A copper tetrathiovanadate anode for ultra-stable potassium-ion storage†
Qifei
Li‡
a,
Xiangxiang
Ye‡
a,
Siling
Cheng
a,
Hong
Yu
 b,
Weiling
Liu
c,
Cheng-Feng
Du
b,
Weiling
Liu
c,
Cheng-Feng
Du
 *b and
Xianhong
Rui
*b and
Xianhong
Rui
 *a
*a
aSchool of Materials and Energy, Guangdong University of Technology, Guangzhou 510006, China. E-mail: xhrui@gdut.edu.cn
bCenter of Advanced Lubrication and Seal Materials, Northwestern Polytechnical University, 127 West Youyi Road, Xi’an 710072, P. R. China. E-mail: cfdu@nwpu.edu.cn
cSchool of Materials Science and Engineering, Nanyang Technological University, Singapore, 639798, Singapore
First published on 12th November 2021
Abstract
Given the abundant natural reserves of potassium and its similar properties to lithium, potassium-ion batteries (KIBs) are expected to be an emerging energy storage system to replace lithium-ion batteries (LIBs). Here, to overcome the sluggish diffusion kinetics and structure collapse of the anode in current KIBs, copper tetrathiovanadate (Cu3VS4) nanoparticles uniformly loaded in carbon nanofibers (CVS/CNF) are designed as a new anode for KIBs through electrospinning and subsequent carbonization/sulfidation treatment. Benefiting from the highly conductive Cu species and CNF network, an outstanding charge transfer kinetics is achieved in CVS/CNF composites. Meanwhile, the nanosized CVS particles and robust CNF networks are believed to be beneficial for enhanced structural stability during potassium insertion, and thus endow the composite anode with an excellent long-term capability. The CVS/CNF composite anode displays a high capacity (486 mA h g−1 at 0.1 A g−1), great rate feature (162 mA h g−1 at 6 A g−1) and outstanding cycling performance (193 mA h g−1 after 2000 cycles at 1 A g−1), which are superior to those of most of the reported transition metal sulfide anodes.
Introduction
Currently, lithium-ion batteries (LIBs) are receiving increasing popularity in portable equipment and hybrid/pure electric vehicles. However, limited lithium reserves block their further application in the field of large-scale energy storage.1–3 Therefore, the development of new and abundant energy storage media has received a growing focus. Potassium, as another element in the same main group with lithium, shares similar physical and chemical properties to lithium, but is much more abundant on earth. Particularly, K has a low redox potential of −2.93 V vs. a standard hydrogen electrode (SHE), which is close to that of Li (−3.04 V vs. SHE) and results in a high working voltage and large energy density of potassium ion batteries (KIBs).4,5 With these advantages, KIBs are expected to be a potential replacement for LIBs as large-scale energy storage equipment. Unfortunately, the larger ionic radius of K+ ions (1.38 Å) leads to sluggish diffusion kinetics in electrode materials and lower power density in KIBs.6–8 Furthermore, during K+ ion insertion, the electrode materials always suffer from large volume expansion and thus collapse, especially the anode material.9–12 Therefore, it is crucial to seek highly reversible anode materials to accommodate the large K+ ions.Recently, a variety of anode materials have been reported in KIBs, mainly including carbon-based materials (e.g., graphene, hard carbon, porous carbon, etc.),13–16 metallic materials (e.g., Sb, Sn, Bi, etc.),17–19 transition metal compounds (e.g., oxides, phosphides, chalcogenides, etc.),20–25 MXene derivatives26–28 and so on. For example, Lian et al. firstly fabricated alkalized Ti3C2 MXene nanoribbons possessing expanded interlayer spacing, with three-dimensional porous frameworks, which obtained the capacities of 136 and 78 mA h g−1 at 20 and 200 mA g−1 as anode materials for KIBs, respectively.27 Dong et al. reported the transformation of accordion-like Ti3C2 MXene into ultrathin nanoribbons of K2Ti4O9 as a high-performance anode material for KIBs, which achieved capacities of 151 and 88 mA h g−1 at 50 and 300 mA g−1, respectively.28 Compared with them, transition metal chalcogenides (TMCs) possess a large theoretical specific capacity and moderate volumetric change upon the charge/discharge process. Vanadium sulfides (such as V5S8 and VS2), as a member of the TMC family, not only carry the common character, but also have been widely explored as anode materials for KIBs because of their multiple valence states (+2 to +5) and rich spatial structures.22–24 For instance, Li et al. reported a honeycomb-like V5S8@C as the anode for KIBs, which achieved an acceptable K+ ion storage property of 351.9 mA h g−1 at 0.1 A g−1.22 Chen et al. reported a nanoflower-like VS2, showing a decent capacity of 383 mA h g−1 at 0.1 A g−1.24 Xie et al. synthesized ultra-enlarged interlayer carbon-containing VS2, which exhibited an enhanced electrochemical performance (439.2 mA h g−1 at 0.1 A g−1) for KIBs.23 Although these achievements have been made, there is still a long way to go before reaching a satisfactory rate performance because of the poor intrinsic conductivity and volume expansion during charge/discharge. Up to now, the combination of vanadium sulfides with a highly electronic conductive carbonaceous substrate, such as carbon nanofibers (CNFs), has been proved to be an effective route to enhance the electrochemical properties of vanadium sulfide materials.29 Meanwhile, atomic doping has also shown great potential towards enhanced conductivity and reaction kinetics for energy storage devices in recent years.30–32 Remarkably, with an ordered atom distribution, the bimetallic compounds provide a buffering substrate to ease volume expansion as well. Due to the introduction of Cu, the electrical conductivity and structural stability of some other metal sulfides can be significantly improved, such as Cu2SnS3 and Cu3SnS4.33,34
Inspired by the above views, using electrospinning and subsequent one-step carbonization/sulfidation treatment, a novel composite of bimetallic sulfide and carbon nanofibers, i.e., copper tetrathiovanadate nanoparticles (Cu3VS4) uniformly dispersed in carbon nanofibers (denoted as CVS/CNF), was designed and synthesized as the anode for KIBs. Derived from the good electrical conductivity of Cu and the CNF network, the CVS/CNF with high Cu content has the characteristic of high electrical conductivity and fast charge transfer. Additionally, the Cu atoms accompanied by CNFs can act as buffer matrixes for easing volume expansion during cycling, which further contributes to the excellent stability. Supported by all these merits, the CVS/CNF composite anode shows an outstanding K+ ion storage performance in terms of a large K+ ion storage capacity (486 mA h g−1 at 0.1 A g−1) and impressive rate property (162 mA h g−1 at 6 A g−1). Remarkably, the CVS/CNF composite anode delivered an ultra-stable long-term cycling capability (193 mA h g−1 after 2000 cycles at a relatively high rate of 1 A g−1), which is better than those of most of the reported transition metal sulfide KIB anodes.
Experimental
Synthesis of CVS/CNF
Electrospinning combined with one-step carbonization/sulfidation treatment was proposed to fabricate the CVS/CNF sample. Firstly, 135 mg of CuCl2 and 795 mg of C10H14O5V were weighed and poured into 20 mL of a mixed solvent of DMF and absolute ethanol in the volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and magnetically stirred for 20 min. When a homogeneous solution was formed, 2 g of PVP (Mw = 1
1 and magnetically stirred for 20 min. When a homogeneous solution was formed, 2 g of PVP (Mw = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was introduced and stirred for 12 h to form the thick and homogeneous precursor solution. In the next process of electrospinning, a 25 mL plastic syringe with a 0.5 mm diameter needle was used at a high voltage of 18 kV and a feed rate of 0.5 mL h−1. The distance between the aluminum foil collector and the tip of the needle was 15 cm. After electrospinning, the resultant textiles were transferred into a 60 °C oven overnight to thoroughly remove the residual DMF and stabilize the nanofiber structures. Finally, the carbonization and sulfidation reactions of the dried textiles were conducted to form CVS/CNF in the tube furnace, which was supplied with argon gas containing CS2 and heated at 500 °C for 6 h. As a comparison, we also synthesized two kinds of monometallic sulfides using the same routing, i.e., copper sulfide composites (CS/CNF) and vanadium sulfide composites (VS/CNF), but only a copper source or vanadium source was added in the corresponding precursor solution.
000) was introduced and stirred for 12 h to form the thick and homogeneous precursor solution. In the next process of electrospinning, a 25 mL plastic syringe with a 0.5 mm diameter needle was used at a high voltage of 18 kV and a feed rate of 0.5 mL h−1. The distance between the aluminum foil collector and the tip of the needle was 15 cm. After electrospinning, the resultant textiles were transferred into a 60 °C oven overnight to thoroughly remove the residual DMF and stabilize the nanofiber structures. Finally, the carbonization and sulfidation reactions of the dried textiles were conducted to form CVS/CNF in the tube furnace, which was supplied with argon gas containing CS2 and heated at 500 °C for 6 h. As a comparison, we also synthesized two kinds of monometallic sulfides using the same routing, i.e., copper sulfide composites (CS/CNF) and vanadium sulfide composites (VS/CNF), but only a copper source or vanadium source was added in the corresponding precursor solution.
Materials characterization
The phase composition of the as-prepared samples was assessed by X-ray diffraction (XRD) technology (Rigaku D/max 2500, Cu Kα radiation). The morphology and microstructure of the samples were observed using a scanning electron microscope (SEM, JEOL, Model JSM-7600F) and transmission electron microscope (TEM, JEOL, Model JEM-2100). The chemical composition and valence states of various constituent elements were explored using X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi).Electrochemical measurements
To evaluate the K+ ion storage property of the CVS/CNF anode, we thoroughly mixed it with carbon nanotubes (CNTs) and polyvinylidene fluoride (PVDF) in a certain proportion of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in an N-methyl-pyrrolidone (NMP) solvent. Subsequently, the obtained homogeneous paste was evenly applied to copper foils and placed in a 60 °C vacuum oven for more than 12 h to remove NMP. After that, 3 M potassium bis(fluorosulfonyl)imide (KFSI) in 1,2-dimethoxyethane (DME) and potassium foil as the electrolyte and counter electrode, respectively, were used to assemble CR2032-type coin cells in a glove box with extremely low levels of water and oxygen (<0.01 ppm). Finally, the galvanostatic charge/discharge tests were conducted with a NEWARE battery test system in a potential window of 0.01–3.00 V vs. K+/K. Additionally, the electrochemical reaction kinetics were studied by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements using a CHI660E electrochemical workstation.
1 in an N-methyl-pyrrolidone (NMP) solvent. Subsequently, the obtained homogeneous paste was evenly applied to copper foils and placed in a 60 °C vacuum oven for more than 12 h to remove NMP. After that, 3 M potassium bis(fluorosulfonyl)imide (KFSI) in 1,2-dimethoxyethane (DME) and potassium foil as the electrolyte and counter electrode, respectively, were used to assemble CR2032-type coin cells in a glove box with extremely low levels of water and oxygen (<0.01 ppm). Finally, the galvanostatic charge/discharge tests were conducted with a NEWARE battery test system in a potential window of 0.01–3.00 V vs. K+/K. Additionally, the electrochemical reaction kinetics were studied by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements using a CHI660E electrochemical workstation.
Results and discussion
The composites of copper tetrathiovanadate/carbon nanofibers (CVS/CNF) were prepared through electrospinning coupled with one-step carbonization/sulfidation treatment. The X-ray diffraction (XRD) pattern of the CVS/CNF sample is shown in Fig. 1a, in which all the sharp diffraction peaks correspond to cubic Cu3VS4 (JCPDS No. 88-1318) and no impurities are detected. The broad diffraction peak at around 23° corresponds to the (002) plane of amorphous carbon.35 As shown in Fig. S1 (ESI†), Cu3VS4 possesses a 3D framework consisting of VS4 and CuS4 tetrahedrons by sharing edges, which is distinctively different from the VS4 structure consisting of a one-dimensional (1D) chain by van der Waals forces and might be beneficial for an improved charge transfer. XRD patterns of the copper sulfide composite (CS/CNF) and vanadium sulfide composite (VS/CNF) are also given in Fig. S2 (ESI†). As shown, the CS/CNF sample contains two phases (Fig. S2a, ESI†), CuS (JCPDS NO. 89-2073) and Cu7.2S4 (JCPDS NO. 24-0061). Besides, the XRD pattern of the VS/CNF sample shows amorphous characteristics (Fig. S2b, ESI†).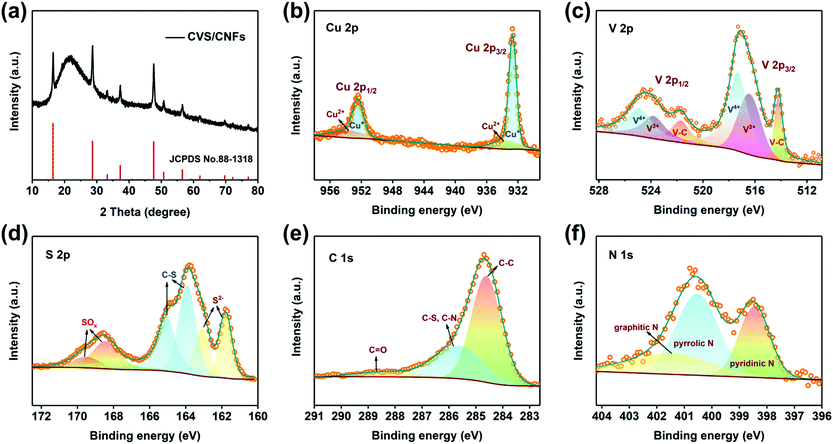 | ||
| Fig. 1 (a) XRD pattern of CVS/CNF. High resolution XPS spectra of (b) Cu 2p, (c) V 2p, (d) S 2p, (e) C 1s and (f) N 1s. | ||
Meanwhile, to examine the chemical compositions and valence state of the elements in all samples, X-ray photoelectron spectroscopy (XPS) techniques were performed (Fig. 1b–f and Fig. S2–S4, ESI†). From the full XPS spectrum (Fig. S3, ESI†), Cu, V, S, C, N and O elements are present in the CVS/CNF sample, in which the O element comes from the surface oxidation and adsorption. As for the other elements, the high-resolution spectrum of Cu 2p can be decomposed into two sets of peaks, as given in Fig. 1b. The two peaks located at 932.7 and 952.4 eV belong to the characteristic peaks of 2p3/2 and 2p1/2 for Cu+, respectively, while the other two peaks at 933.5 and 953.7 eV are appointed to Cu2+.36 The V 2p core-level spectrum reveals three states of V species, as shown in Fig. 1c. The two peaks at 517.3 and 516.4 eV correspond to the 2p3/2 of V4+ and V3+, respectively. Moreover, the two obvious peaks at around 514.2 and 521.7 eV are identified as the V–C bond, which is associated with bonding between Cu3VS4 and carbon nanofibers.37 The S 2p high-resolution spectrum is divided into three sets of characteristic peaks, representing three types of S elements of S2− (161.7 and 163.0 eV), C–S (163.8 and 165.0 eV), and SOx (168.4 and 169.5 eV), as shown in Fig. 1d, which are derived from the bonds between S and metal, S doped carbon nanofibers and surface oxidation, respectively.38 As for the C 1s spectrum (Fig. 1e), it consists of three peaks, i.e., 284.6 eV for C–C, 285.6 eV for C–N and C–S, and 288.4 eV for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, where the formation of the bonds of C–N and C–S may result from that N element in PVP and excess S element in CS2 gas are doped into CNFs during the high-temperature carbonization/sulfidation process.39 The high-resolution spectrum of the N 1s region was also analysed (Fig. 1f), and it can be divided into three fitted peaks at 398.4, 400.5 and 401.5 eV, which correspond to three types of N elements, i.e., pyridinic, pyrrolic, and graphitic, respectively. It has been proved that pyridinic N and pyrrolic N can deliver more abundant vacancies, resulting in more diffusion channels and defects for K+ ions.40,41 In contrast, the XPS spectra of CS/CNF are given in Fig. S4 (ESI†), which show similar characteristic spectra of Cu 2p, S 2p and C 1s with CVS/CNF. Moreover, the XPS spectra of VS/CNF are also shown in Fig. S5 (ESI†), in which only V, S, C and N elements are detected, and the V, S, and C elements show a similar state to CVS/CNF. The S2− and C–S peaks are detected at 161.1/162.1 and 163.8/165.1 eV (Fig. S3c, ESI†), respectively, demonstrating the successful synthesis of VS species on CNFs.
O, where the formation of the bonds of C–N and C–S may result from that N element in PVP and excess S element in CS2 gas are doped into CNFs during the high-temperature carbonization/sulfidation process.39 The high-resolution spectrum of the N 1s region was also analysed (Fig. 1f), and it can be divided into three fitted peaks at 398.4, 400.5 and 401.5 eV, which correspond to three types of N elements, i.e., pyridinic, pyrrolic, and graphitic, respectively. It has been proved that pyridinic N and pyrrolic N can deliver more abundant vacancies, resulting in more diffusion channels and defects for K+ ions.40,41 In contrast, the XPS spectra of CS/CNF are given in Fig. S4 (ESI†), which show similar characteristic spectra of Cu 2p, S 2p and C 1s with CVS/CNF. Moreover, the XPS spectra of VS/CNF are also shown in Fig. S5 (ESI†), in which only V, S, C and N elements are detected, and the V, S, and C elements show a similar state to CVS/CNF. The S2− and C–S peaks are detected at 161.1/162.1 and 163.8/165.1 eV (Fig. S3c, ESI†), respectively, demonstrating the successful synthesis of VS species on CNFs.
The scanning electron microscope (SEM) and transmission electron microscope (TEM) technologies were used to investigate the morphologies and microstructures of the as-prepared CVS/CNF sample (Fig. 2). As exhibited in the low-magnification SEM images (Fig. 2a and b), the CVS/CNF exhibits a uniform 1D nanofiber structure with lengths of about 10–20 μm. Magnified SEM (Fig. 2c) and TEM (Fig. 2d) images reveal that the diameter of the nanofibers is about 250 nm. CVS nanoparticles with a size of dozens of nanometers are uniformly distributed in the CNFs. Meanwhile, the morphologies of both VS/CNF and CS/CNF composites also show a similar 1D nanofiber appearance (Fig. S6, ESI†). As for the high-resolution TEM (HRTEM) images (Fig. 2e and f), two groups of lattice fringes with an interplanar distance of 0.56 nm and 0.32 nm are observed from the nanoparticles, which are coincident with the (100) and (111) planes of Cu3VS4, respectively. In the selected area electron diffraction (SAED) image, as displayed in Fig. 2g, the obvious diffraction rings of the (111) and (220) faces can be observed, proving the polycrystalline nature of Cu3VS4. Moreover, the scanning TEM (STEM) image and the corresponding elemental mapping of a nanofiber are depicted in Fig. 2h. In the regions of the nanoparticles, the dominant elements are Cu, V, and S. As for the other regions of nanofibers, S, C and N dominate, further clarifying that the CNFs are successfully doped with S and N elements.
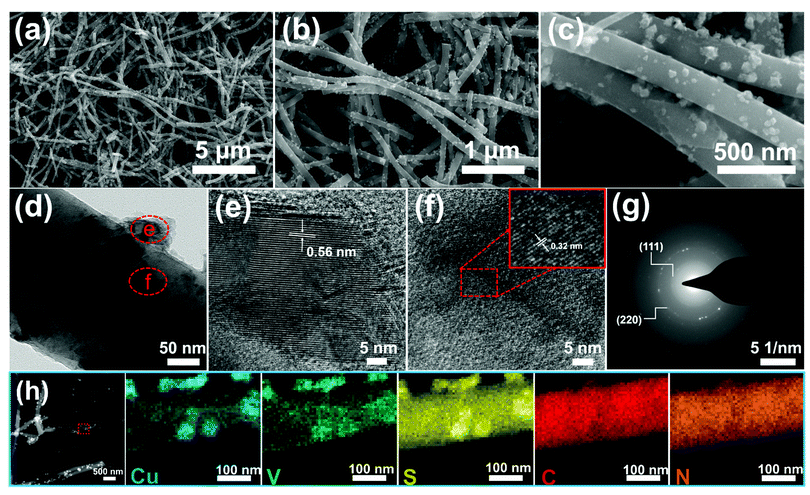 | ||
| Fig. 2 (a–c) SEM, (d) TEM, (e and f) HRTEM pictures, (g) SAED pattern and (h) STEM image and corresponding elemental mapping images of CVS/CNF. | ||
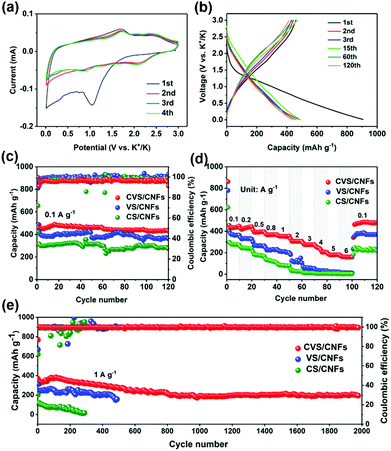
To evaluate the K storage properties of the CVS/CNF anode, the coin-type batteries were packed using K foil and 3 M KFSI in DME as the counter electrode and electrolyte, respectively. Fig. 3a shows the cyclic voltammetry (CV) curves under a relatively low sweep rate of 0.1 mV s−1. During the first reduction process, a broad reduction peak located at 1.1 V is detected and vanishes in the subsequent cycles, which can be described as the generation of a solid electrolyte interphase (SEI) membrane and irreversible K+ ion insertion.42,43 In the next three cycles, the CV curves well overlap, indicating the strong reversibility and cycling stability of the CVS/CNF anode. Fig. 3b exhibits the galvanostatic charging-discharging profiles of the CVS/CNF anode under 0.1 A g−1 for different cycles. During the first cycle, the discharge/charge capacities of 906/459 mA h g−1 are delivered, corresponding to a relatively low coulomb efficiency (CE) of 50.7%, which can be explained by the large consumption of K+ ions by SEI membrane generation and irreversible reactions.43 In the subsequent several cycles, owing to the stepwise stabilization of the SEI membrane and the gradual activation of the CVS/CNF anode, the charge/discharge profiles change slightly and stabilize gradually, and basically follow the same trail after the first 15 cycles, indicating the excellent reversibility and structural stability of the CVS/CNF anode. As presented in Fig. 3c, the CVS/CNF anode delivers a high K+ storage capacity of 430 mA h g−1 after 120 cycles, outdistancing 364 mA h g−1 for VS/CNF and 275 mA h g−1 for CS/CNF under the same conditions. Moreover, the specific capacity delivered in the CVS/CNF anode is also higher than that of recently reported bimetallic sulfides, such as FeCoS2@rGO (372 mA h g−1 at 0.1 A g−1),44 NiCo2S4 (264 mA h g g−1 at 0.1 A g−1),30 and Ni–Fe sulfide (297 mA h g−1 at 0.1 A g−1),45 showing great potential for K+ ion storage.
The rate performance of the CVS/CNF composite was also investigated, as displayed in Fig. 3d. The reversible capacities of 435, 427, 390, 368, 353, 307, 275, 241 and 179 mA h g−1 are delivered at various rates of 0.1, 0.2, 0.5, 0.8, 1, 2, 3, 4 and 5 A g−1, respectively. It is noteworthy that a large K+ ion storage capacity of 162 mA h g−1 is maintained even at a high charging-discharging rate of 6 A g−1, and the capacity can recover to 463 mA h g−1 when the rate decreases to 0.1 A g−1, reflecting the excellent rate adaptability and structural stability of CVS/CNF. As a comparison, VS/CNF shows only a tiny capacity of 13 mA h g−1 left at 6 A g−1. Even worse, the CS/CNF anode is no longer able to charge/discharge properly when the rate exceeds 3 A g−1. The long-term cycling performance of CVS/CNF electrodes was also assessed at a relatively large current density of 1 A g−1. As displayed in Fig. 3e, the rising reversible capacities in the initial several laps can be ascribed to the hysteretic activation behaviours under high current density.46,47 After 2000 cycles, the CVS/CNF anode can still maintain a large specific capacity of 193 mA h g−1 with a stable CE of nearly 100%. In contrast, VS/CNF and CS/CNF anodes exhibit poor cycling stability, in which the former can only retain a low capacity of 142 mA h g−1 upon 500 cycles, and CS/CNF was even damaged in 300 cycles.
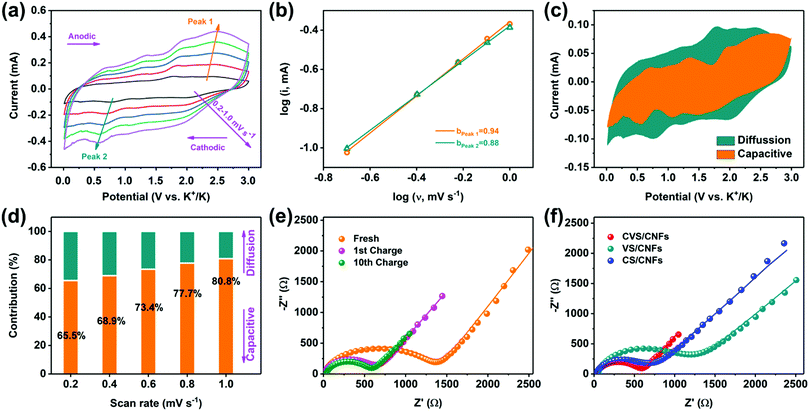
The high capacity and superior rate performance of CVS/CNF anodes arouse our interest. To investigate the electrochemical reaction kinetics of K+ ions in a CVS/CNF anode, the CV technique was performed during the sweep rates of 0.2–1.0 mV s−1 (Fig. 4a). As the scanning rate increased, the redox peaks shifted slightly, but the general shapes of the CV curves are maintained. Theoretically, the peak current (i) follows a power-law relationship with a sweep rate (ν), as follows: i = aνb.48 The b-value is related to the K+ ion storage behaviour, where b = 1.0 represents the capacitive process, and b = 0.5 stands for the diffusion behavior. As shown in Fig. 4b, the b values, calculated from the marked peaks in Fig. 4a, are 0.88 and 0.94, respectively, which illustrates that the main capacity is contributed by the capacitive process. The capacitive contribution of the electrode material during the quantified chemical reaction is then calculated based on the relationship: i = k1ν + k2ν1/2, in which the K+ ion storage behaviour can be divided into two types, i.e., capacitive control behaviour (k1ν) and diffusion control behavior (k2ν1/2).49 At a sweeping rate of 0.2 mV s−1, 65.5% of the capacity is contributed by the capacitive behaviour (Fig. 4c). Furthermore, the capacitive contributions grew from 65.5% to 80.8% as the sweep speed increased (Fig. 4d). Such a large proportion of capacitive-controlled capacity might be in charge with the fast reaction kinetics and excellent high-rate charging/discharging property.
Moreover, electrochemical impedance spectroscopy (EIS) technologies were also used to further explore the charge transfer and reaction kinetics of K+ ions in the CVS/CNF anode. In the Nyquist diagram, the charge transfer resistance (Rct) at the interface between the electrode and electrolyte is represented by a semicircle formed in the high-frequency range, and the K+ ion diffusion resistance (Warburg resistance, W) is represented by a sloping line formed in the low-frequency range. As exhibited in Fig. 4e, the value of Rct for the fresh KIBs assembled with CVS/CNF is relatively large (up to 1411 Ω), and surprisingly drops to 639 Ω after the initial fully charging step, indicating the rapid generation of the fresh SEI membrane on the surface of the negative electrode.35 Furthermore, the cell presents a reduced Rct value of 523 Ω after 10 cycles, indicating the gradual stabilization of the SEI film and CVS/CNF anode.44Fig. 4f shows the Nyquist diagrams of CVS/CNF, VS/CNF, and CS/CNF anodes after 10 cycles under the same conditions. The Rct value of CVS/CNF (523 Ω) is much smaller than that of VS/CNF (1069 Ω) and CS/CNF (650 Ω), indicating the faster charge transfer of CVS/CNF than that of VS/CNF and CS/CNF. In addition, the apparent diffusion coefficients of the K+ ions (DK+) of all samples were analysed and determined using the following formula: DK+ = 0.5 R2T2/S2n4F4C2σ2.36 Here, R, T, F, S, n, C, and σ stand for the gas constant, the absolute temperature, the Faraday constant, the electrochemical reaction area, the number of charges transferred, the K+ ion concentration and the Warburg coefficient, respectively. Moreover, the σ is determined by the formula: Z′ = R + σω−1/2,50 where ω is associated with the low-frequency region, R is a kinetic parameter independent of frequency, which is numerically equal to Rct in the Nyquist plot. Based on the Z′–ω−1/2 curves in Fig. S7 (ESI†), the K+ ion diffusion coefficients are calculated to be 1.82 × 10−19, 7.68 × 10−21, and 4.61 × 10−20 cm2 s−1 for the CVS/CNF, VS/CNF and CS/CNF anodes, respectively, which further confirms the rapid diffusion kinetics of the K+ ions in the CVS/CNF anode.
In addition, to explore the K-storage mechanism of the CVS/CNF anode, the ex situ XRD and XPS under full-discharge/charge states were carried out. As shown in the results of ex situ XRD (Fig. S8, ESI†), only one diffraction peak is observed at the full discharge state, corresponding to the VSx phase. During the subsequent charging process, instead of returning to its original state, the electrode material forms a mixture of CuSy and VSx. The XRD patterns of the electrode during the 5th cycle also show the same transformation, implying the high reversibility of the CVS/CNF anode. Therefore, during the first K+ ion insertion process, we believe that the CVS/CNF anode converts to the composite of VSx and CuSy (i.e., Cu3VS4 → VSx + CuSy). After that, the VSx phase undergoes an intercalation reaction (i.e., VSx + me− + mK+ ↔ KmVSx) and the CuSy phase undergoes a conversion reaction (i.e., CuSy + nye− + nyK+ ↔ Cu + yKnS), which are also confirmed by ex situ XPS. As shown in Fig. S9 (ESI†), the strong intensity of the K element at full discharge demonstrates the insertion of large amounts of K+ ions. Subsequently, the weak signal of the K element at full charge mainly comes from the SEI film and implies the reversible extraction of K+ ions. For Cu spectra (Fig. S10, ESI†), the copper metal is detected at the full discharge state, demonstrating the conversion reaction from CuSy to Cu and KnS. Additionally, the Cu2+ peaks are mainly due to the incomplete conversion reaction. And at the subsequent full charge state, the copper metal returns to the Cu2+ state, implying the reversible conversion reaction. For V spectra (Fig. S11, ESI†), V3+ and V4+ are dominant at full discharge and charge states, respectively, and no vanadium metal is detected, denying the conversion reaction and indicating the insertion reaction of K+ in the VSx phase.
Conclusions
In conclusion, a composite of bimetallic sulfide and carbon nanofibers (CVS/CNF) as a novel anode for KIBs was successfully designed and fabricated using electrospinning and subsequent sulfidation/carbonization treatment. The introduction of Cu leads to a 3D connection of VS4 and CuS4 tetrahedrons, combined with robust CNF networks as conductive supports, the CVS/CNF composite presents an enhanced charge transfer kinetics and outstanding K+ ion storage property (486 mA h g−1 at 0.1 A g−1). Particularly, the CVS/CNF anode delivered an impressive rate capacity (remained 162 mA h g−1 even at 6 A g−1) and an ultra-stable long-term cycling performance (193 mA h g−1 after 2000 cycles at 1 A g−1), revealing ultra-stability for reversible accommodating K+ ions. In view of the excellent K+ ion storage performance of the bimetallic sulfide composite anode, more efforts should be devolved to illuminate the detailed roles of each component during K+ ion storage in the future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China (No. 51972067, 51802265, 51802044, 51902062 and 51802043), the Guangdong Natural Science Funds for Distinguished Young Scholar (No. 2019B151502039), and the Natural Science Basic Research Plan in Shaanxi Province of China (No. 2020JQ-165).Notes and references
- J. Liu, S. Wang, K. Kravchyk, M. Ibanez, F. Krumeich, R. Widmer, D. Nasiou, M. Meyns, J. Llorca, J. Arbiol, M. V. Kovalenko and A. Cabot, SnP nanocrystals as anode materials for Na-ion batteries dagger, J. Mater. Chem. A, 2018, 6, 10958–10966 RSC.
- J. C. Pramudita, D. Sehrawat, D. Goonetilleke and N. Sharma, An initial review of the status of electrode materials for potassium-ion batteries, Adv. Energy Mater., 2017, 7, 1602911 CrossRef.
- J. B. Goodenough and K.-S. Park, The Li-ion rechargeable battery: a perspective, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- J. Zheng, Y. Wu, Y. Sun, J. Rong, H. Li and L. Niu, Advanced anode materials of potassium ion batteries: from zero dimension to three dimensions, Nano-Micro Lett., 2021, 13, 12 CrossRef.
- J.-Y. Hwang, S.-T. Myung and Y.-K. Sun, Recent progress in rechargeable potassium batteries, Adv. Funct. Mater., 2018, 28, 1802938 CrossRef.
- M. Zhou, P. Bai, X. Ji, J. Yang, C. Wang and Y. Xu, Electrolytes and interphases in potassium ion batteries, Adv. Mater., 2021, 33, 2003741 CrossRef CAS PubMed.
- M. Ma, S. Zhang, Y. Yao, H. Wang, H. Huang, R. Xu, J. Wang, X. Zhou, W. Yang, Z. Peng, X. Wu, Y. Hou and Y. Yu, Heterostructures of 2D molybdenum dichalcogenide on 2D nitrogen-doped carbon: superior potassium-ion storage and insight into potassium storage mechanism, Adv. Mater., 2020, 32, 2000958 CrossRef CAS.
- C. Zhang, H. Pan, L. Sun, F. Xu, Y. Ouyang and F. Rosei, Progress and perspectives of 2D materials as anodes for potassium-ion batteries, Energy Storage Mater., 2021, 38, 354–378 CrossRef.
- Y. Liang, C. Luo, F. Wang, S. Hou, S.-C. Liou, T. Qing, Q. Li, J. Zheng, C. Cui and C. Wang, An organic anode for high temperature potassium-ion batteries, Adv. Energy Mater., 2019, 9, 1802986 CrossRef.
- J. Wang, B. Wang, Z. Liu, L. Fan, Q. Zhang, H. Ding, L. Wang, H. Yang, X. Yu and B. Lu, Nature of bimetallic oxide Sb2MoO6/RGO anode for high-performance potassium-ion batteries, Adv. Sci., 2019, 6, 1900904 CrossRef.
- L. Tao, Y. Yang, H. Wang, Y. Zheng, H. Hao, W. Song, J. Shi, M. Huang and D. Mitlin, Sulfur-nitrogen rich carbon as stable high capacity potassium ion battery anode: Performance and storage mechanisms, Energy Storage Mater., 2020, 27, 212–225 CrossRef.
- Z. Shi, Q. Ru, S. Cheng, X. Hou, F. Chen and F. C.-C. Ling, Hierarchically Rambutan-like Zn3V3O8 hollow spheres as anodes for lithium-/potassium-ion batteries, Energy Technol., 2020, 8, 2000010 CrossRef CAS.
- K. Share, A. P. Cohn, R. Carter, B. Rogers and C. L. Pint, Role of nitrogen-doped graphene for improved high-capacity potassium ion battery anodes, ACS Nano, 2016, 10, 9738–9744 CrossRef CAS PubMed.
- D. Li, X. Cheng, R. Xu, Y. Wu, X. Zhou, C. Ma and Y. Yu, Manipulation of 2D carbon nanoplates with a core-shell structure for high-performance potassium-ion batteries, J. Mater. Chem. A, 2019, 7, 19929–19938 RSC.
- S. Alvin, C. Chandra and J. Kim, Controlling intercalation sites of hard carbon for enhancing Na and K storage performance, Chem. Eng. J., 2021, 411, 128490 CrossRef CAS.
- S. Zeng, X. Zhou, B. Wang, Y. Feng, R. Xu, H. Zhang, S. Peng and Y. Yu, Freestanding CNT-modified graphitic carbon foam as a flexible anode for potassium ion batteries, J. Mater. Chem. A, 2019, 7, 15774–15781 RSC.
- K. Song, C. Liu, L. Mi, S. Chou, W. Chen and C. Shen, Recent progress on the alloy-based anode for sodium-ion batteries and potassium-ion batteries, Small, 2021, 17, 1903194 CrossRef CAS.
- K. Lei, C. Wang, L. Liu, Y. Luo, C. Mu, F. Li and J. Chen, A porous network of bismuth used as the anode material for high-energy-density potassium-ion batteries, Angew. Chem., Int. Ed., 2018, 57, 4687–4691 CrossRef CAS.
- Y. Li, Q. Zhang, Y. Yuan, H. Liu, C. Yang, Z. Lin and J. Lu, Surface amorphization of vanadium dioxide (B) for K-ion battery, Adv. Energy Mater., 2020, 10, 2000717 CrossRef CAS.
- L. Yang, W. Hong, Y. Zhang, Y. Tian, X. Gao, Y. Zhu, G. Zou, H. Hou and X. Ji, Hierarchical NiS2 modified with bifunctional carbon for enhanced potassium-ion storage, Adv. Funct. Mater., 2019, 29, 1903454 CrossRef CAS.
- H. Liu, Y. He, K. Cao, S. Wang, Y. Jiang, X. Liu, K.-J. Huang, Q.-S. Jing and L. Jiao, Stimulating the reversibility of Sb2S3 anode for high-performance potassium-ion batteries, Small, 2021, 17, 2008133 CrossRef CAS PubMed.
- J. Li, S. Zhang, S. Zhang, C. An and L. Cao, Templated constructing honeycomb-like V5S8@C anode with multi-scale interfacial coactions and high pseudocapacitive contribution for enhanced potassium storage capability, J. Alloys Compd., 2021, 851, 156920 CrossRef CAS.
- X.-C. Xie, H.-L. Shuai, X. Wu, K.-J. Huang, L.-N. Wang, R.-M. Wang and Y. Chen, Engineering ultra-enlarged interlayer carbon-containing vanadium disulfide composite for high-performance sodium and potassium ion storage, J. Alloys Compd., 2020, 847, 156288 CrossRef CAS.
- J. Chen, Z. Tang, Z. Pan, W. Shi, Y. Wang, Z. Q. Tian and P. K. Shen, Template-free growth of spherical vanadium disulfide nanoflowers as efficient anodes for sodium/potassium ion batteries, Mater. Des., 2020, 192, 108780 CrossRef CAS.
- A. S. Murali, D. S. Baji, S. Nair and D. Santhanagopalan, Vapour phase conversion of metal oxalates to metal phosphide nanostructures and their use as anode in rechargeable Li, Na and K-ion batteries, Electrochim. Acta, 2021, 388, 138643 CrossRef CAS.
- Y. Dong, H. Shi and Z.-S. Wu, Recent advances and promise of MXene-based nanostructures for high-performance metal ion batteries, Adv. Funct. Mater., 2020, 30, 2000706 CrossRef CAS.
- P. Lian, Y. Dong, Z.-S. Wu, S. Zheng, X. Wang, S. Wang, C. Sun, J. Qin, X. Shi and X. Bao, Alkalized Ti3C2 MXene nanoribbons with expanded interlayer spacing for high-capacity sodium and potassium ion batteries, Nano Energy, 2017, 40, 1–8 CrossRef CAS.
- Y. Dong, Z.-S. Wu, S. Zheng, X. Wang, J. Qin, S. Wang, X. Shi and X. Bao, Ti3C2 MXene-derived sodium/potassium titanate nanoribbons for high-performance sodium/potassium ion batteries with enhanced capacities, ACS Nano, 2017, 11, 4792–4800 CrossRef CAS PubMed.
- X. Li, W. Chen, Q. Qian, H. Huang, Y. Chen, Z. Wang, Q. Chen, J. Yang, J. Li and Y.-W. Mai, Electrospinning-based strategies for battery materials, Adv. Energy Mater., 2021, 11, 2000845 CrossRef CAS.
- X. Zhang, Q. He, X. Xu, T. Xiong, Z. Xiao, J. Meng, X. Wang, L. Wu, J. Chen and L. Mai, Insights into the storage mechanism of layered VS2 cathode in alkali metal-ion batteries, Adv. Energy Mater., 2020, 10, 1904118 CrossRef CAS.
- P. He, M. Yan, G. Zhang, R. Sun, L. Chen, Q. An and L. Mai, Layered VS2 nanosheet-based aqueous zn ion battery cathode, Adv. Energy Mater., 2017, 7, 1601920 CrossRef.
- T. Jiao, Q. Yang, S. Wu, Z. Wang, D. Chen, D. Shen, B. Liu, J. Cheng, H. Li, L. Ma, C. Zhi and W. Zhang, Binder-free hierarchical VS2 electrodes for high-performance aqueous Zn ion batteries towards commercial level mass loading, J. Mater. Chem. A, 2019, 7, 16330–16338 RSC.
- C. Wang, H. Tian, J. Jiang, T. Zhou, Q. Zeng, X. He, P. Huang and Y. Yao, Facile synthesis of different morphologies of Cu2SnS3 for high-performance supercapacitors, ACS Appl. Mater. Interfaces, 2017, 9, 26038–26044 CrossRef CAS PubMed.
- N. Sharma, D. Phase, M. O. Thotiyl and S. Ogale, Single-phase Cu3SnS4 nanoparticles for robust high capacity lithium-ion battery anodes, ChemElectroChem, 2019, 6, 1371–1375 CrossRef CAS.
- G. Yao, P. Niu, Z. Li, Y. Xu, L. Wei, H. Niu, Y. Yang, F. Zheng and Q. Chen, Construction of flexible V3S4@CNF films as long-term stable anodes for sodium-ion batteries, Chem. Eng. J., 2021, 423, 130229 CrossRef CAS.
- Y. Fang, X.-Y. Yu and X. W. Lou, Bullet-like Cu9S5 hollow particles coated with nitrogen-doped carbon for sodium-ion batteries, Angew. Chem., Int. Ed., 2019, 58, 7744–7748 CrossRef CAS PubMed.
- L.-q. Yu, S.-X. Zhao, Q.-l. Wu, J.-W. Zhao and G.-d. Wei, Strengthening the interface between flower-like VS4 and porous carbon for improving its lithium storage performance, Adv. Funct. Mater., 2020, 30, 2000427 CrossRef CAS.
- H. Li, Y. Su, W. Sun and Y. Wang, Carbon nanotubes rooted in porous ternary metal sulfide@N/S-doped carbon dodecahedron: bimetal-organic-frameworks derivation and electrochemical application for high-capacity and long-life lithium-ion batteries, Adv. Funct. Mater., 2016, 26, 8345–8353 CrossRef CAS.
- X. Xie, Y. Hu, G. Fang, X. Cao, B. Yin, Y. Wang, S. Liang, G. Cao and A. Pan, Towards a durable high performance anode material for lithium storage: stabilizing N-doped carbon encapsulated FeS nanosheets with amorphous TiO2, J. Mater. Chem. A, 2019, 7, 16541–16552 RSC.
- D. M. Zhang, J. H. Jia, C. C. Yang and Q. Jiang, Fe7Se8 nanoparticles anchored on N-doped carbon nanofibers as high-rate anode for sodium-ion batteries, Energy Storage Mater., 2020, 24, 439–449 CrossRef.
- J. Han, K. Zhu, P. Liu, Y. Si, Y. Chai and L. Jiao, N-doped CoSb@C nanofibers as a self-supporting anode for high-performance K-ion and Na-ion batteries, J. Mater. Chem. A, 2019, 7, 25268–25273 RSC.
- S. Zhang, G. Wang, B. Wang, J. Wang, J. Bai and H. Wang, 3D carbon nanotube network bridged hetero-structured Ni-Fe-S nanocubes toward high-performance lithium, sodium, and potassium storage, Adv. Funct. Mater., 2020, 30, 2001592 CrossRef CAS.
- Q. Peng, S. Zhang, H. Yang, B. Sheng, R. Xu, Q. Wang and Y. Yu, Boosting potassium storage performance of the Cu2S anode via morphology engineering and electrolyte chemistry, ACS Nano, 2020, 14, 6024–6033 CrossRef CAS.
- X. Chen, N. Cheng, L. Zhang, G. Xiang, Y.-L. Ding and Z. Liu, Flower-like spherical FeCoS2 coated by reduced graphene oxide as anode for high performance potassium ion storage, J. Alloys Compd., 2021, 861, 158458 CrossRef CAS.
- S. H. Yang, S.-K. Park, G. D. Park, J. H. Kim and Y. C. Kang, Rational synthesis of uniform yolk-shell Ni-Fe bimetallic sulfide nanoflakes@porous carbon nanospheres as advanced anodes for high-performance potassium-/sodium-ion batteries, Chem. Eng. J., 2021, 417, 127963 CrossRef CAS.
- S. Niu, Z. Wang, T. Zhou, M. Yu, M. Yu and J. Qiu, A polymetallic metal-organic framework-derived strategy toward synergistically multidoped metal oxide electrodes with ultralong cycle life and high volumetric capacity, Adv. Funct. Mater., 2017, 27, 1605332 CrossRef.
- C. A. Etogo, H. Huang, H. Hong, G. Liu and L. Zhang, Metal-organic-frameworks-engaged formation of Co0.85Se@C nanoboxes embedded in carbon nanofibers film for enhanced potassium-ion storage, Energy Storage Mater., 2020, 24, 167–176 CrossRef.
- Q. He, J. Jiang, J. Zhu, Z. Pan, C. Li, M. Yu, J. Key and P. K. Shen, A facile and cost effective synthesis of nitrogen and fluorine Co-doped porous carbon for high performance Sodium ion battery anode material, J. Power Sources, 2020, 448, 227568 CrossRef CAS.
- H. Bian, Z. Li, J. Pan, F. Lyu, X. Xiao, J. Tang, P. Schmuki, C. Liu, J. Lu and Y. Y. Li, Anodic self-assembly method for synthesizing hierarchical FeS/FeOx hollow nanospheres, J. Power Sources, 2021, 484, 229268 CrossRef CAS.
- S. Wang, F. Gong, S. Yang, J. Liao, M. Wu, Z. Xu, C. Chen, X. Yang, F. Zhao, B. Wang, Y. Wang and X. Sun, Graphene oxide-template controlled cuboid-shaped high-capacity VS4 nanoparticles as anode for sodium-ion batteries, Adv. Funct. Mater., 2018, 28, 1801806 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Schematic crystal structures, XRD patterns, XPS spectra, SEM images, TEM images, Z′–ω−1/2 curve, in situ XRD and in situ XPS. See DOI: 10.1039/d1qm01096d |
| ‡ Q. Li and X. Ye contributed equally to this work. |
| This journal is © the Partner Organisations 2022 |