The critical role of inter-component hydrogen bonds in the formation of reversibly interlocked polymer networks
Mei Rui
Fu
,
Yang
You
 *,
Min Zhi
Rong
* and
Ming Qiu
Zhang
*,
Min Zhi
Rong
* and
Ming Qiu
Zhang
 *
*
Key Laboratory for Polymeric Composite and Functional Materials of MOE, GD HPPC Lab, School of Chemistry, Sun Yat-sen University, Guangzhou 510275, China. E-mail: youy23@mail2.sysu.edu.cn; cesrmz@mail.sysu.edu.cn; ceszmq@mail.sysu.edu.cn
First published on 9th November 2021
Abstract
Recently developed reversibly interlocked polymer networks are made from two pre-formed crosslinked polymers containing reversible covalent bonds via topological rearrangement. Unlike interpenetrating polymer networks, the resultant is rather homogeneous regardless of the miscibility between the parent single networks, and can be repeatedly self-healed and unlocked/re-locked. The present work focuses on revealing the critical role of the hydrogen bonds between the single networks in the construction of the interlocked networks, cooperating with dissociation and re-association of the built-in reversible covalent bonds. Based on the systematic investigations of the small molecules, single networks and interlocked networks, the inter-component hydrogen bonds of the interlocked networks are found to be able to be built-up, which are more stable than the intra-molecular hydrogen bonds of the single networks. They help to pull the chains from different single networks together, preventing the occurrence of phase separation during interlocking and implementing the valuable forced miscibility. Besides, abundant but weak inter-component hydrogen bonds are preferred. The outcomes not only show the hitherto unknown part of the mechanism involved in the formation of the interlocked networks, but also benefit the design of new members of interlocked networks.
Introduction
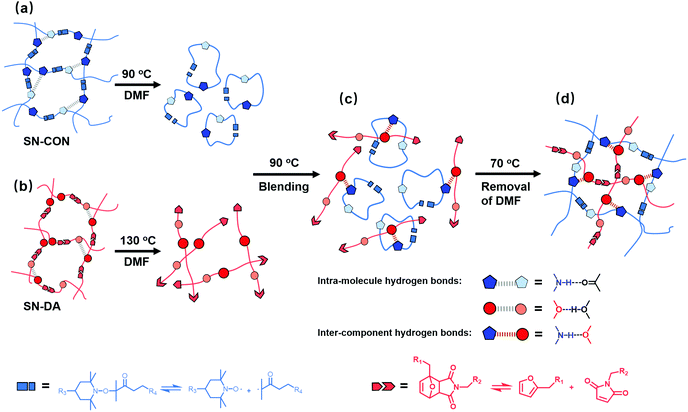
Hybridization of different materials proved to be a facile and effective method to produce new materials with improved performances.1 Nevertheless, unwanted phase separation used to occur because of the thermodynamic immiscibility among most materials,2,3 which often resulted in a poor hybridizing effect except for a few cases like immiscible phase separation of high impact polystyrene (HIPS).4 So far, great efforts have been made to suppress systems, including grafting,5 blocking copolymerization,6 incorporation of processing aids,7 and interpenetrating polymer networks (IPNs),8 but elimination of micro-phase separation still remains a challenge.Recently, we developed a novel solution by taking advantage of the topological rearrangement of two pre-formed polymer networks containing orthogonal reversible covalent bonds.9–12 The resultant reversible interlocked polymer networks (ILNs) were rather homogeneous owing to the forced miscibility, and possess overall improved mechanical performances as well as advantages such as self-healing, reprocessibility and unlocking-relocking ability,10 regardless of the immiscibility of the parent networks. It was believed that the keys to achieve the above goal laid in (i) the synergy among the reversible association/dissociation of the dynamic covalent bonds, (ii) the inter-component secondary interactions and (iii) co-solvent, which were all indispensable. Typically, the parent single networks were firstly dissolved in their co-solvent as a result of the disconnection of the included reversible bonds upon exposure to appropriate stimuli. Then, the solutions containing the fragments of the single networks in the form of molecular segments or nanogels were well mixed, and the fragments from one network embraced together with those from the other networks via the inter-component secondary interactions. Afterwards, the stimuli were removed, leading to reformation of the dissociated reversible bonds, while the co-solvent also gradually evaporated. Eventually, the two single networks were re-built in the intertwined state, giving birth to interlocked networks.
Although the dynamic behaviors of the reversible bonds and their contributions during the preparation of the interlocked polymer networks have been carefully investigated,9,10 understanding the effect of the secondary interactions (represented by hydrogen bonds) stays in the guessing stage, lacking experimental proof. As a continuation of our study, therefore, the present work is focused on revealing the role of hydrogen bonds in helping the construction of interlocked networks. In general, the miscibility of polymer pairs decreases with increasing molecular weight,13 so that the trend of phase separation may appear before the gel point in the current case. This means that suppression of phase separation via the constraints of inter-component hydrogen bonding prior to the establishment of interlocked architecture is critically important.
Hydrogen bonding is a kind of non-covalent interaction between electron-deficient hydrogen (proton donor) and the region with high electron density (proton accepter).14 Extensive research studies have demonstrated that hydrogen bonds between different components in polymer blends could pull the immiscible polymer chains together. Because the chemical structures of hydrogen bonding moieties are diverse, the hydrogen bond strengths can be tuned through appropriate molecular design. For example, the multiple hydrogen bonds between the moieties like 2-ureido-4[1H]-pyrimidone (UPy) and nucleobase pairing are very strong and can effectively link the macromolecular chains,15–19 but they are not ideal candidates for the current system since self-assembly of the moieties would lead to micro-phase separation,20–22 which poses an obstacle to homogenization and unlocking of the target interlocked networks. In contrast, introduction of the weaker but abundant single hydrogen bonds would be more feasible because (i) single hydrogen bonds can be easily incorporated without complex chemical modification; (ii) they can be broken under mild conditions accompanying the dissociation of the reversible covalent bonds during unlocking of the interlocked networks; and (iii) precise self-assembly of hydrogen bonding moieties can be avoided.
Fig. 1 shows the chemical structures of the two single networks acting as the parent polymers of the target interlocked networks in this work. For the single networks with reversible C–ON bonds (SN-CON), the alkoxyamine moieties carry three build-in amido groups (–NH–, proton donors), and the fission and recombination of the C–ON bonds take place at moderate temperature (60–70 °C).9 With respect to the single networks with reversible Diels–Alder (DA) bonds (SN-DA), we chose polyether amine (ED-900) as the crosslinker, which contains ∼eighteen ether groups (–C–O–C–, proton accepters) on each macromolecular chain. The forward and retro-DA reactions occur at 60–80 °C and >120 °C, respectively.9 In this context, plenty of inter-component hydrogen bonds (C–O⋯H–N) are expected to be created by combining SN-CON and SN-DA.
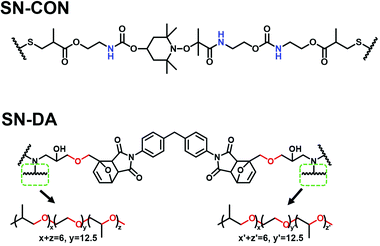 | ||
| Fig. 1 Chemical structures of the single networks (SN-CON and SN-DA) for fabricating the interlocked networks. | ||
Hereinafter, the thermal-responsive hydrogen bonds in the single networks (i.e. SN-CON and SN-DA) and their interlocked networks are investigated mainly using temperature-dependent Fourier transform infrared (FTIR) spectroscopy accompanied by two-dimensional correlation and perturbation correlation moving window (PCMW) technologies. Two-dimensional correlation technology is a mathematical data processing method by spreading the characteristic peaks along a second dimension, which reveals some detailed information not observable in conventional 1D spectroscopy.23 Additionally, model compounds are also involved for studying the intercomponent hydrogen bonds. The results may provide an in-depth description of the formation mechanism of the homogeneous phase structure in interlocked polymer networks, guiding the rational molecular design of multi-component polymer materials.
Experimental
Materials
4-Hydroxy-2,2,6,6-tetramethylpiperidinyloxy (TEMPO) was supplied by Acmec. 2,2′-Azobis(2-methyl-N-(2-hydrox-yethyl)) propionamide (AMHEPA) was supplied by Macklin. Pentaerythritol tetra(3-mercaptopropionate) (PETMP), dibutyltindilaurate (DBTDL), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 2-iso-cyanatoethyl methacrylate (IEM), furfuryl alcohol, 4,4′-methylenebis(N-phenylmaleimide (DPMBMI)), epichlorohydrin (ECH), tetrabutylammonium bromide (TBAB), furoyl chloride, N-methylurethane (NMU) and N-methylacetamide (NMA) were supplied by Aladdin Reagent Ltd. Jeffamine polyether amine (ED900, molecular weight = 900 g mol−1) was supplied by Huntsman. Chloroform and N,N-dimethylformamide (DMF) were dried over activated molecular sieves (4 Å) prior to use. All the other chemicals and solvents were used as received.Sample preparation
The single networks, SN-CON and SN-DA (Fig. 1), which were respectively crosslinked by reversible C–ON bonds and DA bonds, served as the starting materials. The interlocked polymer networks (ILNs) were prepared according to our previous report.9 Typically, SN-DA was soaked in the co-solvent DMF for 2 h, and then the mixture was heated to 130 °C for triggering the retro-DA reaction (temperatures of retro-DA reaction and forward DA reaction are 120 °C and ∼80 °C, respectively), and stirred under nitrogen for 15 min to get the SN-DA solution. Similarly, SN-CON was also soaked in DMF for 2 h, and then the mixture was heated to 90 °C, which is higher than the homolysis temperature of C–ON bonds (∼70 °C), and stirred under nitrogen for 1 h to obtain the SN-CON solution. Next, the two types of solutions were mixed at 90 °C and stirred for an additional 30 min, followed by vacuum drying. Afterwards, the concentrated solution mixture was poured into a silicon mold, and kept at 70 °C for 2 h. Finally, the resultant interlocked networks were moved into a vacuum oven at 70 °C and dried for 10 h to produce a sheet-like sample, which was denoted as ILN-XY, where ‘XY’ means the mass ratio of SN-DA to SN-CON.To prepare the control samples, the hydrogen bonds in the ILNs were weakened by adding different amounts of LiBr24 into the solutions of the single networks before making ILN-11. The products were symbolized by ILN-LiBr-Z%, where Z% represents the parts of LiBr per hundred ILN-11 by weight.
Characterization
Temperature-dependent FTIR spectra of the solutions of SN-DA, SN-CON and ILN-11 were recorded on the equipment composed of a Nicolet Nexus 670 spectrometer (Thermo Electron Co., USA) and an electrically heated jacket (Specac). The solutions were filled in KBr sample cells. The absorption spectra were collected within the wavenumber region of 4000–400 cm−1 at a resolution of 4 cm−1 with 32 scans at a heating or cooling rate of 1 °C min−1. In the meantime, the spectra of the solid samples were recorded by the attenuated total reflectance (ATR) mode. 2DIR was applied to process the data to generate two-dimensional correlation spectra. The correlation peaks were indicated by contour maps, and the red (blue) regions were defined as positive (negative) intensities.To investigate the hydrogen bonds between model compounds, proton nuclear magnetic resonance (1H-NMR) spectra were collected by a Bruker Avance III-400 (400 MHz) spectrometer with deuterated dimethyl sulfoxide (DMSO-d6) as the solvent.
The interaction between the single networks was characterized by differential scanning calorimetry (DSC) tests, which were carried out on Netzsch DSC-204 between −60 and 100 °C at identical heating and cooling rates of 10 °C min−1.
The phase structures of ILN-11 and the controls were evaluated by micro-Raman spectroscopy with a Thermo Fisher DXR3xi Raman imaging microscope at room temperature. The wavelength of the exciting laser beam was 785 nm. The Raman spectroscopy mapping over a 100 × 100 μm2 area was recorded.
Results and discussion
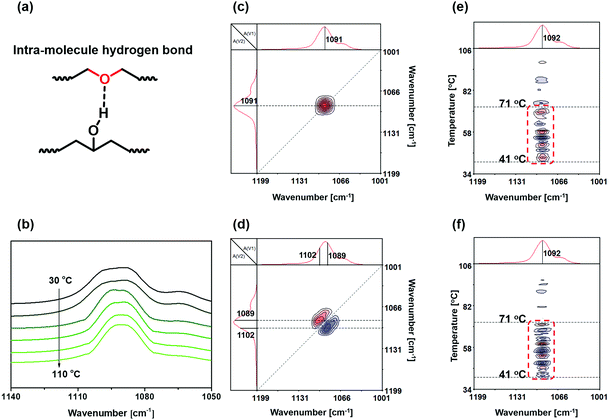
Our earlier works9 demonstrated that topological rearrangement of the single polymer networks (SN-DA and SNCON) in the presence of the co-solvent (DMF) produced the interlocked networks. Therefore, the variations in the intramolecular hydrogen bonds of SN-DA and SN-CON in DMF solutions before mixing and interlocking should be known at the beginning, by collecting their FTIR spectra along with heating according to the preparation procedures described in the Experimental section. Meanwhile, the temperature dependent FTIR spectroscopic data are converted to two-dimensional correlation spectrograms for the convenience of discussion in consideration of the high resolution and sensitivity to the dynamic response of hydrogen bonds of the latter.25–27Generally, the ether group of SN-DA can form an intramolecular hydrogen bond with the hydroxyl group (C–O⋯H–O, refer to Fig. 1 and Fig. 2a). The FTIR spectra of the DMF solution of SN-DA measured with a rise in temperature from 30 to 110 °C (Fig. 2b) indicate that the absorption of –C–O–C– at around 1092 cm−1 gradually shifts to a lower wavenumber regime with heating due to the partial dissociation of the intramolecular hydrogen bonds. This finding agrees with the result of the synchronous two-dimensional correlation spectra (Fig. 2c), which show that there is a strong autopeak at [1091 cm−1, 1091 cm−1], meaning that the change of –C–O–C– groups is rather prominent when temperature increases. Moreover, the asynchronous 2D correlation FTIR spectra (Fig. 2d) show that there are cross-peaks at [1089 cm−1, 1102 cm−1] and [1102 cm−1, 1089 cm−1]. Clearly, the absorption of –C–O–C– is composed of two parts, and those at 1089 cm−1 and 1102 cm−1 are attributed to the free and associated –C–O–C– moieties,28 respectively, which is difficult to be disclosed simply from the conventional spectra (Fig. 2b). Evidently, not all –C–O–C– groups in SN-DA have participated in the formation of the intra-molecular hydrogen bonds.
To exactly identify the specific response temperature range of the intra-molecular hydrogen bonds, the perturbation correlation moving window (PCMW) technology that can quickly determine the tipping points is utilized to treat the FTIR spectra. Since hydrogen bonds are temperature-responsive and continuously combine and open in the response range, the darkest and the most obvious peaks highlighted in the synchronous and asynchronous PCMW2D correlation FTIR spectra (Fig. 2e and f) are selected as the main responsive ones. It is thus known that the response temperature of the intra-molecular hydrogen bonds mainly range between 41 °C and 71 °C, which is much lower than the dissolving temperature of SN-DA (130 °C, refer to the Experimental section). In other words, the intra-molecular hydrogen bonds have been dissociated long before the retro-DA reaction of SN-DA.
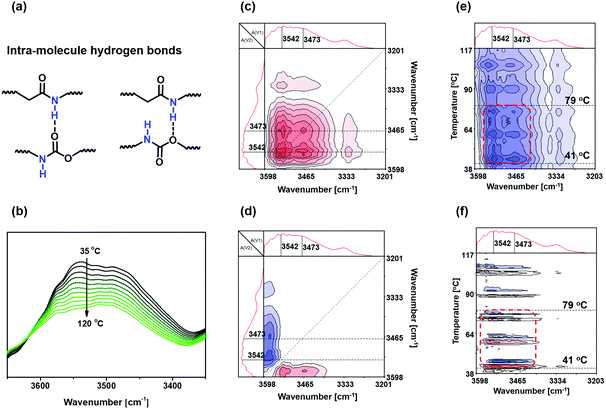
In the case of SN-CON, the –NH– group may form intramolecular hydrogen bonds with the carbonyl group or urethane group (i.e. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N or C–O⋯H–N, refer to Fig. 1 and Fig. 3a). As can be seen from the FTIR spectra of SN-CON solution (Fig. 3b), the broad absorption at 3600–3400 cm−1 originates from the stretching mode of –NH–, and it factually consists of two peaks at 3473 cm−1 and 3542 cm−1 representing free and associated –NH– moieties, respectively.29–32 As a result of the partial dissociation of the intra-molecular hydrogen bonds with increasing temperature, the peak of the associated –NH– weakens and that of the free –NH– intensifies. The strong autopeak at [∼3500 cm−1, ∼3500 cm−1] in Fig. 3c further confirms that the –NH– groups change remarkably throughout this period. As for the asynchronous 2D correlation spectra (Fig. 3d), the crosspeaks at [3473 cm−1, 3542 cm−1] and [3542 cm−1, 3473 cm−1] verify the above analysis of the temperature dependent spectra in Fig. 3b.
O⋯H–N or C–O⋯H–N, refer to Fig. 1 and Fig. 3a). As can be seen from the FTIR spectra of SN-CON solution (Fig. 3b), the broad absorption at 3600–3400 cm−1 originates from the stretching mode of –NH–, and it factually consists of two peaks at 3473 cm−1 and 3542 cm−1 representing free and associated –NH– moieties, respectively.29–32 As a result of the partial dissociation of the intra-molecular hydrogen bonds with increasing temperature, the peak of the associated –NH– weakens and that of the free –NH– intensifies. The strong autopeak at [∼3500 cm−1, ∼3500 cm−1] in Fig. 3c further confirms that the –NH– groups change remarkably throughout this period. As for the asynchronous 2D correlation spectra (Fig. 3d), the crosspeaks at [3473 cm−1, 3542 cm−1] and [3542 cm−1, 3473 cm−1] verify the above analysis of the temperature dependent spectra in Fig. 3b.
Similar to the treatment of Fig. 2e and Fig. 2f, the darkest parts in the synchronous (Fig. 3e) and asynchronous (Fig. 3f) PCMW2D correlation FTIR spectra of SN-CON solution are used to determine the temperature range where the intra-molecular hydrogen bonds vary most. Accordingly, the response temperatures of the intra-molecular hydrogen bonds based on –NH– are found to predominantly range from 41 °C to 79 °C, which are also lower than the dissolving temperature of SN-CON (90 °C, refer to the Experimental section).
The above results confirm that the –C–O–C– groups of SN-DA and –NH– groups of SN-CON can act as proton accepters and donors to form intra-molecular hydrogen bonds, respectively, and the latter are broken with a rise in temperature before blending of the DMF solutions of the two types of single networks at 90 °C for preparation of the interlocked networks. Because the intra-molecular hydrogen bonds are dissociated in advance, the subsequent dissolution of the single networks in DMF at elevated temperature is not hindered. As a result, the three-dimensional networks of SN-DA and SN-CON split into oligomers or nanogels, which can be further mixed with each other on a molecular level. In this context, the prerequisite for the creation of inter-component hydrogen bonds between –C–O–C– groups of fragmented SN-DA and –NH– groups of fragmented SN-CON is satisfied.
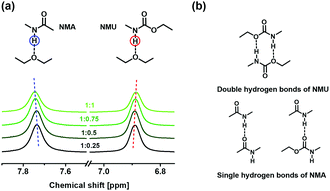
To validate this assumption, model experiments of small molecules are carried out with 1H-NMR spectroscopy. This is because the electron densities of proton accepters or donors would be affected in case hydrogen bonds are produced and the chemical shifts of the protons implicated in the hydrogen bonds are different at free and associated states. Moreover, the observed chemical shifts in a rapid-change system are correlated to the relative amounts of the free and associated protons.33,34 Accordingly, the mixture of isomolar NMA and NMU, which contain the two types of amido groups of SN-CON (Fig. 1), and ethyl ether that simulates the ether bonds of SN-DA (Fig. 1), are employed. When ethyl ether is incorporated into the mixture of NMA and NMU, the balance of free and associated protons included in the hydrogen bonds of NMA and NMU would be broken so long as new hydrogen bonds are built up between ethyl ether and NMA, and ethyl ether and NMU. Under the circumstances, the chemical shifts of the protons of amido groups would change as a function of the relative dosage of ethyl ether.
The 1H-NMR spectra in Fig. 4a show the peaks of the protons of amido groups involved in different hydrogen bonds. Their chemical shifts are dependent on the molar ratio of ethyl ether to NMA and NMU as expected. For NMU, they can form double hydrogen bonds on their own (Fig. 4b), which is stronger and more stable than the single hydrogen bonds. After the addition of ethyl ether, partial double hydrogen bonds of NMU are broken, accompanying the formation of single hydrogen bonds (C–O⋯H–N) because of the presence of excess proton accepter (–C–O–C–). The newborn single hydrogen bonds (C–O⋯H–N) are less stable, so that the concentration of free NMU tends to increase, leading to an upfield shift of δNMU. As for NMA, they can only form single hydrogen bonds at the beginning (Fig. 4b). The appearance of ethyl ether leads to more single hydrogen bonds. Unlike the case of NMU, the newborn hydrogen bonds are as stable as the initial state, and the concentration of free NMA has to slightly decrease as characterized by the downfield shift of δNMA. Therefore, the model experiments of small molecules prove that inter-component hydrogen bonds can be similarly formed between fragmented SN-DA and SN-CON containing ether and amino groups.
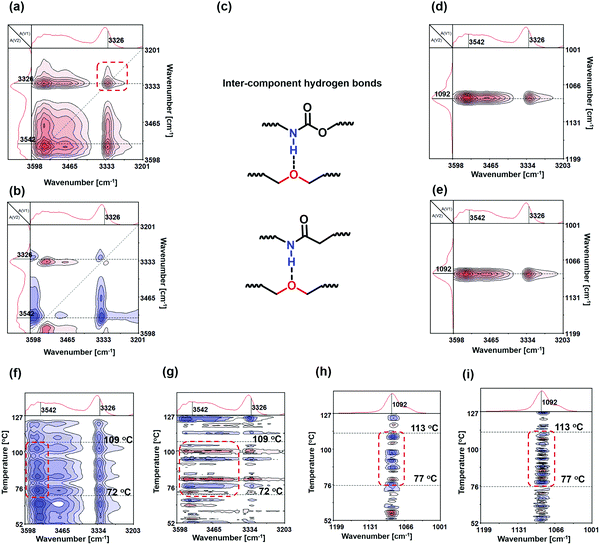
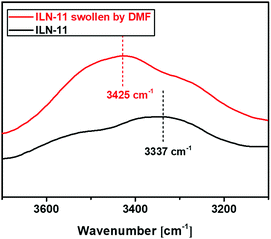
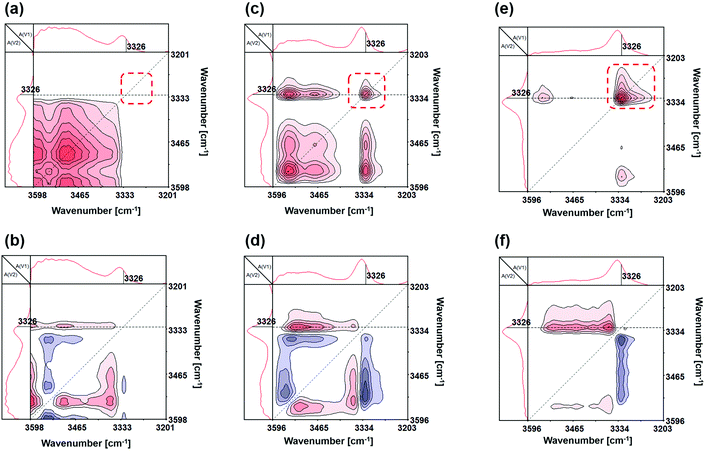
Accordingly, we can proceed to the study of the system containing the solutions of SN-DA and SN-CON by using temperature-dependent FTIR spectroscopy during heating. It is found from Fig. 5a and b that a new peak appears at [3326 cm−1, 3326 cm−1] after mixing of the solutions of the two single networks, which is absent on the spectra of SN-CON (Fig. 3c and d). To identify the origin of this peak, FTIR spectra of the finished interlocked networks ILN-11 in bulk state and swollen state are measured. As shown in Fig. 6, a board peak at 3337 cm−1 is observed for the dry ILN-11, while an absorption at 3425 cm−1 arises after being swollen in DMF. Ref. 35 indicates that the absorption of –NH– hydrogen bonded with ether emerges at around 3300 cm−1, which means that the peaks at 3337 cm−1 and 3425 cm−1 result from the intercomponent hydrogen bonds between SN-DA and SN-CON, and DMF-implicated hydrogen bonds, respectively. It can thus be deduced that inter-component hydrogen bonds (Fig. 5c) are created in the mixed solutions, as evidenced by the new peak at [3326 cm−1, 3326 cm−1] (Fig. 5a and b).
On the other hand, Fig. 5d and e depict that the crosspeaks at [3326 cm−1, 1092 cm−1], [∼3542 cm−1, 1092 cm−1], [3326 cm−1, 1092 cm−1] and [∼3542 cm−1, 1092 cm−1] are positive, suggesting the synergy of –NH– and –C–O–C–, and their identical directions of action. This proves the formation of intercomponent hydrogen bonds between –NH– of SN-CON and –C–O–C– of SN-DA from another angle. Furthermore, the response sequence order of the chemical groups under temperature perturbation can be deduced following Noda's rule. That is, peak υ1 responds prior to peak υ2 when the crosspeak [υ1, υ2] reflects the same signs in both synchronous and asynchronous spectra and vice versa.36,37 Accordingly, it is known from the results of Fig. 5d and e that the peak at 3326 cm−1 varies prior to that at 1092 cm−1, and the peak at 3524 cm−1 also varies prior to that at 1092 cm−1. This can be explained by the fact that the –NH– groups are attached to the dynamic C–ON bonds, while the –C–O–C– groups mainly reside at the segments of polyether amine (i.e. the main chain of SN-DA) far away from the reversible DA bonds. When the reversible bonds are stimulated by the rising temperature, –NH– groups must have higher mobility than –C–O–C– and take the initiative in constructing an inter-component with the latter.
By using Fig. 5f and h, the main temperature response intervals of the mixed solutions are found to be 72–109 °C (Fig. 5f and g) and 77–113 °C (Fig. 5h and i), which are roughly the same and obviously higher than those of the solutions of the single networks (i.e. 41–71 °C for SN-DA and 41–79 °C for SN-CON). The difference manifests that the inter-component hydrogen bonds of the interlocked networks are more stable than the intra-molecular ones of the two single networks. The new-born and more stable inter-component hydrogen bonds pull the macromolecular chains from different single networks together and avoid phase separation during formation of the interlocked networks.
As described in the Experimental section, the last step of producing the interlocked networks includes gradual removal of the cosolvent DMF and decreasing temperature. In this case, the fragmented chains from the single networks would further approach each other and re-crosslink via reversible reactions (i.e. forward DA reaction and recombination of homolytically cleft C–ON bonds). To monitor the corresponding variation of the inter-component hydrogen bonds, FTIR spectra of the mixed DMF solutions of SN-DA and SN-CON of different concentrations (that simulate evaporation of the co-solvent) are measured in the course of cooling from 90 °C to 70 °C. Fig. 7a and b demonstrate that when the co-solvent content is high, there is only a strong autopeak at [∼3554 cm−1, ∼3554 cm−1], as the contact probability of the fragments of the two single networks is relatively low and the intra-component hydrogen bonds with the co-solvent play the leading role. In the case of less DMF, there are autopeaks at [∼3554 cm−1, ∼3554 cm−1] and [3326 cm−1, 3326 cm−1] (Fig. 7c and d), suggesting that the inter-component hydrogen bonds start to be formed. When the co-solvent content is further reduced, only a single autopeak is perceived at [3226 cm−1, 3226 cm−1] (Fig. 7e and f). This means that the inter-component hydrogen bonds are more active at this time. In other words, with the reduction in the amount of the co-solvent and temperature, the main response peak changes from ∼3554 cm−1 to ∼3330 cm−1. Consequently, the inter-component hydrogen bonds between the fragmented single networks dominate and inhibit phase separation during construction of the interlocked networks.
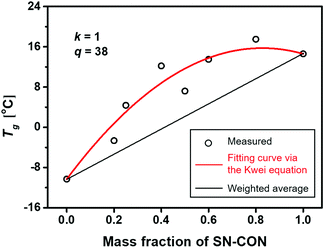
When the fragments of the counterpart single networks move closer together, as driven by the inter-component hydrogen bonds, their re-cosslinking via the re-connection of the reversible bonds occurs with cooling in the meantime. The inter-component hydrogen bonds should still exist in the resultant interlocked networks. Fig. 8 plots the glass transition temperatures, Tg, of the interlocked networks as a function of the mass fraction of SN-CON. The results coincide with those of our earlier explorations.9 The interlocked networks only possess single glass transition temperatures owing to the homogeneous microstructure without detectable microphase separation. Moreover, the Tg data of the interlocked networks positively deviate from the linear relationship between the Tg values of the single networks, reflecting the strong interaction between the components of the interlocked networks.
In fact, the measured Tg values of the interlocked networks well follow the fitting based on the Kwei equation38–40 (Fig. 8):
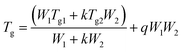 | (1) |
For purposes of characterizing the strength of the inter-component hydrogen bonds, temperature-dependent FTIR spectra of the ILN-11 bulk are measured from 140 °C to 70 °C (Fig. 9a). The broad peak of –NH– (3400–3200 cm−1) can be split into two secondary peaks (Fig. 9b), corresponding to free (3373 cm−1) and associated (3267 cm−1) –NH– groups, respectively.29 Assuming that the extinction coefficients are the same for the free and associated –NH–, the areas under the secondary peaks are used to calculate proportions of the associated –NH– groups and the association constants from:21,41
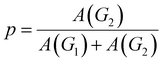 | (2) |
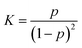 | (3) |
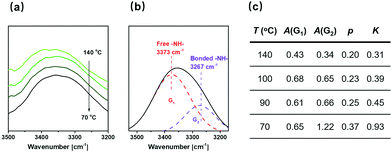 | ||
| Fig. 9 (a) Temperature-dependent FTIR spectra of ILN-11 film recorded from 140 to 70 °C. (b) Example of resolving of the –NH– peak measured at 70 °C. (c) Association constants of –NH– under different temperatures. Note: Calibration of the peak areas based on the ratio of absorptivity coefficient of free –NH– to associated –NH– (∼1/3.242) is conducted when calculating viaeqn (2). | ||
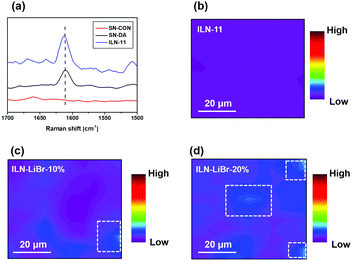
The above results manifest that abundant but weak hydrogen bonds are allowed to be formed between SN-DA and SN-CON during the preparation of the interlocked networks. The resultant benefit of phase separation suppression brought by the inter-component hydrogen bonds is further evaluated by elimination of the hydrogen bonds to certain degrees through introducing different contents of LiBr.24,44 Considering that the characteristic absorption of benzene rings at 1610 cm−1 on the Raman spectrum SN-DA45 is absent on the spectrum of SN-CON (Fig. 10a), the very peak can serve as the mark of SN-DA in the interlocked networks. Accordingly, distributions of benzene rings on the surfaces of ILN-11 containing different amounts of LiBr are detected using a micro-Raman mapping technique. Fig. 10b indicates that SN-DA is uniformly dispersed in ILN-11, but the SN-DA rich domains become more and more obvious with increasing loading of LiBr (Fig. 10c and d). The negative examples indicate the importance of the inter-component hydrogen bonds.
Conclusions
The critical effect of inter-component hydrogen bonds on the formation of interlocked polymer networks from two single networks (SN-DA and SN-CON) respectively containing reversible DA bonds and C–ON bonds is carefully studied. As illustrated in Fig. 11, when the single networks are separately mixed with the co-solvent (DMF), the intra-molecular hydrogen bonds are broken with an increase in temperature, followed by the disconnection of the reversible bonds. Both actions contribute to the dissolution of the single networks. Subsequently, the DMF solution of the fragmented SN-DA and that of the fragmented SN-C–ON are blended, and the –NH– groups of SN-CON approach the –C–O–C– groups of SN-DA generating inter-component hydrogen bonds. Because the inter-component hydrogen bonds are more stable than the intra-molecular hydrogen bonds in the single networks, the fragments derived from the counterpart single networks are allowed to preferentially hold together, and then the reversible crosslinkages are re-constructed during cooling while tangling with each other. The possible phase separation is thus prevented in the resultant interlocked networks no matter whether the parent single networks are miscible or not.In view of molecular design, the present work further reveals that the number of the groups that can form inter-component hydrogen bonds should be large, but the strength of the inter-component hydrogen bonds should not be too high. Otherwise, self-assembling of the molecular chains would lead to unwanted phase separation.20–22
Author contributions
M. R. F. performed the experiments. M. R. F. and Y. Y, analyzed the experimental data. M. Z. R. and M. Q. Z. conceived and supervised the project. All authors contributed to the writing of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the support from the Natural Science Foundation of China (Grants: 52033011, 51773229 and 51873235). The permission of using the software 2DIR by Professor Tao Zhou of Sichuan University, China, is greatly appreciated.Notes and references
- J. L. Qiao, Elastomeric nano-particle and its applications in polymer modifications, Adv. Ind. Eng. Polym. Res., 2020, 3, 47–59 Search PubMed.
- P. J. Flory, Thermodynamics of high polymer solutions, J. Chem. Phys., 1941, 9, 660–661 CrossRef CAS.
- M. L. Huggins, Solutions of long chain compounds, J. Chem. Phys., 1941, 9, 440 CrossRef CAS.
- G. Morales, R. D. de León, P. Acuña, R. F. Flores and A. M. Robels, Improved toughness in HIPS obtained from different styrene/butadiene-graded block copolymers through modification of the polydispersity index of the PS block, Polym. Eng. Sci., 2006, 46, 1333–1341 CrossRef CAS.
- S. H. Deng, H. W. Bai, Z. W. Liu, Q. Zhang and Q. Fu, Toward supertough and heat-resistant stereocomplex-type polylactide/elastomer blends with impressive melt stability via in situ formation of graft copolymer during one-pot reactive melt blending, Macromolecules, 2019, 52, 1718–1730 CrossRef CAS.
- K. Masutani and Y. Kimura, Macromolecular design of specialty polylactides by means of controlled copolymerization and stereocomplexation, Polym. Int., 2017, 66, 260–276 CrossRef CAS.
- A. Taguet, P. Cassagnau and J.-M. Lopez-Cuesta, Structuration, selective dispersion and compatibilizing effect of (nano)fillers in polymer blends, Prog. Polym. Sci., 2014, 39, 1526–1563 CrossRef CAS.
- K. Klempner, L. H. Sperling and L. A. Utracki, Interpenetrating Polymer Networks, American Chemical Society, Washington DC, 1994 Search PubMed.
- Y. You, W. L. Peng, P. Xie, M. Z. Rong, M. Q. Zhang and D. Liu, Topological rearrangement-derived homogeneous polymer networks capable of reversibly interlocking: From phantom to reality and beyond, Mater. Today, 2020, 33, 45–55 CrossRef CAS.
- W. L. Peng, Y. You, P. Xie, M. Z. Rong and M. Q. Zhang, Adaptable interlocking macromolecular networks with homogeneous architecture made from immiscible single networks, Macromolecules, 2020, 53, 584–593 CrossRef CAS.
- W. L. Peng, Z. P. Zhang, M. Z. Rong and M. Q. Zhang, Reversibly interlocked macromolecule networks with enhanced mechanical properties and wide pH range of underwater self-healability, ACS Appl. Mater. Interfaces, 2020, 12, 27614–27624 CrossRef CAS.
- Y. You, M. Z. Rong and M. Q. Zhang, Adaptable reversibly interlocked networks from immiscible polymers enhanced by hierarchy-induced multilevel energy consumption mechanisms, Macromolecules, 2021, 54, 4802–4815 CrossRef CAS.
- K. Dean and W. D. Cook, Effect of curing sequence on the photopolymerization and thermal curing kinetics of dimethacrylate/epoxy interpenetrating polymer networks, Macromolecules, 2002, 35, 7942–7954 CrossRef CAS.
- Y. He, B. Zhu and Y. Inoue, Hydrogen bonds in polymer blends, Prog. Polym. Sci., 2004, 29, 1021–1051 CrossRef CAS.
- Y. H. Lin, S. B. Darling, M. P. Nikiforov, J. Strzalka and R. Verduzco, Supramolecular conjugated block copolymers, Macromolecules, 2012, 45, 6571–6579 CrossRef CAS.
- X. H. Chang, C. L. Ma, G. R. Shan, Y. Z. Bao and P. J. Pan, Poly(lactic acid)/poly(ethylene glycol) supramolecular diblock copolymers based on three-fold complementary hydrogen bonds: Synthesis, micellization, and stimuli responsivity, Polymer, 2016, 90, 122–131 CrossRef CAS.
- M. Wu, Q. Y. Peng, L. B. Han and H. B. Zeng, Self-healing hydrogels and underlying reversible intermolecular interactions, Chinese, J. Polym. Sci., 2021, 39, 1246–1261 CAS.
- X. Huang, D. Lv, L. Q. Ai, S. H. Cheng and X. Yao, Aggregate engineering in supramolecular polymers via extensive non-covalent networks, Chinese, J. Polym. Sci., 2021, 39, 1310–1318 CAS.
- H. Y. Jin, J. K. Dou, J. Y. Xu, X. Y. Huang and D. Y. Chen, Prohibition of interchain cross-linking by h-bonding grafting of the polymer chain, Acta Polym. Sin., 2020, 51, 1021–2018 CAS.
- N. Hosono, M. A. J. Gilissen, Y. C. Li, S. S. Sheiko, A. R. A. Palmans and E. W. Meijer, Orthogonal self-assembly in folding block copolymers, J. Am. Chem. Soc., 2013, 135, 501–510 CrossRef CAS PubMed.
- R. Agnaou, M. Capelot, S. Tencé-Girault, F. Tournilhac and L. Leibler, Supramolecular thermoplastic with 0.5 Pa·s melt viscosity, J. Am. Chem. Soc., 2014, 136, 11268–11271 CrossRef CAS PubMed.
- N. E. Botterhuis, D. J. M. van Beek, G. M. L. van Gemert, A. W. Bosman and R. P. Sijbesma, Self-assembly and morphology of polydimethylsiloxane supramolecular thermoplastic elastomers, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3877–3885 CrossRef CAS.
- L. Hou, K. Feng, P. Y. Wu and H. Gao, Investigation of water diffusion process in ethyl cellulose-based films by attenuated total reflectance Fourier transform infrared spectroscopy and two-dimensional correlation analysis, Cellulose, 2014, 21, 4009–4017 CrossRef CAS.
- L. F. Fan, M. Z. Rong, M. Q. Zhang and X. D. Chen, A very simple strategy for preparing external stress-free two-way shape memory polymers by making use of hydrogen bonds, Macromol. Rapid Commun., 2018, 39, e1700714 CrossRef PubMed.
- S. T. Sun and P. Y. Wu, Spectral insights into microdynamics of thermoresponsive polymers from the perspective of two-dimensional correlation spectroscopy, Chin. J. Polym. Sci., 2017, 35, 700–712 CrossRef CAS.
- S. Morita, H. Shinzawa, I. Noda and Y. Ozaki, Perturbation-correlation moving-window two-dimensional correlation spectroscopy, Appl. Spectrosc., 2006, 60, 398–406 CrossRef CAS PubMed.
- Y. Peng, P. Y. Wu and Y. L. Yang, Two-dimensional infrared correlation spectroscopy as a probe of sequential events in the diffusion process of water in poly(ε-caprolactone), J. Chem. Phys., 2003, 119, 8075–8079 CrossRef CAS.
- L. Salmén and E. Bergström, Cellulose structural arrangement in relation to spectral changes in tensile loading FTIR, Cellulose, 2009, 16, 975–982 CrossRef.
- E. Yılgör, İ. Yılgör and E. Yurtsever, Hydrogen bonding and polyurethane morphology. I. Quantum mechanical calculations of hydrogen bond energies and vibrational spectroscopy of model compounds, Polymer, 2002, 43, 6551–6559 CrossRef.
- B. Yang, H. Z. Lang, Z. Liu, S. H. Wang, Z. W. Men and C. L. Sun, Three stages of hydrogen bonding network in DMF-water binary solution, J. Mol. Liq., 2021, 324, 114996 CrossRef CAS.
- B. J. Sun, Y. N. Lin and P. Y. Wu, Structure analysis of poly(N-isopropylacrylamide) using near-infrared spectroscopy and generalized two-dimensional correlation infrared spectroscopy, Appl. Spectrosc., 2007, 61, 765–771 CrossRef CAS PubMed.
- L. b. Luo, X. H. Ye, J. Yi, K. Li and X. Y. Liu, Heat resistance and dimensional stability of polyimide improved by inhibiting the dissociation of hydrogen bonds at high temperatures through crosslinking, Acta Polym. Sin., 2021, 52, 363–370 Search PubMed.
- F. H. Beijer, R. P. Sijbesma, J. A. J. M. Vekemans, E. W. Meijer, H. Kooijman and A. L. Spek, Hydrogen-bonded complexes of diaminopyridines and diaminotriazines: Opposite effect of acylation on complex stabilities, J. Org. Chem., 1996, 61, 6371–6380 CrossRef CAS PubMed.
- J. Cortese, C. Soulié-Ziakovic and L. Leibler, Binding and supramolecular organization of homo and heterotelechelic oligomers in solutions, Polym. Chem., 2014, 5, 116–125 RSC.
- E. Yılgör, E. Burgaz, E. Yurtsever and İ. Yılgör, Comparison of hydrogen bonding in polydimethylsiloxane and polyether based urethane and urea copolymers, Polymer, 2000, 41, 849–857 CrossRef.
- I. Noda, Advances in two-dimensional correlation spectroscopy, Vib. Spectrosc., 2004, 36, 143–165 CrossRef CAS.
- I. Noda, Cyclical asynchronicity in two-dimensional (2D) correlation spectroscopy, J. Mol. Struct., 2006, 799, 41–47 CrossRef CAS.
- T. K. Kwei, The Effect of hydrogen bonding on the glass transition temperatures of polymer mixtures, J. Polym. Sci., Polym. Lett. Ed., 1984, 22, 307–313 CrossRef CAS.
- S. W. Kuo and F. C. Chang, Studies of miscibility behavior and hydrogen bonding in blends of poly(vinylphenol) and poly(vinylpyrrolidone), Macromolecules, 2001, 34, 5224–5228 CrossRef CAS.
- Y. C. Su, S. W. Kuo, D. R. Yei, H. Y. Xu and F. C. Chang, Thermal properties and hydrogen bonding in polymer blend of polybenzoxazine/poly(N-vinyl-2-pyrrolidone), Polymer, 2003, 44, 2187–2191 CrossRef CAS.
- S. W. Kuo, C. F. Huang and F. C. Chang, Study of hydrogen-bonding strength in poly(e-caprolactone) blends by DSC and FTIR, J. Polym. Sci., Part B: Polym. Phys., 2001, 39, 1348–1359 CrossRef CAS.
- D. J. Skrovanek, P. C. Painter and M. M. Coleman, Hydrogen bonding in polymers. 2. Infrared temperature studies of nylon 11, Macromolecules, 1986, 19, 699–705 CrossRef CAS.
- S. W. Kuo, C. T. Lin, J. K. Chen, F. H. Ko, F. C. Chang and K. U. Jeong, Substituent-induced delocalization effects on hydrogen-bonding interaction in poly(N-phenyl methacrylamide) derivatives, Polymer, 2011, 52, 2600–2608 CrossRef CAS.
- N. Manin, M. C. da Silva, I. Zdravkovic, O. Eliseeva, A. Dyshin, O. Yasar, D. R. Salahub, A. M. Kolker, M. G. Kiselev and S. Yu, Noskov, LiCl solvation in N-methyl-acetamide (NMA) as a model for understanding Li+ binding to an amide plane, Phys. Chem. Chem. Phys., 2016, 18, 4191–4200 RSC.
- X. L. Lu, G. X. Fei, H. S. Xia and Y. Zhao, Ultrasound healable shape memory dynamic polymers, J. Mater. Chem. A, 2014, 2, 16051–16060 RSC.
| This journal is © the Partner Organisations 2022 |