Open Access Article
Open Access ArticleFabrication of gelatin Bi2S3 capsules as a highly sensitive X-ray contrast agent for gastrointestinal motility assessment in vivo†
Ya Wen‡
a,
Wang Zhu‡b,
Xuejun Zhang*a and
Shao-Kai Sun *a
*a
aDepartment of Medical Imaging, Tianjin Medical University, Tianjin 300203, China. E-mail: zhangxj@tmu.edu.cn; shaokaisun@tmu.edu.cn
bDepartment of Radiographic Center, Wuhan Children's Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, 430015, China
First published on 5th May 2022
Abstract
Tiny BaSO4 rod-based X-ray imaging is the most frequently-used method for clinical diagnosis of gastrointestinal motility disorders. The BaSO4 rods usually have a small size to pass through the gastrointestinal tract smoothly, but suffer from unavoidably low sensitivity. Herein, we developed Bi2S3 capsules as a high-performance X-ray contrast agent for gastrointestinal motility assessment for the first time. The Bi2S3 capsules were synthesized by the encapsulation of commercial Bi2S3 powder into commercial gelatin capsules and subsequent coating of ultraviolet-curable resin. The prepared Bi2S3 capsules showed excellent biocompatibility in vitro and in vivo and superior X-ray attenuation ability due to the large atomic number and high K-edge value of Bi. The developed Bi2S3 capsules can serve as a small but highly sensitive X-ray contrast agent to quantitatively assess gastrointestinal motility in a vincristine-induced gastrointestinal motility disorder model in vivo by X-ray, CT and spectral CT imaging successfully, solving the intrinsic drawbacks of clinically used BaSO4.
Introduction
Gastrointestinal motility disorders are common complications of a variety of diseases, such as diabetes, obesity, hypertension, and adverse reactions during treatment with chemotherapy drugs like vincristine, which may lead to constipation or diarrhea.1–5 For instance, nearly one-third of patients receiving vincristine treatment will have symptoms such as constipation and paralytic intestinal obstruction induced by autonomic nervous system dysfunction, which can last for several months and seriously affects the patient's quality of life and treatment compliance.6–11 Eventually, gastrointestinal motility disorders lead to the decreased absorption of nutrients and changes in the intestinal barrier function and strongly affect the normal function of the body. Thus, timely and accurate assessment of gastrointestinal function has far-reaching clinical significance.Non-invasive imaging examinations, such as radiopaque marker tests, radionuclide scintigraphy and wireless motility capsules,12 have been frequently used in the diagnosis of various gastrointestinal diseases due to the advantage of non-invasiveness.13,14 Among these clinical imaging technologies, radiopaque marker test15 owns the merits of simple operation, easy analysis and low cost.16 The use of the first radiopaque agent most likely dates back to 1896, when BaSO4 was used to study gastrointestinal motility of animals17 and various morphologies of BaSO4 agents have been developed to meet the requirement of different diseases during the past decades.18–21 Currently, for gastrointestinal motility assessment in clinic, the patients orally take 2 capsules (containing 20 BaSO4 rods) after breakfast on the day of examination. Then the whole gut transit time was evaluated through observing the change of the position and the number of BaSO4 rods at different time points by X-ray imaging.15,22–25 To satisfy the need of normal gastrointestinal transport, BaSO4 rods are usually small in size. While size is a double-edged sword, which results in inherent low sensitivity of BaSO4 rods and makes it difficult to definite their position in gastrointestinal tract. However, BaSO4 rods have been used for gastrointestinal motility assessment for several decades, and there were few new X-ray contrast agents reported up to now. Thus, it is our great desire to develop a highly sensitive and biocompatible X-ray contrast agent for gastrointestinal motility assessment.
Besides X-ray imaging, X-ray computed tomography (CT) imaging, which is capable of three-dimensional reconstruction, is increasingly used in gastrointestinal examinations, and provides abundant diagnosis information that X-ray imaging fails to give.26–28 Especially, the principle of spectral CT is to collect two data sets from the same anatomical location using different kilovolt peaks, possesses differentiation ability towards materials with different absorption under various X-ray energies. It can reduce image artifacts, distinguish tissue components, and improve the contrast effect of high atomic number elements-based contrast agents to surrounding tissues, which provides a promising way to assess gastrointestinal motility.5,29–34 Similar to X-ray imaging, high-performance contrast agents are also the key to sensitive and accurate evaluation of gastrointestinal motility by CT and spectral CT. However, BaSO4 and iodine-based small molecules commonly used clinically are not suitable for CT and spectral CT imaging due to the low K-edge energies of Ba and I and relatively poor X-ray attenuation ability.5,17,35
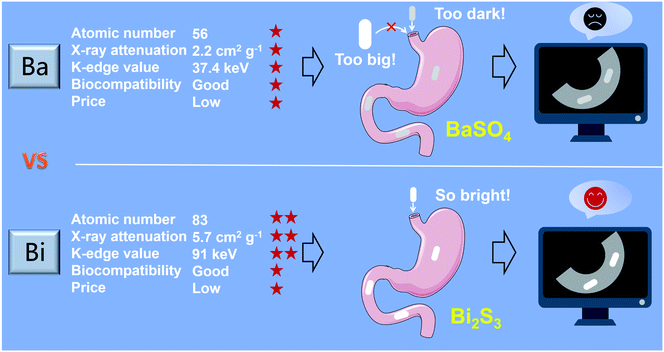
Bi has the highest atom number among non-radioactive elements (Z = 83)36–38 and possesses an outstanding X-ray attenuation coefficient (Bi: 5.74, Au: 5.16, Pt: 4.99, and Ta: 4.3 cm2 g−1 at 100 keV).39 In addition, Bi is the cheapest element among the heavy metal elements suitable for X-ray imaging. Besides, Bi is a highly biocompatible element,36 and many Bi-based compounds, known as “bismuth therapy”,40–43 have served as drugs for gastrointestinal diseases for more than three hundred years.44 In the past decades, various Bi-based nanomaterials, such as Bi,44–47 Bi2O3,48–51 Bi2Se3,52–54 Bi2S3,55–58 have been developed as the new generation of X-ray contrast agents for diagnosis of various diseases. However, a Bi-based contrast agent for gastrointestinal motility assessed by X-ray, CT and spectral CT has not been reported so far (Scheme 1).
 | ||
| Scheme 1 Schematic illustration of synthesis of Bi2S3 capsules as a X-ray contrast agent for gastrointestinal tract visualization in vivo. | ||
Herein, we proposed a highly sensitive method for gastrointestinal transmission assessment using Bi2S3 capsules by X-ray, CT, and spectral CT imaging in vivo. The radiopaque capsules rather than water-soluble nanoparticles are needed in assessment gastrointestinal tract transmission. Commercial Bi2S3 powder was selected as Bi precursor due to its excellent chemical stability, high content of Bi element, good X-ray absorption capacity and low price. It was encapsulated in commercial gelatin capsules and then coated with ultraviolet-curable resin to generate the uniform Bi2S3 capsules. The prepared Bi2S3 capsules had good stability and superior X-ray (especially high-energy X-ray) attenuation ability compared to BaSO4. High sensitive gastrointestinal transmission assessment by X-ray imaging was successfully achieved using Bi2S3 capsules, which was much more sensitive than that using BaSO4. Besides, Bi2S3 capsules were also employed in CT and spectral CT imaging in vivo, and real-time, sensitive, and high-spatial resolution visualization of the gastrointestinal motility was realized, providing abundant three mention information. In vitro and in vivo toxic studies proved the excellent biocompatibility of Bi2S3 capsules. To the best of our knowledge, it was the first time that a Bi-based contrast agent was used to evaluate gastrointestinal motility, which showed great potential as an alternative to clinic BaSO4.
Results and discussion
Synthesis and characterization of Bi2S3 capsules
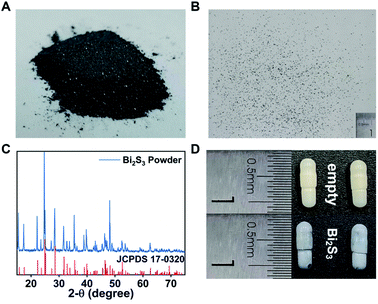
The commercial Bi2S3 powder with tiny size was used as the precursor for the synthesis of Bi2S3 capsules to achieve high imaging sensitivity (Fig. 1A and S2†). The X-ray diffraction pattern indicated the Bi2S3 belonged to the orthorhombic system (Fig. 1C).59,60 Despite the size of Bi2S3 powder showed the evident variability on micrometer scale (Fig. S3†), it was uniform on millimeter scale, which was sufficient to produce uniform Bi2S3 capsules (Fig. 1B). Synthesizing biomaterials with biocompatible macromolecules can improve their biosafety, so we used gelatin capsules as a carrier to prepare Bi2S3 capsules61,62 (Fig. 1D). The resin coating was essential to ensure the stability of Bi2S3 capsules in gastrointestinal tract environments. The synthesized Bi2S3 capsules had a uniform size with 3 mm in diameter and 8 mm in length and there were 25 mg Bi2S3 in each capsule. Thus, the synthesis of Bi2S3 capsules was extremely and reproducible.To investigate the stability of Bi2S3 capsules in stimulated gastrointestinal tract environments, the Bi2S3 capsules were immersed into diluted HCl solution (pH = 1) and artificial small intestinal fluid for 12 h. The morphology of the capsule had no obvious change, and there was no leakage of Bi2S3 power. The results indicated the Bi2S3 capsules could resist the gastrointestinal tract environments and keep stable for potential applications (Fig. S4†).
X-ray, CT and spectral CT imaging in vitro
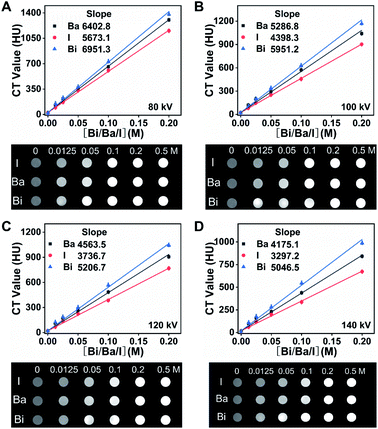
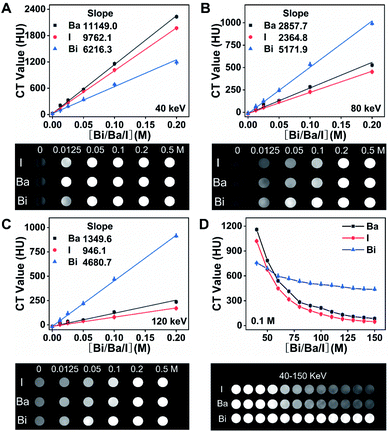
To compare the X-ray absorbance ability of Bi2S3 with clinical BaSO4 and iohexol in vitro, different suspensions were synthesized by dispersing Bi2S3, BaSO4 and iohexol power into alginate–Ca2+ hydrogel. In addition, BaSO4 and iohexol capsules were also prepared according to the synthesis procedures of Bi2S3 capsules. X-ray images of various suspensions indicated their brightness increased with the increase of concentrations of radiopaque elements (Bi, Ba, and I), and the Bi2S3 suspension showed a higher brightness than BaSO4/iohexol suspensions at an equivalent mass or molar concentrations of radiopaque elements (Fig. S5†). And the Bi2S3 capsule was much brighter than that of BaSO4 and iohexol capsule (Fig. S6†). It should be noted that there was serious interference from food in gastrointestinal tract imaging, and iohexol was used to visualize the gastrointestinal tract profile sometimes, so the imaging ability of the capsules should also be evaluated in the presence of food or iohexol. These capsules all showed much higher brightness than food or iohexol solution visually, and the boundary between capsules and surrounding food or iohexol can be seen obviously (Fig. S7†). Quantitative analysis indicated Bi2S3 capsules exhibited the highest brightness among them. These results indicated Bi2S3 capsules had great potential in serving as a superior X-ray contrast agent.In CT imaging, with the increasing concentrations of radiopaque elements, the CT images of Bi2S3 suspensions were brighter than the corresponding images of BaSO4 and iohexol suspensions at various tube voltages (80, 100, 120, and 140 kV). Meanwhile, the Hounsfield unit (HU) values linearly increased under the same voltage for Bi2S3, BaSO4 and iohexol suspensions and capsules in a concentration dependent manner and the slope discrepancy among Bi2S3, BaSO4 and iohexol became more and more distinct along with the increasing tube voltage from 80 to 140 kV (Fig. 2A–D, S8 and S9†). The CT imaging ability of Bi2S3 capsules in food and iohexol were also investigated, and the results confirmed that Bi2S3 capsules had the best CT imaging performance even in the high background induced by food and iohexol (Fig. S10A and S11†). In order to study the feasibility of spectral CT imaging using Bi2S3, monochromatic images of Bi2S3, BaSO4, and iohexol suspensions were obtained (Fig. 3A–C and S12†). Under each X-ray energy, there is a linear relationship between HU value and concentration of contrast agents. At low X-ray energies (40–50 keV), the HU values of BaSO4 and iohexol suspensions were higher than those of Bi2S3 suspensions. As the tissues were mainly composed of low atomic number elements, they showed similar X-ray absorption properties to BaSO4 or iohexol and also have a relatively high X-ray absorption at low X-ray energies. Hence it is useless to perform spectral CT imaging using BaSO4 or iohexol as contrast agents at low X-ray energies. When the energy is larger than about 60 keV, the HU values of Bi2S3 suspensions became higher than those of BaSO4 and iohexol suspensions, and the slope (HU value per unit concentration) difference between Bi2S3 suspensions and BaSO4/iohexol suspensions becomes more and more obvious when monochromatic energy increased.
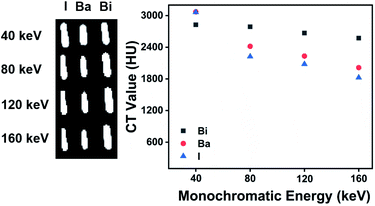
Compared with the sharp decrease of the HU value of BaSO4 and iohexol suspensions with the increase of monochromatic energy, Bi2S3 suspensions exhibit relatively stable HU value due to the high K-edge energy of Bi (91 keV) (Fig. 3D). These results indicated Bi2S3 exhibited excellent spectral CT imaging ability at high X-ray energies. Then we evaluated the spectral CT imaging ability of Bi2S3 capsule. Similar to Bi2S3 suspension, Bi2S3 capsule showed better CT imaging performance when the energy increased from 80 keV to 160 keV, and the energies were higher, the performance was better (Fig. 4). In the presence of food, three kinds of capsules all showed a good contrast effect, and the boundary between capsules and food was very clear, but a quantitative analysis indicated the HU value of Bi2S3 capsule was the highest (Fig. S10B†). In the presence of iohexol, it is hard to distinguish capsules from iohexol only if the concentration of iohexol was very low (5 mg mL−1) at low X-ray energies. However, when the X-ray energies were larger, the contrast of HU values of capsules and iohexol solution became significant, and the boundary between them can be seen obviously (Fig. S13†). It should be noted the size of Bi2S3 capsule kept the same in spectral CT images at various X-ray energies while the size of BaSO4 capsule became smaller along with the increase of X-ray energies. Besides, the HU values of Bi2S3 capsule were higher than those of BaSO4 capsule at various X-ray energies. These results demonstrated Bi2S3 capsule can serve as a spectral CT imaging contrast agent with high sensitivity, which was capable of minimizing the interference of surrounding substance.
Cytotoxicity assessment
The cytotoxicity of Bi2S3 capsules was evaluated by a standard MTT test. Firstly, Bi2S3 capsules were dispersed in various media (ultrapure water, phosphate buffered saline (PBS) solution (pH = 7.4) and diluted hydrochloric acid (pH = 1)) for 24 h, and the leaching solutions were acquired by removing the capsules. Then, 3T3-L1 cells were incubated with various leach solutions for 12 h and the cell viabilities were determined. The results indicated Bi2S3 capsule leach solutions had little influence on the proliferation of 3T3-L1 cells, and the cell viability remained above 80% (Fig. S14†), indicating the low cytotoxicity of Bi2S3 capsules.In vivo toxicity of Bi2S3 capsule
To investigate the in vivo toxicity of Bi2S3 capsules, hematoxylin and eosin (H&E) staining analysis of the main organs (heart, liver, spleen, lung, kidney, and the intestinal tract) at different time points was carried out after oral administration of three Bi2S3 capsules (Fig. S15†). H&E staining results demonstrated there were no obvious histopathological damages in major organs of rats after the treatment of Bi2S3 capsules, further proving their good biocompatibility.Gastrointestinal transmission assessment by X-ray imaging in vivo
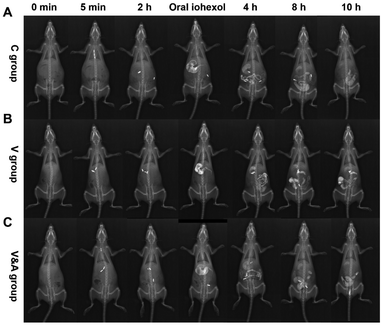
Based on the excellent X-ray absorption ability of Bi2S3 capsules, we investigated the feasibility of gastrointestinal transmission assessment using Bi2S3 capsules by X-ray imaging on vincristine-induced gastrointestinal motility disorder models. Paralytic ileus caused by vincristine can be prevented/treated by CB1 antagonists, such as AM251.63The rats were divided into three groups including a control group, vincristine-treated group and vincristine & AM251-treated group (n = 8 in each group). After oral administration of three Bi2S3 capsules, the X-ray imaging was carried out at different time points (0 min, 5 min, 2 h, 4 h, 8 h, and 10 h) (Fig. 5A–C). For all the groups of rats, three Bi2S3 capsules can be seen clearly in esophagus and stomach after the administration of capsules in X-ray imaging, and then the capsules moved into small intestinal gradually. At 2 h, the rats were orally administrated with iohexol to visualize the gastrointestinal tract profiles, benefiting the determination of the location of Bi2S3 capsules. For the control group, the capsules were mainly located in small intestinal before 8 h, and then moved into cecum and colon at about 8 h. The majority of capsules can be excreted from the body at 10 h. In contrast, for the vincristine-treated group, the movement speed of capsules slowed down obviously, and the majority of capsules were still located into small intestinal at 10 h due to the weakened gastrointestinal motility. While the metabolism of the capsules in gastrointestinal tract became normal after vincristine-treated rats were treated with AM251, as shown in the X-ray imaging. In addition, BaSO4 capsules were also used to assess gastrointestinal motility in the rats with various treatments. However, the contrast effect of BaSO4 capsules in X-ray imaging was much worse than that of Bi2S3 capsules, which demonstrated Bi2S3 can serve as an excellent alternative X-ray contrast agent to BaSO4 (Fig. S17†).
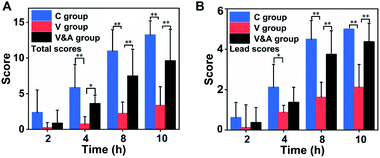
Then we quantitatively assessed the gastrointestinal motility based on a standard rating scale (Table 1),64,65 and two kinds of scores (total score and lead capsule score) were calculated for the assessment. The three capsules are given numerical scores based on their position in the gastrointestinal tract, and the total score is calculated by adding the score of each capsule (if all are excreted, the maximum is equal to 15). The lead capsule score was calculated based on its position in the gastrointestinal tract according to the rating scale (if it is excreted, the maximum is equal to 5). As shown in Fig. 6A, the average total scores of the vincristine group were 7.8 times at 4 h, 4.6 times at 8 h and 3.9 times at 10 h lower than those of the control group, respectively. After treated by AM251, the average total scores of vincristine & AM251 group increased by 4.8 times at 4 h, 3.2 times at 8 h and 2.8 times at 10 h compared with those of the VCR group, which indicated the gastrointestinal motility disorder caused by vincristine was significantly restored by AM251.
| Position | Stomach | Proximal small intestine | Distal small intestine |
| Score | 0 | 1 | 2 |
| Position | Cecum | Colon | Excretion of the gastrointestinal tract |
| Score | 3 | 4 | 5 |
Besides, the lead capsule score was also used to evaluate the gastrointestinal motility. The lead capsules score of vincristine group were 2.4 times at 4 h, 2.7 times at 8 h, and 2.3 times at 10 h lower than those of the control group, respectively (Fig. 6B). The lead capsules in vincristine group only reached the distal small intestine area, while most of lead capsules in the control group were excreted at 10 h after oral administration. The lead capsules scores of vincristine & AM251 group were 1.6 times at 4 h, 2.3 times at 8 h and 2 times at 10 h higher than those of vincristine group. The lead capsules in the gastrointestinal tract were either in the descending colon or excreted at 10 h due to the recovery of gastrointestinal motility function.
Gastrointestinal transmission evaluation by CT and spectral CT imaging in vivo
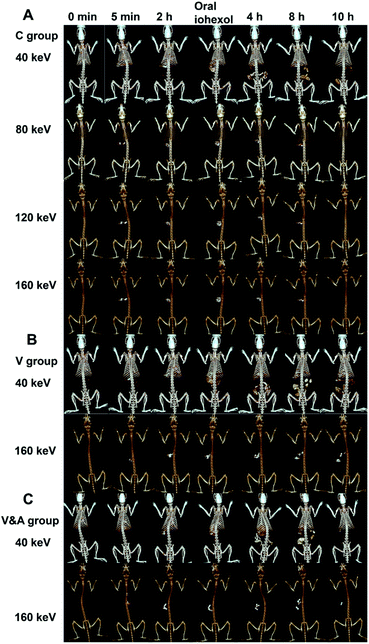
Then the gastrointestinal motility assessment was evaluated by CT and spectral CT imaging using Bi2S3 capsules. The metabolism behavior of Bi2S3 capsules in the gastrointestinal tract of rats in various groups can be monitored in three dimensions clearly, providing more abundant diagnosis information. The gastrointestinal motility disorder and function recovery can be diagnosed by the visualized CT images and rating scores (Fig. S18 and S19†), and the results were consistent with those obtained from X-ray imaging. Besides, the spectral CT imaging with the charming advantage of distinguishing the materials by different atomic numbers was also carried out (Fig. 7A–C and S20†). In common CT imaging or spectral CT imaging at low monochromatic energies, food, faeces, iohexol and other tissues all can be seen besides Bi2S3 capsules, and which resulted in troublesome interferences for the clear visualization of Bi2S3 capsules. While in spectral CT imaging at high monochromatic energies, the CT signal of other subjects decreased significantly, even disappeared. In contrast, Bi2S3 capsules still kept the constant strong brightness in spectral CT imaging regardless of the monochromatic energies, so the Bi2S3 capsules can be distinguished from the background very obviously at high monochromatic energies. Combined spectral CT imaging of Bi2S3 capsules at low and high monochromatic energies, the location information of Bi2S3 capsules and anatomical information of surrounding tissues can be obtained high sensitively and accurately. Therefore, the developed Bi2S3 capsules can serve as an excellent multifunctional X-ray contrast agent for gastrointestinal motility assessment based on X-ray, CT and spectral CT.Conclusions
In summary, we reported the facile fabrication of Bi2S3 capsules as a high-performance X-ray contrast agent for gastrointestinal motility assessment for the first time. Bi2S3 capsules were synthesized by encapsulating commercial Bi2S3 powder into gelatin capsules, followed by the coating of ultraviolet-curable resin. In the harsh environment of the gastrointestinal system, the synthesized Bi2S3 capsules can still keep intact morphology. The prepared Bi2S3 capsules showed superior X-ray absorption ability than BaSO4 capsules at various conditions in X-ray and CT imaging in vitro. In particular, the large atomic number and high K-edge value of Bi ensure the outstanding contrast performance of Bi2S3 capsules at high monochromatic energies. The cellular studies proved the low cytotoxicity of Bi2S3 capsules, and the in vivo toxicity evaluation also confirmed the good biocompatibility of Bi2S3 capsules. Taking the vincristine-induced gastrointestinal motility disorder model as an example, the developed Bi2S3 capsules were successfully employed to quantitatively and sensitively assess gastrointestinal motility through X-ray, CT and spectral CT imaging in vivo, and the imaging performance of Bi2S3 capsules was much better than that of BaSO4 capsules. Our study demonstrated the feasibility of a highly sensitive gastrointestinal motility assessment using a Bi-based contrast agent with great clinical translation potential, which can serve as an excellent alternative contrast agent to clinically used BaSO4.Author contributions
S.-K. Sun and X.-J. Zhang supervised the project. Y. Wen and W. Zhu performed the experiments. Y. Wen principally wrote the manuscript. All authors proof-read, provided comments, and approved the final version of this manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21874101, 21934002, and 82071982) and the Natural Science Foundation of Tianjin City (19JCJQJC63700) and Young Elite Scientists Sponsorship Program by Tianjin (TJSQNTJ-2018-08).Notes and references
- R. M. McQuade, V. Stojanovska, R. Abalo, J. C. Bornstein and K. Nurgali, Front. Pharmacol., 2016, 7, 414 CrossRef CAS PubMed.
- P. Bytzer, N. J. Talley and M. Leemon, Arch. Intern. Med., 2001, 161, 1989–1996 CrossRef CAS PubMed.
- E. M. Richards, C. J. Pepine, M. K. Raizada and S. Kim, Curr. Hypertens. Rep., 2017, 19, 36 CrossRef PubMed.
- X. Fu, Z. Li, Z. Na, H. Yu and J. Liu, Nutr. Metab., 2014, 11, 3 CrossRef PubMed.
- B. M. Yeh, P. F. FitzGerald, P. M. Edic, J. W. Lambert, R. E. Colborn, M. E. Marino, P. M. Evans, J. C. Roberts, Z. J. Wang, M. J. Wong and P. J. Bonitatibus Jr, Adv. Drug Delivery Rev., 2017, 113, 201–222 CrossRef CAS PubMed.
- E. M. Lavoie Smith, D. L. Barton, R. Qin, P. D. Steen, N. K. Aaronson and C. L. Loprinzi, Qual. Life Res., 2013, 22, 2787–2799 CrossRef PubMed.
- E. Y. Ibrahim and B. E. Ehrlich, Crit. Rev. Oncol. Hematol., 2020, 145, 102831 CrossRef PubMed.
- S. B. Park, D. Goldstein, A. V. Krishnan, C. S. Y. Lin, M. L. Friedlander, J. Cassidy, M. Koltzenburg and M. C. Kiernan, Ca-Cancer J. Clin., 2013, 63, 419–437 CrossRef PubMed.
- B. W. Hancock and A. Naysmith, Br. Med. J., 1975, 3, 207 CrossRef CAS PubMed.
- S. Dudeja, S. Gupta, S. Sharma, A. Jain, S. Sharma, P. Jain, S. Aneja and J. Chandra, Pediatr. Hematol. Oncol., 2019, 36, 344–351 CrossRef CAS PubMed.
- S. Quasthoff and H. P. Hartung, J. Neurol., 2002, 249, 9–17 CrossRef CAS PubMed.
- E. R. Kim and P. L. Rhee, J. Neurogastroenterol. Motil., 2012, 18, 94–99 CrossRef PubMed.
- J. T. Fell and G. A. Digenis, Int. J. Pharm., 1984, 22, 1–15 CrossRef CAS.
- R. M. McQuade, V. Stojanovska, R. Abalo, J. C. Bornstein and K. Nurgali, in Colonic Transit Study by Radio-Opaque Markers, Springer, New Delhi, 2016, pp. 23–29 Search PubMed.
- H. Sharif, N. Abrehart, C. L. Hoad, K. Murray, A. C. Perkins, M. Smith, P. A. Gowland, R. C. Spiller, R. Harris, S. Kirkham, S. Loganathan, M. Papadopoulos, K. Frost, D. Devadason, L. Marciani and G. Young Persons Advisory, J. Pediatr. Gastroenterol. Nutr., 2020, 71, 604–611 CrossRef CAS PubMed.
- R. B. Kjeldsen, M. N. Kristensen, C. Gundlach, L. H. E. Thamdrup, A. Mullertz, T. Rades, L. H. Nielsen, K. Zor and A. Boisen, ACS Biomater. Sci. Eng., 2021, 7, 2538–2547 CrossRef CAS PubMed.
- A. Jakhmola, N. Anton and T. F. Vandamme, Adv. Healthcare Mater., 2012, 1, 413–431 CrossRef CAS PubMed.
- R. Bomma, R. A. S. Naidu, M. R. Yamsani and K. Veerabrahma, Acta Pharm., 2009, 59, 211–221 CAS.
- A. S. Narang, A. Balakrishnan, J. Morrison, J. Li, J. Wang, H. Gu, K. Taylor, K. Santone, J. Ehrmann, S. Beyer, X. Lu, R. Ketner, J. Pizzano, T. Orcutt, E. Shields, H. Dulac, S. Aborn, M. Batchelder and K. Lentz, Eur. J. Pharm. Biopharm., 2017, 117, 333–345 CrossRef CAS PubMed.
- K. Kikuchi, M. Kusano and O. Kawamura, Dig. Dis. Sci., 2000, 45, 242–247 CrossRef CAS PubMed.
- S. Saphier, A. Rosner, R. Brandeis and Y. Karton, Int. J. Pharm., 2010, 388, 190–195 CrossRef CAS PubMed.
- Y. B. Wang, G. Li, Y. F. Wang, Y. J. Ding, G. Z. Yan, D. Han, Z. W. Wang and X. H. Zhao, Int. J. Colorectal Dis., 2020, 35, 29–34 CrossRef PubMed.
- S. S. Rao, K. Rattanakovit and T. Patcharatrakul, Nat. Rev. Gastroenterol. Hepatol., 2016, 13, 295–305 CrossRef CAS PubMed.
- H. C. Lin, C. Prather, R. S. Fisher, J. H. Meyer, R. W. Summers, M. Pimentel, R. W. McCallum, L. M. Akkermans and V. Loening-Baucke, Dig. Dis. Sci., 2005, 50, 989–1004 CrossRef PubMed.
- S. S. Rao, M. Camilleri, W. L. Hasler, A. H. Maurer, H. P. Parkman, R. Saad, M. S. Scott, M. Simren, E. Soffer and L. Szarka, Neurogastroenterol. Motil., 2011, 23, 8–23 CrossRef CAS PubMed.
- P. C. Naha, J. C. Hsu, J. Kim, S. Shah, M. Bouche, S. Si-Mohamed, D. N. Rosario-Berrios, P. Douek, M. Hajfathalian, P. Yasini, S. Singh, M. A. Rosen, M. A. Morgan and D. P. Cormode, ACS Nano, 2020, 14, 10187–10197 CrossRef CAS PubMed.
- N. Lee, S. H. Choi and T. Hyeon, Adv. Mater., 2013, 25, 2641–2660 CrossRef CAS PubMed.
- Y. Zu, Y. Yong, X. Zhang, J. Yu, X. Dong, W. Yin, L. Yan, F. Zhao, Z. Gu and Y. Zhao, RSC Adv., 2017, 7, 17505–17513 RSC.
- T. M. Coupal, P. I. Mallinson, S. L. Gershony, P. D. McLaughlin, P. L. Munk, S. Nicolaou and H. A. Ouellette, Am. J. Roentgenol., 2016, 206, 119–128 CrossRef PubMed.
- X. F. Luo, X. Q. Xie, S. Cheng, Y. Yang, J. Yan, H. Zhang, W. M. Chai, B. Schmidt and F. H. Yan, Radiology, 2015, 277, 95–103 CrossRef PubMed.
- A. N. Primak, J. G. Fletcher, T. J. Vrtiska, O. P. Dzyubak, J. C. Lieske, M. E. Jackson, J. C. Williams Jr and C. H. McCollough, Acad. Radiol., 2007, 14, 1441–1447 CrossRef PubMed.
- E. Pessis, R. Campagna and J. M. Sverzut, RadioGraphics, 2013, 33(2), 573–583 CrossRef PubMed.
- Y. Y. Jin, D. L. Ni, L. Gao, X. F. Meng, Y. Lv, F. Han, H. Zhang, Y. Y. Liu, Z. W. Yao, X. Y. Feng, W. B. Bu and J. W. Zhang, Adv. Funct. Mater., 2018, 28, 1802656 CrossRef.
- Y. L. Liu, K. L. Ai, J. H. Liu, Q. H. Yuan, Y. Y. He and L. H. Lu, Angew. Chem., Int. Ed., 2012, 51, 1437–1442 CrossRef CAS PubMed.
- D. Pan, E. Roessl, J.-P. Schlomka, S. D. Caruthers, A. Senpan, M. J. Scott, J. S. Allen, H. Zhang, G. Hu, P. J. Gaffney, E. T. Choi, V. Rasche, S. A. Wickline, R. Proksa and G. M. Lanza, Angew. Chem., Int. Ed., 2010, 49, 9635–9639 CrossRef CAS PubMed.
- H. Li and H. Sun, Curr. Opin. Chem. Biol., 2012, 16, 74–83 CrossRef CAS PubMed.
- Y. Cheng and H. Zhang, Chem.–Eur. J., 2018, 24, 17405–17418 CrossRef CAS PubMed.
- Y. Xuan, X. Q. Yang, Z. Y. Song, R. Y. Zhang, D. H. Zhao, X. L. Hou, X. L. Song, B. Liu, Y. D. Zhao and W. Chen, Adv. Funct. Mater., 2019, 29, 1900017 CrossRef.
- M. A. Shahbazi, L. Faghfouri, M. P. A. Ferreira, P. Figueiredo, H. Maleki, F. Sefat, J. Hirvonen and H. A. Santos, Chem. Soc. Rev., 2020, 49, 1253–1321 RSC.
- X. Zheng, J. Shi, Y. Bu, G. Tian, X. Zhang, W. Yin, B. Gao, Z. Yang, Z. Hu, X. Liu, L. Yan, Z. Gu and Y. Zhao, Nanoscale, 2015, 7, 12581–12591 RSC.
- K. D. Mjos and C. Orvig, Chem. Rev., 2014, 114, 4540–4563 CrossRef CAS PubMed.
- S. L. Gorbach, Gastroenterology, 1990, 99, 863–875 CrossRef CAS.
- D. M. Keogan and D. M. Griffith, Molecules, 2014, 19, 15258–15297 CrossRef PubMed.
- B. Wei, X. Zhang, C. Zhang, Y. Jiang, Y. Y. Fu, C. Yu, S. K. Sun and X. P. Yan, ACS Appl. Mater. Interfaces, 2016, 8, 12720–12726 CrossRef CAS PubMed.
- N. Yu, Z. Wang, J. Zhang, Z. Liu, B. Zhu, J. Yu, M. Zhu, C. Peng and Z. Chen, Biomaterials, 2018, 161, 279–291 CrossRef CAS PubMed.
- P. Lei, R. An, P. Zhang, S. Yao, S. Song, L. Dong, X. Xu, K. Du, J. Feng and H. Zhang, Adv. Funct. Mater., 2017, 27, 1702018 CrossRef.
- X. Yu, A. Li, C. Zhao, K. Yang, X. Chen and W. Li, ACS Nano, 2017, 11, 3990–4001 CrossRef CAS PubMed.
- F. Du, J. Lou, R. Jiang, Z. Fang, X. Zhao, Y. Niu, S. Zou, M. Zhang, A. Gong and C. Wu, Int. J. Nanomed., 2017, 12, 5973–5992 CrossRef CAS PubMed.
- H. Aviv, S. Bartling, I. Grinberg and S. Margel, J. Biomed. Mater. Res., Part B, 2013, 101, 131–138 CrossRef PubMed.
- Y. Xujiang, L. Xinyi, Y. Kai, C. Xiaoyuan and L. Wanwan, ACS Nano, 2021, 15, 2038–2067 CrossRef PubMed.
- W. Xu, P. Cui, E. Happonen, J. Leppanen, L. Liu, J. Rantanen, D. Majda, A. Saukko, R. Thapa, T. Nissinen, T. Tynkkynen, J. Toyras, L. Fan, W. Liu and V. P. Lehto, ACS Appl. Mater. Interfaces, 2020, 12, 47233–47244 CrossRef CAS PubMed.
- F. Mao, L. Wen, C. Sun, S. Zhang, G. Wang, J. Zeng, Y. Wang, J. Ma, M. Gao and Z. Li, ACS Nano, 2016, 10, 11145–11155 CrossRef CAS PubMed.
- X. Niu, Y. Liu, X. Li, W. Wang and Z. Yuan, Adv. Funct. Mater., 2020, 30, 2006883 CrossRef CAS.
- X.-D. Zhang, J. Chen, Y. Min, G. B. Park, X. Shen, S.-S. Song, Y.-M. Sun, H. Wang, W. Long, J. Xie, K. Gao, L. Zhang, S. Fan, F. Fan and U. Jeong, Adv. Funct. Mater., 2014, 24, 1718–1729 CrossRef CAS.
- L. Li, Y. Lu, C. Jiang, Y. Zhu, X. Yang, X. Hu, Z. Lin, Y. Zhang, M. Peng, H. Xia and C. Mao, Adv. Funct. Mater., 2018, 28, 1704623 CrossRef PubMed.
- Y. Wang, Y. Wu, Y. Liu, J. Shen, L. Lv, L. Li, L. Yang, J. Zeng, Y. Wang, L. W. Zhang, Z. Li, M. Gao and Z. Chai, Adv. Funct. Mater., 2016, 26, 5335–5344 CrossRef CAS.
- K. Ai, Y. Liu, J. Liu, Q. Yuan, Y. He and L. Lu, Adv. Mater., 2011, 23, 4886–4891 CrossRef CAS PubMed.
- O. Rabin, J. Manuel Perez, J. Grimm, G. Wojtkiewicz and R. Weissleder, Nat. Mater., 2006, 5, 118–122 CrossRef CAS PubMed.
- D. Wang, C. Hao, W. Zheng, X. Ma, D. Chu, Q. Peng and Y. Li, Nano Res., 2010, 2, 130–134 CrossRef.
- J. Arumugam, A. D. Raj, A. A. Irudayaraj and M. Thambidurai, Mater. Lett., 2018, 220, 28–31 CrossRef CAS.
- S. Ding, A. I. Khan, X. Cai, Y. Song, Z. Lyu, D. Du, P. Dutta and Y. Lin, Mater. Today, 2020, 37, 112–125 CrossRef CAS PubMed.
- A. B. Bello, D. Kim, D. Kim, H. Park and S.-H. Lee, Tissue Eng., Part B, 2020, 26, 164–180 CrossRef CAS PubMed.
- G. Vera, A. E. Lopez-Perez, J. A. Uranga, R. Giron, M. I. Martin-Fontelles and R. Abalo, Front. Pharmacol., 2017, 8, 37 Search PubMed.
- J. E. Dalziel, W. Young, P. Bercik, N. J. Spencer, L. J. Ryan, K. E. Dunstan, C. M. Lloyd-West, P. K. Gopal, N. W. Haggarty and N. C. Roy, Neurogastroenterol. Motil., 2016, 28, 1241–1251 CrossRef CAS PubMed.
- D. E. Reed, M. Pigrau, J. Lu, P. Moayyedi, S. M. Collins and P. Bercik, Neurogastroenterol. Motil., 2014, 26, 1663–1668 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra00993e |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2022 |