 Open Access Article
Open Access ArticleMetal–organic frameworks (MOFs) based nanofiber architectures for the removal of heavy metal ions
Heja Ibrahim Adil
a,
Mohammad R. Thalji
 f,
Suhad A. Yasin
a,
Ibtisam A. Saeed
a,
Mohammed A. Assiri
cd,
Kwok Feng Chong
b and
Gomaa A. M. Ali
f,
Suhad A. Yasin
a,
Ibtisam A. Saeed
a,
Mohammed A. Assiri
cd,
Kwok Feng Chong
b and
Gomaa A. M. Ali
 *e
*e
aCollege of Science, University of Duhok, Duhok, 42001, Iraq
bFaculty of Industrial Sciences & Technology, Universiti Malaysia Pahang, Gambang, 26300 Kuantan, Malaysia
cResearch Center for Advanced Materials Science (RCAMS), King Khalid University, Abha, Kingdom of Saudi Arabia
dDepartment of Chemistry, Faculty of Science, King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia
eChemistry Department, Faculty of Science, Al-Azhar University, Assiut 71524, Egypt. E-mail: gomaasanad@azhar.edu.eg
fIndependent Researcher, Amman, Jordan
First published on 7th January 2022
Abstract
Environmental heavy metal ions (HMIs) accumulate in living organisms and cause various diseases. Metal–organic frameworks (MOFs) have proven to be promising and effective materials for removing heavy metal ions from contaminated water because of their high porosity, remarkable physical and chemical properties, and high specific surface area. MOFs are self-assembling metal ions or clusters with organic linkers. Metals are used as dowel pins to build two-dimensional or three-dimensional frameworks, and organic linkers serve as carriers. Modern research has mainly focused on designing MOFs-based materials with improved adsorption and separation properties. In this review, for the first time, an in-depth look at the use of MOFs nanofiber materials for HMIs removal applications is provided. This review will focus on the synthesis, properties, and recent advances and provide an understanding of the opportunities and challenges that will arise in the synthesis of future MOFs–nanofiber composites in this area. MOFs decorated on nanofibers possess rapid adsorption kinetics, a high adsorption capacity, excellent selectivity, and good reusability. In addition, the substantial adsorption capacities are mainly due to interactions between the target ions and functional binding groups on the MOFs–nanofiber composites and the highly ordered porous structure.
1. Introduction
Heavy metal ions (HMIs) are the most common environmental pollutants found in water sources. Toxic HMIs with densities above 5 g cm−3 in water, including arsenic (As), lead (Pb), mercury (Hg), cobalt (Co), chromium (Cr), copper (Cu), cadmium (Cd), antimony (Sb), and uranium (U), are considered pollutants and represent a significant global problem.1,2 Owing to their incorporation into the atmosphere, these ions are hazardous and harmful to human health.3 The HMIs in aquatic life can damage the foundations of life, especially the food system.4 As a result, their removal has become a major requirement.5 Most of these HMIs are present in the cationic form in water (Mn+), and some of them are present in the anionic form, such as As (arsenate AsO43−, arsenite AsO33−),6 and Cr (in which HCrO4− and Cr2O72− are the main forms for Cr(VI) below pH = 3).6 Many strategies have been used to remove HMIs from water, including nanofiltration,7 solvent extraction and ion exchange,8,9 the use of resins,10 photocatalytic degradation,11,12 chemical precipitation, ion exchange, cooling, chemical deposition, biological treatment, reverse osmosis, and adsorption.4,13–16 Adsorption is one of the most effective treatment strategies as it does not require high temperatures and can simultaneously adsorb many chemicals.12,17,18 Activated carbon (AC), clay minerals, chitosan, and bi-layer hydroxides are among the porous natural resource materials that have been reported.19–21 However, these materials show poor processing and selectivity. On the other hand, it is generally insufficient from a social, ecological, and commercial point of view.22 For example, some metal ions oxidize and dissolve porous adsorbents containing organic acids, such as organic synthetic polymers, leading to secondary pollutants and, more importantly, the inability to achieve a low representation of metal ions. Therefore, to overcome these difficulties and limitations, a liquid is required.Metal–organic frameworks (MOFs) are low-density crystalline structures containing metal ion units or clusters to form compounds and they have recently attracted significant interest.13,23 The hydro (solvo) thermal, mechanochemical, sonochemical, electrochemical, layer-by-layer development, and micro-processing methods can enable processing of these compounds at temperatures up to 220 °C and low air pressures with a pH in the range of 1 to 10.24 Element modules and organic linkers can be combined in an infinite number of ways to create novel structures with fascinating properties for various applications, such as heterogeneous catalysis and photocatalysis,25 drug delivery,26 energy collection,27 gas storage,28 sensing,29 adsorption,30 and the identification of dangerous chemicals.31
MOFs-based materials are effective adsorbents for HMIs removal owing to their large surface areas, good physicochemical properties, high porosity, and intelligent structure32. MOFs can be employed directly as heavy metal ion (HMI) adsorbents. However, the organic ligands in pure MOFs usually lack active functional groups, which leads to an inadequate adsorption capability. For example, MIL-100(Fe) could remove Pb(II) and Cd(II) with an adsorption capacity of 28.29 and 8.79 mg g−1, respectively.33 Various formulations of MOFs materials, such as composite beads, are used to remove HMIs owing to the tunable nature of MOFs. Boix et al.34 reported microbead composites obtained by incorporating inorganic nanoparticles into MOFs (CeO2@UiO-66 and CeO2@UiO-66-(SH)2) for the removal of various metals from water, including As(III) and Cr(VI), with a 31% and 68% adsorption efficiency, respectively. In another work, the adsorption performance of Pb(II) in an aqueous solution was assessed by Jin et al. using carboxymethyl cellulose–coated metal–organic material (MOF-5–CMC) as a possible adsorbent with a 79% adsorption efficiency within 120 min at an optimal pH of 5–6.35
Metal organic framework (MOF) and nanofiber designs have been combined in recent years to improve the overall performance of MOF–nanofiber composites structures for the removal of HMIs.36 MOFs nanofibers have a high porosity, a large specific surface area, and numerous active sites, making them ideal for the adsorption of HMIs. MOF nanoparticles can also form on the nanofiber substrate, reducing aggregation and exposing additional adsorption sites. In addition, their membranes offer the advantages of flexibility and a high loading capacity. Owing to their organic–inorganic nature and low density, MOFs are more compatible with polymer fibers than other MOF-based composites, including metals, metal oxides, and clays.37 Accordingly, the combination of MOFs with polymer fibers enables the formation of fiber composites with advantages over conventional materials. They offer improved molecular transport through the material and easier access to the active sites of MOFs. These properties make composites attractive for air filtration, biomedical delivery, water treatment, and sensor applications.37 For example, the limitations of some catalysts are associated with limited diffusion to active sites, and MOF–nanofiber composites can overcome these limitations owing to their open networks and tunable functionality.38
Ostermann et al. produced the MOF polymer–nanofiber composite from a zeolitic imidazolate framework-8 (ZIF-8) and polyvinylpyrrolidone (PVP) in the electrospinning process.39 This combination took advantage of both types of material to create a unique class of hierarchical nanostructures that makes the composite structures highly attractive for environmental applications. Efome et al. published the first innovative work on developing a nanofiber MOF membrane to separate heavy metals (Pb and Hg) from an aqueous medium.40
The removal of HMIs from water by MOFs has been the subject of several excellent reviews. In particular, to the best of our knowledge, no review articles have focused on MOFs–nanofiber composites for removing HMIs. Kobielska et al.41 summarized several research papers covering various areas that remove HMIs from water using MOFs. Yan et al.42 focused on recent advances in MOF materials and MOF membranes to explore their applications for removing Hg ions from water. Chen et al.43 mainly highlighted the recent developments in applying water-stable MOFs and MOF-based materials for the decontamination of HMIs. The most recent review by Li et al.44 focused on the recent advances in using water-stable MOFs to reduce HMIs in water through adsorption and photocatalysis. However, there is no discussion of the substantial removal of HMIs by MOFs–nanofiber composites, or the information provided is not clear and easily accessible. A literature review was performed by scanning the published materials using the Scopus database. The keywords “MOFs, nanofiber, and metal ions” were used as the search keywords. Therefore, for the first time, this review focuses on recent developments of MOFs on nanofibers for the removal of HMIs with many illustrative examples and a thorough investigation of the influencing factors and interaction processes. There are also debates about the challenges and potential of using MOFs in this area.
2. Overview of metal–organic frameworks
Metal organic frameworks consist of diversified metal centers and various organic ligands and have adjustable structures.45,46 They have attracted significant attention owing to their unique properties and numerous functions.47 The use of MOFs as self-templates or analogs for producing porous metal oxides by thrombolysis within regulated calcination situations has long been a controversial research topic.47 Materials have been used in catalytic solutions, either as salts or compounds, to accelerate various chemical processes.48 MOFs are a novel porous layer material that differ from typical porous materials in terms of the porosity and thermal stability. To date, over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 different MOFs structures have been identified and researched.49
000 different MOFs structures have been identified and researched.49
Many toxins are released into the atmosphere owing to industry and the growth of production that are seriously harmful to human health. It is, therefore, necessary to implement successful strategies for dealing with these pollutants.50 They offer great flexibility in construction and are good choices for many different applications.51 The intrinsic diversity of MOFs enables precise monitoring of the physio-chemical properties of the structures.50 The poor insulation performance of MOF membranes is usually caused by inconsistent growth at the auxiliary membrane gateway, poor moisture balance, and poor control over porous material pathways.52
Metal organic frameworks have a large surface area, a good pore volume configuration, and a wide range of chemical properties, frameworks, and post-synthetic mechanisms that can profoundly alter the chemical structure of the MOF. Thus, MOFs are ideal for various applications owing to these properties. Gas isolation, adsorption, selective catalysis, sensors, and potential drugs carriers are just a few examples .53 In addition, by adding chemical bonds to linkers, specific MOFs can be further tailored to the desired application. Owing to their versatility, MOFs are effective in various fields, including natural gas and insulation.54 However, among these purposes, the composition and microstructure of MOFs are not the only factors that should be considered. The specific surface area and the number of material defects can significantly influence their behavior. Coordination modulation can be used to intelligently monitor the material morphology, density, defects, and crystallinity of the MOFs.55 In addition, the incorporation of unique nanoparticles into MOFs creates a much more stable substance owing to their constant micro-porosity.56
2.1 Synthesis of metal–organic frameworks on nanofibers
Natural polymers, such as carbon nanotubes (CNTs), minerals, metal oxides, and multicomponent hybrids are crucial for environmental applications. Depending on the application, porous materials can be processed using various synthetic methods, such as coarse gelling and soft templating.57 Here, MOF model pyrolysis can be used to develop nanostructured carbon-based products.58 On the other hand, soft synthesis is based on even more complex physical or chemical associations between the system source and the prototype to guide the specific formation and better regulate the mechanical properties.57 Weak fabrication is a more active way of fabricating ordered and disordered porous matrices than the other two structure-directing approaches.57 Amorphous materials are typically produced using the solvothermal process as this is a gentle structure-directing method. MOFs can be used as a pure precursor metal to fabricate various nanoparticles made from MOFs, and the organic components are being used as a carbon source.57 Based on their final microstructures, MOFs can be made using various synthetic routes, including slow diffusion, hydrothermal (solvothermal), electrochemical mechanochemical, microwave-assisted heating, and ultrasound methods.59 MOFs consist of two main components, metal centers and organic linkers. For this reason, their configurations can be tailored to achieve optimal properties for the particular application. Synthesis strategies for the fabrication of MOFs nanofiber materials can be broadly classified as in situ MOFs on nanofiber surfaces and synthetic composites from pre-synthesized MOFs.Liu et al. used electrospun nanofibers made of poly(acrylic acid), poly(vinyl), and SiO2 in a MOF precursor to increase the loading of the MOFs on the surfaces.62 The precursor then supported four different MOFs. HKUST-1 and MIL-53(Al) were applied to fiber substrates using a conventional solvothermal method, while ZIF-8 and MIL-88B(Fe) were integrated into a microwave solvothermal synthesis. They achieved minimal MOF growth after one cycle of the solvothermal synthesis, even though functional groups (–OH and –COOH) were presented on their fiber surface. The repetition of the solvothermal synthesis process produced composite samples with the designations HKUST-1-m1, HKUST-1-m2, HKUST-1-m3, and HKUST-1-m4 (Fig. 1a–d). As the growth cycles were repeated, more MOF crystals were formed in the fiber, as shown in the powder X-ray diffraction pattern (PXRD), as shown in Fig. 1e.
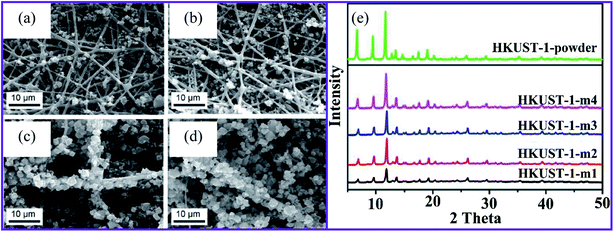 | ||
| Fig. 1 SEM images of HKUST-1 on nanofibers after (a) one, (b) two, (c) three, and (d) four repetitions of the growth cycles, (e) XRD patterns for the HKUST-1 MOF composites, this figure has been adapted from ref. 62 with permission from American Chemical Society, copyright 2016. | ||
In another study, HKUST-1 MOF, also known as Cu-BTC, was synthesized on pretreated anionic electrospun cellulose using a layer-by-layer approach that resulted in a nanoporous material.63 The anionic pretreatment improved the synthesis of HKUST-1 on the surface of the nanofibers. The carboxymethylated electrospun cellulose was best suited for HKUST-1 functionalization because the HKUST-1 crystals were evenly distributed on the nanofiber surface. The approach described for the surface modification of cellulose for the synthesis of MOF/cellulose materials presented in this work opens up possibilities for MOF synthesis on other cellulose materials, such as nanocrystalline cellulose and micro-fibrillated cellulose.
Ma et al.64 developed a green, aqueous, and template-free synthetic approach for textile-fiber production to produce zirconium MOFs (UiO-708 and MOF-66-NH2). They found that trifluoroacetic acid (TFA) plays a crucial role as a modulator in synthesizing a consistent growth morphology on a fiber surface. Owing to the direct application of TFA, the fibrous carrier was used exclusively in MOF crystallization and not as an open-flowing solvent suspended powder. Interestingly, most MOF particles were replaced by TFA with other organic acids, such as acetate acid and formic acid, resulting in a non-homogeneous MOF coating on the fiber. The conductive nickel-2,3,6,7,10,11-hexahydroxytriphenylene (Ni-HHTP) and nickel-2,3,6,7,10,11-hexaaminotriphenylene (Ni-HITP) nanosheets on CNFs have been successfully synthesized using interfacial synthesis for the formation of nanofibrillar CNF@ c-MOF (Fig. 2a).65 With this strategy in mind, we proposed that the fabrication of continuous nucleated c-MOFs nanosheets on CNFs would increase the electrical conductivity of nanocomposites by reducing the grain boundaries and contact resistance, the c-MOFs also increased the flexibility of the nanocomposite.
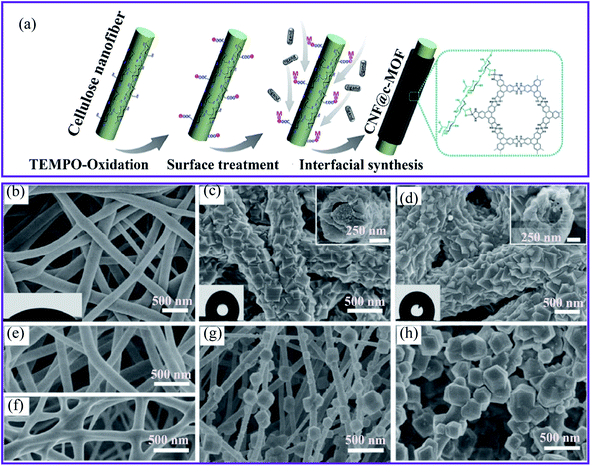 | ||
| Fig. 2 (a) Schematic diagram of the synthesis of CNF@c-MOF nanofibers.65 SEM images of (b) the electrospun silk nanofibers, (c) ZIF-8/nanofibers, the inset shows the sectional view from the top, (d) ZIF-67/nanofibers with the sectional view shown in the inset, (e) polyacrylonitrile, (f) polyurethane, (g) PAN@ZIF-8 and (h) PU@ZIF-8 membranes, this figure has been adapted from ref. 36 with permission from the Royal Society of Chemistry, copyright 2018. | ||
The second method of growing MOFs on nanofibers is known as biomineralization synthesis. It refers to the process of increasing the mineralization of inorganic materials to make the biomineral shells and the generation of skeletal tissue more robust, thereby providing a solid anchoring surface. For example, two zeolitic imidazole framework (ZIF-8 and ZIF-67) MOFs were grown on electrospun silk nanofibers as a biomacromolecule substrate to produce MOFs–fiber composites.36 The composites were covered using crystalline coatings (Fig. 2b–d) and PXRD was used to verify the structures of the desired ZIFs. In addition, the SEM image recordings of sectional views revealed a core–shell structure. In particular, the MOF layer had a unique intergrown architecture, which indicates the excellent stability of the layer. Furthermore, the authors investigated two synthetic nanofibers, polyacrylonitrile (PAN) and polyurethane. Nonetheless, the manufacture of these synthetic polymer substrates resulted in loosely developed patterns of poorly homogeneous fibers (Fig. 2e–h), underscoring the importance of chemical structures for inducing MOF nucleation and the formation of silk bio-substrates.
The layer-by-layer process (LbL) improves the growth of MOFs on fiber substrates with functional groups, such as COOH and NH2. For example, Morsali and colleagues investigated an ultrasound-assisted LbL technique for growing CuBTC MOF66 and zinc terephthalate MOF (MOF-5)67 on a silk fiber surface. The silk substrates were successively dipped into a metal salt solution, and they were washed using an organic ligand solution to remove any unreacted precursors. In both cases, the sonication increased the LbL growth process and enhanced the homogeneity of the MOF coating on silk fibers. However, this technique hampered the production of MOF–silk composites with a high mass loading and full coverage. The lack or low density of functional groups on the untreated surface of the fiber made it impossible for MOF anchoring events to occur during the LbL process, which probably caused the inadequate coverage.
Another technique was developed to fabricate MOFs–nanofiber composites that directly took advantage of the coordination replication from an inexpensive, insoluble precursor. Fig. 3a shows the method established by Kitagawa and colleagues.68 The authors succeeded in creating a well-structured MOF architecture [Al(OH)(ndc)]n by morphological substitution of a shaped sacrificial metal oxide (Al2O3), which served both as a metal ion source and as an architecture-controlling agent. As can be seen in Fig. 3b, the field emission scanning electron microscopy (FESEM) image demonstrated that the fibrous architectures were retained even after the conversion of aluminum oxide to aluminum-based [Al(OH)(ndc)]n. Characteristic block crystals with a size of several hundred nanometers were grown on the surface of the aluminum oxide nanofibers. In addition, this method provided 1D nanofibers based on Al–MOF. A simple solution-processed technique was used to make alumina nanofibers for 1-D Al–MOF nanofiber manufacture.
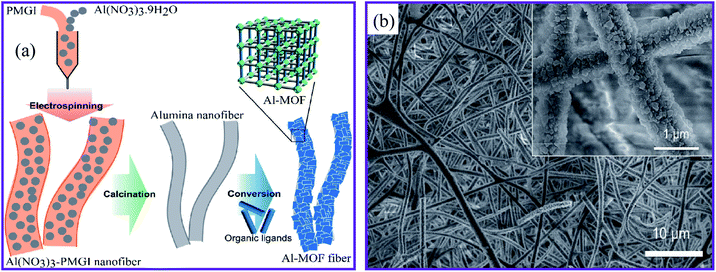 | ||
| Fig. 3 (a) Schematic presentation of the synthesis of the Al–MOF fibrous architectures. (b) FESEM image of the Al–MOF nanofibers at pH 4.3, this figure has been adapted from ref. 68 with permission from the Chemical Society of Japan, copyright 2014. | ||
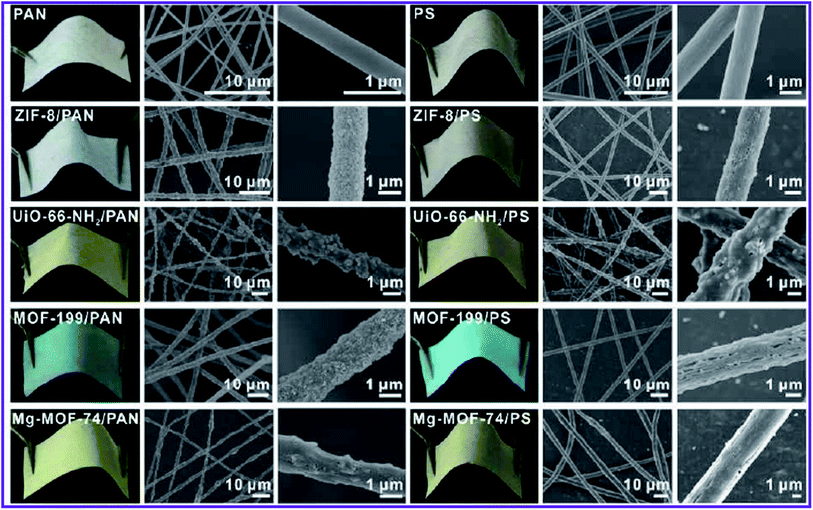 | ||
| Fig. 4 Photographs and SEM images of MOF–PAN nanofibers and MOF–PS nanofibers composites supported on a piece of fabric, this figure has been adapted from ref. 71 with permission from American Chemical Society, copyright 2016. | ||
Interestingly, MOF nanoparticles retained their porosity and surface area after post-synthetic deposition on the fiber. They also used various carrier substrates, such as metal mesh or fleece, to reinforce the structure of the MOFs–nanofiber composite and increase its resilience. Although structurally improved, electrospun composites could not be used in other media such as DMF owing to the solubility of polymer substrates in these polar solvents.
The electrospinning approach to post-synthetic MOF deposition on fibers was developed by López-Maya et al.72 They developed a method by which MOF nanoparticles can be inserted into silk nanofibers, a novel approach for doping nanofibers during electrospinning. The suspension of zirconium 1,4-dicarboxybenzene MOF (UiO-66 MOF), which is an acronym for Universiteteti (Oslo),73 was scattered over the silk nanofibers during the electrospinning process. In the empty silk nanofiber substrate, the incorporated MOF nanoparticles created a UiO-66–nanofiber composite. Although this method provides significant mass loading of the MOF loading in the composite, the low interactions between the MOF and the fiber led to an unstable composite. The leaching of the functional MOF nanoparticle limits the economic viability of this manufacturing process.
The microwave heating approach was used by Jamshidifard et al. to create UiO-66-NH2 MOF. The UiO-66-NH2 MOF was then electrospun onto the surface of the PAN-chitosan nanofibers to produce the UiO-66-NH2–PAN-chitosan nanofiber composite. The XRD patterns show that the UiO-66-NH2 MOF was effectively integrated into the PAN-chitosan nanofibers, and the electrospinning process did not affect the crystal structure UiO-66-NH2 MOF.74 There was no significant change in the observed peak spectrum for UiO-66-NH2 and PAN-chitosan in the Fourier transform infrared spectroscopy (FTIR) spectra, indicating the combination of UiO-66-NH2 and PAN-chitosan did not influence the chemical structure of UiO-66-NH2 MOF.
2.2 MOFs–nanofiber composite characteristics
Porous morphologies exist in many materials, varying from carbon-based to metal-based materials (oxides, carbides, chalcogenides, and phosphides).75–77 Porous materials contain voids that are interconnected to form channels that positively affect the design of materials with attractive properties. MOFs have a high porosity that can reach up to 90% of the free volume, and possess large internal surface areas, approaching 7000 m2 g−1, and concentrated metal sites.78,79 Therefore, MOFs have shown significant promise in various applications, including sensing, gas separation, storage, catalysis, and electrical and optical devices.80,81 Owing to the high porosity of the MOF structure, the choice of supporting substrate can influence the flexibility, robustness, and processability of the MOF-based composite. Fiber materials are among the least expensive and most abundant substrates for MOF-based composites. Tuning the polymer composition, fiber thickness, permeability, porosity, and flexural rigidity allows us to target perfect characteristics for different applications. For example, the diameter and porous structure of the PAN-chitosan–UiO-66-NH2 nanofiber composite can be effectively adjusted by changing the loading ratio of the UiO-66-NH2 MOF.74 Fig. 5 shows scanning electron microscopy (SEM) images of nanofiber composites with different UiO-66-NH2 ratios. For PAN-chitosan nanofibers, smooth nanofibers with an average diameter of 235 nm were produced. The SEM images of 5 and 10 wt% of UiO-66-NH2 MOF-loaded nanofibers showed a well-defined shape with average diameters of 275 and 345 nm, respectively.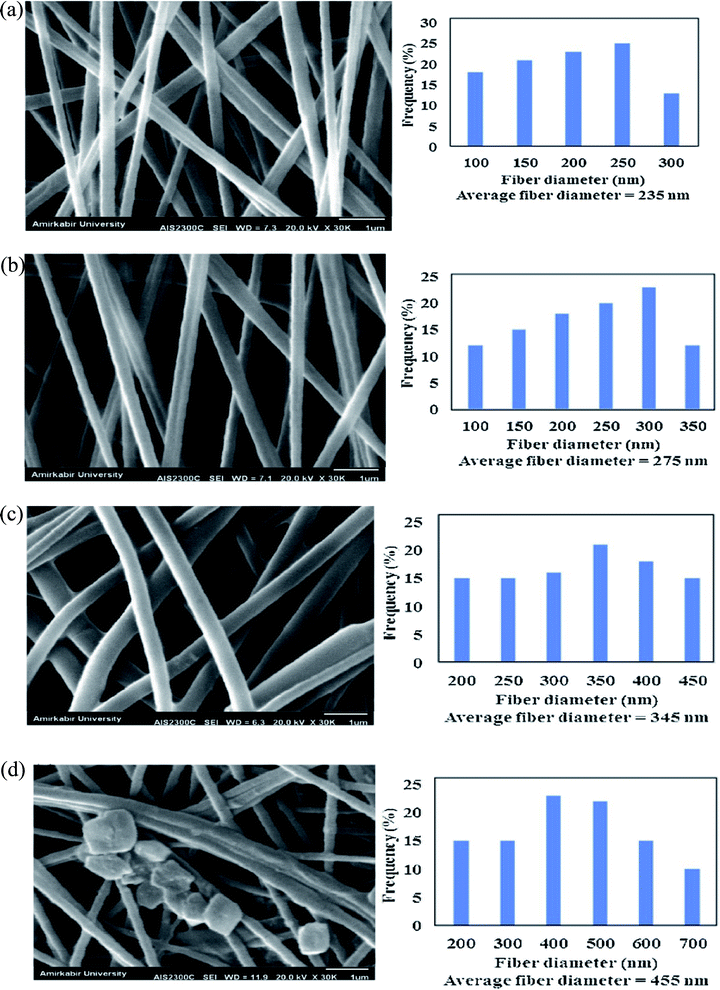 | ||
| Fig. 5 SEM images and fiber diameter distribution of (a) PAN-chitosan nanofiber and nanofibers containing (b) 5 wt% UiO66-NH2 MOF, (c) 10 wt% UiO66-NH2 MOF and (d) 15 wt% UiO66-NH2 MOF, this figure has been adapted from ref. 74 with permission from Elsevier, copyright 2019. | ||
Compared to pure PAN-chitosan nanofibers, the fiber diameter of PAN-chitosan increases by 5 and 10 wt% UiO-66 MOF, enhancing the solution viscosity. MOF crystals were discovered on the surface of PAN-chitosan–UiO-66-NH2 nanofibers with 15 wt% MOF, the behavior of which is attributed to the dispersion of the MOF crystals, both in the inner pores of the nanofibers and the surface of the nanofibers. The aggregation of UiO-66-NH2 MOF crystals at higher MOF concentrations (15 wt%) can lead to the formation of MOF crystals with larger sizes than those of the PAN-chitosan nanofiber diameter and, as a result, exposed UiO-66-NH2 MOF crystals on the surface of the nanofibers. The porosity of the nanofibrous membranes containing 0, 5, 10, and 15 wt% of UiO-66-NH2 MOF were determined to be 87, 82, 78, and 71%, respectively.
3. HMIs removal via MOF-based nanofibers materials
Controlling HMIs in water is challenging owing to their bioavailability and non-degradability. Owing to their longevity, HMIs are more important than other pollutants, such as organic compounds. Researchers have studied various materials and methods of removing HMIs for many years.Adsorption is popular owing to its adaptability, simplicity, cost-effectiveness, and easy replication.82,83 Activated carbons, molecular sieves, chitosan, polymers, and biomass materials are examples of materials with a traditional adsorption capacity.84 However, they have a low scalability, sensitivity, and, above all, adsorptive capacity. This creates the opportunity to develop innovative adsorbents with a good specificity and adsorption potential for environmental and water detoxification applications.
3.1 Removal of bivalent heavy metal ions
Peng et al.85 reported the fabrication of ZIF-8 MOF on electrospun PAN nanofiber membranes (ZIF-8–PAN nanofibers) using an in situ growth technique that includes electrospinning and hot-pressing (Fig. 6a). The ZIF-8–PAN nanofiber membrane exhibited a high water flux of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1 with a high filtration efficiency of 96.5% for Cu(II) removal. In addition, the ZIF-8–PAN nanofibers showed a strong dynamic adsorption and remarkable filtering efficiency (96.5%). In addition, the removal rate of Cu(II) in 4 min reached 34.1% using a combination of adsorption and electrochemistry. The adsorption results demonstrated that the adsorption kinetics of Cu(II) ions on ZIF-8–PAN nanofibers agree well with the second-order model. The maximum capacity was 225.62 mg g−1. Furthermore, the excellent durability of the ZIF-8/PAN nanofiber makes it a suitable membrane for real water treatment applications. The investigations showed that the high specific surface area, abundant active sites of the ZIF-8 nanoparticles, and the reduced equivalent apparent pore size of the membrane by hot pressing contributed to the superior filtration performance of ZIF-8–PAN for Cu(II) ions.
000 L m−2 h−1 with a high filtration efficiency of 96.5% for Cu(II) removal. In addition, the ZIF-8–PAN nanofibers showed a strong dynamic adsorption and remarkable filtering efficiency (96.5%). In addition, the removal rate of Cu(II) in 4 min reached 34.1% using a combination of adsorption and electrochemistry. The adsorption results demonstrated that the adsorption kinetics of Cu(II) ions on ZIF-8–PAN nanofibers agree well with the second-order model. The maximum capacity was 225.62 mg g−1. Furthermore, the excellent durability of the ZIF-8/PAN nanofiber makes it a suitable membrane for real water treatment applications. The investigations showed that the high specific surface area, abundant active sites of the ZIF-8 nanoparticles, and the reduced equivalent apparent pore size of the membrane by hot pressing contributed to the superior filtration performance of ZIF-8–PAN for Cu(II) ions.
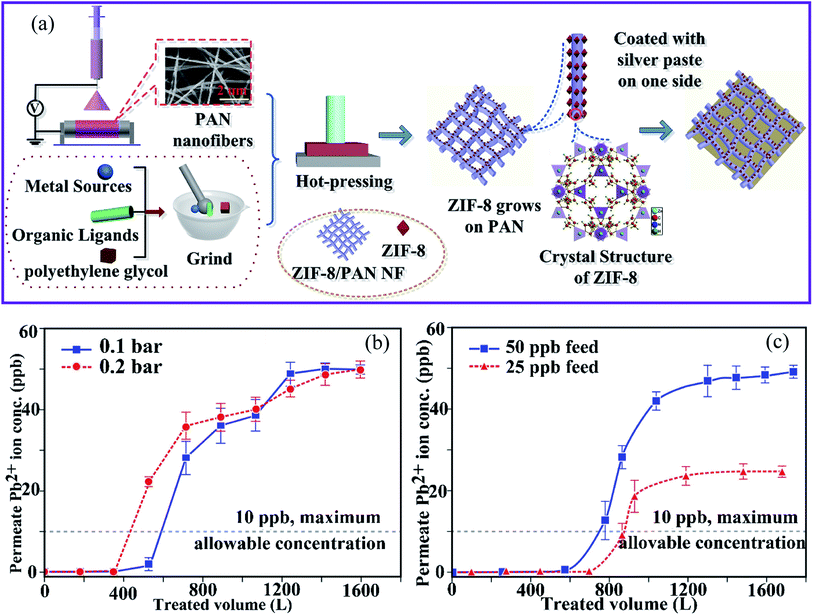 | ||
| Fig. 6 (a) Schematic diagram of the preparation of ZIF-8–PAN–Ag, this figure has been adapted from ref. 85 with permission from Elsevier, copyright 2020. Effects of (b) changes in TMP relative to the permeate quality and (c) initial lead ion concentration in the feed on the permeate lead ion concentration (TMP = 0.1 bar, flux = 348.9 kg m−2 h−1 and TMP = 0.2 bar, flux = 693.6 kg m−2 h−1, membrane thickness = 560 μm, feed concentration = 41.7 × 10−3 mg L−1, and ambient temperature), this figure has been adapted from ref. 86 with permission from Elsevier, copyright 2019. | ||
Similarly, Efome et al.86 reported on the fabrication of PAN–MOF-808 nanofibers using co-electrospinning of MOF-808 and the PAN polymer. These were then used as adsorption filters to remove Pb(II) ions from an aqueous medium to investigate the effect of various process parameters, such as transmembrane pressure (TMP), concentration, and membrane thickness. It was found that a decrease in TMP and in the initial feed concentration, and an increase in the membrane thickness have the greatest influence on increasing the treated feed volume (Fig. 6b and c). On the other hand, the moderate adsorption capacity (18–37%) was attributed to the existence of other coexisting ions in water (e.g., Na+, Mg2+, and Ca2+), as well as the influence of their electronegativity and the ionic radius of Pb(II).
The UiO-66-NH2 MOF crystal was developed and integrated by Jamshidifard et al.74 into PAN-chitosan nanofiber membranes to produce the UiO-66-NH2–PAN-chitosan composite. The synthesized MOFs and nanofibers were used to remove the Pb(II) and Cd(II) ions through adsorption and membrane filtration. The UiO-66-NH2 MOF crystals were observed on the surface of the nanofiber, and this behavior can be attributed to the distribution of the MOF crystals in the inner pores and surface of the nanofibers. The capacity for metal ion sorption was improved by increasing the initial Pb(II) and Cd(II) levels and therefore increasing the numbers of metal ions available for chelation with the adsorbent binding sites. Saturation at a high concentration of the active metal ion centers of the PAN-chitosan–UiO-66-NH2 nanofiber adsorbent resulted in a slight change in the Pb(II) and Cd(II) adsorbent capacity for aqueous solutions. In addition, the slight decrease in the adsorptive capacity of Pb(II) and Cd(II) ions using the composite nanofiber adsorbent after five regeneration cycles demonstrated the excellent reusability of the adsorbent for practical applications. The sorption data could be well fitted using the Redlich–Peterson isotherm model, indicating that the monolayer adsorption of Pb(II) and Cd(II) ions was the predominant reaction. In addition, the high water flux and good removal of ions within 18 h of the filtration time showed the high potential of the UiO-66-NH2–PAN-chitosan membrane for the removal of metal ions from aqueous solutions.
The MOF composite membrane was fabricated by co-electrospinning of the Zr-based MOF-808 ([Zr6O4(OH)4(COOH)6 (BTC)2]) and hydrophilic PAN nanofibers after using different routes to activate MOF-808 to increase the adsorption capacity (Fig. 7a) for the removal of Cd(II) and Zn(II) ions from an aqueous solution.87 The composite membrane containing 20 wt% MOF was used for the removal of Cd(II) and Zn(II) ions from an aqueous solution (Fig. 7b and c). Owing to the incorporation of the MOF particles, the PAN–MOF-808 composite had a significantly larger fiber diameter than that of the PAN, with pore diameters ranging from 0.5–1 μm, which contributed to an increase in the surface roughness. The highest adsorptive capacities of Cd(II) and Zn(II) were 225.05 and 287.06 mg g−1, respectively.
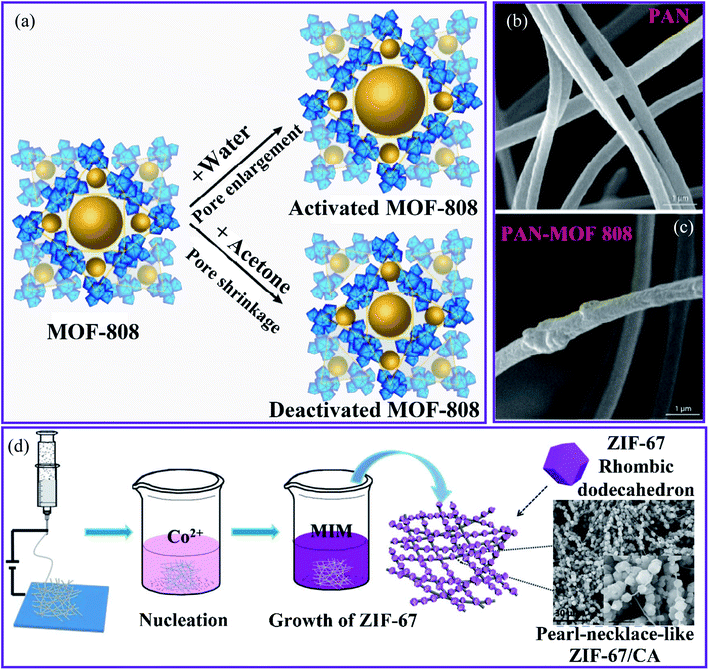 | ||
| Fig. 7 (a) The activation routes of MOF-808. SEM images of the nanofibrous membranes of (b) PAN and (c) PAN–MOF-808, this figure has been adapted from ref. 71 and 87 with permission from American Chemical Society, copyright 2018. (d) Schematic representation of the synthesis of the ZIF-67–CA composite, this figure has been adapted from ref. 88 with permission from Willey Online Library, copyright 2018. | ||
The MOF-808–PAN membrane exhibited a high water flux of 348 L m2 h−1 for Cd(II) owing to the high adsorptive capacity of the MOF and its ability to be accessed in the enclosed form. In addition, the Langmuir adsorption isotherm model is more suitable than the Freundlich and Temkin models in terms of consistent experimental data. Thus, the highest adsorptive capacities found for Cd(II) and Zn(II) were 225.05 and 287.06 mg g−1, respectively. The MOF-808–PAN membrane has been suggested as a suitable membrane for water treatment because of its excellent separation performance, reusability, and superior water stability.
Hou et al.88 used a simple in situ growth technique to generate a zeolitic imidazolate framework-67 (ZIF-67) MOF that was fused onto the surface of electrospun nanofibers made from 2-methylimidazole–cellulose acetate (MIM–CA) (Fig. 7c). The ZIF-67 particles adhere to the CA fibers in an orderly manner, similar to the structure of a pearl chain. The unique composite was used to remove Cu(II) from wastewater. The composite had a high Cu(II) adsorption capacity of 18.9 mg g−1, and the adsorption results were in good agreement with the pseudo-second-order kinetics. This was attributed to the increased specific surface area and the adsorption sites on the adsorbent provided by the pearl-necklace-like structure, which prevented the agglomeration of ZIF-67 and promoted contact between the composite and Cu(II) ions. Using an ultrasound-assisted method, Mahmoodi et al. synthesized ZIF-67 crystals that grew and were stabilized on the surface of a magnetic eggshell membrane (Fe3O4@ESM) to produce the ZIF-67@Fe3O4@ESM composite.89 Owing to the large surface area (1263.9 m2 g−1), the composite has a faster absorption rate and a higher performance for removing Cu(II) ions from wastewater. The results indicated that the composite had a maximum adsorption capacity of 344.82 mg g−1. Kinetic and isotherm results indicated that the adsorption process followed the pseudo-second-order model using the Langmuir adsorption isotherm model.
Shooto et al.90 described an electrospinning method for producing PVA–Co-MOF sorbents by combining novel PVA nanofibers with a cobalt metal–organic framework. SEM images showed that homogeneous, bead-free nanofibers were developed. The FTIR spectra showed changes in certain functional group positions, suggesting cobalt metal–organic frameworks were implanted into the interconnected nanofibers. The ability of these novel PVA–Co-MOF nanofibers to remove Pb(II) ions from the solution was investigated. The sorption of Pb(II) ions on integrated nanofibers (PVA-Co-MO) was twice as high as that of pure PVA nanofibers. Moreover, kinetic studies revealed that Pb(II) ion absorption by nanofibers at low temperatures is favored and is dominated by the physisorption process. Pb(II) ion adsorption processes have also been observed using pseudo-second-order model kinetics and the Langmuir adsorption isotherm model.
Using a simple in situ loading technique, a ZIF-8 hybrid nanofiber film was effectively fabricated on a polymeric substrate material.91 A hydrolysis duration of 1 h, a two-cut film, and 0.3% by weight of poly(4-styrenesufonate sodium) (PSS) were identified as optimal conditions. The film prepared under optimal conditions was used to extract the Pb(II) from water, and a high removal efficiency of 99.5% was demonstrated. In addition, compared to the pure PAN film, the removal capacity increased up to three times, and a high permeation flow of 180 L m2 h−1 psi was observed. The process of the adsorption isotherm followed the Langmuir model. Other heavy metals, including Cr(II), Hg(II), Cu(II), and Cd(II), were examined for removal from water using the hybrid film, and the film still exhibited a high removal efficiency. This improvement can be attributed to the unique properties of microporous ZIF-8 as active sites for Pb(II) adsorption with a high surface area of electrospun PAN.
3.2 Removal of variable-valence heavy metals ions
Ashour et al. described the production of a hybrid nanocomposite consisting of MIL-100(Fe) MOF immobilized in bacterial cellulose (BC) nanofibers, as shown in Fig. 8a.92 The resulting nanocomposite reveals a good, mechanically robust, flexible, and ultralight structure. However, small MIL-100(Fe) particles were found in a consistent pattern on the surface of the BC nanofibers (Fig. 8b), and these remained stable enough to form the nanocomposite. The nanocomposite MIL-100(Fe)@BC was used to remove As(III) from an aqueous solution and reached an adsorption capacity of 4.81 mg g−1 with a removal efficiency of 85% after 72 h (Fig. 8c). Furthermore, the pseudo-second-order nonlinear fit can accurately represent the adsorption process. This suggests that the BC had no effect on the accessibility of the MIL-100(Fe) pores and that the majority of MIL-100(Fe) particles were active in the chemical process of the adsorption of As(III) onto the MIL-100(Fe)@BC nanocomposite through surface complexation.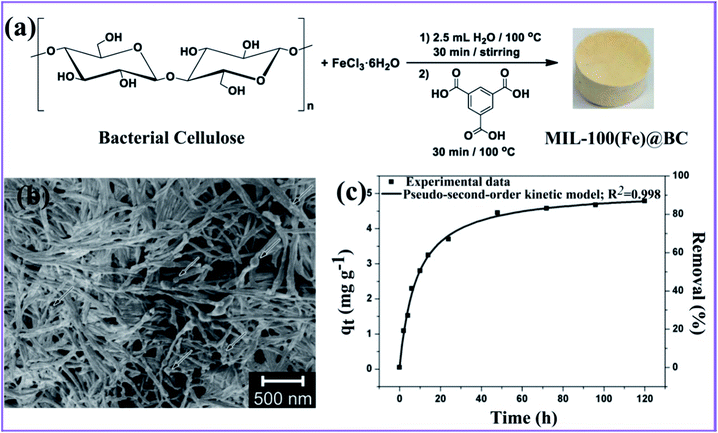 | ||
| Fig. 8 (a) General approach for synthesizing the MIL-100(Fe)@BC nanocomposite. (b) SEM image of the hybrid MIL-100(Fe)@BC nanocomposite. (c) Adsorption capacity versus time, and the nonlinear pseudo-second-order fitting for As(III), this figure has been adapted from ref. 92 with permission from MDPI, copyright 2020. | ||
Wang et al.93 produced a ZIF-8–PAN nanofiber composite using in situ hydrothermal treatment. SEM images reveal that the ZIF-8 nanocrystals grow well in the PAN nanofibers (Fig. 9a and b). The accompanying transmission electron microscopy (TEM) images (Fig. 9c and d) show that the ZIF-8 nanocrystals develop on the surface and inside the PAN fibers. Interestingly, the enlarged TEM image (Fig. 9d) indicates that the mesoporous structures are created during the reaction by PVP etching. The presence of a type IV hysteresis loop in the N2 adsorption/desorption patterns supports this mesoporous property (Fig. 9e). Owing to their porous architectures, the ZIF-8–PAN filter displayed a significant U(VI) (530.3 mg g−1 at pH = 3.0). The adsorption process largely corresponds to the pseudo-second-order model and is mainly chemical adsorption. Furthermore, the results show that the Langmuir model fits the experimental data better than the Freundlich model, confirming that it is a monolayer adsorption process. The ZIF-8–PAN composite exhibited a high U(VI) adsorption capacity of 530.3 mg g−1 at pH 3 by taking advantage of its porous properties.
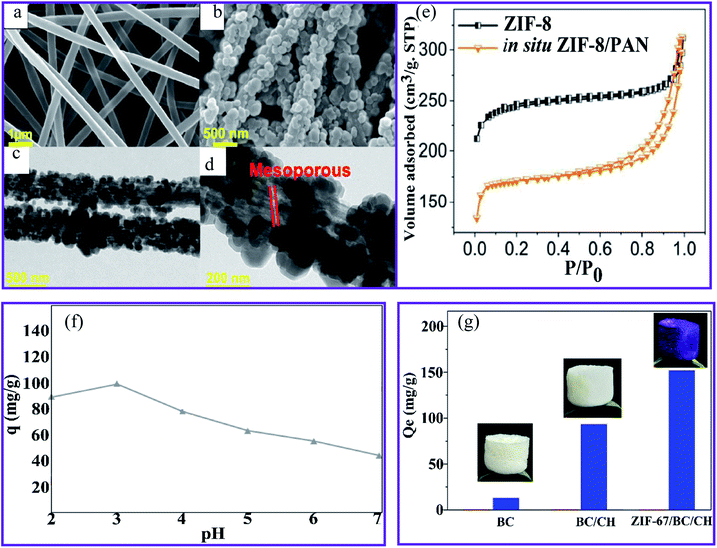 | ||
| Fig. 9 SEM images of (a) the PAN nanofibers and (b) the ZIF-8–PAN composite. (c) and (d) TEM images of the ZIF-8–PAN composite. (e) N2 adsorption/desorption measurements of ZIF-8 and the ZIF-8–PAN composite, this figure has been adapted from ref. 93 with permission from American Chemical Society, copyright 2018. (f) The effect of pH on the Cr(VI) ions sorption using PAN-chitosan–UiO-66-NH2 nanofibers, this figure has been adapted from ref. 74 with permission from Elsevier, copyright 2019. (g) The adsorption capacity of BC, BC–CH, and ZIF-67–BC–CH aerogels for Cr(VI) ions, this figure has been adapted from ref. 71 and 94 with permission from Elsevier, copyright 2020. | ||
Cr(VI) ions were effectively removed from the aqueous solution at an initial concentration of 50 mg L−1 metal ions by the adsorption and membrane filtration processes using the UiO 66-NH2–PAN-chitosan nanofiber composite.74 Fig. 9f shows that the capacity of Cr(VI) reached the highest value at pH 3. The electrostatic attraction of the positive charge (protonated amino and carbonated adsorbent groups with a lower pH) of the nanofibers and the negative charge HCrO4− can be achieved through the use of PAN-chitosan–UiO-66-NH2 nanofibers at a maximum Cr(VI) absorption.95 In comparison, a decrease in the charge density at higher pH values of nanofibers led to a decrease in the effectiveness of the Cr(VI) ion adsorption. Li et al.94 have prepared a zeolitic frame-67 (ZIF-67) MOF modified by a simple technique involving physical mixing, in situ synthesis, and lyophilization in a cellulose–chitosan (BC–CH) composite aerogel. The water stability of the CH aerogel was improved by using BC nanofibers instead of using chemical linking agents. The loading rate of ZIF-67 was 46.1%, and the Brunauer–Emmett–Teller (BET) specific surface area of the ZIF-67–BC–CH aerogel was 268.7 m2 g−1, which was considerably higher than that of the BC–CH aerogel area (8.4 m2 g−1). The aerogel ZIF-67–BC–CH was used to remove the Cr(VI) ions from an aqueous solution with an adsorption capacity of 152.1 mg g−1, corresponding to a higher adsorption capacity than that of the BC and BC–CH aerogel, as observed in Fig. 9g. In addition, the adsorption process fits well with the pseudo-second-order kinetic model, suggesting that chemical adsorption was involved in the overall adsorption process. Coordination, ion exchange, and electrostatic interactions were all involved in the adsorption process of the ZIF-67–BC–CH aerogels. The Cr(VI) ions could be physically adsorbed onto the aerogel via electrostatic attraction, and they could coordinate with the pyridinic N of ZIF-67. The ion exchange also took place between the Cr(VI) ions and the hydroxyl linked to the uncoordinated Co(II) of the ZIF-67 crystals.
In another study on the removal of Cr(VI) ions, Pishnamazi et al. integrated PVDF nanofibers into UiO-66 NH2 and ZIF-8 MOF nanoparticles.96 Then, the PVDF nanofibers were coated with chitosan nanofibers to produce PVDF-chitosan nanofibers. The incorporation of ZIF-8 decreased the hydrophilicity of PVDF and PVDF-chitosan nanofiber membranes. The performance of the ultrafiltration membrane technology for the recovery of Cr(VI) ions was investigated. Interestingly, the highest water flow observed was 470 L m−2 h−1, and the efficiency reached 95.6% when a PVDF-chitosan nanofiber membrane with 20 wt% of UiO-66-NH2 was used.
The maximum sorption capacities of Cr(VI) were determined and found to be 602.3 mg g−1. The pseudo-second-order kinetic model accurately explained the kinetic data for the Cr(VI) sorption. Furthermore, the Redlich–Peterson isotherm model fits the equilibrium data better than the Freundlich and Langmuir isotherm models. The results revealed that the Cr(VI) sorption took place at the synthesized nanofibers' homogeneous and heterogeneous sorption sites. However, sorption of Cr(VI) was the most prominent in the nanofiber monolayer. Wei et al.95 investigated the use of the UiO-66–poly(arylene sulfide sulfone) nanofiber membrane for treating Cr(VI) ions. The nanofibrous membrane exposed a high specific surface area and hydrophobicity structure with a high removal efficiency of 98.44% and a flux of 636 L m−2 h−1. Interestingly, this membrane maintained an excellent stable morphology and good removal performance for a range of pollutants after storage in a corrosive and harsh solution, indicating that it could be widely used in complicated wastewater treatment, particularly in hostile conditions. The key results for the removal of HMIs by MOF–nanofiber composites are summarized in Table 1.
| MOFs–nanofiber composite | Adsorption capacity (mg g−1) | Metal ion | Equilibrium time | pH | Adsorbent dose (g L−1) | Isotherm/kinetic | Removal (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| ZIF-8–PAN NF | 225.62 | Cu(II) | 600 min | — | — | Pseudo-second-order | 97.75 | 85 |
| MOF-808–PAN | 48.67 | Pb(II) | — | — | — | 47 | 86 | |
| Zr-MOF-808–PAN | 225.05 | Cd(II) | 10 min | 4.5 | — | Langmuir, pseudo-second-order | ∼55 | 87 |
| 287.06 | Zn(II) | ∼60 | ||||||
| ZIF-67–MIM/CA | 14.5 | Cr(VI) | 24 h | 6.5 | — | Pseudo-second-order | ∼88 | 88 |
| ZIF-67@Fe3O4@ESM | 344.82 | Cu(II) | 150 min | 5 | 0.006 | Langmuir, pseudo-first order | 93 | 89 |
| Co-MOF–PVA | 55.23667 | Pb(II) | 30 min | 5.03 | — | Langmuir | 59.41 | 90 |
| ZIF-8–PAN | 74.1 | Pb(II) | 3 h | — | — | Langmuir | 99.4 | 91 |
| MIL-100(Fe)@BC | 4.81 | As(III) | 72 h | — | Pseudo-second-order | 85 | 92 | |
| In situ ZIF-8/PAN | 530.3 | U(VI) | 60 min | 3 | — | Langmuir, pseudo-second-order | 85 | 93 |
| ZIF-67–(BC/CH) | 200.6 | Cu(II) | 24 h | 6 | — | Pseudo-second-order | ∼96 | 94 |
| 152.1 | Cr(VI) | ∼93 | ||||||
| UiO-66-NH2–PVDF/chitosan | 602.3 | Cr(VI) | 25 min | 5.5 | 0.5 | Redlich–Peterson/pseudo-second-order | — | 96 |
| ZIF-8–PVDF/chitosan | 569.3 | |||||||
| UiO-66–poly(arylene sulfide sulfone) | 89.12 | Cr(VI) | 100 min | 3.5 | — | Pseudo-second-order | — | 95 |
| ZIF-67–ANF | 250.95 | Cu(II) | 25 h | 6.0 | — | Pseudo-second-order | — | 97 |
| 183.29 | Cr(VI) |
3.3 Removal mechanism and kinetics
The effective removal by MOFs can be attributed to the large specific area and functional groups of nanofibers and MOFs such as carboxylic, amine, hydroxyl, and oxygen for chelation with metal ions resulting in the higher potential adsorbent towards metal ions sorption. Based on the type of interaction between the MOFs and HMIs, the adsorption can be divided into physical and chemical adsorption, as depicted in Fig. 10a. Physical adsorption is known as adsorptive adsorption, in which chemical adsorption is known as reactive adsorption.98 A typical experimental method used to investigate the possible mechanism is to study the adsorption isotherm of the MOFs-based nanofiber structures.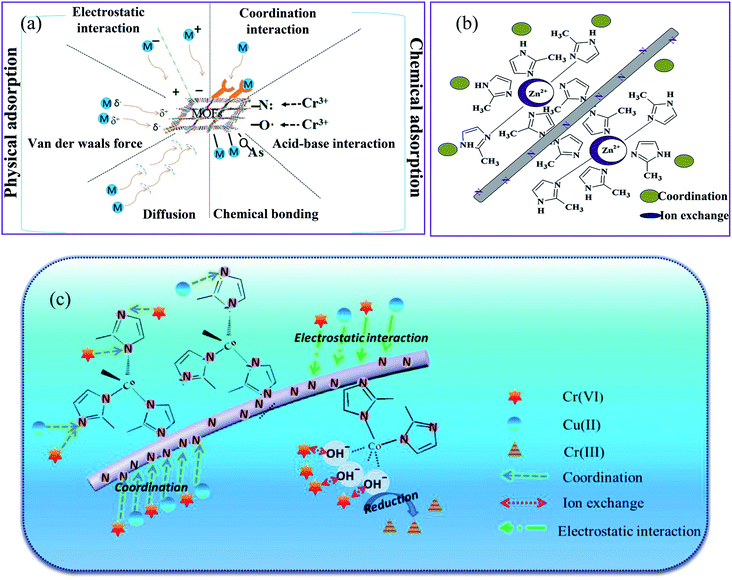 | ||
| Fig. 10 Schematic diagrams of the possible mechanisms for (a) adsorptive removal of HMIs by MOFs, this figure has been adapted from ref. 98 with permission from Elsevier, copyright 2018, (b) adsorption mechanism for Cu(II) on the ZIF-8–PAN surface, this figure has been adapted from ref. 85 with permission from Elsevier, copyright 2020, and (c) adsorption mechanism for Cr(VI) on the ZIF-67–MIM/CA surface, this figure has been adapted from ref. 88 with permission from Willey Online Library, copyright 2018. | ||
The Langmuir and Freundlich models often fit the adsorption equilibrium data. The Langmuir model is suitable for the sorption process on homogeneous surfaces, and the Freundlich model is suitable for the sorption process on heterogeneous surfaces.99,100 Langmuir adsorption is a form of chemical adsorption that is more stable and has a greater capacity than basic physical adsorption.
Adsorption kinetics are a powerful method used to study the adsorption mechanism between MOFs-based nanofibers and HMIs. Almost all adsorption kinetics are better matched with the pseudo-second-order model, as evidenced by the better correlation coefficients compared to the pseudo-first-order model.89,90,93 In a study by Peng et al.,85 the adsorption data of ZIF-8–PAN NF for Cu(II) were adapted to a pseudo-second-order model (R2 = 0.967). The agreement of the kinetic data with this kinetic model suggests that the adsorption of HMIs on MOFs in aqueous solutions can be controlled by chemical adsorption. ZIF-8 nanoparticles adsorbed Cu(II) in wastewater by the adsorption process, as shown in Fig. 10b. The adsorption process proceeds as follows, first, ion diffusion occurs through different Cu(II) concentrations on either side of the ZIF-8–PAN nanofiltration membrane. Second, heavy metal ions coordinate to the pyridinic N in dimethylimidazole. The metal source and the ligand of ZIF-8 have different capacities for the uptake and loss of electrons, which ultimately leads to a bias in the electron cloud in the direction of the ligand. Thus, it contributed to a more ideal interaction between the hydrogen bond on the N atom and Cu(II). Third, the open metal zinc site in ZIF-8 enables ion exchange between the zinc and copper ions. The pH of the heavy metal ion solution is less than 9.3, indicating that no electrostatic adsorption occurs during the Cu(II) degradation process by ZIF-8–PAN NF.
The ZIF-67–MIM/CA composite was used to remove Cr(VI) ions through the interactions shown in Fig. 10c,88 including physical adsorption driven by the concentration gradient and electrostatic attraction, the coordination between the metal ions and the pyridinic N of 2-methylimidazole, the ion-exchange between the heavy metal ions and the hydroxyl that bonded with the uncoordinated Cu(II) sites on the adsorbent surface, and the partial reduction of Cr(VI) to Cr(III) during the adsorption of Cr(VI). Generally, pristine MOFs have a lower adsorption capacity for most HMIs than that of modified MOFs. Therefore, coordination interactions mainly occur between HMIs and the modified MOFs with different functional groups (e.g., amino group (–NH2), carboxyl group (–COOH), and thiol group (–SH)). For example, Wang et al.93 compared the FTIR, XPS, X-ray absorption near edge structure (XANES), and extended X-ray absorption fine structure (EXAFS) characterizations of the ZIF-8–PAN nanocomposite before and after adsorption with U(VI) ions and revealed an obvious coordination interaction between the U(VI) ions and the nitrogen atom in 2-methylimidazole.
4. Conclusions and future perspective
In conclusion, MOFs decorated on nanofiber materials have recently achieved substantial progress. A comprehensive review of recent advances in the fabrication of MOFs–nanofiber composites and their applications in removing HMIs from wastewater and aqueous solutions is presented. We focused on two strategies for producing MOF–nanofiber composites: in situ MOFs on nanofiber surfaces and pre-synthesized MOF composites. MOFs decorated on nanofiber materials show various attractive adsorbents for removing HMIs, including rapid adsorption kinetics, a high adsorption capacity, excellent selectivity, and good reusability. However, some critical information is missing from the previously published reports. We conclude that the substantial adsorption capacities of MOFs decorated on nanofiber materials are mainly a result of interactions between target ions and functional binding groups on the composites and the highly ordered porous structure that can facilitate the diffusion of metal ions. Despite significant progress, there are still some problems and unresolved issues. In general, experimental conditions such as the pH, ionic strength, competing ions, and organic material significantly influence the surface properties and the adsorption behaviors of HMIs, however, less specific information is available. Another aspect to consider is that the active pH range is often limited (pH 3.0–6.5). Therefore, the stabilization of more MOFs decorated on nanofibers over a wider pH range is very desirable in future studies. As a result, more research is needed into the adsorption behavior of MOFs and the processes of the nanofiber materials that are decorated on the surface as adsorbents. For practical uses of these materials in the control of pollutants, the stability, high adsorption capacity, high selectivity, good reusability, and synthesis by large-scale, inexpensive, and environmentally friendly processes should be considered.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for financial support from USAID Partnerships for Enhanced Engagement in Research (PEER) Program (an international grants program that funds scientists and engineers in developing countries who partner with U.S. government-funded researchers to address global development challenges) and the U.S. National Academies of Sciences Administered Engineering and Medicine (NASEM), PEER/Iraq project/cycle 6. In addition, the authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the research group project under grant number (KKU/RCAMS/G0014/21).References
- M. A. Barakat, Arabian J. Chem., 2011, 4, 361–377 CrossRef CAS
.
- H. Shayegan, G. A. M. Ali and V. Safarifard, ChemistrySelect, 2020, 5, 124–146 CrossRef CAS
.
- E. Khezerloo, S. M. Mousavi-khoshdel and V. Safarifard, Polyhedron, 2019, 166, 166–174 CrossRef CAS
.
- H. Sadegh, G. A. M. Ali, A. S. H. Makhlouf, K. F. Chong, N. S. Alharbi, S. Agarwal and V. K. Gupta, J. Mol. Liq., 2018, 258, 345–353 CrossRef CAS
.
- B. Dhal, H. N. Thatoi, N. N. Das and B. D. Pandey, J. Hazard. Mater., 2013, 250–251, 272–291 CrossRef CAS PubMed
.
- F. Lu and D. Astruc, Coord. Chem. Rev., 2018, 356, 147–164 CrossRef CAS
.
- L. N. Nthunya, M. F. Bopape, O. T. Mahlangu, B. B. Mamba, B. Van der Bruggen, C. A. Quist-Jensen and H. Richards, J. Environ. Manage., 2022, 301, 113922 CrossRef CAS PubMed
.
- Z. Lv, C. Liang, J. Cui, Y. Zhang and S. Xu, RSC Adv., 2015, 5, 18213–18217 RSC
.
- L. L. Li, X. Q. Feng, R. P. Han, S. Q. Zang and G. Yang, J. Hazard. Mater., 2017, 321, 622–628 CrossRef CAS PubMed
.
- R. Liang, F. Jing, L. Shen, N. Qin and L. Wu, J. Hazard. Mater., 2015, 287, 364–372 CrossRef CAS PubMed
.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed
.
- M. Tanhaei, A. R. Mahjoub and V. Safarifard, Inorg. Chem. Commun., 2019, 102, 185–191 CrossRef CAS
.
- H. Shayegan, G. A. M. Ali and V. Safarifard, J. Inorg. Organomet. Polym. Mater., 2020, 30, 3170–3178 CrossRef CAS
.
- H. Sadegh, G. A. M. Ali, V. K. Gupta, A. S. H. Makhlouf, R. Shahryari-ghoshekandi, M. N. Nadagouda, M. Sillanpää and E. Megiel, J. Nanostruct. Chem., 2017, 7, 1–14 CrossRef CAS
.
- L. Nthunya, M. Masheane, M. George, M.-B. Kime and S. Mhlanga, J. Water Chem. Technol., 2019, 41, 81–86 CrossRef
.
- B. Makgabutlane, L. N. Nthunya, N. Musyoka, B. S. Dladla, E. N. Nxumalo and S. D. Mhlanga, RSC Adv., 2020, 10, 2416–2427 RSC
.
- L. N. Nthunya, L. Gutierrez, S. Derese, B. B. Mamba, A. R. Verliefde and S. D. Mhlanga, J. Environ. Chem. Eng., 2019, 7, 103254 CrossRef
.
- G. A. M. Ali, A. Barhoum, V. K. Gupta, A. A. Nada, H. El-Maghrabi, R. Kanthasamy, E. R. Shaaban, H. Algarni and K. F. Chong, Curr. Nanosci., 2020, 16, 226–234 CrossRef
.
- M. Tanhaei, A. R. Mahjoub and V. Safarifard, Mater. Lett., 2018, 227, 318–321 CrossRef CAS
.
- O. A. Habeeb, K. Ramesh, G. A. M. Ali and R. M. Yunus, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2017, 32, 305–320 CrossRef
.
- O. A. Habeeb, K. Ramesh, G. A. M. Ali and R. M. Yunus, Desalin. Water Treat., 2017, 84, 205–214 CrossRef CAS
.
- L. Esrafili, V. Safarifard, E. Tahmasebi, M. D. Esrafili and A. Morsali, New J. Chem., 2018, 42, 8864–8873 RSC
.
- Z. S. Rozveh, S. Kazemi, M. Karimi, G. A. M. Ali and V. Safarifard, Polyhedron, 2020, 183, 114514 CrossRef CAS
.
- Y. D. Farahani and V. Safarifard, J. Solid State Chem., 2019, 270, 428–435 CrossRef CAS
.
- L. Zeng, X. Guo, C. He and C. Duan, ACS Catal., 2016, 6, 7935–7947 CrossRef CAS
.
- C. Y. Sun, C. Qin, X. L. Wang and Z. M. Su, Expert Opin. Drug Delivery, 2013, 10, 89–101 CrossRef CAS PubMed
.
- H. Wang, Q. L. Zhu, R. Zou and Q. Xu, Chem, 2017, 2, 52–80 CAS
.
- S. Ma and H. C. Zhou, Chem. Commun., 2010, 46, 44–53 RSC
.
- E. Moradi, R. Rahimi and V. Safarifard, Polyhedron, 2019, 159, 251–258 CrossRef CAS
.
- X. Y. Li, Z. J. Li, Y. Z. Li, L. Hou, Z. Zhu and Y. Y. Wang, Inorg. Chem., 2018, 57, 12417–12423 CrossRef CAS PubMed
.
- S. Beheshti, V. Safarifard and A. Morsali, Inorg. Chem. Commun., 2018, 94, 80–84 CrossRef CAS
.
- Y. Bai, Y. Dou, L. H. Xie, W. Rutledge, J. R. Li and H. C. Zhou, Chem. Soc. Rev., 2016, 45, 2327–2367 RSC
.
- B.-L. Zhang, W. Qiu, P.-P. Wang, Y.-L. Liu, J. Zou, L. Wang and J. Ma, Chem. Eng. J., 2020, 385, 123507 CrossRef CAS
.
- G. Boix, J. Troyano, L. Garzón-Tovar, C. Camur, N. Bermejo, A. Yazdi, J. Piella, N. G. Bastus, V. F. Puntes, I. Imaz and D. Maspoch, ACS Appl. Mater. Interfaces, 2020, 12, 10554–10562 CrossRef CAS PubMed
.
- H.-X. Jin, H. P. Xu, N. Wang, L.-Y. Yang, Y.-G. Wang, D. Yu and X.-K. Ouyang, Materials, 2019, 12, 942 CrossRef CAS PubMed
.
- Z. Li, G. Zhou, H. Dai, M. Yang, Y. Fu, Y. Ying and Y. Li, J. Mater. Chem. A, 2018, 6, 3402–3413 RSC
.
- G. W. Peterson, D. T. Lee, H. F. Barton, T. H. Epps and G. N. Parsons, Nat. Rev. Mater., 2021, 6, 605–621 CrossRef CAS
.
- J. Zhao, D. T. Lee, R. W. Yaga, M. G. Hall, H. F. Barton, I. R. Woodward, C. J. Oldham, H. J. Walls, G. W. Peterson and G. N. Parsons, Angew. Chem., Int. Ed., 2016, 55, 13224–13228 CrossRef CAS PubMed
.
- R. Ostermann, J. Cravillon, C. Weidmann, M. Wiebcke and B. M. Smarsly, Chem. Commun., 2011, 47, 442–444 RSC
.
- J. E. Efome, D. Rana, T. Matsuura and C. Q. Lan, J. Mater. Chem. A, 2018, 6, 4550–4555 RSC
.
- P. A. Kobielska, A. J. Howarth, O. K. Farha and S. Nayak, Coord. Chem. Rev., 2018, 358, 92–107 CrossRef CAS
.
- X. Yan, P. Li, X. Song, J. Li, B. Ren, S. Gao and R. Cao, Coord. Chem. Rev., 2021, 443, 214034 CrossRef CAS
.
- Y. Chen, X. Bai and Z. Ye, Nanomaterials, 2020, 10, 1–23 Search PubMed
.
- Z. Li, L. Wang, L. Qin, C. Lai, Z. Wang, M. Zhou, L. Xiao, S. Liu and M. Zhang, Chemosphere, 2021, 285, 131432 CrossRef CAS PubMed
.
- H. Yu, S. Gao, X. Cheng, P. Wang, X. Zhang, Y. Xu, H. Zhao and L. Huo, Sens. Actuators, B, 2019, 297, 126744 CrossRef CAS
.
- S. Zhang, J. Wang, Y. Zhang, J. Ma, L. Huang, S. Yu, L. Chen, G. Song, M. Qiu and X. Wang, Environ. Pollut., 2021, 291, 118076 CrossRef CAS PubMed
.
- C. Vaitsis, G. Sourkouni and C. Argirusis, Ultrason. Sonochem., 2019, 52, 106–119 CrossRef CAS PubMed
.
- L. Hu, Y. Huang, F. Zhang and Q. Chen, Nanoscale, 2013, 5, 4186–4190 RSC
.
- G. R. Xu, Z. H. An, K. Xu, Q. Liu, R. Das and H. L. Zhao, Coord. Chem. Rev., 2021, 427, 213554 CrossRef CAS
.
- M. Hao, M. Qiu, H. Yang, B. Hu and X. Wang, Sci. Total Environ., 2021, 760, 143333 CrossRef CAS PubMed
.
- A. Krajnc, B. Bueken, D. De Vos and G. Mali, J. Magn. Reson., 2017, 279, 22–28 CrossRef CAS PubMed
.
- S. R. Venna and M. A. Carreon, Chem. Eng. Sci., 2015, 124, 3–19 CrossRef CAS
.
- G. H. Albuquerque and G. S. Herman, Cryst. Growth Des., 2017, 17, 156–162 CrossRef CAS
.
- V. V. Butova, V. A. Polyakov, E. A. Bulanova, M. A. Soldatov, I. S. Yahia, H. Y. Zahran, A. F. Abd El-Rehim, H. Algarni, A. M. Aboraia and A. V. Soldatov, Microporous Mesoporous Mater., 2020, 293, 109685 CrossRef CAS
.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Eur. J., 2011, 17, 6643–6651 CrossRef CAS PubMed
.
- J. Yu, C. Mu, B. Yan, X. Qin, C. Shen, H. Xue and H. Pang, Mater. Horiz., 2017, 4, 557–569 RSC
.
- M. H. Yap, K. L. Fow and G. Z. Chen, Green Energy Environ., 2017, 2, 218–245 CrossRef
.
- V. K. Abdelkader-Fernández, D. M. Fernandes, S. S. Balula, L. Cunha-Silva, M. J. Pérez-Mendoza, F. J. López-Garzón, M. F. Pereira and C. Freire, ACS Appl. Energy Mater., 2019, 2, 1854–1867 CrossRef
.
- J. Y. Wu, T. C. Chao and M. S. Zhong, Cryst. Growth Des., 2013, 13, 2953–2964 CrossRef CAS
.
- P. Küsgens, S. Siegle and S. Kaskel, Adv. Eng. Mater., 2009, 11, 93–95 CrossRef
.
- D. Britt, D. Tranchemontagne and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11623–11627 CrossRef CAS PubMed
.
- C. Liu, Y. N. Wu, C. Morlay, Y. Gu, B. Gebremariam, X. Yuan and F. Li, ACS Appl. Mater. Interfaces, 2016, 8, 2552–2561 CrossRef CAS PubMed
.
- E. Laurila, J. Thunberg, S. P. Argent, N. R. Champness, S. Zacharias, G. Westman and L. Öhrström, Adv. Eng. Mater., 2015, 17, 1282–1286 CrossRef CAS
.
- K. Ma, T. Islamoglu, Z. Chen, P. Li, M. C. Wasson, Y. Chen, Y. Wang, G. W. Peterson, J. H. Xin and O. K. Farha, J. Am. Chem. Soc., 2019, 141, 15626–15633 CrossRef CAS PubMed
.
- X. K. B. Z. F. H. M. S. Shengyang Zhou and X. Chao, ACS Nano, 2019, 13, 9578–9586 CrossRef PubMed
.
- A. R. Abbasi, K. Akhbari and A. Morsali, Ultrason. Sonochem., 2012, 19, 846–852 CrossRef CAS PubMed
.
- S. Khanjani and A. Morsali, Ultrason. Sonochem., 2014, 21, 1424–1429 CrossRef CAS PubMed
.
- M. Nakahama, J. Reboul, K. I. Kamei, S. Kitagawa and S. Furukawa, Chem. Lett., 2014, 43, 1052–1054 CrossRef CAS
.
- A. X. Lu, M. McEntee, M. A. Browe, M. G. Hall, J. B. Decoste and G. W. Peterson, ACS Appl. Mater. Interfaces, 2017, 9, 13632–13636 CrossRef CAS PubMed
.
- K. Roongraung, S. Chuangchote, N. Laosiripojana and T. Sagawa, ACS Omega, 2020, 5, 5862–5872 CrossRef CAS PubMed
.
- Y. Zhang, S. Yuan, X. Feng, H. Li, J. Zhou and B. Wang, J. Am. Chem. Soc., 2016, 138, 5785–5788 CrossRef CAS PubMed
.
- E. López-Maya, C. Montoro, L. M. Rodríguez-Albelo, S. D. Aznar Cervantes, A. A. Lozano-Pérez, J. L. Cenís, E. Barea and J. A. R. Navarro, Angew. Chem., Int. Ed., 2015, 54, 6790–6794 CrossRef PubMed
.
- M. Nasrabadi, M. A. Ghasemzadeh and M. R. Z. Monfared, New J. Chem., 2019, 43, 16033–16040 RSC
.
- S. Jamshidifard, S. Koushkbaghi, S. Hosseini, S. Rezaei, A. Karamipour, A. Jafari rad and M. Irani, J. Hazard. Mater., 2019, 368, 10–20 CrossRef CAS PubMed
.
- M. R. Thalji, G. A. M. Ali, H. Algarni and K. F. Chong, J. Power Sources, 2019, 438, 227028 CrossRef CAS
.
- W. Tian, H. Zhang, X. Duan, H. Sun, G. Shao and S. Wang, Adv. Funct. Mater., 2020, 30, 1909265 CrossRef CAS
.
- M. R. Thalji, G. A. M. Ali, P. Liu, Y. L. Zhong and K. F. Chong, Chem. Eng. J., 2021, 409, 128216 CrossRef CAS
.
- Z. Hu and D. Zhao, CrystEngComm, 2017, 19, 4066–4081 RSC
.
- K. Ma, K. B. Idrees, F. A. Son, R. Maldonado, M. C. Wasson, X. Zhang, X. Wang, E. Shehayeb, A. Merhi, B. R. Kaafarani, T. Islamoglu, J. H. Xin and O. K. Farha, Chem. Mater., 2020, 32, 7120–7140 CrossRef CAS
.
- K. J. Lee, J. H. Lee, S. Jeoung and H. R. Moon, Acc. Chem. Res., 2017, 50, 2684–2692 CrossRef CAS PubMed
.
- Y. T. A. Wong, V. Martins, B. E. G. Lucier and Y. Huang, Chem.–Eur. J., 2019, 25, 1848–1853 CrossRef CAS PubMed
.
- L. Yin, B. Hu, L. Zhuang, D. Fu, J. Li, T. Hayat, A. Alsaedi and X. Wang, Chem. Eng. J., 2020, 381, 122744 CrossRef CAS
.
- L. N. Nthunya, N. P. Khumalo, A. R. Verliefde, B. B. Mamba and S. D. Mhlanga, Phys. Chem. Earth, Parts A/B/C, 2019, 112, 228–236 CrossRef
.
- J. Huang, Y. Cao, Q. Shao, X. Peng and Z. Guo, Ind. Eng. Chem. Res., 2017, 56, 10689–10701 CrossRef CAS
.
- L. Peng, X. Zhang, Y. Sun, Y. Xing and C. Li, Environ. Res., 2020, 188, 109742 CrossRef CAS PubMed
.
- J. E. Efome, D. Rana, T. Matsuura and C. Q. Lan, Sci. Total Environ., 2019, 674, 355–362 CrossRef CAS PubMed
.
- J. E. Efome, D. Rana, T. Matsuura and C. Q. Lan, ACS Appl. Mater. Interfaces, 2018, 10, 18619–18629 CrossRef CAS PubMed
.
- X. Hou, H. Zhou, J. Zhang, Y. Cai, F. Huang and Q. Wei, Part. Part. Syst. Charact., 2018, 35, 1–8 CrossRef
.
- N. M. Mahmoodi, M. Taghizadeh, A. Taghizadeh, J. Abdi, B. Hayati and A. A. Shekarchi, Appl. Surf. Sci., 2019, 480, 288–299 CrossRef CAS
.
- N. D. Shooto, D. Wankasi, L. M. Sikhwivhilu and E. D. Dikio, J. Residuals Sci. Technol., 2016, 13, 233–242 CrossRef CAS
.
- G. Wang, H. Zhang, J. Wang, Z. Ling and J. Qiu, Sep. Purif. Technol., 2017, 177, 257–262 CrossRef CAS
.
- R. M. Ashour, A. F. Abdel-Magied, Q. Wu, R. T. Olsson and K. Forsberg, Polymers, 2020, 12, 1–10 CrossRef PubMed
.
- C. Wang, T. Zheng, R. Luo, C. Liu, M. Zhang, J. Li, X. Sun, J. Shen, W. Han and L. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 24164–24171 CrossRef CAS PubMed
.
- D. Li, X. Tian, Z. Wang, Z. Guan, X. Li, H. Qiao, H. Ke, L. Luo and Q. Wei, Chem. Eng. J., 2020, 383, 123127 CrossRef CAS
.
- Z. Wei, Q. Su, Q. Lin, X. Wang, S. Long, G. Zhang and J. Yang, Chem. Eng. J., 2022, 430, 133021 CrossRef CAS
.
- M. Pishnamazi, S. Koushkbaghi, S. S. Hosseini, M. Darabi, A. Yousefi and M. Irani, J. Mol. Liq., 2020, 317, 113934 CrossRef CAS
.
- G. Zhao, H. Zhao, L. Shi, B. Cheng, X. Xu and X. Zhuang, Sep. Purif. Technol., 2021, 274, 119054 CrossRef CAS
.
- J. Wen, Y. Fang and G. Zeng, Chemosphere, 2018, 201, 627–643 CrossRef CAS PubMed
.
- H. Sadegh, G. A. M. Ali, S. Agarwal and V. K. Gupta, Int. J. Environ. Res., 2019, 13, 523–531 CrossRef CAS
.
- S. M. Seyed Arabi, R. S. Lalehloo, M. R. T. B. Olyai, G. A. M. Ali and H. Sadegh, Phys. E Low-dimens. Syst. Nanostruct., 2019, 106, 150–155 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2022 |







