 Open Access Article
Open Access ArticleAdvanced applications of cerium oxide based nanozymes in cancer
Na Fengab,
Ying Liu†
b,
Xianglin Daiab,
Yingying Wangb,
Qiong Guo *ab and
Qing Li
*ab and
Qing Li *ab
*ab
aDepartment of Molecular Pathology, Application Center for Precision Medicine, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450052, China. E-mail: 1515012032lq@sina.com
bCenter for Precision Medicine, Academy of Medical Sciences, Zhengzhou University, Zhengzhou 450001, China
First published on 10th January 2022
Abstract
Cerium oxide nanozymes have emerged as a new type of bio-antioxidants in recent years. CeO2 nanozymes possess enzyme mimetic activities with outstanding free radical scavenging activity, facile synthesis conditions, and excellent biocompatibility. Based on these extraordinary properties, use of CeO2 nanozymes has been demonstrated to be a highly versatile therapeutic method for many diseases, such as for inflammation, rheumatoid arthritis, hepatic ischemia-reperfusion injury and Alzheimer's disease. In addition to that, CeO2 nanozymes have been widely used in the diagnosis and treatment of cancer. Many examples can be found in the literature, such as magnetic resonance detection, tumour marker detection, chemotherapy, radiotherapy, photodynamic therapy (PDT), and photothermal therapy (PTT). This review systematically summarises the latest applications of CeO2-based nanozymes in cancer research and treatment. We believe that this paper will help develop value-added CeO2 nanozymes, offering great potential in the biotechnology industry and with great significance for the diagnosis and treatment of a wide range of malignancies.
Introduction
Enzymes are proteinaceous biomolecules with high specificity and high catalytic efficiency to their substrates. Natural enzymes may present some disadvantages, such as unstable properties, low biological content, high price, etc. However, with the rapid development of nanoscience, nanozymes have attracted the attention of many scholars and clinicians, as they can improve the stability of natural enzymes as well as reduce production costs.1 Cerium oxide nanoparticles (CeNPs) are considered a promising candidate in nanomedicine due to their redox regulation and enzyme-like activity.2 CeNPs have been shown to mimic a range of natural redox enzymes, including superoxide dismutase (SOD)3 and catalase (CAT),4 which remove detrimental reactive oxygen species (ROS) from the body.Cerium has two different oxidation states in nature, Ce3+ and Ce4+, and the enzymatic activity of CeO2-x scavenging ROS is thought to be due to the self-regeneration cycle of Ce3+/Ce4+ and the oxygen vacancy on the cerium oxide surface5 (Fig. 1). Most researchers considered that the antioxidant property of CeO2-x is closely related to Ce3+/Ce4+ redox cycling. The potential role of oxygen vacancies in the fast redox cycling of CeO2-x is a center of debate as well.6 X-ray photoelectron spectroscopy analysis suggested a reliable dependence of Ce3+/Ce4+ ratio on the size of ceria nanoparticles, which can reach as high as 44% for 3 nm nanoceria.3 This ratio changes as a result of ROS–nanoceria interaction suggesting the possibilities of the fast redox cycling to the judge of CeO2-x redox activities.
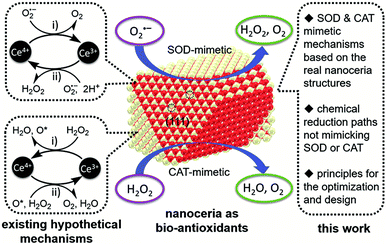 | ||
| Fig. 1 Schematic illustrating the bio-antioxidant activity of nanoceria (middle), mechanisms hypothesized for it by drawing an analogy with those of natural superoxide dismutase (SOD) and catalase (CAT). Reproduced from ref. 5 with permission from the Royal Society of Chemistry. | ||
It is widely recognized that cancer is a major worldwide public health problem with few effective treatment choices, poor prognosis, and high mortality rates.6 Nanotechnology has emerged as the latest approach to diagnose and treat cancer, and CeNPs are a great candidate as they exhibit an exceptional potential as a catalyst and antioxidant. Nanostructured metal oxides have a large surface area, good reactivity, high sensitivity, and specificity. Hence, they can be used to prepare nano-sensors to detect biomarkers, such as cancer-related proteins,7 ctDNA,8 etc. In particular, CeNP-based sensors present several advantages, such as good biocompatibility and high chemical stability. At present, resistance has become a serious challenge for anticancer therapies. In general, drug resistance mechanisms in tumours can be divided into three categories: inadequate pharmacokinetics, intrinsic factors of cancer cells, and tumour microenvironments (TMEs).9 Nanoparticles are specific and effective in delivering drugs to target cells, tissues or organs, reducing drug resistance and minimizing the risk of side effects.10 Furthermore, hypoxia is an essential factor in the formation of multidrug resistance, recurrence, and metastasis in solid tumours.11 Recent studies have shown that CeNPs with catalase activity can catalyze H2O2 to produce oxygen, improving the hypoxia of the TMEs to execute a synergistic anticancer effect. It is reported that CeNPs are protective as antioxidants in the neutral pH environment of normal cells, whereas they are toxic as pro-oxidants in the acidic pH environment of cancer cells.12 Therefore, CeNPs have great potential in cancer treatment as both cytotoxic and protective agents. In addition, there is growing evidence that pro-oxidants are increasingly seen as potential chemotherapeutic drugs due to the high base levels of ROS in cancer cells13–15 (Fig. 2). In conclusion, CeNPs have a broad application prospect in the diagnosis and treatment of cancer.
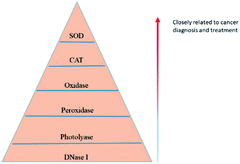 | ||
| Fig. 2 Summarization of the discovered enzyme mimetic activities of CeNPs and the correlation with cancer diagnosis and treatment. | ||
On this account, we elucidate the application of CeNPs in cancer diagnosis and treatment. By sharing a brief personal overview about the challenges and perspectives in CeNPs-based biomedical application, we expect that this review will open new research orientations for CeNPs-related theranostics.
Application of nanoparticles in cancer diagnosis
Early diagnosis of cancer offers the best opportunity for appropriate therapeutic intervention strategies and significantly increases the success rate of treatment and recovery. Imaging, haematology pathology and genetics are the main techniques used in oncology, and the specificity and sensitivity of the examination tools are vital for accurate cancer detection. Abundant oxygen vacancy on the surface of CeNPs is reported to be suitable for use as a nanometer contrast agent for imaging examination.16 CeNPs can significantly immobilize enzymes or proteins on the surface of biological electrodes and improve the electron transfer efficiency between electrodes and electrode surface modified materials. Due to their biocompatibility, high chemical stability, excellent electron transfer capability, large surface area, strong adsorption capacity and catalytic performance, CeNPs can be used to prepare the substrate for immune biosensors and electrochemical DNA biosensors. It has been reported that some researchers have used CeNPs-based biosensors to detect cancer biomarkers. For example, CeNPs can be used as a luminescent material to detect carcinoembryonic antigen (CEA),17 improving the sensitivity of the immune sensor to detect tumour-specific growth factor (TSGF) through its catalytic properties18 and be used for electrochemical biosensor detection of the BCR/ABL fusion gene.19 According to the above illustrations, CeNPs are widely used in medicine, and they deserve further research and development. Table 1 summarises some examples of CeNPs applications in the detection of tumour markers.| Markers | Features | Nanoparticle | Years (reference) | Broad linear range | Detection limit |
|---|---|---|---|---|---|
| CEA | Electrochemical immunosensor | GO/MWCNTs-COOH/Au@CeO2 | 2015 (ref. 17) | 0.05–100 ng mL−1 | 0.02 ng mL−1 |
| TSGF | Electrochemical immunosensor | Ab2-Ag@CeO2 | 2016 (ref. 18) | 0.500–100 pg mL−1 | 0.2 pg mL−1 |
| SCCA | Electrochemical immunosensor | Co3O4@CeO2–Au@Pt | 2017 (ref. 25) | 100 fg mL−1 to 80 ng mL−1 | 33 fg mL−1 |
| Cyfra-21-1 | Electrochemical immunosensor | ncCeO2-RGO | 2018 (ref. 27) | 0.625 pg mL−1 to 0.01 ng mL−1 | 0.625 pg mL−1 |
| CA19-9 | Electrochemical immunosensor | CeO2/FeOx@mC | 2019 (ref. 29) | 0.1–10 U mL−1 | 10 μU mL−1 |
Currently, diffusion-weighted imaging combined with dynamic contrast-enhanced perfusion-weighted imaging (DWI/DCE-PWI) technology is used to detect cancer microvessel permeability and water diffusion to assess the degree of cancer malignancy. Before DCE-PWI examination, it is necessary to use gadolinium (Gd) chelate to improve the sensitivity of DWI. Still, the Gd–DTPA commonly used in clinical practice can cause MRI artefacts and is not sensitive enough. Chulun Shao et al. developed gadolinium doped (CeO2:Gd) CeO2 nanoparticles as contrast agents.16 The lattice oxygen vacancies on the surface of CeO2-x not only combine with a large number of water molecules to increase the R1 value but also limit the diffusion motion of water molecules to further enhance the DWI signal and high-sensitivity detection on the tumour vascular microenvironment.16 Therefore, CeNPs provide a new method for the design of magnetic resonance contrast agents.
Tumour markers have crucial practical value in screening, diagnosis, and efficacy evaluation.20,21 Carcinoembryonic antigen (CEA) is one of the most well-known tumour markers as it is overexpressed in many cancers, especially colorectal cancer.22 Xuehui Pang et al.17 synthesized a chemiluminescent immunosensor based on GO/MWCNTs-COOH/Au–CeO2-x nanocomposite. The immunosensor showed satisfactory performance in CEA analysis of human serum samples, demonstrating high sensitivity and excellent repeatability. Tumour specific growth factor (TSGF) is a novel tumour marker, and it was reported to be significantly increased in the early stage of malignant tumours.23 Siqi Yu et al. modified TSGF antigen and AB2-Ag@CeO2-x onto the electrode surface and designed a super-sensitive electrochemical immune sensor for the detection of TSGF.18 Under optimal conditions, the immune sensor has a wide linear range, low detection limit, good repeatability, selectivity, and stability. As an early diagnosis marker of various cancers, squamous cell carcinoma antigen (SCCA) has a remarkable specificity of up to 90–96%. SCCA is mainly used as a tumour marker to diagnose squamous cell carcinoma, including cervical cancer.24 SCCA should be diluted before detection, so high-sensitivity immunoassay is of great significance for early detection of SCCA. Yueyun Li and colleagues made an ultrasensitive electrochemical immunosensor for quantitative detection of SCCA using Co3O4@CeO2–Au@Pt nanocomposite as enzyme-mimetic labels with low detection limit rate and wide linear range.25 Among various oral cancer biomarkers, cytokeratin fragment-21-1 (Cyfra-21-1) has vital clinical applications due to its high concentration in the saliva samples of the patients.26 Namrata Pachauri et al. used CeNPs cubes (ncCeO2)-reduced graphene oxide (RGO) based nanocomposite to detect Cyfra-21-1 (ref. 27) that showed improved sensitivity and detection compared with the previous work. The cancer marker carbohydrate antigen 19-9 (CA19-9) achieved the highest sensitivity and specificity in patients with pancreatic cancer.28 Minghua Wang et al. successfully developed an electrochemical immune sensor based on nanometer CeO2-x for sensitive detection of CA19-9.29 The immune sensor based on nanometer CeO2-x shows outstanding reproducibility, high selectivity, and stability. Human serum sample analysis results are satisfactory and have a broad application prospect in clinical tumour monitoring.
With the development of biomedical research on genetic diseases, DNA sequence detection has attracted more and more attention, especially in cancer diseases. Electrochemical methods have been widely used to detect DNA hybridisation due to their simplicity, low cost, and high sensitivity.30 Ke-jun Feng et al. developed an effective DNA fixation matrix based on the nanoparticle CeO2-x/chitosan composite membrane to manufacture colorectal cancer DNA biosensors.31 The biosensor is characterized by high detection sensitivity and wide linear range and can perfectly identify complementary target sequences and tetra-base mismatched sequences. Shenfeng Li et al. developed an effective DNA electrochemical biosensor for the detection of BCR/ABL based on gold nanoparticles (GNP) synthesized in situ on the surface of multi-walled carbon nanotubes (MWCNT), CeO2-x and Chits composite membranes.19 The detection of the BCR/ABL gene is of great significance for the early diagnosis, prognosis and assessment of chronic myelogenous leukaemia (CML) patients.32 This method has been successfully used to detect real PCR samples with favourable selectivity, stability, and reproducibility.
Application of nanoparticles in cancer treatment
Antitumour therapies include traditional surgery, chemotherapy, and radiation therapy, as well as emerging immunotherapies, targeted therapies, photodynamic therapy (PDT) and photothermal therapy (PTT). In terms of drug delivery systems, CeNPs with pharmacological potential can be used as nanocarriers. Studies have shown that CeNPs halt the invasion of tumor cells by preventing the formation of myofibroblasts, a key component of cancer progression.33 CeNPs can also cause the death of cancer cells by increasing the production of ROS.34 The mimetic activity of CeNPs such as SOD, catalase, DNase I, photolyase, oxidase and peroxidase endows the ability of CeNPs to regulate ROS levels, leading to their exploration for the enhancement of PDT and PTT. Moreover, the dual capabilities to act as an oxidant in cancer cells, yet antioxidant in normal cells, makes the role of CeNPs as an adjuvant for radiation therapy (RT) which will dramatically benefit the patient quality of life.33 It is reported that CeO2-x leads to DNA fragmentation by enhancing the production of ROS and ultimately leads to cell apoptosis through the p53-dependent mitochondrial signalling pathway.14 It is worth mentioning that CeNPs are protective as antioxidants in neutral pH environments of normal cells,12 whereas they are toxic as pro-oxidants in acidic pH environments of cancer cells. Therefore, CeNPs can be used as cytotoxic drugs and protective agents, which has great potential in cancer treatment. To summarise, CeNPs have many functions in tumour therapy and has significant application value. Table 2 summarises some examples of CeNPs application in oncology.| Treatment | Nanoparticle | Nanoparticle size (nm) | Years (reference) | Mainly role |
|---|---|---|---|---|
| PDT + chemotherapy | MSN-HP-DOX@CeO2 | 100 | 2016 (ref. 39) | Nanocarrier |
| PDT + chemotherapy | PPCNPs-Ce6/FA | 36.1 | 2019 (ref. 40) | Nanocarrier |
| PDT | HA@CQDs-Ce6 | 3–5 | 2018 (ref. 41) | Radiation-protective and nanocarriers |
| PDT + PTT | Bi2S3@Ce6–CeO2NC | 280–340 | 2020 (ref. 42) | Produce oxygen and nanocarrier |
| PTT + chemotherapy | Ru@CeO2-RBT/Res-DPEG | 78 | 2020 (ref. 43) | Produce oxygen and nanocarrier |
| Radiotherapy | NGA-CNPs | 3–5 | 2019 (ref. 44) | Radiation-protective agent and nanocarrier |
| Radiotherapy | CuS@CeO2 | 3–5 | 2020 (ref. 45) | ROS scavengers and nanocarrier |
| Chemotherapy | DNR-CeO2/TiO2NPs | 9.12 | 2020 (ref. 46) | Nanocarrier |
| Chemotherapy | C-TherMods | <25 | 2018 (ref. 47) | Synergistic anticancer |
Chemotherapy
Chemotherapy remains one of the most critical cancer treatments, but its effectiveness is often compromised by the rising incidence of multidrug resistance (MDR).35 The hallmarks of the TME are hypoxia, high H2O2 concentration, glucose deficiency and low pH value, all of these directly affecting the chemotherapy effect and outcome of the treatment.36 In addition, chemotherapy has various well-known side effects that affect the patient's lifestyle, such as hair loss, bone marrow suppression, mucositis, nausea, and vomiting. Therefore, a compromise between killing the cancer cells and minimising the side effects on the system is the critical goals to be achieved. Among carriers for chemotherapy, CeNPs have been characterised by good biocompatibility, low cytotoxicity, and unique catalytic properties that make it a preferable choice for drug loading.Wu et al. found that CeNPs can act as a chemical sensitiser because the pre-treated cells enhance the toxicity of the chemotherapy drug doxorubicin (DOX).37 The intrinsic mechanism is that ROS produced by the CeNPs reduces the mitochondrial membrane potential (MMP), leading to the disruption of the mitochondrial function to inhibit chemotherapeutic drug efflux, and CeNPs consumes GSH to reduce DOX detoxification. In addition, CeNPs are more chemically sensitive to cancer cells than to normal cells, reducing the side effects due to the loss of healthy tissue. Xu et al. developed DOX loaded hollow CeNPs coated with polydopamine (PDA) and ammonium bicarbonate (NH4HCO3) (Fig. 3). Under laser irradiation, the PDA shell would be destroyed due to the hyperthermia effect induced by the conversion of light into heat with PDA. The DOX and CeNPs would be released after PDA destruction. On the one hand, CeNPs could enhance the chemotherapy effect of DOX, as discussed above. On the other hand, CeNPs could also degrade hydrogen peroxide into hydroxyl radical to elicit chemodynamic therapy.38 Besides, another research used cerium doped titanium dioxide nanoparticles (CeO2-x/TiO2NPs) to form DNR- CeO2-x/TiO2NPs complexes as a drug delivery system (DDS) for daunorubicin (DNR). Test results in B lymphocyte cultures showed that this DDS was superior to TiO2 NPs alone, and it had good biocompatibility and load efficiency, increasing drug accumulation in cells.46 Pro-oxidants are increasingly seen as potential chemotherapy drugs because of the high base levels of reactive oxygen species (ROS) in cancer cells. It is well known that the normal tissue microenvironment is neutral and the TMEs is acidic, and CeNPs plays a pro-oxidant role in the acidic microenvironment.12 Studies have shown that CeO2-x induces apoptosis in human colorectal cancer cell lines but has no effect on normal cells.14 Christos Tapeinos et al. developed a pH-sensitive drug delivery system consisting of calcium carbonate and Type I collagen, loaded with CeNPs and the anticancer drug adriamycin. This system showed an enhanced chemotherapeutic effect on osteosarcoma SaOS-2 cells and reduced toxicity on cardiac myoblasts H9C2 compared to adriamycin alone.47 At a pH of 6.0, the synergistic effect of the oxidant CeNPs and encapsulated adriamycin resulted in almost 100% cell death, even at the lowest concentrations of the drug. Ying Zhang et al. coated dithio-polydopamine (PDS) on the porous CeO2-x nanorod (CeONPs) surface to prepare a new drug delivery carrier capable of carrying DOX, which was then coupled to the surface of the nanorod using a lactose derivative (lac-NH2).48 pH is one of the few factors proved to drive whether CeNPs act as oxidants or antioxidants. The antioxidant abilities of CeNPs have also resulted in the exploration of these particles as a promising therapy for cancer. Therefore, pH is a vital factor for CeNPs' appearance as nontoxic in normal cells due to various pH in normal cells compared to tumour cells.49 In the specific microenvironment of cancer cells, such DDS can be used as nanocarriers and degrade PDS through high GSH concentration and low pH to expose cytotoxic CeONRs to cancer cells and have a synergistic anticancer effect on malignant cells.48
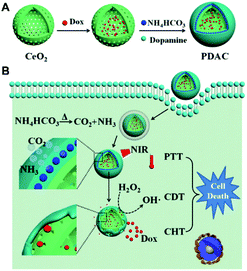 | ||
| Fig. 3 (A) Schematic illustrating the design and synthesis of PDAC NPs for tumor therapy. The preparation process of PDAC NPs. (B) The synergic effect among PTT, chemodynamic therapy and chemotherapy of cancer: under NIR laser irradiation, PDA shell firstly generates the photothermal performance, subsequently leading to the shell collapse and exposed CeO2-x surface, which can catalyze H2O2 into hydroxyl radical for chemodynamic therapy, meanwhile the leakage of PDA shell can further release DOX to present chemotherapy against cancer. Reproduced from ref. 38 with permission from Springer Nature. | ||
Notably, the effect of nanoparticles depends on the dose, application time, nanometer diameter, cell type, intracellular environment, etc50,51. Furthermore, the toxicity of CeNPs is dose-dependent and time-dependent in cancer cells.34 However, studies have shown that high doses of CeNPs may promote the proliferation of hepatocellular carcinoma cells in a dose-dependent manner.50 Therefore, the selection of CeNPs in cancer treatment should consider various circumstances.
Radiotherapy
Radiotherapy (RT) is a clinically effective treatment strategy for malignancies, with more than half of all tumours suitable for radiotherapy.52 The main mechanism of radiotherapy is DNA damage, including direct ionization damage and indirect induction by stimulating ROS production.53 However, radiation resistance and damage to normal tissue are the cardinal obstacles to be overcome. Hypoxia-induced resistance to radiotherapy is a pivotal factor in radiation resistance.54 CeNPs can catalyse endogenous H2O2 to O2 in malignant tissues, thus re-modelling the anoxic microenvironment to an RT-sensitive environment. Therefore, Zhou et al. designed a multiple radiosensitisation strategy to resist the main causes of radioresistance, such as hypoxia in the tumour microenvironment and upregulation of DNA repair proteins.55 By anchoring CeNPs on the surface of a novel 2D graphdiyne (GDY), the fabricated GDY-CeNPs could exhibit desirable CAT activity to catalyse H2O2 into O2 to alleviate hypoxia. Simultaneously, through encapsulation of miR181a in the nanosystem, the sensitivity of RT could be enhanced by targeting the RAD17 and Chk2 pathways (Fig. 4).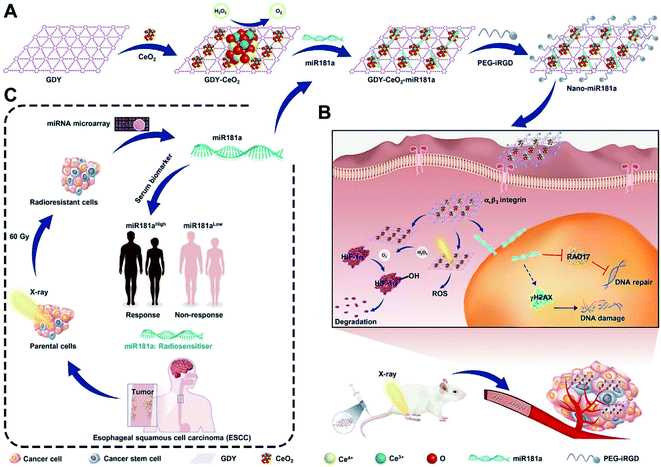 | ||
| Fig. 4 (A) Schematic illustration of the successive synthetic procedures of the multiple radiosensitizer with catalase activity, (B) enhancing intracellular radiation deposition, (C) and RNA interference for the highly efficient radiotherapy of ESCC. Reproduced from ref. 55 with permission from John Wiley and Sons. | ||
In addition, by regulating the number of antioxidant enzymes and ROS, CeNPs have been reported to provide nearly 99% protection against radiation-induced cell death in normal cells but not in tumour cells.56,57 Studies have shown that CeNPs can protect the gastrointestinal epithelium from radiation damage56 and prevent radiation-induced pneumonia57 or dermatitis.58 Therefore, CeNPs are an ideal material for radiosensitiser and radioprotectant. The experimental results suggested a striking effect of these nanocomposites in overcoming hypoxia-induced radioresistance and in the therapy of oesophageal squamous cell carcinoma (ESCC) both in vivo and in vitro.55 Further studies have shown the use of CeNPs before RT can significantly enhance the apoptosis of cancer cells and inhibit the growth of pancreatic tumours in mice without damaging healthy tissues.59 A novel radiation sensitiser (NGA-CNPs) was synthesised by coating ceria nanoparticles (CNPs) with the anticancer drug neogambogic acid (NGA).44 Compared with RT alone, NGA and CNPs, the combined application of NGA-CNPs and RT has better clinical effects and reduces damage to surrounding tissues. Wei Jiang et al. synthesised spindle-shaped CuS@CeO2-x core–shell nanoparticles that could be used in combination with PTT/RT therapy for hypoxic tumours.45 CeO2-x alters the anoxic tumour environment, while CuS nanoparticles encapsulated in CeO2-x undergo stable release and deep tissue penetration. In vitro and in vivo studies have shown that CuS@CeO2-x not only reduces the dose of RT but, more importantly, enables the entire tumour to be treated without recurrence. In short, as a kind of auxiliary material for RT, CeNPs have a broad application prospect in clinical treatment.
Photodynamic therapy and photothermal therapy
As a novel strategy for cancer treatment, PDT involves light, photosensitisers (PSs), and oxygen and has excellent potential in treating drug-resistant malignancies. The mechanism of PDT is that the PSs accumulated in tumour cells are stimulated by light at appropriate wavelengths and transfer photon energy to biological substrates to produce ROS, which destroys biomolecules and eventually causes the destruction and death of tumour cells.60,61 The number of PSs in tumours is directly related to ROS generation; thus, the effective localization of PSs in tumours is crucial. Some limitations of clinical application of PDT include low PSs delivery efficiency, poor targeting, and hypoxic environment in tumours. Due to its spontaneous circulation between Ce3+ and Ce4+ in the redox reaction, CeO2-x can react with the high level of endogenous H2O2 in tumour cells to generate H2O and O2 simultaneously.62,63 At the same time, the increased surface area, selective targeting, and long cycle time of CeNPs increase the effectiveness of PSs delivery, thus improving the utility of PDT. Porphyrin derivatives and chloramphenicol e6 (Ce6) are typical PSs of PDT,64,65 but their poor water solubility and low targeting ability severely limit their applications in vivo. Researchers have designed a response system based on CeNPs coated DOX and photosensitised hemorporphyrin (HP) double-loaded mesoporous silica nanoparticles (MSN).39 After entering into cancer cells, the high concentration of intracellular glutathione and low pH environment will reduce CeNPs to cerium ions. With the degradation of CeNPs and conformational changes of HP under light irradiation, preloaded DOX is released. This change exacerbates its cytotoxicity on cancer cells (Fig. 5). Hong Li et al. synthesised a novel drug delivery platform based on chlorin e6 (Ce6)/folic acid (FA)-loaded branched polyethylenimine-PEGylation CeNPs (PPCNPs-Ce6/FA) to overcome drug-resistant breast cancer via targeted PDT.40 The nanosystem promotes cell uptake of PSs with the assistance of CeNPs and FA, resulting in better tumour inhibition. A recent report41 illustrated that H2O2 assisted HA@ceria nanoquantum dots (HA@CQDs-Ce6/H2O2), a unique particle synthesised with hyaluronic acid (HA) and CeNPs on its surface, increased the efficacy of PDT due to the targeted action between HA and cancer cells66 and the ability of CeNPs to catalyse H2O2 to produce more O2 (ref. 41). In conclusion, various surface modifications based on the CeNPs technology improve adjuvant therapies for PDT and constitute a leap forward to their foreseeable clinical application.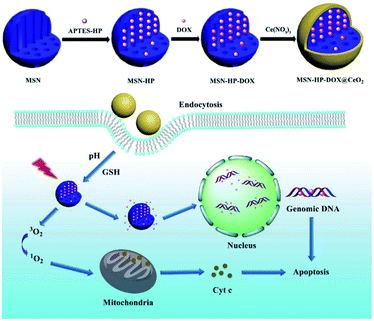 | ||
| Fig. 5 Schematic demonstration of synthetic and working protocol for triple-stimuli-responsive drug delivery system. DOX was assembled into MSN-HP and then coated with CeO2 to form the triple-stimuli-responsive drug delivery system. After endocytosed by cells, the system could respond under the intracellular environment and ultimately, targeted the nucleus and mitochondria to induce apoptosis of cancer cells. Reproduced from ref. 39 with permission from Springer Nature. | ||
PTT is a highly effective and non-invasive cancer therapy that benefits from the photothermal effects of photothermal transducers (PTA) to collect energy from light and convert it into heat. Thereby, the heat generation raises the ambient temperature and triggering cancer cell death.67 The cardinal problem with PTT lies in the limited depth of light penetration, and recurrent tumours often occur at the edge of the tumour beyond the limit of laser penetration.68 Other disadvantages include the relatively low efficiency of PTA delivery in tumours, excessive heat leading to unnecessary damage to healthy tissue, and resistance to PTT due to overexpression of heat shock proteins in some cancers. Thus, current strategies have focused on combining PTT with other cancer therapies, such as PDT.69,70 On the one hand, PTT-induced heating can improve blood flow and O2 content, providing more O2 for PDT; on the other hand, PDT increases the sensitivity of cancer cells to heat. However, PTT/PDT alone does not kill cancer cells completely because the heat and O2 are not evenly distributed within the tumour. Therefore, it is critical to design a nanosystem with an O2 supply function in situ. Lingwan Zeng et al. synthesised a nanosystem named Bi2S3@Ce6–CeO2-x for PTT/PDT treatment.42 CeNPs can react with excessive H2O2 in the TMEs, producing a large amount of O2 to improve the hypoxia condition and thus enhance the efficacy of PTT/PDT. According to experimental results in vitro and in vivo, PTT/PDT therapy of Bi2S3@Ce6–CeO2-x NC has a synergistic therapeutic effect, therefore being better than any single treatment. Another example was reported by Zeng et al., in which recycled CeO2-x catalase nanozymes and indocyanine green (ICG) were co-loaded into hyaluronic acid nanovesicle to alleviate the hypoxic TMEs and realise the tumour-targeted PTT/PDT.71 The in vivo tests indicated that CeO2-x could improve the outcomes of PDT by the recycling of cerium valence state, in combination with the PTT effect induced by ICG, providing a desirable therapeutic efficacy in tumour-bearing mice (Fig. 6). Moreover, it is worth noting that PTT, as a non-invasive treatment mode, can also be used cooperatively with chemotherapy to execute a better treatment effect.72 Studies packed Ru@CeO2YSNs with the anticancer ruthenium complex (RBT) and resveratrol (Res) and used REG to construct a double-layered structure to form a dual drug delivery system Ru@CeO2-RBT/Res-DPEG.43 The system can catalyse endogenous H2O2 to produce oxygen, realizing in situ oxygen supply and enhancing chemotherapy and PTT for colorectal cancer. In vitro research has found that Ru@CeO2-RBT/Res-DPEG has an ideal tissue penetration depth and anticancer effect and inhibits the metastasis and recurrence of colorectal cancer.
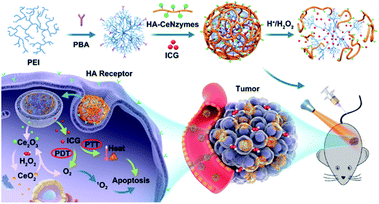 | ||
| Fig. 6 Schematic illustration of in vivo regenerable cerium oxide nanozyme-loaded pH/H2O2-responsive nanovesicle for tumor-targeted PDT and PTT (PEI, poly(ethylene imine); PBA, 4-carboxylphenylboronic acid pinacol ester; HA, hyaluronate; CeNzymes, CeO2 nanozyme). PDT and PTT were both marked with red ellipses. This figure was reproduced from ref. 71 with permission from the American Chemical Society. | ||
Conclusions
In this review, we intensely focus on the application and mechanism of CeNPs in cancer diagnosis and treatment. CeNPs are a great tool as they show excellent potential in biological diagnosis, drug delivery and oncology. The effect of CeNPs depends on the dose, application time, nanometer diameter, cell type and intracellular environment, to cite a few. Furthermore, the toxicity of CeNPs is also dose-dependent and time-dependent in cancer cells, as some studies have shown that high doses of CeNPs may promote the proliferation of hepatocellular carcinoma cells in a dose-dependent manner. Therefore, a compromise is needed to destroy the malignant tissue without affecting the healthy tissue around it. CeNPs face an important issue in conducting clinical trials to explore effectiveness and toxicity, so therefore the selection of CeNPs in cancer treatment should take into account the various circumstances here reviewed. In this regard, extensive research is needed to further investigate the absorption, distribution, metabolism, and related immune responses of nanoparticles. This guide provides advice for the application of biological antioxidant nanomaterials to cancer treatment.Author contributions
Na Feng and Ying Liu: literature collection and evaluation, and manuscript preparation; Xianglin Dai and Yingying Wang: figure legend preparation, references insertion, and graphic abstract preparation; Qing Li and Qiong Guo: concept development idea generation, and manuscript editing and revision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We greatly acknowledge the financial support from the National Natural Science Foundation of China (No. 81901882), China postdoctoral science foundation (2019M663062), and Education Department of Henan Province (20A430026).Notes and references
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- A. Asati, S. Santra, C. Kaittanis, S. Nath and J. Perez, Angew. Chem. Int. Ed. Engl., 2009, 48, 2308–2312 CrossRef CAS PubMed.
- C. Korsvik, S. Patil, S. Seal and W. Self, Chem. Commun., 2007, 1056–1058 RSC.
- T. Pirmohamed, J. M. Dowding, S. Singh, B. Wasserman, E. Heckert, A. S. Karakoti, J. E. S. King, S. Seal and W. T. Self, Chem. Commun., 2010, 46, 2736–2738 RSC.
- Z. Wang, X. Shen, X. Gao and Y. Zhao, Nanoscale, 2019, 11, 13289–13299 RSC.
- F. Bray, J. Ferlay, I. Soerjomataram, R. Siegel, L. Torre, A. Jemal and C. A. Cancer, J. Clin., 2018, 68, 394–424 Search PubMed.
- S. Akbari Nakhjavani, H. Afsharan, B. Khalilzadeh, M. Ghahremani, S. Carrara and Y. Omidi, Biosens. Bioelectron., 2019 DOI:10.1016/J.BIOS.2019.111439.
- P. Hu, S. Zhang, T. Wu, D. Ni, W. Fan, Y. Zhu, R. Qian and J. Shi, Adv. Mater., 2018 DOI:10.1002/ADMA.201801690.
- M. Gottesman, Annu. Rev. Med., 2002, 53, 615–627 CrossRef CAS PubMed.
- D. Peer, J. Karp, S. Hong, O. Farokhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- X. Jing, F. Yang, C. Shao, K. Wei, M. Xie, H. Shen and Y. Shu, Mol. Cancer, 2019 DOI:10.1186/S12943-019-1089-9.
- A. Asati, S. Santra, C. Kaittanis and J. Perez, ACS Nano, 2010, 4, 5321–5331 CrossRef CAS PubMed.
- N. Sisubalan, V. Ramkumar, A. Pugazhendhi, C. Karthikeyan, K. Indira, K. Gopinath, A. Hameed and M. Basha, Environ. Sci. Pollut. Res. Int., 2018, 25, 10482–10492 CrossRef CAS PubMed.
- A. Datta, S. Mishra, K. Manna, K. Saha, S. Mukherjee and S. Roy, ACS omega, 2020, 5, 9714–9723 CrossRef CAS PubMed.
- G. Fernández-Varo, M. Perramón, S. Carvajal, D. Oró, E. Casals, L. Boix, L. Oller, L. Macías-Muñoz, S. Marfà, G. Casals, M. Morales-Ruiz, P. Casado, P. Cutillas, J. Bruix, M. Navasa, J. Fuster, J. Garcia-Valdecasas, M. Pavel, V. Puntes and W. Jiménez, Hepatology, 2020, 72, 1267–1282 CrossRef PubMed.
- C. Shao, A. Shen, M. Zhang, X. Meng, C. Song, Y. Liu, X. Gao, P. Wang and W. Bu, ACS Nano, 2018, 12, 12629–12637 CrossRef CAS PubMed.
- X. Pang, J. Li, Y. Zhao, D. Wu, Y. Zhang, B. Du, H. Ma and Q. Wei, ACS Appl. Mater. Interfaces, 2015, 7, 19260–19267 CrossRef CAS PubMed.
- S. Yu, G. Zou and Q. Wei, Talanta, 2016, 156–157, 11–17 CrossRef CAS PubMed.
- S. Li, L. Wang, Y. Li, X. Zhu, L. Zhong, L. Lu, W. Zhang, B. Liu, G. Xie and W. Feng, Colloids Surf. B. Biointerfaces, 2013, 112, 344–349 CrossRef CAS PubMed.
- D. Di Gioia, I. Blankenburg, D. Nagel, V. Heinemann and P. Stieber, Clin. Chim. Acta., 2016, 461, 1–7 CrossRef CAS PubMed.
- M. Duffy, R. Lamerz, C. Haglund, A. Nicolini, M. Kalousová, L. Holubec and C. Sturgeon, Int. J. cancer, 2014, 134, 2513–2522 CrossRef CAS PubMed.
- R. Blumenthal, E. Leon, H. Hansen and D. Goldenberg, BMC Cancer, 2007 DOI:10.1186/1471-2407-7-2.
- L. Huang, W. Huang and Y. Chen, Lin Chuang Er Bi Yan Hou Ke Za Zhi, 2005, 19, 201–202 Search PubMed.
- Y. Chen, J. Feng, L. Mei, C. Shi and A. Wang, J. Colloid Interface Sci., 2019, 555, 647–654 CrossRef CAS PubMed.
- Y. Li, Y. Zhang, F. Li, J. Feng, M. Li, L. Chen and Y. Dong, Biosens. Bioelectron., 2017, 92, 33–39 CrossRef CAS PubMed.
- L. Zhong, C. Zhang, J. Zheng, J. Li, W. Chen and Z. Zhang, Arch. Oral Biol., 2007, 52, 1079–1087 CrossRef CAS PubMed.
- N. Pachauri, K. Dave, A. Dinda and P. Solanki, J. Mater. Chem. B, 2018, 6, 3000–3012 RSC.
- K. Goonetilleke and A. Siriwardena, Eur. J. Surg. Oncol., 2007, 33, 266–270 CrossRef CAS PubMed.
- M. Wang, M. Hu, B. Hu, C. Guo, Y. Song, Q. Jia, L. He, Z. Zhang and S. Fang, Biosens. Bioelectron., 2019, 135, 22–29 CrossRef CAS PubMed.
- H. Xie, C. Zhang and Z. Gao, Anal. Chem., 2004, 76, 1611–1617 CrossRef CAS PubMed.
- K. Feng, Y. Yang, Z. Wang, J. Jiang, G. Shen and R. Yu, Talanta, 2006, 70, 561–565 CrossRef CAS PubMed.
- P. Bartley, D. Ross, S. Latham, M. Martin-Harris, B. Budgen, V. Wilczek, S. Branford, T. Hughes and A. Morley, Int. J. Lab. Hematol., 2010 DOI:10.1111/J.1751-553X.2010.01236.X.
- L. Alili, M. Sack, A. Karakoti, S. Teuber, K. Puschmann, S. Hirst, C. Reilly, K. Zanger, W. Stahl, S. Das, S. Seal and P. Brenneisen, Biomaterials, 2011, 32, 2918–2929 CrossRef CAS PubMed.
- W. Lin, Y. Huang, X. Zhou and Y. Ma, Int. J. Toxicol., 2006, 25, 451–457 CrossRef CAS PubMed.
- C. Holohan, S. Van Schaeybroeck, D. Longley and P. Johnston, Nat. Rev. Cancer, 2013, 13, 714–726 CrossRef CAS PubMed.
- M. Kartal-Yandim, A. Adan-Gokbulut and Y. Baran, Crit. Rev. Biotechnol., 2016, 36, 716–726 CrossRef CAS PubMed.
- G. Wu, Z. Zhang, X. Chen, Q. Yu, X. Ma and L. Liu, Ecotoxicol. Environ. Saf., 2019, 167, 301–308 CrossRef CAS PubMed.
- K. Xu, Y. Cheng, J. Yan, Y. Feng, R. Zheng, X. Wu, Y. Wang, P. Song and H. Zhang, Nano Res, 2019, 12, 2947–2953 CrossRef CAS.
- J. Wen, K. Yang, Y. Xu, H. Li, F. Liu and S. Sun, Sci. Rep., 2016 DOI:10.1038/SREP38931.
- H. Li, C. Liu, Y. Zeng, Y. Hao, J. Huang, Z. Yang and R. Li, ACS Appl. Mater. Interfaces, 2016, 8, 31510–31523 CrossRef CAS PubMed.
- Z. Yiping, Z. Weinan, Z. Qing, J. Xiaolin, L. Juan, Y. Zhangyou, H. Yuhui and L. Junli, J. Mater. Chem. B, 2019, 7, 3210–3219 RSC.
- L. Zeng, H. Zhao, Y. Zhu, S. Chen, Y. Zhang, D. Wei, J. Sun and H. Fan, J. Mater. Chem. B, 2020, 8, 4093–4105 RSC.
- X. Zhu, Y. Gong, Y. Liu, C. Yang, S. Wu, G. Yuan, X. Guo, J. Liu and X. Qin, Biomaterials, 2020 DOI:10.1016/J.BIOMATERIALS.2020.119923.
- F. Chen, X. Zhang, X. Hu, W. Zhang, Z. Lou, L. Xie, P. Liu and H. Zhang, Int. J. Nanomedicine, 2015, 10, 4957–4969 CrossRef CAS PubMed.
- W. Jiang, X. Han, T. Zhang, D. Xie, H. Zhang and Y. Hu, Adv. Healthc. Mater., 2020 DOI:10.1002/ADHM.201901303.
- A. Torres-Romero, M. Cajero-Juárez, R. Nuñez-Anita and M. Contreras-García, J. Nanosci. Nanotechnol., 2020, 20, 3971–3980 CrossRef CAS PubMed.
- C. Tapeinos, M. Battaglini, M. Prato, G. La Rosa, A. Scarpellini and G. Ciofani, ACS omega, 2018, 3, 8952–8962 CrossRef CAS PubMed.
- Y. Zhang, X. Wu, C. Hou, K. Shang, K. Yang, Z. Tian, Z. Pei, Y. Qu and Y. Pei, Int. J. Nanomedicine, 2018, 13, 2161–2173 CrossRef CAS PubMed.
- Y. Gao, K. Chen, J. L. Ma and F. Gao, Onco. Targets. Ther., 2014, 7, 835–840 CrossRef CAS PubMed.
- H. Cheng, Z. Liao, L. Ning, H. Chen, S. Wei, X. Yang and H. Guo, Cancer Med, 2017, 6, 374–381 CrossRef CAS PubMed.
- J. Vassie, J. Whitelock and M. Lord, Acta Biomater, 2017, 50, 127–141 CrossRef CAS PubMed.
- G. Delaney, S. Jacob, C. Featherstone and M. Barton, Cancer, 2005, 104, 1129–1137 CrossRef PubMed.
- J. Ward, Prog. Nucleic Acid Res. Mol. Biol., 1988, 35, 95–125 CAS.
- M. Horsman and J. Overgaard, J. Radiat. Res., 2016, 57(Suppl 1), i90–i98 CrossRef PubMed.
- Z. Xuantong, Y. Min, W. Fuhui, W. Zhenzhen, G. Xingfa, J. Chao, L. Jiaming, G. Mengyu, L. Jiayang, L. Aiping, L. Huibiao, L. Zhihua and C. Chunying, Adv. Mater., 2021, 33, 2100556 CrossRef PubMed.
- J. Colon, N. Hsieh, A. Ferguson, P. Kupelian, S. Seal, D. Jenkins and C. Baker, Nanomedicine, 2010, 6, 698–705 CrossRef CAS PubMed.
- J. Colon, L. Herrera, J. Smith, S. Patil, C. Komanski, P. Kupelian, S. Seal, D. Jenkins and C. Baker, Nanomedicine, 2009, 5, 225–231 CrossRef CAS PubMed.
- R. Madero-Visbal, B. Alvarado, J. Colon, C. Baker, M. Wason, B. Isley, S. Seal, C. Lee, S. Das and R. Mañon, Nanomedicine, 2012, 8, 1223–1231 CrossRef CAS PubMed.
- M. Wason, J. Colon, S. Das, S. Seal, J. Turkson, J. Zhao and C. Baker, Nanomedicine, 2013, 9, 558–569 CrossRef CAS PubMed.
- D. Dolmans, D. Fukumura and R. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed.
- A. Juarranz, P. Jaén, F. Sanz-Rodríguez, J. Cuevas and S. González, Clin. Transl. Oncol., 2008, 10, 148–154 CrossRef CAS PubMed.
- C. Xu, Y. Lin, J. Wang, L. Wu, W. Wei, J. Ren and X. Qu, Adv. Healthc. Mater., 2013, 2, 1591–1599 CrossRef CAS PubMed.
- C. Yao, W. Wang, P. Wang, M. Zhao, X. Li and F. Zhang, Adv. Mater., 2018 DOI:10.1002/ADMA.201704833.
- M. Ethirajan, Y. Chen, P. Joshi and R. Pandey, Chem. Soc. Rev., 2011, 40, 340–362 RSC.
- J. Celli, B. Spring, I. Rizvi, C. Evans, K. Samkoe, S. Verma, B. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795–2838 CrossRef CAS PubMed.
- G. Mattheolabakis, L. Milane, A. Singh and M. Amiji, J. Drug Target., 2015, 23, 605–618 CrossRef CAS PubMed.
- J. Beik, Z. Abed, F. Ghoreishi, S. Hosseini-Nami, S. Mehrzadi, A. Shakeri-Zadeh and S. Kamrava, J. Control. Release, 2016, 235, 205–221 CrossRef CAS PubMed.
- Y. Chen, K. Ai, J. Liu, X. Ren, C. Jiang and L. Lu, Biomaterials, 2016, 77, 198–206 CrossRef CAS PubMed.
- C. Xu, Q. Feng, H. Yang, G. Wang, L. Huang, Q. Bai, C. Zhang, Y. Wang, Y. Chen, Q. Cheng, M. Chen, Y. Han, Z. Yu, M. Lesniak and Y. Cheng, Adv. Sci., 2018 DOI:10.1002/ADVS.201800382.
- B. Wilson and R. Weersink, Photochem. Photobiol., 2020, 96, 219–231 CrossRef CAS PubMed.
- Z. Li, C. Hui, D. Yuwei, S. Zhipeng, W. Chengde, L. Lei, L. Deqing, L. Xingyi, C. Hao, F. Kelong and S. Shuai, ACS Appl. Mater. Interfaces, 2020, 13, 233–244 Search PubMed.
- Y. Wang, G. Wei, X. Zhang, X. Huang, J. Zhao, X. Guo and S. Zhou, Small, 2017 DOI:10.1002/SMLL.201702994.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |
