Open Access Article
Open Access ArticleBimetal phosphide as high efficiency and stable bifunctional electrocatalysts for hydrogen and oxygen evolution reaction in alkaline solution†
Yutong Liu‡
a,
Meng Ding‡ *a,
Xiaolong Dengb,
Yafang Zhanga and
Gang Zhao
*a,
Xiaolong Dengb,
Yafang Zhanga and
Gang Zhao a
a
aSchool of Physics and Technology, University of Jinan, 336 Nanxinzhuang West Road, Jinan, 250022, People's Republic of China. E-mail: sps_dingm@ujn.edu.cn
bSchool of Mathematics and Physics, Anhui University of Technology, Ma'anshan 243032, Anhui, People's Republic of China
First published on 22nd March 2022
Abstract
The development of low-cost, high-efficiency, and stable bifunctional electrocatalysts for large-scale water electrolysis is very important for the sustainable development of energy. In this paper, the nickel cobalt phosphide (CoNiP) microstructure was prepared by the “in situ growth-ion exchange-phosphating” method. Due to the flake structure and the synergistic effect of the bimetal, the synthesized CoNiP microstructure exhibited high electrocatalytic activity and stability for hydrogen and oxygen evolution in alkaline electrolyte. The optimized CoNiP showed low overpotential of 116 mV at 10 mA cm−2 for hydrogen evolution reaction and 400 mV at 50 mA cm−2 for oxygen evolution reaction in KOH solution. In addition, it exhibited long-term stability at a high constant current density of 100 mA cm−2 for 48 hours at room temperature and for 65 hours at 80 °C without significant degradation. Theoretical results showed that the introduction of Co and P atoms could reduce the reaction barrier and improve the electron transfer ability. This work provides a simple and economical way for the synthesis of electrocatalytic bimetal phosphide catalysts.
1. Introduction
With the development of global industrialization, energy becomes an indispensable resource in human life. As traditional energy is greatly consumed, which mainly comes from non-renewable resources, human beings are already facing the problem of energy depletion.1 Hydrogen has the apparent advantages of being a clean, broad resource, having high energy density, and being sustainable,2 and also it is an ideal energy carrier. The production of hydrogen as an important research direction of hydrogen energy application has been widely investigated by researchers all over the world. Electrochemical decomposition of water is considered as one of the cleanest ways to produce hydrogen.3 It is an important research topic in the field of decomposing water to produce hydrogen and oxygen with low electric energy by creating a highly efficient electrocatalyst. Overall, water decomposition is divided into anodic oxygen evolution reaction and cathodic hydrogen evolution reaction.4 At present, Ir/Ru and Pt are considered to be much better catalysts for the OER and HER.5,6 However, the scarcity and high cost of noble metals limit their practical application as efficient electrocatalysts. In order to overcome these problems, non-precious metal electrocatalysts with excellent electrocatalytic performance and low cost are chosen to replace these precious metal catalysts. The bifunctional catalysts are active for both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) reactions, which are more conducive to practical application.7 Therefore, it is of great practical significance to develop economical, efficient and bifunctional electrocatalysts.Transition metals and their oxide, sulfide,8 selenide,9 phosphide,10 nitride11 are considered as promising bifunctional electrocatalytic materials because of their abundant reserves and low cost. Especially, transition metal phosphides have been extensively studied due to their high catalytic activity and electrical conductivity.12–16 For example, Yang et al. synthesized nanosheet self-assembled NiCoP microflowers as efficient bifunctional catalysts in alkaline medium.17 Yin et al. prepared NiCoP on nickel foam with intertwined and porous columnar morphology, which showed superior HER and OER performance.18 Zeng et al. fabricated 3D flower-like clusters of CoNiP nanofoils grown on randomly-dispersed rGO-nanosheets with superior electrocatalysis for hydrogen evolution reactions.19 Furthermore, some research groups have studied the electrocatalytic activity of compounds based on CoNiP. Shao et al. synthesized CoNiP@layered double hydroxides core–shell nanosheets arrays as bifunctional electrocatalyst.20 Although some achievements have been achieved with nanostructured metallic phosphides, their electrocatalytic activity can be improved by adjusting geometric structures.21 Additionally, researchers prefer to prepare self-supporting electrodes for direct application in water electrolysis cells, which can simplify the equipment of water electrolysis and potentially reduce the cost.22,23
In this paper, the self-supporting bimetal phosphide CoNiP catalyst is prepared through three steps (etching, ion exchange and phosphating) on foamed nickel. The layered structure of the microstructure affords abundant active sites and provides enough space for the diffusion of the electrolyte to accelerate the release of bubbles. In addition, CoNiP shows much better electrocatalytic performance after ion exchange in cobalt nitrate solution. Furthermore, the CoNiP catalyst shows superior durability with little change after constant current measurement for 48 hours at room temperature and 65 hours at 80 °C.
2. Results and discussion
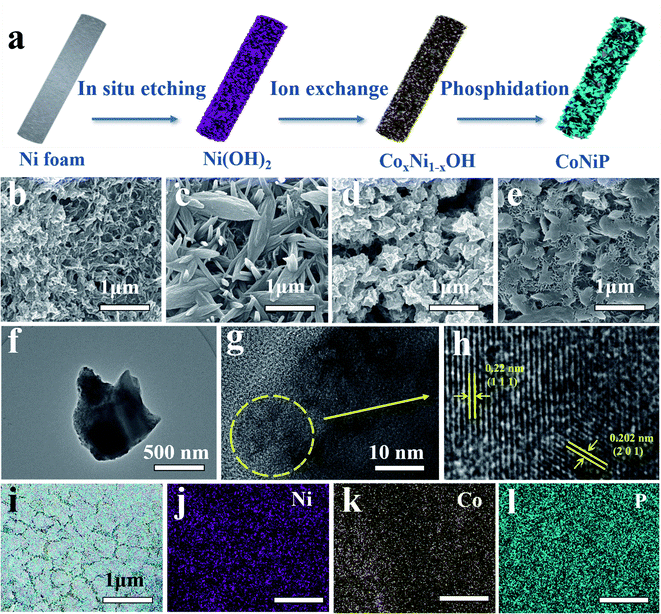
The CoNiP/NF electrocatalyst is synthesized by three steps of “in situ growth-ion exchange-phosphating”, as shown in Fig. 1a. The SEM images of Ni(OH)2, NixCo1−xOH, Ni2P and CoNiP are displayed in Fig. 1b–e. It can be seen that Ni(OH)2 with strip structure is formed after etching by hydrochloric acid and immersing in deionized water. Note that after ion exchange, the morphology of product changes to spike-like structure. Finally, a lamellar layered structure appears after phosphating, and the thickness of CoNiP nanosheets is about 21 nm, which is displayed in Fig. S1.† The TEM images of CoNiP with high magnification in Fig. 1f–h display the fringe spacings of 0.22 nm and 0.202 nm, corresponding well to the (111) and (201) crystal plane of CoNiP,24,25 which further confirms successful synthesis of CoNiP. The EDS element mapping images (Fig. 1i–l) verify the existent of Ni, Co, and P elements, and distributed uniformly in the lamellar layered structure.The X-ray diffraction (XRD) is used to study the crystal structure of as-synthesized samples. As shown in Fig. S2,† the diffraction peaks of CoNiP/NF-0.15 M centered at 40.99°, 44.90° and 47.58° can be assigned to the (111), (201) and (210) plane of hexagonal CoNiP (marked with triangle, PDF: 71-2336). The result is consistent with the TEM image in Fig. 1h, which could further confirm the existence of CoNiP. For comparison, the crystalline Ni2P sample is prepared. The diffraction peak of Ni2P (Fig. 2b) located at 40.71°, 44.61° and 54.20° could be well indexed to (111), (201), (002) crystal planes of hexagonal Ni2P (marked with asterisk, PDF: 03-0953).
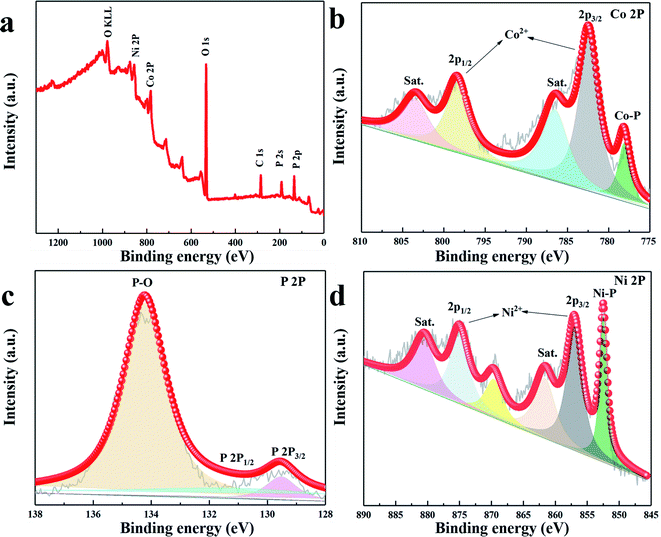 | ||
| Fig. 2 (a) XPS survey spectrum of CoNiP-0.15 M. High-resolution (b–d) XPS spectra of the Co 2p, P 2p and Ni 2p for the CoNiP-0.15 M microstructure. | ||
In order to study the surface chemical composition of CoNiP microstructure, X-ray photoelectron spectroscopy depth profile analysis was carried out. As shown in Fig. 2a, the characteristic peaks of nickel, cobalt and phosphorus elements clearly appear in the XPS survey spectrum of CoNiP. The high-resolution XPS spectrum of Co element is depicted in Fig. 2b, and two obvious peaks located at 782.5 eV and 798.5 eV in the Co 2p spectrum can be assigned to Co 2p2/3 and Co 2p1/2, which are accompanied by two satellite peaks at 786.7 eV and 803.5 eV. Moreover, the peak located at 778.2 eV can be attributed to the Co–P bond.26 As presented in Fig. 2c, the binding energy of about 130.1 and 129.4 eV can be ascribed to P–Ni or P–Co bond in P 2p1/2 and P 2p3/2, respectively. In addition, the strong oxidation peak located at 134.2 eV is assigned to the P–O bond because of the air exposure.24,26 In Fig. 2d, the two obvious peaks at 857.1 eV and 875.1 eV are attributed to Ni 2p2/3 and Ni 2p1/2, meanwhile, the satellite peaks of Ni 2p2/3 and Ni 2p1/2 at 861.7 eV and 880.6 eV are observed. Furthermore, the peak with binding energy at about 853.7 eV is ascribed to the Ni–P bond.26,27 The XPS result corresponds well to the XRD of the sample, which further illustrates the successful synthesis of CoNiP.
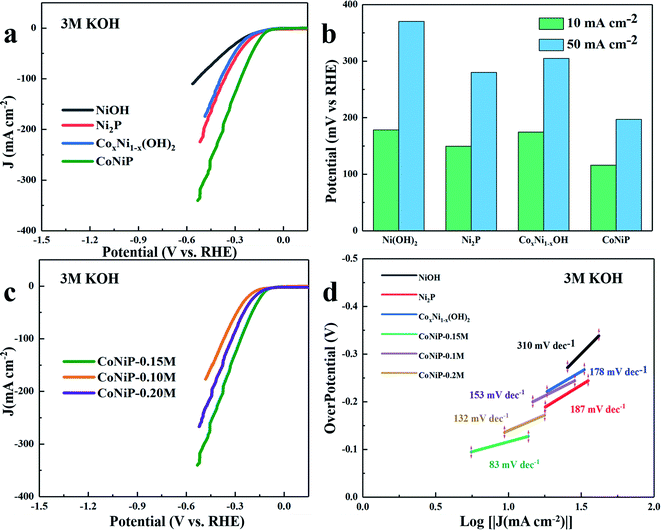
The HER and OER electrocatalytic activity of all samples are evaluated in 3.0 M KOH at room temperature. For HER electrocatalytic activity, as shown in Fig. 3a, the LSV curves exhibit that the CoNiP/NF-0.15 M electrocatalyst possesses excellent catalytic activity compared with other samples. As Fig. 3b shown, the overpotential of the prepared CoNiP/NF-0.15 M is 116 mV at 10 mA cm−2, which is lower than that of Ni2P (149.5 mV), CoxNi1−x(OH) (174.5 mV), Ni(OH)2 (178.5 mV), indicating that the introduction of Co ions and P ions has significantly improved catalytic performance of samples.28,29 For bimetal phosphide, the content of metal ions has influence on the performance of electrocatalyst. Therefore, in order to investigate the effect of Co amount on the HER performance, the LSV curves of CoNiP with three different concentrations of cobalt nitrate solution are studied (shown in Fig. 3c). Among these catalysts, the CoNiP-0.15 catalyst exhibits the best HER activity, and the overpotential is 61 and 24 mV lower than that of CoNiP-0.1 M, CoNiP-0.2 M, respectively. In order to further evaluate catalytic activity of as-fabricated samples, the catalytic kinetics are analysed using the corresponding Tafel plots computed from LSV data in Fig. 3a. The Tafel slope is fitted by the following Tafel equation:
η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log|j| + a log|j| + a |
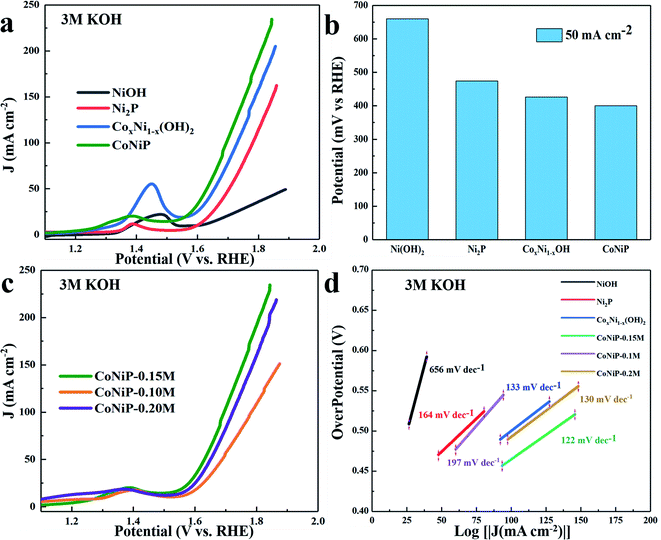
The OER catalytic activity of CoNiP is also investigated in alkaline solution (3 M KOH), and the LSV curve is compared with Ni2P, CoxNi1−x(OH), Ni(OH)2. As shown in Fig. 4a, an obvious oxidation peak at about 1.4 V in the LSV curves of CoNiP/NF-0.15 M, Ni2P/NF, CoxNi1−xOH/NF and Ni(OH)2/NF samples can be observed, which is attributed to the oxidation–reduction reaction of Co2+/Co3+ or Ni2+/Ni3+ in these catalysts.26,27,31 Similarly, CoNiP/NF-0.15 M electrocatalyst possesses superior catalytic activity for OER. Furthermore, the CoNiP/NF-0.15 M requires overpotential of about 400 mV to achieve the current density of 50 mA cm−2, which is smaller than 474 mV of Ni2P and 426 mV of CoxNi1−xOH, indicating that CoNiP/NF-0.15 M has relatively excellent OER catalytic activity (Fig. 4b). The LSV curves of CoNiP synthesized with different concentrations of cobalt nitrate are observed in Fig. 4c. Obviously, the synthesized CoNiP-0.15 M has a lower overpotential at the same current density, which is 57 and 20 mV lower than that of CoNiP-0.1 M, CoNiP-0.2 M, implying that it possesses much better OER performance. As shown in Fig. 4d, the Tafel slopes of CoNiP/NF-0.15 M, Ni2P/NF, CoxNi1−x(OH)/NF and Ni(OH)2/NF are 122, 164, 133 mV dec−1, respectively. The improved electrocatalytic activity of CoNiP is probably due to the synergistic effects between Ni and Co, which can strengthen the polarization of Ni and Co electrons and make them easier to adsorb OH− ions, and then accelerate reaction kinetics that is in favour of OER performance.32
The electrochemical impedance spectroscopy analyses of as-grown samples are shown in Fig. 5a, the CoNiP/NF-0.15 M exhibits smaller Nyquist semicircle diameter compared with other electrocatalysts, indicating much smaller charge transfer resistance and faster electron transport rate. In order to evaluate the durability of the CoNiP/NF-0.15 M electrocatalyst in 3 M KOH, sequential chronopotentiometric measurement at 100 mA cm−2 is performed at room temperature (as shown in Fig. 5b), and there is only negligible voltage changes after the 48 hour continuous test. Especially, the durability of the CoNiP/NF-0.15 M at 80 °C is evaluated, and the overpotential increases by about 18 mV after 65 hours of continuous testing, which indicates that the material has excellent stability.
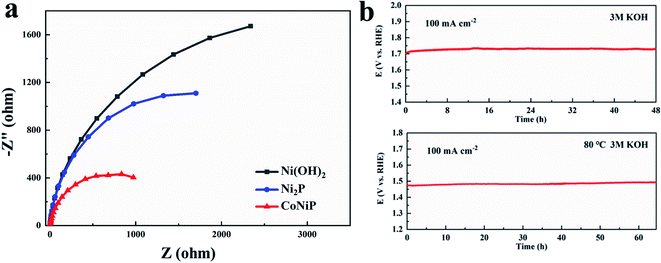 | ||
| Fig. 5 (a) Nyquist plots of Ni(OH)2/NF, Ni2P/NF, and CoNiP/NF samples. (b) Chronopotentiometric curve of CoNiP/NF at 100 mA cm−2 in 3 M KOH electrolyte at room temperature and 80 °C, respectively. | ||
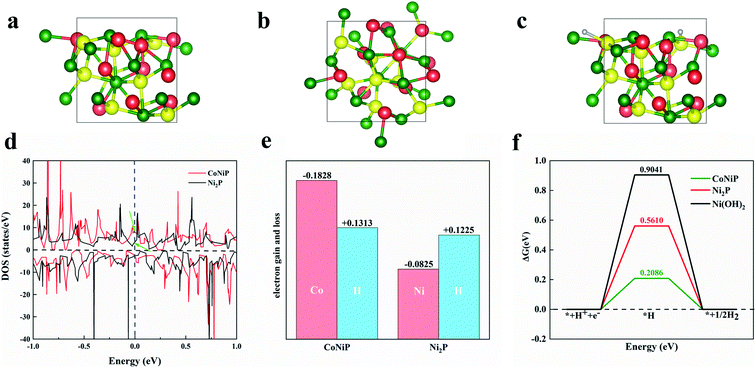
Density functional theory calculations are carried out to investigate the mechanism of enhanced HER performance of CoNiP. Fig. 6a and b shows the side and top view of the geometric structure of CoNiP, respectively. In our calculation, the optimization of structure shows that the best adsorption position of hydrogen atom in CoNiP is the top site of cobalt atom. We calculate the density of states (DOS) of the non-adsorbed surface to analyze their electronic structures. Compared with Ni2P, CoNiP shows significant increase in DOS at Fermi level (Fig. 6d), inferring that the introduction of Co element can improve the ability of electric conduction of the material. Moreover, we perform Bader's charge population analysis on both CoNiP and Ni2P. As shown in Fig. 6e, The adsorption site of Co (CoNiP) loses more electrons than that of Ni (Ni2P), and the *H on CoNiP gains more electrons than that on Ni2P, which indicates that CoNiP has stronger electron transfer ability than Ni2P, and enhances the bonding between the adsorption site and *H. The Gibbs free energy is also calculated and plotted in Fig. 6f. It can be seen that Ni2P has a higher (positive) Gibbs free energy (ΔG = 0.5610 eV) for adsorption of H, while CoNiP has a relatively lower potential barrier (ΔG = 0.2086 eV), which indicates that the bonding between Ni2P and *H is too weak for the HER, and the introduction of Co atom could enhance the bonding and therefore reduce the overpotential of HER. According to the analysis above, it is the improvement of electric conduction and enhancement of charge transfer, both of which are due to the doping of Co atoms, that promote the formation of *H on CoNiP surface.
3. Conclusion
A facile strategy to prepare CoNiP microstructure by the three-step method of “in situ growth-ion exchange-phosphating” was proposed. In the catalytic process, CoNiP-0.15 M showed the best catalytic performance. The overpotential of HER at 10 mA cm−2 is 116 mV, and that of OER at 50 mA cm−2 is 400 mV. In addition, it can be electrolyzed at a constant high current density of 100 mA cm−2 for 48 hours at room temperature and for 65 hours for 80 °C without significant degradation, showing that it has excellent stability. Theoretical results show that the introduction of Co and P atoms can obviously reduce the reaction barrier and improve the electron transfer ability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Y. T. Liu and M. Ding contributed equally to this work. The authors thanks eceshi (www.eceshi.com) for the SEM, XRD, XPS, TEM experiments.References
- G. T. Xiang, Y. Meng, G. M. Qu, J. M. Yin, B. Teng, Q. Wei and X. J. Xu, Sci. Bull., 2020, 65, 443–451 CrossRef CAS.
- J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS PubMed.
- Y. Liang, Y. Li, H. Wang and H. Dai, J. Am. Chem. Soc., 2013, 135, 2013–2036 CrossRef CAS PubMed.
- L. A. Stern, L. Feng, F. Song and X. Hu, Energy Environ. Sci, 2015, 8, 2347–2351 RSC.
- G. Chen, T. Wang, J. Zhang, P. Liu, H. Sun, X. Zhuang, M. Chen and X. Feng, Adv. Mater., 2018, 30, 1706279 CrossRef PubMed.
- Y. Wei, X. Zhang, Z. Wang, J. Yin, J. Huang, G. Zhao and X. Xu, Chinese Chem. Lett., 2021, 32, 119–124 CrossRef CAS.
- X. Chia and M. Pumera, Nat. Catal., 2018, 1, 909–921 CrossRef CAS.
- D. Kong, J. J. Cha, H. Wang, H. R. Lee and Y. Cui, Energy Environ. Sci, 2013, 6, 3553–3558 RSC.
- K. Li, J. Zhang, R. Wu, Y. Yu and B. Zhang, Adv. Sci., 2016, 3, 1500426 CrossRef PubMed.
- Q. Liu, J. Tian, W. Cui, P. Jiang, N. Cheng, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 6710–6714 CrossRef CAS PubMed.
- X. Jia, Y. Zhao, G. Chen, L. Shang, R. Shi, X. Kang, G. I. Waterhouse, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Energy Mater., 2016, 6, 1502585 CrossRef.
- Y. Li, Z. Dong and L. Jiao, Adv. Energy Mater., 2020, 10, 1902104 CrossRef CAS.
- J. Tian, Q. Liu, A. M. Asiri and X. Sun, J. Am. Chem. Soc., 2014, 136, 7587–7590 CrossRef CAS PubMed.
- L. Gan, M. Heggen, R. O'Malley, B. Theobald and P. Strasser, Nano Lett., 2013, 13, 1131–1138 CrossRef CAS PubMed.
- F. Yu, H. Zhou, Y. Huang, J. Sun, F. Qin, J. Bao, W. A. Goddard, S. Chen and Z. Ren, Nat. Commun., 2018, 9, 1–9 CrossRef PubMed.
- Y. Xin, X. Kan, L. Y. Gan and Z. Zhang, ACS Nano, 2017, 11, 10303–10312 CrossRef CAS PubMed.
- T. Chen, M. Qian, X. Tong, W. Liao, Y. Fu, H. Dai and Q. Yang, Int. J. Hydrogen Energy, 2021, 46, 29889–29895 CrossRef CAS.
- J. Guo, Z. Zhan, T. Lei and P. Yin, Int. J. Hydrogen Energy, 2022, 47, 5974–5989 CrossRef CAS.
- M. Huang, K. Ge, G. Dong, Z. Zhou and Y. Zeng, Int. J. Hydrogen Energy, 2019, 44, 13195–13204 CrossRef CAS.
- L. Zhou, S. Jiang, Y. Liu, M. Shao, M. Wei and X. Duan, ACS Appl. Energy Mater., 2018, 1, 623–631 CrossRef CAS.
- G. Li, X. Zhang, H. Zhang, C. Liao and G. Jiang, Appl. Catal. B Environ., 2019, 249, 147–154 CrossRef CAS.
- X. Zhang, W. Gu and E. Wang, Nano Res., 2017, 10, 1001–1009 CrossRef CAS.
- Y. P. Zhu, Y. P. Liu, T. Z. Ren and Z. Y. Yuan, Adv. Funct. Mater., 2015, 25, 7337–7347 CrossRef CAS.
- R. Zhang, X. Wang, S. Yu, T. Wen, X. Zhu, F. Yang, X. Sun, X. Wang and W. Hu, Adv. Mater., 2017, 29, 1605502 CrossRef PubMed.
- W. Li, D. Xiong, X. Gao, W. G. Song, F. Xia and L. Liu, Catal. Today, 2017, 287, 122–129 CrossRef CAS.
- Y. Lin, K. Sun, S. Liu, X. Chen, Y. Cheng, W. C. Cheong, Z. Chen, L. Zheng, J. Zhang and X. Li, Adv. Energy Mater., 2019, 9, 1901213 CrossRef.
- Z. Wang, J. Yang, W. Wang, F. Zhou, H. Zhou, Z. Xue, C. Xiong, Z.-Q. Yu and Y. Wu, Sci. China Mater., 2021, 64, 861–869 CrossRef CAS.
- P. He, X. Y. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2017, 56, 3897–3900 CrossRef CAS PubMed.
- L. Wu, L. Yu, F. Zhang, B. McElhenny, D. Luo, A. Karim, S. Chen and Z. Ren, Adv. Funct. Mater., 2021, 31, 2006484 CrossRef CAS.
- Y. Zhao, J. Zhang, Y. Xie, B. Sun, J. Jiang, W.-J. Jiang, S. Xi, H. Y. Yang, K. Yan and S. Wang, Nano Lett., 2021, 21, 823–832 CrossRef CAS PubMed.
- Y. Jin, S. Huang, X. Yue, H. Du and P. K. Shen, ACS Catal., 2018, 8, 2359–2363 CrossRef CAS.
- K. Zhan, C. Feng, X. Feng, D. Zhao, S. Yue, Y. Li, Q. Jiao, H. Li and Y. Zhao, ACS Sustain. Chem. Eng., 2020, 8, 6273–6281 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00099g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |