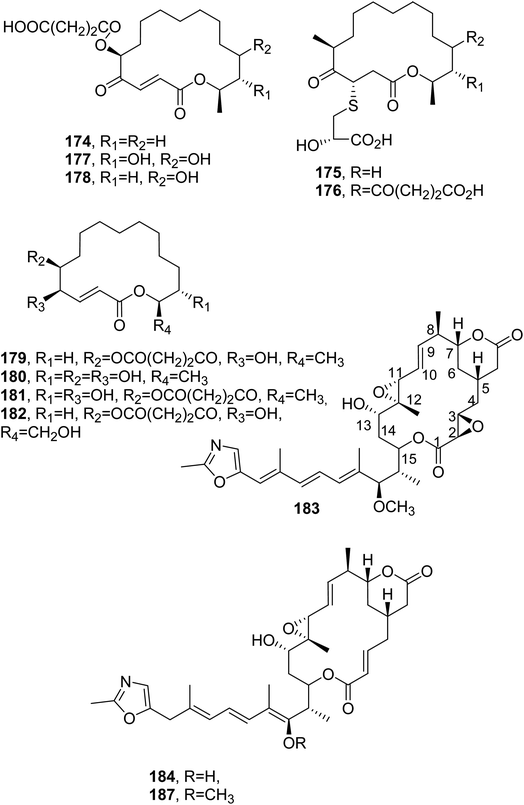 | ||
| Fig. 14 The structures of 16-membered macrolides isolated from Penicillium turbatum, Penicillium fuscum, and P. camembertii/clavigerum (174 and 175–182) and Rhizopus chinensis (183, 184 and 187). | ||
The antibiotic A26671B (174) was also subsequently isolated together with other 16-membered macrolides named berkeleylactone A–H (175–182, Fig. 14), patulin, and citrinin from the culture filtrate of co-cultured Penicillium fuscum and P. camembertii/clavigerum. These two extremophilic fungi were isolated from the surface of the water of Berkeley Pit lake. All compounds isolated were tested against a panel of gram-positive and gram-negative bacteria and three Candida species at concentrations from 1 μM to 1 mM per well. Among the macrolides tested, compound 175 showed the strongest antimicrobial activity, with a MIC of 1–2 μg mL−1 against four MRSA strains, including Bacillus anthracis, S. pyogenes, C. albicans, and Candida glabrata. Studies of its mode of action showed that compound 175 did not inhibit protein synthesis nor target ribosomes; thus, its antibiotic activity could be associated with a new mode of action.130
Rhizoxin (183, Fig. 14) is a toxin isolated from Rhizopus chinensis, which is a pathogenic fungus inducing rice seedling blight. Compound 183 caused the abnormal swelling of rice seedling roots when tested at 10 ng mL−1. Furthermore, it exhibited strong inhibiting activity against a variety of phytopathogenic fungi, with MIC values less than 1 μg mL−1 toward 10 tested fungi, except for Rhizopus chinensis and Fusarium oxysporum.131 Subsequently, four analogues of rhizoxin, named WF-1360B, WF-1360C, WF-1360E, and WF-1360F (184 and 187, Fig. 14 and 185 and 186, Fig. 15) were isolated from Rhizopus sp. no. F 1360. These macrolides showed cytotoxicity when tested against P388 leukemia cells in vitro. Compound 183 exhibited the strong inhibition of leukemia L1210 and melanoma B16 cells. The macrolides also showed significant antifungal activity but only weak antibiotic activity against some gram-positive and -negative bacteria.132
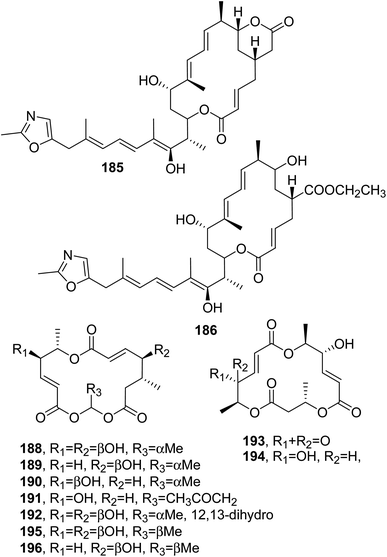 | ||
| Fig. 15 The structures of 16-membered macrolides isolated from Rhizopus (185 and 186) and Periconia byssoides (188–196). | ||
Macrosphelides E–I (188–192, Fig. 15) were isolated together with the already known macrosphelide A and C (193 and 195, Fig. 15) from a strain of Periconia byssoides, which was obtained from the sea hare Aplysia kurodai. Compounds 188–193 and 195 inhibited the adhesion of human-leukemia HL-60 cells to HUVEC.133 Macrospelides A and B (193 and 194, Fig. 15) and C and D (195 and 196, Fig. 15), which showed anti-cell adherence, were previously isolated from Macrosphaeropsis sp. FO 5050, although their absolute configurations were not reported at that time.134,135
3.7. 18-membered macrolides
Myrothecines A–C (197–199, Fig. 16), together with the already known roridin E and mytoxin B, were isolated from the two Myrothecium roridum strains IFB-E009 and FB-E012. These two isolates are endophytic fungi obtained from Trachelospermum jasminoides and Artemisia annua, which are two plants used in Chinese folk medicine. When tested against the human tumor cell line nasopharyngeal epidermoid KB, all the macrolides showed strong cytotoxic activity, with IC50 values of 8.5, 0.76, 32.21, 0.034 and 0.0022 μg mL−1 for 197–199, roridin E, and mytoxin B, respectively.136 Myrothecine H and I (200 and 201, Fig. 16) were isolated together with other already known trichothecene macrolides from Paramyrothecium roridum (syn. Myrothecium roridum), an endophytic fungus obtained from the medicinal plant Morinda officinalis. The already known compounds were identified as 14′-hydroxymytoxin B, mytoxin B, roridin A, verrucin A, verrucarin J, 12,13-deoxyroridin E (202, Fig. 16), 12′-hydroxyroridin E (203, Fig. 16), miophytocen A, trichoverrol B, and myrothecine A. The cytotoxic activities of all the isolated macrolides were tested against SF-268, NCI-H460, and HepG-2 tumor cell lines, and all compounds showed strong cytotoxicity, with IC50 values ranging from 0.0002 to 16.2 μM. Furthermore, compounds 202 and 203 induced the phosphorylation of JNK (c-Jun N-terminal protein kinase) protein and PARP (polyADP-ribose polymerase) cleavage, eventually inducing the apoptosis of HepG-2 cells.137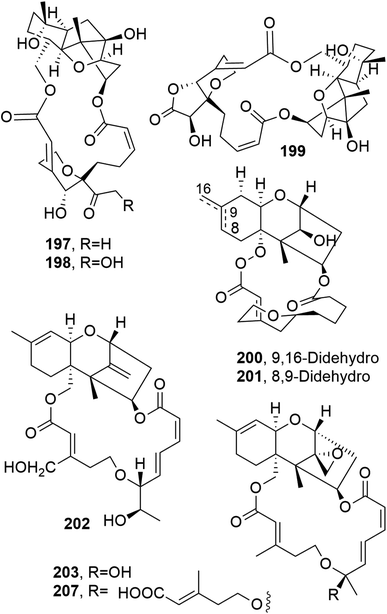 | ||
| Fig. 16 The structures of 18-membered macrolides isolated from Myrothecium roridum (197–203 and 207). | ||
12,13-Deoxyroridin E (202), roridin E (204, Fig. 17), verrucarin A (205, Fig. 17), and verrucarin J (207, Fig. 16) were isolated from the marine-derived fungus Myrothecium roridum, collected in Palau, a small Pacific island. The cytotoxic activities of the compounds were assayed against HL-60 L1210 human and murine leukemia cell lines, showing IC50 values of 25, 0.3, 0.2, and 2.5 ng mL−1, respectively, and 15, 0.2, 0.35, and 2.5 ng mL−1, respectively. Compound 202 showed reduced cytotoxicity: about 80-times less than that of roridin E (204).138
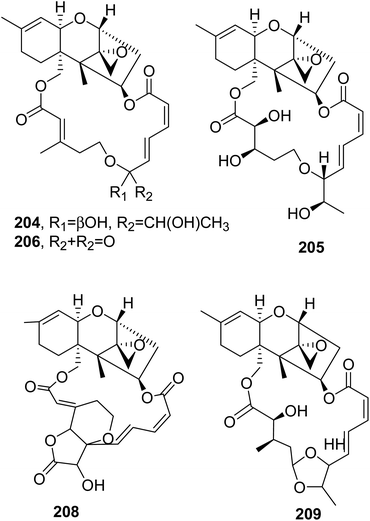 | ||
| Fig. 17 The structures of 18-membered macrolides isolated from Myrothecium roridum (204–206 and 208) and Myrothecium sp. (209). | ||
12′-Hydroxyroridin E (203), roridin Q (207, Fig. 16), and 2′,3′-deoxyroritoxin D (208, Fig. 17) were isolated from the marine-derived fungus Myrothecium roridum TUF 98F42. Roridin R (209, Fig. 17) was isolated together with roridin A and H and isororidin E from Myrothecium sp. TUF 02F6. Compounds 203, 207, and 209 were assayed for their ability to inhibit L1210 cell growth, showing IC50 values of 0.19, 31.2, and 0.45 μM, respectively. In the same test, roridin A, E, and H exhibited IC50 values of 0.11, 0.11, and 0.12 μM, respectively. Compound 208 inhibited the growth of Saccharomyces cerevisiae IAM 14383T at 1 μg per disc (inhibition zone: 12.2 mm); thus, it was about 10 times more active than roritoxin D.139
Trichobotryside A (210, Fig. 18) is 16-membered macrolide that was isolated together with two 18-membered macrolides named trichobotrysides B and C (211 and 212, Fig. 18) from the deep-sea-derived fungus Trichobotrys effuse DFFSCS021, which was collected from the South China Sea. Compounds 210–212 were assayed for their antifouling activities against larvae settlements of Bugula neritina and Balanus amphitrite. Compound 210 strongly inhibited the larvae settlement of both marine organisms, with EC50 values of 7.3 and 2.5 μg mL−1 and LC50/EC50 ratios greater than 40.5 and 37.4, respectively, while compound 211 only exhibited the inhibition of B. amphitrite larvae, with an EC50 value of 9.2 μg mL−1 and an LC50/EC50 ratio greater than 100. Finally, compound 212 only showed 41% lethality towards larvae at a concentration of 25 μg mL−1. The same compounds were also tested for cytotoxicity and antibacterial activity, showing weak cytotoxicity against the human carcinoma cell lines HepG2, Eca109, PC-3, Hep2, and KG-1a, with IC50 values ≥100 μM, and weak antibacterial activity against Pseudomonas aeruginosa, S. aureus, and Shewanella oneidensis.140
Borrelidin and borrelidin F (213 and 214, Fig. 18) were isolated together with borrelidin J and K and 7-methoxy-2,3-dimethylchromone-4-one from a co-culture of Streptomyces rochei MB037 with a gorgonian-derived fungus Rhinocladiella similis.141 When tested against S. aureus, borrelidin J and K showed stronger antibiotic activity than compounds 213 and 214. Borrelidin J exhibited a MIC value of 0.195 μg mL−1, stronger than that of the positive control ciprofloxacin. As expected, the same compounds showed weak antibacterial activity against E. coli and P. aeruginosa.142
3.8. 20-membered macrolides and larger lactone rings
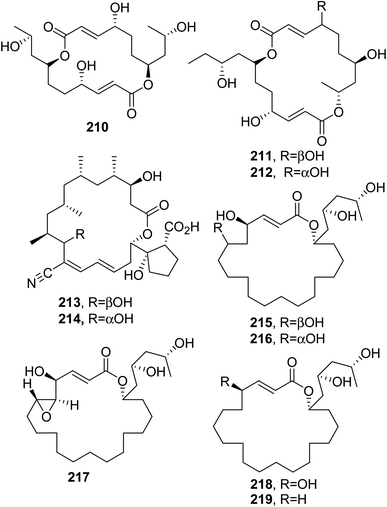
Rickiols, which are 20-, 22-, and 24-membered macrolides, were isolated from Hypoxylon rickii collected in 2010 from the Caribbean island of Martinique. The macrolides named rickiols A-E; A2 and E2; and A3 and E3 were 20-membered (215–219, Fig. 18); 22-membered (220 and 221, Fig. 19); and 24-membered (222 and 223, Fig. 19) macrolides, respectively. The biological activities of compounds 215–223 were tested against a broad group of bacteria, fungi, and yeasts. The measured MIC values against the gram-positive bacteria Micrococcus luteus, B. subtilis, S. aureus, and Mycobacterium sp., the fungus Mucor hiemalis, and the yeast Rhodotorula glutinis were noteworthily low. The compounds 219 and 221 showed strong antibiotic activity against S. aureus (MIC = 4.2 μg mL−1) and M. luteus (MIC = 4.2 μg mL−1), respectively. When assayed for cytotoxicity against the mouse fibroblast cell line L-929 and human HeLa cell line KB3.1, all the compounds showed moderate activity, except rickiol E2 (217) which showed IC50 values of 8.3 and 5.8 μg mL−1, respectively.143Wortmannilactones A–D (224–227, Fig. 19), which are 22-membered macrolides, were isolated from Talaromyces wortmannii. This fungus was obtained from a soil sample collected in the Yunnan province of China. Macrolides 222–225 were assayed for cytotoxicity against different human cancer cell lines, such as HCT-5, HCT-115, A549, MDA-MB-231, and K562 and they showed IC50 values in the range from 28.7 to 130.5 μM.144
Eushearilide (228, Fig. 19), which is a 24-membered macrolide, was isolated from a solid culture of Eupenicillium shearii IFM54447. Compound 228 showed antifungal activity against various fungi and yeasts, including human pathogens such as Aspergillus fumigatus, Trichophyton spp., and Candida spp., but it exhibited negligible antibacterial activity.145
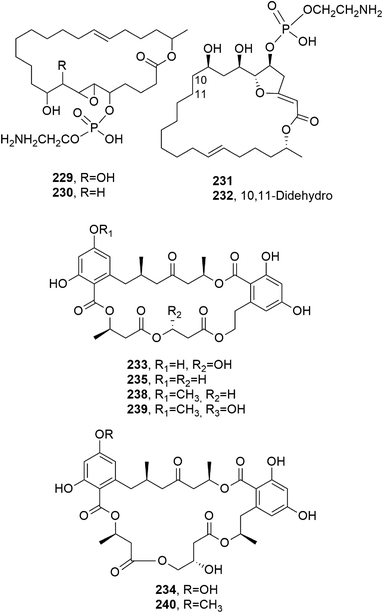
Two other 24-membered macrolides, named JBIR-19 and JBIR-20 (229 and 230, Fig. 20), were isolated from the entomopathogenic fungus Metarhizium sp. fE61, which was collected in Kyoto Prefecture, Japan. Compounds 229 and 230 induced S. cerevisiae to display striking elongated morphology when tested at concentrations of 3.1 and 13 μM, respectively, but they showed weak antimicrobial activity at concentrations of 200 and >200 μM, respectively.146
Preussolide A and B (231 and 232, Fig. 20), which are two phosphoethanolamine-substituted 24-membered macrolides, were isolated together with leptosin C from Preussia typharum. This fungus was collected near Fayetteville, Washington Co., Arkansas, USA. Compounds 231 and 232 moderately inhibited the growth of the pathogen Cryptococcus neoformans H99.147
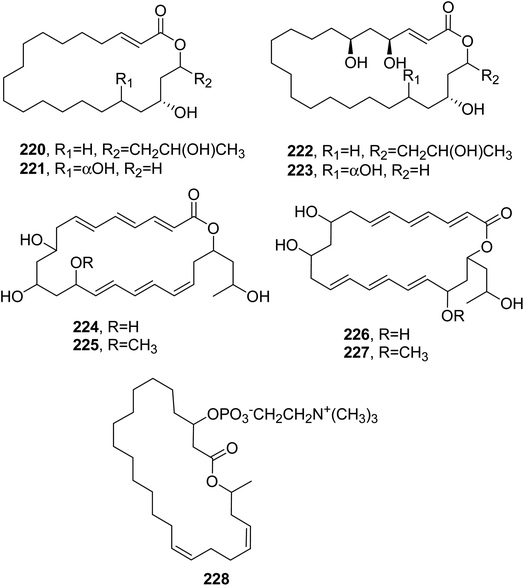
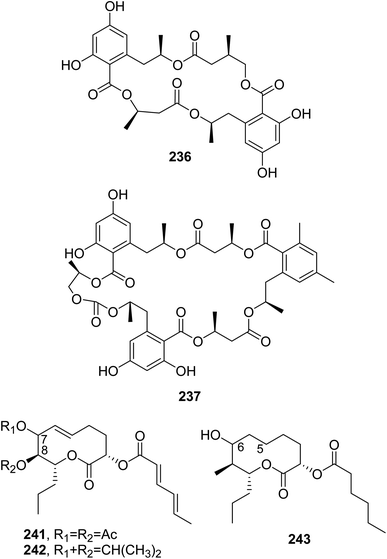
The macrocyclic lactide polyesters 15G256α, 15G256α-1, 15G256β, 15G256t, and 15G256ω (233–237, Fig. 20 and 21) were isolated together with some closely related linear polyesters and lipodespsipeptides from Hypoxylon oceanicum. When these compounds were tested for their antifungal activity against Neurospora crassa OS-110, using nikkomycin with a MIC value of 2 μg mL−1 as a positive control, compound 235 showed the strongest activity, with a MIC value of 0.5 μg mL−1, followed by compound 234, which showed a MIC value of 2 μg mL−1.148
Calcarides A–C (238–240, Fig. 20) were isolated together with the polyesters 15G256α, and 15G256β (233 and 235), three linear polyesters named calcaride C–E, and three other examples from Calcarisporium sp. KF525. This fungus was obtained from German Wadden Sea water samples.
All the compounds were tested against Staphylococcus epidermidis, Xanthomonas campestris, and Propionibacterium acnes, which are a biofilm-forming pathogen, a plant pathogen, and an agent of acne skin disease strains, respectively. The macrocyclic polyesters 233 and 235 and calcaride A and C (238 and 240) showed the inhibition of S. epidermidis and X. campestris, while the linear polyesters did not exhibit any activity at concentrations below 100 μM. Among the tested compounds, 233 and 238 showed the strongest activities against S. epidermidis and X. campestris, with MIC values of 12.9 ± 3.6 and 5.5 ± 1.3 μM, respectively.149
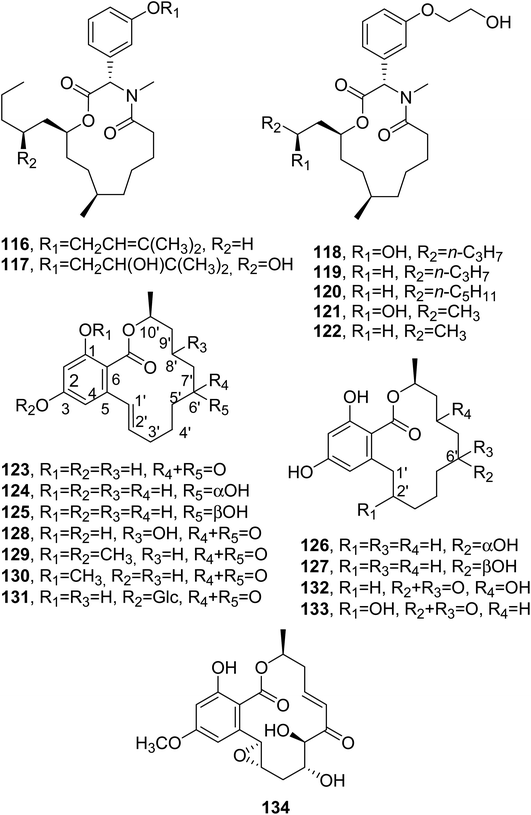
4 SAR (structure–activity relationship) results for some macrolides, natural analogues, and hemisynthetic derivatives
In this section the results of studies relating to the structure–activity relationships (SARs) of some macrolides belonging to different subgroups are reported in ring-size and chronological order.Pinolidoxin and putaminoxin (11 and 18), as reported above, are phytotoxic 10-membered macrolides produced, respectively, by D. pinodes and P. putaminum, pathogens of peas (P. sativum L.) and the weed annual fleabane (E. annuus L.). Both fungi produce closely related nonenolides. D. pinodes produced three nonenolides structurally related to 11, 7-epi-, 5,6-dihydro-, and 5,6-epoxy-pinolidoxin (12–14, Fig. 2). Similarly, P. putaminum produced nonenolides close to 18, in the form of putaminoxin B, D, and E and formylputaminoxin (19, 21, 22, and 23, Fig. 3), and putaminoxin C (20, Fig. 3), which is a closely related disubstituted cyclononendione. Considering these natural analogues, some hemisynthetic derivatives were prepared to further modify the key functionalities of both nonenolides and carry out SAR studies. Thus, starting from 11, its 7,8-O,O′-diacetyl-, 7,8-O,O′-isopropylidene-, and hexahydro-derivatives (241–243, Fig. 21) were prepared, while putaminoxin was converted into 5-O-acetyl- and the 6,7-dihydro-derivative (244 and 245, Fig. 22). Then, the phytotoxic, antifungal, and zootoxic activities of the natural analogs and hemisynthetic derivatives of compounds 11 and 18 were assayed. The phytotoxicity was tested against weeds such as annual fleabane (E. annus L.), annual mercury (Mercurialis annua L.), showy crotalaria (Crotalaria spectabilis), Canada thistle (Cirsium arvense L.), fat hen (Chenopodium album L.), buttercup oxalis (Oxalis pes-caprae L.), and Noogoora burr (Xanthium occidentale Bertol.). The crop plants used were sugar beet (Beta vulgaris L.), cucumber (Cucumis sativus), pea (Pisum sativum L.), tomato (Lycopersicon esculentum L.), sorghum (Sorghum bicolor L.), and globe artichoke (Cynara cardunculus L.). The antifungal and zootoxic activities were assayed toward Geotrichum candidum and brine shrimp (Artemia salina L.) larvae, respectively. Compounds 11 and 18 showed the highest phytotoxicity, showing that the important structural features required to induce this activity were the integrity and conformational freedom of the nonenolide ring and the presence of hydroxy groups and an unmodified propyl side chain. All tested compounds exhibited antifungal activity, while the pinolidoxin analogs and derivatives showed high to weak zootoxicity. In particular, the most zootoxic pinolidoxin derivatives appeared to be compounds 241 and 242, probably due to their lipophilicity, which allows them to penetrate the larvae membrane and then to be activated via the hydrolysis of the protective group of the diol system between C-7 and C-8 (lethal metabolism). Similarly, the derivative 243 showed strong toxicity, as the hydrogenation of the double bonds of both the macrocyclic ring and the side chain noteworthily increased the lipophilicity. All compounds showed antifungal activity.152 Later, as reported above, pinolide (15, Fig. 2) was isolated together pinolodoxin, herbarumin II (25, Fig. 3), and its 2-epimer from a more aggressive strain of D. pinodes. When tested on leaves of the host plant and other legumes, pinolide (15) and both herbarumins did not show phytotoxicity. The lack of toxicity is attributed to the stereochemistry of the hydroxy group at C-7, which is located in the α-position, in pinolide and 7-epi-pinolidoxin (12), which had zero or weak activity; however, pinolidoxin (11), herbarumin I (24, Fig. 3) and II (25), and 2-epi-herbarumin II, having the same hydroxyl group in a β-position, showed strong activity. In addition, the configuration of the hydroxy group at C-2 seems not to affect the phytotoxicity. In fact, it is located in an α-position for all the nonenolides tested, while it is located in the β-position only in the case of herbarumin II. Finally, it is important to note that the plants previously used to assay the phytotoxicity of herbarumins are different, and this could explain the lack of activity observed. Consequently, the possibility that pinolide and herbarumins could show toxicity when applied to different weed species or when different application methods are used cannot be excluded.
Furthermore, the esterification of the hydroxy group at C-2 with 2,4-hexadienoic acid seems to be important for the activity of 11.150
Stagonolide (31, Fig. 4), stagonolide B–F, stagonolide G–I, and modiolide A (32–36, 37–39, and 29, Fig. 4), as reported above, were isolated as phytotoxic nononelides from S. cirsium. When tested on the host plant at a concentration of 1 mg mL−1, compound 31 was highly toxic, while the others did not show phytotoxicity. These results appeared to be in agreement with the phytotoxic activity previously reported for other nonenolides. The hydroxylation of the lactone core at C-2 and C-4 induces a decrease in phytotoxicity, as reported for herbarumin I (24)48 and stagonolide C (33) with respect to stagonolide (31). Furthermore, these results could also be due to the substitution of the n-propyl group at C-9 in 31 with a methyl group in 33. The importance of this structural feature was confirmed by the lack of activity shown by stagonolide B–F (32–36), which have a methyl group at C-9, when compared to the phytotoxic herbarumin I-III (24–26)48 and putaminoxins (18–23), having an n-propyl group at the same position. The functionalization and the conformational freedom of the macrocyclic ring are important for the activity, as already observed for putaminoxins and pinolidoxins. In fact, stagonolide D and E (34 and 35), which were not toxic with respect to compound 31, not only substituted the n-propyl group at C-9 with a methyl group but also possessed marked changes in both the functionalization and conformational freedom of the nonenolide ring.58 Stagonolide G-I and modilide A (37–39 and 29) showed different phytotoxic activities when tested at a concentration of 1 mg mL−1 toward C. arvense. In fact, stagonolide H (38) showed phytotoxic activity at same level as compound 31 while stagonolide G (37) was inactive and stagonolide I and modiolide A (39 and 29) showed a significant decrease in toxicity. Stagonolide H (39) exhibited both strong phytotoxicity and high selectivity, inhibiting root growth by 85% at 1 mg mL−1 in chicory seedlings, while the other nonenolides were inactive. Compound 39 affected the leaves of eight plant species differently, with C. arvense leaves appearing to be significantly more sensitive than the other plants.60 Stagonolide J and K (40 and 41, Fig. 4), isolated from S. cirsii, both having an n-propyl group at C-9 but with macrocyclic rings with different functionalization and conformational freedom, were non-toxic and significantly phytotoxic, respectively, when assayed on the host plant (S. arvensis) compared to stagonolide 31 and herbarumin I (24), which are the most toxic compounds tested.60
Among the 14-membered macrolides, the resorcylic lactones cochliomycin A–C (147 and 148, Fig. 11, and 149, Fig. 12) and their four analogues, zeaenol, LL-Z1640-1, LL-Z1640-2 (150–152, Fig. 12), and paecilomycin F (158, Fig. 12), isolated as reported above from Cochliobolus lanatus, and two 2,4′-O,O′- and 2-O-acetyl derivatives of cochliomycin A (246 and 247, Fig. 22) and 2,4′,5′,6′-O,O′,O′′,O′′′′-tetracetylzealenol (248, Fig. 22), when tested at a concentration of 20.0 μg mL−1, completely inhibited larval settlements of the barnacle Balanus amphitrite. Compound 147 showed the significant inhibition of larval settlements even at a concentration of 5.0 μg mL−1, but it also had some toxic activity, which was lost at concentrations lower than 2.5 μg mL−1. The acetyl derivatives of compounds 147 and 150 (246, 247, and 248) were always less toxic than their parent compounds. Further investigations showed that 147, its derivatives 246 and 247, and compounds 150, 151, and 158 had potent antifouling activity at non-toxic concentrations. These results demonstrate that the functionality and conformational freedom of the macrocyclic ring are features that are important for the activity: the partial or total acetylation of the hydroxyl groups reduced the activity, while the presence of an isopropylidene ring induced a noteworthy increase in toxicity.115
Pestalotioprolides B–H (162, Fig. 12, and 165–170, Fig. 13) and 7-O-methylnigrosporolide (171, Fig. 13), isolated as reported above from Pestalotiopsis sp. and Pestalotiopsis microspore, were assayed for their cytotoxic activity against L5178Y mouse lymphoma cells. Compound 171 exhibited strong cytotoxicity, while compounds 166–168 and 170 were less active. These results demonstrated that the epoxy group at C-5/C-6 affected the loss of toxicity and the double bond from C-2/C-3 and the C-4 ketone induced a decrease in cytotoxicity. Whether an 8Z or 8E and 4R or 4S configuration was adopted by the double bond and hydroxyl group barely affected the toxicity. Finally, the methylation of the hydroxyl group at C-7 noteworthily increased the cytotoxicity. When the cytotoxic activity was tested against the human ovarian cancer cell line A2780, compound 167 was the only one that showed significant toxicity; compound 168 was less active, while the others exhibited weak or no activity. These differences in activity may be species specific (murine vs. human) or cell-line specific (blood cancer vs solid cancer), affecting the susceptibility of the assayed compounds.127
Mutolide and 6,7,9,10-tetrahydromutolide (163 and 164, Fig. 13), isolated as reported above from the endophytic fungus A. javeedii,128 were tested against the L5178Y mouse lymphoma cell line. Mutolide (161) showed strong cytotoxicity, with an IC50 value of 0.4 μM, while compound 164 was not toxic, and the positive control kahalalide F exhibited an IC50 value of 4.3 μM. These results demonstrate that the double bonds between C(6)–C(7) and C(9)–C(10) are structural features determining the cytotoxicity.126
Rhizoxin (RZX, 183, Fig. 14) and its four analogues WF-1360B, WF-1360C, WF-1360E, and WF-1360F (184 and 185, Fig. 14, and 186 and 187, Fig. 15) were isolated, as reported above from Rhizopus sp. No. F 1360. Later, some other analogues (249–252, Fig. 23) were isolated from the same fungus and three derivatives (253–255, Fig. 24) of ZRX were prepared. All the compounds were tested with respect to cell division in sea urchin eggs to test their inhibitory effects on tubulin. When tested at a concentration of l0−4 M, RZX-treated tubulin preformed microtubules showed only small granules. The activities of the analogues 249–252 were similar to that of ZRX, showing that the two epoxy groups were not essential for the inhibitory activity. Furthermore, molecular geometry, either in the presence of epoxy or olefinic groups, should be important for the interaction with the protein. Finally, the lack of activity observed when testing derivatives 254 and 255 suggests that the free hydroxyl group at C-13 is important for the activity.151
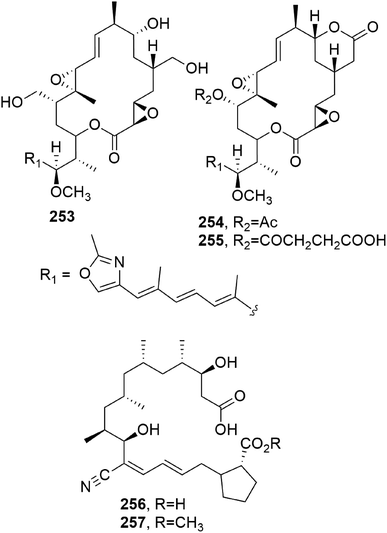 | ||
| Fig. 24 The structures of three derivatives prepared from rhizoxin (253–255) and those of borrelidin J and K (256 and 257) isolated from a co-culture of S. rochei MB037 and the fungus R. similis. | ||
The 18-membered macrolides borrelidin and borrelidin F (213 and 214, Fig. 18), as reported above, were isolated together with borrelidin J and K (256 and 257, Fig. 24), two fatty acids with a rare nitrile group, and 7-methoxy-2,3-dimethylchromone-4-one from a co-culture of S. rochei MB037 and the fungus R. similis. When assayed against S. aureus, compounds 256 and 257 showed stronger antibiotic activities than the corresponding 18-membered macrolides 213 and 214, with a MIC value of 0.195 μg mL−1. Compounds 256 and 257 are probably generated as long-chain fatty acids via the hydrolysis of the lactone group of borrelidins. As demonstrated in previous studies, the antibiotic activities of long-chain unsaturated fatty acids can be attributed to their ability to inhibit the enoyl-acyl carrier protein reductase, which is essential in bacterial fatty acid synthesis.152 Furthermore, the latter compounds showed weak antibiotic activity against E. coli and P. aeruginosa, with the increased resistance being attributed to the impermeability of their outer membranes.141
The 24-membered macrocyclic antifungal polyesters 15G256α, 15G256α-1, and 15G256β, and 15G256t and 15G256ω lactides (233–235, Fig. 20, and 236 and 237, Fig. 21), as reported above, were isolated from H. oceanicum. Similarly, calcarides (238–240, Fig. 20), other 24-membered macrolide polyesters, were isolated, as reported above, together with polyesters 233 and 235, three linear polyesters named calcaride C–E, and three other examples close to 233 from Calcarisporium sp. KF525.151 The 15G256 lactides showed antifungal activity against several phytopathogenic fungi and a detailed study of their antifungal activities was carried out using Neurospora crassa OS-1 with nikkomycin (MIC: 2 μg mL−1) as a positive control. The results demonstrated that compounds 233, 235, and 238–240 inhibited Staphylococcus epidermis and Xanthomonas campestris, while the linear polyesters were inactive. Compound 233 had the strongest antifungal activity, with MICs of 12.9 and 5.5 μg mL−1, respectively, when tested against S. epidermis and X. campestris. As the linear polyesters were inactive, it is possible to hypothesize that the ring structure is an important feature for determining the activity.151 In addition, calcaride A–C (238–240) showed antibiotic activities that were four-fold those of the 15G256-type lactone series and, in particular, compounds 233 and 235 demonstrate that the methylation of the hydroxy group of the resorcinol located with p-orientation with respect to the ester group of the macrocyclic ring moiety plays a role in imparting activity.149
5 Conclusions
This review reports on fungal macrolides. After a brief introduction to macrolides and their biosynthesis, a critical description of the most recent reviews in the field was included. The subsequent and main section describes the sources, structures, and biological activities of macrolides, organized according to ring size and chronology and including the corresponding literature. All this content is summarized in Table 1. For some macrolide groups with different ring sizes and different biological activities, the results of SAR studies are also described. The content in this review is of interest to chemists of natural substances, plant pathologists and physiologists, botanists, mycologists, biologists, and pharmacologists. Farmers and agri-food workers and those in the medicinal and cosmetic industries may also be interested in the potential practical application of bioactive macrolides. Politicians could also be interested in the practical application of these compounds in the above-cited fields, as their employment could reduce environmental pollution and satisfy the increasing demands of consumers for food with reduced or zero risk for human and animal health and increased nutraceutical value. These practical potential applications of fungal macrolides in different fields and the idea that very small percentages of plants, marine organisms, and fungi have been investigated to date strongly support the continuation of studies involving the isolation and chemical and biological characterization of specialized metabolites from new fungal sources. Furthermore, in-depth investigations of their chemical and biological aspects and modes of action should also be performed.7 Conflicts of interest
There are no conflicts to declare.8 Acknowledgements
Prof. A. E. is associated with the Institute of Biomolecular Chemistry, CNR, Pozzuoli, Italy.9 Notes and references
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2020, 83, 770–803 CrossRef CAS PubMed.
- K. D. Lenz, K. E. Klosterman, H. Mukundan and J. Z. Kubicek-Sutherland, Toxins, 2021, 13, 347 CrossRef CAS PubMed.
- P. M. Dewick, Medicinal natural products: A biosynthetic approach, John Wiley & Sons Ltd, Chichester, UK, 2019 Search PubMed.
- R. B. Woodward, Angew. Chem., 1957, 69, 50–58 CrossRef CAS.
- J. W. Corcoran, in Biosynthesis, ed. J. W. Corcoran, Springer, Berlin, Heidelberg, 1981, pp. 132–174 Search PubMed.
- C. Peano, A. Talà, G. Corti, D. Pasanisi, M. Durante, G. Mita, S. G. De Bllis and P. Alifano, Microb. Cell Fact., 2012, 11, 1–25 CrossRef PubMed.
- H. Zhang, H. Zou, J. Yan, X. Chen, J. Cao, X. Wu, J. Liu and T. Wang, Mar. Drugs, 2021, 19, 180 CrossRef CAS PubMed.
- T. M. Karpiński, Mar. Drugs, 2019, 17, 241, DOI:10.3390/md17040241.
- G. Dräger, A. Kirschning, R. Thiericke and M. Zerlin, Nat. Prod. Rep., 1996, 13, 365–375 RSC.
- A. Janas and P. Przybylski, Eur. J. Med. Chem., 2019, 182, 111662 CrossRef CAS PubMed.
- B. Arsic, J. Barber, A. Čikoš, M. Mladenovic, N. Stankovic and P. Novak, Int. J. Antimicrob. Agents, 2018, 51, 283–298 CrossRef CAS PubMed.
- J. A. Walla, Plant Dis. Rep., 1979, 63, 464 Search PubMed.
- M. Nukina, M. Ikeda and T. Sassa, Agric. Biol. Chem., 1980, 44, 2761–2762 CAS.
- M. Nukina, M. Ikeda and T. Sassa, Tetrahedron Lett., 1980a, 21, 301–302 Search PubMed.
- M. Nukina and H. Hirota, Bull. Biochem. Biotechnol., 1992, 56, 1158–1159 CrossRef CAS PubMed.
- R. A. Bucknall, H. Moores, R. Simms and B. Hesp, Antimicrob. Agents Chemother., 1973, 4, 294–298 CrossRef CAS PubMed.
- R. P. Mabelis, A. H. Ratcliffe, M. J. Ackland, J. R. Hanson and P. B. Hitchcock, J. Chem. Soc., Chem. Commun., 1981, 19, 1006–1007 RSC.
- M. J. Ackland, J. R. Hanson, P. B. Hitchcock, R. P. Mabelis and A. H. Ratcliffe, J. Chem. Soc., Perkin Trans. 1, 1984, 2755–2757 RSC.
- M. J. Ackland, J. R. Hanson, P. B. Hitchcock and A. H. Ratcliffe, J. Chem. Soc., Perkin Trans. 1, 1985, 843–847 RSC.
- A. Evidente, R. Lanzetta, R. Capasso, M. Vurro and A. Bottalico, Phytochemistry, 1993, 34, 999–1003 CrossRef CAS.
- A. Evidente, R. Capasso, M. A. Abouzeid, R. Lanzetta, M. Vurro and A. Bottalico, J. Nat. Prod., 1993, 56, 1937–1943 CrossRef CAS.
- A. Cimmino, M. Masi, M. Evidente, S. Superchi and A. Evidente, J. Pharm. Biomed. Anal., 2017, 144, 59–89 CrossRef CAS PubMed.
- L. de Napoli, A. Messere, D. Palomba, V. Piccialli, A. Evidente and G. Piccialli, J. Org. Chem., 2000, 65, 3432–3442 CrossRef CAS PubMed.
- A. Fürstner, K. Radkowski, C. Wirtz, R. Goddard, C. W. Lehmann and R. Mynott, J. Am. Chem. Soc., 2002, 124, 7061–7069 CrossRef PubMed.
- M. Vurro and B. E. Ellis, Plant Sci., 1997, 126, 29–38 CrossRef CAS.
- A. Evidente, R. Capasso, A. Andolfi, M. Vurro and M. C. Zonno, Phoma and Ascochyta species, Nat. Toxins, 1998, 6, 183–188 CrossRef CAS PubMed.
- A. Arnone, G. Assante, M. Montorsi, G. Nasini and E. Raggi, Gazz. Chim. Ital., 1993, 123, 71–73 CAS.
- A. Cimmino, A. Andolfi, S. Fondevilla, M. A. Abouzeid, D. Rubiales and A. Evidente, J. Agric. Food Res., 2012, 60, 5273–5278 CrossRef CAS PubMed.
- A. S. Ratnayake, W. Y. Yoshida, S. . L. Mooberry and T. Hemscheidt, Organic Lett, 2001, 3, 3479–3481 CrossRef CAS PubMed.
- K. S. Yeung and I. Paterson, Angew. Chem., Int. Ed., 2002, 41, 4632–4653 CrossRef CAS PubMed.
- K. S. Yeung and I. Paterson, Angew. Chem., 2002a, 114, 4826–4847 Search PubMed.
- J. R. Peterson and T. J. Mitchison, Chem. Biol., 2002, 9, 1275–1285 CrossRef CAS PubMed.
- A. Fürstner, T. Nagano, C. Müller, G. Seidel and O. Müller, Chem.–Eur. J., 2007, 13, 1452–1462 CrossRef PubMed.
- E. M. Johnson and W. D. Valleau, Rev. Appl. Mycol., 1933, 13, 32 Search PubMed.
- E. G. Yarmola, T. Somasundaram, T. A. Boring, I. Spector and M. R. Bubb, J. Biol. Chem., 2000, 275, 28120–28127 CrossRef CAS PubMed.
- G. Assante, U. Brambilla, G. Nasini and G. Farina, Rivista Patil. Veg., 1994, 25–38 Search PubMed.
- J. García-Fortanet, J. Murga, E. Falomir, M. Cardanand and J. Marco, J. Org. Chem., 2005, 70, 9822–9827 CrossRef PubMed.
- A. Farooq, J. Gordon, J. R. Hanson and J. A. Takahashi, Phytochemistry, 1995, 38, 557–558 CrossRef CAS.
- A. Evidente, R. Lanzetta, R. Capasso, A. Andolfi, A. Bottalico, M. Vurro and M. C. Zonno, Phytochemistry, 1995, 40, 1637–1641 CrossRef CAS.
- A. Evidente, R. Lanzetta, R. Capasso, A. Andolfi, M. Vurro and M. C. Zonno, Phytochemistry, 1997, 44, 1041–1045 CrossRef CAS.
- A. Evidente, R. Capasso, A. Andolfi, M. Vurro and M. C. Zonno, Phytochemistry, 1998, 48, 941–945 CrossRef CAS.
- E. Gil-Av and D. Nurok, Adv. Chromatogr., 1974, 10, 99–172 CAS.
- J. F. Rivero-Cruz, G. García-Aguirre, C. M. Cerda-García-Rojas and R. Mata, Tetrahedron, 2000, 56, 5337–5344 CrossRef.
- K. H. Domsh, W. Gams and T. H. Anderson, Compendium of Soil Fungi, Academic Press, London, 1980, vol. 1 Search PubMed.
- S. N. Brebaum, G. J. Borland, S. Neumann Brebaum and G. J. Boland, Plant Dis., 1999, 83, 200 Search PubMed.
- A. Fürstner and K. Radkowski, Chem. Commun., 2001, 671–672 RSC.
- A. Fürstner, K. Radkowski, C. Wirtz, R. Goddard, C. W. Lehmann and R. Mynott, J. Am. Chem. Soc., 2002, 124, 7061–7069 CrossRef PubMed.
- J. F. Rivero-Cruz, M. Macías, C. M. Cerda-García-Rojas and R. Mata, J. Nat. Prod., 2003, 66, 511–514 CrossRef CAS PubMed.
- J. Kumla, N. Suwannarach and S. Lumyong, Can. J. Plant Pathol., 2016, 38, 103–106 CrossRef CAS.
- S. Boonphong, P. Kittakoop, M. Isaka, D. Pittayakhajonwut, M. Tanticharoen and Y. Thebtaranonth, J. Nat. Prod., 2001, 64, 965–967 CrossRef CAS PubMed.
- R. A. Edrada, M. Heubes, G. Brauers, V. Wray, A. Berg, U. Gräfe, M. Wohlfarth, J. Muhlbacher, K. Schaumann, Sudarsono, G. Bringmann and P. Proksch, J. Nat. Prod., 2002, 65, 1598–1604 CrossRef CAS PubMed.
- M. Tsuda, T. Mugishima, K. Komatsu, T. Sone, M. Tanaka, Y. Mikami and J. I. Kobayashi, Nat. Prod., 2003, 66, 412–415 CrossRef CAS PubMed.
- N. F. Mikhailova and A. V. Tarasov, Botanicheskiy J., 1989, 74, 509–514 Search PubMed.
- W. W. Donald, Rev. Weed Sci., 1990, 5, 193–250 CAS.
- J. Herschenhorn, M. Vurro, M. C. Zonno, A. Stierle and G. Strobel, Plant Sci., 1993, 94, 227–234 CrossRef.
- A. Evidente, A. Cimmino, A. Andolfi, M. Vurro, M. C. Zonno, C. L. Cantrell and A. Motta, Tetrahedron, 2008, 64, 1612–1619 CrossRef CAS.
- M. C. Zonno, M. Vurro, M. Lucretti, A. Andolfi, C. Perrone and A. Evidente, Plant Sci., 2008, 175, 818–825 CrossRef CAS.
- A. Evidente, A. Cimmino, A. Andolfi, M. Vurro, M. C. Zonno and A. Motta, J. Agric. Food Chem., 2008, 56, 884–888 CrossRef CAS PubMed.
- O. Yuzikhin, G. Mitina and A. Berestetskiy, J. Agric. Food Chem., 2007, 55, 7707–7711 CrossRef CAS PubMed.
- A. Evidente, A. Cimmino, A. Berestetskiy, G. Mitina, A. Andolfi and A. Motta, J. Nat. Prod., 2008, 71, 31–34 CrossRef CAS PubMed.
- A. Evidente, A. Cimmino, A. Berestetskiy, A. Andolfi and A. Motta, J. Nat. Prod., 2008, 71, 1897–1901 CrossRef CAS PubMed.
- A. Dalinova, V. Dubovik, L. Chisty, D. Kochura, A. Ivanov, S. Smirnov, M. Petrova, A. Zolotarev, A. Evidente and A. Berestetskiy, J. Agric. Food Chem., 2019, 67, 13040–13050 CrossRef CAS PubMed.
- K. Götz, J. C. Liermann, E. Thines, H. Anke and T. Opatz, Org. Biomol. Chem., 2010, 8, 2123–2130 RSC.
- W. Ebrahim, A. H. Aly, A. Mándi, F. Totzke, M. H. Kubbutat, M. V. Wray, W.-H. Lin, H. Dai, P. Proksch, T. Kurtán and A. Debbab, Eur. J. Org. Chem., 2012, 3476–3484 CrossRef CAS.
- L. Yanez-Espinosa, T. Terrazas, L. Lopez-Mata and J. I. Valdez-Hernandez, Wood Sci. Technol., 2004, 38, 217–226 CrossRef CAS.
- H. Ferraz, F. I. Bombonato, M. K. Sano and L. S. Longo Jr, Quim. Nova, 2008, 31, 885–900 CrossRef CAS.
- S. B. Singh, H. Jayasuriya, D. L. Zink, J. D. Polishook, A. W. Dombrowski and H. Zweerink, Tetrahedron Lett., 2004, 45, 7605–7608 CrossRef CAS.
- Y. H. Wu, Z. H. Zhang, Y. Zhong, J. J. Huang, X. X. Li, J. Y. Jiang, Y. Y. Deng, L. H. Zhanga and F. He, RSC Adv., 2017, 7, 40015–40019 RSC.
- O. C. Musgrave, J. Chem. Soc., 1956, 4301–4305 RSC.
- H. D. Munro, 0. C. Musgrave and R. Templeton, J. Chem. Soc., 1967, 947–948 CAS.
- A. J. Birch, O. C. Musgrave, R. W. Rickards and H. Smith, J. Chem. Soc., 1959, 3146–3152 RSC.
- S. Lai, Y. Shizuri, S. Yamamura, K. Kawai, Y. Terada and H. Furukawa, Tetrahedron Lett., 1989, 30, 2241–2244 CrossRef CAS.
- S. Lai, Y. Shizuri, S. Yamamura, K. Kawai and H. Furukawa, Bull. Chem. Soc. Jpn., 1991, 64, 1048–1050 CrossRef CAS.
- S. B. Hyeon, A. Ozaki, A. Suzuki and S. Tamura, Agric. Biol. Chem., 1976, 40, 1663–1664 CAS.
- A. Hirota, H. Sakai and A. Isogai, Agric. Biol. Chem., 1985, 49, 731–735 CAS.
- Y. Fujii, A. Fukuda, T. Hamasaki, I. Ichimoto and H. Nakajima, Phytochemistry, 1995, 40, 1443–1446 CrossRef CAS.
- H. Zhang, H. Tomodai, N. Tabata, H. Miura, M. Namikoshi, Y. Yamaguchi, R. Masuma and S. Omura, J. Antibiot., 2001, 54, 635–641 CrossRef CAS PubMed.
- C. Y. Chou and D. R. Hou, J. Org. Chem., 2006, 71, 9887–9890 CrossRef CAS PubMed.
- D. Rodphaya, J. Sekiguchi, Y. Sekiguchi and Y. Yamada, J. Antibiot., 1986, 39, 629–635 CrossRef CAS PubMed.
- J. Sekiguchi and G. M. Gaucher, Appl. Environ. Microbiol., 1977, 33, 147–158 CrossRef CAS PubMed.
- E. Diao, K. Ma, S. Qian, H. Zhang, P. Xie, R. Mao and H. Song, Toxicon, 2022, 205, 31–37 CrossRef CAS PubMed.
- J. Sekiguchi, H. Kuroda, Y. Yamada and H. Okada, Tetrahedron Lett., 1985, 26, 2341–2342 CrossRef CAS.
- H. Matsuura, K. Nakamori, E. A. Omer, C. Hatakeyama, T. Yoshihara and A. Ichihara, Phytochemistry, 1998, 49, 579–584 CrossRef CAS.
- K. Nakamori, H. Matsuura, T. Yoshihara, A. Ichihara and Y. Koda, Phytochemistry, 1994, 35, 835–839 CrossRef CAS.
- Q. Yang, M. Asai, H. Matsuura and T. Yoshihara, Phytochemistry, 2000, 54, 489–494 CrossRef CAS PubMed.
- C. J. Smith, D. Abbanat, V. S. Bernan, W. M. Maiese, M. Greenstein, J. Jompa, A. Tahir and C. M. Ireland, J. Nat. Prod., 2000, 63, 142–145 CrossRef CAS PubMed.
- R. Jadulco, P. Proksch, V. W. Sudarsono, A. Berg and U. Gräfe, J. Nat. Prod., 2001, 64, 527–530 CrossRef CAS PubMed.
- H. Shigemori, Y. Kasai, K. Komatsu, M. Tsuda, Y. Mikami and J. I. Kobayashi, Mar. Drugs, 2004, 2, 164–169 CrossRef CAS.
- R. Y. Yang, C. Y. Li, Y. C. Lin, G. T. Peng, Z. G. She and S. N. Zhou, Bioorg. Med. Chem. Lett., 2006, 16, 4205–4208 CrossRef CAS PubMed.
- J. Dai, K. Krohn, U. Flörke, G. Pescitelli, G. Kerti, T. Papp, K. E. Kövér, A. C. Bényei, S. Sigfried Draeger, B. Schulz and T. Kurtán, Eur. J. Org. Chem., 2010, 6928–6937 CrossRef CAS.
- P. Sun, D. X. Xu, A. Mandi, T. Kurtan, T. J. Li, B. Schulz and W. Zhang, J. Org. Chem., 2013, 78, 7030–7047 CrossRef CAS PubMed.
- E. Oppong-Danquah, P. Budnicka, M. Blümel and D. Tasdemir, Mar. Drugs, 2020, 18, 73 CrossRef CAS PubMed.
- L. H. Meng, X. M. Li, C. T. Lu, C. S. Li, G. M. Xu, C. G. Huang and B. G. Wang, J. Nat. Prod., 2013, 76, 2145–2149 CrossRef CAS PubMed.
- L. Zhang, S. I. Niaz, D. Khan, Z. Wang, Y. Zhu, H. Zhou, Y. Lin, J. Li and L. Liu, Mar. Drugs, 2017, 15, 35, DOI:10.3390/md15020035.
- W. Wang, H. Feng, C. Sun, Q. Che, G. Zhang, T. Zhu and D. Li, Phytochemistry, 2020, 178, 112462, DOI:10.1016/j.phytochem.2020.112462.
- V. Betina, Folia Microbiol., 1992, 37, 3–11 CrossRef CAS PubMed.
- M. Vurro, A. Evidente, A. Andolfi, M. C. Zonno, F. Giordano and A. Motta, Plant Sci., 1998, 138, 67–79 CrossRef CAS.
- J. Wang, Y. Huang, M. Fang, Y. Zhang, Z. Zheng, Y. Zhao and W. Su, FEMS Immunol. Med. Microbiol., 2002, 34, 51–57 CrossRef CAS PubMed.
- J. P. Guo, C. Y. Zhu, C. P. Zhang, Y. S. Chu, Y. L. Wang, J. X. Zhang, D. K. Wu, K. Q. Zhang and X. M. Niu, J. Am. Chem. Soc., 2012, 134, 20306–20309 CrossRef CAS PubMed.
- C. T. Walsh, Science, 2004, 303, 1805–1810 CrossRef CAS PubMed.
- M. A. Fischbach and C. T. Walsh, Chem. Rev., 2006, 106, 3468–3496 CrossRef CAS PubMed.
- C. Hertweck, Angew. Chem., Int. Ed., 2009, 48, 4688–4716 CrossRef CAS PubMed.
- M. Okabe, T. Sugita, K. Kinoshita and K. Koyama, J. Nat. Prod., 2016, 79, 1208–1212 CrossRef CAS PubMed.
- C. J. Mirocha, C. M. Christensen and G. J. Nelson, in Microbial Toxins, ed. S. J. Ají, S. Kadis and A. Ciegler, Academic Press, New York, 1971, vol. 7, pp. 107–138 Search PubMed.
- C. J. Mirocha, and C. M. Christensen, in Mycotoxins, ed. J. F. H. Purchase, Elsevier, Amsterdam, 1974, pp. 129–149 Search PubMed.
- S. H. El-Sharkawy and Y. J. Abul-Hajj, J. Org. Chem., 1988, 53, 515–519 CrossRef CAS.
- X. Yang, T. T. Khong, L. Chen, H. D. Choi, J. S. Kang and B. W. Son, Chem. Pharm. Bull., 2008, 56, 1355–1356 CrossRef CAS PubMed.
- M. S. R. Nair and S. T. Carey, Tetrahedron Lett., 1980, 21, 2011–2012 CrossRef CAS.
- M. S. R. Nair, S. T. Carey and J. C. James, Tetrahedron, 1981, 37, 2445–2449 CrossRef CAS.
- T. Agatsuma, A. Takashi, C. Kabuto and S. Nozoe, Chem. Pharm. Bull., 1993, 41, 373–375 CrossRef CAS.
- J. A. O'Neill, J. Chem. Soc., Perkin Trans. 1, 1994, 17, 2493–2497 Search PubMed.
- U. Höller, G. M. König and A. D. Wright, Eur. J. Org. Chem., 1999, 2949–2955 CrossRef.
- M. Isaka, C. Suyarnsestakorn, M. Tanticharoen, P. Kongsaeree, Y. Thebtaranonth and A.-E. Aigialomycins, new resorcylic macrolides from the marine mangrove fungus Aigialus parvus, J. Org. Chem., 2002, 67, 1561–1566 CrossRef CAS PubMed.
- M. Isaka, A. Yangchum, S. Intamas, K. Kocharin, E. G. Jones, P. Kongsaeree and S. Prabpai, Tetrahedron, 2009, 65, 4396–4403 CrossRef CAS.
- C. L. Shao, K. H. Wu, C. Y. Wang, Q. A. Liu, Y. Xu, M. Y. Wei, P. Y. Qian, Y. C. Gu, C. J. Zheng, Z. G. She and Y. C. Lin, J. Nat. Prod., 2011, 74, 629–633 CrossRef CAS PubMed.
- C. L. Shao, K. H. Wu, C. Y. Wang, Q. A. Liu, Y. Xu, M. Y. Wei, P. Y. Qian, Y. C. Gu, C. J. Zheng, Z. G. She and Y. C. Lin, J. Nat. Prod., 2013, 76, 302 CrossRef CAS.
- F. Sugawara, K. M. Kim, K. Kobayashi, J. Uzawa, S. Yoshida, N. Murofushi, N. Takahashi and G. A. Strobel, Phytochemistry, 1992, 31, 1987–1990 CrossRef CAS.
- G. A. Ellestad, F. M. Lovell, N. A. Perkinson, R. T. Hargreaves and W. J. McGahren, J. Org. Chem., 1978, 43, 2339–2343 CrossRef CAS.
- L. Xu, Z. He, J. Xue, X. Chen and X. Wei, J. Nat. Prod., 2010, 73, 885–889 CrossRef CAS PubMed.
- E. Adelin, C. Servy, S. Cortial, H. Lévaique, M. Martin, T. P. Retailleau, G. Le Gof, B. Bussaban, S. Lumyong and J. Ouazzani, Phytochemistry, 2011, 72, 2406–2412 CrossRef CAS PubMed.
- A. Ballio, A. Evidente, A. Graniti, G. Randazzo and L. Sprapano, Phytochemistry, 1988, 27, 3117–3121 CrossRef CAS.
- A. Graniti, EPPO Bull., 1986, 16, 479–486 CrossRef.
- C. Bartolucci, S. Cerrini, D. Lamba, A. Evidente and G. Randazzo, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1992, 48, 83–86 CrossRef.
- V. Rukachaisirikul, A. Rodglin, S. Phongpaichit, J. Buatong and J. Sakayaroj, Phytochem. Lett., 2012, 5, 13–17 CrossRef CAS.
- M. Shah, S. K. Deshmukh, S. A. Verekar, A. Gohil, A. S. Kate, V. Rekha and A. Kulkarni-Almeida, SpringerPlus, 2015, 4, 1–10 CrossRef CAS PubMed.
- Y. Gao, F. Stuhldreier, L. Schmitt, S. Wesselborg, L. Wang, W. E. Müller, R. Kalscheuera, Z. Guod, K. Zoud, Z. Liu and P. Proksch, Fitoterapia, 2020, 146, 104652 CrossRef CAS PubMed.
- S. Liu, H. Dai, G. Makhloufi, C. Heering, C. Janiak, R. Hartmann, A. Mándi, T. Kurtán, W. E. G. Müller, M. U. Kassack and W. Lin, J. Nat. Prod., 2016, 79, 2332–2340 CrossRef CAS PubMed.
- K. H. Michel, M. O. Chaney, N. D. Jones, M. M. Hoehn and R. Nagarajan, Epipolythiopiperazinedione antibiotics from Penicillium turbatum, J. Antibiot., 1974, 27, 57–64 CrossRef CAS PubMed.
- K. H. Michel, P. V. Demarco and R. Nagarajan, J. Antibiot., 1977, 30, 571–575 CrossRef CAS PubMed.
- A. A. Stierle, D. B. Stierle, D. Decato, N. D. Priestley, J. B. Alverson, J. Hoody, K. McGrath and D. Klepacki, J. Nat. Prod., 2017, 80, 1150–1160 CrossRef CAS PubMed.
- S. Iwasaki, H. Kobayashi, J. Furukawa, M. Namikoshi, S. Okuda, Z. Sato, I. Matsuda and T. Noda, J. Antibiot., 1984, 37, 354–362 CrossRef CAS PubMed.
- S. Kiyoto, Y. Kawai, T. Kawakita, E. King, M. Okuhara, I. Uchida, H. Tanaka, M. Hashimoto, H. Terano, M. Kohsaka, H. Aoki and H. Imanaka, J. Antibiot., 1986, 39, 762–772 CrossRef CAS PubMed.
- T. Yamada, M. Iritani, M. Doi, K. Minoura and T. I. A. Numata, J. Chem. Soc., Perkin Trans. 1, 2001, 22, 3046–3053 RSC.
- S. Takamatsu, Y. P. Kim, M. Hayashi, H. Hiaoka, M. Natori, K. Komiyama and S. Omura, J. Antibiot., 1996, 49, 95–98 CrossRef CAS PubMed.
- S. Takamatsu, H. Hiraoka, Y. P. Kim, M. Hayashi, M. Natori, K. Komiyama and S. Omura, Macrosphelides C and D, novel inhibitors of cell adhesion, J. Antibiot., 1997, 50, 878–880 CrossRef CAS PubMed.
- L. Shen, R. H. Jiao, Y. H. Ye, X. T. Wang, C. Xu, Y. C. Song, H. L. Zhu and R. X. Tan, Chem.–Eur. J., 2006, 12, 5596–5602 CrossRef CAS PubMed.
- H. Liu, Y. Chen, S. Li, W. Zhang, Z. Liu, H. Tan and W. Zhang, Fitoterapia, 2020, 147, 104768 CrossRef CAS PubMed.
- M. Namikoshi, K. Akano, S. Meguro, I. Kasuga, Y. Mine, T. Takahashi and H. Kobayashi, J. Nat. Prod., 2001, 64, 396–398 CrossRef CAS PubMed.
- J. Xu, A. Takasaki, H. Kobayashi, T. Oda, J. Yamada, R. E. Mangindaan, K. Ukai, H. Nagai and M. Namikoshi, J. Antibiot., 2006, 59, 451–455 CrossRef CAS PubMed.
- Y. L. Sun, X. Y. Zhang, N. H. Nong, X. Y. Xu and S. H. Qi, Tetrahedron Lett., 2016, 57, 366–370 CrossRef CAS.
- M. Yu, Y. Li, S. P. Banakar, L. Liu, C. Shao, Z. Li and C. Wang, Front. Microbiol., 2019, 10, 915 CrossRef PubMed.
- C. J. Zheng, J. S. Yoo, T. G. Lee, H. Y. Cho, Y. H. Kim and W. G. Kim, FEBS Lett., 2005, 579, 5157–5162 CrossRef CAS PubMed.
- F. Surup, E. Kuhnert, A. Böhm, T. Pendzialek, D. Solga, D. Wiebach, H. Engler, A. Berkessel, M. Stadler and M. Kalesse, Chem.–Eur. J., 2018, 24, 2200–2213 CrossRef CAS PubMed.
- Y. Dong, J. Yang, H. Zhang, J. Lin, X. Ren, M. Liu, X. Lu and J. He, J. Nat. Prod., 2006, 69, 128–130 CrossRef CAS PubMed.
- T. Hosoe, K. Fukushima, K. Takizawa, T. Itabashi, N. Kawahara, V. Vidotto and K. I. Kawai, J. Antibiot., 2006, 59, 597–600 CrossRef CAS PubMed.
- I. Kozone, J. Y. Ueda, M. Watanabe, S. Nogami, A. Nagai, S. Inaba, Y. Ohya, M. Takagi and K. Shinya, J. Antibiot., 2009, 62, 159–162 CrossRef CAS PubMed.
- B. Perlatti, N. Lan, M. Xiang, C. E. Earp, J. E. Spraker, C. J. Harvey, C. B. Nichols, J. A. Alspaugh, J. B. Gloe and G. F. Bills, J. Ind. Microbiol. Biotechnol., 2021, 48, kuab022 CrossRef CAS PubMed.
- G. Schlingmann, L. Milne and G. T. Carter, Tetrahedron, 2002, 58, 6825–6835 CrossRef CAS.
- J. Silber, B. Ohlendorf, A. Labes, A. Erhard and J. F. Imhoff, Mar. Drugs, 2013, 11, 3309–3323 CrossRef PubMed.
- A. Evidente, R. Capasso, A. Andolfi, M. Vurro and M. C. Zonno, Nat. Toxins, 1998, 6, 183–188 CrossRef CAS PubMed.
- M. Takahashi, S. Iwasaki, H. Kobayashi, S. Okuda, T. Murai, Y. Sato, H. H. Tokuko and H. Nagano, J. Antibiot., 1987, 40, 66–72 CrossRef CAS PubMed.
- C. J. Zheng, J. S. Yoo, T. G. Lee, H. Y. Cho, Y. H. Kim and W. G. Kim, FEBS Lett., 2005, 579, 5157–5162 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2np00025c |
| This journal is © The Royal Society of Chemistry 2022 |