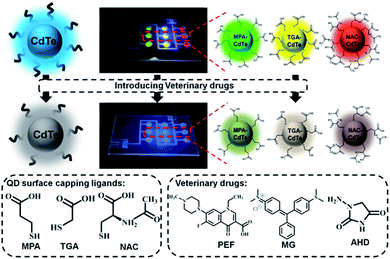
Open Access Article
Open Access ArticleMicrofluidic fluorescent platform for rapid and visual detection of veterinary drugs†
Ge Li,
Hao Li,
Jiang Zhai,
Jiazhuang Guo,
Qing Li,
Cai-Feng Wang * and
Su Chen
* and
Su Chen *
*
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemical Engineering, Jiangsu Key Laboratory of Fine Chemicals and Functional Polymer Materials, Nanjing Tech University, Nanjing 210009, China. E-mail: caifengwang@njtech.edu.cn; chensu@njtech.edu.cn; Tel: +86-25-83172258
First published on 16th March 2022
Abstract
The overuse of veterinary drugs and veterinary drug residues is increasingly becoming an obstacle to sustainable development worldwide. It is therefore imperative to establish a quantitative, sensitive and efficient method for the detection of veterinary drugs. Herein, we developed a visual microfluidic detection platform for rapid and sensitive detection of veterinary drugs using CdTe quantum dots (QDs) with three different ligands as the sensing units. Green-emissive 3-mercaptopropionic acid (MPA)-CdTe QDs, yellow-emissive thioglycolic acid (TGA)-CdTe QDs and orange-emissive N-acetyl-L-cysteine (NAC)-CdTe QDs were synthesized by a sulfhydryl aqueous phase method. These CdTe QDs show selective rapid fluorescence response to pefloxacin (PEF), malachite green (MG), and 1-aminohydantoin hydrochloride (AHD). With the concentration of veterinary drugs increasing, the CdTe QDs reveals a fluorescence color variation from bright to dark until quenched and the response degree of CdTe QDs with different ligands to veterinary drugs is different. Specifically, the limits of detection (LODs) of MPA-CdTe, TGA-CdTe and NAC-CdTe QDs probes for PEF were 7.57 μM, 1.75 μM and 2.90 μM, respectively, and the response was complete in a few seconds, realizing the sensitive and rapid detection of PEF. The three kinds of CdTe QDs could also be used in the detection of other veterinary drugs such as MG and AHD. Finally, a microfluidic detection platform was constructed for visual sensing and rapid detection towards veterinary drugs. The sensor platform holds the advantages of simple operation, low cost, rapid sensing and good sensitivity, and is potentially useful for visual quantitative detection of veterinary drug residues in aquatic products and the environment.
Introduction
Veterinary drugs are widely used in modern agriculture and animal husbandry to prevent the devastating losses of large-scale diseases, pests and epidemics.1 However, the use of large amounts of veterinary drugs has brought about the problem of drug residues in environmental water and food.2 These residues bring risks to human health, such as cancers, birth defects, or hormone disruption.3,4 Veterinary drug residues in environmental water and food are not only harmful to people's health, but also affect the export of agricultural and aquatic products.5 As there is a growing demand for environmental protection and food safety, many countries have promulgated various constraints and regulations to strictly limit veterinary drug residues in environmental water and food.Currently, the detection methods for veterinary drug residues mainly contain instrumental analysis and immunoassay,6 including high performance liquid chromatography-ultraviolet detection (HPLC-UV),7 high performance liquid chromatography-fluorescence (HPLC-FL),8 high performance liquid chromatography-mass spectrometry (HPLC-MS),9 enzyme-linked immunosorbent assay (ELISA),10,11 high resolution mass spectrometry (HRMS)12 and so on. Although instrumental analysis holds good accuracy and sensitivity, its high cost and complex operation steps deter people.13 The merits of immunoassay include simple sample processing, high specificity, low cost and simple operation, while the high false positive and poor repeatability become big problems.14,15 It is still of great practical significance to develop rapid, sensitive and efficient detection methods for veterinary drug residues.
With the rapid development of nano-science, quantum dots (QDs) have emerged as active fluorescent probes. Due to their unique optical characteristics, excellent photochemical stability, easy synthesis and rapid detection of target analytes, QDs are widely used in life science,16 environmental detection,17 biochemical sensing,18 bioimaging,19 immunoassay,20 medical diagnosis21 and many other research fields.22 In recent years, QDs have attracted extensive attention and greatly promoted the development of fluorescence immunoassay.23 In particular, it was found that QDs modified with different surface functional groups exhibited photoluminescence or chemiluminescence response to external stimulation, indicating their excellent sensing potential.24 Fluorescence correlation detection technology is becoming high-profile and also show prospects in the field of veterinary drug residue detection.25,26
In this study, we constructed a simple microfluidic sensing system as a fluorescent probe platform, based on the optical properties of CdTe QDs with three different ligands, allowing the detection of veterinary drugs. Three kinds of CdTe QDs with good water solubility, high fluorescence intensity, uniform dispersion and narrow size distribution were synthesized by an aqueous phase method. CdTe QDs show a clear fluorescence intensity and color variation within a short time after mixing with veterinary drugs. A microfluidic chip was designed for the rapid detection of veterinary drugs using different colored CdTe QDs capped with different ligands as fluorescent probes. It is reusable, cost-saving and beneficial for environmental protection. Combined with the visualized microfluidic detection platform, a simple, rapid and intuitive method for the detection of veterinary drugs on samples can be implemented. Significantly, on this visualized microfluidic detection platform, CdTe QDs with different ligands could be used for the detection of different kinds of veterinary drugs such as pefloxacin (PEF), malachite green (MG), and 1-aminohydantoin hydrochloride (AHD), with sensitive detection effect, good correlation and low LOD. The probe showed sensitive response to PEF, MG and AHD, and was successfully applied to the detection of unknown concentrations of veterinary drugs. The response is rapid and visual owing to the reaction between surface functional groups of CdTe QDs and veterinary drugs molecules. More importantly, benefitting from the merits of microfluidic chip microchannels, the surface functional groups of CdTe QDS can fully react with veterinary drugs molecules, which further improves the reaction time and sensitivity. In addition, due to the advantages of visualization of the microfluidic device, the fluorescence intensity information can be captured in time. This sensor platform shows a broad application prospect in real-time quantitative detection of veterinary drug residues with rapid, sensitive and visual features, meaningful for food and environmental safety.
Experimental
Materials and reagents
Cadmium chloride hemi (pentahydrate) (CdCl2·2.5H2O, 99%), 3-mercaptopropionic acid (MPA, 99%), thioglycolic acid (TGA, 99%) and N-acetyl-L-cysteine (NAC, 99%) were purchased from Tianjin Chemical Reagents Co. Ltd. Tellurium (Te) powder (99.99%), sodium borohydride (NaBH4, 99.5%) and sodium hydroxide (NaOH, ≥85%) were purchased from Sinopharm Chemical Reagents Co. Ltd. Pefloxacin (PEF, 99%), malachite green (MG, 99%), 1-aminohydantoin hydrochloride (AHD, 99%) and chloramphenicol (CAP, 99%) were purchased from Shanghai Macklin Biochemical Technology Co. Ltd. All chemicals were of analytical reagent grade and required no further purification prior to use. All solutions were prepared using ultrapure water with a resistivity of 18.2 mΩ, obtained from Sinopharm Chemical Reagent Co. Ltd. Pure water was provided by Hangzhou Wahaha Group Co., Ltd, China.Synthesis of NaHTe
NaHTe was prepared according to a method described previously in the literature,27 with some modifications. Briefly, 0.5 mmol of Te powder and 3.0 mmol of NaBH4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 molar ratio of Te to NaBH4) were loaded into a 5 mL strain flask and 2 mL of pure water was added. The strain flask was sealed with a rubber stopper into which a small needle was inserted to allow the release of the hydrogen (H2) gas produced by the reaction. After 8 hours of reaction in an ice bath, the bottom of the strain bottle formed white sodium tetraborate crystals and the black Te powder disappeared completely. The upper clarified layer was a lavender solution of NaHTe, which was used as a Te precursor in the subsequent preparation.
6 molar ratio of Te to NaBH4) were loaded into a 5 mL strain flask and 2 mL of pure water was added. The strain flask was sealed with a rubber stopper into which a small needle was inserted to allow the release of the hydrogen (H2) gas produced by the reaction. After 8 hours of reaction in an ice bath, the bottom of the strain bottle formed white sodium tetraborate crystals and the black Te powder disappeared completely. The upper clarified layer was a lavender solution of NaHTe, which was used as a Te precursor in the subsequent preparation.
Synthesis of CdTe QDs with different ligands
The synthesis of CdTe QDs was performed according to a previously reported procedure.27 In a typical synthesis, 1 mmol CdCl2·2.5H2O was weighed into a 100 mL three-necked flask with a condenser and 40 mL of pure water was added to dissolve it. Then 5 mL of aqueous solution containing organic ligand (2.5 mmol MPA or 0.5 mmol TGA or 1.5 mmol NAC) was injected under vigorous stirring. The pH value of the solution was adjusted to 9–10 with use of an aqueous solution of NaOH (5 M), introduced with nitrogen (N2) gas and stirred for 30 minutes. After that, the solution was heated and kept at a constant temperature of 95 °C. The freshly prepared NaHTe solution was quickly injected into the above solution using a syringe. In order to obtain different sizes of CdTe QDs, the reaction mixture was taken in equal amounts at different intervals of time for PL spectra measurements. The solution obtained was precipitated with ethanol and centrifuged at 8000 rpm for 10 min. The resulting precipitate was washed with ethanol and then reprecipitated 3 times, dried under vacuum and stored in a sealed container away from light.Fluorescence detection for veterinary drugs residues based on CdTe QDs with different ligands
Fresh aqueous solutions of MPA-CdTe, TGA-CdTe and NAC-CdTe QDs were prepared (1 mg mL−1). Different concentrations of veterinary drug solutions were prepared. A series of known concentrations of veterinary drug solutions were added to the prepared CdTe QDs solutions, respectively, and the change of fluorescence intensity with the concentration of veterinary drug solution was measured by fluorescence spectrophotometer. According to the fluorescence intensity of the tested solution, a relationship between the fluorescence intensity and the concentration of the veterinary drug solution was established.Detection for veterinary drugs via visual microfluidic detection platform
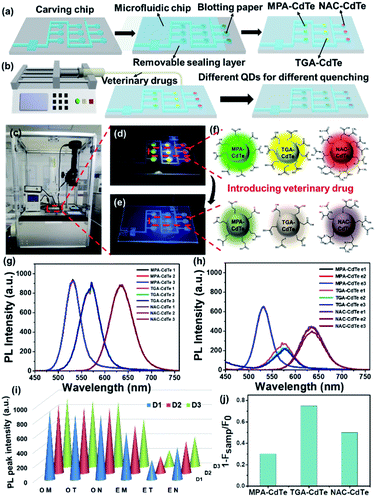
First, microfluidic chips were manufactured by carving 2 mm thick polymethyl methacrylate (PMMA) substrates with parameters as follows: inlet pipe diameter of 0.8 mm, serpentine pipe diameter of 1.6 mm, circular holes diameter of 4 mm, depth of circular holes of 1.6 mm, spacing distance of circular holes of 4 mm and buffer pool of 5 × 10 mm. Subsequently, a blotting paper with a diameter of 3 mm was placed into each circular hole of the detection chamber, and then the chip was covered with a detachable sealing layer. Then, CdTe QDs with different ligands (5 μL) were separately injected into circular holes filled with blotting papers as fluorescent probes. Finally, the prepared microfluidic fluorescence detection chip was put into the microfluidic device and connected with the injection pump for subsequent veterinary drug detection. The solution containing unknown concentration of veterinary drugs was flowed into the chip through a simple microfluidic device. The sample solution was flowed through a serpentine micro-channel into the detection chamber, where different QDs were quenched to varying degrees. According to the linear relationship between fluorescence intensity and veterinary drug concentration, the veterinary drug concentration in the tested solution was determined.Measurements
The microstructure of CdTe QDs was characterized by transmission electron microscopy (TEM) (FEI TECNAI G2 F20). X-ray diffraction (XRD) patterns were collected using a ARL X'TRA powders X-ray diffractometer. Fourier transform infrared (FT-IR) spectra were performed on a Nicolet 6700 FT-IR spectrophotometer. Ultraviolet-visible (UV-Vis) absorption spectra of the CdTe QDs were performed on a PerkinElmer Lambda 900 UV-Vis spectrophotometer. The PL spectra were performed on a Varian Cary Eclipse spectrophotometer. The lifetime decay curves of CdTe QDs were measured using an Edinburgh FLS 980-STM.Results and discussion
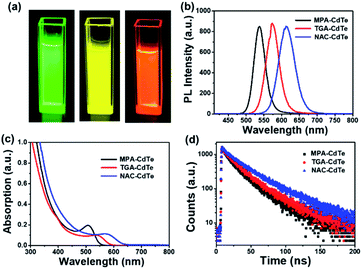
Three kinds of highly fluorescent multicolor CdTe QDs were successfully prepared via an aqueous phase method using 3-mercaptopropionic acid (MPA), thioglycolic acid (TGA) and N-acetyl-L-cysteine (NAC) as ligands, respectively, and then we applied them to the field of veterinary drugs detection. This method has the advantages of simple synthesis, easy controllable of experimental conditions and can be used as fluorescent probes without further surface hydrophilic modification, together with providing high-quality CdTe QDs.28,29 CdTe QDs can interact with many molecules owing to external carboxyl groups, thus achieving direct application in analytical chemistry. Meanwhile, the carboxyl group is the electron-absorbing group, the surface of QDs has a negative charge, which can be combined with the target molecule with a positive charge through electrostatic interaction, so as to achieve the purpose of detection.30,31 Upon the addition of veterinary drugs, the increase of acidity of the system or the change of the surface energy state of the QDs will lead to fluorescence attenuation or quenching of the QDs. A visual microfluidic fluorescent detection platform was designed for rapid and sensitive detection of different veterinary drugs based on PL sensing ability of multicolor CdTe QDs with multiple capping ligands as fluorescent probes shown in Scheme 1. Combined with the visualized microfluidic QDs platform, this allows simple, fast and intuitive detection of veterinary drugs on samples.Green-emissive MPA-CdTe QDs, yellow-emissive TGA-CdTe QDs and orange-emissive NAC-CdTe QDs were prepared in the study. As shown in Fig. 1a, the prepared MPA-CdTe, TGA-CdTe and NAC-CdTe QDs emitted bright green, yellow and orange fluorescence under UV (365 nm) irradiation. The photoluminescence (PL) spectra of MPA-CdTe, TGA-CdTe and NAC-CdTe QDs exhibit high fluorescence intensity. At 400 nm excitation wavelength, PL centers were located at 537 nm, 574 nm and 615 nm, respectively (Fig. 1b). Fig. 1c shows the ultraviolet-visible (UV-Vis) absorption spectra of CdTe QDs with different ligands. In the UV-Vis absorption spectra, the optimum absorption peaks for CdTe QDs with different ligands can be observed at 508 nm, 542 nm and 576 nm, respectively. The time-resolved PL decay was tested to explore the PL lifetime and fluorescence decay of CdTe QDs (Fig. 1d). The average PL decay lifetime of MPA-CdTe, TGA-CdTe and NAC-CdTe QDs were fitted by a biexponential decay function as 23.19 ns, 26.24 ns and 34.27 ns, respectively. The long PL decay life lays a foundation for the sensitive detection of QDs in veterinary drugs. In a word, CdTe QDs with good water-solubility, high fluorescence intensity, strong absorption, and long PL decay life were synthesized by using a sulfhydryl water phase method, which is essential for rapid and sensitive detection of veterinary drugs content to meet the needs of practical application.
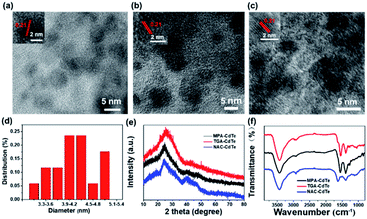
The microstructures of these CdTe QDs were studied by transmission electron microscopy (TEM). As shown in Fig. 2a–c, the MPA-CdTe, TGA-CdTe and NAC-CdTe QDs are monodisperse and uniform in size. Clear and identical lattice spacing can be seen in high-resolution TEM (HRTEM) observations (inset in Fig. 2a–c), and the lattice stripe with a planar spacing of 0.21 nm matches the (111) plane of CdTe QDs,27,32 confirming the good crystallinity of these CdTe QDs. The sizes of the three CdTe QDs are distributed in the regions of 3–6 nm, 3–5 nm and 3–7 nm, with average diameters of 4.2 nm, 5.1 nm and 3.7 nm, respectively (Fig. 2d, S1a and b†). The crystalline structure of these CdTe QDs was further characterized using XRD measurement. As shown in Fig. 2e, the CdTe QDs have a typical zinc blende structure with planes at 111, 220 and 311 and peaks at 24.29°, 40.72° and 46.84°.33 The good solubility of CdTe QDs in water is attributed to their hydrophilic groups, such as –COOH and –NH2. As shown in FT-IR spectra (Fig. 2f), 3429 cm−1 indicates the O–H vibration, 2968 cm−1 and 2922 cm−1 represent the presence of C–H. Wave numbers at 1625 cm−1, 1389 cm−1 and 1294 cm−1 are peaks of characteristic absorption of –COOH. The peaks at 1531 cm−1 and 1458 cm−1 are attributed to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –NH, respectively. While the characteristic absorption band of –SH at 2548 cm−1 is not seen, which is due to the formation of covalent bonds between Cd2+ and –SH.34,35 The above results show that the ligand has been successfully covered on the surface of CdTe QDs. All these structural characterization results indicate the successful preparation of well-defined CdTe QDs with different ligands.
O and –NH, respectively. While the characteristic absorption band of –SH at 2548 cm−1 is not seen, which is due to the formation of covalent bonds between Cd2+ and –SH.34,35 The above results show that the ligand has been successfully covered on the surface of CdTe QDs. All these structural characterization results indicate the successful preparation of well-defined CdTe QDs with different ligands.
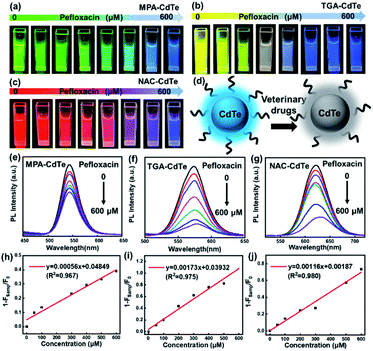
CdTe QDs show PL response towards a variety of veterinary drugs and can be used directly as fluorescent probes. The degree of PL quenching correlates with the concentration of the veterinary drug and can be used as an index for veterinary drug residues. Taking the response of CdTe QDs to PEF as an example, we studied the fluorescence changes of CdTe QDs with different ligands to PEF. Firstly, the fresh MPA-CdTe, TGA-CdTe and NAC-CdTe QDs solutions were centrifugally-purified and prepared into aqueous solutions with a concentration of 1 mg mL−1, respectively. Different concentrations of PEF solutions were added into the above series of known concentrations of CdTe QD solutions, and the fluorescence intensity of the mixture changed significantly on the vision. As shown in Fig. 3a–c, under UV (365 nm) irradiation, different fluorescence colours of CdTe QDs with different ligands gradually weaken until quenching with the gradual increase of PEF solution concentration. The mixed detection solution emits weak blue fluorescence under UV (365 nm) irradiation when the concentration of PEF solution is large, which is mainly attributed to the weak blue fluorescence of PEF solution under UV (365 nm) irradiation (Fig. S2†). The weak fluorescence of PEF solution is helpful to visually observe the flow of sample solution in microchannels. So far, there is no unified conclusion about the fluorescence quenching mechanism of QDs, which can be summarized as the following hypotheses: energy transfer, electron transfer and surface adsorption of other substances lead to changes in the surface energy state of QDs, resulting in fluorescence quenching of QDs.36,37 Studies have shown that the sulfhydryl-containing ligands on the surface of QDs form complexes with Cd2+, reducing the suspended bonds of Te atoms on the surface of QDs. If the acidity of the system increases, the protonation of sulfhydryl groups is enhanced, which leads to the decomposition of the complex and the formation of more suspended bonds on the surface of QDs, reducing the fluorescence intensity of QDs.38 We hypothesized that the acidity of PEF enhanced the protonation of sulfhydryl groups, decomposed the complex and resulted in the formation of more dangling bonds on the surface of CdTe QDs, which reduced the fluorescence intensity of CdTe QDs (Fig. 3d). The variation of fluorescence intensity with the concentration of PEF solution was determined by fluorescence spectrophotometer. PL spectra directly shows the changes in PL intensity of CdTe QDs with MPA, TGA and NAC as ligands at different concentrations of PEF in the range of 0–600 μM, respectively (Fig. 3e–g). As can be seen in the Stern–Volmer plot in Fig. 3h–j, a good linear relationship can be seen between the 1 − Fsamp/F0 ratio (F0 and Fsamp represent the fluorescence intensity of the probe solution before and after the addition of PEF, respectively) and the PEF concentration. The detection limits (LOD) were 7.57 μM, 1.75 μM and 2.90 μM, respectively. The response times were all completed within a few seconds, realizing the sensitive rapid detection of PEF.
In addition, we also studied the fluorescence changes of CdTe QDs with different ligands on veterinary drugs MG and AHD. As shown from the detection results, MG can effectively quench the fluorescence of CdTe QDs. As the concentration of MG increases from 0 μM to 100 μM, the fluorescence intensity of CdTe QDs gradually decreases (Fig. S3a–c†). In Fig. S3d–f,† the ratio of PL intensity between 1 − Fsamp and F0 (1 − Fsamp/F0) increases linearly from 0 μM to 100 μM with the increase of MG solution concentration. The LODs were 9.20 × 10−4 μM 1.16 × 10−4 μM and 3.04 × 10−4 μM, and the correlation coefficients were R2 = 0.988, R2 = 0.995 and R2 = 0.983, respectively. Fig. S4† shows the detection results of AHD. As the concentration of AHD increases, the fluorescence intensity of QDs gradually decreases (Fig. S4a–c†), and the PL intensity ratio of 1 − Fsamp and F0 (1 − Fsamp/F0) exhibits a linear correlation. The LODs were 2.22 μM, 0.69 μM and 1.89 μM, respectively (Fig. S4d–f†). These three kinds of CdTe QDs with different ligands show different linear relationship in the detection of PEF, MG and AHD and possess the advantages of rapid and sensitive detection, good linear relationship and low LOD. Different linear relationship between 1 − Fsamp/F0 and CAP concentration cloud also be observed (Fig. S5†). Therefore, based on the different PL response degree of CdTe QDs with different ligands towards different veterinary drugs, the detection of various veterinary drugs might be available.
Based on the sensitive response of CdTe QDs with different ligands to veterinary drugs, we developed a visual microfluidic detection platform. Fig. 4a and S6† show a microfluidic chip using CdTe QDs of different colors and ligands as fluorescent probes for rapid detection of veterinary drugs. Polymethyl methacrylate (PMMA), which is optically transparent, low-cost and easy to process, was selected as the substrate for microfluidic chips. The microfluidic chip is mainly composed of inlet, serpentine microchannel, detection chamber (circular holes) and buffer pool. The blotting-paper with a diameter of 3 mm is placed in the circular holes and then covered with a detachable sealing layer. CdTe QDs with different ligands were injected separately into circular holes of the detection chamber as fluorescent probes. More details are presented in the Experimental Section. Due to the removable sealing layer, the microfluidic detection chip has the advantages of reusable, cost-saving and beneficial for environmental protection. CdTe QDs with different ligands show different emission peaks and different PL response towards veterinary drugs, and three parallel detection groups could determine the content of veterinary drugs more accurately. As shown in Fig. 4b, the sample solution containing veterinary drugs flows into the chip through a simple microfluidic device. When the sample solution flows through the serpentine microchannel, it enters the detection chamber, in which different CdTe QDs are quenched to varying degrees. Fig. 4c–e show the response process of veterinary drug (PEF) containing unknown concentration under the microfluidic device. It can be seen that with the entry of the veterinary drug, the CdTe QDs in the detection chamber undergone significant quenching and could be distinguished by the naked eye (Fig. 4d and e). This is mainly due to the enhanced protonation of sulfhydryl groups due to the acidity of PEF, which leads to the decomposition of the complex, resulting in more suspended bonds on the surface of QDs, so that the fluorescence intensity of CdTe QDs is weakened or even quenched (Fig. 4f). It can be seen from the changes of the fluorescence intensity of the initial CdTe QDs (Fig. 4g) and the CdTe QDs after the veterinary drug is injected (Fig. 4h), the concentration of PEF can weaken the CdTe QDs with different ligands to varying degrees. The conical histogram shown in Fig. 4i of the maximum fluorescence peak more intuitively and clearly shows the effect of PEF concentration on the fluorescence of CdTe QDs. The PL intensity ratio of 1 − Fsamp and F0 (1 − Fsamp/F0) of MPA-CdTe, TGA-CdTe and NAC-CdTe QDs are 0.30, 0.75 and 0.50, respectively (Fig. 4j). According to the relationship between the PL intensity and the concentration of veterinary drug solution, the concentration of PEF in the sample is about 420 μM. The parallel three detection groups further increase the accuracy of detection. As shown in Movie S1,† clear visual fluorescence sensing and detection towards veterinary drugs could be achieved via this microfluidic detection platform. The visual microfluidic detection platform has the advantages of simplicity, speed and high sensitivity. It has broad application prospects in the real-time quantitative detection of veterinary drug residues. This work promises an effective platform for rapid, sensitive and visual detection of veterinary drug residues, circumventing expensive equipment and complicated operations.
Conclusions
In summary, multicolor CdTe QDs with different ligands were synthesized via a sulfhydryl aqueous phase method to show fluorescence sensing toward different veterinary drugs, including PEF, MG, and AHD. Based on the sensitive fluorescence response of CdTe QDs with different ligands to veterinary drugs, we developed a visual fluorescent microfluidic detection platform for veterinary drugs utilizing green MPA-CdTe QDs, yellow TGA-CdTe QDs and red NAC-CdTe QDs as fluorescent probes. With the increase of veterinary drugs concentration, the fluorescence of the probe gradually decreases to a different degree, which can achieve rapid visual sensitive detection of veterinary drugs within a few seconds of ultra-short response time, with good correlation and low LOD. The microfluidic chip with microscale, reusable and three-parallel detectable has the advantages of speed, sensitivity, visualization and increased accuracy, as well as cost saving and environmental protection. Hence, the designed visual fluorescent microfluidic detection platform offers an attractive way for visual quantitative detection of veterinary drug residues quickly and accurately, which possesses important implications for the development of fluorescent nanosensing in the field of food and environmental safety detection.Author contributions
Ge Li: methodology, formal analysis, data curation, writing – original draft. Hao Li: investigation, formal analysis, writing – original draft. Jiang Zhai: formal analysis, visualization. Jiazhuang Guo: investigation, validation. Qing Li: resources, writing – review & editing. Cai-Feng Wang: conceptualization, validation, writing – review & editing, funding acquisition. Su Chen: conceptualization, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFC1602800 and 2016YFB0401700), the National Natural Science Foundation of China (21736006 and 21978132), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- B. M. Bohrer, Trends Food Sci. Technol., 2017, 65, 103–112 CrossRef CAS.
- A. E. Douglas, Annu. Rev. Plant Biol., 2018, 69, 637–660 CrossRef CAS PubMed.
- Z. Yin, T. Chai, P. Mu, N. Xu, Y. Song, X. Wang, Q. Jia and J. Qiu, J. Chromatogr. A, 2016, 1463, 49–59 CrossRef CAS PubMed.
- A. Margalida, G. Bogliani, C. G. R. Bowden, J. A. Donazar, F. Genero, M. Gilbert, W. B. Karesh, R. Kock, J. Lubroth, X. Manteca, V. Naidoo, A. Neimanis, J. A. Sanchez-Zapata, M. A. Taggart, J. Vaarten, L. Yon, T. Kuiken and R. E. Green, Science, 2014, 346, 1296–1298 CrossRef CAS PubMed.
- N. Gilbert, Nature, 2012, 481, 125 CrossRef CAS PubMed.
- M. Gonzalez Ronquillo and J. C. Angeles Hernandez, Food Control, 2017, 72, 255–267 CrossRef CAS.
- S. Wang, Y. Li, X. Wu, M. Ding, L. Yuan, R. Wang, T. Wen, J. Zhang, L. Chen, X. Zhou and F. Li, J. Hazard. Mater., 2011, 186, 1513–1519 CrossRef CAS PubMed.
- L. Giannetti, A. Giorgi, F. Necci, G. Ferretti, F. Buiarelli and B. Neri, Anal. Chim. Acta, 2011, 700, 11–15 CrossRef CAS PubMed.
- C. Nebot, A. Iglesias, R. Barreiro, J. M. Miranda, B. Vázquez, C. M. Franco and A. Cepeda, Food Control, 2013, 31, 102–107 CrossRef CAS.
- F. F. Zhu, J. Peng, Z. Huang, L. M. Hu, G. G. Zhang, D. F. Liu, K. Y. Xing, K. Y. Zhang and W. H. Lai, Food Chem., 2018, 257, 382–387 CrossRef CAS PubMed.
- Y. Zhang, B. Duan, Q. Bao, T. Yang, T. Wei, J. Wang, C. Mao, C. Zhang and M. Yang, J. Mater. Chem. B, 2020, 8, 8607–8613 RSC.
- F. Sun, H. Tan, Y. Li, M. De Boevre, H. Zhang, J. Zhou, Y. Li and S. Yang, J. Hazard. Mater., 2021, 401, 123266 CrossRef CAS PubMed.
- J. Alcantara-Duran, D. Moreno-Gonzalez, B. Gilbert-Lopez, A. Molina-Diaz and J. F. Garcia-Reyes, Food Chem., 2018, 245, 29–38 CrossRef CAS PubMed.
- B. Wang, K. Xie and K. Lee, Foods, 2021, 10, 555 CrossRef CAS PubMed.
- J. Points, D. Thorburn Burns and M. J. Walker, Food Control, 2015, 50, 92–103 CrossRef CAS.
- Y. Xiao and X. Qian, Coord. Chem. Rev., 2020, 423, 213513 CrossRef CAS.
- P. K. Mehta, J. Jeon, K. Ryu, S.-H. Park and K.-H. Lee, J. Hazard. Mater., 2022, 427, 128161 CrossRef CAS PubMed.
- S. Mehta and J. Zhang, Acc. Chem. Res., 2021, 54, 2409–2420 CrossRef CAS PubMed.
- S. Wang, B. Li and F. Zhang, ACS Cent. Sci., 2020, 6, 1302–1316 CrossRef CAS PubMed.
- L. Van Hoovels, S. Schouwers, S. Van den Bremt and X. Bossuyt, Ann. Rheum. Dis., 2019, 78, e48 CrossRef PubMed.
- C. Y. Lee, I. Degani, J. Cheong, J. H. Lee, H. J. Choi, J. Cheon and H. Lee, Biosens. Bioelectron., 2021, 178, 113049 CrossRef CAS PubMed.
- S. H. Park, N. Kwon, J. H. Lee, J. Yoon and I. Shin, Chem. Soc. Rev., 2020, 49, 143–179 RSC.
- X. Tao, Y. Peng and J. Liu, J. Food Drug Anal., 2020, 28, 576–595 CrossRef.
- J. Zhou, Y. Liu, J. Tang and W. Tang, Mater. Today, 2017, 20, 360–376 CrossRef CAS.
- Y. Wu, J. Sun, X. Huang, W. Lai and Y. Xiong, Trends Food Sci. Technol., 2021, 118, 658–678 CrossRef CAS.
- H. Li, H. G. Ye, R. Cheng, J. Z. Guo, Z. B. Liang, G. Li, Q. Li, C. F. Wang and S. Chen, J. Lumin., 2021, 236, 118092 CrossRef CAS.
- G. Li, T.-B. Chen, Z. Zhao, L. Ling, Q. Li and S. Chen, J. Lumin., 2020, 228, 117625 CrossRef CAS.
- K. Ma, T. Fang, J. Bai and H. Guo, RSC Adv., 2013, 3, 4935–4939 RSC.
- C. Hunsur Ravikumar, R. Shwetharani and R. G. Balakrishna, J. Photochem. Photobiol., B, 2020, 204, 111799 CrossRef CAS PubMed.
- F. Scholz, L. Ruttinger, T. Heckmann, L. Freund, A. M. Gad, T. Fischer, A. Gutter and H. H. Soffing, Biosens. Bioelectron., 2020, 164, 112324 CrossRef CAS PubMed.
- M. Mahbubur Rahman, D. Liu, N. Siraj Lopa, J.-B. Baek, C.-H. Nam and J.-J. Lee, Electroanal. Chem., 2021, 898, 115628 CrossRef CAS.
- L. Jing, S. V. Kershaw, T. Kipp, S. Kalytchuk, K. Ding, J. Zeng, M. Jiao, X. Sun, A. Mews, A. L. Rogach and M. Gao, J. Am. Chem. Soc., 2015, 137, 2073–2084 CrossRef CAS PubMed.
- Y. Xu, J. Hao, X. Niu, S. Qi, H. Chen, K. Wang, X. Chen and T. Yi, Chem. Eng. J., 2016, 299, 201–208 CrossRef CAS.
- F. O. Silva, M. S. Carvalho, R. Mendonça, W. A. A. Macedo, K. Balzuweit, P. Reiss and M. A. Schiavon, Nanoscale Res. Lett., 2012, 7, 536 CrossRef PubMed.
- Q. Wang, T. Fang, P. Liu, B. Deng, X. Min and X. Li, Inorg. Chem., 2012, 51, 9208–9213 CrossRef CAS PubMed.
- T. Senden, R. J. A. van Dijk-Moes and A. Meijerink, Light: Sci. Appl., 2018, 7, 8 CrossRef PubMed.
- M. D. Peterson, S. C. Jensen, D. J. Weinberg and E. A. Weiss, ACS Nano, 2014, 8, 2826–2837 CrossRef CAS PubMed.
- J. Hottechamps, T. Noblet, M. Erard and L. Dreesen, J. Colloid Interface Sci., 2021, 594, 245–253 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00626j |
| This journal is © The Royal Society of Chemistry 2022 |