Open Access Article
Open Access ArticleNanocomposites based on the graphene family for food packaging: historical perspective, preparation methods, and properties
Vinicius Rossa a,
Luanne Ester Monteiro Ferreira
a,
Luanne Ester Monteiro Ferreira a,
Sancler da Costa Vasconcelos
a,
Sancler da Costa Vasconcelos a,
Eric Thomas Tai Shimabukuroa,
Vinicius Gomes da Costa Madriagaa,
Anna Paula Carvalhob,
Sibele Berenice Castellã Pergherc,
Fernando de Carvalho da Silva
a,
Eric Thomas Tai Shimabukuroa,
Vinicius Gomes da Costa Madriagaa,
Anna Paula Carvalhob,
Sibele Berenice Castellã Pergherc,
Fernando de Carvalho da Silva d,
Vitor Francisco Ferreira
d,
Vitor Francisco Ferreira e,
Carlos Adam Conte Junior
e,
Carlos Adam Conte Junior b and
Thiago de Melo Lima
b and
Thiago de Melo Lima *a
*a
aDepartamento de Química Inorgânica, Campus Do Valonguinho, Instituto de Química, Universidade Federal Fluminense - IQ-UFF, 24020-150, Niterói, RJ, Brazil. E-mail: tmlima@id.uff.br
bFood Science Program, Instituto de Química, Universidade Federal Do Rio de Janeiro, 21941-909, Rio de Janeiro, Brazil
cLaboratory Molecular Sieves - LABPEMOL, Chemistry Institute - Federal University of Rio Grande do Norte - IQ-UFRN, Natal, RN, Brazil
dDepartamento de Química Orgânica, Campus Do Valonguinho, Instituto de Química, Universidade Federal Fluminense, 24020-150, Niterói, RJ, Brazil
eDepartamento de Tecnologia Farmacêutica, Faculdade de Farmácia, Universidade Federal Fluminense, 24241-000, Niterói, RJ, Brazil
First published on 11th May 2022
Abstract
Nanotechnology experienced a great technological advance after the discovery of the graphene family (graphene – Gr, graphene oxide – GO, and reduced graphene oxide-rGO). Based on the excellent properties of these materials, it is possible to develop novel polymeric nanocomposites for several applications in our daily routine. One of the most prominent applications is for food packaging, offering nanocomposites with improved thermal, mechanical, anti-microbial, and barrier properties against gas and water vapor. This paper reviewed food packaging from its inception to the present day, with the development of more resistant and intelligent packaging. Herein, the most common combinations of polymeric matrices (derived from non-renewable and renewable sources) with Gr, GO, and rGO and their typical preparation methods are presented. Besides, the interactions present in these nanocomposites will be discussed in detail, and their final properties will be thoroughly analyzed as a function of the preparation technique and graphene family-matrix combinations.
1. Introduction
The nanotechnology field has been expanding with nanomaterials' development regarding their different structural, morphological, and chemical characteristics, guaranteeing enormous versatile applications. One of these features includes the ability to link molecules of different natures and functionalities that can be used for diverse applications for various products: pharmaceutical nanoformulations, ointments/creams for therapeutic or aesthetic purposes, curative films, active packaging, food additives, food packaging, and others.1–3 Considering these cutting-edge nanotechnology applications, food packaging is of great interest since several physical and chemical properties can be tuned through the formulation of nanocomposites.1–3 The application of nanomaterials in the food industry – mainly in the packaging market – increased its market value from $20.4 billion in 2006 to more than $3 trillion in 2020.4 Moreover, nanotechnology contributes significantly to reducing food lost amount and thus offers an alternative for the increasing food demand as the worldwide population increases. Food packaging acts as a physical barrier against the interaction of food and the environment and thus preventing its contamination with microorganisms, dust, or even spoiling as a consequence of moisture, light, and shock. Considering that, several strategies for food packaging have been developed over the centuries, most notably in the last century, in which materials chemistry exerted a pivotal role in the search for smart packaging. The combination with conventional polymers used for packaging with other materials offered a wide range of new materials (composites) with improved properties.4In this way, graphene (Gr), graphene oxide (GO), and reduced graphene oxide (rGO)5–23 are examples of these nanomaterials which are used in composites formulations with polymers, such as polypropylene (PP),24 polystyrene (PS),25 polyethylene terephthalate (PET),26 polyvinyl alcohol (PVA),27 poly lactic acid (PLA),28 polyhydroxybutanoate (PHB),29 chitosan (CS),30 starch (ST),31 cellulose32 and others polymeric matrix.33–36 The interaction between Gr or GO with these polymers occurs through chemical bonds or molecular interactions and thus improves their chemical,37 mechanical, thermal, optical, anti-microbiological, and barrier properties.16,19,38–45
In light of the considerations mentioned above, this review will focus on the recent developments of the graphene family (graphene, graphene oxide, and reduced graphene oxide)-based nanocomposites used as food packaging systems. We will provide an overview of the historical use of food packaging materials and present the most common preparation methods of graphene-based nanocomposites – with several fossils and renewable-derived polymeric matrices – their physicochemical interactions, final properties as a food packaging material, and most common applications found in the market.
2. Food packaging
2.1. Historical perspective
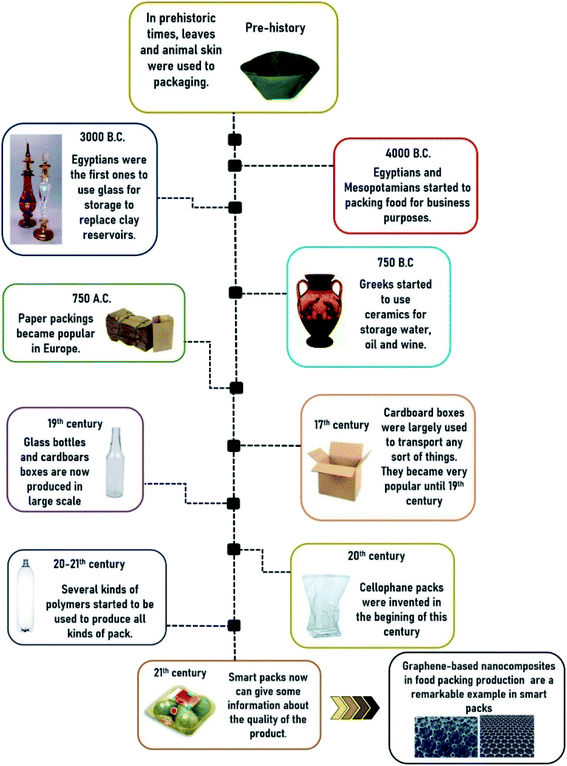
The first types of food packaging arose between prehistory and ancient history. People stored and protected their food in plant leaves, clay artifacts, seashells, baskets, gourds, skin bags, and animal organs (stomach and bladder), coats, hooves, and horns.46 Years later, in A.D. 751, paper became known and made throughout Europe, but its use for food packaging became famous many years later. The development of food and beverage storage techniques was essential to transition from nomadic activities to more complex societies.46The discovery of novel technologies applied to food packaging is of great importance for preserving and maintaining the shelf life of foods in general. Many techniques have been employed to manufacture packaging and have continued to change over the centuries. The correct selection of the type of packaging for each food is essential for maintaining the integrity and quality of the food, as they will keep the food protected, fresh and healthy since they leave the industries until they reach the consumer's table. Several types of material have been used to pack food, such as glass, aluminum, tin-free steel, paper, cardboard, and rigid and flexible plastics.47
The use of glass for food storage dates back to 3000 B.C. The glass containers have characteristics to be impermeable to gases and vapors, keeping food and drink fresh, and offer the possibility of storage for long periods without interfering with the food taste. The main disadvantage of using glass containers is their difficulty transporting because they are fragile and bulky. The glass package may break if there are impacts, thermal shocks or if the internal pressure increases.47,48
Metal packaging is generally produced from aluminum, tin-free steel, and tinplate. These materials are versatile and have good protection and barrier properties, and also possess a high decorative potential and more resistance, being lighter and more malleable than glass packaging.47–49
In that sense, aluminum packaging is resistant to corrosion because it is naturally coated with aluminum oxide, creating an effective barrier to gases and vapors, chemical attacks, variations in humidity and temperature, odors, light, and microorganisms.47–49 Steel packages without tin or chrome steel coated with oxide or electrolytic chromium require the coating with organic material to be completely resistant to corrosion.47–49
Paper-based packaging is also important for the food industry and is widely applied. Paper and cardboard use as food packaging began in the 17th century and expanded in the late 19th century.47,50 Kraft paper is the most resistant paper packaging used to pack vegetables, dried fruits, sugar, and flour. Also, glassine is a paper that undergoes a more extreme hydration process than the previously mentioned and produces a denser, smoother, and shiny paper used to pack fried foods, baked goods, and fast foods. Currently, laminated papers are coated or not with cellulose and kraft sulfite. They can also be laminated with plastic or aluminum to improve their gas and moisture barrier properties, substantially increasing the cost of paper.47,48,50
The rise of modern food packaging began in the 19th century after the industrial revolution, with the studies of can-food by Appert, based on the food microbiology of Pasteur, Prescott, and Underwood.51 In this way, Fig. 1 shows the types of food packaging used by humanity over the centuries.
In the early 20th century, some food packaging appeared from unexpected sources due to unsuccessful attempts, such as the transparent tablecloths by Jacques Brandenberger, a Swiss textile engineer. However, cellophane on regenerated cellulose kind was created through this unsuccessful attempt.51
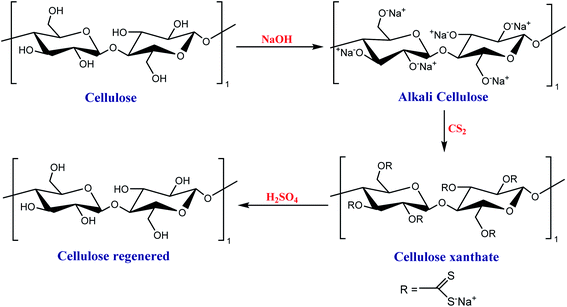
For cellophane production, cellulose is first dissolved in sodium hydroxide solution. It is treated with carbon sulfide producing viscose (cellulose xanthate), which is extruded in a sulfuric acid and sodium sulfate solution to convert the viscose into regenerate cellulose. The regenerated cellulose (cellophane) goes through several baths: sulfur removal, bleaching, and glycerin to avoid fragility,52,53 as shown in Scheme 1. Cellophane is transparent, with a high permeability barrier to oils, greases, and air; it is used to pack sweets, generally.54
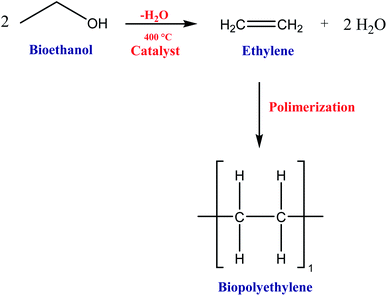
During the first (1914–1918) and the second (1939–1945) World wars, there was a lot of innovation in food packagings, such as oil products and wax for dry cereals and cookies. In this way, plastics like polyethylene (PE) and polyvinylidene chloride (PVDC) emerged as aseptic packaging and flexible metal cans for bottling beer. The development of polymers such as polyethylene (PE), polyethylene terephthalate (PET), polypropylene (PP), and polyvinyl alcohol allowed a partial replacement of glass, metal, and rigid plastic packaging by these new flexible plastic packaging.48,50,51,55 Since the end of the 20th century, many polymers produced from microorganisms, plants, and animals have been studied. Furthermore, in the 20th century, intelligent packaging capable of controlling oxygen diffusion, being a breath mediator, scent controller, and antimicrobial was developed.48,50,51,54–56
In 2007 Kalaitizidou and co-workers24 were the first group of researchers to create a polymer of polypropylene and graphene. They observed that the addition of graphene to the polymer matrix improved the physical, thermal, and mechanical properties and that the polymeric nanocomposite could be used to produce food packaging. Item 3.4 presents more details about graphene-based nanocomposites applied to food packaging.
Considering the pivotal role that polymers-based packaging possesses in our modern society, the following sections will cover the non-renewable and renewable polymers most used in food packaging.
2.2. Non-renewable-based polymers applied to food packaging
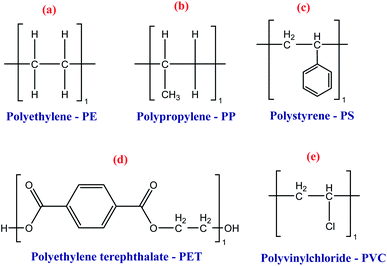
Since the 20th century, the main polymers used in food packaging have been polyethylene, polyethylene terephthalate, polystyrene, polypropylene, and polyvinyl because of their high applicability and availability low molecular weight, and low cost.51 Most of these materials have some drawbacks to be used as food packaging. In most cases, they are not biodegradable and take several years to completely degrade by nature.33,48,502.3. Renewable-based polymers applied to food packages
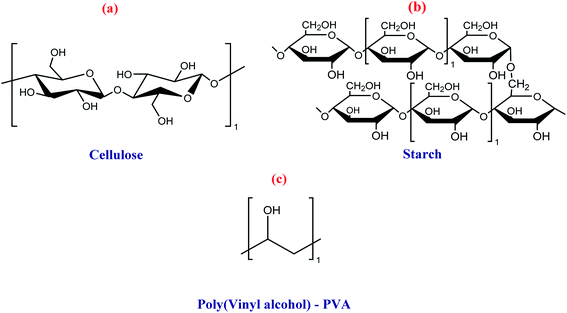
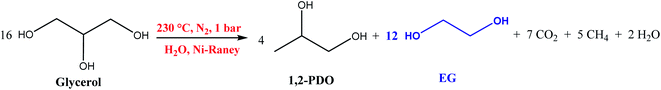
Several polymeric materials from renewable feedstocks are already accessible, representing an evolution towards a more sustainable society, allowing at least a partial replacement of fossil-based polymers. These polymers can be classified into four groups: polymers from vegetal sources (cellulose and starch); polymers from animal sources (chitin and chitosan); polymers from microbiological sources (polyhydroxyalkanoates – PHAs); and polymers chemically synthesized from agricultural raw materials (poly(vinyl alcohol – PVA; polylactic acid – PLA; biopolyethylene – bioPE; biopolyethylene terephthalate – bioPET).54,56Cellulose is highly hydrophilic, insoluble in most solvents, and non-thermoplastic, i.e., it does not melt and cannot be moldable at a specific temperature. Besides, several functional groups, such as carbonyl and carboxyl, can be intercalated in its structure.34,35,54,56,61 The changes made in the cellulose structure usually involve the intrinsic hydroxyl groups through esterification and etherification reactions, thus changing their physicochemical properties.
Superhydrophobic cellulose-based materials are on the micrometric scale and have low surface energy. These materials are in evidence because they are abundant on Earth. In addition to being biodegradable, non-toxic, and renewable, they have chemical, physical and mechanical properties superior to non-renewable materials. In other words, they are a more sustainable and environmentally friendly alternative to polymers based on fossil fuels. Traditional hydrophobicity treatments do not insert the functionalities found in superhydrophobic cellulose materials. One of the methods used to manufacture cellulose-based superhydrophobic materials is wet chemistry, which produces more durable coatings (important for materials with abrasion resistance and wash cycles). The other method is the dry method, which has the main advantage of simplicity by using one-step processes without using organic solvents. Its promising features have potential application in self-cleaning, self-curing, oil and water separation, shielding against electromagnetic interference, etc. The perspective is that the subsequent studies aim to seek these materials for more practical and suitable applications in different areas.62,63
The most known cellulose-derived materials are carboxymethyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, ethylcellulose, and cellulose acetate. Their uses are being investigated for numerous purposes in food packaging, such as fruit, vegetables, fast food, bread, and others.54,56,64
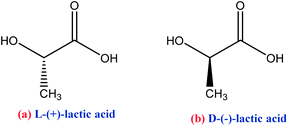
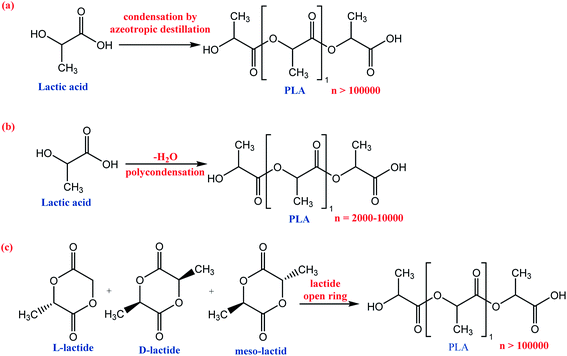
PLA can be synthesized by different polymerization routes,70 Scheme 4, (a) LA condensation by azeotropic distillation; (b) LA polycondensation and dehydration; (c) lactide ring-opening polymerization. The main properties of PLA are good protection barrier, high mechanical and chemical resistance, low toxicity, transparency, and insolubility in water. However, it presents lower thermal stability compared to PET. Besides, the PLA barrier property to gases such as O2 and CO2 is lower than PS and approximately equal to PET polymers. PLA mechanical properties are superior to those observed in PP, HDPE, and PS due to their chemical structure and hardness, and Tm between 173–178 °C. The commercial PLA consists of a copolymer formed between the stereoisomers D(−) and L(+) of the lactic acid, as shown in Scheme 4c. Considering its applications, PLA is used to manufacture food packaging such as meat and soft drinks, among other purposes. Furthermore, packages composed of PLA/cellulose and PLA/natural fiber copolymers gain strength in several packaging and material sectors as they are biodegradable.35,54,59,61
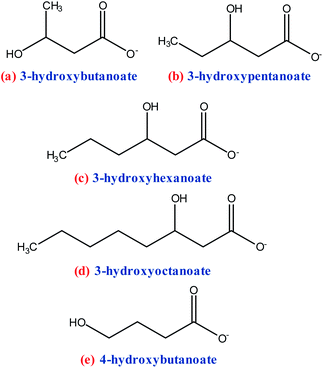
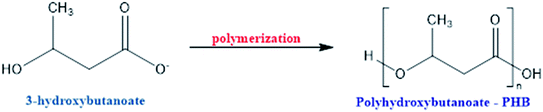
Fig. 5 shows the different monomers used in PHAs production.71 The polymers shown in Fig. 5 also be chemically synthesized with biodegradability similar to those produced by a bacterial pathway. The most common PHAs is polyhydroxybutanoate (PHB), the polymerization product of 3-hydroxybutanoate monomers,35,61,71,72 Scheme 5. PHB has an orthorhombic crystalline structure, with a flat amorphous phase between the lamellae; it is a thermoplastic with high crystallinity (80%) and possesses higher rigidity and fragility compared to PP. It has good gas barrier properties, high hydrophobicity, resistance to organic solvents, and is highly biodegradable. The disadvantages of industrial PHB processing are low deformability and thermal stability, the difficulty of being processed industrially, and being expensive. However, some studies have been reported to modify PHAs, such as the use of their combination with plasticizers, acrylic acid, epoxidized soybean oil, and cellulose acetate to improve properties and facilitate the processing for future applications in food packaging, paper coating, and other purposes.35,54,59,61,64,71,73,74
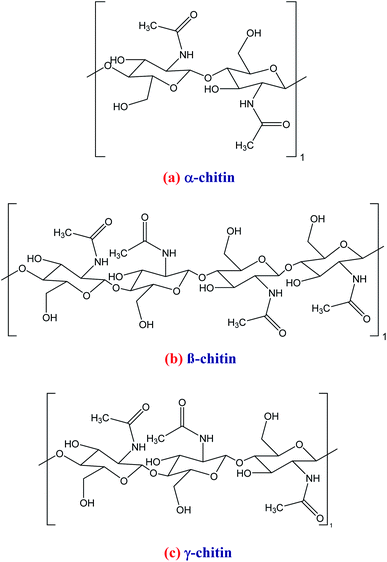
The α-chitin structure has higher physical and chemical resistance because it has an antiparallel arrangement between its oligomers, causing higher strength in the hydrogen bonds of its structure. β-Chitin is characterized by the parallel arrangement of chitin oligomers for the formation of its structure. The N-acetyl groups are structural spacers, facilitating the hydration of this macromolecule. The γ-chitin structure is formed by two parallel and one antiparallel chitin sheets, and the N-acetyl groups play the same role as in β-chitin. The strength and resistance of β- and γ-chitin are similar and weaker compared to α-chitin. Amorphous chitin structures have also been reported and were found in fungi.54,73,74
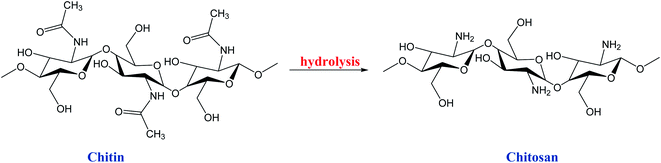
Nature produces billions of tons of crystalline chitin present as the exoskeleton of some insects, arthropods, and mollusks, and in the cell walls of fungi, yeasts, and plants. Chitin is highly hydrophobic, non-toxic, biodegradable, and easily compacted. It is generally soluble only in mixtures of hexafluoroacetone, chloroalcohols, and hexafluoroalcohols with aqueous solutions of strong inorganic acids. Due to its poor chemical reactivity, cellulose is industrially preferred to chitin. However, its derivative chitosan (CS) possesses higher solubility in a wide range of solvents and has gained considerable attention from researchers and industries, resurging the interest in nature chitin. Amines' presence in the chitin and chitosan structure can be chemically modified to improve their properties and hence furnishing several applications in technological and industrial fields.54,74 In this sense, CS is produced by the hot leaching process of chitin, which causes the removal of the acetate group from the chitin molecule by hydrolysis,54,74 Scheme 6.
After this process, chitosan is washed, dried, ground, and ready to be commercialized.34,35,61 The chitosan application for food packaging formulation tests showed excellent mechanical and barrier (O2 and moisture) properties, suitable antimicrobial and antioxidant activities, and good biodegradability, making chitosan a promising polymer in the packaging industry.41–44,75–78
Although biopolymers based on renewable sources consist a step forward for food packaging to achieve a more sustainable society, we are still highly dependent on polymers derived from non-renewable sources for such purposes. In addition, the current packaging technologies lack several points, such as higher resistance barrier properties, hence the research of new technologies for food packaging. In this way, nanocomposites (mixture of two different nanomaterials with distinct properties) endure a promising pathway for high-tech packaging. They might improve mechanical, thermal, barrier properties, cytotoxicity, microbiology, and biodegradable properties.41–44,75
2.4. Nanocomposites-based packaging
The first example of composites for food packaging was reported at the beginning of this century (21st). Metal cans were covered with a multilayer plastic barrier preventing the contact of the food with the metal and thus increasing its shelf life. Also, other technological changes in food packaging were reported, such as the change in the internal atmosphere, the resistance of the packaging to the microwave oven, trays for gas and electric ovens, and retractable film labels, among other examples.51Due to the several combinations of polymer matrix/dispersed nanomaterials, polymer-based nanocomposites can provide different properties for a wide range of applications.33,79
Graphene-based nanocomposites have been utilized to improve UV resistance as a barrier against gases and still have good thermal, mechanical, and electrical properties compared to their polymeric matrices.80–83 Furthermore, graphene-based nanomaterials combined with biodegradable polymers offer high potentials to be used as antimicrobial and antioxidant in active packaging technology resulting in more quality, safety, extended shelf-life, and added value.84–86
Some examples of nanocomposites based on polymer matrix/nanomaterials can be found in the literature. For example, polyvinyl alcohol (PVA)/graphene-based nanocomposites are just one example of polymeric nanocomposites with considerable potential in the food packaging industry. They have excellent gas and moisture barrier properties and good thermal resistance. Besides protecting the product, the new concept of packaging technologies brings technological innovations to adding novel features for barrier performance. An example is the active packaging technologies and modified atmosphere packaging for fresh vegetables and fruits.87
The interaction between the packaging, the product, and the environment through partial gas permeability increases shelf-life providing oxygen to cellular respiration.33
Biobased polymer packaging generally are films made from biopolymers and biodegradable polymers, such as starch,88 pullulan,81 PVA,85,86 and PLA.89 These nanocomposites can be used in food packaging as nanocoating films to control moisture transfer and gas exchange, provide security, and preserve nutrition and sensory features.79
Furthermore, in smart packaging based on polymer nanocomposites, graphene can be used as a sensor for biochemical or microbial changes in the product packed to detect specific food-borne pathogens or gases. Thus, this type of packaging can serve as oxygen and spoilage indicators for food safety and quality monitoring.90 Due to the advanced features required for the food packaging industry, we highlight in this review graphene-based nanocomposites. Specially graphene oxide is a promising packaging material due to its unique characteristics, such as high barrier properties; good mechanical, electric, and thermal properties; large specific surface area; high electrical conductivity; carrier mobility; antibacterial, antifungal antioxidant, and biocompatibility.84,87,89,90,92–101
The electrical properties mean that the food in the package can be quickly heated and provide a package that “speaks to the consumer”. The gas barrier means that the food can be preserved for longer, preventing oxidation and external contamination by microorganisms.80,83,91–95
Since all physicochemical properties of food packaging materials can be improved using graphene-based composites, a closer look at the graphene family exerts a pivotal role in better understanding the final product. The following section will present a detailed discussion regarding the graphene family and their nanocomposites.
3. Graphene-based nanocomposites applied to food packaging
3.1. Graphene, graphene oxide, and reduced graphene oxide
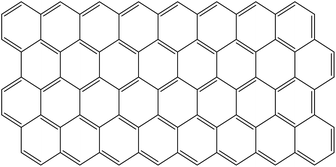
Graphene (Gr) was introduced in 1986 by Bohen's group, aiming to understand a two-dimensional atomic sheet of graphite. However, in the 21st century, discoveries concerning graphene were made, and it was observed that graphene was thermodynamically unstable under ambient conditions. In 2004, Novoselov's group isolated and characterized a graphene monolayer obtained by mechanical exfoliation under ambient conditions. IUPAC defines graphene as a single layer of aromatic polycyclic carbon of almost infinite size, a single flat layer of carbon atoms with sp2 hybridization, compacted together in a dense shape-shaped grid in two ordered dimensions (2D),11,12,39,40,99,100 Fig. 7.The graphene unit cell has a hexagonal shape and comprises two equivalent sub-networks of carbon atoms, connected by sigma (σ) bonds, where the length of this C–C bond is 0.142 nm. Each carbon has a pi (π) orbital in this network that generates a delocalized electronic effect. These bonds and structures provide excellent properties that are highly superior compared to other nanomaterials such as clays, zeolites, zinc oxide, and silicate-based.11,12,39,40,99,100
Graphene presents several interesting electronic properties due to its two-dimensional framework.101–103 High carrier mobility is observed, reaching 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1 at room temperature (or 200
000 cm2 V−1 s−1 at room temperature (or 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1 when impurity scattering is minimized). The high carrier is explained by promoting electrons in the valence band to the conduction band, forming a positively charged space that allows the current formation under an external electric field.101–103
000 cm2 V−1 s−1 when impurity scattering is minimized). The high carrier is explained by promoting electrons in the valence band to the conduction band, forming a positively charged space that allows the current formation under an external electric field.101–103
The structure of graphene can be modified to generate different structures derived from graphene (graphene-based materials), thus presenting other physicochemical properties.7–13,19–23
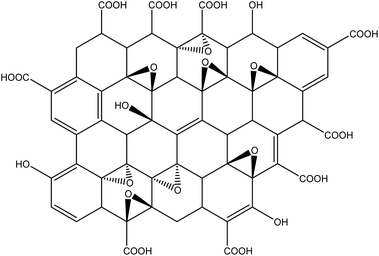
GO (Fig. 8) possesses reduced thermal and electrical properties than Gr. However, the formation of polar groups in the GO structure favors its application in composites and polymeric nanocomposites.11 The structure of the GO is still the target of several discussions in the literature,7–13,19–23 in which physicochemical studies indicate that epoxy and hydroxyl groups are created – usually in the basal planes of the GO leaves – and carboxyl groups commonly in the leaf edges or the pores of the GO structure. Also, it a variation in the amount, location, and functional available in the GO structure is observed, which can be controlled by the synthesis conditions and methods as a function of the specific application and thus reflected the desired properties.11
Graphene oxide may present insulating or semiconducting behavior, possessing sp2 and sp3-bonded carbon atoms in its honeycomb structure. Oxygenated groups such as epoxy and hydroxyl groups are usually covalently bonded with sp3 hybridized carbons, hindering their conductivity and leading to higher hydrophilicity.103–106 Recent works stated that depending on the oxygen content, GO might present a bandgap from 1.7 eV (semiconductor) to 2.1 eV (insulator), having a higher electrical resistance than graphene.107
Generally, graphene oxide (GO) synthesis uses well-known methods described by Staudenmaier,108 Brudie,109 and Hummers.110 In this manner, Staudenmaier,108 and Brodie,109 in a pivotal study, oxidized graphite with a combination of HNO3 and KClO3. Furthermore, H2SO4, HNO3, and HClO4 have also been used to promote graphite oxidation, as described by Staudenmaier,108 Brudie,109 and Hummers' method.110,111
However, the most used GO preparation methods considering biomedical and food packing purposes are the Hummers' method110 and the modified Hummers' method.108,112 Classical Hummers' process involves the treatment of graphite with H2SO4, NaNO3, KMnO4 and H2O2.110 The modified Hummers' method uses H2SO4, KMnO4, and H2O2 without the addition of NaNO3, which generates less toxic gases during the procedure.112 KMnO4 is one of the most potent oxidizing agents in an acidic medium. In concentrated H2SO4, this oxidizing agent provides complete graphite intercalation, forming graphite bisulfate intercalated in all the graphene layers. This intercalation favors the diffusion of the KMnO4 and thus leads to effective graphene oxidation to graphene oxide without the need for NaNO3.113,114 Furthermore, other variations of the Hummers' method are reported, by the replacement of H2SO4 with a mixture of H2SO4/H3PO4, causing a less exothermic process without the formation of hazardous gases and also leading to more considerable oxidation in graphite, higher hydrophilicity and a more regular structure due to the cyclic phosphates creation.115
From a sustainable point of view, greener methods for synthesizing graphene and graphene oxide are reported, such as the synthesis using pyrolysis biochar from the lignocellulosic biomass leaves of Cinnamomum Camphora, in which the π–π interaction of the biochar suspension with D-tyrosine solution promotes the separation of Gr layers by centrifugation.22 Other graphene-like carbons from biomass pyrolysis methods found in the literature are the salt-based method, chemical blowing technique, model-based confinement, coupling to hydrothermal carbonization treatment, and post-exfoliation.23
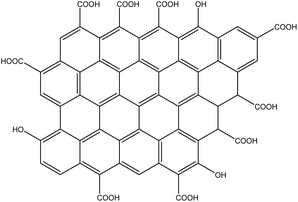
Notwithstanding, changes in GO structure can also be carried out by a reduction reaction and generating rGO. This reduction consists of partially removing oxygenated groups, thus conferring excellent structural stability compared to GO,10 Fig. 9.
GO reduction to rGO can be carried out by chemical17,18 or thermal19,116 routes. Several reducing agents are used in the chemical reduction, such as hydrazine, sulfur compounds, sodium borohydride, ascorbic acid, tyrosine, dimethylhydrazine, ethylenediamine, and others reducing agents and reducing atmosphere.17,18 The first publication regarding GO reduction by chemical route was in 2006, in which it was used hydrazine and dimethylhydrazine as reducing agents. The nitrogen atom from hydrazine possesses a nucleophilic character, essential to reducing GO. In addition, other examples of reducing nucleophilic compounds are amines, hydrazine derivatives, and sodium borohydride. The reducing agents can also be electrophilic, such as borane in THF solution, one of the most common electrophiles for GO reduction.17 Furthermore, another way to reduce GO is to treat the suspension in an acid solution under reflux. The number of oxygenated species to be reduced can be easily controlled by the reflux time.17 Still, considering the chemical methods for rGO preparation, most of them use a lot of solvents and hazardous reductants, thus making the process expensive and non-sustainable due to the large amount of waste produced. In this way, other processes of chemical reduction of GO that use more sustainable reducers, such as tryptophan and ascorbic acid, have been reported. However, in these reduction processes, the agglomeration of rGO particles can occur, reducing their surface area.19 The thermal reduction process involves submitting GO to rapid heating under an inert atmosphere (Ar, N2). Firstly, GO the is dried, placed in a quartz tube and subjected to high temperatures (in the range of 700 to 1000 °C) for a short time, usually less than 1 h. During this thermal decomposition, H2O, CO2 and CO are released, which increases the internal pressure, causing the separation of the leaves, forming the rGO.11 The amount of oxygenated groups remaining in the rGO depends on the initial oxygen concentration in the GO, the proportion of epoxy and hydroxyl groups in the GO, and the temperature used to reduce the GO.117
In this way, chemical and thermal reduction methods generate rGO with similar electronic and structural properties. However, the thermal protocol does not require a purification step or solvents since it only uses temperature as a reducing agent, making it a faster and cheaper protocol.17,18,116 Notwithstanding, decomposition gases (CO2 and CO) can be collected and used as reagents in several green chemical processes.118–121
Thus, graphene-based materials have a high potential for application in the production of new energy storage batteries, magnetic sensors, materials, superconductors, medication administration, ultra-sensitive biosensors, tissue engineering, catalysis, nanocomposites and polymeric nanocomposites, and food packing.21 Considering the encouraging features of GO and rGO, such as chemical, mechanical and antimicrobiological properties, these materials are highly promising to be used in food packing composites.
3.2. Graphene-based nanocomposites – properties
It is essential to select the most suitable food packaging for storage and transportation to maintain food quality, i.e., from the moment they leave the industry until reaching consumer usage. Also, proper packaging provides food with safety, lifetime extension, and protection against insects, microorganisms, light, and chemical contaminants. Furthermore, appropriate packaging can control the humidity, oxidation, diffusion of gases, and taste-odor maintenance.57,70The molecular structure of the polymeric materials used for food packaging directly influences the polarity, rigidity, malleability, crystallinity, and intermolecular forces present in the polymeric chain, controlling the diffusion of the external molecules into the packaging. The chemical polymer composition can alter the barrier properties, impacting the characteristics of resistance, rigidity, cost, efficiency, ease of processing, and increasing its useful life.57,70
Polar polymers have good gas barrier properties. However, they are hydrophilic, and they allow water vapor permeation. On the other hand, nonpolar polymers do not allow the water vapor passage but have weak barrier properties, facilitating gas permeation independently of the polymer thickness. Copolymerization or additives incorporation in the polymeric matrix for food packaging formulations may affect these barrier properties as they fill and reinforce the polymeric matrix-free spaces. However, this will depend on the degree of adhesion and polymer and additive compatibility.57,70
Knowing the polymers' thermal properties is essential to understanding the physical and chemical transformation in nanocomposites structure with the temperature variation. The glass transition temperature, crystalline melting temperature, and crystallization temperature are the most critical transformations in these materials.70
3.3. Graphene-based preparation for nanocomposite production
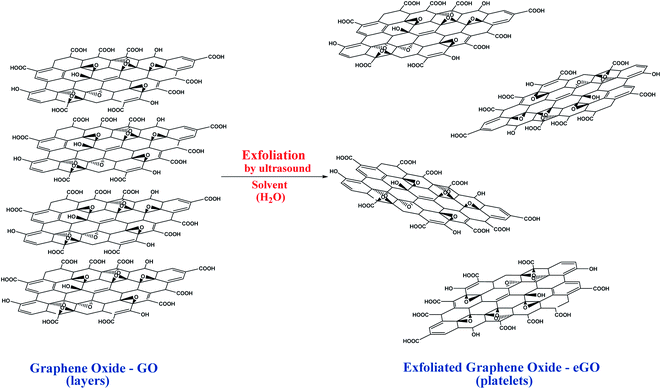
In this way, the encouraging features of GO and rGO, such as chemical, mechanical and antimicrobiological properties, these materials are highly promising to be used in food packaging composites.21 Generally, before producing graphene-based nanocomposites, the Gr should be in the GO or exfoliated forms (eGO). Therefore, incorporating the polymeric matrix into the structure of the eGO can be carried out, generating nanocomposites of commercial interest. GO exfoliation can be performed by two main routes: (i) thermal route; (ii) using polar solvents (water, ethanol, dimethylformamide – DFM, methyl pyrrolidone – NMP, propylene carbonate – PC) under stirring or ultrasonication.19,37 In the thermal process, the powdered GO is subjected to high temperatures (400–1000 °C) and a high heating rate for a few seconds. Then, the decomposition of the –OH and –COOH groups on the GO surface into CO, CO2, and H2O occurs. This process is advantageous since it produces a thermally exfoliated GO (TeGO) with a high surface area (700–1500 m2 g−1), low apparent density, and platelets similar to the eGO platelets produced using the sonication process.19The suspension is electrostatically stabilized in the solvent and ultrasound-assisted method by the carboxylated groups present in the GO lamellae peripheries.19 The GO exfoliation performed by the sonication process in aqueous suspension furnishes GO monolayer platelet with high yield and hence forming eGO monolayers. However, the exfoliation process with agitation formed eGO monolayers with larger platelets, and this process is time-consuming and presents a low yield.19,37 Fig. 10 shows the graphene oxide (GO) and exfoliated graphene oxide (eGO) structures, layers, and platelets.19
The exfoliated platelets by stirring or ultrasound can be later reduced by chemical processes. These processes involve the formation of a suspension between eGO and a reducing agent, such as sodium borohydride, hydrazine monohydrate, or dimethylhydrazine, in a polar solvent (water, NMP, DMF, DMSO) or a mixture of these solvents.19
Generally, chemically reduced eGO platelets result in materials (erGO) with a C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio = 10
O ratio = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in which the oxygen content is related to the –OH and –COOH non-reduced groups in the eGO structure.19,37 Biochemical reducing agents such as vitamin C (ascorbic acid) and tryptophan have also been tested but have resulted in the aggregation and reduction of the eGO platelet surface area.19,122,123
1, in which the oxygen content is related to the –OH and –COOH non-reduced groups in the eGO structure.19,37 Biochemical reducing agents such as vitamin C (ascorbic acid) and tryptophan have also been tested but have resulted in the aggregation and reduction of the eGO platelet surface area.19,122,123
GO, rGO, eGO, and erGO structures have interesting functional groups, such as hydroxyl and epoxy, carboxyl, carbonyl, ketone, phenol, and lactone groups. These moieties allow the insertion of new atoms, molecules, or macromolecules (thermoplastic, thermosetting and biodegradable polymers) and thus creating the graphene-based nanocomposites.19,37,124
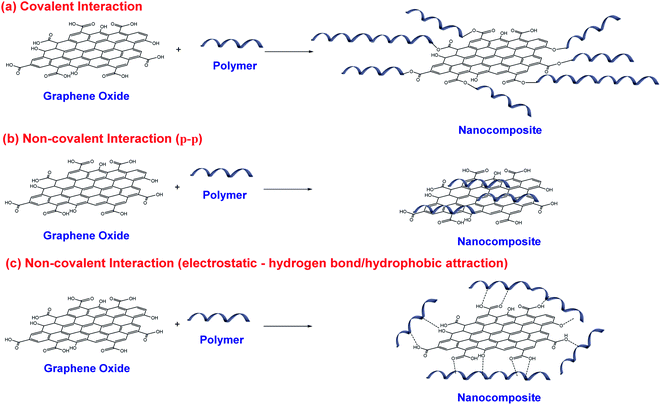
The functionalization of graphene-based nanomaterials can be performed by covalent or non-covalent pathways. The covalent functionalization occurs from creating the covalent bonds between functional groups on the eGO platelets surface with the polymeric matrix,16,19,37,38 (Fig. 11(a)). This pathway can be carried out by nucleophilic substitution reactions, electrophilic substitution, condensation, cycloaddition, esterification, and hydrolytic condensation. In this manner, covalent functionalization is a propitious procedure for adjusting the charge transfer interface but impairs the electrical properties of the formed nanocomposite. On the other hand, non-covalent functionalization involves π–π interactions (Fig. 11(b)), hydrogen bonding (Fig. 11(c)), and hydrophobic attraction.16,19,37,38
Covalent and non-covalent functionalization generates stable dispersions in organic solvents and can improve the compatibility of GO platelets with the polymers to be inserted in the nanocomposite structure.16,19,37,38
Graphene-based/polymer nanocomposites can be prepared by different methods such as: mixing (MX), melt-mixing (MM), in situ polymerization (ISP), and layer-by-layer (LBL) assembling. Here, it is worth mentioning that the chosen method will influence the properties of the final nanocomposite, such as surface area, molecular interaction, dispersion, and other properties.16,19,37,38
The MX method consists of adding a polymeric matrix solution to a graphene-based suspension. The resulting mixture is subjected to sonication, mechanical stirring and solvent evaporation. This method effectively obtains a fine dispersion of the polymeric material, but it uses non-benign solvents, and removing the solvent is difficult. As an example, the synthesis of graphene-based/poly(vinyl)alcohol nanocomposite.16,19,37,38
The MM technique is the most efficient and environmentally benign since solvents are not used. This method is commonly applied when in situ polymerization is not indicated, generally for thermoplastic polymers. The synthesis consists of melting the polymer and dispersing the graphene-based material through an extrusion and injection molding. However, the polymer can undergo thermal degradation, and the structure of the graphene-based nanocomposite can be disrupted by the high shear force involved during synthesis. For example, MM is applied to synthesize the graphene-based/polypropylene, graphene-based/poly(lactic acid), and graphene-based/polyethylene terephthalate nanocomposites.16,19,37,38
ISP protocol is used to disperse the graphene-based material homogeneously in the polymeric matrix. In this technique, the graphene-based material is dispersed in the monomer followed by the polymerization reaction, with subsequent polymer grafting over the graphene-based surface. In situ polymerization uses heat or microwave radiation to form nanocomposites. Using ISP, a more homogeneous and fine dispersion occurs between graphene-based and the polymeric matrix, being very effective for both heating techniques. For example, this protocol is used to synthesize the graphene-based/polystyrene nanocomposite.16,19,37,38
The LBL technique offers the possibility of building nanocomposite alternating layers between the graphene-based material and polymeric matrix. This synthesis method creates a nanomaterial with good dispersion and graphene-based orientation. Also, it allows the possibility of controlling the amount of graphene added between the polymer layers and the morphology of the generated nanocomposite. For example, the GO/poly(vinyl) alcohol nanocomposite construction can be mentioned.16,19,37,38
Graphene-based nanocomposites have several applications in biosensors, energy-related devices, food packaging, and biomedical, among others. However, many challenges are still encountered, especially for materials used in health and food. However, many challenges are still faced, especially for health and food packaging materials. There are already successes in using graphene nanocomposites with polymeric matrices of renewable sources in drug delivery. The low solubility of graphene in an aqueous solution makes its implementation difficult. The aqueous stability and the solubility of graphene can be modified by the induction of π–π interactions with drugs, aromatic molecules, or other organic molecules. However, it is possible to circumvent it by replacing graphene with graphene oxide in the nanocomposite assembly. The advantages of using GO and rGO to construct polymeric nanocomposites consist of oxygenated groups (hydroxyl, epoxide, carboxyl, and carbonyl) on both GO and rGO nanosheets that favor the assembly of new nanometric structures and the new polymeric nanocomposite is formed. This assembly occurs through the stacking system between the nanomaterial and the polymeric matrix and between the hydrogen bonds and π–π interactions that will significantly improve the properties of the polymeric nanocomposite formed as biocompatibility, low toxicity, thermal detachment, and mechanical stability.125,126
3.4 Graphene-based nanocomposites – types
Gr, GO, and rGO are used as polymeric matrices additives for several nanocomposites' formulations. In this sense, these polymeric nanocomposites formulations often present improved properties for food packaging.127,128Several research groups have been developing new materials for the manufacture of packaging based on nanocomposites, such as clays, tourmaline, zeolites, oxides, and carbon-based materials, such as graphene-based, to improve and control food packaging properties.25,33–36,51,61 In this sense, no polymeric matrix has all the necessary/desired properties in food packaging production. However, with the nanotechnology advent, polymeric nanocomposites (polymer matrix + nanomaterial) arise, improving their thermal, mechanical, electrical, optical, and barrier properties of food packaging.70
The matrix/graphene-based nanocomposites will be divided into two groups in the next section: (a) non-renewable polymeric matrix/graphene-based group (petroleum-derived polymers) and (b) renewable polymeric matrix/graphene-based group (biomass-derived polymers).
Table 1 shows that melt-mixing is the most used synthesis method for constructing polymeric nanocomposites (non-renewable origin). The addition of graphene-based nanomaterials increases the nanocomposite's thermal, barrier, and other properties.
| Nanocomposite types | Matrix![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphene-based ratio and others graphene-based ratio and others |
Synthesis method | Polymers properties modifications and improvements | Important comments and applications | Ref. |
|---|---|---|---|---|---|
| a Gr: graphene; GO: graphene oxide; PP: polypropylene; PS: polystyrene; PET: polyethylene terephthalate; MM: melt-mixing; ISPs: in situ polymerization with solvent; IM: injection molding. ↑: increase; ↓: decrease. | |||||
| PP-Gr | -Gr-PP 3 vol%; extrusion at 180 °C for 3 min; injection molding at Tbarrel = 180 °C and Tmold = 80 °C, injection pressure at 1.1 MPa | MM/IM | -↑Thermal conductivity | -Viscoelastic properties allow the fabrication of composites with desired properties at high loadings | Kalaitizidou and co-workers (2007)24 |
| -↑O2 barrier | -Improvement of physical, thermal, and mechanical properties | ||||
| -Can be applied to food packaging | |||||
| PET-Gr | -Gr 1.5 wt%, sandwiched between two PET films, hot pressed, and chopped to granules | MM | -↑Thermal stability | -Gr uniformly dispersed in the matrix | Harel and co-workers (2012)26 |
| -Melt compound at 260 °C for 5 min | -↑Young's modulus, 1.16 to 1.40 GPa | -Enhanced crystallinity | |||
| -Molded into thin films (0.17 mm thick) | -↓Elongation at break, 40% | -The formed films present Gr exfoliated morphology | |||
| -Dried under vacuum at 120 °C for 4 h | -↓Tensile strength, 56% | -Gr addition enhanced brittleness | |||
| -↑O2 barrier | -Can be applied to food packaging | ||||
| PS-GO | -2 g of PS, GO 20 wt%, and 4-vinyl benzyl chloride (VBC) | ISPs | -↓Crystallinity | The nanocomposite showed high barrier and antimicrobial properties and could be utilized in yogurt containers and bottles for medicine capsules | Ghanem and co-workers (2020)25 |
| -Stirring | -↑Hydrophobicity | -Applied to food packaging | |||
| -Dried for 24 h on a glass plate | -↑Thermic stability | ||||
| -↑Tg | |||||
| -↑Young modulus, 60% | |||||
| -↑Impact resistance | |||||
| -↓Water vapor transmission rate, 50 to 16.6 wt | |||||
In this sense, Table 2 shows that MX and MM are the methods for synthesizing polymeric nanocomposites (from renewable sources) based on graphene most used by most research groups. It is due to their ease and simplicity of handling on a small scale. It is indisputable that the addition of graphene-based nanomaterials (Gr, GO, and rGO) generates a significant improvement in the various properties of these nanomaterials when compared to their pure polymeric matrices.
| Nanocomposite types | Matrix![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphene-based ratio and others graphene-based ratio and others |
Synthesis method | Polymers properties modifications and improvements | Important comments and applications | Ref |
|---|---|---|---|---|---|
| a Gr: graphene; GO: graphene oxide, rGO: reduced graphene oxide; CS: chitosan; PLA: poly lactic acid; PVA: poly(vinyl alcohol); PEI: polyethyleneimine; ST: starch; GA: glutaraldehyde; CNC: cellulose nanocrystals; EVOH: poly(ethylene-co-vinyl alcohol); PAA: poly(acrylic acid); OS: oxidized starch; PHB: polyhydroxybutanoate; MX: mixing; MM: melt-mixing; MC: melt compounding; PEP: pickering emulsion polymerization; ISPs: in situ polymerization with solvent; LBL: layer-by-layer; PS: plasticized-starch; SP: solution processing; SC: solution casting method. ↑: increase; ↓: decrease. | |||||
| CS-GO | -GO + water, sonication for 45 min | MX | -↑Young's modulus, 64% | -Good GO dispersion on CS matrix | Li and co-workers (2010)129 |
| -CS + acetic acid solution 0.5% v/v (water) | -↑Tensile strength, 88% | -The CS-GO nanocomposite formation occurred by H bonding between CS molecules with GO oxygenated groups and by electrostatic interaction between polycationic CS and the negative charge of GO surface | |||
-GO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CS ratio = 1 CS ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, GO suspension was added on CS solution and stirring for 24 h 1, GO suspension was added on CS solution and stirring for 24 h |
-↑Tg, 175.4 to 180.4 °C | -Can be applied to food packaging | |||
| -Dried at 40 °C, in glass plate until weight equilibration | |||||
| PVA/GO and PVA/rGO | -PVA/GO and PVA/rGO: 0.3 wt% | MX | -↓O2 permeability, 17–87 times bigger | -Promising for the development of transparent high-gas-barrier films | Lee and co-workers (2011)27 |
| -PET: a substrate | -73% light transmittance at 550 nm | -The H-bonding interaction between GO and PVA is not very large to change the PVA thermal properties | |||
| -Hybrids solutions were cast onto PET film at 90 °C | -Can be applied to food packaging and bottles | ||||
| PLA/GO | -GO 0.4 wt%, dispersed in acetone, sonicated for 5 h | SC | -↑Young's modulus | -Environmentally benign | Magalhães and co-workers (2013)130 |
| -GO + PLA/chloroform (plasticizer) solution, sonicated for 15 min | -↑Tensile strength | -Possible biomedical application | |||
| -PLA/GO films formed in polytetrafluoroethylene, coated plate | -↑O2 and N2 barrier | -Formation of transparent films | |||
| -Dried at room temperature for 7 days | -No detectable effects in the elongation at break | ||||
| -Can be applied to food packaging | |||||
| PVA-rGO | -GOr 0.8 wt% | MM | -↓Elongation at break, 451.9 to 229.1% | -GO reduction process result in a good dispersion in PVA matrix | Hu and co-workers (2013)131 |
| -PVA aqueous solution (1.0 g mL−1), 90.2 wt% | -↑Tensile strength, 35.7 to 39.9 Mpa | -Can be applied to food packaging | |||
| -Glycerol (plasticizer), 9 wt% | -↑Thermal stability | ||||
| -Stirred at 95 °C for 24 h | -↑O2 barrier | ||||
| -Dried 2 days, 45 °C, in a glass plate, after 80 °C for 24 h | |||||
| PVA-CS-rGO | -rGO 0.8 wt% | MM | -↓Elongation at break, 102.3 to 76.5% | -GO reduction process result in a good dispersion in CS-PVA matrix | Hu and co-workers (2013)131 |
| -PVA 63.1 wt% | -↑Tensile strength, 46.2 to 64.5 Mpa | -PVA/CS/rGO showed improvement in mechanical and thermal properties | |||
| -CS 27.1 wt% | -↑Thermal stability | -Can be applied to food packaging | |||
| -Glycerol (plasticizer), 9 wt% | -↑O2 barrier | ||||
| -Stirred at 95 °C for 24 h | |||||
| -Dried 2 days at 45 °C, in glass plate, after 80 °C for 24 h | |||||
| PLA/PEI-GO | -PLA: 99.84 wt% | LBL | -↓O2 permeability, ≈99% | -Use on biodegradable packaging materials | He and co-workers (2014)132 |
| -PEI: 0.1 wt% in deionized water | -↑Tensile strength 93.2 to 120.2 MPa | -Increasing GO concentration in the nanocomposite structure can reduce oxygen permeation | |||
| -GO: 0.06 wt% | -↑Elongation at break, 57.5 to 63.3% | -Can be applied to food packaging | |||
| PVA-XGO | -XGO is GO functionalized with oxygen groups | MM | -↑Young's modulus, 27.6 to 37.8 MPa | -The PVA-XGO mechanical and barrier properties depend on the dispersion and the alignment of graphene-based in PVA matrix | Loryuenyog and co-workers (2015)133 |
| -XGO 0.3 wt% | -↑Tensile strength, 25.4 to 37.9 MPa | -Can be applied to food packaging | |||
| -PVA solution aqueous 10wt%, 20 g | -↑Elongation at break, 260 to 317% | ||||
| -↑O2 barrier | |||||
| -↑Water vapor barrier | |||||
| -↑Thermic stability | |||||
| -↑Tg: 51.8 to 53.5 °C | |||||
| -↑Tm: 175.7 to 178.2 °C | |||||
| -↑Crystallinity: 24.8 to 28.0% | |||||
| CH-PAA-rGO | Solvent: deionized water | LBL | -↓O2 permeability: 3.9 × 10−20 cm3 cm−2 Pa−1 s−1 | -Different pH's may affect the thickness of the film and also the permeability of O2 | Grunlan and co-workers (2015)134 |
| -CS: 0.1 wt% (pH = 3.5; 4.5; 5.5) | cm−2 Pa−1 s−1 | -In comparison with PET, the O2 barrier of this nanocomposite is 20 times higher | |||
| -PAA: 0.2 wt% (pH = 3, 4 and 5) | ↓O2 transmission rate: 0,34 cm3 per m2 per day per atm | -The authors performed a thermal reduction of GO to increase the barrier and gas selectivity of this material by increasing the hydrophobicity of the film | |||
| -Expholiated GO: 0.1 wt% (by sonication) | -↑Gas selectivity (H2 and CO2) | -Can be applied to food packaging | |||
| -A support was used to deposite a thin layer of each polymer and GO. | |||||
| -Thermal reduction was performed at 175 °C for 90 min | |||||
| PLA-rGO | -The assembling process comprised a freestanding rGO film between two pieces of PLA film with heat pressing compression: 2000 pounds-force at 65 °C for 30 min | LBL | -↑Hydrophobicity | -Promising solution to food packaging with outstanding environmental sustainability | Chen and co-workers (2016)135 |
| -↑Rresistance towards moisture penetration of 87.6% | |||||
| -↑O2 barrier, 99% | |||||
| GO-CS | -50 mL GO solution (by sonication): obtain a homogeneous and stable dispersion solution | MX | -The spectrum of GO-CS nanocomposites exhibited neither a peak at 1596 cm−1 related to –NH2 absorbance vibration nor a peak at 1730 cm−1 related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of the carboxylic group O stretch of the carboxylic group |
-Can be applied to food packaging | Xu & Liu (2017)93 |
| -CS 1 wt%: dissolving CS in 0.5% (v/v) aqueous acetic acid solution | |||||
| -GO was dropwise into the CS solution | |||||
| -Stirring for 24 h | |||||
| GO-CS-TiO2 | -Ratio GO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CS CS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 (1 TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4): NPs2 4): NPs2 |
MX | -Absorption bands of oxygen-containing functional groups were dramatically reduced | -Non-toxic | Xu & Liu (2017)93 |
| -50 mL GO solution (by sonication, 60 W): obtain a homogeneous and stable dispersion solution | -C–OH and carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands were observed at 1200 and 1729 cm−1, respectively O bands were observed at 1200 and 1729 cm−1, respectively |
-Exhibited significant antimicrobial effects against B. subtilis and A. niger biofilm formation | |||
| -CS 1 wt%: dissolving CS in 0.5% (v/v) aqueous acetic acid solution | -Strong absorption bands at 450 and 670 cm−1, indicating the presence of the Ti–O–Ti bond in TiO2 | -Can be employed as a cling film that effectively delays the loss of moisture in fruits and vegetables | |||
| -GO was dropwise into the CS solution | -Can be applied to food packaging | ||||
| -Stirring for 24 h | |||||
| -NaOH solution: adjusted pH to 6.5 | |||||
| -200 μL 25% glutaraldehyde solution (magnetic stirring overnight) | |||||
| -Centrifuged at 6000 rpm for 1 h | |||||
| -Washed three times | |||||
| -The supernatant was discarded, and the deposit was freeze-dried for 12 h in freeze dryer | |||||
| Cellulose-GO | -Cellulose/16 wt% GO and cellulose/4 wt% GO | MX | -↓O2 permeability, 99.85% | -CPAM prevents the GO self-aggregation in the fiber matrix | Huang and co-workers (2016)32 |
| -Cationic polyacrylamide (CPAM) | -↑Burst strength 81.4% (4 wt% GO) | -Potential packaging applications | |||
-CPAM/GO: 0.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt% 1 wt% |
-Permit the large scale preparation of GO/cellulose paper with high doping amount of GO | ||||
| -Can be applied to food packaging | |||||
| CS0.5CA-Gr (6 wt%) | -2 g CS was suspending in acetic acid solution (0.1 M) | MX | -↑Elastic modulus, 2.5 to 3.8 GPa | -Presence of expanded graphite led to higher values of mechanical properties, likely due to the increase in chain entanglements | Demitri and co-workers (2016)136 |
| -Immersion in thermostatic bath: 25 °C | -↑Fracture strength, 50 to 90 MPa | -Small concentrations of CA are sufficient to functionalize chitosan and are effective against the natural proliferation of mold | |||
| -Mechanical stirrer: 2 h | -↓Elongation at break, 15 to 9% | -Can be applied to food packaging | |||
| -Gr: 6 wt% | -↑Efficiency against mold | ||||
| -CA: 0.5 wt% | |||||
| -Under controlled temperature: 25 °C | |||||
| CS-GO | -Chitosan (CS): water solution 1 v/v% mixed with acetic acid solution 2 wt% | MM | -↑Young's modulus, 22.7 to 5843.7 MPa | -Potential application for food packaging | Advincula and co-workers (2017)137 |
| -GO 0.6 wt% | -↑Tensile strength, 32.4 to 43.27 MPa | -CS-GO showed higher antimicrobial properties than CS | |||
| -Sonication at 60 °C for 1 h, dried at 120 °C overnight | -↑Thermal stability | -CS does not show toxicity for bacteria | |||
| -Can be applied to food packaging | |||||
| PLA-GO | -PLA + GO, 1 wt% | MX | -↑Tensile strength 32.4 to 40.6 Mpa | -Demonstrated antibacterial activity in food packaging | Ahmed and co-workers (2017)89 |
| -GO and PLA mixed and sonicated for 30 min (was added poly ethylene glycol as plasticizer) | -↓Elongation at break, 41 to 31.6% | -Can be applied to food packaging | |||
| -↑Tg, +5 °C | |||||
| -↑Tc, 81.5 to 84.8 °C | |||||
| -↓O2 barrier, 40% | |||||
| Gly-nanocellulose-GO (Glycerol/Nanocellulose/GO = GGN) | - GO 0.5% wt | MM | -↑Tensile strength, 18.4 to 25.0 MPa | -Glycerol and GO in the nanocellulose matrix led to synergistic effects | Arcot and co-workers (2017)138 |
| - GO aqueous solution sonicated for 3 h | -↓Thermal stability | -GGN films show potential to be used in the food package since they can bear heavier products than a zip-lock bag | |||
| -Glycerol 40 wt% (plasticizer) | -↑Elasticity | -Can be applied to food packaging | |||
| -GO, glycerol, and cellulose were mixed | -↑Elongation at break, 1.9 to 9.4% | ||||
| -Films formed in Petri dishes; dried at 40 °C for 2 days | -↑Contact angle of the films | ||||
| -↑Moisture sorption | |||||
| -↑Water vapor permeability | |||||
| -↑O2 barrier | |||||
| OS-rGO (OS: oxidized starch) | -rGO: 1.0 wt% | MM | -↑Tensile strength 58.5 to 17.2 MPa | -The nanocomposite mechanical property can be controlled by the rGO reduction time | Jiang and co-workers (2017)139 |
| -Plasticizing the OS: mixture and stirring (OS- rGO) at 90 °C, 300 rpm for 60 h | -↓O2 permeability | -Can be applied to food packaging | |||
| PLA-CNC/Gr | -PLA/CNC/Gr: 95/0.5/0.5 wt%, prepared by melt compounding | MC | -↑Young's modulus, 8% | -Good GO/matrix dispersion | Montes and co-workers (2018)140 |
| -Stirring 70 rpm at 185 °C for 10 min | -↑Tensile strength, 11% | -Significant improvement in the antifungal activity by Gr | |||
| -↑O2 barrier, 23% | -↑Transparency loss on materials | ||||
| -↑Hydrophobicity | -Potential applications for food packaging | ||||
| -↑Thermic stability | |||||
| -↑Tg: 51.8 to 53.5 °C | |||||
| -↑Tm: 175.7 to 178.2 °C | |||||
| -↑Crystallinity: 24.8 to 28.0% | |||||
| EVOH-GO | -GO: 0.5 wt% | MM | -↑Electrical conductivity | -Excellent GO dispersion on polymeric matrix | Lagaron and co-workers (2018)141 |
| -EVOH: 99.5 wt% | -↑Tg | -Can be used in smart food packaging | |||
| -GO/EVOH sonication 15 min | -↑Tc | ||||
| -Electrospinning process | |||||
| GO-CS | -50 mL GO solution (by sonication): obtain a homogeneous and stable dispersion solution | MX | -The spectrum of GO-CS nanocomposites exhibited neither a peak at 1596 cm−1 related to –NH2 absorbance vibration nor a peak at 1730 cm−1 associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of the carboxylic group O stretch of the carboxylic group |
-Can be applied to food packaging | Xu & Liu (2017)93 |
| -CS 1 wt%: dissolving CS in 0.5% (v/v) aqueous acetic acid solution | |||||
| -GO was dropwise into the CS solution | |||||
| -Stirring for 24 h | |||||
| PLA/starch-Gr | -PLA/starch-Gr, 0.1 wt% of Gr | MM | -↑Elongation at break, 103.4% | -Suitable for food packaging application | Auras and co-workers (2018)142 |
| -Vacuum oven overnight | -↑Toughness, 500–900% | -Can be applied to food packaging | |||
| -The extrusion was pelletized at 50 °C for 4 h | -↓Young's modulus, 1.2 to 0.8 GPa | ||||
| -↓O2 permeability, 50% | |||||
| Starch/gelatina-GO | -Starch/gelatin-GO matrix, 99.15 wt% | MM | -↑Tensile strength 57.97 to 76.09 MPa | -Good candidate for biodegradable food packaging production | Baniasadi and co-workers (2018)31 |
-Starch/gelatin ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
-↑Young's modulus, 20.59 to 35.91 MPa | ||||
| -GO 0.85 wt% | -↓Elongation at break, 6.6 to 3.13% | ||||
| -Sonication for 30 min | -↑Thermal stability | ||||
| -↓Water vapor permeability | |||||
| PVA/Cu2O/TiO2-rGO | -Cu2O–TiO2/rGO | SC | -↑Zone of inhibition | -Environmentally benign | Venkatapras and co-workers (2018)143 |
| -Synthesized by ultrasonic reduction and wet impregnation method using TiO2 NPs, Cu(NO3)2 3H2O, and GO | -↑Antibacterial activity under visible light | -Uniform distribution of the particles in the PVA films | |||
| -PLA-Cu2O–TiO2/rGO | -↑Antimicrobial activity | -Effective antimicrobial activity under visible light against four different microorganisms | |||
| -PVA 3 wt% | -Potential for ambient light food packaging | ||||
| -Cu2O–TiO2/rGO 12.5 mg mL−1 | |||||
| -PLA + Cu2O–TiO2/rGO, solutions were stirred | |||||
| -Dried in Petri plates at room temperature for 48 h | |||||
| PLA-GO | -PLA/0.5 wt% GO (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) 90) |
MM | -↓Water vapor permeability | -Lightweight and strong packaging materials for food and industrial applications | Peijs and co-workers (2018)28 |
| -↑Tm, 165.9 to 170.2 °C | |||||
| -↑Young's modulus, 30% | |||||
| PLA-ST-fGO | -fGO: GO functionalized with maleic anhydride and dodecyl amine | SC | -↑Thermal stability | -Homogeneous dispersion of fGO in PLA-ST matrix | Sheng & Xiong (2019)144 |
| -4 g of PLA + 0.45 g of starch dissolved in chloroform | -↑Crystallization capacity | -Potential versatile nanohybrids for food packaging and pharmaceutical industries | |||
| -5 wt% tributyl citrate | -↓Plasticizer migration rates | ||||
| -Stirred for 1 h | -↑UV shielding capacity | ||||
| -0.4 wt% fGO, added and stirred for 1 h | -↑Hydrophobicity | ||||
| -Dried at 40 °C for 4 days in a polytetrafluorethylene frame | -↑Aging resistance | ||||
| -↑Storage modulus | |||||
| CS-rGO and CS-GO | -GO or rGO + acetic acid solution 1% v/v (water), 20 mL sonication for 9 min | MX | -↓Electric conductivity, graphite 1200 to GO 3.1 S cm−1 and to rGO 60 S cm−1 | -The addition of rGO did not affect the CS matrix hydrophilicity | Malmonge & Basso (2019)30 |
| -CS (0.4 mg) + acetic ácid solution 1% v/v (water), 20 mL | -↓GO and rGO dispersion on CS matrix | -The GO and rGO addition on the CS matrix did not accelerate the composite degradation process | |||
| -GO or rGO suspension was added to CS solution, stirring for 5 min and sonication for 9 min | -Can be applied to food packaging | ||||
| -Solvent remotion and dried | |||||
| Starch/PVA-GO | -Starch/5 wt%, PVA-GO | MX | -↑Tensile strength 18.40 to 25.28 MPa | -Starch/PVA-GO composite film can resist the water molecules migration | Lin & Pu (2019)145 |
| -GO: 2 mg mL | -↓Elongation at break, 184.03 to 143.78% | -Can be applied to food packaging | |||
| -All films should be adjusted before being tested for temperature (T ≈ 24 °C) and relative humidity (≈50%) | - ↑ thermal stability | ||||
| PVA-GA-GO | -22.5 mg GO in water, 2.71 mL, sonication 1 h | MX | -↑Young's modulus 0.66 to 1.55 MPa | -PVA-GA-GO demonstrated antibacterial activity and can be applied to food packaging | Chowdhury & Mah (2020)146 |
| -GO/water suspension was added in PVA-GA matrix and stirred at 6000 rpm 15 min | -↑Tensile strength, 0.66 to 1.51 MPa | ||||
| -Dried overnight, at room temperature, on a glass plate | -↑Thermic stability | ||||
| -↓Water vapor transmission rate, 38.49 to 32.13% | |||||
| PHB-Gr | -0.7 wt% Gr on PHB | MX | -↓O2 permeability, 1.53 to | -Was found to be environmentally safe and highly biodegradable | Pakshirajan & Pugazhenthi (2020)29 |
| -Solvent: chloroform | 0.4 mm per m2 per day2 per atm | -Applications in packaging of light-sensitive food products | |||
| -Gr nanoparticles were dispersed in chloroform (1 mL) by sonication for 45 min (15 s on cycle: 45 s off/cycle) | -↓Water vapor permeability, 9.26 to 4 mm per m2 per day2 per atm | -High strength is desired for the packaging of heavy food items | |||
| -Stirring at 100 rpm for 60 min | -↑Thermal stability | ||||
| -↑Tm, 172.8 to 182.5 °C | |||||
| -↑Tensile strength, 4.5 to 9.0 MPa | |||||
| -↓Elongation at break, 15 to 12.2% | |||||
| -↓Transparency to UV and visible light | |||||
Improvements in polymeric nanocomposites occurred in their thermal, mechanical (high tensile strength, low elongation at break, increase in Young's modulus), and barrier properties (low permeability to water vapor; low permeability to O2). Generally, there is also an excellent dispersion of Gr, GO, or rGO in the polymer matrix, generating environmentally safe and biodegradable nanocomposites. However, the addition of GO or rGO in the polymeric matrices did not accelerate the degradation process of the composite. Many graphene-based polymeric nanocomposites have demonstrated antimicrobial activity and can apply to food packaging.
A comparison between Tables 1 and 2 shows that several studies have been devoted to using renewable polymeric matrix/graphene compared to non-renewable-based composites. This perspective shows the great scientific concern for environmental causes. The creation of new packaging from nanocomposites is one of the most impressive and promising packaging technology results for the near future challenges.124
4. Microbiological properties of graphene-based nanocomposites
Fungal and bacterial transmission in contaminated objects and surfaces is one of the vectors of disease transmission by these microorganisms, although it is not the main transmission route. They adhere to surfaces and colonize the plants, insects, animals, fabrics, food, medical apparatus, packaging, and hard materials. This transmission by adhesion to solid organic and inorganic surfaces is a relevant challenge to public health, epidemiological significance, and economic losses.147 Depending on the surface type and its humidity condition, the microorganisms can survive and grow for extended storage periods, sometimes even months. Removing them is necessary to disinfect and destroy microorganisms by using organic substances and chemical disinfectants that can effectively combat these pathogenic microorganisms.148Another strategy is to use organic and inorganic compounds with fungal and bactericidal activities incorporated in polymeric matrices (fabrics, paper, paints, etc.) to eliminate microorganisms by contact. In this way, graphene and graphene-based materials emerge as a practical pathway to minimize the proliferation of these microorganisms.
Graphene is a biocompatible material with low or no cytotoxicity and promising for food packaging composites, and its oxidized form has different properties and antimicrobial effects in solution and when deposited on surfaces. In that perspective, several studies reported in the literature involving the graphene family and reporting its antibacterial activity148–157 (e.g., fungi and bacteria) – making these materials highly promising for food packaging nanocomposites.158–161
Regarding graphene-family composites, their antibacterial activity can be related to several intrinsic properties emerging from geometric and electronic structures.162 The most common mechanism accepted for the antibacterial activities of graphene is oxidative stress caused in the microorganism by transferring electrons to their membrane and deactivating its protein functions and lipids through the reactive oxygen species (ROS). Furthermore, also can be found studies in the literature reporting that antibacterial activity can occur when phospholipids are extracted by supramolecular interactions with graphene and damage in the permeability of the bacterial cell wall and/or membrane.163,164
Moreover, the antimicrobial properties of the graphene family might be improved by the insertion of molecules by covalent bonds and the deposition of metal ions on its surface. These graphene-based nanocomposites materials may contain nanoparticles of metal ions/oxides/sulfides (manganese disulfide, cadmium sulfide), titanium dioxide, zinc oxide, copper oxide, polymers, antibiotics, among others.163,164
Nanotechnology involving the graphene family and its composites can be a solution for coping with bacteria in various situations. Table 3 shows some selected most recent examples of graphene-based-nanomaterials with high efficiency against bacteria, such as Escherichia coli, Klebsiella pneumoniae, Pseudomonas pneumonia, and P. aeruginosa, Staphylococcus aureus, Staphylococcus pyogenes, and Bacillus subtilis.
| Material | Microorganism | Ref |
|---|---|---|
| Reduced graphene oxide/Ag/Ag2S | E. coli | 165 |
| Reduced graphene oxide/ZnO | E. coli | 166 |
| Graphene oxide | S. aureus | 159 |
| Graphene oxide-catechol | E. coli/S. aureus | 165 |
| Reduced graphene oxide/CuO | E. coli/S. aureus | 166 |
| Graphene oxide cellulose/CuO | S. aureus, B. subtilis, E. coli/P. aeruginosa | 167 |
| Graphene oxides NiS–MoO3 | E. coli/S. pyogenes | 168 |
| Reduced graphene oxide/Ag | E. coli/K. pneumoniae | 169 |
| Reduced graphene oxide/Ag | S. aureus/E. coli/P. aeruginosa | 170 |
| Graphene oxide | E. coli K12 | 171 |
| Graphene oxide/p-aminophenol | E. coli/S. aureus | 172 |
| Graphene oxide/modified sodium anthraquinone-2-sulfonate | E. coli | 173 |
| Graphene oxide chloramine bromosuccinimide/FeCl3/KIO3 | P. pneumonia/S. aureus | 174 |
| Graphene Oxide-chitosan/Ag | Pseudomonas sp | 175 |
| Graphene oxide/Ag | E. coli/S. aureus | 176 |
| Graphene oxide/ampicillin, chloramphenicol, or tetracycline | P. aeruginosa | 177 |
| Graphene oxide/NiS–In2O3 | E. coli/S. aureus | 178 |
Despite the importance of graphene-based nanocomposites for safer packaging with resistance against microorganisms, it is noteworthy to mention that the specialized literature lacks in filling the gaps regarding the risks of nanomaterials used. In this way, information regarding their interaction with cellular components, migration to the food, tolerance dosage levels, toxicity to the human organism, long-term exposure, and toxicity to ecologically organisms when disposed of incorrectly.
5. Graphene-based nanocomposites – toxicity and (bio)degradability
Graphene and its derivatives (GD) have chemical, mechanical, electrical, and optical properties of interest for different applications such as biotechnology, biosensors, biomedicine, nanocomposites, catalysis, and drug delivery systems, metal detection, and removal, among others. Because of this, many studies are being developed to assess the effects of GD toxicity on the environment and the human body.29 GD toxicity is influenced by the shape, size, concentration, synthesis methods, and type of functional groups. For example, the PHB/graphene nanocomposite showed promising results in improving the food packaging life and high biodegradability. However, graphene in polymer nanocomposites can be released into the air and groundwater depending on size, shape, and concentration.155,179,180In the formulation of nanocomposite PVA/gelatin was added to cellulose nanocrystals to make food packaging. The addition of cellulose nanocrystals in the nanocomposite showed improved properties (mechanical, thermal, and barrier). The results indicate that PVA-gelatin films reinforced with cellulose nanocrystals can be considered a potential biodegradable packaging material, especially for food packaging.63,181 They can be tested in the construction of graphene-based polymeric nanocomposites.
Although the excellent adsorption capacity of graphene allows the capture of organic contaminants in aqueous media, DGs can promote the spread of these contaminants in groundwater, where DGs are more unstable and have greater mobility. Because of this, some research has been developed to evaluate the effects of these materials on aquatic organisms. It has been observed that the cell division of photosynthetic microorganisms is inhibited as the concentration of GD is high. On the other hand, invertebrates present several changes: changes in biochemical performances, the survival rate and inhibition of the swimming behavior of crustaceans, and alterations in membranes. The impact observed in zebrafish was from inhibition of embryo growth to cardiac development.182
Graphene and its derivatives have excellent properties such as good dispersibility and stability in human physiological environments, making them promising for application in biomedicine, drug delivery systems, and food packaging. However, studies of GD toxicity in human biological organisms are not conclusive. Yang's group (2013) performed biodistribution and toxicology nanographene oxide analyses in mice, by oral and intraperitoneal administration. The researchers noted that the GDs were not adsorbed by the organs but were easily excreted. Despite this, they emphasize that toxicity will depend on the surface coating, size, and routes of administration.183 Liu et al. (2012) conducted a study of size and dose on the biodistribution of GO in mice. The results showed that sizes between 1 and 5 μm accumulate in the lungs and between 110 and 500 nm in the liver. Therefore, they concluded that GO is not suitable for human use.184,185
On the other hand, some research shows that GD effectively fights cancer cells and is safe for healthy cells. The administered dose influences the cytotoxicity and apoptosis of human cells; that is, the use of small doses (<50 mg L−1) of these materials proved to be safe.186–188 In addition, Manikandan et al. (2020) considered that PHB/graphene nanocomposites have a negligible cytotoxic effect.1 Thus, the toxicity of GD in human cells still needs thorough research, as there are few publications on genotoxicity tests and lots of contradictory information.
One of the biggest challenges in this area is producing Gr, GO and rGO by greener processes and on an industrial scale to formulate polymeric nanocomposites that are increasingly less toxic and aggressive to the environment through greener and more sustainable routes, Fig. 12.
Final considerations
The development of novel packaging from polymeric nanocomposites based on graphene opens several possibilities for the food packaging industry and reduces production costs. It also brings lightness and higher resistance to these novel packaging resulting in numerous applications. The Gr, GO, and rGO application in nanocomposites significantly improves the packaging's thermal, mechanical, and barrier properties, reducing waste and increasing food safety. The more significant interest in using renewable polymers in the novel polymeric nanocomposites creation with the use of origin natural and biodegradable polymeric matrices and a smaller number of investigations using polymers is also noticeable of fossil origin, showing the concern with the environment. In general, studies are still lacking on the application of graphene nanocomposite packaging in wet foods and also on its toxicity and biodegradability, suggesting that further research in this regard should be carried out to ensure the safety and efficiency application of graphene and graphene-based in the formulation of new food packaging.Author contributions
The authors confirm contribution to the paper as follows: conceptualization: Vinicius Rossa and Thiago M. Lima. investigation: Vinicius Rossa, Luanne E. Monteiro, Sancler C. Vasconcelos and Vinicius G. Madriaga. Data curation: Vinicius Rossa, Luanne E. Monteiro, Sancler da Costa Vasconcelos, Vinicius G. Madriaga, Eric Thomas Tai Shimabukuro, Anna Paula Carvalho, Vitor Francisco Ferreira and Thiago M. Lima. Writing – original draft preparation: Vinicius Rossa, Luanne E. Monteiro, Sancler C. Vasconcelos, Anna Paula Carvalho, Sibele Berenice Castellã Pergher and Thiago M. Lima. Visualization: Thiago M. Lima, Vinicius Rossa, Vinicius G. Madriaga, Sancler C. Vasconcelos, Sibele Berenice Castellã Pergher and Thiago M. Lima. Writing – review & editing: Thiago M. Lima, Vinicius Rossa, Sibele Berenice Castellã Pergher, Fernando de Carvalho da Silva, Vitor Francisco Ferreira, Anna Paula Carvalho and Carlos Adam Conte Junior. Supervision: Thiago M. Lima and Vinicius Rossa.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 and FAPERJ (E-26/010.000.984/2019; E-26/211.575/2019, E-26/201.358/2021), IQ/UFRJ, IQ/UFF and IQ/UFRN.References
- H. N. Lim, N. M. Huang and C. H. Loo, J. Non-Cryst. Solids, 2012, 358, 525–530 CrossRef CAS.
- D. Han, L. Yan, W. Chen and W. Li, Carbohydr. Polym., 2011, 83, 653–658 CrossRef CAS.
- J. Jia, Y. Gai, W. Wang and Y. Zhao, Ultrason. Sonochem., 2016, 32, 300–306 CrossRef CAS PubMed.
- Ecosustainable Polymer Nanomaterials for Food Packaging, ed. C. Silvestre and S. Cimmino, CRC Press, 2013 Search PubMed.
- V. G. L. Souza and A. L. Fernando, Food Packag. Shelf Life, 2016, 8, 63–70 CrossRef.
- H. Bouwmeester, S. Dekkers, M. Y. Noordam, W. I. Hagens, A. S. Bulder, C. de Heer, S. E. C. G. ten Voorde, S. W. P. Wijnhoven, H. J. P. Marvin and A. J. A. M. Sips, Regul. Toxicol. Pharmacol., 2009, 53, 52–62 CrossRef CAS PubMed.
- R. Mahfouz, F. J. Cadete Santos Aires, A. Brenier, E. Ehret, M. Roumié, B. Nsouli, B. Jacquier and J. C. Bertolini, J. Nanopart. Res., 2010, 12, 3123–3136 CrossRef CAS.
- D. Cade, E. Ramus, M. Rinaudo, R. Auzély-Velty, T. Delair and T. Hamaide, Biomacromolecules, 2004, 5, 922–927 CrossRef CAS PubMed.
- A. Haase, A. Mantion, P. Graf, J. Plendl, A. F. Thuenemann, W. Meier, A. Taubert and A. Luch, Arch. Toxicol., 2012, 86, 1089–1098 CrossRef CAS PubMed.
- P. R. Sivashankari and M. Prabaharan, in Biopolymer-Based Composites, Elsevier, 2017, pp. 381–397 Search PubMed.
- C. Botas, P. Álvarez, P. Blanco, M. Granda, C. Blanco, R. Santamaría, L. J. Romasanta, R. Verdejo, M. A. López-Manchado and R. Menéndez, Carbon, 2013, 65, 156–164 CrossRef CAS.
- S. S. Nanda, G. C. Papaefthymiou and D. K. Yi, Crit. Rev. Solid State Mater. Sci., 2015, 40, 291–315 CrossRef CAS.
- H. Ahmad, M. Fan and D. Hui, Composites, Part B, 2018, 145, 270–280 CrossRef CAS.
- X. Wang, M. Su, C. Liu, C. Shen and X. Liu, J. Renewable Mater., 2020, 8, 89–99 Search PubMed.
- M. J. Nine, M. A. Cole, D. N. H. Tran and D. Losic, J. Mater. Chem. A, 2015, 3, 12580–12602 RSC.
- T. Gatti, N. Vicentini, M. Mba and E. Menna, Eur. J. Org. Chem., 2016, 2016, 1071–1090 CrossRef CAS.
- S. Park and R. S. Ruoff, Curr. Opin. Colloid Interface Sci., 2015, 20, 322–328 CrossRef CAS.
- C. K. Chua and M. Pumera, Chem. Soc. Rev., 2014, 43, 291–312 RSC.
- J. R. Potts, D. R. Dreyer, C. W. Bielawski and R. S. Ruoff, Polymer, 2011, 52, 5–25 CrossRef CAS.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- T. Sattar, Top. Curr. Chem., 2019, 377, 10 CrossRef PubMed.
- S. S. Shams, L. S. Zhang, R. Hu, R. Zhang and J. Zhu, Mater. Lett., 2015, 161, 476–479 CrossRef CAS.
- X. Kong, Y. Zhu, H. Lei, C. Wang, Y. Zhao, E. Huo, X. Lin, Q. Zhang, M. Qian, W. Mateo, R. Zou, Z. Fang and R. Ruan, Chem. Eng. J., 2020, 399, 125808 CrossRef CAS.
- K. Kalaitzidou, H. Fukushima and L. T. Drzal, Carbon, 2007, 45, 1446–1452 CrossRef CAS.
- A. F. Ghanem, A. M. Youssef and M. H. Abdel Rehim, J. Mater. Sci., 2020, 55, 4685–4700 CrossRef CAS.
- A. Al-Jabareen, H. Al-Bustami, H. Harel and G. Marom, J. Appl. Polym. Sci., 2012 Search PubMed.
- H. M. Kim, J. K. Lee and H. S. Lee, Thin Solid Films, 2011, 519, 7766–7771 CrossRef CAS.
- Y. Gao, O. T. Picot, W. Tu, E. Bilotti and T. Peijs, J. Appl. Polym. Sci., 2018, 135, 46041 CrossRef.
- N. A. Manikandan, K. Pakshirajan and G. Pugazhenthi, Int. J. Biol. Macromol., 2020, 154, 866–877 CrossRef CAS PubMed.
- T. G. Maraschin, R. da S. Correa, L. F. Rodrigues, N. M. Balzarettid, J. A. Malmonge, G. B. Galland and N. R. de S. Basso, Mater. Res., 2019, 221–10 Search PubMed.
- S. Afshar and H. Baniasadi, Int. J. Biol. Macromol., 2018, 109, 1019–1028 CrossRef CAS PubMed.
- Q. Huang, M. Xu, R. Sun and X. Wang, Ind. Crops Prod., 2016, 85, 198–203 CrossRef CAS.
- A. Allahbakhsh, in Food Packaging, Elsevier, 2017, pp. 699–737 Search PubMed.
- T. B. Rouf and J. L. Kokini, J. Mater. Sci., 2016, 51, 9915–9945 CrossRef CAS.
- T. B. Rouf and J. L. Kokini, in Bionanocomposites for Packaging Applications, Springer International Publishing, Cham, 2018, pp. 149–177 Search PubMed.
- S. S. Sablani, Polymer Nanocomposites for Food Packaging Applications, in Food Nanoscience and Nanotechnology, Food Engineering Series, ed. H. Hernández-Sánchez and G. Gutiérrez-López, Springer, Cham, 2015, pp. 205–212 Search PubMed.
- Graphene Properties, Preparation, Characterization and Applications, N. C. Chhetri, S. Kuila and T. Murmu, Wiley-VCH Verlag GmbH & Co. KGaA, Published 2016 by Wiley-VCH Verlag GmbH & Co. KGaA., 1st edn, 2016, pp. 63–111 Search PubMed.
- M. Bera and P. K. Maji, MOJ Polym. Sci, 2017, 1, 94–97 Search PubMed.
- Z. Terzopoulou, G. Kyzas and D. Bikiaris, Materials, 2015, 8, 652–683 CrossRef CAS PubMed.
- J. Byun, J. Microbiol. Biotechnol., 2015, 25, 145–151 CrossRef CAS PubMed.
- S. Cesur, C. Köroğlu and H. T. Yalçın, J. Vinyl Addit. Technol., 2018, 24, 376–387 CrossRef CAS.
- S. Tripathi, G. K. Mehrotra and P. K. Dutta, e-Polymers, 2008, 93, 1–7 Search PubMed.
- V. Manigandan, R. Karthik, S. Ramachandran and S. Rajagopal, in Biopolymers for Food Design, Elsevier, 2018, pp. 469–491 Search PubMed.
- M. Aider, LWT--Food Sci. Technol., 2010, 43, 837–842 CrossRef CAS.
- S. Omidi and A. Kakanejadifard, RSC Adv., 2018, 8, 12179–12189 RSC.
- C. Cavalcanti and C. Chagas, História Da Embalagem No Brasil, 1st edn, 2006 Search PubMed.
- K. Marsh and B. Bugusu, J. Food Sci., 2007, 72, R39–R55 CrossRef CAS PubMed.
- A. Ojha, A. Sharma, M. Sihag and S. Ojha, Agric. Rev., 2015, 36, 241 CrossRef.
- B. Page, in Packaging Technology, Elsevier, 2012, pp. 122–162 Search PubMed.
- D. Raheem, Emir. J. Food Agric., 2013, 25, 177 CrossRef.
- A. L. Brody, B. Bugusu, J. H. Han, C. K. Sand and T. H. McHugh, J. Food Sci., 2008, 73, R107–R116 CrossRef CAS PubMed.
- L. McKeen, in The Effect of Sterilization Methods on Plastics and Elastomers, Elsevier, 2018, pp. 417–435 Search PubMed.
- B. Rodgers and W. Waddell, in The Science and Technology of Rubber, Elsevier, 2013, pp. 653–695 Search PubMed.
- A. Sharif and M. E. Hoque, in Bio-based Polymers and Nanocomposites, Springer International Publishing, Cham, 2019, pp. 1–28 Search PubMed.
- A. P. M. Landim, C. O. Bernardo, I. B. A. Martins, M. R. Francisco, M. B. Santos and N. R. de Melo, Polímeros, 2016, 26, 82–92 CrossRef.
- H. Moustafa, A. M. Youssef, N. A. Darwish and A. I. Abou-Kandil, Composites, Part B, 2019, 172, 16–25 CrossRef CAS.
- A. Akelah, Functionalized Polymeric Materials in Agriculture and the Food Industry, Springer US, Boston, MA, 2013, vol. 9781461470 Search PubMed.
- F. Ciardelli, M. Bertoldo, S. Bronco and E. Passaglia, Polymers from Fossil and Renewable Resources, Springer International Publishing, Cham, 2019 Search PubMed.
- V. Siracusa, P. Rocculi, S. Romani and M. D. Rosa, Trends Food Sci. Technol., 2008, 19, 634–643 CrossRef CAS.
- F. A. MEDEIROS and H. WIEBECK, Polim.: Cienc. Tecnol., 2013, 23, 636–643 CrossRef CAS.
- D. Turan, G. Gunes and A. Kilic, in Bionanocomposites for Packaging Applications, Springer International Publishing, Cham, 2018, pp. 1–32 Search PubMed.
- D. W. Wei, H. Wei, A. C. Gauthier, J. Song, Y. Jin and H. Xiao, J. Bioresour. Bioprod., 2020, 5, 1–15 CrossRef CAS.
- L. Zhao, G. Duan, G. Zhang, H. Yang, S. Jiang and S. He, Nanomaterials, 2020, 10, 150 CrossRef CAS PubMed.
- N. Kumar, P. Kaur and S. Bhatia, Nutr. Food Sci., 2017, 47, 591–606 CrossRef.
- A. Morschbacker, Polym. Rev., 2009, 49, 79–84 CrossRef CAS.
- M. Akiyama, S. Sato, R. Takahashi, K. Inui and M. Yokota, Appl. Catal., A, 2009, 371, 60–66 CrossRef CAS.
- J. Chaminand, L. auren. Djakovitch, P. Gallezot, P. Marion, C. Pinel and C. Rosier, Green Chem., 2004, 6, 359 RSC.
- D. G. Lahr and B. H. Shanks, Ind. Eng. Chem. Res., 2003, 42, 5467–5472 CrossRef CAS.
- A.-Y. Yin, X.-Y. Guo, W.-L. Dai and K.-N. Fan, Green Chem., 2009, 11, 1514 RSC.
- Nanotechnologies in Food and Agriculture, ed. M. Rai, C. Ribeiro, L. Mattoso and N. Duran, Springer International Publishing, Cham, 2015 Search PubMed.
- U. Bhardwaj, P. Dhar, A. Kumar and V. Katiyar, Food Additives and Packaging, 2014, ch. 19, pp. 275–314 Search PubMed.
- R. Sindhu, P. Binod and A. Pandey, in Industrial Biorefineries & White Biotechnology, Elsevier, 2015, pp. 575–605 Search PubMed.
- K. B. Rufato, J. P. Galdino, K. S. Ody, A. G. B. Pereira, E. Corradini, A. F. Martins, A. T. Paulino, A. R. Fajardo, F. A. Aouada, F. A. La Porta, A. F. Rubira and E. C. Muniz, in Hydrogels - Smart Materials for Biomedical Applications, IntechOpen, 2019 Search PubMed.
- S. Talegaonkar, H. Sharma, S. Pandey, P. K. Mishra and R. Wimmer, in Food Packaging, Elsevier, 2017, pp. 79–110 Search PubMed.
- H. Wang, J. Qian and F. Ding, J. Agric. Food Chem., 2018, 66, 395–413 CrossRef CAS PubMed.
- M. Sabzevari, D. E. Cree and L. D. Wilson, ACS Omega, 2018, 3, 13045–13054 CrossRef CAS PubMed.
- U. Siripatrawan and W. Vitchayakitti, Food Hydrocolloids, 2016, 61, 695–702 CrossRef CAS.
- Y. Peng, Y. Wu and Y. Li, Int. J. Biol. Macromol., 2013, 59, 282–289 CrossRef CAS PubMed.
- B. Kuswandi, Nanotechnology in Food Packaging, in Nanoscience in Food and Agriculture 1. Sustainable Agriculture Reviews, ed. S. Ranjan, N. Dasgupta and E. Lichtfouse, Springer, Cham, 2016, vol. 20, pp. 151–183 Search PubMed.
- Y. Huang, T. Wang, X. Zhao, X. Wang, L. Zhou, Y. Yang, F. Liao and Y. Ju, J. Chem. Technol. Biotechnol., 2015, 90, 1677–1684 CrossRef CAS.
- I. U. Unalan, C. Wan, Ł. Figiel, R. T. Olsson, S. Trabattoni and S. Farris, Nanotechnology, 2015, 26, 275703 CrossRef PubMed.
- S. F. Abdellah Ali, IOP Conf. Ser.: Mater. Sci. Eng., 2016, 137, 012035 Search PubMed.
- S. Kashi, R. K. Gupta, N. Kao, S. A. Hadigheh and S. N. Bhattacharya, J. Mater. Sci. Technol., 2018, 34, 1026–1034 CrossRef CAS.
- A. P. A. de Carvalho and C. A. Conte Junior, Trends Food Sci. Technol., 2020, 103, 130–143 CrossRef.
- J. Wang, X. Wang, C. Xu, M. Zhang and X. Shang, Polym. Int., 2011, 60, 816–822 CrossRef CAS.
- R. Zhang, Y. Wang, D. Ma, S. Ahmed, W. Qin and Y. Liu, Ultrason. Sonochem., 2019, 59, 104731 CrossRef PubMed.
- M. A. Cerqueira, A. A. Vicente and L. M. Pastrana, in Nanomaterials for Food Packaging, Elsevier, 2018, pp. 1–11 Search PubMed.
- A. Usman, Z. Hussain, A. Riaz and A. N. Khan, Carbohydr. Polym., 2016, 153, 592–599 CrossRef CAS PubMed.
- Y. A. Arfat, J. Ahmed, M. Ejaz and M. Mullah, Int. J. Biol. Macromol., 2018, 107, 194–203 CrossRef CAS PubMed.
- A. K. Sundramoorthy, T. H. Vignesh Kumar and S. Gunasekaran, in Graphene Bioelectronics, Elsevier, 2018, pp. 267–306 Search PubMed.
- A. Barra, N. M. Ferreira, M. A. Martins, O. Lazar, A. Pantazi, A. A. Jderu, S. M. Neumayer, B. J. Rodriguez, M. Enăchescu, P. Ferreira and C. Nunes, Compos. Sci. Technol., 2019, 173, 53–60 CrossRef CAS.
- A. L. Rivera-Briso, F. L. Aachmann, V. Moreno-Manzano and Á. Serrano-Aroca, Int. J. Biol. Macromol., 2020, 143, 1000–1008 CrossRef CAS PubMed.
- W. Xu, W. Xie, X. Huang, X. Chen, N. Huang, X. Wang and J. Liu, Food Chem., 2017, 221, 267–277 CrossRef CAS PubMed.
- N. Pal, S. Banerjee, P. Roy and K. Pal, Mater. Sci. Eng., C, 2019, 104, 109956 CrossRef CAS PubMed.
- D. Olteanu, A. Filip, C. Socaci, A. R. Biris, X. Filip, M. Coros, M. C. Rosu, F. Pogacean, C. Alb, I. Baldea, P. Bolfa and S. Pruneanu, Colloids Surf., B, 2015, 136, 791–798 CrossRef CAS PubMed.
- J. Du and H.-M. Cheng, Macromol. Chem. Phys., 2012, 213, 1060–1077 CrossRef CAS.
- J. Jose, M. A. Al-Harthi, M. A.-A. AlMa’adeed, J. Bhadra Dakua and S. K. De, J. Appl. Polym. Sci., 2015, 132, 41827 CrossRef.
- F. Li, H.-Y. Yu, Y.-Y. Wang, Y. Zhou, H. Zhang, J.-M. Yao, S. Y. H. Abdalkarim and K. C. Tam, J. Agric. Food Chem., 2019, 67, 10954–10967 CrossRef CAS PubMed.
- E. C. Romani, D. G. Larrude, L. Nachez, C. Vilani, J. B. de Campos, S. B. Peripolli and F. L. Freire, Tribol. Lett., 2017, 65, 96 CrossRef.
- A. P. Kauling, A. T. Seefeldt, D. P. Pisoni, R. C. Pradeep, R. Bentini, R. V. B. Oliveira, K. S. Novoselov and A. H. Castro Neto, Adv. Mater., 2018, 30, 1803784 CrossRef PubMed.
- J. Wang, F. Ma, W. Liang and M. Sun, Mater. Today Phys., 2017, 2, 6–34 CrossRef.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- Z. U. Khan, A. Kausar, H. Ullah, A. Badshah and W. U. Khan, J. Plast. Film Sheeting, 2016, 32, 336–379 CrossRef CAS.
- G. Venugopal, K. Krishnamoorthy, R. Mohan and S.-J. Kim, Mater. Chem. Phys., 2012, 132, 29–33 CrossRef CAS.
- Y. Yao, X. Chen, J. Zhu, B. Zeng, Z. Wu and X. Li, Nanoscale Res. Lett., 2012, 7, 363 CrossRef PubMed.
- M. Jin, H.-K. Jeong, W. J. Yu, D. J. Bae, B. R. Kang and Y. H. Lee, J. Phys. D. Appl. Phys., 2009, 42, 135109 CrossRef.
- A. Haque, M. A.-A. Mamun, M. F. N. Taufique, P. Karnati and K. Ghosh, IEEE Trans. Semicond. Manuf., 2018, 31, 535–544 Search PubMed.
- L. Staudenmaier, Eur. J. Inorg. Chem., 1898, 31, 1481–1487 CAS.
- B. C. Brodie, Proc. R. Soc. London, 1860, 10, 11–12 CrossRef.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- D. Li, M. B. Müller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- J. Chen, B. Yao, C. Li and G. Shi, Carbon, 2013, 64, 225–229 CrossRef CAS.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- N. E. Sorokina, M. A. Khaskov, V. V. Avdeev and I. V. Nikol’skaya, Russ. J. Gen. Chem., 2005, 75, 162–168 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- M. J. McAllister, J. Li, D. H. Adamson, H. C. Schniepp, A. a Abdala, J. Liu, M. Herrera-Alonso, D. L. Milius, R. Car, R. K. Prud’homme and I. a Aksay, Chem. Mater., 2007, 19, 4396–4404 CrossRef CAS.
- A. Bagri, C. Mattevi, M. Acik, Y. J. Chabal, M. Chhowalla and V. B. Shenoy, Nat. Chem., 2010, 2, 581–587 CrossRef CAS PubMed.
- J. George, Y. Patel, S. M. Pillai and P. Munshi, J. Mol. Catal. A: Chem., 2009, 304, 1–7 CrossRef CAS.
- J. Li and T. Wang, J. Chem. Thermodyn., 2011, 43, 731–736 CrossRef CAS.
- C. K. Rofer-DePoorter, Chem. Rev., 1981, 81, 447–474 CrossRef CAS.
- A. P. Amrute, K. Jeske, Z. Łodziana, G. Prieto and F. Schüth, Chem. Mater., 2020, 32, 4369–4374 CrossRef CAS.
- J. Zhang, H. Yang, G. Shen, P. Cheng, J. Zhang and S. Guo, Chem. Commun., 2010, 46, 1112–1114 RSC.
- J. Gao, F. Liu, Y. Liu, N. Ma, Z. Wang and X. Zhang, Chem. Mater., 2010, 22, 2213–2218 CrossRef CAS.
- I. Uysal Unalan, G. Cerri, E. Marcuzzo, C. A. Cozzolino and S. Farris, RSC Adv., 2014, 4, 29393–29428 RSC.
- R. Miraftab and H. Xiao, J. Bioresour. Bioprod., 2019, 4, 200–201 CAS.
- H. Qian, J. Wang and L. Yan, J. Bioresour. Bioprod., 2020, 5, 204–210 CrossRef CAS.
- X. Ji, Y. Xu, W. Zhang, L. Cui and J. Liu, Composites, Part A, 2016, 87, 29–45 CrossRef CAS.
- Y. Joo, V. Agarkar, S. H. Sung, B. M. Savoie and B. W. Boudouris, Science, 2018, 359, 1391–1395 CrossRef CAS PubMed.
- X. Yang, Y. Tu, L. Li, S. Shang and X. Tao, ACS Appl. Mater. Interfaces, 2010, 2, 1707–1713 CrossRef CAS PubMed.
- A. M. Pinto, J. Cabral, D. A. P. Tanaka, A. M. Mendes and F. D. Magalhães, Polym. Int., 2013, 62, 33–40 CrossRef CAS.
- X. Feng, X. Wang, W. Xing, B. Yu, L. Song and Y. Hu, Ind. Eng. Chem. Res., 2013, 52, 12906–12914 CrossRef CAS.
- L.-L. Wu, J. Wang, X. He, T. Zhang and H. Sun, Packag. Technol. Sci., 2014, 27, 693–700 CrossRef CAS.
- R. A. Hurley, A. Ouzts, J. Fischer and T. Gomes, Packag. Technol. Sci., 2013, 26, 399–412 CrossRef.
- P. Tzeng, B. Stevens, I. Devlaming and J. C. Grunlan, Langmuir, 2015, 31, 5919–5927 CrossRef CAS PubMed.
- K. Goh, J. K. Heising, Y. Yuan, H. E. Karahan, L. Wei, S. Zhai, J.-X. Koh, N. M. Htin, F. Zhang, R. Wang, A. G. Fane, M. Dekker, F. Dehghani and Y. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 9994–10004 CrossRef CAS PubMed.
- C. Demitri, V. M. De Benedictis, M. Madaghiele, C. E. Corcione and A. Maffezzoli, Measurement, 2016, 90, 418–423 CrossRef.
- C. D. Grande, J. Mangadlao, J. Fan, A. De Leon, J. Delgado-Ospina, J. G. Rojas, D. F. Rodrigues and R. Advincula, Macromol. Symp., 2017, 374, 1600114 CrossRef.
- R. H. F. Faradilla, G. Lee, J. Roberts, P. Martens, M. Stenzel and J. Arcot, Cellulose, 2018, 25, 399–416 CrossRef CAS.
- X. Ge, H. Li, L. Wu, P. Li, X. Mu and Y. Jiang, J. Appl. Polym. Sci., 2017, 134, 44910 CrossRef.
- S. Montes, A. Etxeberria, V. Mocholi, A. Rekondo, H. Grande and J. Labidi, eXPRESS Polym. Lett., 2018, 12, 543–555 CrossRef CAS.
- S. Torres-Giner, Y. Echegoyen, R. Teruel-Juanes, J. Badia, A. Ribes-Greus and J. Lagaron, Nanomaterials, 2018, 8, 745 CrossRef PubMed.
- A. Bher, I. Uysal Unalan, R. Auras, M. Rubino and C. Schvezov, Polymers, 2018, 10, 95 CrossRef PubMed.
- M. Dhanasekar, V. Jenefer, R. B. Nambiar, S. G. Babu, S. P. Selvam, B. Neppolian and S. V. Bhat, Mater. Res. Bull., 2018, 97, 238–243 CrossRef CAS.
- P. Wang, S. Tang, F. Sheng, J. Cai, P. Fei, A. Nawaz, N. Walayat, A. B. Javaid and H. Xiong, Int. J. Biol. Macromol., 2019, 132, 1208–1220 CrossRef CAS PubMed.
- Z. Wu, Y. Huang, L. Xiao, D. Lin, Y. Yang, H. Wang, Y. Yang, D. Wu, H. Chen, Q. Zhang, W. Qin and S. Pu, Int. J. Biol. Macromol., 2019, 123, 569–575 CrossRef CAS PubMed.
- S. Chowdhury, Y. L. Teoh, K. M. Ong, N. S. Rafflisman Zaidi and S.-K. Mah, Food Packag. Shelf Life, 2020, 24, 100463 CrossRef.
- M. Moritz and M. Geszke-Moritz, Chem. Eng. J., 2013, 228, 596–613 CrossRef CAS.
- Q. Yu, Z. Wu and H. Chen, Acta Biomater., 2015, 16, 1–13 CrossRef CAS PubMed.
- A. M. Pinto, I. C. Gonçalves and F. D. Magalhães, Colloids Surf., B, 2013, 111, 188–202 CrossRef CAS PubMed.
- G. Wu and P. Xu, in Multifunctional Nanocomposites for Energy and Environmental Applications, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2018, pp. 203–230 Search PubMed.
- X. Ren, H. Guo, J. Feng, P. Si, L. Zhang and L. Ci, Chemosphere, 2018, 191, 389–399 CrossRef CAS PubMed.
- S. C. Smith and D. F. Rodrigues, Carbon, 2015, 91, 122–143 CrossRef CAS.
- K. Tadyszak, J. Wychowaniec and J. Litowczenko, Nanomaterials, 2018, 8, 944 CrossRef PubMed.
- S. Asha, A. N. Ananth, S. P. Jose and M. A. J. Rajan, Appl. Nanosci., 2018, 8, 395–405 CrossRef CAS.
- Q. Zhang, Z. Wu, N. Li, Y. Pu, B. Wang, T. Zhang and J. Tao, Mater. Sci. Eng., C, 2017, 77, 1363–1375 CrossRef CAS PubMed.
- S. Goenka, V. Sant and S. Sant, J. Control. Release, 2014, 173, 75–88 CrossRef CAS PubMed.
- X. Zhu, A. F. Radovic-Moreno, J. Wu, R. Langer and J. Shi, Nano Today, 2014, 9, 478–498 CrossRef CAS PubMed.
- G. Franci, A. Falanga, S. Galdiero, L. Palomba, M. Rai, G. Morelli and M. Galdiero, Molecules, 2015, 20, 8856–8874 CrossRef CAS PubMed.
- I. Sengupta, P. Bhattacharya, M. Talukdar, S. Neogi, S. K. Pal and S. Chakraborty, Colloid Interface Sci. Commun., 2019, 28, 60–68 CrossRef CAS.
- S. Aliamradni, V. Abolmaali and S. S. Borandeh, J. Nanostruct., 2019, 3, 402–413 Search PubMed.
- P. Kumar, P. Huo, R. Zhang and B. Liu, Nanomaterials, 2019, 9, 737 CrossRef CAS PubMed.
- S. Gurunathan, J. Woong Han, A. Abdal Daye, V. Eppakayala and J. Kim, Int. J. Nanomed., 2012, 5901 CrossRef CAS PubMed.
- S. Romero-Vargas Castrillón, F. Perreault, A. F. de Faria and M. Elimelech, Environ. Sci. Technol. Lett., 2015, 2, 112–117 CrossRef.
- Y. Tu, M. Lv, P. Xiu, T. Huynh, M. Zhang, M. Castelli, Z. Liu, Q. Huang, C. Fan, H. Fang and R. Zhou, Nat. Nanotechnol., 2013, 8, 594–601 CrossRef CAS PubMed.
- P. Huo, C. Liu, D. Wu, J. Guan, J. Li, H. Wang, Q. Tang, X. Li, Y. Yan and S. Yuan, J. Ind. Eng. Chem., 2018, 57, 125–133 CrossRef CAS.
- L. T. Trinh, L. A. B. Quynh and N. H. Hieu, Int. J. Nanotechnol., 2018, 15, 108 CrossRef.
- Q. Tu, Q. Zhang, Y. Wang, Y. Jiao, J. Xiao, T. Peng and J. Wang, Prog. Org. Coatings, 2019, 129, 247–253 CrossRef CAS.
- Z. Yang, X. Hao, S. Chen, Z. Ma, W. Wang, C. Wang, L. Yue, H. Sun, Q. Shao, V. Murugadoss and Z. Guo, J. Colloid Interface Sci., 2019, 533, 13–23 CrossRef CAS PubMed.
- Y.-Y. Xie, X.-H. Hu, Y.-W. Zhang, F. Wahid, L.-Q. Chu, S.-R. Jia and C. Zhong, Carbohydr. Polym., 2020, 229, 115456 CrossRef CAS PubMed.
- M. A. Ashraf, Y. Yang and A. Fakhri, Ceram. Int., 2020, 46, 8379–8384 CrossRef CAS.
- S. Tan, X. Wu, Y. Xing, S. Lilak, M. Wu and J. X. Zhao, Colloids Surf., B, 2020, 185, 110616 CrossRef CAS PubMed.
- N. M. Dat, P. N. B. Long, D. C. U. Nhi, N. N. Minh, L. M. Duy, L. N. Quan, H. M. Nam, M. T. Phong and N. H. Hieu, Synth. Met., 2020, 260, 116260 CrossRef CAS.
- R. K. Matharu, T. A. Tabish, T. Trakoolwilaiwan, J. Mansfield, J. Moger, T. Wu, C. Lourenço, B. Chen, L. Ciric, I. P. Parkin and M. Edirisinghe, J. Colloid Interface Sci., 2020, 571, 239–252 CrossRef CAS PubMed.
- Y. Zhang, H. Ruan, C. Guo, J. Liao, J. Shen and C. Gao, Sep. Purif. Technol., 2020, 234, 116017 CrossRef.
- L. Zhang, P. Chen, Y. Xu, W. Nie and Y. Zhou, Appl. Catal., B, 2020, 265, 118572 CrossRef CAS.
- A. Hashmi, A. K. Singh, B. Jain and S. A. C. Carabineiro, Nanomaterials, 2020, 10, 105 CrossRef CAS PubMed.
- G. Jena, B. Anandkumar, S. C. Vanithakumari, R. P. George, J. Philip and G. Amarendra, Prog. Org. Coatings, 2020, 139, 105444 CrossRef CAS.
- T. Vi, S. Kumar, J.-H. Pang, Y.-K. Liu, D. Chen and S. Lue, Nanomaterials, 2020, 10, 366 CrossRef CAS PubMed.
- H. Velichkova, S. Kotsilkov, E. Ivanov, R. Kotsilkova, S. Gyoshev, N. Stoimenov and N. K. Vitanov, Food Addit. Contam., Part A: Chem., Anal., Control, Exposure Risk Assess., 2017, 34, 1072–1085 CrossRef CAS PubMed.
- H. Velichkova, I. Petrova, S. Kotsilkov, E. Ivanov, N. K. Vitanov and R. Kotsilkova, J. Appl. Polym. Sci., 2017, 134, 1–12 CrossRef.
- H. C. Oyeoka, C. M. Ewulonu, I. C. Nwuzor, C. M. Obele and J. T. Nwabanne, J. Bioresour. Bioprod., 2021, 6, 168–185 CrossRef CAS.
- L. De Marchi, C. Pretti, B. Gabriel, P. A. A. P. Marques, R. Freitas and V. Neto, Sci. Total Environ., 2018, 631–632, 1440–1456 CrossRef CAS PubMed.
- K. Yang, H. Gong, X. Shi, J. Wan, Y. Zhang and Z. Liu, Biomaterials, 2013, 34, 2787–2795 CrossRef CAS PubMed.
- X. Guo and N. Mei, J. Food Drug Anal., 2014, 22, 105–115 CrossRef CAS PubMed.
- J. H. Liu, S. T. Yang, H. Wang, Y. Chang, A. Cao and Y. Liu, Nanomedicine, 2012, 7, 1801–1812 CrossRef CAS PubMed.
- L. Q. Chen, P. P. Hu, L. Zhang, S. Z. Huang, L. F. Luo and C. Z. Huang, Sci. China Chem., 2012, 55, 2209–2216 CrossRef CAS.
- Z. Ding, Z. Zhang, H. Ma and Y. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 19797–19807 CrossRef CAS PubMed.
- Z. Singh, Nanotechnol. Sci. Appl., 2016, 9, 15–28 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |