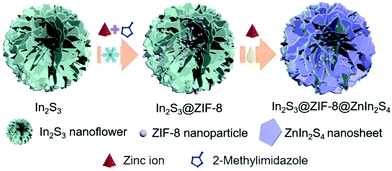
Construction of In2S3@ZIF-8@ZnIn2S4 hierarchical nanoflower heterostructures to promote photocatalytic reduction activity†
Chengyuan
Yang
a,
Rong
Wang
a,
Wenxin
Zhu
a,
Jing
Wang
a,
Liang
Zhang
a,
Ting
Du
a,
Zhaoli
Liu
a,
Linxuan
Xie
a,
Jing
Sun
b and
Jianlong
Wang
 *a
*a
aCollege of Food Science and Engineering, Northwest A&F University, Yangling, 712100, Shaanxi, P. R. China. E-mail: wanglong79@nwsuaf.edu.cn
bQinghai Key Laboratory of Qinghai-Tibet Plateau Biological Reources, Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Qinghai, 810008, China
First published on 28th October 2021
Abstract
Current technologies for photocatalytic reduction in wastewater suffer from the limitations of the material. In this study, we demonstrate that ZIF-8 can be utilized as an interfacial substance in the preparation of heterostructured In2S3@ZnIn2S4 nanoflowers. This structure effectively promotes carrier movement and inhibits the recombination of photogenerated carriers by spatial separation at the edge of nanoflowers and the reduced energy loss of the charge-transfer state in the heterostructure, thus achieving ultrahigh photoreduction efficiency. Consequently, the photoreduction rate of the final In2S3@ZIF-8@ZnIn2S4 can reach as high as 97.8% toward Cr(VI) under visible-light irradiation within 30 min, almost 2 times higher than that of the pristine heterojunction.
Introduction
Improper discharge of industrial waste from energy production and mining operations results in significant environmental pollution including heavy metal ions, metal oxo-hydroxo anions and other types of wastewater.1–4 Therein, chromium is regarded as a carcinogen due to its high acute toxicity to organisms, and this state is more difficult to remove from wastewater using conventional methods like chemical sedimentation, physical adsorption or chemical reduction.5–7 Compared to such conventional strategies, photocatalytic reduction serves as an eco-friendly, sustainable and economical method that directly utilizes solar energy to effectively reduce heavy metals from wastewater.1,8,9 However, many photocatalytic semiconductors used for photocatalytic detoxification suffer from severe drawbacks, including low light-harvesting and adsorption efficiencies, slow reduction rates and poor cycling stability, which are mainly because of the limited levels of heavy metal accumulation and the sluggish separation/transfer kinetics of electron–hole pairs.10,11 Therefore, it is essential to optimize photocatalysts with enhanced light-harvesting abilities, efficient adsorption capacities, and excellent photo-generated charge separations and migrations for use in photocatalytic reduction.Thus far, many synthetic approaches to heterojunction structures have been proposed and the fabrication of a stacked-growth structure is promising for maximizing the function of heterojunctions, as this kind of structure could allow for lower bulk-to-surface diffusion lengths, larger surface areas, and opportunities for hybridization with other compounds within the hierarchical heterostructures.12–15 However, most of these structures are generated by epitaxial-growth in source substrates with poorly controlled nanodomains of different species and high binding energy of intra/interlayer excitons.16,17 In other words, the electrons transferring across the epitaxial-growth hierarchical heterojunction are expected to be accompanied by a large momentum change and high Coulomb barrier, resulting in a significantly retarded charge transfer process.18,19 In this case, unique heterostructures with the incoming D–A structure might improve the interfacial charge transfer to fortify the photocatalytic efficiency.6,19–21 With such structures, there are two main issues that need to be considered: the selection of interfacial compounds and how to efficiently promote the charge separation and migration.22 Most metal–organic frameworks (MOFs) can only absorb ultraviolet light due to their large band gap, which limits the scope of application. In order to modify MOFs, many new strategies have been developed, such as metal loading, organic linker decoration, semiconductor combination, etc., to reduce the band gap and expand the utilization of visible light. In 2016, Wang and co-workers developed a ZnO@ZIF-8 heterostructure photocatalyst, which can selectively reduce Cr(VI) by light and increase the degradation rate.23 In addition, Zhang and co-workers developed a MoO3@ZIF-8 heterostructure photocatalyst that can efficiently reduce Cr(VI) with light, and its degradation efficiency of Cr(VI) (15 mg L−1) can reach 100% in 45 min.5 The combination with semiconductors is commonly used to improve the photocatalytic performance, by building a coherent interface connection between the MOF and semiconductors to prevent photo-induced electron–hole pair recombination, which makes it a promising candidate for use in the design of photocatalysts.5,24 This encouraged us to systematically study the impact of ZIF-8 as an interface with regard to electron transfer in heterostructure systems in an effort to gain insight into the relationship between the MOF interface and heterostructure for photocatalytic efficiency.
Among the catalytic semiconductors currently being investigated, the heterostructures formed by In2S3 nanoflowers and ZnIn2S4 nanosheets have displayed advantages including high specific surface areas, light reflection and scattering, rapid mass transfer and shortened transmission distance.25–30 Herein, we demonstrate the rational design and fabrication of In2S3@ZIF-8@ZnIn2S4 hierarchical nanoflower heterostructures by assembling In2S3 nanoflowers and ZnIn2S4 nanosheets (as the inner and outer layers) and ZIF-8 nanoparticles (as the middle-layer) as an efficient photocatalyst for photocatalytic Cr(VI) reduction. This unique heterojunction not only ensures the advantages of ZIF-8 nanoparticles in the heterostructure, but also utilizes ZIF-8 as an interface, which can greatly eliminate the negative effects of momentum mismatch and excitonic localization by the D–A structure. Due to these merits, the optimized In2S3@ZIF-8@ZnIn2S4 heterostructure displays the highest photocatalytic efficiency of up to 97.8% toward Cr(VI) within 30 min. The results reveal that the MOF interface strongly affects the transfer and separation of the photo-generated electrons and holes in this heterostructure system. As such, the MOF appears to function as an appropriate interfacial material, and this could provide a new perspective to design a high-performance photocatalyst with a hierarchical heterostructure.
Results and discussion
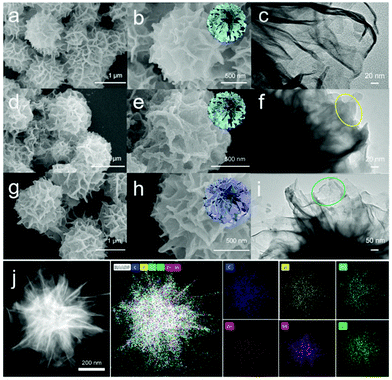
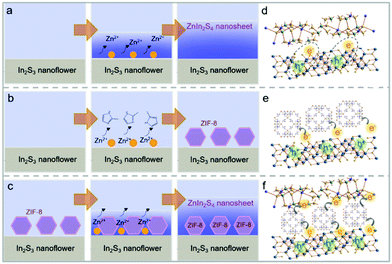
In this work, the In2S3@ZIF-8@ZnIn2S4 composite was fabricated via hydrothermal synthesis of In2S3 nanoflowers, ice-path synthesis of ZIF-8, and growth of ZnIn2S4 nanosheets using an oil-path (Fig. 1). The scanning electron microscopy (SEM) images illustrate the synthesis process of the In2S3@ZIF-8@ZnIn2S4 samples including the structure after treating the In2S3 nanoflower precursors with the growth of ZIF-8 and subsequent ZnIn2S4 in Fig. 2. Fig. 2a and b present the morphology of the In2S3 sample and clearly show their nanoflower structures with an average size of 1.2 μm. Fig. 2d, e and g, h display the obtained In2S3@ZIF-8 and In2S3@ZIF-8@ZnIn2S4 composite after ZIF-8 had been coated on the surface of the In2S3 nanoflowers and the subsequent growth of ZnIn2S4 nanosheets. Representative images of In2S3@ZIF-8 are displayed in Fig. 2d and e, which depict the fabricated ZIF-8 nanoparticles on the entire surface of the In2S3 nanoflowers. The images of the In2S3@ZIF-8 nanoflowers indicate that the average size is maintained. As illustrated in Fig. 2g and h, the nanoflower structures of In2S3@ZIF-8@ZnIn2S4 are still maintained and then the size increased to 1.5 μm after the ZnIn2S4 ultrathin nanosheets were stacked grown onto the In2S3@ZIF-8 nanoflowers, further indicating that the In2S3@ZIF-8@ZnIn2S4 composite was successfully synthesized in a hierarchical nanoflower configuration.Transmission electron microscopy (TEM) provides further details regarding the different microscopic structures of the obtained In2S3@ZIF-8 and In2S3@ZIF-8@ZnIn2S4 composites.
Fig. 2c indicates that the In2S3 sample displays obvious, smooth, and transparent crystal edges with a diameter of 1.2 μm. In Fig. 2f, it is apparent that the entire surface of the In2S3 nanoflower is homogeneously covered with ZIF-8 nanoparticles, further indicating that the In2S3@ZIF-8 composite possesses a hierarchical heterojunction configuration. Compared with the pure In2S3 and In2S3@ZIF-8 composite, it can be observed that the size of the In2S3@ZIF-8@ZnIn2S4 nanoflower was clearly increased with the different surface crystal edges, which is strong evidence for the formation of a hierarchical nanoflower heterostructure (Fig. 2i). The hierarchical heterojunction between the In2S3@ZIF-8 composite and the layered ZnIn2S4 nanosheets is shown in the HRTEM images (Fig. S4†). The inner layer of the heterostructure indicates two types of clear lattice fringes ca. 0.32 nm and ca. 0.62 nm corresponding to the (311) and (111) planes of In2S3, respectively.31 ZIF-8 shows indistinct lattice fringes in the middle of In2S3@ZnIn2S4 nanoflowers.32 In addition, the outer lattice spacing is 0.322 nm, which corresponds to the (102) plane of ZnIn2S4.26 EDX analysis of a single In2S3@ZIF-8@ZnIn2S4 nanoflower shows the even distribution of C, N, O, Zn, In, and S (Fig. 2j). As a result, these conclusions indicate that the In2S3@ZIF-8@ZnIn2S4 composite was completely decorated with ZIF-8 to form an intimate interfacial contact that would be favourable to the photocatalytic process.
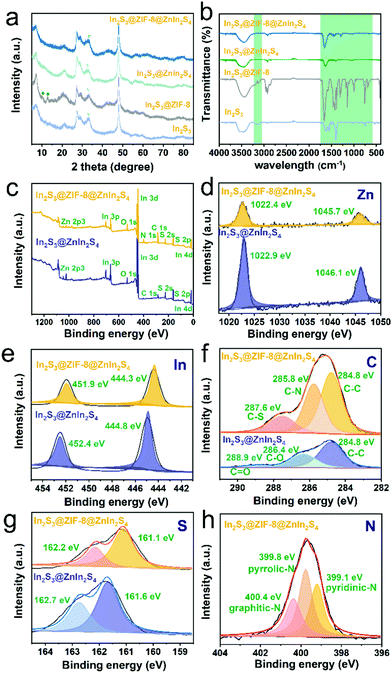
The crystalline structure and phase composition of In2S3, In2S3@ZIF-8, In2S3@ZnIn2S4 and In2S3@ZIF-8@ZnIn2S4 were explored via X-ray diffraction analysis. The peaks of In2S3 nanoflowers at 14.3°, 27.4°, 28.7°, 33.2°, 43.6°, 47.7°, 56.5°, and 59.4° (Fig. 3a) correspond well to the (111), (311), (222), (400), (511), (440), (622) and (444) planes, respectively.31 In2S3@ZIF-8 could be distinguished by three peaks positioned at 2θ = 7.4°, 12.7°, and 18.0° which resulted from the reflections of the (022), (112) and (222) planes, respectively.33 Although the content of ZIF-8 is low, the main diffraction peaks of the In2S3@ZIF-8 hybrids display both characteristic peaks of In2S3 and ZIF-8, indicating the presence of ZIF-8. For In2S3@ZIF-8@ZnIn2S4, no characteristic ZIF-8 diffraction peak is observed, which is most likely due to the low content of ZIF-8 in the complex and the growth of ZnIn2S4 on the surface. In addition, characteristic peaks of In2S3@ZnIn2S4 are observed at 21.6°, 27.7°, 32.5°, and 47.2°, which can be readily assigned to the (006), (102), (104) and (110) planes, respectively.34 Meanwhile, the In2S3@ZIF-8@ZnIn2S4 composite also presents characteristic diffraction peaks at 21.6°, 27.7°, 32.5°, and 47.2°.35 These findings emphasize that the ZIF-8 nanosheets are grown in the In2S3@ZnIn2S4 heterostructure.
The Fourier-transform infrared (FTIR) spectra of pure In2S3, In2S3@ZIF-8, In2S3@ZnIn2S4, and In2S3@ZIF-8@ZnIn2S4 are shown in Fig. 3b, which further demonstrate the existing ZIF-8 interface in the hierarchical heterojunctions. The peaks at 1306, 1145, 759 and 1444 cm−1 of In2S3@ZIF-8 match the typical stretching modes of ZIF-8, and present the three main characteristic absorption regions of In2S3. Moreover, the In2S3@ZIF-8@ZnIn2S4 composite also displays absorption peaks at around 1306, 1145, 759 and 1444 cm−1 for the samples corresponding to the bending signals and stretching vibrations of the imidazole ring. Meanwhile, the peaks located at 1610 and 1396 cm−1 of In2S3@ZIF-8@ZnIn2S4 identify the existence of ZnIn2S4, which confirm the presence of ZnIn2S4 nanosheets on the surface of In2S3@ZIF-8 nanoflowers.
The N2 adsorption–desorption curves and pore size distributions of In2S3@ZnIn2S4 and In2S3@ZIF-8@ZnIn2S4 are presented (Fig. S5†). The isotherms are categorized as type III with H3 hysteresis based on the Brunauer–Deming–Deming–Teller classification which is thought to be a slit hole formed by the accumulation of flake materials. In2S3@ZnIn2S4 and In2S3@ZIF-8@ZnIn2S4 show the BET values of 88.45 and 123.43 m2 g−1, illustrating the impact of the introduction of ZIF-8. This is because of the partial increase in mesopores due to the loading of ZIF-8 at the interface of the hierarchical heterostructure. As shown in Fig. S6,† the TGA curves depict the decomposition of In2S3@ZnIn2S4 and In2S3@ZIF-8@ZnIn2S4 under nitrogen flow. In particular, the different mass loss at up to 220 °C from the two materials implied the removal of ZIF-8, which is consistent with the weight loss from 98.76% to 87.85% in In2S3@ZIF-8@ZnIn2S4. The sharp mass loss of 10.91% also demonstrates the successful synthesis of the ZIF-8 interface in the hierarchical heterostructure.
The X-ray photoelectron spectroscopy (XPS) analysis of the In2S3@ZnIn2S4 and In2S3@ZIF-8@ZnIn2S4 composites is shown in Fig. 3c–h. The XPS survey spectrum confirms the existence of In, S, Zn, C, and N in the In2S3@ZIF-8@ZnIn2S4 composites. The In 3d spectrum with two peaks at 444.9 eV (In 3d5/2) and 452.4 eV (In 3d3/2) and the peaks of S 2p3/2 at 161.7 eV is simultaneously attributed to the S coordinated to Zn and In within ZnIn2S4. The S 2p core-level spectrum (Fig. 3g) indicates peaks at 161.2 eV and 162.5 eV, which are attributed to the S 2p3/2 and S 2p1/2 transitions, respectively. Two peaks in the Zn 2p spectrum (Fig. 3d) associated with Zn–O (1044.5 eV) and Zn-S (1021.5 eV) were detected in the Zn 2p1/2 and Zn 2p3/2 regions, indicating the Zn(II) sulphuration state of In2S3, ZIF-8 and ZnIn2S4.36 Correspondingly, the C 1s and N 1s spectra present Zn–C (284.6 eV) and Zn–N (399.2 eV) signals (Fig. 3f and h), confirming the formation of ZIF-8 after ZnIn2S4 nanosheets were added. The observation of C–N (285.6 eV) indicates the generation of ZIF-8 (Fig. 3f), which was further demonstrated by the signals representing pyridinic-N at 399.1 eV, pyrrolic-N at 399.8 eV and graphitic-N at 400.4 eV (Fig. 3h).37 Together, these results indicate that the main chemical states of the elements in the four heterostructures were In3+, O2−, Zn2+ and S2−. Therefore, the XPS results of Zn 2p, S 2p, C 1s and N 2p combined with the XRD spectrum further confirmed the existence of ZIF-8.
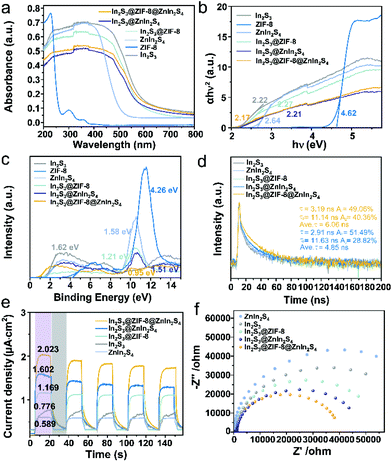
An in-depth investigation of the optical properties of the materials using UV/Vis diffuse reflectance spectroscopy (DRS) is shown in Fig. 4a. On the one hand, we intuitively observe that the In2S3@ZnIn2S4 heterojunction displays stronger visible-light absorption to ca. 500 nm in the visible-light region relative to pure In2S3 and In2S3@ZIF-8, indicating the excellent visible light response of these heterostructures. On the other hand, the absorption edge of the In2S3@ZnIn2S4 heterojunction is transformed by the introduction of the ZIF-8 nanoparticles and the absorption intensity produced a substantial red shift. Such findings confirm that the In2S3@ZIF-8@ZnIn2S4 heterojunction absorbed more light over the solar spectrum, which would be favorable for the generation of electron–hole pairs for photocatalytic reductions.38 Meanwhile, the bandgap energies of In2S3, ZIF-8 and ZnIn2S4 were calculated to be 2.22, 4.62 and 2.64 eV based on the Tauc plots, respectively (Fig. 4b). Furthermore, the band edges of all the samples were explored via XPS valence band spectroscopy, and the VBs (the valence bands) of the In2S3, ZIF-8 and ZnIn2S4 are +1.62 eV, +4.26 eV and +1.58 eV, respectively (Fig. 4c). The CBs (the conduction bands) of the In2S3 and ZnIn2S4 parts from the two heterostructures were calculated on the basis of the formula used for calculating the band gap.38 Then the CBs (the conduction bands) of the In2S3, ZIF-8 and ZnIn2S4 parts were calculated on the basis of the formula used for calculating the band gap. The CB edge potentials were derived as −0.60 eV in In2S3, −0.36 eV in ZIF-8 and −1.06 eV in ZnIn2S4. As a result, the band structure analysis of In2S3@ZnIn2S4 and In2S3@ZIF-8@ZnIn2S4 is schematically described in Fig. S7.† The above observations indicate that In2S3@ZIF-8@ZnIn2S4 significantly optimized the electron transport path and the photocatalytic reduction compared with the In2S3@ZnIn2S4 heterostructure.
It was clearly observed that the individual lifetime components (τ1 and τ2) of the In2S3@ZIF-8@ZnIn2S4 sample are longer than those of In2S3@ZnIn2S4 (Fig. 4d and S8†). After insertion of the ZIF-8 nanoparticles as the interface of the heterostructure, the average photoluminescence (PL)lifetime (τave) is significantly extended from 5.78 ns (In2S3@ZnIn2S4) to 9.77 ns (In2S3@ZIF-8@ZnIn2S4). Meanwhile, the PL lifetime of In2S3@ZnIn2S4 and In2S3@ZIF-8 were prolonged which supported the positive effect of the heterostructure, compared with pure In2S3 and ZnIn2S4. Furthermore, the prolonged τave indicates the embedded ZIF-8 nanoparticles at the In2S3@ZnIn2S4 interface function as an electron donor receptor and allow for a lower binding energy of the intra/interlayer excitons compared to the pristine heterojunction, thus inhibiting the charge recombination of the heterostructure, and accelerating the photo-generated electron migration from the CB of In2S3 to the VB of ZnIn2S4. As a result, In2S3@ZIF-8@ZnIn2S4 shows a much more remarkable charge-carrier transfer scenario compared to the original heterostructure.25,27
In order to investigate the impact of the ZIF-8 interface with regard to the separation–recombination efficiency of the photo-generated charge, room-temperature photoluminescence (PL) characterization of these composites was performed. These results reveal that the In2S3@ZIF-8@ZnIn2S4 sample exhibits prominent fluorescence quenching compared to the In2S3, In2S3@ZIF-8 and In2S3@ZnIn2S4 samples in Fig. S9.† This result indicates that In2S3@ZIF-8@ZnIn2S4 displays a more efficient charge separation and migration of the In2S3@ZIF-8@ZnIn2S4 heterostrcture.13 To characterize the photoelectrochemical properties of the samples, the photocurrent response and electrochemical impedance were determined using the as-prepared materials. The effect of ZIF-8 incorporation is clearly demonstrated from the observation that In2S3@ZIF-8@ZnIn2S4 exhibits the highest photocurrent among all the tested materials (Fig. 4e).39 It has been demonstrated that the ZIF-8 interface facilitates the anisotropic electron flow and separation and improves the charge separation on In2S3@ZIF-8@ZnIn2S4 compared to In2S3@ZnIn2S4. When ZIF-8 was coupled with the hierarchical heterostructure, In2S3@ZIF-8@ZnIn2S4 exhibits the smallest arc radius in the Nyquist plot analysis, which is consistent with its demonstrated highest photocurrent (Fig. 4f).40
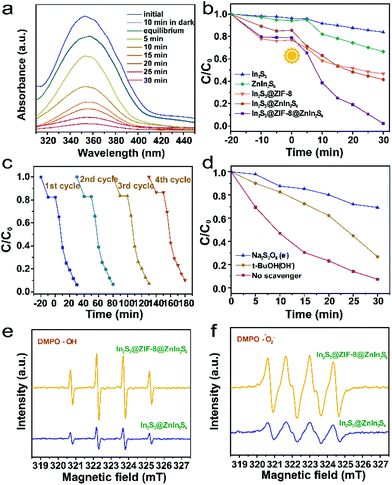
To shed light on the advantage of the hierarchical heterostructure system incorporating ZIF-8, the photocatalytic reduction efficiencies of all as-prepared products were assessed. The typical temporal evolution spectrum of the Cr(VI) solutions over In2S3@ZIF-8@ZnIn2S4 at the absorption peak of 350 nm is presented in Fig. 5a, which almost disappeared after 30 min. Compared to the reported hierarchical heterostructure, the In2S3@ZIF-8@ZnIn2S4 heterostructures display satisfactory photocatalytic reduction activity (Table S1†). The photocatalytic reduction activities of the samples were initiated after the adsorption–desorption equilibria between Cr(VI) (Fig. 5b). For comparison, the adsorption capacity and photocatalytic effectivity of In2S3@ZnIn2S4 for Cr(VI) were both less than that of In2S3@ZIF-8@ZnIn2S4 (97.8%) after 30 min due to its small BET specific surface area and the rapid recombination of the electron–hole pairs. Furthermore, the adsorption capacity and photocatalytic reduction effectivity of In2S3@ZIF-8@ZnIn2S4 for Cr(VI) simultaneously increased, indicating the positive impact of the hierarchical heterostructure with the introduction of ZIF-8. Meanwhile, the photocatalytic reduction performances of In2S3, ZnIn2S4 and In2S3@ZIF-8 over the course of 40 min were all lower than that of In2S3@ZnIn2S4, suggesting that the enhanced capacity of the hierarchical heterostructure and the nanoflower structure also increased the photocatalytic reduction effectivity of Cr(VI). These results serve as direct evidence that the existence of the ZIF-8 interface improves the adsorption capacity and accelerates the electron migration of the In2S3@ZnIn2S4 heterostructure because of the enhanced BET surface area and electron donor receptor optimized electron transfer process. In addition, stability and reusability are important for judging the quality of photocatalysts and their further applications. The In2S3@ZIF-8@ZnIn2S4 composite material also exhibits good cycling performance, which is proved by ten consecutive cycles of the photocatalytic degradation of Cr(VI) samples. As shown in Fig. 5c and S10a,† the removal efficiency of In2S3@ZIF-8@ZnIn2S4 declines to 78.06% after 10 cycles. We can clearly see that the photocatalytic efficiency of In2S3@ZIF-8@ZnIn2S4 decreased slightly, but there was almost no deactivation. In order to further study the stability of the prepared photocatalyst, the XRD patterns, FTIR and XPS spectra and TEM images of In2S3@ZIF-8@ZnIn2S4 are shown in Fig. S10 and S11† before and after ten consecutive experiments of degradation of the Cr(VI) solution. The XRD, FTIR, XPS and TEM data after 10 cycles did not change significantly, indicating that In2S3@ZIF-8@ZnIn2S4 has good chemical stability. To further examine the mechanism of photoreduction reduction, active species (˙OH and e−) trapping experiments (Fig. 5d) and DMPO spin-trapping ESR (Fig. 5e, f and S12, 13†) were performed. The photocatalytic reduction efficiency was suppressed by the addition of t-BuOH and Na2S2O8. Meanwhile, the ESR signals of DMPO-˙OH and DMPO-˙O2− are enhanced with increasing time, suggesting that In2S3@ZIF-8@ZnIn2S4 can generate electrons and holes under visible light. Furthermore, the ESR signals of ˙OH and ˙O2− are observed for In2S3@ZIF-8@ZnIn2S4, which are almost 2 times higher than those of the In2S3@ZnIn2S4 heterojunction; an increase in charge separation has promoted In2S3@ZIF-8@ZnIn2S4 for the effective utilization of electrons to generate more ˙OH and ˙O2− to enhance the efficiency of photocatalytic reduction.
The efficiency of the In2S3@ZnIn2S4 heterostructure with ZIF-8 nanoparticles leads to the speculation that the impact of the interface in the hierarchical heterostructure in relation to photo-generated carrier recombination induces this enhanced photocatalysis. As depicted in Fig. 6, the construction type of the heterostructure for In2S3 nanoflowers and ZnIn2S4 nanosheets has been determined. In the photocatalytic reduction, incident photons are absorbed by In2S3via photoelectron transition, and the photoexcited electrons are then injected into the ZnIn2S4 conduction band.41 This heterojunction type reveals a lack of spontaneous growth on the nanosheet, although it does partly improve the carrier separation efficiency. In this case, the electron transfer rate of the stacked heterostructure is lower, and this is probably due to the high binding energy of the excitons and large momentum change in electron transfer, and therefore, the photo-generated carriers can be easily recombined.18,20,22 However, inspired by the rapid charge transfer of the donor–acceptor heterojunction, this D–A construction has been applied to boost the charge mobility of the hierarchical heterojunction by controlling the local structure of the heterostructure.6,13,19 Under the introduction of the ZIF-8 interface, the heterostructure modified with a ZIF-8 nanoparticle layer allows for a lower binding energy of the intra/interlayer excitons compared to that determined by the conduction band minimum and valence band maximum of the epitaxial-growth hierarchical heterostructure (Fig. 6d and f). Furthermore, the charges can persistently transfer from the electron donor to the electron acceptor when the final charge state transfer is still larger than the acceptor. Moreover, the electron transfer rate in In2S3@ZIF-8@ZnIn2S4 might depend on ZIF-8 owing to their D–A structure and overcome the high Coulomb potential by spatial separation at the edge of nanoflowers. As a result, the electron transfer process from In2S3 is greatly accelerated, and most of the photo-generated electrons can drift to the ZIF-8 interface instead of undergoing recombination. After H2O is reduced to O2, subsequent electron–hole pairs produce hydroperoxide and peroxide anionic species. As shown in Fig. S7,† further abstraction of an electron from the In2S3 group to the ZIF-8 interface takes place and then ZnIn2S4 gives rise to the target Cr(III). These mechanisms suggest that the catalytic reaction occurring at the ZIF-8 interface upon visible light irradiation optimizes the hierarchical heterostructure, followed by the promotion of the electron transfer process within the heterojunction.
On the basis of the above-mentioned observations, ZIF-8 (as the electron donor–acceptor) plays a pivotal role in the optimized hierarchical heterostructure and enhanced photocatalytic reduction performance. Firstly, ZIF-8 improves the In2S3@ZnIn2S4 heterostructure adsorption capacity because of its large internal surface areas to enhance the photocatalytic performance. Secondly, the electrons of the In2S3@ZnIn2S4 heterostructure transferring across the epitaxial-growth hierarchical heterojunction are expected to be accompanied by a large momentum change, resulting in a significantly retarded charge transfer process. The presence of ZIF-8 leads to charge transfers, resulting in an intermediate state of the electron/hole pair with excess energy before the formation of tightly bound interlayer excitons. The excess energy of the intermediate state available allows the sampling of a broader range of momentum space, thus allowing them to easily dissociate and hindering the photocurrent recombination.18,19 And then, In2S3@ZnIn2S4 with poorly controlled nanodomains of different species leads to high binding energy of intra/interlayer excitons. These tightly bound electron–hole pairs also need to overcome a high Coulomb barrier to dissociate and contribute to the photocurrent. The presence of ZIF-8 makes the charges tend to spontaneously migrate to the acceptor by spatial separation at the edge of nanoflowers because of the larger energy of the intra/interlayer excitons of the in situ heterostructure and this accelerates the electron transfer from In2S3 to ZIF-8 and from ZIF-8 to the ZnIn2S4 surface, leading to efficient Cr(VI) reduction on the ZnIn2S4 surface.19–21 Furthermore, to understand the photoresponse mechanism of In2S3@ZIF-8@ZnIn2S4 under visible light irradiation, the electronic structure analysis was carried out. The bandgap energies of In2S3, ZIF-8 and ZnIn2S4 were calculated to be 2.22, 4.62 and 2.64 eV based on the Tauc plots, respectively (Fig. 4). According to the experimental data and comparison of other references DFT analysis, the band structure analysis of In2S3@ZnIn2S4 and In2S3@ZIF-8@ZnIn2S4 is schematically described in Fig. S7.†21,22 In addition, the band gap of In2S3@ZIF-8@ZnIn2S4 decreased to 2.17 eV; this calculation result may explain the reason for the enhanced photocatalysis performance after forming the heterojunction. The above observations indicate that In2S3@ZIF-8@ZnIn2S4 optimized the electron transport path and the photocatalytic reduction compared with the In2S3@ZnIn2S4 heterostructure.
Experimental
Synthesis of In2S3 nanoflowers
Firstly, 90 mg of In(NO3)3·xH2O and 110 mg of 1,4-benzenedicarboxylic acid (H2BDC) were dissolved in 20 mL of DMF, respectively, and then mixed and stirred for 5 min. Later the resultant solution was placed in an oil bath at 120 °C for 30 min. After that, the final precipitate was filtered and washed six times with ethanol. The obtained products were added to a 25 mL ethanol solution containing 375 mg of thiourea, and then stirred for 5 min. Subsequently, the resultant mixture was transferred into a Teflon-lined autoclave (50 mL in capacity) and maintained at 180 °C for 3 h. Then, the final yellow precipitate was filtered and washed at least three times with ethanol and H2O, respectively.Synthesis of In2S3@ZIF-8 nanoflowers
In the second step, the as-prepared In2S3 nanoflowers were homogeneously dispersed in 9 mL of ethanol via ultrasonication. The mixture was subsequently placed in an ice bath. A total of 4.96 mg of zinc acetate hexahydrate, 2.48 mg of PVP, and 14.76 mg of 2-methylimidazole were added to 16 mL of ethanol and mixed with In2S3 solution via magnetic stirring for 1 h. The yellow samples were centrifuged and rinsed three times in ethanol.Synthesis of hierarchical In2S3@ZIF-8@ZnIn2S4
Conversion of the In2S3 and In2S3@ZIF-8 nanoflowers into hierarchical In2S3@ZnIn2S4 and In2S3@ZIF-8@ZnIn2S4 structures was realized via an efficient in situ growth method. The In2S3 and In2S3@ZIF-8 nanoflowers were dispersed in 20 mL of H2O (pH = 2.5), respectively, followed by the addition of 27.2 mg of ZnCl2. The resultant mixture was stirred for 30 min and then maintained at 80 °C for 2 h in an oil bath. The obtained yellow precipitates were then filtered, washed with ethanol and H2O, and dried under vacuum at 60 °C for 12 h. The products obtained were designated as In2S3@ZnIn2S4 and In2S3@ZIF-8@ZnIn2S4, respectively.Photocatalysis test
A series of photodegradation tests using Cr(VI) (20 mg L−1) solutions as target pollutants were evaluated for comparing the photocatalytic activities of In2S3, ZnIn2S4, In2S3@ZIF-8, In2S3@ZnIn2S4 and In2S3@ZIF-8@ZnIn2S4. Briefly, 0.005 g of photocatalysts in 50 mL of Cr(VI) solution were stirred under dark conditions for 20 min to ensure that the adsorption–desorption balance was reached. Samples were then taken from the suspension at a given 10 min interval under visible-light irradiation. For cyclic utilization, the photocatalysts were centrifugally separated for removal.Conclusions
In summary, we demonstrated that a ZIF-8 interface with In2S3@ZnIn2S4 heterostructure nanoflowers exhibits unique structural characteristics and compositional features, which allow for efficient dissociation to form the photocurrent, such as excellent electron transfer efficiency and highly reliable operation. Such a photocatalytic process enabled ultrahigh photocatalytic activity in the photoreduction of Cr(VI) under visible-light illumination. By simply incorporating a ZIF-8 interface in the In2S3@ZnIn2S4 heterostructure in the form of nanoflowers, two positive effects, overcoming the interlayer momentum mismatch in the edge of the nanoflowers and promoting the charge migration as the D–A structure, helped facilitate the intrinsic heterostructure efficiency, resulting in noteworthy performance for this photocatalysis. It should be noted that these results illustrate the remarkable impact of ZIF-8 as a typical MOF for photocatalytic reduction with the combination of theoretical and experimental fundamental insight. The designed MOF as an interface with the In2S3@ZnIn2S4 heterostructure has great potential for realizing new-generation high-performance photoreductions compared with traditional heterostructured photocatalysts.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (21675127), the Shaanxi Provincial Science Fund for Distinguished Young Scholars (2018JC-011), the Qinghai Special Project of Innovation Platform for Basic Conditions of Scientific Research of China (No. 2020-ZJ-T05), and the Qinghai Provincial Key Laboratory of Qinghai-Tibet Plateau Biological Resource (2021-ZJ-Y14).Notes and references
- Y. Wang, L. Rao, P. Wang, Z. Shi and L. Zhang, Photocatalytic activity of N-TiO2/O-doped N vacancy g-C3N4 and the intermediates toxicity evaluation under tetracycline hydrochloride and Cr(VI) coexistence environment, Appl. Catal., B, 2020, 262, 118308 CrossRef CAS.
- X. Sun, S. Yao, C. Yu, G. Li, C. Liu, Q. Huo and Y. Liu, An ultrastable Zr-MOF for fast capture and highly luminescence detection of Cr2O72− simultaneously in an aqueous phase, J. Mater. Chem. A, 2018, 6, 6363–6369 RSC.
- Z. Fang, Q. Li, L. Su, J. Chen, K.-C. Chou and X. Hou, Efficient synergy of photocatalysis and adsorption of hexavalent chromium and rhodamine B over Al4SiC4/rGO hybrid photocatalyst under visible-light irradiation, Appl. Catal., B, 2019, 241, 548–560 CrossRef CAS.
- J. Cai, J. Huang, S. Wang, J. Iocozzia, Z. Sun, J. Sun, Y. Yang, Y. Lai and Z. Lin, Crafting Mussel-Inspired Metal Nanoparticle-Decorated Ultrathin Graphitic Carbon Nitride for the Degradation of Chemical Pollutants and Production of Chemical Resources, Adv. Mater., 2019, 31, e1806314 CrossRef PubMed.
- Y. Zhang and S.-J. Park, Facile construction of MoO3@ZIF-8 core-shell nanorods for efficient photoreduction of aqueous Cr(VI), Appl. Catal., B, 2019, 240, 92–101 CrossRef CAS.
- W. Chen, Z. Yang, Z. Xie, Y. Li, X. Yu, F. Lu and L. Chen, Benzothiadiazole functionalized D–A type covalent organic frameworks for effective photocatalytic reduction of aqueous chromium(VI), J. Mater. Chem. A, 2019, 7, 998–1004 RSC.
- Y. Wang, G. Tan, T. Liu, Y. Su, H. Ren, X. Zhang, A. Xia, L. Lv and Y. Liu, Photocatalytic properties of the g-C3N4/{010} facets BiVO4 interface Z-Scheme photocatalysts induced by BiVO4 surface heterojunction, Appl. Catal., B, 2018, 234, 37–49 CrossRef CAS.
- M.-h. Wu, L. Li, Y.-c. Xue, G. Xu, L. Tang, N. Liu and W.-y. Huang, Fabrication of ternary GO/g-C3N4/MoS2 flower-like heterojunctions with enhanced photocatalytic activity for water remediation, Appl. Catal., B, 2018, 228, 103–112 CrossRef CAS.
- S. Wang, M. Xu, T. Peng, C. Zhang, T. Li, I. Hussain, J. Wang and B. Tan, Porous hypercrosslinked polymer-TiO2-graphene composite photocatalysts for visible-light-driven CO2 conversion, Nat. Commun., 2019, 10, 676 CrossRef CAS PubMed.
- L. Jing, Y. Xu, M. Xie, J. Liu, J. Deng, L. Huang, H. Xu and H. Li, Three dimensional polyaniline/MgIn2S4 nanoflower photocatalysts accelerated interfacial charge transfer for the photoreduction of Cr(VI), photodegradation of organic pollution and photocatalytic H2 production, Chem. Eng. J., 2019, 360, 1601–1612 CrossRef CAS.
- F. Mu, Q. Cai, H. Hu, J. Wang, Y. Wang, S. Zhou and Y. Kong, Construction of 3D hierarchical microarchitectures of Z-scheme UiO-66-(COOH)2/ZnIn2S4 hybrid decorated with non-noble MoS2 cocatalyst: A highly efficient photocatalyst for hydrogen evolution and Cr(VI) reduction, Chem. Eng. J., 2020, 384, 123352 CrossRef CAS.
- Y. Chao, P. Zhou, N. Li, J. Lai, Y. Yang, Y. Zhang, Y. Tang, W. Yang, Y. Du, D. Su, Y. Tan and S. Guo, Ultrathin Visible-Light-Driven Mo Incorporating In2O3-ZnIn2Se4 Z-Scheme Nanosheet Photocatalysts, Adv. Mater., 2019, 31, e1807226 CrossRef PubMed.
- Y. Li, Y. Fang, Z. Cao, N. Li, D. Chen, Q. Xu and J. Lu, Construction of g-C3N4/PDI@MOF heterojunctions for the highly efficient visible light-driven degradation of pharmaceutical and phenolic micropollutants, Appl. Catal., B, 2019, 250, 150–162 CrossRef CAS.
- Y. P. Zheng, X. M. Hou, Q. Li, Z. Fang, T. Yang, T. X. Liang, X. M. Duan, M. H. Shang and W. Y. Yang, Electrostatic interaction assisted synthesis of a CdS/BCN heterostructure with enhanced photocatalytic effects, J. Mater. Chem. C, 2020, 8, 1803–1810 RSC.
- Q. Li, X. M. Hou, Z. Fang, T. Yang, J. H. Chen, X. Z. Cui, T. X. Liang and J. L. Shi, Construction of layered h-BN/TiO2 hetero-structure and probing of the synergetic photocatalytic effect, Sci. China Mater., 2020, 63, 276–287 CrossRef CAS.
- S. Zhang, M. Du, Z. Xing, Z. Li, K. Pan and W. Zhou, Defect-rich and electron-rich mesoporous Ti-MOFs based NH2-MIL-125(Ti)@ZnIn2S4/CdS hierarchical tandem heterojunctions with improved charge separation and enhanced solar-driven photocatalytic performance, Appl. Catal., B, 2020, 262, 118202 CrossRef CAS.
- B. Liu, X. Liu, J. Liu, C. Feng, Z. Li, C. Li, Y. Gong, L. Pan, S. Xu and C. Q. Sun, Efficient charge separation between UiO-66 and ZnIn2S4 flowerlike 3D microspheres for photoelectronchemical properties, Appl. Catal., B, 2018, 226, 234–241 CrossRef CAS.
- H. Chen, X. Wen, J. Zhang, T. Wu, Y. Gong, X. Zhang, J. Yuan, C. Yi, J. Lou, P. M. Ajayan, W. Zhuang, G. Zhang and J. Zheng, Ultrafast formation of interlayer hot excitons in atomically thin MoS2/WS2 heterostructures, Nat. Commun., 2016, 7, 12512 CrossRef CAS PubMed.
- Z. A. Lan, G. Zhang, X. Chen, Y. Zhang, K. A. I. Zhang and X. Wang, Reducing the Exciton Binding Energy of Donor-Acceptor-Based Conjugated Polymers to Promote Charge-Induced Reactions, Angew. Chem., Int. Ed., 2019, 58, 10236–10240 CrossRef CAS PubMed.
- J. Jiang, X. Zou, Y. Lv, Y. Liu, W. Xu, Q. Tao, Y. Chai and L. Liao, Rational design of Al2O3/2D perovskite heterostructure dielectric for high performance MoS2 phototransistors, Nat. Commun., 2020, 11, 4266 CrossRef CAS PubMed.
- S. Mao, J.-W. Shi, G. Sun, D. Ma, C. He, Z. Pu, K. Song and Y. Cheng, Au nanodots@thiol-UiO66@ZnIn2S4 nanosheets with significantly enhanced visible-light photocatalytic H2 evolution: The effect of different Au positions on the transfer of electron-hole pairs, Appl. Catal., B, 2021, 282, 119550 CrossRef CAS.
- B. Sun, W. Zhou, H. Li, L. Ren, P. Qiao, W. Li and H. Fu, Synthesis of Particulate Hierarchical Tandem Heterojunctions toward Optimized Photocatalytic Hydrogen Production, Adv. Mater., 2018, 30, e1804282 CrossRef PubMed.
- X. B. Wang, J. Liu, S. Leong, X. C. Lin, J. Wei, B. Kong, Y. F. Xu, Z. X. Low, J. F. Yao and H. T. Wang, Rapid Construction of ZnO@ZIF-8 Heterostructures with Size-Selective Photocatalysis Properties, ACS Appl. Mater. Interfaces, 2016, 8, 9080–9087 CrossRef CAS.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H. C. Zhou, Stable Metal-Organic Frameworks: Design, Synthesis, and Applications, Adv. Mater., 2018, 30, e1704303 CrossRef.
- S. Wang, B. Y. Guan, Y. Lu and X. W. D. Lou, Formation of Hierarchical In2S3-CdIn2S4 Heterostructured Nanotubes for Efficient and Stable Visible Light CO2 Reduction, J. Am. Chem. Soc., 2017, 139, 17305–17308 CrossRef CAS.
- X. Peng, L. Ye, Y. Ding, L. Yi, C. Zhang and Z. Wen, Nanohybrid photocatalysts with ZnIn2S4 nanosheets encapsulated UiO-66 octahedral nanoparticles for visible-light-driven hydrogen generation, Appl. Catal., B, 2020, 260, 118152 CrossRef CAS.
- S. Wang, B. Y. Guan and X. W. D. Lou, Construction of ZnIn2S4-In2O3 Hierarchical Tubular Heterostructures for Efficient CO2 Photoreduction, J. Am. Chem. Soc., 2018, 140, 5037–5040 CrossRef CAS PubMed.
- Y. Li, P. Han, Y. Hou, S. Peng and X. Kuang, Oriented ZnmIn2Sm+3@In2S3 heterojunction with hierarchical structure for efficient photocatalytic hydrogen evolution, Appl. Catal., B, 2019, 244, 604–611 CrossRef CAS.
- H. S. Huang, X. Jiang, N. J. Li, D. Y. Chen, Q. F. Xu, H. Li, J. H. He and J. M. Lu, Noble-metal-free ultrathin MXene coupled with In2S3 nanoflakes for ultrafast photocatalytic reduction of hexavalent chromium, Appl. Catal., B, 2021, 284, 119754 CrossRef CAS.
- J. H. Qiu, M. Li, Y. Yang and J. F. Yao, Facile construction of three-dimensional netted ZnIn2S4 by cellulose nanofibrils for efficiently photocatalytic reduction of Cr(VI), Chem. Eng. J., 2019, 375, 121990 CrossRef CAS.
- J. Li, Y. Ma, Z. Ye, M. Zhou, H. Wang, C. Ma, D. Wang, P. Huo and Y. Yan, Fast electron transfer and enhanced visible light photocatalytic activity using multi-dimensional components of carbon quantum dots@3D daisy-like In2S3/single-wall carbon nanotubes, Appl. Catal., B, 2017, 204, 224–238 CrossRef CAS.
- K. Jayaramulu, K. K. Datta, C. Rosler, M. Petr, M. Otyepka, R. Zboril and R. A. Fischer, Biomimetic Superhydrophobic/Superoleophilic Highly Fluorinated Graphene Oxide and ZIF-8 Composites for Oil-Water Separation, Angew. Chem., Int. Ed., 2016, 55, 1178–1182 CrossRef CAS PubMed.
- X. Li, G. Liu, D. Xu, X. Hong and S. C. E. Tsang, Confinement of subnanometric PdZn at a defect enriched ZnO/ZIF-8 interface for efficient and selective CO2 hydrogenation to methanol, J. Mater. Chem. A, 2019, 7, 23878–23885 RSC.
- Y. He, H. Rao, K. Song, J. Li, Y. Yu, Y. Lou, C. Li, Y. Han, Z. Shi and S. Feng, 3D Hierarchical ZnIn2S4 Nanosheets with Rich Zn Vacancies Boosting Photocatalytic CO2 Reduction, Adv. Funct. Mater., 2019, 29, 1905153 CrossRef CAS.
- B. Xu, P. He, H. Liu, P. Wang, G. Zhou and X. Wang, A 1D/2D helical CdS/ZnIn2S4 nano-heterostructure, Angew. Chem., Int. Ed., 2014, 53, 2339–2343 CrossRef CAS PubMed.
- S. Wang, Y. Wang, S. L. Zhang, S. Q. Zang and X. W. D. Lou, Supporting Ultrathin ZnIn2S4 Nanosheets on Co/N-Doped Graphitic Carbon Nanocages for Efficient Photocatalytic H2 Generation, Adv. Mater., 2019, 31, e1903404 CrossRef PubMed.
- L. Zhang, Z. Su, F. Jiang, L. Yang, J. Qian, Y. Zhou, W. Li and M. Hong, Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions, Nanoscale, 2014, 6, 6590–6602 RSC.
- L. Yang, P. Wang, J. Yin, C. Wang, G. Dong, Y. Wang and W. Ho, Engineering of reduced graphene oxide on nanosheet–g-C3N4/perylene imide heterojunction for enhanced photocatalytic redox performance, Appl. Catal., B, 2019, 250, 42–51 CrossRef CAS.
- G. Zhang, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity, Appl. Catal., B, 2019, 250, 313–324 CrossRef CAS.
- D. Majhi, K. Das, A. Mishra, R. Dhiman and B. G. Mishra, One pot synthesis of CdS/BiOBr/Bi2O2CO3: A novel ternary double Z-scheme heterostructure photocatalyst for efficient degradation of atrazine, Appl. Catal., B, 2020, 260, 118222 CrossRef CAS.
- Z. Wu, X. Yuan, G. Zeng, L. Jiang, H. Zhong, Y. Xie, H. Wang, X. Chen and H. Wang, Highly efficient photocatalytic activity and mechanism of Yb3+/Tm3+ codoped In2S3 from ultraviolet to near infrared light towards chromium(VI) reduction and rhodamine B oxydative degradation, Appl. Catal., B, 2018, 225, 8–21 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1qi00973g |
| This journal is © the Partner Organisations 2022 |