Open Access Article
Open Access ArticleReviewing a plethora of oxidative-type reactions catalyzed by whole cells of Streptomyces species
Sara Salama
 *a,
Mohamed H. Habib
*a,
Mohamed H. Habib
 b,
Rajni Hatti-Kaul
b,
Rajni Hatti-Kaul
 c and
Yasser Gaber
c and
Yasser Gaber
 de
de
aBiotechnology and Life Sciences Department, Faculty of Postgraduate Studies for Advanced Sciences, Beni-Suef University, Beni-Suef, 62517, Egypt. E-mail: sarasalama@psas.bsu.edu.eg
bDepartment of Microbiology and Immunology, Faculty of Pharmacy, Cairo University, Cairo, 11562, Egypt
cDivision of Biotechnology, Department of Chemistry, Center for Chemistry and Chemical Engineering, Lund University, Sweden
dDepartment of Pharmaceutical Microbiology and Immunology, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, 62511, Egypt
eDepartment of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Mutah University, Al-Karak, 61710, Jordan
First published on 1st March 2022
Abstract
Selective oxidation reactions represent a challenging task for conventional organic chemistry. Whole-cell biocatalysis provides a very convenient, easy to apply method to carry out different selective oxidation reactions including chemo-, regio-, and enantio–selective reactions. Streptomyces species are important biocatalysts as they can catalyze these selective reactions very efficiently owing to the wide diversity of enzymes and enzymatic cascades in their cell niche. In this review, we present and analyze most of the examples reported to date of oxidative reactions catalyzed by Streptomyces species as whole-cell biocatalysts. We discuss 33 different Streptomyces species and strains and the role they play in different oxidative reactions over the past five decades. The oxidative reactions have been classified into seven categories that include: hydroxylation of steroids/non-steroids, asymmetric sulfoxidations, oxidation of aldehydes, multi-step oxidations, oxidative cleavage, and N-oxidations. The role played by Streptomyces species as recombinant hosts catalyzing bio-oxidations has also been highlighted.
Introduction
Biocatalysis contributes greatly to a growing number of transformations aimed at the conversion of natural and synthetic compounds under benign conditions, and detection and analysis of several compounds.1 It is turning out to be a key enabling technology in the chemical and pharmaceutical industries, and various industrial bioeconomy sectors. Biocatalysts do not only facilitate the development of new drugs but also play a role in improving the synthesis of existing drugs using selective, ‘green’ methods.2 For drug design aspects, biocatalysis is mainly focused in the study of early stage metabolism, which contributes to selection of the final form of a drug to ensure correct design and structure–activity relationship.3Biocatalysis using whole microbial cells is preferably used when substrate and product structures are insensitive to other enzymatic functions present in the cells and when cofactor regeneration systems are required.4 In general, the use of whole cells is easier and cheaper than the pure enzymes since enzyme isolation and purification are avoided and the enzymes retain better stability in their natural environment of cells and are also protected from shear forces in stirred bioreactors.5,6 The use of whole cells is further motivated if the target enzyme is membrane-bound (intracellular) and in processes involving a cascade of enzymatic reactions e.g. for biosynthesis of secondary metabolites like antibiotics.6 Furthermore, the stoichiometric consumption of nicotinamide cofactors such as NAD(P)H or NAD(P)+ during the enzymatic reaction makes the use of whole cells important as it is capable of continuous cofactor regeneration.5,6
Oxidation is an important reaction in drug metabolism, hence a critical step in drug development processes.7 Carbonyl compounds, dihydroxy compounds and epoxides, resulting from carbon–carbon oxidation, are among the valuable intermediates in the synthesis of pharmaceutical agents.8 Pharmaceutical industries have started to rely on biocatalysis and to get access to effective enzymes for catalysis.9 Synthesis of chiral compounds for various pharmaceutical preparations is challenging to achieve through chemical approaches, while the use of biocatalytic approaches provide routes to stereo-, regio-, and enantioselectivity.10,11 In contrast to chemical oxidation reactions, biocatalytic counterparts can be carried out under ambient conditions giving rise to pure compounds without consuming high energy that affects selectivity.12,13 Biocatalysts avoid the use of metals and complex chemicals that are problematic for waste treatment, and thus provide economic and environmental benefits over chemical catalysts.9,12,14
Oxidation via microbial approaches was developed long time ago to constitute key steps in the production of pharmaceutically important vitamins and steroids as well as several other organic compounds.1 Consequently, such approaches were considered to be of most fundamental scientific interest and a starting point for innovative synthetic pathways. The great diversity of oxidative biocatalysts either in the form of natural producer organisms or as recombinant microbial cells has made the microbial oxidation toolbox quite handy for organic chemistry. Asymmetric oxidations are enabled through various microbial biocatalysts with inherent selective enzymes. In addition, methods of process optimization increase opportunities to enhance the conversion rates and selectivity, and reduce the substrate/product inhibition, while protein engineering also allows tailoring of the biocatalyst to the given reaction. Many microbial oxidations use mild oxidants (i.e., molecular oxygen or hydrogen peroxide) that are benign and harmless, and minimize undesirable side reactions, unlike other oxidants (e.g., peroxyacids). Therefore, replacing strong, non-selective oxidants with simple molecular oxygen represents an excellent practical choice.4,15 The ability of microbial biocatalysts to conduct difficult oxidation reactions without protection/deprotection of the reactants is highly advantageous as compared to conventional chemical methods.4,16,17
Importance of Streptomyces species in whole-cell biocatalysis
Streptomyces is the most extensively studied genus of the actinobacteria phylum which represents one of the most diverse phyla in the bacterial domain.18–21 Their secondary metabolism has been a focus of attention for many years,19 as they represent three-fourths of the total bioactivity produced by Actinobacteria.22,23 Streptomyces species produce different bioactive molecules including antibiotics such as tetracenomycin and streptomycin, pigments like melanin, and extracellular enzymes like tyrosinase.24 Their versatile metabolism has further made Streptomyces species an important source of enzymes for the biocatalysis toolbox for advanced biotechnological applications.25 Several Streptomyces species contribute to the processes of antibiotics, anticancer drugs, immunosuppressants, steroids, and anthelmintic agents.26 Furthermore, together with other actinobacterial species, including Rhodococcus and Corynebacterium, they were use as whole-cell biocatalysts in different studies.22 Genome mining approach and bioinformatic tools play vital roles in the search for novel biocatalysts within the different genomes of Streptomyces species. This helps in identifying enzyme functions, efficient enzyme production systems as well as many tools that are useful for rapid prototyping of the biocatalytic reaction.1,25According to other review studies on related topics, there has been a long-term focus on secondary metabolism of Streptomyces species for finding novel antibiotics as well as developments for their production. This has overshadowed investigations on their potential for other biotechnological applications.19,26 Additionally, there is one review that has discussed the applications of various enzymes derived from different Streptomyces strains;25 and another has focused on the contributions of Streptomyces P450 enzymes in drug metabolism applications.27 In the current review, we take a close look on the published works since the 1980s of using whole cells of different Streptomyces species as oxidative biocatalysts and highlight the main experimental tricks that allowed successful selective oxidation reactions.
Streptomyces culture conditions for induction of relevant enzymes
The application of Streptomyces as a whole-cell biocatalyst is in the form of either growing or as resting cells. Growing cultures maintain cofactors needed for recycling or regeneration systems, which ensure completion of reaction such as those dependent on P450 enzymatic systems.28 On the other hand, the resting cells allow easier isolation of products during subsequent purification steps as the occurrence of side products and bacterial metabolites is much less than in growing cells.5 It is also possible that the most favorable conditions for growth may not be suitable for the putative reaction. Therefore, optimization of biotransformation conditions is important, to induce and express the target enzymatic system, and to positively influence the biotransformation process.29 This includes modification of the culture media or fermentation effectors such as pH, temperature, time, use of co-solvents, type of bioreactor, and speed of stirring. For example, the oxidative P450 enzyme system was induced in S. griseus upon growing on soybean flour as enriched media.30–34 Genistein, the major component of soybean flour, was responsible for induction of enzyme expression although it inhibited cell growth due to its bacteriostatic effect.35 In a study related to hydroxylation of vitamin D3 derivatives catalyzed by S. sclerotialus FERM BP-1370 and S. roseosporus FERM BP-1574 grown in two different media, P450 enzyme was produced only in the enriched medium containing bacto-soytone, corn steep liquor and a high concentration of glucose.36The use of low concentrations of substrates as inducers in primary pre-cultures is useful in several cases e.g., with toxic substrates, as this helps in the adaptation of microbial cells. This evokes cells to express enzymes responsible for substrate conversion or metabolism to less toxic compounds as has been reported for transformation of several substrates using the whole cells of Streptomyces.32,37–39 Streptomyces species might transform low concentrations of substrates unless the cells are induced by specific substrates. Remarkably, the resting (recombinant) cells of S. avermitilis, expressing isoflavone O-methyl transferase, oxidized daidzein at a concentration not exceeding 100 μM.40
Enzyme production is induced in vivo upon addition of specific substrates. Hernandez and coworkers examined a laccase-type phenol-oxidase produced by S. cyaneus CECT 3335,39 where 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonate) (ABTS) was used as a substrate to induce expression of laccase activity in submerged cultures. The enzyme was applied for bleaching of eucalyptus Kraft pulps using ABTS as a mediator.
Co-solvents can help increase the solubility of hydrophobic or poorly water-soluble substrates, hence the permeability into bacterial cells. However, the choice of the type of co-solvents is crucial to ensure the safety of the process and maintenance of enzyme activity. So low concentrations of polar organic solvents help increase solubility of substrates and reaction rates and maintain selectivity.4,41,42
Detergents play critical role in some biotransformations, thanks to their effect on cell permeability, as reported in earlier and recent studies.37,43–45 Triton X100 (1% v/v) increased the relative hydroxylation activity of S. avermitilis MA-4680 to transform daidzein by about 170%, while Brij 35 had no significant effect.44 On the other hand, 1% (v/v) Brij-35 increased the hydroxylation activity of S. avermitilis MA-4680 for the biotransformation of phloretin to the respective regiospecific hydroxylated product.45 Regarding the biotransformation of adamantane and its derivatives, Tween 60, at a concentration of 3% (v/v) increased the activity of S. griseoplanus AC122 for its hydroxylation to 1-adamantanol.43 In a following study, the authors indicated that such detergents could have effect on the induced enzyme system responsible for the reaction.37 Among the other detergents tested, Tween 20 doubled the activity of Streptomyces sp. SA8 cells for hydroxylation of 1-adamantanol although the substrate is poorly soluble in the detergent. Triton X-100 and Nymeen S-215 inhibited the activity probably due to the denaturation of the responsible enzyme system.37
Culture characteristics of Streptomyces species
The morphology of Streptomyces species was widely studied four decades ago, and they were classified into subgroups based on the nature and flexibility of their hairy spores.46,47 Most Streptomyces exhibit the mycelial growth feature,48 which represents a major disadvantage for industrial applications due to the formation of highly viscous cultures. This may cause problems in stirred tank reactors and contribute to mass and heat transfer problems. The morphology of Streptomyces mycelia grown in liquid media is affected by external factors including: (a) the medium composition and fermentation conditions, (b) chemical signaling molecules (i.e., A-factor),49 and (c) genetic factors, like the ssgA expression level.50 The latter is critical for septum formation in aerial hyphae according to earlier reports.51The cell morphology of the host also has a significant effect on the level of heterologous protein production since the filamentous nature of the microorganism impedes the fermentation process due to the formation of large clumps, highly viscous culture, slow growth rates, and stirring problems.52 Some genetic manipulation strategies positively impacted the morphology of Streptomyces species. S. lividans 1326 was selected to overexpress a morphogene, ssgA, that pleiotropically affects bacterial growth and cell division. This resulted in enhanced septation in vegetative hyphae and the formation of much wider hyphae that is in turn favorable for protein production and secretion.52 Another species, S. coelicolor, has never been utilized in large-scale fermentation due to the formation of large pellets. A variant overproducing ssgA, S. coelicolor GSA2, produced smaller average-sized mycelia with many protruding hyphae, which enhanced growth rates and reduced lag phase.50
The type of culture medium has an essential role, as some proteins that induce hyphal growth were reported to be produced only in rich media. For instance, SapB, a surfactant peptide important for differentiation, is produced by S. coelicolor to induce efficient formation of aerial hyphae.53 However, aerial hyphae are formed in minimal medium by other pathways mediated by substitute proteins like chaplins and rodlins. The overexpression of gene encoding SapB in chaplin-lacking mutant strains hardly restored aerial formation in minimal medium, indicating that chaplin alone is responsible for aerial morphogenesis on minimal medium. SapB and the chaplins are both essential for formation of aerial hyphae on rich medium since strains that did not produce both were bald under all examined growth conditions.54
Notably, morphology has a great effect on productivity in Streptomyces species. Mycelial mats with reduced branching rates and strong cell walls (which make nutrients be easily available to all hyphae) are more appropriate for efficient enzyme production. In comparison, for production of antibiotics, growth as pellets is more suitable, and greatly depends on the growth phase.50,55 It was also reported in previous studies (in relation to antibiotic production from S. antibioticus) that the mode of growth from adopting different media, was taken into consideration to avoid producing too many mycelial clumps. Cultures containing carbon sources such as glucose and glycerol resulted in high biomass levels, but the oleandomycin production was not high. Contrarily, in cases of growth on nitrogen sources such as aspartic acid, the biomass levels were not as high as in the former case, but the oleandomycin production was higher.56
Fermentation parameters, including dissolved oxygen tension, rotation, and rates of oxygen uptake have critical impact on the differentiation of Streptomyces cell growth in bioreactors. There is a strong correlation between the hyphal growth and oxygen demand for respiration needed for maintenance of saturation, hence enhancing the catalytic efficiency.57,58 In-depth studies on factors affecting growth and morphology have helped crucially in the optimization of protein production.50
Immobilized whole cells of Streptomyces
Application of immobilized cells enable easy removal and reusability, reduction of operational cost, use of continuous flow reactors, and low formation of secondary products due to the non-growing state of the cells. In addition, higher flow rate of reagents can be obtained due to the confinement of cells in a small region resulting in increased specific productivity.5 However, Streptomyces sp. MTCC 7546 directly converted acrylonitrile into acrylic acid using the immobilized or free cells. Unlike other actinobacteria (e.g., Rhodococcus and other genera), the acrylonitrile biotransformation was enhanced only by using their immobilized bacterial cells.22 Other Actinobacteria exhibited higher activity in immobilized form as observed for Gordonia terrae catalyzing selective oxidation of prochiral sulfides.59 As a preliminary test, we performed immobilization of Streptomyces glaucescens GLA.0 in calcium alginate beads. To avoid mycelial growth that might hinder confinement of cells on immobilized beads, basal mineral media containing 0.1% (v/v) of cyclohexanol, as a sole carbon source, was used instead of an enriched medium. The cells maintained viability and catalytic activity for up to two weeks compared to free cells exposed to the same conditions of growth (unpublished data).Recombinant whole cells of Streptomyces
The use of recombinant microorganisms is an alternative system that serves for the overexpression of genes of the desired enzymes. This technique ensures the regeneration of cofactors required for reaction of interest. Secondary reactions can be avoided by knocking out genes expressing enzymes responsible for the unwanted pathway(s) or even by selection of another proper host. Such design can be tailored to be suitable at industrial scale levels with regard to the maximum substrate concentration and the physical and chemical environment.5Streptomyces strains were successfully applied as good heterologous hosts for expression and production of target protein.52 Many advantages of Streptomyces as recombinant hosts are that they do not form inclusion bodies, have a high secretion capacity, relatively low activity levels of extracellular proteases,60 and are well suited for expressing GC-rich genes.52
The deletion of genes encoding for specific transcriptional repressors is a good strategy for enhanced protein expression in Streptomyces as reported for xylanase enzyme production.52 As a prominent example, S. lividans is one of the most flexible species to be manipulated genetically, among the actinomycetes61 and is thus considered the appropriate host for proper folding and efficient production of active enzymes.50,52,62–64 Bifunctional plasmids formed from multi-copy number E. coli plasmids and Streptomyces shuttle plasmids were constructed and developed with inducible promoters, which enhanced the production of some proteins of interest.52,65
Streptomyces species as recombinant hosts for biocatalytic oxidations
Some Streptomyces strains have been exploited as heterologous hosts for cytochrome P450 genes from various wild type Streptomycetes. They are better than E. coli due to high GC contents of the native Streptomyces genes (up to more than 70%), which make functional expression in E. coli more complicated.40 S. ahygroscopicus ZB01 has high catalytic activity for the regiospecific hydroxylation of avermectin (1) to 4′′-oxo-avermectin (2),65 which is a key step in the synthesis of emamectin benzoate, a potent semisynthetic insecticide.89 A cytochrome P450 gene, CYP107Z13, presumed to be responsible for the reaction, was cloned into a shuttle vector pKC1139 to generate pKCZ1, and then transformed into S. lividans TK54. The growing cells had higher catalytic activity for avermectin oxidation than resting cells due to effective coenzyme regeneration, electron transformation, and energy production. As cytochrome P450s require cofactor regeneration and exhibit poor stability in isolated forms,90 use of recombinant whole-cell systems is an efficient approach for biotransformation processes. Since the direct chemical regiospecific oxidation of the 4-OH group in avermectin requires protection–deprotection steps, the biological approach is a greener and cheaper option for conducting such a reaction (Scheme 1).65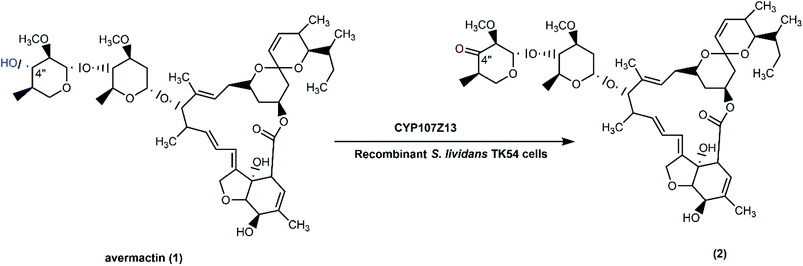 | ||
| Scheme 1 Biotransformation of avermectin (1) with whole cells (resting or growing) of S. lividans TK54, as a recombinant host expressing CYP107Z13 gene from S. ahygroscopicus ZB01. | ||
S. lividans TK24, was again exploited as a recombinant host for heterologous expression of two cytochrome P450 genes, CYP105D1 derived from S. griseus ATCC 13273, and CYP107B1 from Saccharopolyspora erythraea NRRL 2338.67 To evaluate the activity of both enzymes, the biotransformation of 7-ethoxycoumarin (3) was performed using a whole-cell system upon expression in S. lividans TK24 (Scheme 2). Dealkylation of the substrate was directly observed with heterologously expressed CYP107B1, which depended on an endogenous electron transfer partner from the host. On the other hand, CYP105D1 exhibited oxidative activity (O-dealkylation activity) upon co-expression with a redox partner (ferredoxin reductase, FdR1) derived from a closely related strain, S. coelicolor A3 (II).67,91 Remarkably, another cytochrome P450 enzyme, CYP107P3, is responsible for mediating the O-dealkylation of 7-ethoxycoumarin in the wild-type biotransformation of S. griseus.89
 | ||
| Scheme 2 Biotransformation of 7-ethoxycoumarin (3) with the growing cells of S. lividans TK24 overexpressing CYP105D1 gene derived from S. griseus ATCC 13273. This scheme has been adapted, with amendments, from Ueno et al.;67 with permission from Elsevier, copyright 2005. | ||
In addition, S. avermitilis is a useful host strain with various enzymes capable of useful transformations.40,92 Unnatural O-methyl-isoflavone was produced, for the first time, with a genetically-modified construct of S. avermitilis.40 Isoflavone O-methyltransferases were isolated from Streptomyces species and transformed into S. avermitilis ΔSaOMT2 strain (a deletion mutant of the gene encoding for O-methyltransferase). This was done in order to cancel the background O-methylation activity of the host. Among other constituted recombinant strains of S. avermitilis, only SeOMT3 showed the highest conversion yield of 4′-hydroxy-7-methoxyisoflavone (6). Upon coexpression with SAM synthetase gene (metK), the novel 4′,7-dihydroxy-3′-methoxyisoflavone (8) was produced due to 3′O-methylation of 3′,4′,7-trihydroxyisoflavone (7), the hydroxylated product of daidzein formed by other oxygenases found in the host (Scheme 3).
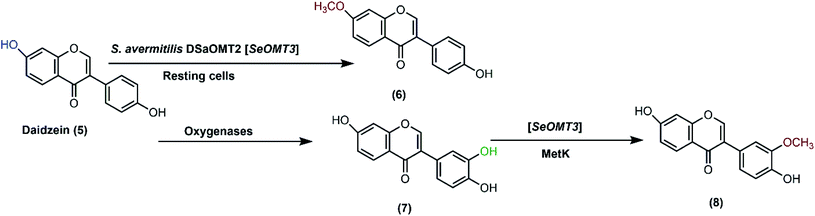 | ||
| Scheme 3 Biotransformation pathways for daidzein (5) catalyzed by the resting cells of S. avermitilis ΔSaOMT2 to 4′-hydroxy-7-methoxyisoflavone (6). Daidzein (5) was converted by the endogenous oxygenases of the host to the corresponding 3′O-methylated derivatives of 3′,4′,7-trihydroxyisoflavone (7), then further methylated to 4′,7-dihydroxy-3′-methoxyisoflavone (8) upon coexpression with SAM synthetase gene (metK). This scheme has been adapted, with amendments, from Choi et al.;40 with permission from John Wiley and Sons, copyright 2013. | ||
Another species, S. albus J1074 was used as a heterologous host for two genes, supposed to encode for crucial enzymes involved in the synthesis of borrelidin,68 a polyketide macrolide produced by various Streptomycetes. To determine the metabolic pathway, the biosynthetic gene cluster was cloned from a producing strain, S. parvulus Tü4055.93 Later, borrelidin non-producing mutants were generated to define and characterize the genes responsible for formation of nitrile moiety in borrelidin, about which little is known concerning the structural characteristics. According to the metabolites detected, one gene was supposed to code for a P450 monooxygenase (borI), and the other for an aminotransferase (borJ). An additional gene was likely encoding for a putative dehydrogenase. The activities of borI and borJ were confirmed by their heterologous expression in S. albus J1074, and bioconversion of the starting substrate, 12-desnitrile-12-methyl-borrelidin (9), to borrelidin (15). The reaction also included oxidation steps. As a result, the pathway for the formation of the nitrile moiety was proposed during borrelidin biosynthesis (Scheme 4).68
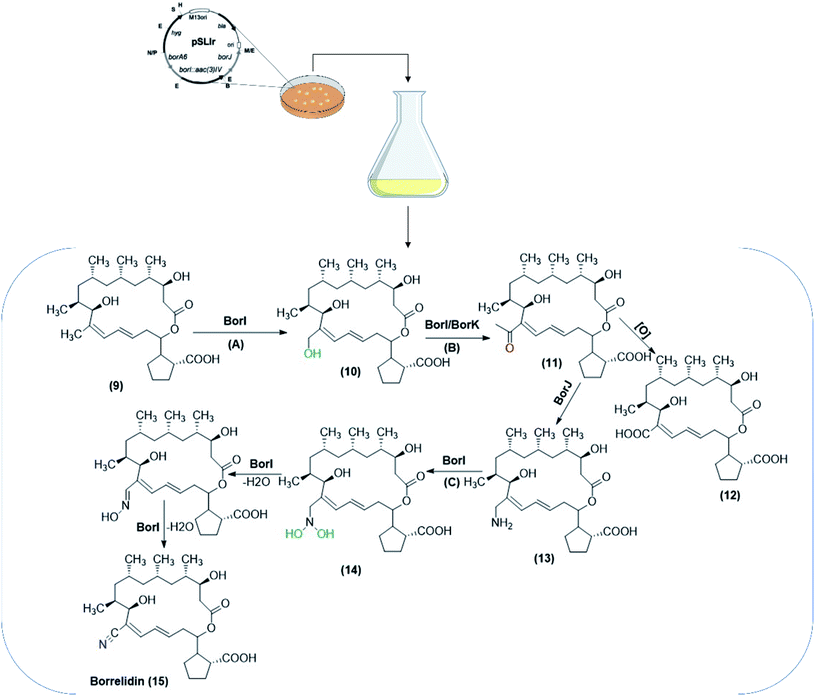 | ||
| Scheme 4 The biotransformation pathway for the formation of nitrile moiety included in the borrelidin biosynthesis. The biocatalytic conversions were performed utilizing S. albus J1074 co-expressing biosynthetic genes (borI, borJ and borK), isolated from S. parvulus Tü4055. The process was performed through incorporation of substrates/intermediates in a solid medium for bacterial growth. The pathway involved S. albus J1074 co-expressing borI (A), S. albus J1074 co-expressing borI/borK (B) and S. albus J1074 co-expressing borI (C) as oxidation steps included in the biosynthetic pathways. The scheme has been adapted, with minor modifications, from Olano et al.;68 with permission from John Wiley and Sons, copyright 2004. | ||
Role of cytochrome P450 enzymes in the oxidative transformations catalyzed by Streptomycetes
Streptomyces species are among the microorganisms possessing similar route for drug metabolism as humans, which involves hydroxylation at a saturated carbon atom.27 Whole-cell reactions using Streptomyces species serve to produce good quantities of hydroxylated derivatives that can be scaled for pharmacokinetic analysis since such metabolites are equivalent to those of mammalian ones.3 As one of the richest producers of natural bioactives, several studies related to the biosynthetic pathways within Streptomyces have been set up indicating that P450s are key enzymes in such pathways,74 which also represent excellent biocatalysts for various selective oxidations.25,94 Bacterial P450s are more stable and allow for heterologous expression than membrane bound eukaryotic P450s. This enables not only feasible applications in organic and pharmaceutical industries, but also in pharmacological and toxicological studies for the produced drug metabolites.95 It was noted that P450 enzymes are a major class of biocatalysts responsible for the oxidative metabolism of various drugs depending on electron transport systems.67,91The genome sequences of Streptomyces species have revealed multiple cytochrome P450 genes.96 Species such as S. coelicolor A3 (2), S. avermitilis, S. scabies, S. hygroscopicus and S. peucetius contain 18–33 P450s within their respective sequences.97,98 P450s are heme-containing monooxygenases involved in the oxidative transformations in the secondary metabolism for production of bioactive molecules.91 The oxidative steps catalyzed by P450s enzymes in biosynthetic pathways of antibiotics from different Streptomyces species have been recently reviewed.97 Streptomyces P450s were also detected to be responsible for the stereo- and regio-specific oxidation of antibiotics such as oleandomycin.91 Multiple P450 genes in Streptomyces aid in the stereochemistry required for preparing various therapeutic agents and can contribute to engineering strategies in the future.97
P450s possess highly strict substrate specificities displaying stereo-specific reactions including deamination, dealkylations, dehalogenation, epoxidation, N-oxidation, heteroatom oxidation, desaturation and peroxidation98–100 in addition to hydroxylation as a common Streptomycetes catalyzed oxidative reactions.97 However, P450s are also reported to have promiscuous activities, which enable interaction with diverse xenobiotics,27,96 especially when involved in the bioactive synthetic pathways.94
Many previous studies on steroid biotransformations, catalyzed by Streptomycetes verified P450s to be the essential catalytic enzymes. S. griseus ATCC 13273 and S. setonii ATCC 39116, among others, catalyzed oxidation of five xenobiotics mimicking the mammalian rat liver P450s.101 Diazepam and testosterone were subjected to aliphatic and aromatic hydroxylation, while warfarin and theophyllin were subjected to N-demethylation, but further oxidation of a hydroxyl group and an unsaturated carbon occurred to yield theophyllin metabolites.76 In another study reported in 2007, S. virginiae IBL-14, isolated from soil, transformed diosgenone (16), a spiro steroid, to a rare nuatigenin-type spiro steroid, called isonuatigenone (17).81 The latter is of pharmacological value in traditional medicine. In a subsequent study, a cytochrome P450 monooxygenase, FcpC, from S. virginiae IBL-14, was identified as the enzyme responsible for the tertiary hydroxylation activity at C-25 atom in the F-ring (Scheme 5).82
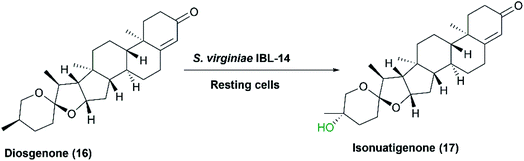 | ||
| Scheme 5 Biotransformation of diosgenone to the corresponding hydroxylated derivative, isonuatigenone, by the resting cells of S. virginiae IBL-14. This scheme has been adapted, with amendments, by permission from Springer, Applied Microbiology and Biotechnology; Wang, FQ., Li, B., Wang, W. et al. Biotransformation of diosgenin to nuatigenin-type steroid by a newly isolated strain, Streptomyces virginiae IBL-14.,81 Copyright 2007. | ||
Recently, a novel cytochrome P450 enzyme, 154C2, from S. avermitilis has been identified and characterized for regioselective 2α-hydroxylation of testosterone.102 Hydroxylation of flavonoids by S. avermitilis MA4680 is attributed to such enzymes,45 as indicated in the examples below. A cytochrome P450, CYP154C3, was identified from S. griseus and characterized as a 16α-specific hydroxylase. It was overproduced in E. coli and catalyzed the 16α hydroxylation of the D-ring of a range of steroids such as testosterone, estrone and deoxycorticosterone. Ferredoxin and ferredoxin reductase either acted as electron transporters or the isolated enzyme was fused with a P450 reductase from a Rhodococcus species.103
Präg and co-workers studied the ability of S. afghaniensis NC 5228 and S. aurantiacus JA 4570 to catalyze the regio- and stereoselective intermolecular oxidative phenol coupling.104 This is the first detected activity performed by bacteria thus far. In vitro analysis and heterologous expression proved that a cytochrome P450 monooxygenase catalyzed such a selective reaction to produce axially chiral biaryl compounds. This enzyme could be comparable to the analogous ones from fungi or plants.104 Moreover, P450s are believed to have essential role in biooxidation of chemicals such as coumarins, retinoids and alkaloids catalyzed by Streptomyces.91
Studies on electron transport systems of Streptomyces P450s involved in secondary metabolism have been done in the last fifteen years to evaluate their roles in biocatalysis of exogenous substrates.91 The primary electron transfer pathway for a cytochrome P450 from S. coelicolor, P450105D5, which catalyzes the hydroxylation of endogenous fatty acids utilizing specific ferredoxin and ferredoxin reductase as redox partners, was established.105,106 CYP107Z13 was found earlier to be responsible for regio-specific oxidation of avermectin to 4′′-oxo-avermectin in S. ahygroscopicus ZB01.65 In a following study, whole-cell recombinant systems were constructed co-expressing CYP107Z13 genes and the putative electron transporter proteins (i.e., ferredoxin (Fd68) and ferredoxin reductases (FdR18/FdR28)). Both FdR18 and FdR28 were found to be critical for electron transfer in the oxidation of avermectin since the conversion was lowered by up to 60% in the gene-deleted mutants compared to the wild-type strain.89
CYP105 and CYP107 are the most prevalent and well-studied P450 families from Streptomyces species.98 These enzymes tolerate a broad range of substrates correlating with the biosynthesis of natural products, such as antibiotics (e.g. erythromycin).27,95 The CYP105 subfamilies have been detected in the genomes of all Streptomycetes associated with either biosynthesis of natural products or biotransformation of xenobiotics, which proves the structural variation within the members of this family.100 CYP105D7, involved in the biosynthesis of pentalenic acid in S. avermitilis, has also exhibited regiospecific hydroxylation of isoflavones in both whole cells and as an isolated enzyme.100,107,108
To verify the role of P450s in hydroxylation activity catalyzed by whole Streptomyces cells, many studies relied on the action of different P450 inhibitors on the biotransformation process. Mitsukura, and co-workers found that menadione and 1-aminobenzotriazole inactivated the hydroxylation of adamantane catalyzed by S. griseoplanus AC122, which proved that the reaction proceeded via P450 cooperating systems.43 Among other inhibitors, ketoconazole significantly inhibited the activity of S. avermitilis MA-4680 towards the hydroxylation of daidzein,44 while quinidine completely blocked the hydroxylation of phloretin catalyzed by the organism.45 Transformation of 1-adamantanol catalyzed by Streptomyces sp. SA8 was attributed to P450-dependent oxidizing enzymes, since the reaction was completely inactivated by specific or non-specific P450 inhibitors such as clotrimazole, menadione, and 1-aminobenzotriazole.37 In an earlier study, carbon monoxide, metyrapone, and SKF-525-A, as specific P450 inhibitors inactivated the hydroxylating activity of S. roseosporus FERM BP-1574 and S. sclerotialus FERM BP-1370 for biotransformation of vitamin D3 derivatives, which indicated that such enzyme systems are involved in the oxidation process.36
Examples of bio-oxidation using Streptomyces species
(1) Whole-cell hydroxylation of steroids catalyzed by Streptomycetes
Microbial biotransformation of natural steroids into pharmaceutically active intermediates has been practiced for many years.22 Regarding hydroxylation of steroids, various Streptomyces species have been reported as excellent biocatalysts. The growing cells of S. roseochromogenes NCIB 10984 were used for the transformation of progesterone (18) to the respective monohydroxy and dihydroxy products.74 16α-Monohydroxy progesterone (19) and 2β, 16α-dihydroxyprogesterone (20) were produced as metabolites as shown in Scheme 6.5 The progesterone16α-hydroxylase activity was determined via the sodium periodate method and the enzyme was purified for characterization.5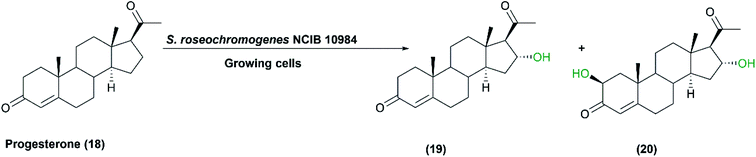 | ||
| Scheme 6 Biotransformation of progesterone (18) with the growing cells of S. roseochromogenes NCIB 10984. This scheme has been adapted, with minor modifications, from Carballeira et al.;5 with permission from Elsevier, copyright 2005. | ||
In a recently published study, Streptomyces sp. W2233-SM was checked for its capability to transform steroids, and for the occurrence of a P450 enzyme prior to proceeding to cloning and expression in E. coli as a host.76 Progesterone (18), testosterone (21), and androstenedione (23) were converted to the respective mono-hydroxylated products (19), (22) and (24), using the resting bacterial culture (Scheme 7). CYP154C8 gene was cloned and over expressed in E. coli and confirmed as a steroid hydroxylating cytochrome P450.76
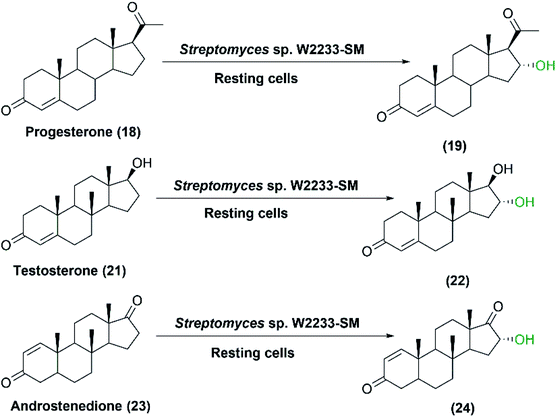 | ||
| Scheme 7 Biotransformation of progesterone, testosterone, and androstenedione with resting cells of Streptomyces sp. W2233-SM. | ||
Hydroxylation of steroid compounds via microbial approaches has been widely used to obtain anti-inflammatory agents with high glucocorticoid activities. S. roseochromogenes ATCC 13400 was exploited by Restaino and coworkers in the hydroxylation of hydrocortisone (25) at the 16α position75 (Scheme 8). The ability of the strain to convert steroid substrates was studied earlier,109 and was found to depend on a P450 cytochrome multi-enzymatic complex including a specific 16α-hydroxylase and two other proteins, roseoredoxin and roseoredoxin reductase, involved in electron transportation. The process parameters were designed wisely to achieve the highest conversion to 16α-hydroxy hydrocortisone (26), a key intermediate in the production of the anti-inflammatory agent desfluorotriamcinolone. Screening of various cultivation parameters showed higher concentration of glucose, malt extract and yeast extract in a newly formulated media led to formation of 0.23 ± 0.01 g L−1 of 16α-hydroxy hydrocortisone in shake flasks.75 Also, supplementation of hydrocortisone at the beginning of the experiment increased the conversion rates due to the induction of the P450 cytochrome expression as proved earlier.109 Furthermore, maximum product concentration was obtained in a pulsed-batch strategy, reaching levels up to 0.508 ± 0.01 g L−1 starting from 1 g L−1 of the substrate.75
 | ||
| Scheme 8 Biotransformation of hydrocortisone with the growing cells of S. roseochromogenes ATCC 13400. | ||
Later in 2016, another study was performed to improve the transformation of hydrocortisone (25) by the whole cells of S. roseochromogenes.29 Physiological parameters were first studied in shake flasks to maximize conversion rates and to minimize the formation of by-products. The biomass density of 10.8 ± 0.2 g cell dry weight/L was obtained at pH 6 and 26 °C on rich medium reaching conversion rates above 70% on small scale. Besides, a conversion of approximately 77.5%, equivalent to 0.387 ± 0.01 g L−1 of 16α-hydroxy hydrocortisone (26), was obtained in batch fermentation (2.5 L) at the same pH and temperature. Fed-batch methods were employed with agitation at 400–600 rpm and air flow at 4–8 min−1 to maintain the dissolved oxygen (DO) concentration greater than 20%. This resulted in the highest production yield (0.804 ± 0.02 g L−1) with lower by-product formation (5%), which was unprecedented according to steroid biotransformation by Streptomyces species. This could be due to the enhanced performance of the P450 enzymatic complex by a constant amount of oxygen compared to the pulsed batch strategy performed previously. The product was purified with a yield of 85% by reverse phase chromatography directly from the broth supernatant and separated from the substrate to reach a purity grade of 93%.
Coupled biocatalytic reactions are attractive for improving individual processes since they can overcome incomplete bioconversions.1 In a recent study, the whole-cell system of S. roseochromogenes ATCC13400 was coupled to that of Arthrobacter simplex ATCC31652 sequentially or concurrently for transformation of hydrocortisone (25) at different temperatures.28 S. roseochromogenes was responsible for the 16α-hydroxylation, while A. simplex performed 1,2-dehydrogenation reaction. Starting from hydrocortisone, 16α-hydroxy prednisolone (28) was obtained at a yield of 68.8% after 120 h of the coupled reactions catalyzed by the two strains at pH 6.0 and 26 °C (Scheme 9).28
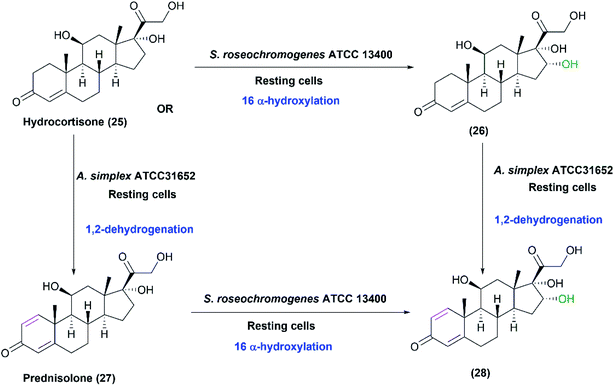 | ||
| Scheme 9 Biotransformation of hydrocortisone (25) by coupling the resting cultures of S. roseochromogenes ATCC13400 and Arthrobacter simplex ATCC31652. This scheme has been adapted, with amendments, with permission from Restaino et al.,28 Molecules; published by MDPI, 2020. | ||
(2) Whole-cell hydroxylation of non-steroids catalyzed by Streptomycetes
Streptomyces species can hydroxylate various compounds other than steroids such as iso-flavones, admantane derivatives, and daidzein and its analogues. In a study reported in 2007, growing cultures of S. griseus NRRL B8090 catalyzed the oxidation of naphthalene (29) to 4-hydroxy-1-tetralone (30). The latter is a natural product isolated from Juglans mandshurcia Maximowicz, which has anti-leishmanial and anti-diabetic activities.70 Among different cultures, S. griseus NRRL B8090 gave the highest conversion, and the subsequent trials with the whole cells for a preparative scale conversion of 150 mg naphthalene resulted in 81 mg of the pure product after 72 h. Furthermore, another derivative, 2-methyl-1,4-naphthoquinone (31), was converted to the corresponding hydroxyl compounds (32) with the whole cells of the same Streptomyces strain (Scheme 10).70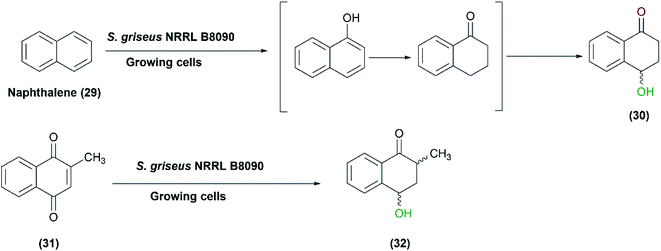 | ||
| Scheme 10 Biotransformation of naphthalene (29) and 2-methyl-1,4-naphthoquinone (31) to 4-hydroxy-1-tetralone (30) and 2-methyl-4-hydroxy-1-tetralone (32), respectively, with the growing cells of S. griseus NRRL B8090. This scheme has been adapted, with minor modifications, from Gopishetty et al.;70 with permission from Elsevier, copyright 2006. | ||
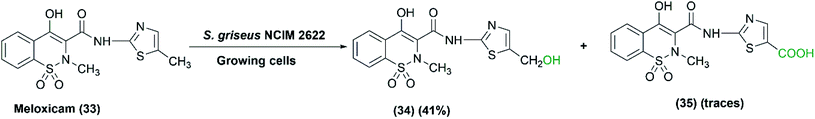
Gurram et al. (2009) reported the use of S. griseus NCIM 2622, among five tested actinomycetes, to catalyze the oxidation of meloxicam (33), a non-steroidal anti-inflammatory drug, into two metabolites: 5-hydroxymethyl meloxicam (34) and trace amounts of 5-carboxy meloxicam (35) (Scheme 11), with a higher biological activity.69 A moderate amount of 5-hydroxymethyl meloxicam was obtained at a yield of 41% utilizing growing cultures of S. griseus NCIM 2622.
Mori et al. conducted a study in 1996 on screening the activity of proline hydroxylases in different microbial cultures.73 Among 3000 strains isolated from soil, few strains including Streptomyces sp. strain TH1 and Streptomyces canus ATCC12647 exhibited high specific and regio-hydroxylation. L-Proline (36) was converted to cis-3-hydroxy-L-proline (37) with a yield of 63% employing the resting cultures (Scheme 12). Proline 3-hydroxylase activity was preliminarily characterized in cell extracts. Notably, hydroxyl prolines are useful as chiral synthons in organic synthesis.110
 | ||
| Scheme 12 Biotransformation of L-proline (36) with resting cells of Streptomyces sp. strain TH1 or S. canus ATCC 12647. | ||
In 1991, the first study was conducted on the microbial hydroxylation of vitamin D3 derivatives.36 Among 300 Streptomyces species strains examined, S. sclerotialus FERM BP-1370 and S. roseosporus FERM BP-1574 could convert 25-hydroxyvitamin D3 (38) and l α-hydroxyvitamin D3 (39), respectively, to l-α,25-dihydroxyvitamin D3 (40) with a productivity of approximately 7 μg L−1 min−1 (Scheme 13). Cytochrome P450 inhibitors, including carbon monoxide and metyrapone influenced the hydroxylation process, indicating that P450 oxidizing enzyme system was responsible for mediating the reaction in the whole-cell suspensions.36
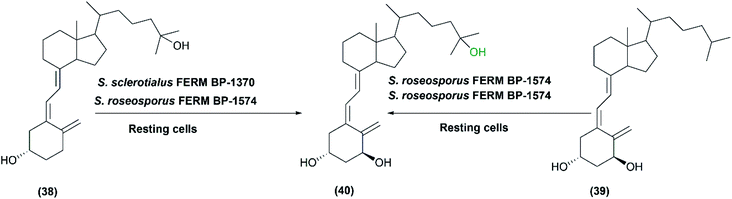 | ||
| Scheme 13 Biotransformation of 25-hydroxyvitamin D3 (38) and l α-hydroxyvitamin D3 (39) with the resting cells of S. sclerotialus FERM BP-1370 and S. roseosporus FERM BP-1574. This scheme has been adapted, with minor modifications, from Sasaki et al., Applied and environmental microbiology;36 with permission from America Society for Microbiology, copyright 1991. | ||
Hydroxylated adamantane, pharmaceutical intermediates, polymers, and materials for electronic industry were obtained upon conjugation of admantane (41) with S. griseoplanus AC122 cells. Among 470 examined strains, S. griseoplanus was found to be highly regioselective to give 1-adamantanol (42) from adamantane with up to 32% conversion yield (Scheme 14). Eventually, the products, 1-adamantanol (42) and 2-adamantanol were separated by silica gel chromatography, and obtained in purified forms.43
 | ||
| Scheme 14 Biotransformation of adamantane (41) with the growing cells of S. griseoplanus AC122 to 1-adamantanol. | ||
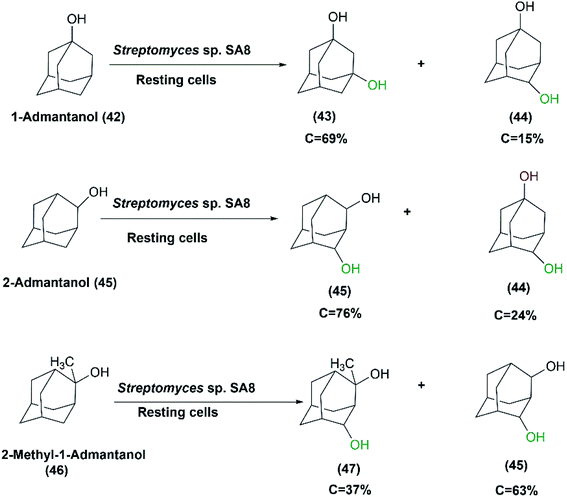
In another study aimed at screening actinomycetes displaying high hydroxylation activity with high regio-selectivity, Streptomyces sp. SA8 was one of the identified strains possessing the highest 1,3-admantanediol (43) producer from 1-adamantanol (42).37 The resting culture produced 2.3 g L−1 of (43) at 69% conversion after 96 hours of incubation. Cycloketones such as adamantane, 1-admantanol, cyclohexane, cyclohexanol, or cyclooctane were utilized as inducers of hydroxylation activity during cultivation. 1-Admantanol at a concentration of 0.3% (w/v) exhibited the highest level of induction, and in combination with 1% (v/v) Tween 20, was most effective for preparing high activity resting cells. Also, glucose or glycerol was added at 100 mM acting as carbon sources for coenzyme-recycling systems. The growing cells of Streptomyces sp. SA8 produced 5.9 g L−1 of 1,3-admantane diol (43) from 6.2 g L−1 1-admantanol (42) after 120 hours under optimized conditions. However, purification of the products by silica gel column chromatography was easier from the reaction mixture of the resting cultures than from culture broth owing to higher productivity. The ratio of the product 1,3-admantanediol (43) to the byproduct 1,4-admantanediol (44) was 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. This made Streptomyces sp. SA8 superior to other studied species for the regio-selective hydroxylation of 1-admantol (42) to the corresponding diol. The position, number, and steric effect of the hydroxyl group on the adamantane skeleton could influence the activity of the oxidizing enzymes of Streptomyces sp. SA8. meso-2,4-Admantanediol (46) and 1,4ax-admantanediol (44) were produced from 2-admantanol (45) at a ratio of 76
15. This made Streptomyces sp. SA8 superior to other studied species for the regio-selective hydroxylation of 1-admantol (42) to the corresponding diol. The position, number, and steric effect of the hydroxyl group on the adamantane skeleton could influence the activity of the oxidizing enzymes of Streptomyces sp. SA8. meso-2,4-Admantanediol (46) and 1,4ax-admantanediol (44) were produced from 2-admantanol (45) at a ratio of 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 at 32% conversion rate. Also, 2-Me-2-adOH (46) was transformed into meso-2,4-ad (OH)2 (45) and a diastereomeric mixture of 2-Me-2,4-ad(OH)2 (47) at a ratio of 63
24 at 32% conversion rate. Also, 2-Me-2-adOH (46) was transformed into meso-2,4-ad (OH)2 (45) and a diastereomeric mixture of 2-Me-2,4-ad(OH)2 (47) at a ratio of 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37, and at a 22% conversion rate (Scheme 15).37
37, and at a 22% conversion rate (Scheme 15).37
Daidzein and genistein are the major isoflavone compounds of phytoestrogen found in plants and soybeans. They act as antioxidants, antimicrobials, free radical scavengers, and metal chelators. Roh et al. reported that Streptomyces avermitilis MA-4680, among different screened microorganisms, exhibited regiospecific hydroxylation of the two isoflavones.44 Daidzein (5) and genistein (48) were converted at highest rates to 3′,4′,7-trihydroxyisoflavone (7) and 3′,4′,5,7-tetrahydroxyisoflavone (49), respectively (Scheme 16). It is noteworthy that the detected antioxidant activities of the hydroxylated products are higher than the original isoflavones.111 Upon using 100 g L−1 of S. avermitilis wet cell mass, ortho-dihydroxylated isoflavones, more potent antioxidants, were produced at a yield of 2.03 mg L−1 from daidzein and genistein as substrates. Strikingly, the ortho–dihydroxylation activity for isoflavones was approximately 300–500 times higher for the wild type S. avermitilis MA-4680 than the E. coli recombinant system expressing P450 genes from various microorganisms.44
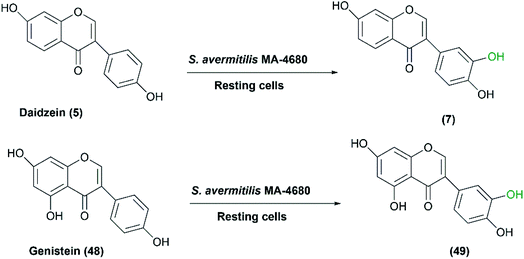 | ||
| Scheme 16 Biotransformation of daidzein and genistein to the corresponding selective hydroxylated compounds with the resting cells of S. avermitilis MA-4680. This scheme has been adapted, with minor modifications, from Roh et al.;44 with permission from Elsevier, copyright 2009. | ||
However, 3′,4′,7-trihydroxyisoflavone (7), the product produced from daidzein biotransformation, was subjected to degradation by the bacterial cells. Genetic manipulation of the producer microorganism is a possible approach to prevent degradation and also to avoid secondary reactions by blocking side pathways.5,71 CYP105D7, the P450 presumably responsible for the oxidative activity, was overexpressed with its electron transfer proteins in S. avermitilis, resulting in higher yield of (7) (112.5 mg L−1), however degradation was observed within only 15 hours, which limited its industrial scale application.108,112 The enzyme tyrosinase catalyzes oxidation of phenolic compounds into corresponding quinone forms. An extracellular tyrosinase, involved in melanin production in S. avermitilis, was thought to contribute to the modification of 3′,4′,7-trihydroxyisoflavone (7).71 Tyrosinase deletion mutant (ΔmelC2), constructed to evaluate the effect on the production of 3′,4′,7-trihydroxyisoflavone (7), and regulation of CYP105D7 and its redox partners, prevented the degradation of 3′,4′,7-trihydroxyisoflavone (7), but the hydroxylation activity was decreased by 40% compared to the wild-type strain. This was due to a decrease in the expression level of the responsible P450 enzyme, CYP105D7, and its electron transfer protein counterparts.
In a study reported in 2012,72 the melanin-producing S. avermitilis MA4680 was utilized to produce piceatannol from trans-resveratrol (50) (Scheme 17). By generating deletion mutants, extracellular tyrosinase (MelC2) was detected as the enzyme responsible for the ortho-specific hydroxylation. Phenolic compounds such as catechol and hydroquinone were crucial additives for production of piceatannol in high yield. Catechol acted as a competitive inhibitor of the dioxygenase pathway involved in tyrosinase functioning for melanin production, which blocked subsequent oxidation, hence preventing piceatannol degradation. As a result, piceatannnol (51) was produced, with a yield of 78%, via a single monooxygenation pathway using catechol at the optimal concentration of 1 mM for inhibition.72
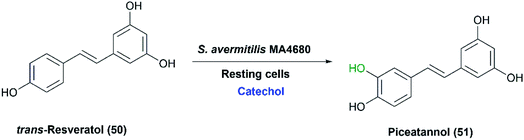 | ||
| Scheme 17 Biotransformation of trans-resveratrol with the resting cells of S. avermitilis MA4680 in the presence of catechol as an inhibitor for the potential dioxygenase activity. This scheme has been adapted with amendments; with permission from Nahum Lee, Eun Jung Kim and Byung-Gee Kim, ACS Chemical Biology, 2012 7 (10), 1687–1692, DOI: 10.1021/cb300222b.72 Copyright 2012 American Chemical Society. | ||
In another study, three P450s, CYP107Y1, CYP125A2 and CYP107P2, from S. avermitilis MA4680 exhibited good hydroxylation activity.66 The strain was developed and utilized as expression host for these enzymes using a self-cloning strategy employing genistein (48), chrysin (52) and apigenin (54), respectively, as substrates (Scheme 18). It is noteworthy that the E. coli host system did not attain enough quantity of products to well determine the entire structure of the hydroxylated products. In the case of S. avermitilis, all the electron transfer proteins (i.e., ferredoxins and ferredoxin reductases) were not well expressed in the E. coli system due to the high GC-content of Streptomyces species. So, S. avermitilis host system was convenient for efficient expression of its own P450s.66
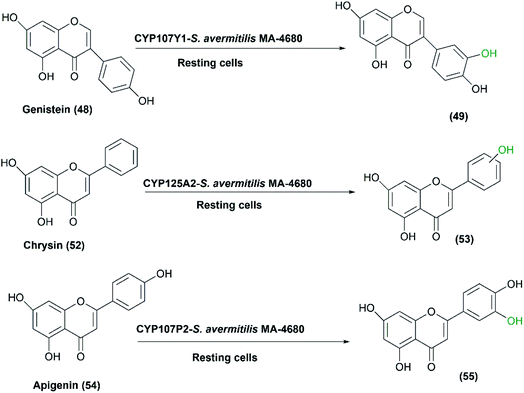 | ||
| Scheme 18 Biotransformation of genistein, chrysin and apigenin with the resting cells of S. avermitilis MA4680 overexpressing three P450 responsible genes. | ||
Recently, S. avermitilis MA4680 was used for regiospecific hydroxylation of phloretin (54), a dihydrochalcone found in apple bark.45 Selective hydroxylated products of phloretin in B ring were obtained utilizing the resting cultures with a maximal conversion of 6.7% (Scheme 19). Remarkably, phloretin and its hydroxylated derivatives (57) and (58) are most known for their therapeutic properties as anti-amyloid, antiarthritic, and anti-inflammatory effects.45,111
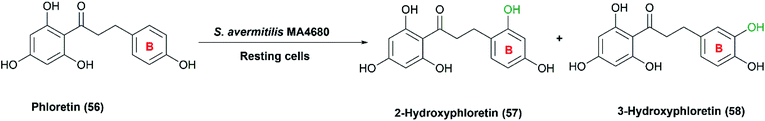 | ||
| Scheme 19 Selective hydroxylation of phloretin in the B ring with the resting cells of S. avermitilis MA4680. | ||
(3) Whole-cell asymmetric oxidation of prochiral sulfides catalyzed by Streptomycetes
The organo-sulfur compounds are crucial biological groups involved in metabolism, and also constitute amino acids and their derivatives i.e., cysteine and methionine. The largest group of sulfur-based compounds are sulfoxides for which several attempts have been established to develop biotechnological synthesis.113,114 Chiral sulfoxides possess many applications; they are used as chiral ligands, chiral auxiliaries, and intermediates in asymmetric synthesis.79,115–117 They are used in the pharmaceutical industry e.g. modafinil and esomeprazole.118 Remarkably, the use of microorganisms in the asymmetric sulfoxidation has recently received significant attention.114Microbial asymmetric sulfoxidation reactions have been successfully performed during the last 50 years. In 1982, Holland and Carter presented results of a study involving the use of Mortierella isabellina to oxidize aryl-substituted phenyl and benzyl-methyl sulfides to the corresponding sulfoxides.119 In 2013, Mascotti et al. examined several bacterial strains to explore their abilities to perform the oxidation of cyclohexyl methyl sulfide (CMS) (59) as a model prochiral sulfide. S. hiroshimensis ATCC27429, S. flavogriseus ATCC33331 and S. phaeochromogenes NCIMB 11741, displayed sulfoxidation of the compound with different enantioselectivities. S. phaeochromogenes showed a characteristic biotransformation profile as the stereochemistry of the process was inverted over the time-course of the experiments as shown in Scheme 20. This was explained by the expression of at least two enzymes that may reach their highest rates at different times. The three Streptomyces strains conducted the sulfoxidation of cyclohexyl methyl sulfide (59), in an enantio-complementary style, to the corresponding sulfoxide, cyclohexyl methyl sulfoxide (CHMO), (60) and (61).41 S. flavogriseus was the only strain that exhibited a constant S enantiopreference with maximal conversion of 73% despite poor optical purity. The effect of solvents like isopropyl alcohol (IPA) and dimethyl sulfoxide (DMSO), as recommended solvents,120,121 on enhancing the biotransformation process catalyzed by S. hiroshimensis ATCC 27429 was tested; 2% (v/v) DMSO caused a decrease of conversion to 18%, while IPA dropped the enantiomeric excess to 66%. This was probably due to inhibition of the enzymes by the co-solvents.
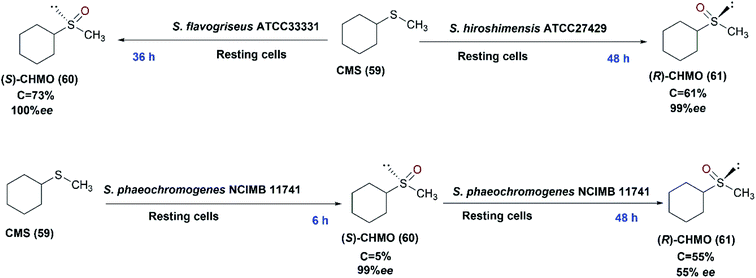 | ||
| Scheme 20 Biotransformation of cyclohexyl methyl sulfide (59) with the resting cells of three different Streptomyces strains (S. hiroshimensis ATCC27429, S. flavogriseus ATCC33331 and S. phaeochromogenes NCIMB 11741) indicating the time detected for each product in blue font. This scheme has been adapted, with amendments, from Mascotti et al.41 with permission from Elsevier, copyright 2013. | ||
We have recently checked the capability of S. glaucescens GLA.0 for asymmetric sulfoxidation using thioanisole (62) as a prochiral aromatic sulfide model.32 The substrate was converted to the pure isomer of phenyl methyl sulfoxide (R-PMSO) (63) at a high yield of 90% and excellent ee exceeding 99% employing the growing bacterial culture. Different parameters were varied to optimize the reaction conditions reaching higher yields of the product and smaller amounts of phenyl methyl sulfone (64) as a byproduct. Lower concentrations (0.2–1%) of IPA as a co-solvent were used to increase substrate solubility (Scheme 21), which contributed to higher product yields compared to the analogous reactions utilizing the sparingly soluble substrate. Moreover, it had negligible effect on the selectivity of the biooxidation reaction. Furthermore, other parameters helped in enhancing the biotransformation including the use of higher yeast extract-containing medium, higher rotation speed (180 RPM), and addition of the substrate at the time of the inoculation (zero time) of the biotransformation reaction medium (BRM).
 | ||
| Scheme 21 Biotransformation of thioanisole (62) with the growing cells of S. glaucescens GLA.0 under optimized conditions. | ||
The enantioselective sulfoxidation is a typical Baeyer–Villiger reaction.114 So, we hypothesized a putative Baeyer–Villiger monooxygenase (BVMO), identified via an in-silico analysis of the genome of S. glaucescens GLA.0, to be responsible for the activity. Notably, BVMOs are part of class B flavoprotein monooxygenases that are among the major enzymes catalyzing the formation of chiral sulfoxides such as albendazole.7,122,123 S. glaucescens GLA.0 cells were primarily screened for BVMO activity using cyclohexanone as a model substrate. Qualitative analysis of cyclohexanone consumption by the resting bacterial cells, by a method based on the reaction of an enolizable ketone (i.e., cyclohexanone) with dinitrobenzoic acid in an alkaline solution leading to the formation of a purple coloured complex,124 showed total disappearance of color within 24–48 hours. Monitoring the bioconversion of cyclohexanone by the bacterial cells over time revealed 6-carbon and 7-carbon esters and traces of ε-caprolactone by GC-MS analysis. This may be due to the action of esterases, lactonases, and/or hydrolases, the presence of which is documented for different Streptomyces strains.125 Such enzymes might be produced by the bacterial cells upon incubation with cyclohexanol (65) or cyclohexanone (Scheme 22) as detected before in the case of cyclohexanol-grown Acinetobacter calcoaceticus.126 This would affect the quantity of the produced caprolactone. As revealed, the putative monooxygenase (BVMO) from S. glaucescens GLA.0 shares 26% identity with cyclohexanone monooxygenase from A. calcoaceticus NCIMB 9871.
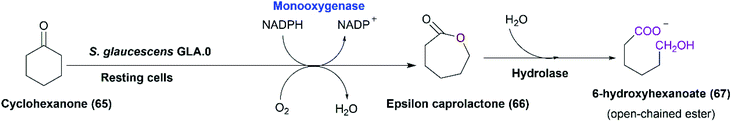 | ||
| Scheme 22 Putative pathway of cyclohexanol and/or cyclohexanone catalyzed by the whole cells of S. glaucescens GLA.0. This scheme has been adapted, with amendments, from Donoghue et al.;127 with permission from John Wiley and Sons, copyright 2008. | ||
(4) Whole-cell oxidation of aldehydes catalyzed by Streptomycetes
Although traditional chemical oxidations of aldehydes to carboxylic acids by oxidants like oxone are highly efficient and straightforward, microbial selective oxidation of aldehydes are still of interest.4 Various examples of aromatic aldehydes biooxidation to the respective carboxylic acids employing the whole cells of Streptomyces species were reported. S. viridosporus T7A catalyzed the oxidation of vanillin (68) to the more valuable, vanillic acid (69) in a two-stage fermentation process.38 Vanillin (68) was used as inducer in the first stage of the bacterial cultivation, to help the production of aromatic aldehyde oxidase in vivo and make the growing cells active for a longer time (Table 1). The product was recovered with high purity and yield exceeding 96% of the initial amount of vanillin, and concentration of 1.6 g L−1 (Scheme 23).| No. | Streptomyces species | Biological application | Type of reaction | Substrate | Scheme number | Reference |
|---|---|---|---|---|---|---|
| a Key points related to the biooxidation reaction conditions are presented, the asterisk mark ‘*’, beside the substrate of the entry 20 and 22, indicates that low concentrations of substrates were used in the first stage of fermentations. | ||||||
| 1 | Streptomyces lividans TK54 | Recombinant whole cells | Regiospecific hydroxylation | Avermectin | 1 | 65 |
| 2 | Streptomyces avermitilis MA4680 | Recombinant whole cells | Regiospecific hydroxylation | Genistein | 17 | 66 |
| 3 | S. avermitilis ΔSaOMT2 | Recombinant whole cells | Regioselective hydroxylation | Daidzein | 3 | 40 |
| 4 | Streptomyces lividans TK24 | Recombinant whole cells | Oxidative dealkylation | 7-Ethoxycoumarin | 2 | 67 |
| 5 | Streptomyces albus J1074 | Recombinant whole cells | Carboxylation | 12-Desnitrile-12-methyl-borrelidin | 4 | 68 |
| 6 | Streptomyces griseus NCIM 2622 | Wild type whole cells | Hydroxylation | Meloxicam | 11 | 69 |
| 7 | Streptomyces griseus NRRL B8090 | Wild type whole cells | Hydroxylation | Naphthalene | 10 | 70 |
| 8 | Streptomyces sclerotialus FERM BP-1370 | Wild type whole cells | Hydroxylation | 25-Hydroxyvitamin D3 | 13 | 36 |
| 9 | Streptomyces griseoplanus AC122 | Wild type whole cells | Regioselective hydroxylation | Adamantane | 14 | 43 |
| 10 | Streptomyces sp. SA8 | Wild type whole cells | Regioselective hydroxylation | 1-Adamantanol | 15 | 37 |
| 11 | Streptomyces avermitilis MA-4680 | Wild type whole cells | Regiospecific hydroxylation | Daidzein | 16 | 44 and 71 |
| 12 | Streptomyces avermitilis MA4680 | Wild type whole cells | Regioselective hydroxylation | Trans-resveratrol | 17 | 72 |
| 13 | Streptomyces canus ATCC 12647 | Wild type whole cells | Stereospecific hydroxylation | L-Proline | 12 | 73 |
| 14 | Streptomyces roseochromogenes NCIB 10984 | Wild type whole cells | Hydroxylation | Progesterone | 6 | 74 |
| 15 | Streptomyces roseochromogenes ATCC 13400 | Wild type whole cells | Hydroxylation | Hydrocortisone | 8 | 75 |
| 16 | Streptomyces roseochromogenes | Wild type whole cells | Hydroxylation | Hydrocortisone | 9 | 28 |
| 17 | Streptomyces avermitilis MA4680 | Wild type whole cells | Regioselective hydroxylation | Phloretin | 19 | 45 |
| 18 | Streptomyces sp. W2233-SM | Wild type whole cells | Hydroxylation | Progesterone | 7 | 76 |
| 19 | Streptomyces phaeochromogenes NCIMB 11741 | Wild type whole cells | Asymmetric sulfoxidation | Cyclohexyl methyl sulfide | 20 | 41 |
| 20 | Streptomyces glaucescens GLA.0 | Wild type whole cells | Enantioselective sulfoxidation | Thioanisole* | 21 | 32 |
| 21 | Streptomyces cattleya | Wild type whole cells | Carboxylation | Fluoroacetaldehyde | 24 | 77 |
| 22 | Streptomyces viridosporus T7A | Wild type whole cells | Carboxylation | Vanillin * | 23 | 38 |
| 23 | Streptomyces griseus ATCC 13273 | Wild type whole cells | Carboxylation followed by hydroxylation (site-selective oxidation) | Ursane triterpenes [ex; ursolic acid] | 31 | 78 |
| 24 | Streptomyces griseus ATCC 13273 | Wild type whole cells | Carboxylation & glycosylation | 3-Oxo oleanolic acid | 30 | 79 |
| 25 | Streptomyces griseus ATCC 13273 | Wild type whole cells | Hydroxylation & carboxylation (site-selective oxidation) | Oleanane triterpenes [ex; oleanolic acid] | 32 | 80 |
| 26 | Streptomyces platensis | Wild type whole cells | Hydroxylation followed by two-step oxidation | Terfenadine | 26 | 31 |
| 27 | Streptomyces virginiae IBL-14 | Wild type whole cells | Hydroxylation | Diosgenone | 5 | 81 and 82 |
| 28 | Streptomyces fulvissimus NRRL B1453 | Wild type whole cells | Hydroxylation & sulfation | 5-Hydroxyflavone | 25 | 83 |
| 29 | Streptomyces griseus (UT 1158, NRRL 8090) | Wild type whole cells | O-Deethylation & hydroxylation | 7-Ethoxycoumarin | 27 | 84 |
| 30 | Streptomyces griseus ATCC 13273 | Wild type whole cells | Oxidation | Precocene II | 28 | 30 and 85 |
| 31 | Streptomyces griseus (SC 1754 and SC 13971) | Wild type whole cells | Selective hydroxylation | Mutilin | 29 | 86 |
| 32 | Streptomyces cinnamonensis | Wild type whole cells | Oxidative cleavage | 1,4-Naphthoquinone (INO5042) | 33 | 87 |
| 33 | S. griseus CECT 3116 | Wild type whole cells | N-oxidation | p-Aminobenzoic acid | 34 | 88 |
In a study published in 2001, S. cattleya NRRL 8057 was screened for the enzyme involved in fluorometabolite biosynthesis.77 The resting cultures transformed fluoroacetaldehyde (70) to the corresponding acid, fluoroacetate (71) (Scheme 24). The enzyme was produced mainly in the late exponential to the stationary phase after around 6 days of growth. Determination of the substrate specificity of the isolated fluoroacetaldehyde dehydrogenase indicated fluoroacetaldehyde and glycoaldehyde to be good substrates.77
 | ||
| Scheme 24 Biotransformation of fluoroacetaldehyde (70) with the resting cells of S. cattleya NRRL 8057. | ||
(5) Multi-step and/or concurrent oxidations catalyzed by the whole cells of Streptomyces
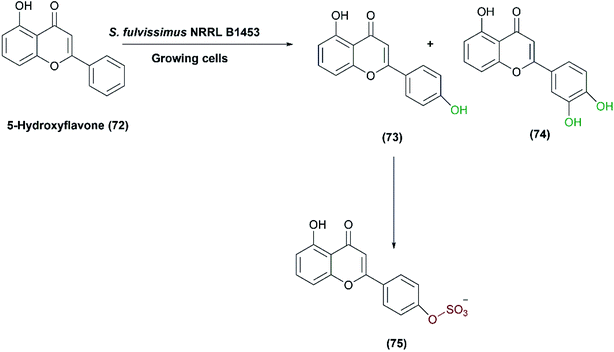
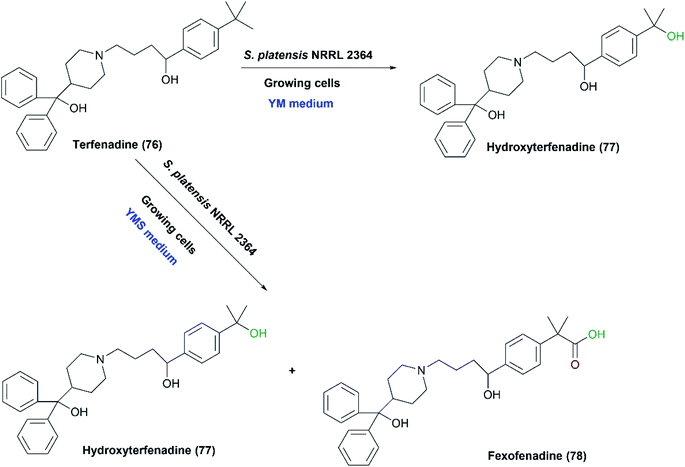
One-pot multi-step reactions catalyzed by microbial cells represent an alternative pathway to others consuming time and materials.1,128 S. fulvissimus NRRL B1453 was reported to catalyze multi-step oxidation reactions;83 the bacteria transformed 5-hydroxyflavone (72) first to 5,4′-dihydroxy- (73) and 5,3′,4′-trihydroxyflavone (74), and subsequently sulfation at the 4′ position of (73), resulting in the formation of 5,4′-dihydroxyflavone-4′-sulfate (75) as shown in Scheme 25.Mazier et al. reported the biooxidation of terfenadine (76) utilizing the whole cells of S. platensis NRRL 2364.31 The formation of the alcohol, hydroxyl terfenadine (77), and the acid, fexofenadine (78) depended on the culture conditions. Fexofenadine (78) was formed together with hydroxy terfenadine (77) in a yeast extract-malt medium containing soybean peptone (YMS), depending mainly on the age of the culture. The highest yield of fexofenadine (78) (25%) was achieved just in 48-hour-old culture rather than older cultures. In contrast, hydroxy terfenadine (77) was solely obtained at a yield up to 51% in a medium devoid of soybean peptone (YM) (Scheme 26). Moreover, the oxygen dependency of the process was studied through performing transformation in aerobic or argon atmospheres. No oxidation reaction occurred under argon and neither upon using terfenadine nor hydroxyterfenadine as substrates, which indicated that the oxidizing activity was due to an oxygenase not a dehydrogenase. Different ratios of hydroxy terfenadine (77) and fexofenadine (78) were observed in the biotransformation process due to the instability of the oxidative enzyme system induced by soybean peptone. Later the purified P450 dependent monooxygenase, CYP107L was found to be responsible for the hydroxylation of the tertiary-butyl group of terfenadine.95,129 S. platensis CYP107L was cloned and expressed in E. coli, and examined for whole-cell conversions of amodiaquine, ritonavir, amitriptyline, and thioridazine. Its behavior resembled that of human P450s involved in the metabolism of these drugs, which may contribute to preparative synthesis of the metabolites.95
Streptomyces griseus has several applications in oxidative transformation of xenobiotics, which could be catalyzed by the same enzyme system. S. griseus (UI 1158, NRRL 8090) was screened for the transformation of 7-ethoxycoumarin (79), a sensitive analytical substrate, to detect and characterize such type of enzymatic activity.84 Conversions included O-deethylation of 7-ethoxycoumarin to 7-hydroxycoumarin (80), followed by hydroxylation of 7-hydroxycoumarin to 6,7-dihydroxycoumarin (81), and finally the latter was further methylated to form 7-hydroxy-6-methoxycoumarin (82) (Scheme 27). The products were isolated at a yield of up to 30%, but trials to obtain an isolated enzyme from the cell-free extract were unsuccessful. However, the findings indicated that the strain has biosynthetic machinery for coumarin derivatives almost resembling eukaryotic systems.84 Later, the enzyme, CYP105D1, was cloned and heterologously expressed, and examined to convert a variety of xenobiotics to equivalent mammalian metabolites.96,130
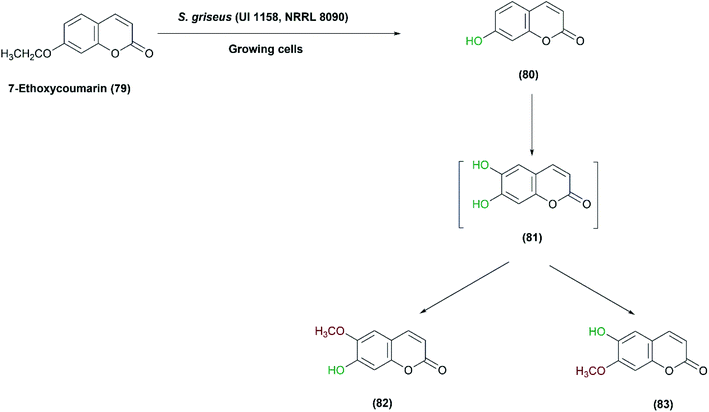 | ||
| Scheme 27 Multistep oxidation of 7-ethoxycoumarin (79) with the growing cells of S. griseus (UI 1158, NRRL 8090). This scheme has been adapted, with minor modifications, from Sariaslani et al.;84 with permission from American Society for Microbiology, copyright 1983. | ||
S. griseus ATCC 13273 was found to possess a similar metabolism for precocene II (84), a fourth-generation pesticide, as in eukaryotic systems.85 On incubation with the growing culture, three oxidized products were obtained, identified, and isolated. An inducible cytochrome P-450 system enzyme was considered responsible for the mono oxygenation pathway as reported in a subsequent study performed by the same authors.30 The high levels of the enzyme were produced in bacterial cultures grown on an enriched medium containing soybean flour, leading to rapid oxidation of precocene II, compared to other media compositions. Furthermore, aerial mycelium-negative variant of S. griseus (AMV cells), that lacked to cytochrome P-450 after growth in the same medium containing soybean flour, failed to oxidize precocene II, which proved that the P-450 system was responsible for the reaction. S. griseus growing cells catalyzed the production of a highly reactive epoxide (85) which acted as an initial intermediate in the process. (−) Cis- and (+) trans-Precocene II 3,4-dihydrodiols (88), (89) and (+)-3-chromenol (87) were then detected as final products (Scheme 28).85
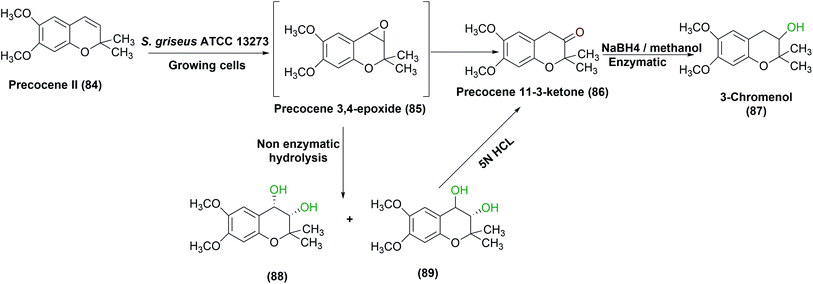 | ||
| Scheme 28 Multistep oxidation of precocene II (84) with the growing cells of S. griseus ATCC 13273 under optimized conditions of growth. This scheme has been adapted, with minor modifications, from Sasaki et al., Applied and environmental microbiology;85 with permission from American Society for Microbiology, copyright 1987. | ||
Hanson et al. checked the ability of S. griseus to hydroxylate mutilin, an antibiotic for poultry and animals.86 Hydroxylated derivatives of mutilin prevent its metabolism (i.e., hydroxylation at definite positions), hence inactivation. Furthermore, their biological activities do not change, and can be the same as the parent molecule, mutilin. Two strains of S. griseus, SC 1754 and SC 13971 (ATCC 13273) transformed mutilin (90) selectively to 2S-hydroxymutilin (91), 7S-hydroxymutilin (92) and 8S-hydroxymutilin (93) (Scheme 29). The process was performed using bacterial cultures grown on media containing soy flour. A typical medium, including 2% soy and 0.5% glucose gave the highest rates of hydroxylation in shake flasks. Glycerol as a carbon source produced lower yields compared to glucose. The reaction was scaled up to 15, 60 and 100 L and larger amounts of hydroxy products, 16–100 mg, were extracted.86
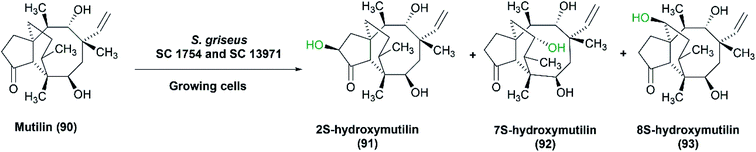 | ||
| Scheme 29 Biotransformation of mutilin (90) with the growing cells of S. griseus SC 1754 and SC 13971 to the corresponding hydroxylated products (91–93). This scheme has been adapted with permission from Ronald L. Hanson, James A. Matson, David B. Brzozowski, Thomas L. LaPorte, Dane M. Springer and Ramesh N. Patel, Organic Process Research & Development, 2002, 6 (4), 482–487, DOI: 10.1021/op020028q.86 Copyright 2002 American Chemical Society. | ||
Zhu et al. set up a study on biotransformation of olean-type pentacyclic triterpenes, the natural products possessing various pharmacological applications, by employing whole cells of S. griseus ATCC 13273, among other microorganisms.79 Four substrates, including 3-oxo oleanolic acid, 3-acetyl oleanolic acid, oleanolic acid, and esculentoside A were used and underwent regio-selective methyl oxidation and glycosylation. The corresponding oxidized products as depicted in Scheme 30, were isolated and structurally elucidated by ESI-MS, 1H NMR, 13C NMR, and 2D-NMR spectroscopy. Such microbial conversion provides an alternative approach to enrich the structural diversity of olean-type pentacyclic triterpenes.79
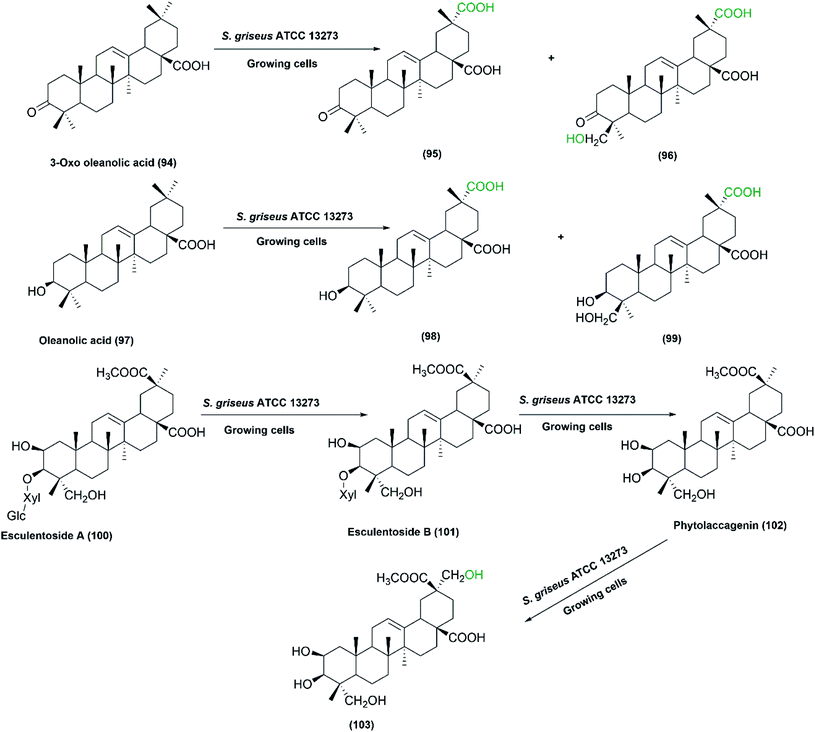 | ||
| Scheme 30 Biotransformation of 3-oxo oleanolic acid (94), oleanolic acid (97), and esculentoside A (100), respectively, with the growing cells of S. griseus ATCC 13273 to the corresponding hydroxylated and carboxylated products. This scheme has been adapted, with minor modifications, from Zhu et al.79 with permission from Elsevier, copyright 2011. | ||
The same authors recently reported a study on the capability of S. griseus in transforming a number of ursane triterpenes.78 Ursolic acid (104), 3-oxo ursolic acid (107), corosolic acid (110), asiatic acid (113) and madasiatic acid (115), as substrates, were converted by the growing cells of S. griseus ATCC 13273 to give moderate yields of hydroxylated and/or carboxylated products via site selective oxidation of the C–H group (Scheme 31). It is essential to make use of readily available approaches for expanding the structural diversity of such natural products to obtain more effective and less toxic derivatives with regard to biological activities. Among the compounds produced, eight metabolites were isolated and structurally elucidated for the first time. The biological processes allowed the production of novel metabolites in a single step under environmentally benign conditions, which was difficult to achieve by chemical methods. The strain selectively catalyzed both carboxylation of the C-30 methyl group and hydroxylation of the C-24 methyl group in (104), (107), and (110), while the hydroxylation of C-24 was blocked in case of asiatic acid (113) and madasiatic acid (115), which bear a hydroxyl group on C-23. The new products (105, 106, 108, 109, 111, 112, 116 and 117) were isolated and structurally elucidated based on 1D and 2D NMR and HR-MS data.78
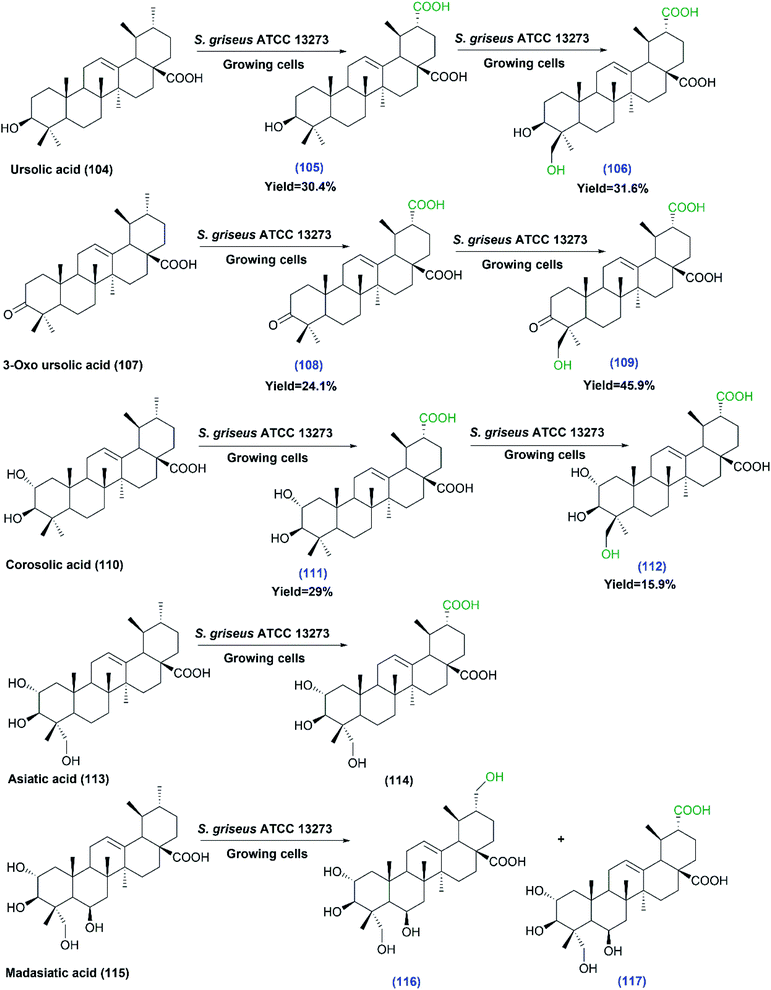 | ||
| Scheme 31 Biotransformation of ursolic acid (104), 3-oxo ursolic acid (107), corosolic acid (110), asiatic acid (113) and madasiatic acid (115) with growing cells of S. griseus ATCC 13273, the first three substrates undergo multistep oxidations. The products' numbers in blue referred to the newly reported compounds. This scheme has been adapted, with minor modifications, from Xu et al.;78 with permission from the Royal Society of Chemistry. | ||
Oleanolic triterpenes have also been recently tested as substrates for the growing cultures of the same strain (S. griseus ATCC 13273).80 The substrates including oleanolic acid (97), hederagenin (120), echinocystic acid (123), quillaic acid (126) and senegenin (129), were selectively carboxylated at the C-29 methyl group. In addition, the hydroxylation of C-24 and C-21 were also detected on some substrates, as indicated by Scheme 32. Most of the produced metabolites were isolated and identified by MS and NMR analyses. All the products did not exhibit any cytotoxicity, in contrast to their corresponding substrates that exhibited cytotoxic activities. Moreover, testing the anti-inflammatory activities of all substrates and products against lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophages showed the products (124), (127) and (128) to have moderate inhibitory effects, while the products (119), (122) and (130) showed enhanced inhibitory activities. The findings from the two studies suggest that S. griseus ATCC 13273 could be a useful biocatalyst to enrich the structural diversity of pentacyclic triterpenes, which will have great impact on medicinal chemistry research.
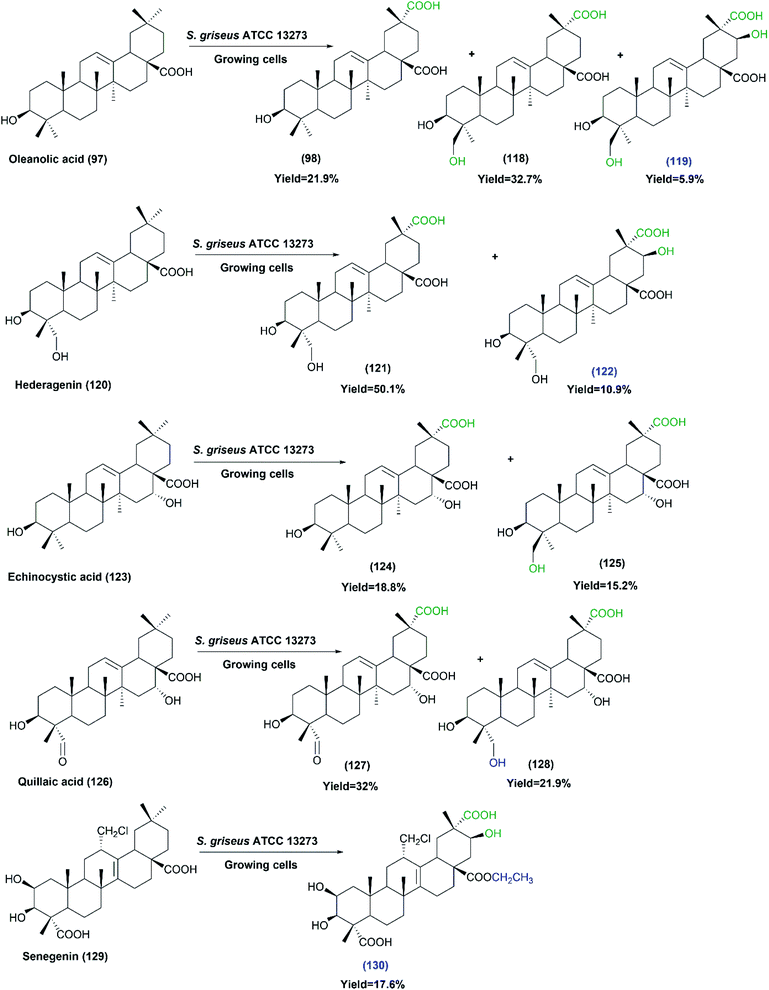 | ||
| Scheme 32 Biotransformation of oleanolic acid (97), hederagenin (120), echinocystic acid (123), quillaic acid (126) and senegenin (129) with the growing cells of S. griseus ATCC 13273. The products' numbers in blue refer to the newly reported compounds. This scheme has been adapted, with minor modifications, from Xu et al.;80 with permission from Elsevier, copyright 2017. | ||
(6) Oxidative cleavage catalyzed by the whole cells of Streptomyces
Fosse et al. studied the oxidative cleavage of heterocyclic naphthoquinone model in a report published in 2004.87 Heterocyclic naphthoquinones are known to have potential pharmacological activities against venous alterations and inflammatory edema, but they are poorly soluble and bioavailable, which hampers their testing. Thiazole fused 1,4-naphthoquinone, INO5042 (131), was transformed by the whole cells of either S. platensis or S. cinnamonensis to the corresponding isomeric phenol-carboxylic acids, (132) and (133), as indicated in Scheme 33. Among other Streptomyces and fungal species, both S. platensis and S. cinnamonensis produced one of the respective oxidized cleaved products in moderate to high yields, which allowed easy purification and further characterization. Optimal production was achieved at pH 6 and 35 °C. Investigation of the mechanism of the bioconversions via oxygen isotope incorporation revealed a dioxygenase pathway related to the hydroquinone-epoxidase system, oxygenating enzymes involved in the polyketide antibiotics' synthesis in Streptomyces.87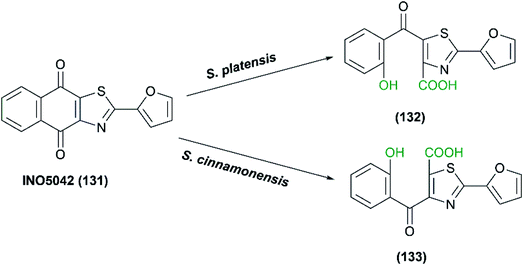 | ||
| Scheme 33 Oxidative cleavage of thiazole fused 1,4-naphthoquinone (INO5042) with the whole cells (resting cells or growing cells) S. platensis or S. cinnamonensis. This scheme has been adapted, with minor modifications, by permission from Springer, Applied Microbiology and Biotechnology; Fosse, C., Le Texier, L., Roy, S. et al. Parameters and mechanistic studies on the oxidative ring cleavage of synthetic heterocyclic naphthoquinones by Streptomyces strains,87 Copyright 2004. | ||
(7) N-Oxidations catalyzed by the whole cells of Streptomyces
Streptomyces species can convert the amino group to the corresponding nitro groups contributing to the production of nitroaromatic compounds such as nitroaromatic antibiotics like everninomycin.88,131 A recently published paper has detected an N-oxygenase activity displayed by the whole cells of S. griseus.88 Based on bioinformatics and experimental analyses, the authors have been able to identify a diiron monooxygenase gene, AurF, from several Streptomyces strains examined. The AurF enzyme was previously characterized from another Streptomyces species, S. thioletus. The enzyme is responsible for the oxidation of p-aminobenzoic acid (pABA) to p-nitrobenzoic acid (pNBA), a building block of several drugs such as aureothin.132 The substrate (pABA) was used to induce the N-oxygenase activity, but all trials lead to growth inhibition and/or low conversion. Alternatively, stressful conditions were applied to induce the responsible enzyme (N-oxygenase) through the activation of secondary metabolism, which helped optimize the biotransformation process. Conducting the process during the stationary phase of growth (starvation conditions) raised the conversion percentage to the product (pNBA) about 8 times. It was thought that the decrease in nutrients that occurred in the stationary phases can make various secondary metabolites increase their concentrations. Another stressful condition strategy was adopted using co-cultures with other bacterial strains such as Agrobacterium tumefaciens CECT 4017, Arthrobacter oxydans CECT 387, and Bacillus cereus. Those organisms occupy the natural habitat of S. griseus and therefore act as natural competitors for nutrients, which in turn affect the production of secondary metabolites.88,133 Co-cultivation of S. griseus with Bacillus cereus, as a stressful organism, at an optimized biomass mixture ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 improved the conversion to 45.5% within 4 hours (Scheme 34).88
30 improved the conversion to 45.5% within 4 hours (Scheme 34).88
 | ||
Scheme 34 N-Oxidation of p-aminobenzoic acid to p-nitrobenzoic acid with the growing cells of S. griseus CECT 3116 under an optimized stimulation strategy of co-culturing with B. cereus in 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratios. This scheme has been adapted, with minor modifications, from Nóbile et al.;88 with permission from Elsevier, copyright 2021. 30 ratios. This scheme has been adapted, with minor modifications, from Nóbile et al.;88 with permission from Elsevier, copyright 2021. | ||
Conclusion
This review has highlighted several examples of Streptomyces species catalyzing diverse oxidative reactions, including both single step and multistep reactions and leading to the formation of bioactive products that would not have been easy to produce by chemical syntheses. The reports in the literature have shown that optimization of the culture conditions and adding a suitable inducer to the medium can have highly positive effect on the catalytic activity of the whole cells for the desired reaction. There could be scope for further improvement by testing different process-engineering strategies, like cell immobilization, cultivation mode, type of bioreactors, etc., which could have a great impact on the oxidative activity of Streptomyces strains. Furthermore, cloning and expression of the enzymes involved in the oxidation reactions will be useful for understanding the enzyme features for activity and stability, and for designing better enzymes. Selective heteroatom oxidations have just been reported for some Streptomyces species,32,88 in addition to specific oxidation of unactivated C–H bonds,78,80 which opens up the possibilities for further studies on identifying the enzymes involved in such species. Development of expression hosts for the genes from Streptomyces species is yet another aspect that would be needed for increasing the utilization of these important biocatalysts. Streptomyces species, sources of eco-friendly catalysts for diverse oxidation reaction including selective ones, can replace chemical approaches that need harsh conditions affecting product selectivity.Abbreviations
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonate) |
| AMV | Aerial mycelium-negative variant |
| BRM | Biotransformation reaction media |
| BVMO | Baeyer–Villiger monooxygenase |
| CHMO | Cyclohexyl methyl sulfoxide |
| CMS | Cyclohexyl methyl sulfide |
| DMSO | Dimethyl sulfoxide |
| DO | Dissolved oxygen |
| FdR18/FdR28 | Ferredoxin (Fd68) and ferredoxin reductases |
| INO5042 | Thiazole fused 1,4-naphthoquinone |
| IPA | Isopropyl alcohol |
| melC2 | Tyrosinase deletion mutant |
| meso-2,4-ad (OH)2 | meso-2,4-Admantanediol |
| metK | SAM synthetase gene |
| 2-Me-2-adOH | 2-Methyl-2-admantanol |
| 2-Me-2,4-ad(OH)2 | 2-Methyl-2,4-admantanediol |
| pABA | p-Aminobenzoic acid |
| pNBA | p-Nitrobenzoic acid |
| PMSO | Phenyl methyl sulfoxide |
| P450 | Cytochrome P450 enzymes |
| SAM | S-Adenosyl-L-methionine |
| YM | Yeast extract-malt medium devoid of soybean peptone |
| YMS | Yeast extract-malt medium containing soybean peptone |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Dr Yasser Gaber acknowledges the Deanship of Scientific Research, Mutah University for the Fund No. [2020/360]. Sara Salama wants to thank Dr Ahmed Zakaria for useful discussions regarding the construction of some schemes.References
- R. Wohlgemuth, New Biotechnol., 2020, 60, 113–123 CrossRef PubMed.
- G. de Gonzalo and A. Franconetti, Enzyme Microb. Technol., 2018, 113, 24–28 CrossRef CAS PubMed.
- J. Shanu-Wilson, L. Evans, S. Wrigley, J. Steele, J. Atherton and J. Boer, ACS Med. Chem. Lett., 2020, 11, 2087–2107 CrossRef CAS PubMed.
- R. Wohlgemuth, in Comprehensive Organic Synthesis II, ed. P. Knochel, Elsevier, Switzerland, 2014, vol. 7, pp. 121–144 Search PubMed.
- J. Carballeira, M. Quezada, P. Hoyos, Y. Simeó, M. Hernaiz, A. Alcantara and J. Sinisterra, Biotechnol. Adv., 2009, 27, 686–714 CrossRef CAS PubMed.
- W. A. Duetz, J. B. Van Beilen and B. Witholt, Curr. Opin. Biotechnol., 2001, 12, 419–425 CrossRef CAS PubMed.
- T. Gul, M. Krzek, H. P. Permentier, M. W. Fraaije and R. Bischoff, Drug Metab. Dispos., 2016, 44, 1270–1276 CrossRef CAS PubMed.
- S. Caron, R. W. Dugger, S. G. Ruggeri, J. A. Ragan and D. H. B. Ripin, Chem. Rev., 2006, 106, 2943–2989 CrossRef CAS PubMed.
- M. D. Truppo, ACS Med. Chem. Lett., 2017, 8, 476–480 CrossRef CAS PubMed.
- S. Wu, R. Snajdrova, J. C. Moore, K. Baldenius and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2021, 60, 88–119 CrossRef CAS PubMed.
- J. P. Adams, M. J. Brown, A. Diaz-Rodriguez, R. C. Lloyd and G. D. Roiban, Adv. Synth. Catal., 2019, 361, 2421–2432 CAS.
- N. Ran, L. Zhao, Z. Chen and J. Tao, Green Chem., 2008, 10, 361–372 RSC.
- H. Puetz, E. Puchľová, K. Vranková and F. Hollmann, Catalysts, 2020, 10, 952 CrossRef CAS.
- J. Dong, E. Fernández-Fueyo, F. Hollmann, C. E. Paul, M. Pesic, S. Schmidt, Y. Wang, S. Younes and W. Zhang, Angew. Chem., Int. Ed., 2018, 57, 9238–9261 CrossRef CAS PubMed.
- F. Hollmann, I. W. Arends, K. Buehler, A. Schallmey and B. Bühler, Green Chem., 2011, 13, 226–265 RSC.
- R. Azerad, in Biocatalysis in the Pharmaceutical and Biotechnology Industries, ed. R. N. Patel, CRC Press, Taylor and Francis, New York, 2006, ch. 9 Search PubMed.
- A. Swizdor, T. Kolek, A. Panek and N. Milecka, Curr. Org. Chem., 2012, 16, 2551–2582 CrossRef CAS.
- V. Ortseifen, J. Kalinowski, A. Pühler and C. Rückert, J. Biotechnol., 2017, 262, 84–88 CrossRef CAS PubMed.
- D. Tischler, W. J. Van Berkel and M. W. Fraaije, Front. Microbiol., 2019, 10, 800 CrossRef PubMed.
- B. L. T. Prosser and N. J. Palleroni, Int. J. Syst. Evol. Microbiol., 1978, 28, 516–522 Search PubMed.
- J. W.-F. Law, V. Letchumanan, L. T.-H. Tan, H.-L. Ser, B.-H. Goh and L.-H. Lee, Progress In Microbes & Molecular Biology, 2020, 3(1) DOI:10.36877/pmmb.a0000064.
- Y. S. Anteneh and C. M. M. Franco, Frontiers in Microbiology, 2019, 10, 77 CrossRef PubMed.
- V. Běhal, in Advances in Applied Microbiology, Academic Press, 2000, vol. 47, pp. 113–156 Search PubMed.
- M. Abou-Dobara, A. El-Sayed, A. El-Fallal and M. Sauf, Journal of Egyptian Academic Society for Environmental Development. D, Environmental Studies, 2019, 20, 79–90 CrossRef.
- J. Spasic, M. Mandic, L. Djokic and J. Nikodinovic-Runic, Appl. Microbiol. Biotechnol., 2018, 102, 3513–3536 CrossRef CAS PubMed.
- S. B. Ferraiuolo, M. Cammarota, C. Schiraldi and O. F. Restaino, Appl. Microbiol. Biotechnol., 2021, 1–18 Search PubMed.
- D. C. Lamb, M. R. Waterman and B. Zhao, Expert Opin. Drug Metab. Toxicol., 2013, 9, 1279–1294 CrossRef CAS PubMed.
- O. F. Restaino, S. Barbuto Ferraiuolo, A. Perna, M. Cammarota, M. G. Borzacchiello, A. Fiorentino and C. Schiraldi, Molecules, 2020, 25, 4912 CrossRef CAS PubMed.
- O. F. Restaino, M. Marseglia, P. Diana, M. G. Borzacchiello, R. Finamore, M. Vitiello, A. D'Agostino, M. De Rosa and C. Schiraldi, Process Biochem., 2016, 51, 1–8 CrossRef CAS.
- F. S. Sariaslani, M. K. Trower and D. A. Kunz, Biocatalysis, 1989, 2, 151–160 CrossRef CAS.
- C. Mazier, M. Lombard, M.-A. Sari and D. Buisson, Biocatal. Biotransform., 2007, 25, 401–407 CrossRef CAS.
- S. Salama, T. Dishisha, M. H. Habib, A. Z. Abdelazem, W. Bakeer, M. Abdel-Latif and Y. Gaber, RSC Adv., 2020, 10, 32335–32344 RSC.
- F. S. Sarlaslani and D. A. Kunz, Biochem. Biophys. Res. Commun., 1986, 141, 405–410 CrossRef.
- M. K. Trower, F. S. Sariaslani and D. P. O'Keefe, J. Bacteriol., 1989, 171, 1781–1787 CrossRef CAS PubMed.
- F. Sariaslani and D. A. Kunz, Biochem. Biophys. Res. Commun., 1986, 141, 405–410 CrossRef CAS PubMed.
- J. Sasaki, A. Mikami, K. Mizoue and S. Omura, Appl. Environ. Microbiol., 1991, 57, 2841–2846 CrossRef CAS PubMed.
- K. Mitsukura, H. Sakamoto, H. Kubo, T. Yoshida and T. Nagasawa, J. Biosci. Bioeng., 2010, 109, 550–553 CrossRef CAS PubMed.
- A. Pometto and D. Crawford, Appl. Environ. Microbiol., 1983, 45, 1582–1585 CrossRef CAS PubMed.
- M. E. Arias, M. Arenas, J. Rodríguez, J. Soliveri, A. S. Ball and M. Hernández, Appl. Environ. Microbiol., 2003, 69, 1953–1958 CrossRef CAS PubMed.
- K. Y. Choi, E. Jung, Y. H. Yang and B. G. Kim, Biotechnol. Bioeng., 2013, 110, 2591–2599 CrossRef CAS PubMed.
- M. L. Mascotti, M. A. Palazzolo, E. Lewkowicz and M. Kurina-Sanz, Biocatal. Agric. Biotechnol., 2013, 2, 399–402 CrossRef.
- J. Gorke, F. Srienc and R. Kazlauskas, Biotechnol. Bioprocess Eng., 2010, 15, 40–53 CrossRef CAS PubMed.
- K. Mitsukura, Y. Kondo, T. Yoshida and T. Nagasawa, Appl. Microbiol. Biotechnol., 2006, 71, 502–504 CrossRef CAS PubMed.
- C. Roh, S.-H. Seo, K.-Y. Choi, M. Cha, B. P. Pandey, J.-H. Kim, J.-S. Park, D. H. Kim, I. S. Chang and B.-G. Kim, J. Biosci. Bioeng., 2009, 108, 41–46 CrossRef CAS PubMed.
- W. Kim, J.-k. Lee, K.-Y. Choi, B.-G. Kim and J. Kim, Biotechnol. Bioprocess Eng., 2020, 25, 272–278 CrossRef CAS.
- A. Dietz and J. Mathews, Int. J. Syst. Evol. Microbiol., 1972, 22, 173–177 Search PubMed.
- A. Dietz and J. Mathews, Int. J. Syst. Evol. Microbiol., 1977, 27, 282–287 Search PubMed.
- K. F. Chater, F1000Research, 2016, 5, 2795 Search PubMed.
- N. Ando, K. Ueda and S. Horinouchi, Microbiology, 1997, 143, 2715–2723 CrossRef CAS PubMed.
- G. P. Van Wezel, P. Krabben, B. A. Traag, B. J. Keijser, R. Kerste, E. Vijgenboom, J. J. Heijnen and B. Kraal, Appl. Environ. Microbiol., 2006, 72, 5283–5288 CrossRef CAS PubMed.
- H. Yamazaki, Y. Ohnishi and S. Horinouchi, J. Bacteriol., 2003, 185, 1273–1283 CrossRef CAS PubMed.
- L. Sevillano, E. Vijgenboom, G. P. van Wezel, M. Díaz and R. I. Santamaría, Microb. Cell Fact., 2016, 15, 28 CrossRef PubMed.
- K. Flärdh and M. J. Buttner, Nat. Rev. Microbiol., 2009, 7, 36–49 CrossRef PubMed.
- D. S. Capstick, J. M. Willey, M. J. Buttner and M. A. Elliot, Mol. Microbiol., 2007, 64, 602–613 CrossRef CAS PubMed.
- B. Zacchetti, J. Willemse, B. Recter, D. van Dissel, G. P. van Wezel, H. A. Wösten and D. Claessen, Sci. Rep., 2016, 6, 27045 CrossRef CAS PubMed.
- C. Vilches, C. Méndez, C. Hardisson and J. A. Salas, Microbiology, 1990, 136, 1447–1454 CrossRef CAS PubMed.
- A. Manteca, B. Rioseras, N. González-Quiñónez, G. Fernández-García and P. Yagüe, in Growing and Handling of Bacterial Cultures, ed. M. Mishra, IntechOpen, England, London, 2019, pp. 129–140 Search PubMed.
- B. Rioseras, M. T. López-García, P. Yagüe, J. Sánchez and Á. Manteca, Bioresour. Technol., 2014, 151, 191–198 CrossRef CAS PubMed.
- T. Kylosova, A. Elkin, V. Grishko and I. Ivshina, J. Mol. Catal. B: Enzym., 2016, 123, 8–13 CrossRef CAS.
- G. Chandra and K. F. Chater, FEMS Microbiol. Rev., 2014, 38, 345–379 CrossRef CAS PubMed.
- A. Martinez, S. J. Kolvek, C. L. T. Yip, J. Hopke, K. A. Brown, I. A. MacNeil and M. S. Osburne, Appl. Environ. Microbiol., 2004, 70, 2452–2463 CrossRef CAS PubMed.
- M. Díaz, E. Ferreras, R. Moreno, A. Yepes, J. Berenguer and R. Santamaría, Appl. Microbiol. Biotechnol., 2008, 79, 1001–1008 CrossRef PubMed.
- J. Anné, K. Vrancken, L. Van Mellaert, J. Van Impe and K. Bernaerts, Biochim. Biophys. Acta, Mol. Cell Res., 2014, 1843, 1750–1761 CrossRef PubMed.
- N. Nakashima, Y. Mitani and T. Tamura, Microb. Cell Fact., 2005, 4, 1–5 CrossRef PubMed.
- X. Jiang, W. Liu, Y. Ji, J. Niu and M. Li, Appl. Microbiol. Biotechnol., 2012, 93, 1957–1963 CrossRef CAS PubMed.
- B. P. Pandey, N. Lee, K.-Y. Choi, E. Jung, D.-h. Jeong and B.-G. Kim, Enzyme Microb. Technol., 2011, 48, 386–392 CrossRef CAS PubMed.
- M. Ueno, M. Yamashita, M. Hashimoto, M. Hino and A. Fujie, J. Biosci. Bioeng., 2005, 100, 567–572 CrossRef CAS PubMed.
- C. Olano, S. J. Moss, A. F. Braña, R. M. Sheridan, V. Math, A. J. Weston, C. Méndez, P. F. Leadlay, B. Wilkinson and J. A. Salas, Mol. Microbiol., 2004, 52, 1745–1756 CrossRef CAS PubMed.
- S. P. Gurram, P. Rama, G. Sivadevuni and M. R. Solipuram, Iran. J. Biotechnol., 2009, 7, 142–147 CAS.
- S. R. Gopishetty, J. Heinemann, M. Deshpande and J. P. Rosazza, Enzyme Microb. Technol., 2007, 40, 1622–1626 CrossRef CAS.
- B. P. Pandey, N. Lee and B.-G. Kim, Appl. Biochem. Biotechnol., 2018, 184, 1036–1046 CrossRef CAS PubMed.
- N. Lee, E. J. Kim and B.-G. Kim, ACS Chem. Biol., 2012, 7, 1687–1692 CrossRef CAS PubMed.
- H. Mori, T. Shibasaki, Y. Uozaki, K. Ochiai and A. Ozaki, Appl. Environ. Microbiol., 1996, 62, 1903–1907 CrossRef CAS PubMed.
- J. R. Berrie, R. A. Williams and K. E. Smith, J. Steroid Biochem. Mol. Biol., 1999, 71, 153–165 CrossRef CAS PubMed.
- O. F. Restaino, M. Marseglia, C. De Castro, P. Diana, P. Forni, M. Parrilli, M. De Rosa and C. Schiraldi, Appl. Microbiol. Biotechnol., 2014, 98, 1291–1299 CrossRef CAS PubMed.
- B. Dangi, K. H. Kim, S. H. Kang and T. J. Oh, ChemBioChem, 2018, 19, 1066–1077 CrossRef CAS PubMed.
- C. D. Murphy, S. J. Moss and D. O'Hagan, Appl. Environ. Microbiol., 2001, 67, 4919–4921 CrossRef CAS PubMed.
- S.-H. Xu, C. Zhang, W.-W. Wang, B.-Y. Yu and J. Zhang, RSC Adv., 2017, 7, 20754–20759 RSC.
- Y.-Y. Zhu, L.-W. Qian, J. Zhang, J.-H. Liu and B.-Y. Yu, Tetrahedron, 2011, 67, 4206–4211 CrossRef CAS.
- S.-H. Xu, W.-W. Wang, C. Zhang, X.-F. Liu, B.-Y. Yu and J. Zhang, Tetrahedron, 2017, 73, 3086–3092 CrossRef CAS.
- F.-Q. Wang, B. Li, W. Wang, C.-G. Zhang and D.-Z. Wei, Appl. Microbiol. Biotechnol., 2007, 77, 771–777 CrossRef CAS PubMed.
- W. Wang, F.-Q. Wang and D.-Z. Wei, Appl. Environ. Microbiol., 2009, 75, 4202–4205 CrossRef CAS PubMed.
- A.-R. Ibrahim and Y. J. Abul-Hajj, Appl. Environ. Microbiol., 1989, 55, 3140–3142 CrossRef CAS PubMed.
- F. S. Sariaslani and J. P. Rosazza, Appl. Environ. Microbiol., 1983, 46, 468–474 CrossRef CAS PubMed.
- F. S. Sariaslani, L. R. McGEE and D. W. Ovenall, Appl. Environ. Microbiol., 1987, 53, 1780–1784 CrossRef CAS PubMed.
- R. L. Hanson, J. A. Matson, D. B. Brzozowski, T. L. LaPorte, D. M. Springer and R. N. Patel, Org. Process Res. Dev., 2002, 6, 482–487 CrossRef CAS.
- C. Fosse, L. Le Texier, S. Roy, M. Delaforge, S. Grégoire, M. Neuwels and R. Azerad, Appl. Microbiol. Biotechnol., 2004, 65, 446–456 CrossRef CAS PubMed.
- M. L. Nóbile, A. M. Stricker, A. M. Iribarren and E. S. J. J. o. B. Lewkowicz, J. Biotechnol., 2021, 327, 36–42 CrossRef PubMed.
- M. Li, Y. Zhang, L. Zhang, X. Yang and X. Jiang, PLoS One, 2014, 9, e98916 CrossRef PubMed.
- S. Ding, D. Yao, Y. Y. Deeni, B. Burchell, C. R. Wolf and T. Friedberg, Biochem. J., 2001, 356, 613–619 CrossRef CAS.
- Y. J. Chun, T. Shimada, M. R. Waterman and F. P. Guengerich, Biochem. Soc. Trans., 2006, 34, 1183–1185 CrossRef CAS PubMed.
- M. Brawner, G. Poste, M. Rosenberg and J. Westpheling, Curr. Opin. Biotechnol., 1991, 2, 674–681 CrossRef CAS PubMed.
- C. Olano, B. Wilkinson, S. J. Moss, A. F. Braña, C. Méndez, P. F. Leadlay and J. A. Salas, Chem. Commun., 2003, 2780–2782 RSC.
- J. D. Rudolf, C.-Y. Chang, M. Ma and B. Shen, Nat. Prod. Rep., 2017, 34, 1141–1172 RSC.
- A. Worsch, F. K. Eggimann, M. Girhard, C. J. von Bühler, F. Tieves, R. Czaja, A. Vogel, C. Grumaz, K. Sohn and S. Lütz, Biotechnol. Bioeng., 2018, 115, 2156–2166 CrossRef CAS PubMed.
- C. D. Murphy, Biotechnol. Lett., 2015, 37, 19–28 CrossRef CAS PubMed.
- M.-A. Cho, S. Han, Y.-R. Lim, V. Kim, H. Kim and D. Kim, Biomol. Ther., 2019, 27, 127 CrossRef CAS PubMed.
- C. W. Lee, J.-H. Lee, H. Rimal, H. Park, J. H. Lee and T.-J. Oh, Int. J. Mol. Sci., 2016, 17, 813 CrossRef PubMed.
- R. Bernhardt, Rev. Physiol., Biochem. Pharmacol., 1995, 127, 137–221 Search PubMed.
- S. C. Moody and E. J. Loveridge, J. Appl. Microbiol., 2014, 117, 1549–1563 CrossRef CAS PubMed.
- D. A. Griffiths, D. J. Best and S. G. Jezequel, Appl. Microbiol. Biotechnol., 1991, 35, 373–381 CrossRef CAS PubMed.
- Q. Wang, B. Ma, S. Fushinobu, C. Zhang and L.-H. Xu, Biochem. Biophys. Res. Commun., 2020, 522, 355–361 CrossRef CAS PubMed.
- T. Makino, Y. Katsuyama, T. Otomatsu, N. Misawa and Y. Ohnishi, Appl. Environ. Microbiol., 2014, 80, 1371–1379 CrossRef PubMed.
- A. Präg, B. r. A. Grüning, M. Häckh, S. Lüdeke, M. Wilde, A. Luzhetskyy, M. Richter, M. Luzhetska, S. Günther and M. Müller, J. Am. Chem. Soc., 2014, 136, 6195–6198 CrossRef PubMed.
- Y.-J. Chun, T. Shimada, R. Sanchez-Ponce, M. V. Martin, L. Lei, B. Zhao, S. L. Kelly, M. R. Waterman, D. C. Lamb and F. P. Guengerich, J. Biol. Chem., 2007, 282, 17486–17500 CrossRef CAS PubMed.
- S. Li, L. Du and R. Bernhardt, Trends Microbiol., 2020, 28, 445–454 CrossRef CAS PubMed.
- V. Jungmann, I. Molnár, P. E. Hammer, D. S. Hill, R. Zirkle, T. G. Buckel, D. Buckel, J. M. Ligon and J. P. Pachlatko, Appl. Environ. Microbiol., 2005, 71, 6968–6976 CrossRef CAS PubMed.
- B. P. Pandey, C. Roh, K. Y. Choi, N. Lee, E. J. Kim, S. Ko, T. Kim, H. Yun and B. G. Kim, Biotechnol. Bioeng., 2010, 105, 697–704 CAS.
- J. R. Berrie, R. A. Williams and K. E. Smith, J. Steroid Biochem. Mol. Biol., 2001, 77, 87–96 CrossRef CAS PubMed.
- L. A. Al-Momani, ARKIVOC, 2012, 2012, 101–111 Search PubMed.
- C. E. Rüfer and S. E. Kulling, J. Agric. Food Chem., 2006, 54, 2926–2931 CrossRef PubMed.
- B. P. Pandey, N. Lee, K.-Y. Choi, J.-N. Kim, E.-J. Kim and B.-G. Kim, Appl. Microbiol. Biotechnol., 2014, 98, 5009–5017 CrossRef CAS PubMed.
- R. Bentley, Chem. Soc. Rev., 2005, 34, 609–624 RSC.
- W. Mączka, K. Wińska and M. Grabarczyk, Catalysts, 2018, 8, 624 CrossRef.
- H. Cotton, T. Elebring, M. Larsson, L. Li, H. Sörensen and S. von Unge, Tetrahedron: Asymmetry, 2000, 11, 3819–3825 CrossRef CAS.
- V. De Sio, M. R. Acocella, R. Villano and A. Scettri, Tetrahedron: Asymmetry, 2010, 21, 1432–1435 CrossRef CAS.
- M. Rachwalski, S. Leśniak and P. Kiełbasiński, Tetrahedron: Asymmetry, 2010, 21, 1890–1892 CrossRef CAS.
- M. Kwiatkowska, I. Janicki and P. Kiełbasiński, J. Mol. Catal. B: Enzym., 2015, 118, 23–28 CrossRef CAS.
- H. L. Holland and I. M. Carter, Can. J. Chem., 1982, 60, 2420–2425 CrossRef CAS.
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- G. de Gonzalo, G. Ottolina, F. Zambianchi, M. W. Fraaije and G. Carrea, J. Mol. Catal. B: Enzym., 2006, 39, 91–97 CrossRef CAS.
- G. Catucci, C. Gao, S. J. Sadeghi and G. Gilardi, Rendiconti Lincei, 2017, 28, 195–206 CrossRef.
- T. Matsui, Y. Dekishima and M. Ueda, Appl. Microbiol. Biotechnol., 2014, 98, 7699–7706 CrossRef CAS PubMed.
- J. A. Linares-Pastén, G. Chávez-Lizárraga, R. Villagomez, G. Mamo and R. Hatti-Kaul, Enzyme Microb. Technol., 2012, 50, 101–106 CrossRef PubMed.
- F. Zocher, M. M. Enzelberger, U. T. Bornscheuer, B. Hauer, W. Wohlleben and R. D. Schmid, J. Biotechnol., 2000, 77, 287–292 CrossRef CAS PubMed.
- A. Watts, J. Beecher, C. Whitcher and J. Littlechild, Biocatal. Biotransform., 2002, 20, 209–215 CrossRef CAS.
- N. A. Donoghue and P. W. Trudgill, Eur. J. Biochem., 1975, 60, 1–7 CrossRef CAS PubMed.
- S. Wu and Z. J. C. Li, ChemCatChem, 2018, 10, 2164–2178 CrossRef CAS.
- M. Lombard, I. Salard, M.-A. Sari, D. Mansuy and D. Buisson, Arch. Biochem. Biophys., 2011, 508, 54–63 CrossRef CAS PubMed.
- M. Taylor, D. C. Lamb, R. Cannell, M. Dawson and S. L. Kelly, Biochem. Biophys. Res. Commun., 1999, 263, 838–842 CrossRef CAS PubMed.
- E. K. McCranie and B. O. J. N. p. r. Bachmann, Nat. Prod. Rep., 2014, 31, 1026–1042 RSC.
- E. Chanco, Y. S. Choi, N. Sun, M. Vu and H. Zhao, Bioorg. Med. Chem., 2014, 22, 5569–5577 CrossRef CAS PubMed.
- M. Köberl, E. M. Ramadan, M. Adam, M. Cardinale, J. Hallmann, H. Heuer, K. Smalla and G. Berg, FEMS Microbiol. Lett., 2013, 342, 168–178 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |