Stable two-dimensional lead iodide hybrid materials for light detection and broadband photoluminescence†
Mohamed Saber
Lassoued
ab,
Yuan-Chao
Pang
ab,
Qian-Wen
Li
ab,
Xinkai
Ding
b,
Bo
Jiao
cd,
Hua
Dong
 cd,
Guijiang
Zhou
cd,
Guijiang
Zhou
 a,
Shujiang
Ding
a,
Shujiang
Ding
 a,
Zhicheng
Zhang
a,
Zhicheng
Zhang
 a,
Zhaoxin
Wu
a,
Zhaoxin
Wu
 cd,
Gaoyang
Gou
*b,
Zongyou
Yin
cd,
Gaoyang
Gou
*b,
Zongyou
Yin
 e,
Ju
Li
e,
Ju
Li
 e and
Yan-Zhen
Zheng
e and
Yan-Zhen
Zheng
 *ab
*ab
aSchool of Chemistry, Xi’an Key Laboratory of Sustainable Energy and Materials Chemistry, MOE Key Laboratory for Nonequilibrium Synthesis and Modulation of Condensed Matter, Xi’an Jiaotong University, Xi’an 710049, China. E-mail: zheng.yanzhen@xjtu.edu.cn
bFrontier Institute of Science and Technology (FIST), State Key Laboratory for Mechanical Behavior of Materials, and School of Physics, Xi’an Jiaotong University, Xi’an 710054, China. E-mail: gougaoyang@xjtu.edu.cn
cKey Laboratory of Photonics Technology for Information, Key Laboratory for Physical Electronics and Devices of the Ministry of Education, Department of Electronic Science and Technology, School of Electronic and Information Engineering, Xi'an Jiaotong University, Xi’an 710049, China
dCollaborative Innovation Center of Extreme Optics, Shanxi University, Taiyuan 030006, China
eDepartment of Nuclear Science and Engineering, Department of Materials Science and Engineering, Massachu-setts Institute of Technology, Cambridge, MA 02139, USA
First published on 23rd November 2021
Abstract
Two-dimensional (2D) organic–inorganic hybrid materials have attracted widespread attention for photodetection. Moreover, such materials with broadband photoluminescence and strong photocurrent response are still rare. Here, we report two new semiconducting 2D organic–inorganic hybrid materials, namely {PbII2I6[PbII(TETA)]}n (1Pb) and {PbII3I8[PbII(TETA)]}n (2Pb), where TETA = triethylenetetramine, with broadband yellow-green emission. Both compounds are soluble in DMF and yield high surface coverage films through spin coating. Strikingly, the photocurrent responses of such thin films show ca. 600 and 700 nA cm−2 difference between Ilight and Idark for 1Pb and 2Pb, respectively. To the best of our knowledge, 1Pb and 2Pb showed among the highest current obtained in 2D lead iodide hybrid materials under a low voltage (0.7 V). Moreover, 1Pb and 2Pb are stable under heat, moisture and light, which may provide realistic applications for light detection.
In recent years, methyl ammonium lead halide materials have become a new path in the photovoltaic field due to their simple manufacturing processes,1 high absorption coefficient2,3 and long charge carrier diffusion lengths.4,5 The interest around these compounds is driven by their fast advancement of the energy conversion efficiency (PCE). Since Miyasaka manufactured the initial device in 2009, its efficiency was 3.8%, and the PCE of organic–inorganic solar cells have been increased to 25.5% in such a short period of time,6 surpassing the most advanced copper indium gallium diselenide (CIGS) solar cells, and approaching single crystal silicon solar cells.7 However, under environmental conditions (moisture, oxygen and UV radiation), their stability is still poor and remains a principal drawback for further commercial use.8–10
In order to address the long-term stability issue for real-world applications, two-dimensional (2D) hybrid materials have become the best candidates due to their superior stability and wider structural diversity.11–13 In addition, 2D organic–inorganic hybrid materials also present exciting optical and unique optoelectronic properties, such as widely tunable bandgap energy, extremely large exciton binding energy, layered characteristic and long decay times.14–18 All these features make those kind of hybrid materials very promising in optoelectronic devices. In particular, 2D lead halide hybrid compounds have been paid extensive attention not only due to their rich structural chemistry (by using different organic cations, a large number of 2D halo-plumbate anions have been investigated such as [PbX4]2−, [Pb3X9]3−, [Pb5X14]4−, and [Pb7X18]4−)19–23 but also because of their interesting stability and photophysical properties. In this context, Dou et al. synthesized stable 2D hybrid perovskite quantum wells (4Tm)2PbI4 and (BTm)2PbI4 using hydrophobic organic semiconducting ligands (4Tm and BTm: thiophene derivatives). Those two compounds showed high stability under harsh conditions (heat and moisture).24 The employment of larger organic cations with strong π–π interactions within a 2D structure will greatly enhance the stability of these compounds.25 Moreover, due to the strong van der Waals interactions between the layers, Li et al. showed that 2D Dion Jacobson lead iodide hybrid perovskite (PA)2(MA)3Pb4I13 and (PDA)(MA)3Pb4I13 (PA = propylamine and PDA = 1,3-propanediamine) exhibited ultrahigh stability for 4000 h under 40–70% relative humidity and for 168 h damp heat at 85 °C.26 Other than that, 2D lead halide hybrid compounds were reported to have excellent luminescence properties; for example, Liu et al.27 prepared two 2D organic–inorganic lead bromide hybrid materials (C7H18N2)PbBr4 and (C9H22N2)PbBr4, exhibiting broadband emission with a long-life time emission of ≃1 ms; Luo et al. also successfully designed a new 2D lead bromide hybrid, (γ-methoxy propyl amine)2PbBr4, which exhibits bright bluish white light emission with high Color Rendering Index and PLQE of 6.85%;28 and [DMEDA]PbCl4 (DMEDA = N,N-dimethylethylenediamine), (C6H5C2H4NH3)2PbCl4, [DMPDA]PbCl4 (DMPDA = N,N-dimethyl-1,3-diaminopropane), [(CH3)4N]4Pb3Cl10 and (C4H9NH3)2PbCl4 have also been reported as luminescent materials.29–32
On the other hand, despite a few literature reports based on 2D lead halide hybrid photodetectors (particularly for 2D lead iodide hybrids), those works showed very interesting and promising results. For example, a 2D bilayered lead iodide hybrid compound reported by Zhang et al. exhibited broadband photoresponsive properties with high photoresponsivity.33 Another important work reported by Huang et al. showed a photodetector with tunable photoresponse by the precise control of the n number of 2D (BA)2(MA)n−1PbnI3n+1 (n = 1, 2, 3) hybrid materials.34 Nevertheless, how to realize stable 2D lead iodide hybrid compounds broadband photoluminescence together with strong photo-response is still a huge challenge and very rarely reported.
In light of this discussion, we report two new 2D lead iodide organic–inorganic hybrid materials, namely {PbII2I6[PbII(TETA)]}n (1Pb) and {PbII3I8[PbII(TETA)]}n (2Pb), which possessed excellent semiconductor properties and broadband yellow-green light emission. In addition to the high solubility and film processable nature, 1Pb and 2Pb showed significant stability against moisture, light and temperature, which made 1Pb and 2Pb suitable for light detection applications.
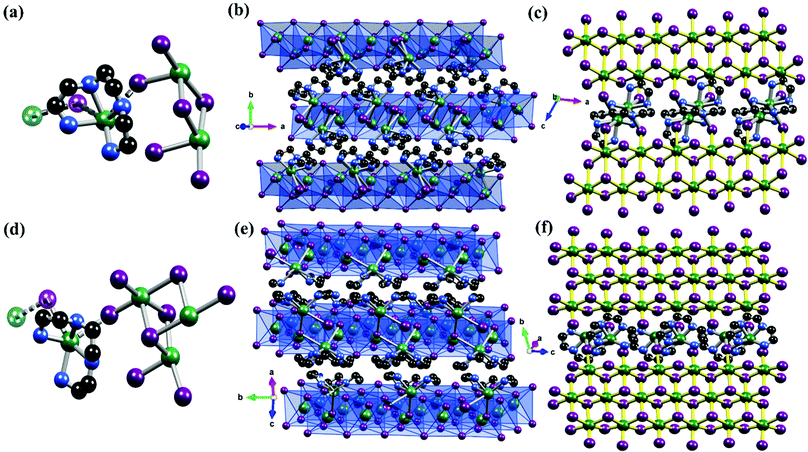
1Pb and 2Pb were synthesized through the hydrothermal technique, in which different stoichiometric amounts of Pb(NO3)2, triethylenetetramine (TETA) and KI in a concentrated hydroiodic acid (HI) were added, and the mixture was kept for 24 h at 130 °C for 1Pb, and at 150 °C for 48 h for 2Pb (see “Materials and sample preparation” in the ESI† for more details). Structural analyses disclosed that 1Pb crystallized in a monoclinic system with a centrosymmetric space group of P21/c at 298 K (Table S1, ESI†). The asymmetric unit comprises one organic triethylenetetramine (TETA), three lead(II) ions and six iodine anions (Fig. 1a).
As shown in Fig. 1b and c, the topography of 1Pb can be described as a 2D structure, in which there are two crystallographically independent Pb(II) atoms, which exhibited two different coordination environments: [PbI6] octahedra for both Pb1 and Pb2, while [PbN4I2] octahedron for Pb3. Thus, for Pb1 and Pb2, each lead atom was connected with six iodine atoms to form PbI6 octahedra, in that Pb1 and Pb2 were linked together by two bridge I atoms. The Pb–I distances varied between 3.0447 (13) Å and 3.3673 (12) Å, while the Ieq–Pb–Ieq (“eq” refers to equatorial) bond angles ranged from 80.41 (3)° to 105.96 (3)°, and the Iax–Pb–Iax (“ax” refers to axial) bond angles varied between 162.51 (3)° and 177.28 (4)°, indicating that the octahedra were distorted. The Pb3 atom was bonded to four N atoms and shared two iodine atoms with [Pb2I6] units to form [PbN4I2] octahedron, in which the Pb–I bond lengths varied between 3.5192 (13) Å and 3.662 (16) Å; the Pb–N distance ranged from 2.4470 (15) Å to 2.628 (17) Å and the N–Pb–N angle ranged from 68.6 (6)° to 122.4 (6)° (Table S2, ESI†). Hence, a 2D {PbII2I6[PbII(TETA)]}n network was formed by sharing two iodine atoms between the [Pb2I6]2− and [Pb(TETA)]2+ units.
Using different stoichiometric amounts from 1Pb, yellow crystals of 2Pb were synthesized. Single-crystal X-ray diffraction studies revealed that 2Pb crystallized in a monoclinic system at room temperature with the space group of C2/c. As shown in Fig. 1d, the asymmetric unit of 2Pb contains four lead(II) ions, eight iodine atoms, and one TETA. Three lead(II) (Pb1, Pb2 and Pb3) ions adopted a geometry of six-coordinated relative regular octahedron (Pb–I: 3.0070 (14) Å−3.3768 (14) Å and I–Pb–I: 85.62 (3)°−178.11 (4)°). The Pb–I bond lengths are closer to the sum of the ionic radii of iodide and lead(II) (ri = 2.2 + 1.03 = 3.23 Å) rather than to that of their covalent radii (re = 1.39 + 1.48 = 2.87 Å), proving that these bonds are ionic in nature. However, the Pb4 ion was coordinated with four nitrogen atoms of the bent TETA (Pb–N: 2.505 (15)–2.596 (19) Å), and two iodine atoms to form [PbN4I2] octahedron (Fig. 1e and f). The coordination modes of 2Pb are quite similar to that of 1Pb. However, the difference between 1Pb and 2Pb was that the Pb–I distance and I–Pb–I angle in the 2Pb complex were slightly broader than those in 1Pb, indicating that 2Pb was a bit distorted from 1Pb. As shown with 1Pb, the [Pb(TETA)]2+ units in 2Pb shared two iodine atoms with [Pb3I8]2− units to connect them into a 2D network.
TETA showed a regular configuration with normal values of C–C and C–N bond lengths varying from 1.41 (3) Å to 1.55 (3) Å, whereas C–C–C, C–N–C and C–C–N angles ranged between 107.7 (19)° and 117.2 (17)° (see Tables S2 and S3, ESI†). Moreover, 1Pb and 2Pb exhibited several intermolecular hydrogen bonding interactions between the cationic and [Pb2I6]2− or [Pb3I8]2− anions of the type N–H⋯I and C–H⋯I listed in Tables S4 and S5 in ESI.† The XRD powder spectra of 1Pb and 2Pb are given in Fig. S1, ESI.† It can be seen that PXRD patterns of these two materials matched perfectly well with the simulated results from the single crystal structure, which indicated the high purity of 1Pb and 2Pb powder samples.
To analyse and obtain additional information about intermolecular interactions within the crystal structure of 1Pb and 2Pb, molecular Hirshfeld surface (MHS) calculations were performed using the crystal explorer 3.1 program. The blue, red and white areas in the MHS represent the largest, shortest and equal to van der Waals separations, respectively (Fig. S2, ESI†). It can be seen from Fig. S3a and c in ESI† that 2D finger print maps of 1Pb and 2Pb provide quantitative contribution of the intermolecular interaction. Two remarkable spikes presented the most abundant interaction of 1Pb and 2Pb, which were from H⋯I and Pb⋯I, consistent with the red area in the MHS. It was very clear that hydrogen bonds played an important role in crystal stabilization. These results are also confirmed by single crystal analysis (Tables S4 and S5, ESI†). Other intercontacts present less to MHS were also calculated, such as H⋯H and I⋯I (Fig. S3b and d, ESI†).
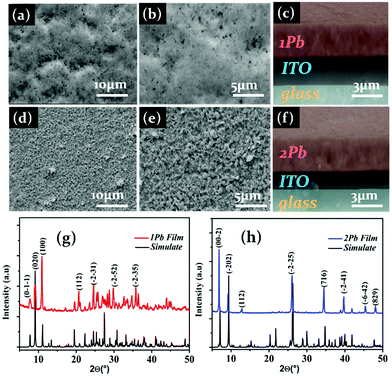
Significantly, 1Pb and 2Pb are highly soluble in dimethylformamide (DMF), and 1 ml DMF can dissolve 0.2 g of each compound (1Pb or 2Pb). Using the one step spin coating technique, we dissolved 20 mg of 1Pb and 2Pb single crystals in 1 ml DMF, and then cooled at ambient temperature (More details can be found in the ESI†). Smooth, high coverage, less pin hole films of 1Pb and 2Pb readily formed (Fig. 2). 1Pb and 2Pb had small grain sizes of 750 nm and 900 nm, respectively ((Fig. 2a and b) and Fig. 2d and e). As shown in Fig. 2c and f, the cross-section images reveal that 1Pb and 2Pb have grain thicknesses of 3 μm and 3.1 μm, respectively. XRD patterns of 1Pb and 2Pb films match very well with the simulated ones, which indicated their high purity (Fig. 2g and h).
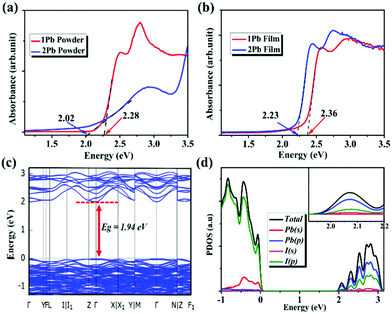
Based on the Kubelka–Munk function,35 (F(R) = α/S = (1 − R)2/(2R), where R is the reflectance, α is the absorption coefficient, S is the scattering coefficient), the diffuse reflectance spectrum was converted to an absorbance spectrum to understand the semiconducting performance of 1Pb and 2Pb. As shown in Fig. 3a, the optical band edges of 1Pb and 2Pb were determined to be 2.28 eV and 2.02 eV, respectively. These results are consistent with their colors, and are very similar to other lead iodide hybrid compounds.36,37 Based on the intercepts of the curves of (F(RN)hν)1/n (n = 2 or n = 1) versus the energy according to the τauc equation, bandgap energies were estimated to be 2.31 eV (indirect) and 2.20 eV (direct) for 1Pb and 1.86 eV (indirect) and 2.08 eV (direct), respectively, for 2Pb (Fig. S4, ESI†). These bandgap values could be considered within the range of a typical semiconductor compound and can be used as the absorber for tandem solar cells when these two materials are coupled with silicon.38 Interestingly, optical absorption spectra were performed on films of 1Pb and 2Pb, respectively, and both are similar for those of powder samples (Fig. 3b).
To get an insight into the electronic properties of these two compounds, DFT calculations of 1Pb and 2Pb were performed. After including spin orbital coupling (SOC) effects, GGA-PBE functional predicts that the valence band maximum (VBM) and conduction band minimum (CBM) are both located at the same k points (Γ for 1Pb and C for 2Pb), indicating that 1Pb and 2Pb are semiconductors with direct bandgaps (Fig. 3c and Fig. S5a, ESI†). In addition, the predicted energy bandgaps are 2.12 eV and 1.94 eV for 1Pb and 2Pb, respectively, which are close to experimental results. Without SOC effect, the larger bandgaps of 2.72 eV for 1Pb and 2.24 eV for 2Pb will be predicted (Fig. S5b and S6a, ESI†). Based on the partial density of states, we will further analyze the orbital nature for electronic states around VBM and CBM. The top of valence bands was mainly contributed by hybridized I-5p and Pb-6s orbitals, while the bottom of conduction bands had dominant Pb/I-5p orbital characters for both two compounds (Fig. 3d, Fig. S5 (c–f) and S6 (b–d), ESI†). These results clearly indicated that optical excitations within 1Pb and 2Pb are mainly determined by the hybridized Pb and I orbitals from the inorganic framework.
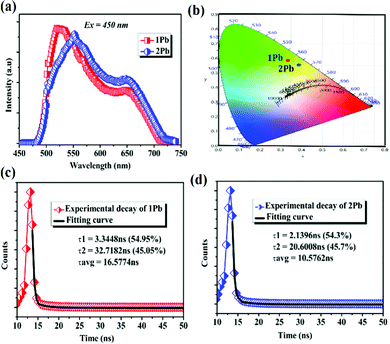
The photoluminescence properties of 1Pb and 2Pb lead iodide hybrid compounds were studied at room temperature using steady state and time-resolved emission spectroscopy. Upon excitation of 450 nm, both compounds exhibited two band emissions, which generally matched very well with the two slopes from the absorption spectra. Indeed, 1Pb and 2Pb display two broadband emissions with the highest bands at 519 nm for 1Pb and 550 nm for 2Pb, and another two weak emissions present in the range of 600–720 nm can be tentatively assigned to lead halide-centered transitions within the inorganic group, as suggested by band structure calculations, and observed recently in other reported iodoplumbate organic–inorganic based hybrid materials (Fig. 4a).39–41 Thus, the broad photoluminescence mainly originated from the inorganic component. We should note that similar emission bands between 1Pb and 2Pb indicate the same radiative recombination route. In addition, the combination of the two emission bands gave rise to the CIE 1931 chromaticity coordinates of (0.39, 0.56) for 1Pb with CCT of 3780 K and of (0.43, 0.53) for 2Pb with CCT of 3825 K. Both compounds exhibited yellow green emission (Fig. 4b). Moreover, the PLQY of 1Pb and 2Pb was measured to be 1% and 1.12%, respectively, which were comparable to those of previous reported 2D hybrid lead materials, such as 0.5% for (N–MEDA)PbI4 and ∼1% for (C6H5C2H4NH3)2PbCl4.42,43
Using a fitting with a double-exponential function I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2
exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2), the decay life times of 1Pb and 2Pb have been calculated (Fig. 4c and d). Interestingly, the average life time for 1Pb was 16.57 ns and for 2Pb was 10.57 ns, which were longer than that of other reported 2D lead hybrid compounds (Table S6, ESI†). The photoluminescence properties for both compounds are summarized in Table 1.
exp(−t/τ2), the decay life times of 1Pb and 2Pb have been calculated (Fig. 4c and d). Interestingly, the average life time for 1Pb was 16.57 ns and for 2Pb was 10.57 ns, which were longer than that of other reported 2D lead hybrid compounds (Table S6, ESI†). The photoluminescence properties for both compounds are summarized in Table 1.
| Compds | λ ex (nm) | λ em (nm) | CIE | τ (ns) |
|---|---|---|---|---|
| 1Pb | 450 | 518/645 | (0.39, 0.56) | 16.57 |
| 2Pb | 450 | 550/645 | (0.43, 0.53) | 10.57 |
The photoconductivity of 1Pb and 2Pb was studied using film samples as the active layer under the illumination from a 350 W Xenon lamp irradiation at 0.7 V bias (more details can be found in the ESI†). As shown in Fig. 5a and c, both materials exhibited a strong photoresponse in that the photocurrent for 1Pb enhanced from 20 to 636 nm cm−2, and increased for 2Pb from 42 to 780 nm cm−2. Those values are higher than that of bismuth halide organic–inorganic materials and comparable to that of lead-based hybrids. However, they were lower than that of 3D MAPbI3 and those of inorganic systems.39,40,44–48 To the best of our knowledge, 1Pb and 2Pb had among the highest current obtained in 2D lead iodide hybrid materials under low voltages. We need to mention that the order of photocurrent for these two compounds was well coherent with their optical bandgaps, which signalized that a small bandgap may be better to generate and separate a photoinduced electron/hole.37,49
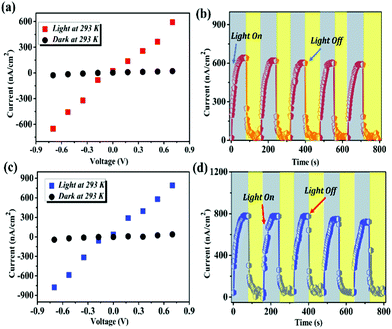 | ||
| Fig. 5 (a, c) I–V plots for dark and light current of 1Pb (a) and 2Pb (c) measured at 298 K. (b, d) I–t plots of several irradiation cycles of 1Pb (b) and 2Pb (d). | ||
Fig. 5b and d show that these devices exhibited a broadband, repeatable and periodical switching (on/off) of the light, which means that 1Pb and 2Pb exhibited an obvious photocurrent reproducibility and high stability. Moreover, the photoresponsivity (R) (it is an important figure of merit to represent the sensitivity of the photodetector to the light signal) was calculated to be 7.04 μA W−1 and 8.57 μA W−1 for 1Pb and 2Pb, respectively.
Other important parameters of photodetector devices, such as detectivity (D*) and external quantum efficiency (EQE), were also determined. A comparative table between 1Pb and 2Pb with other general materials are illustrated in Table S7 in the ESI.† The responsivity, detectivity and external quantum efficiency are obtained using the following equations:
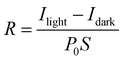 | (1) |
| D* = RS½/(2eId)½ | (2) |
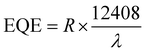 | (3) |
Moreover, we studied the stability of both 1Pb and 2Pb towards moisture, light and heat. The as-prepared films of 1Pb and 2Pb were stored in the dark at 55% relative humidity for 7 days. The XRD patterns of the film samples of 1Pb and 2Pb remained almost the same as those of the freshly prepared sample (see Fig. S1, ESI†) and showed no evidence of peak related to material degradation. However, we noted a decrease in the diffraction peak intensity of 1Pb and 2Pb within 7 days (Fig. S7, ESI†). To explore the UV aging effect, we exposed 1Pb and 2Pb films to UV light for 24 h at room temperature. No obvious change has been observed for the XRD patterns compared to the simulated ones (Fig. S7, ESI†). Moreover, to evaluate the thermal stability of 1Pb and 2Pb, thermogravimetric analyses (TGA) were conducted from 30 °C to 600 °C under nitrogen atmosphere (Fig. S8, ESI†). It can be seen from the TGA curves that the two compounds are stable up to 225 °C. Hence, 1Pb and 2Pb showed high stability towards moisture, light and heat.
Conclusions
In summary, two new 2D organic–inorganic hybrid lead iodide using a tetradentate ligand were successfully synthesized and fully characterized. Both compounds exhibited an excellent semiconductor property and showed broadband yellow-green emission. Interestingly, 1Pb and 2Pb showed high stability and exhibited a strong photocurrent response at 0.7 V. In addition, 1Pb and 2Pb were processed into high coverage films through the one-spin coating method. Thus, this study showed two new broadband 2D lead iodide hybrid materials with potential for light detection applications.Author contributions
M. S. Lassoued, Y.-C. Pang and Q.-W. Li conducted the syntheses, crystallography and photoluminescence. The ab initio calculations were performed by X. Ding, G. Gou and J. Li. The photoresponse measurements were performed by Z. Yin, B. Jiao and H. Dong; G. Zhou, S. Ding, Z. Zhang and Z. Wu drew the pictures and wrote the manuscript; Y.-Z. Zheng supervised the whole project. All authors read and approved the manuscript before submission.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of China (no. 21971203 and 21773130), the State Key Laboratory for Mechanical Behavior of Materials (20182006), the Key Laboratory Construction Program of Xi'an Municipal Bureau of Science and Technology (201805056ZD7CG40), the China Postdoctoral Science Foundation (2018M631138), the Shaanxi Postdoctoral Science Foundation (2018), the Cyrus Chung Ying Tang Foundation and the Fundamental Research Funds for Central Universities, Key Scientific and Technological Innovation Team of Shaanxi Province (2020TD-001).References
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal halide perovskites as visible-light sensitizers for photovoltaic cells, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites, Science, 2012, 338, 643–647 CrossRef CAS.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Graetzel, Sequential deposition as a route to high-performance perovskite-sensitized solar cells, Nature, 2013, 499, 316–319 CrossRef CAS.
- X. Li, D. Bi, C. Yi, J.-D. Decoppet, J. Luo, S. M. Zakeeruddin, A. Hagfeldt and M. Gratzel, A vacuum flash–assisted solution process for high-efficiency large-area perovskite solar cells, Science, 2016, 353, 58–62 CrossRef CAS PubMed.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, High-performance photovoltaic perovskite layers fabricated through intramolecular exchange, Science, 2015, 348, 1234–1237 CrossRef CAS.
- E. L. Unger, E. T. Hoke, C. D. Bailie, W. H. Nguyen, A. R. Bowring, T. Heumueller, M. G. Christoforo and M. D. McGehee, Hysteresis and transient behavior in current–voltage measurements of hybrid-perovskite absorber solar cells, Energy Environ. Sci., 2014, 7, 3690–3698 RSC.
- The National Renewable Energy Laboratory (NREL) Best Research-Cell Record Efficiencies, Best Research-Cell Efficiency Chart (nrel.gov).
- L. Martiradonna, Entropy in halide perovskites, Nat. Mater., 2018, 17, 377–379 CrossRef CAS PubMed.
- L. Etgar, The merit of perovskite's dimensionality; can this replace the 3D halide perovskite?, Energy Environ. Sci., 2018, 11, 234–242 RSC.
- B. W. Park and S. I. Seok, Intrinsic Instability of Inorganic–Organic Hybrid Halide Perovskite, Materials, Adv. Mater., 2019, 3, 1805337 CrossRef.
- M. S. Lassoued, L.-Y. Bi, Z. Wu, G. Zhou and Y.-Z. Zheng, Piperidine-induced Switching of the direct band gaps of Ag(I)/Bi(III) bimetallic iodide double perovskites, J. Mater. Chem. C, 2020, 8, 5349–5354 RSC.
- M. S. Lassoued, L.-Y. Bi, Z. Wu, G. Zhou and Y.-Z. Zheng, Two-dimensional semiconducting Cs(i)/Bi(iii) bimetallic iodide hybrids for light detection, Mater. Chem. Front., 2021, 5, 973–978 RSC.
- L.-Y. Bi, Y.-Q. Hu, M.-Q. Li, T.-L. Hu, H.-L. Zhang, X.-T. Yin, W.-X. Que, M. S. Lassoued and Y.-Z. Zheng, Two-dimensional lead-free iodide-based hybrid double perovskites: crystal growth, thin-film preparation and photocurrent responses, J. Mater. Chem. A, 2019, 7, 19662–19667 RSC.
- I. C. Smith, E. T. Hoke, D. Solis-Ibarra, M. D. McGehee and H. I. Karunadasa, A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability, Angew. Chem., Int. Ed., 2014, 53(42), 11232–11235 CrossRef CAS PubMed.
- D. H. Cao, C. C. Stoumpos, O. K. Farha, J. T. Hupp and M. G. Kanatzidis, 2D Homologous Perovskites as Light-Absorbing Materials for Solar Cell Applications, J. Am. Chem. Soc., 2015, 137(24), 7843–7850 CrossRef CAS.
- H. Tsai, W. Nie, J.-C. Blancon, C. C. Stoumpos, R. Asadpour, B. Harutyunyan, A. J. Neukirch, R. Verduzco, J. J. Crochet, S. Tretiak, L. Pedesseau, J. Even, M. A. Alam, G. Gupta, J. Lou, P. M. Ajayan, M. J. Bedzyk, M. G. Kanatzidis and A. D. Mohite, High efficiency two-dimensional Ruddlesden−Popper perovskite solar cells, Nature, 2016, 536(7616), 312–316 CrossRef CAS PubMed.
- M. Leng, Z. Chen, Y. Yang, Z. Li, K. Zeng, K. Li, G. Niu, Y. He, Q. Zhou and J. Tang, Lead-Free, Blue Emitting Bismuth Halide Perovskite Quantum Dots, Angew. Chem., Int. Ed., 2016, 55, 15012–15016 CrossRef CAS PubMed.
- D. B. Mitzi, Organic−Inorganic Perovskites Containing Trivalent Metal Halide Layers:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The Templating Influence of the Organic Cation Layer, Inorg. Chem., 2000, 39, 6107–6113 CrossRef CAS.
The Templating Influence of the Organic Cation Layer, Inorg. Chem., 2000, 39, 6107–6113 CrossRef CAS. - N. Mercier, S. Poiroux, A. Riou and P. Batail, Unique hydrogen bonding correlating with a reduced band gap and phase transition in the hybrid perovskites (HO(CH2)2NH3)2PbX4 (X = I, Br), Inorg. Chem., 2004, 43, 8361–8366 CrossRef CAS.
- D. G. Billing and A. Lemmerer, Inorganic–organic hybrid materials incorporating primary cyclic ammonium cations: The lead iodide series, CrystEngComm, 2007, 9, 236–244 RSC.
- H. Krautscheid and F. Vielsack, Synthese und Kristallstrukturen kettenförmiger und netzartiger Iodoplumbate, Z. Anorg. Allg. Chem., 1997, 623, 259–263 CrossRef CAS.
- H. Krautscheid and F. Vielsack, Discrete and polymeric iodoplumbates with Pb3I10 building blocks: [Pb3I10]4−, [Pb7I22]8−, [Pb10I28]8−, 1∞[Pb3I10]4− and 2∞[Pb7I18]4−, J. Chem. Soc., Dalton Trans., 1999, 2731–2735 RSC.
- H. Krautscheid, F. Vielsack and N. Klaassen, Polymere Iodoplumbate – Synthese und Kristallstrukturen von (Pr3N–C2H4–NPr3)[Pb6I14(dmf)2] 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF, (Pr3N–C2H4–NPr3)[Pb(dmf)6][Pb5I14] DMF und (Me3N–C2H4–NMe3)2[Pb2I7]I, Z. Anorg. Allg. Chem., 1998, 624, 807–812 CrossRef CAS.
DMF, (Pr3N–C2H4–NPr3)[Pb(dmf)6][Pb5I14] DMF und (Me3N–C2H4–NMe3)2[Pb2I7]I, Z. Anorg. Allg. Chem., 1998, 624, 807–812 CrossRef CAS. - Y. Gao, E. Shi, S. Deng, S. B. Shiring, J. M. Snaider, C. Liang, B. Yuan, R. Song, S. M. Janke, A. L-Peláez, P. Yoo, M. Zeller, B. W. Boudouris, P. Liao, C. Zhu, V. Blum, Y. Yu, B. M. Savoie, L. Huang and L. Dou, Molecular engineering of organic-inorganic hybrid perovskites quantum wells, Nat. Chem., 2019, 11, 1151–1157 CrossRef CAS.
- Y. Gao, Z. Wei, S.-N. Hsu, B. W. Boudouris and L. Dou, Two-dimensional halide perovskites featuring semiconducting organic building blocks, Mater. Chem. Front., 2020, 4, 3400–3418 RSC.
- S. Ahmad, P. Fu, S. Yu, Q. Yang, X. Liu, X. Wang, X. Wang, X. Guo and C. Li, Dion-Jacobson Phase 2D Layered Perovskites for Solar Cells with Ultrahigh Stability, Joule, 2018, 3, 794–806 CrossRef.
- C. Deng, G. Zhou, D. Chen, J. Zhao, Y. Wang and Q. Liu, Broadband Photoluminescence in 2D Organic−Inorganic Hybrid Perovskites: (C7H18N2)PbBr4 and (C9H22N2)PbBr4, The, J. Phys. Chem. Lett., 2020, 11(8), 2934–2940 CrossRef CAS.
- Y. Li, C. Ji, L. Li, S. Wang, S. Han, Y. Peng, S. Zhang and J. Luo, γ-Methoxy propyl amine)2PbBr4: a novel two-dimensional halide hybrid perovskite with efficient bluish white-light emission, Inorg. Chem. Front., 2021, 8, 2119–2124 RSC.
- C.-Q. Jing, J. Wang, H.-F. Zhao, W.-X. Chu, Y. Yuan, Z. Wang, M.-F. Han, T. Xu, J.-Q. Zhao and X.-W. Lei, Improving Broadband White-Light Emission Performances of 2D Perovskites by Subtly Regulating Organic Cations, Chem. – Eur. J., 2020, 26, 10307–10313 CrossRef CAS.
- C. Xue, S. Wang, W.-L. Liu and X.-M. Ren, Two-Step Structure Phase Transition, Dielectric Anomalies, and Thermochromic Luminescence Behavior in a Direct Band Gap 2D Corrugated Layer Lead Chloride Hybrid of [(CH3)4N]4Pb3Cl10, Chem. – Eur. J., 2019, 25, 5280–5287 CrossRef CAS.
- C. Ji, S. Wang, L. Li, Z. Sun and M. Hong, The First 2D Hybrid Perovskite Ferroelectric Showing Broadband White-Light Emission with High Color Rendering Index, Adv. Funct. Mater., 2019, 29, 1805038 CrossRef.
- P. Cai, Y. Huang and H. J. Seo, Anti-Stokes Ultraviolet Luminescence and Exciton Detrapping in the Two-Dimensional Perovskite (C6H5C2H4NH3)2PbCl4, J. Phys. Chem. Lett., 2019, 10(14), 4095–4102 CrossRef CAS PubMed.
- D. Fu, J. Yuan, S. Wu, Y. Yao, X. Zhang and X.-M. Zhang, A two-dimensional bilayered Dion–Jacobson-type perovskite hybrid with a narrow bandgap for broadband photodetection, Inorg. Chem. Front., 2020, 7, 1394–1399 RSC.
- J. Zhou, Y. Chu and J. Huang, Photodetectors Based on Two-Dimensional Layer-Structured Hybrid Lead Iodide Perovskite Semiconductors, ACS Appl. Mater. Interfaces, 2016, 8, 25660–25666 CrossRef CAS PubMed.
- J. Tauc, Absorption edge and internal electric fields in amorphous semiconductors, Mater. Res. Bull., 1970, 5, 721–729 CrossRef CAS.
- C.-Y. Yue, H.-X. Sun, Q.-X. Liu, X.-M. Wang, Z.-S. Yuan, J. Wang, J.-H. Wu, B. Hu and X.-W. Lei, Organic cation directed hybrid lead halides of zero-dimensional to two-dimensional structures with tunable photoluminescence properties, Inorg. Chem. Front., 2019, 6, 2709–2717 RSC.
- B. Zhang, H.-Y. Sun, J. Li, Y.-R. Xu, Y.-P. Xu, X. Yang and G.-D. Zou, Hybrid iodoplumbates with metal complexes: syntheses, crystal structures, band gaps and photoelectric properties, Dalton Trans., 2020, 49, 1803–1810 RSC.
- R. Sheng, A. W. Ho-Baillie, S. Huang, M. Keevers, X. Hao, L. Jiang, Y. B. Cheng and M. A. Green, Four-Terminal Tandem Solar Cells Using CH3NH3PbBr3 by Spectrum Splitting, J. Phys. Chem. Lett., 2015, 6, 3931–3934 CrossRef CAS.
- T.-t. Zhang, X. Liu, J. Zhou and J.-t. Liu, Two Organic Hybrid Iodoplumbates Directed by a Bifunctional Bis(pyrazinyl)triazole, Inorg. Chem., 2021, 60, 5362–5366 CrossRef CAS PubMed.
- Y. He, Y.-R. Huang, Y.-L. Li, H.-H. Li, Z.-R. Chen and R. Jiang, Encapsulating Halometallates into 3-D Lanthanide-Viologen Frameworks: Controllable Emissions, Reversible Thermochromism, Photocurrent Responses, and Electrical Bistability Behaviors, Inorg. Chem., 2019, 58(20), 13862–13880 CrossRef CAS PubMed.
- X.-L. Lin, B. Chen, Y.-R. Huang, K.-Y. Song, P.-K. Zhou, L.-L. Zong, H.-H. Li, Z.-R. Chen and R. Jiang, Achievement of intrinsic white light emission by hybridization-deformable haloplumbates with rigid luminescent naphthalene motifs, Inorg. Chem. Front., 2020, 7, 4477–4487 RSC.
- E. R. Dohner, A. Jaffe, L. R. Bradshaw and H. I. Karunadasa, Intrinsic white-light emission from layered hybrid perovskites, J. Am. Chem. Soc., 2014, 136, 13154–13157 CrossRef CAS.
- C. Ji, S. Wang, L. Li, Z. Sun, M. Hong and J. Luo, The first 2D hybrid perovskite ferroelectric showing broadband white–light emission with high color rendering index, Adv. Funct. Mater., 2018, 29, 1805038 CrossRef.
- J. K. Pious, A. Katre, C. Muthu, S. Chakraborty, S. Krishna and C. Vijayakumar, Zero-Dimensional Lead-Free Hybrid Perovskite-like Material with a Quantum-Well Structure, Chem. Mater., 2019, 31, 1941–1945 CrossRef CAS.
- N. Chen, Y. Gao, Y. Tian, B. Wu, D. Jia and S. Zhao, One-dimensional polymeric iodoplumbate hybrids with lanthanide complex cations: syntheses, crystal structures, and photoelectric and photocatalytic properties, New J. Chem., 2020, 44, 19166–19173 RSC.
- A. Dey, J. Ye, A. De, E. Debroye, S. K. Ha, E. Bladt, A. S. Kshirsagar, Z. Wang, J. Yin, Y. Wang, L. N. Quan, F. Yan, M. Gao, X. Li, J. Shamsi, T. Debnath, M. Cao, M. A. Scheel, S. Kumar, J. A. Steele, M. Gerhard, L. Chouhan, K. Xu, X.-g. Wu, Y. Li, Y. Zhang, A. Dutta, C. Han, I. Vincon, A. L. Rogach, A. Nag, A. Samanta, B. A. Korgel, C.-J. Shih, D. R. Gamelin, D. H. Son, H. Zeng, H. Zhong, H. Sun, H. V. Demir, I. G. Scheblykin, I. M-Seró, J. K. Stolarczyk, J. Z. Zhang, J. Feldmann, J. Hofkens, J. M. Luther, J. P-Prieto, L. Li, L. Manna, M. I. Bodnarchuk, M. V. Kovalenko, M. B. J. Roeffaers, N. Pradhan, O. F. Mohammed, O. M. Bakr, P. Yang, P. M-Buschbaum, P. V. Kamat, Q. Bao, Q. Zhang, R. Krahne, R. E. Galian, S. D. Stranks, S. Bals, V. Biju, W. A. Tisdale, Y. Yan, R. L. Z. Hoye and L. Polavarapu, State of the Art and Prospects for Halide Perovskite Nanocrystals, ACS Nano, 2021, 15, 10775–10981 CrossRef CAS PubMed.
- Y. Tang, M. Liang, Bi. Chang, H. Sun, K. Zheng, T. Pulleritsd and Q. Chi, Lead-free double halide perovskite Cs3BiBr6 with well-defined crystal structure and high thermal stability for optoelectronics, J. Mater. Chem. C, 2019, 7, 3369–3374 RSC.
- Y. Dang, G. Tong, W. Song, Z. Liu, L. Qiu, L. K. Ono and Y. Qi, Interface engineering strategies towards Cs2AgBiBr6 single-crystalline photodetectors with good Ohmic contact behaviours, J. Mater. Chem. C, 2020, 8, 276–284 RSC.
- M.-Q. Li, Y.-Q. Hu, L.-Y. Bi, H.-L. Zhang, Y. Wang and Y.-Z. Zheng, Structure Tunable Organic−Inorganic Bismuth Halides for an Enhanced Two-Dimensional Lead-Free Light-Harvesting Material, Chem. Mater., 2017, 29(13), 5463–5467 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC [2107024, 2107027]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1qm01247a |
| This journal is © the Partner Organisations 2022 |