Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hyperpolarised NMR to aid molecular profiling of electronic cigarette aerosols†
Ben. J. Tickner‡
 a,
Sanna Komulainen‡a,
Sanna Palosaari
a,
Sanna Komulainen‡a,
Sanna Palosaari bc,
Janne Heikkinen
bc,
Janne Heikkinen b,
Petri Lehenkari
b,
Petri Lehenkari bcd,
Vladimir V. Zhivonitko
bcd,
Vladimir V. Zhivonitko *a and
Ville-Veikko Telkki
*a and
Ville-Veikko Telkki *a
*a
aNMR Research Unit, Faculty of Science, University of Oulu, 90014, Finland. E-mail: vladimir.zhivonitko@oulu.fi; ville-veikko.telkki@oulu.fi
bCancer and Translational Medicine Research Unit, Faculty of Medicine, University of Oulu, 90014, Finland
cMedical Research Center Oulu, Faculty of Medicine, University of Oulu and Oulu University Hospital, 90014, Finland
dDivision of Orthopedic Surgery, Oulu University Hospital, 90220, Finland
First published on 10th January 2022
Abstract
Signal amplification by reversible exchange (SABRE) hyperpolarisation is used to enhance the NMR signals of nicotine and acrolein in methanol-d4 solutions of electronic cigarette aerosols. Consequently, detection of 74 μM nicotine is possible in just a single scan 1H NMR spectrum. The first example of an aldehyde hyperpolarised using SABRE is demonstrated and we work towards novel real-world applications of SABRE-hyperpolarised NMR for chemical analysis.
Introduction
Electronic cigarette aerosols are becoming increasingly popular with an estimated 41 million users as of 2018.1,2 Unlike traditional cigarettes that involve tobacco combustion, electronic cigarettes heat and vapourise cigarette fluid. The composition of these inhaled aerosols, which are often studied using techniques such as Gas Chromatography Mass spectrometry (GC-MS) or Nuclear Magnetic Resonance (NMR) is not fully understood.1,2 A greater understanding of the chemical composition of these aerosols, and their solutions, is essential given the role they may play in user's health. NMR is used routinely for molecular identification and is often the method-of-choice for many scientists as it does not use ionising radiation or destroy the sample.3 However, NMR experiments are typically repeated many times to generate discernible signals due to the ppm thermal polarisation of NMR active nuclei.4 This low sensitivity can often make NMR experiments time consuming and necessitates the use of concentrated samples (>ca. 1 mM) for analysis by NMR.Nuclear spin hyperpolarisation can generate NMR signals enhanced by many orders of magnitude compared to those recorded using thermal polarisation.4 Signal Amplification By Reversible Exchange (SABRE) is one such hyperpolarisation technique that uses parahydrogen (pH2) as its source of enhanced magnetisation, which is a spin isomer of dihydrogen that is cheap and easy to produce. The latent magnetism of pH2 can be unlocked and transferred to a target molecule via reversible interactions with a metal catalyst.5 Polarisation transfer occurs via a temporary J-coupled network that exists within the short-lived SABRE catalyst (Fig. 1).6,7 SABRE is a low-cost route to produce molecules in an enhanced nuclear spin state. However, hyperpolarisation soon decays back to thermal equilibrium according to T1 relaxation and must therefore be measured rapidly. The reversibility of SABRE provides an additional benefit as these enhanced NMR signals are easily regenerated upon fresh shaking or bubbling with pH2.5,8 SABRE has been reported to hyperpolarise a wide range of molecular targets such as N-donor ligands9 via catalysts of the form [Ir(H)2(NHC)(substrate)3]Cl where NHC is an N-heterocyclic carbene. SABRE has produced 1H, 15N, and 13C polarisation of 65%,10 79%,11 and 4%12 respectively, and NMR signals of other heteronuclei have also been enhanced.13–18 SABRE-enhanced NMR signals have been involved in a broad range of applications including reaction monitoring,19,20 mechanistic elucidation,21 magneto-optics22 and many others.23 In particular, use of SABRE-hyperpolarised NMR for mixture analysis is promising as it provides a method for boosting the NMR signals of many low concentration analytes.24–30 In this work, the NMR sensitivity improvements that SABRE hyperpolarisation can provide are employed to aid detection of low concentration analytes in electronic cigarette aerosols dissolved in methanol-d4 using NMR.
Results and discussion
Two samples of electronic cigarette aerosol were prepared and studied: Ecig contained electronic cigarette fluid with 18 mg mL−1 nicotine which was vapourised and dissolved in methanol-d4 (see ESI, Section S1.2†). A background reference sample, Ecigdiluent, contained dissolved aerosol from electronic cigarette carrier fluid without nicotine. Thermally polarised 1H and 13C NMR measurements of these aerosol solutions (0.1 mL) in methanol-d4 (0.5 mL) at 298 K and 9.4 T were able to confirm the presence of a wide range of molecules including acetaldehyde, propylene glycol, glycerol, hydroxyacetone, 1-propenol, and formaldehyde (see ESI, Section S2†). These molecules have all been identified previously in electronic cigarette aerosols using LCMS, GCMS, or NMR spectroscopy.1,2 In these concentrated solutions, these molecules are all present at concentrations ca. >5 mM and these thermally polarised measurements require ca. 20 min to acquire. In this work we investigate the use of SABRE-hyperpolarisation to enhance NMR signals for analytes of interest, such as nicotine, and use this sensitivity boost to aid chemical analysis of electronic cigarette aerosol solutions when analyte concentrations are much lower than mM levels.The enhancement of 1H NMR signals for the N-heterocycle nicotine, which is present in many tobacco products,31 has been reported under many different conditions elsewhere.5,29,32–35 We reacted SABRE precatalyst [IrCl(COD)(IMes)] (5 mM) (where COD is cis,cis-1,5-cyclooctadiene and IMes is 1,3-bis(2,4,6-trimethyl-phenyl)imidazol-2-ylidene) and (−)-nicotine (5 equiv.) with H2 (3 bar) overnight at room temperature in methanol-d4 (0.6 mL) and confirmed the formation of the active catalyst [Ir(H)2(IMes)(nicotine)3]Cl by 2D NMR characterisation (see ESI, Section S3.2†). These solutions were shaken vigorously with pH2 (3 bar) for 10 seconds to yield enhanced 1H NMR signals at δ = 8.52, 8.46, 7.45 and 7.88 ppm corresponding to the two inequivalent ortho, meta and para resonances of the free nicotine pyridine ring respectively. These are 66 ± 9, 65 ± 12, 107 ± 19 and 87 ± 17 times larger than those recorded under Boltzmann conditions respectively (see ESI, Fig. S12†). In our experiments, this shaking process was performed inside an electromagnetic coil that generates a magnetic field of ca. 6.5 mT (see ESI, Section S1.4†).36 This field was selected as it has been reported optimal for most efficient SABRE polarisation transfer to 1H sites within nicotine and similar N-heterocyclic targets.5,37 After shaking, samples were rapidly inserted into a 9.4 T NMR spectrometer for collection of a single scan 1H NMR spectrum.
We turn our attention to the detection of small concentrations (<1 mM) of nicotine, rather than the ca. 25 mM substrate concentrations typically used in SABRE measurements, or the ca. 6 mM nicotine concentrations in our electronic cigarette solutions analysed using thermally polarised NMR. For these experiments, the inclusion of a coligand is essential to allow formation of a stable SABRE catalyst of the form [Ir(H)2(IMes)(coligand)3]Cl. Trace analytes can then displace a coligand to form [Ir(H)2(IMes)(analyte)(coligand)2]Cl which catalyses polarisation transfer from pH2 to an analyte giving rise to enhanced analyte NMR signals. This approach facilitates detection of substrate concentrations lower than that of the catalyst as the substrate is no longer required in at least a 3-fold excess relative to the metal centre.24,26 It has also been reported that the hyperpolarised NMR signal intensity of dilute substrates in the presence of a coligand can be proportional to their concentration due to the formation of small amounts of [Ir(H)2(NHC)(coligand)2(analyte)]Cl relative to [Ir(H)2(IMes)(colignad)3]Cl.24,26 Standard additions of known analyte concentrations are made to a sample of interest and the linear increase in hyperpolarised analyte signal is used to determine the original analyte concentration prior to spiking.24,26
It is important to select a coligand that does not dilute significant polarisation away from the analyte of interest and it must ideally contain 1H NMR signals that do not overlap with the analyte of interest. Here, the readily available coligand imidazole is employed as it is known to form stable SABRE complexes21,38 and shows only modest 1H NMR signal enhancements (50–100 fold,39,40 although other studies have increased this by deuteration of the catalyst and other mixed ligand approaches38). Samples were prepared based on previously reported concentrations26 that contained [IrCl(COD)(IMes)] (2 mM) and the coligand imidazole (15 equiv.) activated overnight with H2 (3 bar) in methanol-d4 (0.6 mL). When nicotine concentrations as low as 74 μM were added to this sample and pH2 shaking was performed, hyperpolarised nicotine 1H NMR signals for the two ortho sites could be discerned (the meta and para sites overlap with those of the imidazole coligand) (Fig. 2a). Additions of further amounts of nicotine, followed by fresh pH2 shaking after each addition, yields a linear relationship between nicotine concentration and its hyperpolarised 1H NMR signal intensity at these concentrations (Fig. 2b). This relationship breaks down when [Ir(H)2(IMes)(imidazole)3]Cl is no longer in excess compared to [Ir(H)2(NHC)(imidazole)2(nicotine)]Cl (see ESI, Fig. S13†).24,26 Nicotine concentrations of 34 μM or lower could not be detected in these single scan 1H NMR spectra. Others have reported the detection of ca. 1–2 μM amounts of N-heterocycles in single scan SABRE hyperpolarised 1H NMR measurements that utilise a 1-methyl-1,2,3-triazole coligand.24,26 Our inability to detect these concentrations is likely linked with modest polarisation transfer to the imidazole coligand, which should be reduced or prevented to increase the nicotine detection limit, which is estimated to be ca. 50 μM using this system.
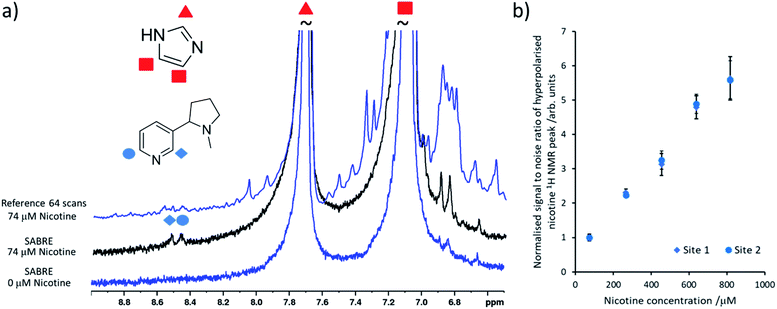 | ||
| Fig. 2 Quantitative low concentration detection of nicotine. (a) Partial single scan 1H NMR spectra recorded at 9.4 T and 298 K after a sample containing [IrCl(COD)(IMes)] (2 mM) and imidazole (15 equiv.) and the indicated nicotine concentration in 0.6 mL methanol-d4 was shaken with 3 bar pH2 for 10 seconds at 6.5 mT. The corresponding reference spectrum (not to scale) is shown above. (b) The hyperpolarised 1H NMR signal to noise ratios for the inequivalent ortho sites of free nicotine increases as its concentration is increased. These are calculated by taking the largest signal intensity value between δ = 8.53–8.49 and 8.42–8.48 ppm for nicotine site 1 and 2 respectively and dividing by spectral noise between δ = −1 and −5 ppm (see ESI, Section S1.4† for more details). Each data point is the average of three repeat measurements, the error bar represents the average of these. | ||
These measurements are next extended to the detection of nicotine, and other molecules, in electronic cigarette aerosol, for which our current detection limit is sufficient for analysis. 50 μL of the samples Ecig and Ecigdiluent were hyperpolarised by addition to a solution of [IrCl(COD)(IMes)] (5 mM) and imidazole (15 equiv.) which had been preactivated with H2 (3 bar) in methanol-d4 (0.5 mL). Shaking with pH2 produced hyperpolarised 1H NMR signals for nicotine in addition to a range of other hyperpolarised signals (Fig. 3b). Enhanced signals at δ = 9.56, 6.58 and 6.41 ppm correspond to acrolein which are enhanced by ca. 150-fold. We are unaware of any examples of aldehydes hyperpolarised using SABRE to date.9 A set of hyperpolarised signals at δ = 5.87, 5.36, 5.22 and 4.92 ppm are also observed immediately after the addition of electronic cigarette aerosol solution (Fig. 3b). 2D NMR characterisation of a reference sample of electronic cigarette aerosol solution confirm that these resonances belong to the same molecule (see ESI, Fig. S7†). A structure for this molecule cannot be assigned definitively, although its chemical shift values are consistent with 1,4-pentadien-3-ol and related molecules.41
Reversible coordination of both aldehyde and alcohol to the SABRE catalyst must be responsible for the observation of these SABRE-enhanced NMR signals. When the hydride region of their SABRE-hyperpolarised spectra are examined more closely, enhanced signals for species at δ = −5.85, −16.28 ppm and δ = −4.92, −16.14 ppm are observed in addition to those of [Ir(H)2(IMes)(imidazole)3]Cl and [Ir(H)2(IMes)(imidazole)2(nicotine)]Cl (Fig. 3b). Unfortunately, these species are of insufficient concentration or lifetime to allow their characterisation using 2D NMR spectroscopy. Nevertheless, the more dominant of these, at δ = −5.85, −16.28 ppm, is expected to correspond to a complex of the form [Ir(H)2(IMes)(imidazole)2(acrolein)]Cl which is responsible for the SABRE effect of the free aldehyde. It is anticipated that substrate coordination through the vinyl group is more likely and is supported by these hydride chemical shift values, which are not consistent with the ca. δ = −28 to −30 ppm range expected for iridium(III) hydrides located trans to oxygen.42–46
Interestingly, when the shaking process is repeated several times following addition of fresh pH2, these enhanced acrolein and hydride signals decrease in intensity until they are no longer visible (Fig. 3d), while signals of nicotine remain enhanced with similar signal intensity. When a fresh 50 μL addition of the electronic cigarette aerosol solution is made, enhanced 1H NMR signals for acrolein, and those we speculate could belong to 1,4-pentadien-3-ol, and the associated hydride signals of their iridium-bound adducts, reappear before decreasing upon fresh pH2 shaking. Control measurements confirm that this behaviour is not due to evaporation of these analytes by sample degassing or replacement of pH2. Rather, this is indicative of a chemical reaction in which the analyte concentration decreases throughout the process. For example, many-scan thermal 1H NMR measurements on this solution after the reaction has occurred show that no acrolein signals remain, despite reference measurements of equivalent concentrations of electronic cigarette aerosol solution confirming its presence (see ESI, Fig. S14†). These observations can be explained by the hydrogenation of these analytes by the SABRE catalyst,47,48 although 1H NMR signals for any additional organic reaction products are not clearly discerned in either SABRE or thermally polarised measurements of this complex mixture. Related hydrogenation of a vinylsulfoxide using this precatalyst has been reported and similar reactivity of unsaturated aldehydes and alcohols is not unexpected.49
This decomposition makes quantification of these analytes challenging as their concentration changes during the measurements. Nevertheless, the diagnostic potential for rapid mixture analysis is highlighted by comparison of these results with those from Ecigdiluent that do not contain nicotine. When these experiments are repeated using Ecigdiluent aerosol solutions, the two samples are clearly distinguished based on the appearance of their SABRE-hyperpolarised 1H NMR spectra, which do not show any peaks for hyperpolarised nicotine. In this mixture, acrolein and the signals tentatively attributed to 1,4-pentadien-3-ol display similar enhanced 1H NMR signals (see ESI, Fig. S15†).
The nicotine concentrations detected in electronic cigarette aerosol solutions using the SABRE-hyperpolarised NMR measurements discussed so far (Fig. 3) are estimated to be on the order of 2–3 mM which are still sufficient for detection using thermally polarised 1H NMR, with only modest time investment required for sufficient signal averaging. Therefore, these measurements are extended to detect much lower nicotine concentrations in Ecig aerosol for which thermally polarised 1H NMR may involve prohibitively long acquisition times. For example, hyperpolarised nicotine 1H NMR signals could be observed when 3 μL of the nicotine-containing Ecig aerosol solution was added to a preactivated sample of [IrCl(COD)(IMes)] (2 mM), imidazole (15 equiv.) and pH2 (3 bar) in methanol-d4 (0.6 mL) and SABRE experiments performed (Fig. 4c). Addition of further portions of known nicotine amounts can be used to determine the unknown concentration of nicotine as previously described (Fig. 4e). This yields a nicotine concentration of 193 ± 12 μM. Signal averaged thermally polarised 1H NMR spectra can be used to estimate a nicotine concentration by comparison of the integral intensity to those of the imidazole signals which correspond to a known concentration. This yields a nicotine concentration of ca. 187 μM which is consistent with SABRE-hyperpolarised data. These measurements contain an average reproducibility error of ca. 5%, which is sufficient for agreement between concentration values determined from hyperpolarised and thermally-polarised NMR measurements. The accuracy of the determined concentrations can likely be increased by improved experiment design, such as automated pH2 bubbling apparatus and/or flow systems that might control the polarisation transfer field more precisely.8,50,51 Smaller incremental increases of nicotine concentration during spiking may also improve accuracy as reproducibility errors increase at larger concentrations. Nevertheless, these SABRE-hyperpolarised NMR spectra involve a total measurement time of ca. 1 minute and can be performed by an experienced researcher in around 45 minutes (signal to noise ratio for nicotine of >35). This provides a time advantage compared to Boltzmann-polarised NMR, for which nicotine signals of a reference sample of equivalent concentration can be discerned with a signal to noise ratio of ca. 9 after 70 minutes of signal averaging. This time saving will become more pronounced when molecules are detected at even lower concentrations, for which the detection limit of our setup must be lowered.
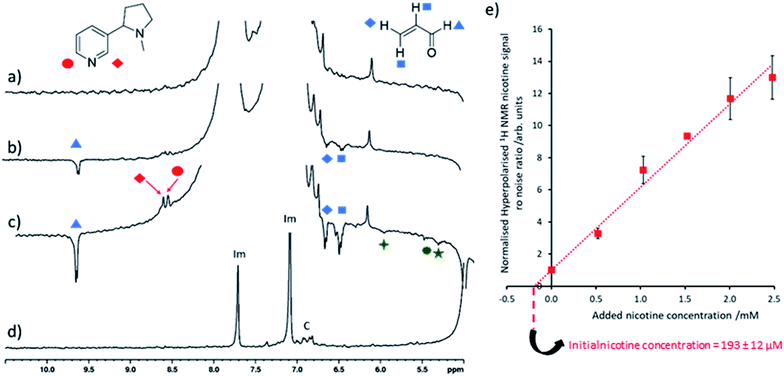 | ||
| Fig. 4 Quantitative low concentration nicotine detection using SABRE hyperpolarised 1H NMR (a) partial single scan 1H NMR spectra recorded at 9.4 T and 298 K after a sample containing [IrCl(COD)(IMes)] (2 mM) and imidazole (15 equiv.) in 0.6 mL methanol-d4 was shaken with 3 bar pH2 for 10 seconds at 6.5 mT. Analogous SABRE hyperpolarised 1H NMR spectrum after addition of (b) 1 μL and (c) 3 μL electronic cigarette aerosol solution to the sample used in (a). (d) Thermally polarised 1H NMR spectrum of the solution used in (c). Note that resonances labelled ‘Im’ and ‘C’ correspond to imidazole coligand and the IMes ligand of the SABRE catalyst respectively. Those signals marked by the green symbols are consistent with 1,4-pentadien-3-ol or related molecules. (e) The hyperpolarised 1H NMR signal to noise ratio of the nicotine ortho signals increase after known nicotine concentrations were added to the sample used in (c) and were used to calculate initial nicotine concentration from the line of best fit x axis intercept. Signal to noise ratios are calculated by taking the largest signal intensity value between δ = 8.53–8.49 and 8.42–8.48 ppm for nicotine site 1 and 2 respectively and dividing by spectral noise between δ = −1 and −5 ppm (see ESI, Section S1.4† for more details). Each data point is the average of three repeat measurements, the error bar represents the average of these. The error value on the initial nicotine concentration has been calculated by propagating the average 5% experimental error on SABRE experiments with the 2% error of the regression fitting. | ||
Conclusions
The SABRE-hyperpolarised NMR approach presented here can clearly make low concentration molecules such as nicotine and acrolein visible to single scan 1H NMR. The enhanced 1H NMR signals of acrolein are novel as, to the best of our knowledge, SABRE has not yet been used to enhance NMR signals of aldehydes.9 We demonstrate how SABRE can be used to enhance chemical analysis of complex mixtures by NMR and work towards a real-world example such as the analysis of electronic cigarette products. In the future, SABRE-hyperpolarised NMR may become a viable complementary method for chemical analysis in addition to established thermally polarised NMR or MS methods, the latter of which can be more sensitive. An average tobacco rod contains 10–14 mg nicotine52 and the electronic cigarette fluids sold for consumer use contain typical nicotine concentrations of 18 mg mL−1. In electronic cigarette aerosols, nicotine concentration is in the mg region.53 Therefore, the current detection range of single scan SABRE-hyperpolarised 1H NMR is more than adequate to observe nicotine and other molecules in tobacco products or electronic cigarette aerosols.In the future these results can be improved by lowering the analyte detection limit. This could be achieved by limitation of the modest (<200-fold) 1H NMR polarisation of the imidazole coligand by approaches such as deuteration,10 or even selection of an alternative coligand,24,26 which could also reduce chemical shift overlap. A lower analyte detection limit will also improve the time savings that SABRE-hyperpolarised NMR can provide compared to other NMR methods. Closely related hyperpolarisation methods, such as relayed polarisation transfer (SABRE-Relay) has recently been developed to enhance the NMR signals of OH-containing molecules that do not ligate to the metal SABRE catalyst.54 These measurements could be extended to low concentration detection of other OH-containing molecules present in these cigarette aerosol solutions in the future. We demonstrate how SABRE-hyperpolarised NMR could be used in a real-world application to aid molecular profiling of electronic cigarette aerosols which may be of great use in rapid distinctions between different aerosol solutions.
Data availability statement
Data for this paper, including 1D and 2D experimental NMR data of the article are available at IDA repository at http://urn.fi/urn:nbn:fi:att:04e3bfee-0fd0-43c5-8bbb-59b7da7ad9a8.Author contributions
BJT: conceptualisation, investigation, validation, visualisation, writing – original draft, review and editing; SK: conceptualisation, investigation, validation, writing –review and editing; SP: providing aerosol solutions, writing –review and editing; JH: providing aerosol solutions; PL: funding acquisition, supervision; VVZ: funding acquisition, resources, synthesis of [IrCl(COD)(IMes)] precatalyst, supervision, writing – review and editing; VVT: conceptualisation, funding acquisition, resources, supervision, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to Dr Petr Štěpánek for assistance in experimental setup. Financial support from the European Research Council (Project number 772110), Academy of Finland (grant no. 323480 and 340099) and the University of Oulu (Kvantum Institute) is gratefully acknowledged.Notes and references
- R. M. Strongin, Annu. Rev. Anal. Chem., 2019, 12, 23–39 CrossRef PubMed.
- J. S. Herrington and C. Myers, J. Chromatogr. A, 2015, 1418, 192–199 CrossRef CAS PubMed.
- J. Keeler, Understanding NMR spectroscopy, John Wiley & Sons, 2011 Search PubMed.
- P. Nikolaou, B. M. Goodson and E. Y. Chekmenev, Chem.–Eur. J., 2015, 21, 3156–3166 CrossRef CAS PubMed.
- R. W. Adams, J. A. Aguilar, K. D. Atkinson, M. J. Cowley, P. I. P. Elliott, S. B. Duckett, G. G. R. Green, I. G. Khazal, J. López-Serrano and D. C. Williamson, Science, 2009, 323, 1708–1711 CrossRef CAS PubMed.
- R. W. Adams, S. B. Duckett, R. A. Green, D. C. Williamson and G. G. R. Green, J. Chem. Phys., 2009, 131, 194505 CrossRef PubMed.
- A. N. Pravdivtsev, K. L. Ivanov, A. V Yurkovskaya, P. A. Petrov, H.-H. Limbach, R. Kaptein and H.-M. Vieth, J. Magn. Reson., 2015, 261, 73–82 CrossRef CAS PubMed.
- P. Štěpánek, C. Sanchez-Perez, V.-V. Telkki, V. v Zhivonitko and A. M. Kantola, J. Magn. Reson., 2019, 300, 8–17 CrossRef PubMed.
- D. A. Barskiy, S. Knecht, A. V. Yurkovskaya and K. L. Ivanov, Prog. Nucl. Magn. Reson. Spectrosc., 2019, 114–115, 33–70 CrossRef CAS PubMed.
- P. J. Rayner, M. J. Burns, A. M. Olaru, P. Norcott, M. Fekete, G. G. R. Green, L. A. R. Highton, R. E. Mewis and S. B. Duckett, Proc. Natl. Acad. Sci., 2017, 201620457 Search PubMed.
- M. Fekete, F. Ahwal and S. B. Duckett, J. Phys. Chem. B, 2020, 124, 4573–4580 CrossRef CAS PubMed.
- D. A. Barskiy, R. V Shchepin, C. P. N. Tanner, J. F. P. Colell, B. M. Goodson, T. Theis, W. S. Warren and E. Y. Chekmenev, ChemPhysChem, 2017, 18, 1493–1498 CrossRef CAS PubMed.
- R. V Shchepin, B. M. Goodson, T. Theis, W. S. Warren and E. Y. Chekmenev, ChemPhysChem, 2017, 18, 1961–1965 CrossRef PubMed.
- N. V Chukanov, O. G. Salnikov, R. V Shchepin, A. Svyatova, K. V Kovtunov, I. V Koptyug and E. Y. Chekmenev, J. Phys. Chem. C, 2018, 122, 23002–23010 CrossRef PubMed.
- A. M. Olaru, T. B. R. Robertson, J. S. Lewis, A. Antony, W. Iali, R. E. Mewis and S. B. Duckett, ChemistryOpen, 2018, 7, 97–105 CrossRef CAS PubMed.
- M. J. Burns, P. J. Rayner, G. G. R. Green, L. A. R. Highton, R. E. Mewis and S. B. Duckett, J. Phys. Chem. B, 2015, 119, 5020–5027 CrossRef CAS PubMed.
- V. V Zhivonitko, I. V Skovpin and I. V Koptyug, Chem. Commun., 2015, 51, 2506–2509 RSC.
- A. M. Olaru, A. Burt, P. J. Rayner, S. J. Hart, A. C. Whitwood, G. G. R. Green and S. B. Duckett, Chem. Commun., 2016, 52, 14482–14485 RSC.
- B. J. Tickner, O. Semenova, W. Iali, P. J. Rayner, A. C. Whitwood and S. B. Duckett, Catal. Sci. Technol., 2020, 10, 1343–1355 RSC.
- H. Chae, S. Min, H. J. Jeong, S. K. Namgoong, S. Oh, K. Kim and K. Jeong, Anal. Chem., 2020, 92, 10902–10907 CrossRef CAS PubMed.
- B. J. Tickner, R. O. John, S. S. Roy, S. J. Hart, A. C. Whitwood and S. B. Duckett, Chem. Sci., 2019, 10, 5235–5245 RSC.
- P. Štěpánek and A. M. Kantola, J. Phys. Chem. Lett., 2019, 10, 5458–5462 CrossRef PubMed.
- N. Arunkumar, D. B. Bucher, M. J. Turner, P. TomHon, D. Glenn, S. Lehmkuhl, M. D. Lukin, H. Park, M. S. Rosen and T. Theis, PRX Quantum, 2021, 2, 10305 CrossRef.
- N. Eshuis, B. J. A. van Weerdenburg, M. C. Feiters, F. P. J. T. Rutjes, S. S. Wijmenga and M. Tessari, Angew. Chem., Int. Ed., 2015, 54, 1481–1484 CrossRef CAS PubMed.
- L. Sellies, I. Reile, R. L. E. G. Aspers, M. C. Feiters, F. P. J. T. Rutjes and M. Tessari, Chem. Commun., 2019, 55, 7235–7238 RSC.
- N. Eshuis, N. Hermkens, B. J. A. van Weerdenburg, M. C. Feiters, F. P. J. T. Rutjes, S. S. Wijmenga and M. Tessari, J. Am. Chem. Soc., 2014, 136, 2695–2698 CrossRef CAS PubMed.
- N. Eshuis, R. L. E. G. Aspers, B. J. A. van Weerdenburg, M. C. Feiters, F. P. J. T. Rutjes, S. S. Wijmenga and M. Tessari, Angew. Chem., Int. Ed., 2015, 54, 14527–14530 CrossRef CAS PubMed.
- V. Daniele, F. Legrand, P. Berthault, J. Dumez and G. Huber, ChemPhysChem, 2015, 16, 3413–3417 CrossRef CAS PubMed.
- N. Reimets, K. Ausmees, S. Vija and I. Reile, Anal. Chem., 2021, 93, 9480–9485 CrossRef PubMed.
- L. Guduff, P. Berthault, C. Van Heijenoort, J. Dumez and G. Huber, ChemPhysChem, 2019, 20, 392–398 CrossRef CAS PubMed.
- R. L. Stedman, Chem. Rev., 1968, 68, 153–207 CrossRef CAS PubMed.
- S. Glöggler, M. Emondts, J. Colell, R. Müller, B. Blümich and S. Appelt, Analyst, 2011, 136, 1566–1568 RSC.
- W. H. Duckworth, Improving NMR Sensitivity: The Synthesis and SABRE Evaluation of Nicotine Isotopologues, PhD thesis, University of York, 2018.
- W. Iali, G. G. R. Green, S. J. Hart, A. C. Whitwood and S. B. Duckett, Inorg. Chem., 2016, 55, 11639–11643 CrossRef CAS PubMed.
- A. J. Ruddlesden and S. B. Duckett, Chem. Commun., 2016, 52, 8467–8470 RSC.
- B. J. Tickner, V. V Zhivonitko and V.-V. Telkki, Phys. Chem. Chem. Phys., 2021, 23, 16542–16550 RSC.
- E. B. Dücker, L. T. Kuhn, K. Münnemann and C. Griesinger, J. Magn. Reson., 2012, 214, 159–165 CrossRef PubMed.
- M. Fekete, P. J. Rayner, G. G. R. Green and S. B. Duckett, Magn. Reson. Chem., 2017, 55, 944–957 CrossRef CAS PubMed.
- K. X. Moreno, K. Nasr, M. Milne, A. D. Sherry and W. J. Goux, J. Magn. Reson., 2015, 257, 15–23 CrossRef CAS PubMed.
- R. V Shchepin, D. A. Barskiy, A. M. Coffey, T. Theis, F. Shi, W. S. Warren, B. M. Goodson and E. Y. Chekmenev, ACS Sens., 2016, 1, 640–644 CrossRef PubMed.
- V. Jäger, D. Schröter and B. Koppenhoefer, Tetrahedron, 1991, 47, 2195–2210 CrossRef.
- W. Iali, S. S. Roy, B. J. Tickner, F. Ahwal, A. J. Kennerley and S. B. Duckett, Angew. Chem., 2019, 131, 10377–10381 CrossRef.
- B. J. Tickner, F. Ahwal, A. C. Whitwood and S. B. Duckett, ChemPhysChem, 2021, 22, 13–17 CrossRef CAS PubMed.
- S. Knecht, S. Hadjiali, D. A. Barskiy, A. Pines, G. Sauer, A. S. Kiryutin, K. L. Ivanov, A. V. Yurkovskaya and G. Buntkowsky, J. Phys. Chem. C, 2019, 123, 16288–16293 CrossRef CAS.
- B. J. Tickner, J. S. Lewis, R. O. John, A. C. Whitwood and S. B. Duckett, Dalton Trans., 2019, 48, 15198–15206 RSC.
- M. E. Gemeinhardt, M. N. Limbach, T. R. Gebhardt, C. W. Eriksson, S. L. Eriksson, J. R. Lindale, E. A. Goodson, W. S. Warren, E. Y. Chekmenev and B. M. Goodson, Angew. Chem., 2020, 132, 426–431 CrossRef.
- K. Tokmic, R. B. Greer, L. Zhu and A. R. Fout, J. Am. Chem. Soc., 2018, 140, 14844–14850 CrossRef CAS PubMed.
- S. R. Muhammad, R. B. Greer, S. B. Ramirez, B. M. Goodson and A. R. Fout, ACS Catal., 2021, 11, 2011–2020 CrossRef CAS.
- B. J. Tickner, R. R. Parker, A. C. Whitwood and S. B. Duckett, Organomet, 2019, 38, 4377–4382 CrossRef CAS PubMed.
- R. E. Mewis, K. D. Atkinson, M. J. Cowley, S. B. Duckett, G. G. R. Green, R. A. Green, L. A. R. Highton, D. Kilgour, L. Lloyd, J. A. B. Lohman and D. C. Williamson, Magn. Reson. Chem., 2014, 52, 358–369 CrossRef CAS PubMed.
- P. TomHon, E. Akeroyd, S. Lehmkuhl, E. Y. Chekmenev and T. Theis, J. Magn. Reson., 2020, 312, 106700 CrossRef CAS PubMed.
- L. T. Kozlowski, N. Y. Mehta, C. T. Sweeney, S. S. Schwartz, G. P. Vogler, M. J. Jarvis and R. J. West, Tob. Control, 1998, 7, 369–375 CrossRef CAS PubMed.
- T. Cheng, Tob. Control, 2014, 23, 11–17 CrossRef PubMed.
- P. J. Rayner, B. J. Tickner, W. Iali, M. Fekete, A. D. Robinson and S. B. Duckett, Chem. Sci., 2019, 10, 7709–7717 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07376a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |