Introducing ytterbium acetate to luminescent CsPbCl3 nanocrystals for enhanced sensitivity of Cu2+ detection†
Xiufeng
Wu
,
Songtao
Hu
,
He
Shao
,
Lifang
Li
,
Wenda
Chen
,
Biao
Dong
 ,
Lin
Xu
,
Wen
Xu
,
Donglei
Zhou
,
Lin
Xu
,
Wen
Xu
,
Donglei
Zhou
 ,
Zhennan
Wu
*,
Hongwei
Song
,
Zhennan
Wu
*,
Hongwei
Song
 * and
Xue
Bai
* and
Xue
Bai
 *
*
State Key Laboratory of Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, 2699 Qianjin Street, Changchun 130012, China. E-mail: wuzn@jlu.edu.cn; songhw@jlu.edu.cn; baix@jlu.edu.cn
First published on 3rd November 2021
Abstract
In this study, we employed a luminescenct agent CsPbCl3 perovskite nanocrystal for Cu2+ detection, and achieved a prominent enhancement in sensitivity by the introduction of ytterbium acetate (Yb(OAc)3). The introduced Yb(OAc)3 is capable of causing a morphology transformation of CsPbCl3 nanocrystals from initial nanocubes into one-dimensional (1D) nanowires (NWs). The formed 1D CsPbCl3 NWs paved a direct charge transport path and exhibited low defect density as well as superior conductivity, which were beneficial to accelerating the rapid electron transport from the luminescent agent to Cu2+, and to give rise to an efficient inhibition of undesired charge trap, respectively. Besides, AcO− rendered a reduced steric hindrance during the formation of a copper-based counter-ion pair, thus improving the adsorption capacity of Cu2+ on the surface of the luminescent agent, CsPbCl3 nanocrystals. As results, the 1D Yb(OAc)3 passivated CsPbCl3 NWs exhibited efficient luminescence quenching for enhanced detection sensitivity. This Yb(OAc)3-involved system exhibited a detection limit as low as 0.06 nM in the Cu2+ detection window range from 0 to 1000 nM, and such value is optimal in the documented works for Cu2+ detection based on luminescent perovskite materials.
Introduction
Heavy metal ion pollution poses a serious threat to the environment and human health due to its high toxicity, carcinogenic effects, and accumulation in the ecosystem and human body.1–4 This situation dictates the lasting pursuit of simultaneous precise and efficient detection of heavy metal ions considering their quantitative and qualitative recognition. In particular, demand for Cu2+ detection with high sensitivity is going up because of their crucial role in biological and industrial systems.5–9 At present, numerous strategies for Cu2+ detection have been well-developed, such as traditional inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectrometry (AAS), and electrochemistry. However, they suffer from a complicated sample pre-treatment, limited universality and unsatisfied sensitivity of detection.10–12 In contrast, luminescent nanomaterials as detection agents towards highly sensitive detection of heavy metal ions have attracted intensive attention due to their low detection limit, desired universality, expected responsiveness, etc. Considering the luminescence responsiveness in the detection of metal ions, several mechanisms have been developed including fluorescence resonance energy transfer (FRET), cation exchange induced by exotic metal ions (static quenching), and electron transfer.13–15 Thus, attaining the desired detection sensitivity is highly dependent on the selection of luminescent species, in which features, including excellent optical properties, large specific surface area, strong ion adsorption capacity, and rapid electron transfer rate, are most important.8,16–19Lead-halide perovskites (APbX3, A is a cation and X is a halide anion) with brilliant photoelectric properties, adjustable bandgaps, and abundant surface landscapes are promising luminescent agents for ion detection.20–23 Recently, there have been some reports on Cu2+ detection by luminescent perovskite materials. For example, Sheng et al. employed CsPbX3 quantum dots (QDs) to detect Cu2+ in cyclohexane,24 in which CsPbBr3 QDs exhibited the optimal performance with a detection limit of 2 nM. In contrast, Liu et al. accomplished a more lower detection limit of 0.1 nM for the detection of Cu2+ in hexane using CsPbBr3 QDs.25 Although these CsPbX3 QD-based probes could realize the sensitive detection of Cu2+, a higher ion detection sensitivity with satisfactory selectivity is still highly expected.
In this study, we performed CsPbCl3 perovskite nanocrystals as luminescence agents to evaluate their detection ability of Cu2+. To gain a significant enhancement in detection sensitivity, ytterbium acetate (Yb(OAc)3) was introduced. The introduction of Yb(OAc)3 drove the anisotropic transformation from initial CsPbCl3 nanocubes (NCs) to one-dimensional (1D) nanowires (NWs). The as-formed 1D CsPbCl3 NWs exhibited lower defect density (0.67 × 1016 cm−3) compared with that of the initial CsPbCl3 NCs (2.1 × 1016 cm−3), which will promote the photoluminescence quantum efficiency (PLQY) and restrain the possibility of charge trap. 1D CsPbCl3 NWs displayed prominent conductivity (15.8 × 10–3 S cm−1), about 10-times higher than that of the initial CsPbCl3 NCs (1.6 × 10–3 S cm−1), due to the direct electron transport path and reduced defects. This enhancement is beneficial to the efficient electron transfer between host CsPbCl3 and Cu2+. Besides, AcO− possess shorter alkyl chains in comparison to oleate ions (OA−), which coordinated with Cu2+ originally in the organic phase, leading to the reduced steric hindrance during the formation of a copper-based counter-ion pair, thus improving the adsorption capacity of Cu2+ on the surface of the luminescent agent, CsPbCl3 nanocrystals. Through the strategy of synergistically optimizing electron transfer capacity and ion adsorption, such Yb(OAc)3-involved system exhibits a detection limit as low as 0.06 nM in the Cu2+ detection window ranging from 0 to 1000 nM, of which the value was the best in the documented works for Cu2+ detection based on luminescent perovskite materials.
Results and discussion
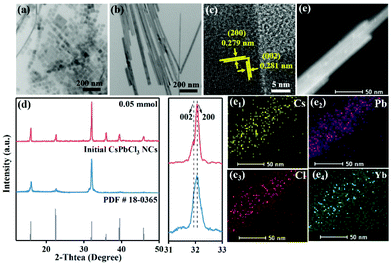
In a typical synthesis, the hot-injection method was used to prepare the long-chain alkylammonium and alkylacid co-capped CsPbCl3 nanocrystals. As displayed in Fig. 1a, b and Fig. S1a–d,† the morphology was observed by transmission electron microscopy (TEM), namely the well-defined NCs and NWs before and after the introduction of AcO−, respectively. The size evolution indicated that the as-formed NWs kept a constant width of ∼16 nm but an increased length of up to ∼500 nm. Further high resolution TEM (HR-TEM) image of such formed CsPbCl3 NWs in Fig. 1c shows the clear lattice distances of 0.281 nm and 0.279 nm, which are assigned to the (002) and (200) lattice planes of tetragonal CsPbCl3 perovskites. This indicated that the NWs grow along a preferred [200] direction, which was further verified by the powder X-ray diffractions analysis (XRD) (Fig. 1d). The XRD patterns of initial NCs and final NWs of CsPbCl3 presented their tetragonal perovskite structure, refering to the standard card PDF # 18-0365. Although the diffraction peaks were unshifted, the relative intensity of the diffraction peaks of (200) and (002) planes increased after the formation of NWs, implying the favored growth orientation along the [200] direction during the morphology transformation.In this morphology transformation system, AcO− plays a key role, and the complete transformation can only occur when the feeding amount of Yb(OAc)3 was up to 0.05 mmol (Fig. 1, S1a, b and Table S1†). Notably, we performed comparative trials by adding AcO− (Pb(OAc)2) and Yb3+ (YbCl3) separately to evaluate the effect of AcO− and Yb3+ on the formation of CsPbCl3 NWs. As a result, only the co-existence of AcO− and Yb3+ enabled by the synergistic effect could promote the formation of CsPbCl3 NWs effectively (Fig. S1c–e†). This directed growth may involve in the AcO− and Yb3+ corelative passivation and activation effect on the crystal plane of the CsPbCl3 perovskites.
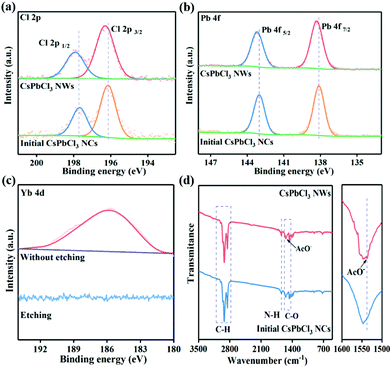
Furthermore, mapping images and inductively coupled plasma mass spectrometry (ICP-MS) were conducted to validate the chemical compositions and elemental distribution of CsPbCl3 NWs. As shown in Fig. 1e, Cs, Pb, Cl and Yb elements were homogeneously distributed in CsPbCl3 NWs. The composition of the initial CsPbCl3 NCs and final CsPbCl3 NWs was further explored via the investigation of X-ray photoelectron spectra (XPS) (Fig. 2a–c and Fig. S3†). The survey spectra displayed that the initial CsPbCl3 NCs and final CsPbCl3 NWs both included Cs, Pb, Cl and Yb elements, and the additional signal peaks of Yb elements emerged (Fig. S3a†). The high-resolution XPS spectra of the Cs, Pb, Cl and Yb elements for initial CsPbCl3 NCs and final CsPbCl3 NWs are revealed in Fig. 2a–c and Fig. S3b.† The binding energies related with Cs (Cs 3d3/2, Cs 3d5/2) exhibited the same position in the initial CsPbCl3 NCs and final CsPbCl3 NWs (Fig. S3b†). However, the high-resolution XPS spectra demonstrate that the binding energies of the Cl 2p and Pb 4f peaks shifted to higher binding energies in the final CsPbCl3 NWs compared with initial CsPbCl3 NCs. This shift verified that the Yb3+ has partially replaced Pb2+ ions in the CsPbCl3 perovskite (Fig. 2a and b). In addition, Fig. 2c shows the depth-resolved XPS measurement results of Yb 4d. Most interestingly, after the etching treatment, the binding energy signal corresponding to Yb 4d in the final CsPbCl3 NWs disappeared, indicating that the Yb3+ ions passivated only on the surface of the final CsPbCl3 NWs.26 In addition, the binding energy of Yb3+ corresponded to YbCl3 that passivated on the surface of NCs, which consisted well with the previous reports.27
Next, the capping ligands on the surface of the CsPbCl3 perovskite were identified by Fourier transform infrared (FTIR) spectroscopy. As shown in Fig. 2d, the asymmetric stretching of C–H of the alkyl chain could be detected at the wavenumber range of 2800–3010 cm−1 in the initial CsPbCl3 NCs and final CsPbCl3 NWs.28 In addition, the peak at 1640 cm−1 can be assigned to the characteristic N–H bending vibrations of alkylammonium cations (RNH3+), and the peak at 1400–1560 cm−1 corresponded to the C–O bending vibrations of carboxylate (RCOO−).29,30 After treatment with AcO−, the C–O bending vibration of AcO− at 1537 cm−1 appeared, which indicated that the introduced AcO− was located on the surface of the final CsPbCl3 NWs.31
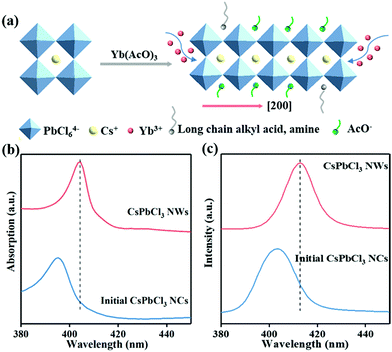
With respect to the formation mechanism of the final CsPbCl3 NWs, we presented a schematic in Fig. 3a. After AcO− was introduced, AcO− was mainly passivated on the (002) facet, which decreased the surface activation energy and inhibited the growth of the CsPbCl3 NWs along the [002] direction. In addition, AcO− also played as a surface passivator, providing a sufficient precursor to promote the NWs grow along the [200] direction. However, Yb3+ mainly activated the (200) crystal planes. The Yb3+ ions with three charges will compete with alkylammonium (RNH3+) ions on the perovskite surface, thus reducing the steric hindrance and promoting the anisotropic growth along the [200] direction.
To investigate the impact of AcO− on the optical properties of CsPbCl3 NCs, we have performed absorption and photoluminescence (PL) spectral measurements (Fig. 3b and c and Fig. S4†). Compared with initial CsPbCl3 NCs, the absorption peak of final CsPbCl3 NWs red-shifted by 11 nm. In addition, the PL peak red-shifted from 403 nm (initial CsPbCl3 NCs) to 412 nm (final CsPbCl3 NWs) (Fig. 3c and d), as verified by the TEM pattern in Fig. 1b. This can be attributed to the enhanced electronic coupling of excitons and energy transfer through nonradiative pathways between adjacent NCs in the perovskite system, which were enhanced after the morphology transformation from CsPbCl3 NCs to NWs.32 The final CsPbCl3 NWs exhibited a larger PLQY value (17.3%) than that of the initial CsPbCl3 NCs (2.1%). We speculated that this may have resulted from the reduced defect of the final CsPbCl3 NWs compared with initial CsPbCl3 NCs caused by the AcO− passivation. However, the Yb3+-related emission was not detectable, which was mainly contributed to the minimum amount of Yb3+ passivated on the surface of CsPbCl3 perovskites.21
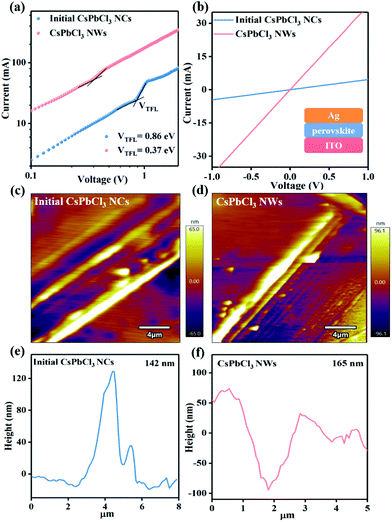
For this consideration, the defect density for the initial CsPbCl3 NCs and the final CsPbCl3 NWs by I–V measurements was further investigated (Fig. 4a). The space-charge-limited current under different bias from capacitor-like devices was measured, as shown in Fig. 4b, and the defect density can be calculated via the subsequent equation:
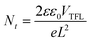 | (1) |
The conductivity of the CsPbCl3 perovskite was also characterized by the I–V measurement under the capacitor-like devices. As shown in Fig. 4b, the resistance (R) values were attained for these devices, which are listed in Table S2, ESI.† The electrical conductivity (σ) of the perovskite NC films has been calculated by the following equation:
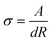 | (2) |
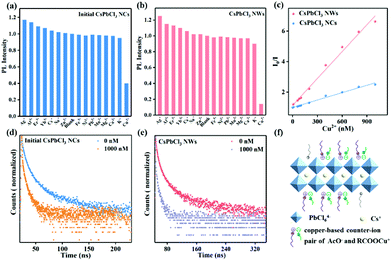
Besides the excellent photoelectric properties, the CsPbCl3 perovskite is equipped with numerous active sites that can adsorb metal ions efficiently, since the formation energy of vacancy in the CsPbCl3 perovskite is lower than that in CsPbBr3 and CsPbI3.36 CsPbCl3 displays brilliant stability than CsPbBr3 and CsPbI3 due to its desirable tolerance factor of 0.87, which is more close to 1 compared to that of CsPbBr3 (0.81) and CsPbI3 (0.80).37 All of these enable them to be an excellent luminescence agent for metal ion detection. We therefore evaluated the practical value by studying their relative PL intensity before and after adding different metal ions, including Ag+, Al3+, Er3+, Yb3+, Cs+, Na+, Zn2+, Fe3+, Ni2+, Pb2+, Mn2+, Mg2+, Ca2+, K+, and Cu2+, at the same concentration level. As shown in Fig. 5a and b, only Cu2+ can effectively quench the luminescence of CsPbCl3 perovskites compared with other cations. Clearly, the final CsPbCl3 NWs exhibited a higher selectivity towards Cu2+ detection than that of initial CsPbCl3 NCs, which can be attributed to the efficient electron transfer from the CsPbCl3 NWs to Cu2+ adsorbed on the surface of the perovskite. The absorption and PL intensity of initial CsPbCl3 NCs and final CsPbCl3 NWs decreased with an increase in the concentration of Cu2+, as shown in Fig. S5 and S6.† In addition, the PL peaks of initial CsPbCl3 NCs and final CsPbCl3 NWs remained unshifted after introducing Cu2+, implying that the quenching mode was independent of the size and the cation exchange process caused by the introduced cations.24Fig. 5c displays the function relation of the luminescent intensity of initial CsPbCl3 NCs and final CsPbCl3 NWs with the Cu2+ concentration. The detection curves present that the luminescent intensity of the CsPbCl3 perovskite was linear with the Cu2+ concentration, demonstrating the reliability of the ion detection measurement.
The luminescence quenching is illustrated by the equation:38
| I0/I = A + K[Q] | (3) |
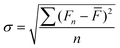 | (4) |
In terms of the detection mechanism, the emission peaks of the CsPbCl3 perovskite remained unchanged with an increase in the concentration of Cu2+, implying that Cu2+ preferred to adsorb on the surface of the perovskite. In addition, the PL decay curves of the CsPbCl3 perovskite are shown in Fig. S7† and Fig. 5d and e, and the corresponding data are listed in Tables S4 and S5.† Both the initial CsPbCl3 NCs and final CsPbCl3 NWs exhibited a short average decay time for the exciton luminescence vs. the Cu2+ concentration. The reduced average decay time after the combination with the Cu2+ indicated an electron transfer process from the CsPbCl3 perovskite to Cu2+ by the dynamic quenching mode.25 In detail, the average PL lifetimes of initial CsPbCl3 NCs and final CsPbCl3 NWs in the absence of Cu2+ were 9.4 ns and 12.5 ns, respectively, while it would drop to 8.4 ns and 7.5 ns once fed with a certain amount of Cu2+ (Table S6†). In particular, compared with the initial CsPbCl3 NCs, the short lifetime of NWs corresponding to the nonradiative transition path became shorter after feeding Cu2+. It suggests that the electron transfer process from the CsPbCl3 NWs to Cu2+ was faster than that from the initial CsPbCl3 NCs. From the previous investigations, the low defect density in the as-prepared 1D CsPbCl3 NWs inhibited the possibility of charge trap through other nonradiative transition paths. The direct charge transport path in the 1D material could promote the rapid transport of electrons. Therefore, the successful preparation of 1D Yb(OAc)3 passivated CsPbCl3 NWs with reduced defect and prominent conductivity mainly promoted the electron transport. Most interestingly, when Cu2+ was adsorbed on the surface of the CsPbCl3 perovskite, the copper-based counter-ion pair (Cl–Cu–OOC–R2) species formed easily.26 The introduced AcO− adsorbed on the surface of the CsPbCl3 perovskite, as confirmed by Fig. 2d, with a shorter alkyl chain can reduce the steric hindrance during the formation of a copper-based counter-ion pair (AcO− and RCOOCu+) (Fig. 5f).39–41 Namely, introduced Yb(OAc)3 can not only accelerate the rapid transfer of electrons from the perovskite to Cu2+, but also promote the Cu2+ adsorption capacity on the perovskite surface. In results, the Cu2+ was more sensitive to the NWs with a remarkable decrease in the detection limit.
Conclusions
In this study, we report that the introduction of Yb(OAc)3 into a luminescence agent, CsPbCl3 perovskite nanocrystals, was beneficial to achieving a significant advancement in sensitivity for Cu2+ detection. On the one hand, the introduced Yb(OAc)3 promoted the morphology transformation of CsPbCl3 from NCs to 1D NWs. 1D CsPbCl3 NWs exhibited low defect density and enhanced conductivity. The decreased defect density in the as-prepared 1D CsPbCl3 NWs inhibited the possibility of charge trap. The direct charge transport path in 1D CsPbCl3 NWs accelerated the electron transfer process. On the other hand, the adsorbed AcO− on the surface of the CsPbCl3 perovskite enhanced the adsorption capacity of Cu2+ by the formation of a stable Copper counter-ion pair. As a result, the PL intensity of the CsPbCl3 perovskite was more sensitive towards Cu2+ in a wide range from 0 to 1000 nM with a detection limit of as low as 0.06 nM by synergistically advancing the electron transfer and ion adsorption capacity. This work is of interest not only because it exemplifies the usefulness of luminescent perovskite nanocrystals for Cu2+ detection, but also represents a useful step towards the rational design of luminescence agents towards high detection sensitivity of metal ions.Author contributions
Xiufeng Wu: Methodology, software, formal analysis, investigation resources writing – original draft. Songtao Hu: Software, formal analysis, resources. He Shao: Formal analysis, investigation, data curation. Lifang Li: Resources, formal analysis. Wenda Chen: investigation, data curation. Biao Dong: Investigation, supervision, project administration. Lin Xu: Supervision, formal analysis. Wen Xu: Funding acquisition, project administration. Donglei Zhou: Investigation, resources. Zhennan Wu: Data curation, visualization, writing – review & editing. Hongwei Song: Data curation, visualization, project administration, funding acquisition. Xue Bai: Conceptualization, validation, writing – review & editing, visualization, supervision, project administration, funding acquisition.Conflicts of interest
There is no conflict of interest for the authors to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 61822506, 11974142, 11874181, 12174151) and the Fundamental Research Funds for the Central Universities.References
- N. A. Khan, A. Niaz, M. I. Zaman, F. A. Khan, M. Nisar-ul-haq and M. Tariq, Sensitive and selective colorimetric detection of Pb2+ by silver nanoparticles synthesized from aconitum violaceum plant leaf extract, Mater. Res. Bull., 2018, 102, 330–336 CrossRef.
- X. Cao, Y. Li, Y. Yu, S. Fu, A. Gao and X. Chang, Multifunctional supramolecular self-assembly system for colorimetric detection of Hg2+, Fe3+, Cu2+ and continuous sensing of volatile acids and organic amine gases, Nanoscale, 2019, 11, 10911–10920 RSC.
- Q. Zhao, X. Rong, L. Chen, H. Ma and G. Tao, Layer-by-layer self-assembly xylenol orange functionalized CdSe/CdS quantum dots as a turn-on fluorescence lead ion sensor, Talanta, 2013, 114, 110–116 CrossRef CAS.
- S. Cui, S. Xu, H. Song, W. Xu, X. Chen, D. Zhou, Z. Yin and W. Han, Highly sensitive and selective detection of mercury ions based on up-conversion FRET from NaYF4:Yb3+/Er3+ nanophosphors to CdTe quantum dots, RSC Adv., 2015, 5, 99099–99106 RSC.
- J. Yan, K. Wang, Q. Liu, J. Qian, X. Dong, W. Liu and B. Qiu, One-pot synthesis of CdxZn1−xS-reduced graphene oxide nanocomposites with improved photoelectrochemical performance for selective determination of Cu2+, RSC Adv., 2013, 3, 7760–7768 Search PubMed.
- N. Ding, D. Zhou, G. Pan, W. Xu, X. Chen, D. Li, X. Zhang, J. Zhu, Y. Ji and H. Song, Europium-doped lead-free Cs3Bi2Br9 perovskite quantum dots and ultrasensitive Cu2+ detection, ACS Sustainable Chem. Eng., 2019, 7, 8397–8404 CrossRef CAS.
- Y. Wang, Y. Zhu, J. Huang, J. Cai, J. Zhu, X. Yang, J. Shen and C. Li, Perovskite quantum dots encapsulated in electrospun fiber membranes as multifunctional supersensitive sensors for biomolecules, metal ions and pH, Nanoscale Horiz., 2017, 2, 225–232 RSC.
- M. Qing, Y. Yuan, W. Cai, S. Xie, Y. Tang, R. Yuan and J. Zhang, An ultrasensitive electrochemical biosensor based on multifunctional hemin/G-quadruplex nanowires simultaneously served as bienzyme and direct electron mediator for detection of lead ion, Sens. Actuators, B, 2018, 263, 469–475 CrossRef CAS.
- Z. Wang, M. Shoji and H. Ogata, Facile low-temperature growth of carbon nanosheets toward simultaneous determination of dopamine, ascorbic acid and uric acid, Analyst, 2011, 136, 4903–4905 RSC.
- X. Luo, W. Zhang, Y. Han, X. Chen, L. Zhu, W. Tang, J. Wang, T. Yue and Z. Li, N,S co-doped carbon dots based fluorescent “on-off-on” sensor for determination of ascorbic acid in common fruits, Food Chem., 2018, 258, 214–221 CrossRef CAS.
- M. Jiao, Y. Li, Y. Jia, Z. Yang and X. Luo, Aqueously synthesized color-tunable quaternary Cu-In-Zn-S quantum dots for Cu(II) detection via mild and rapid cation exchange, Sens. Actuators, B, 2019, 294, 32–39 CrossRef CAS.
- D. Li, W. Xu, D. Zhou, X. Ma, X. Chen, G. Pan, J. Zhu, Y. Ji, N. Ding and H. Song, Cesium tin halide perovskite quantum dots as an organic photoluminescence probe for lead ion, J. Lumin., 2019, 216, 51–58 Search PubMed.
- L.-J. Fan, Y. Zhang, C. B. Murphy, S. E. Angell, M. F. L. Parker, B. R. Flynn and W. E. Jones, Fluorescent conjugated polymer molecular wire chemosensors for transition metal ion recognition and signaling, Coord. Chem. Rev., 2009, 253, 410–422 CrossRef CAS.
- Y. Lou, Y. Zhao, J. Chen and J.-J. Zhu, Metal ions optical sensing by semiconductor quantum dots, J. Mater. Chem. C, 2014, 2, 595–613 RSC.
- P. Wu, T. Zhao, S. Wang and X. Hou, Semicondutor quantum dots-based metal ion probes, Nanoscale, 2014, 6, 43–64 RSC.
- Q.-L. Chen, X. Wu, H. Cheng, Q. Li and S. Chen, Facile synthesis of carbon nanobranches towards cobalt ion sensing and high-performance micro-supercapacitors, Nanoscale Adv., 2019, 1, 3614–3620 RSC.
- H. Zhang, Y. Huang, X. Lin, F. Lu, Z. Zhang and Z. Hu, Lanthanum loaded graphitic carbon nitride nanosheets for highly sensitive and selective fluorescent detection of iron ions, Sens. Actuators, B, 2018, 255, 2218–2222 CrossRef CAS.
- J. Tian, Q. Liu, A. M. Asiri, X. Sun and Y. He, Ultrathin graphitic C3N4 nanofibers: hydrolysis-driven top-down rapid synthesis and application as a novel fluorosensor for rapid, sensitive, and selective detection of Fe3+, Sens. Actuators, B, 2015, 216, 453–460 CrossRef CAS.
- Y. Liu, Y. Sun and M. Yang, A double-potential ratiometric electrochemiluminescence platform based on g-C3N4 nanosheets (g-C3N4 NSs) and graphene quantum dots for Cu2+ detection, Anal. Methods, 2021, 13, 903–909 RSC.
- Y. Zhai, X. Bai, G. Pan, J. Zhu, H. Shao, B. Dong, L. Xu and H. Song, Effective blue-violet photoluminescence through lanthanum and fluorine ions co-doping for CsPbCl3 perovskite quantum dots, Nanoscale, 2019, 11, 2484–2491 RSC.
- G. Pan, X. Bai, D. Yang, X. Chen, P. Jing, S. Qu, L. Zhang, D. Zhou, J. Zhu, W. Xu, B. Dong and H. Song, Doping lanthanide into perovskite nanocrystals: highly improved and expanded optical properties, Nano Lett., 2017, 17, 8005–8011 CrossRef CAS PubMed.
- G. Pan, X. Bai, W. Xu, X. Chen, Y. Zhai, J. Zhu, H. Shao, N. Ding, L. Xu, B. Dong, Y. Mao and H. Song, Bright blue light emission of Ni2+ ion-doped CsPbClxBr3−x perovskite quantum dots enabling efficient light-emitting devices, ACS Appl. Mater. Interfaces, 2020, 12, 14195–14202 CrossRef CAS PubMed.
- H. Zhou, Z. Song, C. R. Grice, C. Chen, J. Zhang, Y. Zhu, R. Liu, H. Wang and Y. Yan, Self-powered CsPbBr3 nanowire photodetector with a vertical structure, Nano Energy, 2018, 53, 880–886 CrossRef CAS.
- X. Sheng, Y. Liu, Y. Wang, Y. Li, X. Wang, X. Wang, Z. Dai, J. Bao and X. Xu, Cesium lead halide perovskite quantum dots as a photoluminescence probe for metal Ions, Adv. Mater., 2017, 29, 342–353 CrossRef.
- Y. Liu, X. Tang, T. Zhu, M. Deng, I. P. Ikechukwu, W. Huang, G. Yin, Y. Bai, D. Qu, X. Huang and F. Qiu, All-inorganic CsPbBr3 perovskite quantum dots as a photoluminescent probe for ultrasensitive Cu2+ detection, J. Mater. Chem. C, 2018, 6, 4793–4799 RSC.
- M. Lu, X. Zhang, Y. Zhang, J. Guo, X. Shen, W. W. Yu and A. L. Rogach, Simultaneous strontium doping and chlorine surface passivation improve luminescence intensity and stability of CsPbI3 nanocrystals enabling efficient light-emitting devices, Adv. Mater., 2018, 30, 1804691 CrossRef.
- S. Zhao, Y. Zhang and Z. Zang, Room-temperature doping of ytterbium into efficient near-infrared emission CsPbBr1.5Cl1.5 perovskite quantum dots, Chem. Commun., 2020, 56, 5811–5814 RSC.
- W. Xu and T. Wang, Synergetic effect of blended alkylamines for copper complex ink to form conductive copper films, Langmuir, 2017, 33, 82–90 CrossRef CAS.
- H. Shao, Y. Zhai, X. Wu, W. Xu, L. Xu, B. Dong, X. Bai, H. Cui and H. Song, High brightness blue light-emitting diodes based on CsPb(Cl/Br)3 perovskite QDs with phenethylammonium chloride passivation, Nanoscale, 2020, 12, 11728–11734 RSC.
- J. Kim, B. Koo, W. H. Kim, J. Choi, C. Choi, S. J. Lim, J.-S. Lee, D.-H. Kim, M. J. Ko and Y. Kim, Alkali acetate-assisted enhanced electronic coupling in CsPbI3 perovskite quantum dot solids for improved photovoltaics, Nano Energy, 2019, 66, 2331–2339 CrossRef.
- S. Mourdikoudis and L. M. Liz-Marzán, Oleylamine in nanoparticle synthesis, Chem. Mater., 2013, 25, 1465–1476 CrossRef CAS.
- N. Soetan, W. R. Erwin, A. M. Tonigan, D. G. Walker and R. Bardhan, Solvent-assisted self-assembly of CsPbBr3 perovskite nanocrystals into one-dimensional superlattice, J. Phys. Chem. C, 2017, 121, 18186–18194 CrossRef CAS.
- N. Ding, W. Xu, D. Zhou, Y. Ji, Y. Wang, R. Sun, X. Bai, J. Zhou and H. Song, Extremely efficient quantum-cutting Cr3+, Ce3+, Yb3+ tridoped perovskite quantum dots for highly enhancing the ultraviolet response of Silicon photodetectors with external quantum efficiency exceeding 70%, Nano Energy, 2020, 78, 105278 CrossRef CAS.
- G. Pan, X. Bai, X. Shen, L. Wang, Y. Mao, X. Chen, W. Xu, H. Shao, D. Zhou, B. Dong, L. Xu, J. Hu and H. Song, Bright red YCl3-promoted CsPbI3 perovskite nanorods towards efficient light-emitting diode, Nano Energy, 2021, 81, 105615 CrossRef CAS.
- J. Pan, Y. Shang, J. Yin, M. De Bastiani, W. Peng, I. Dursun, L. Sinatra, A. M. El-Zohry, M. N. Hedhili, A. H. Emwas, O. F. Mohammed, Z. Ning and O. M. Bakr, Bidentate Ligand-Passivated CsPbI3 Perovskite Nanocrystals for Stable Near-Unity Photoluminescence Quantum Yield and Efficient Red Light-Emitting Diodes, J. Am. Chem. Soc., 2018, 140, 562–565 CrossRef CAS PubMed.
- X. Zhu, L. Ge, Y. Wang, M. Li, R. Zhang, M. Xu, Z. Zhao, W. Lv and R. Chen, Recent advances in enhancing and enriching the optical properties of Cl-based CsPbX3 nanocrystals, Adv. Opt. Mater., 2021, 9, 2100058 CrossRef CAS.
- H. Zhao, Y. Fu, Z. Li, S. Yang, B. Xu, X. Liu, J. Xu, S. Liu and J. Yao, High-efficiency and thermal/moisture stable CsPbI2.84Br0.16 inorganic perovskite solar cells enabled by a multifunctional cesium trimethylacetate organic additive, J. Mater. Chem. A, 2021, 9, 4922–4932 RSC.
- L. Q. Lu, T. Tan, X. K. Tian, Y. Li and P. Deng, Visual and sensitive fluorescent sensing for ultratrace mercury ions by perovskite quantum dots, Anal. Chim. Acta, 2017, 986, 109–114 CrossRef CAS PubMed.
- D. Rocco, G. Manfroni, A. Prescimone, Y. M. Klein, D. J. Gawryluk, E. C. Constable and C. E. Housecroft, Single and double-stranded 1D-coordination polymers with 4′-(4-alkyloxyphenyl)-3,2′:6′,3′′-terpyridines and {Cu2(mu-OAc)4} or {Cu4(mu3-OH)2(mu-OAc)2(mu3-OAc)2(AcO-kappaO)2} Motifs, Polymers, 2020, 12, 112–119 CrossRef.
- J. Chang, S. Yoo, W. Lee, D. Kim and T. Kang, Spontaneous phase transfer-mediated selective removal of heavy metal ions using biocompatible oleic acid, Sci. Rep., 2017, 7, 16727 CrossRef PubMed.
- R. Nazari, H. Golchoubian and G. Bruno, Chromotropism studies on copper(II) compounds. Part II. Dinuclear copper(II) complexes with triply-bridged hydroxo, acetate, and halo ligands, J. Coord. Chem., 2018, 71, 2510–2525 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1qi01235e |
| This journal is © the Partner Organisations 2022 |