Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biocompatible fluorocarbon liquid underlays for in situ extraction of isoprenoids from microbial cultures†
Sebastian Overmans * and
Kyle J. Lauersen
* and
Kyle J. Lauersen
Bioengineering Program, Biological and Environmental Sciences and Engineering Division (BESE), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: sebastian.overmans@kaust.edu.sa
First published on 6th June 2022
Abstract
Microbial production of heterologous metabolites is now a mature technology in many host organisms, opening new avenues for green production processes of specialty chemicals. At lab scale, petroleum-based hydrophobic bio-compatible solvents like dodecane can be used as a second phase on top of microbial cultures to act as a physical sink for heterologous hydrocarbon products like isoprenoids. However, this approach has significant drawbacks at scale due to the difficulty of handling solvents and their potential contamination with unwanted byproducts of their manufacture. We discovered that synthetic perfluorocarbon liquids (FCs), commonly used for heat transfer, can also act as physical sinks for microbially produced isoprenoid compounds. FCs are stable, inert, and are amenable to direct liquid–liquid extraction with alcohols for rapid product isolation. These liquids are more dense than water and form a lower phase to microbial cultures rather than an upper phase as with other solvents. Their ability to form an under-layer or ‘underlay’ also enables the cultivation of microbes directly at the FC–culture medium interface via gravity settling, which could open their application for filamentous or mat-forming organisms. We present comparisons of the isoprenoid extraction potential of three commercial FCs: FC-3283, FC-40, and FC-770 with engineered green microalga cultures producing patchoulol, taxadiene, casbene, or 13R(+) manoyl oxide. We demonstrate that FCs are promising alternatives to traditional solvents and open new avenues in bio-process design for microbial heterologous metabolite milking.
Introduction
Metabolic engineering of microbes for the production of heterologous isoprenoids is now a mature technology, with several commercial enterprises converting base feedstocks into hydrocarbons of various complexities.1 Hemi-(C5), mono-(C10), sesqui-(C15), di-(C20), and tri-(C30), non-canonical terpenoids (C11, C12, C16, C17), and carotenoids2 are now routinely produced in diverse host organisms such as bacteria, yeast, microalgae, and mosses.3–6 These engineering successes leverage the universal need for isoprenoid precursors isopentenyl and dimethylallyl diphosphate (IPP and DMAPP), and their subsequent condensed and dephosphorylated hydrocarbon chains. Core isoprenoids have cellular roles in membrane fluidity, sterol biosynthesis, prenylation, light capture, photoprotection, and as electron carriers.7 The base hydrocarbon backbones of isoprenoids have also served as a substrate for the evolution of complex specialty chemical structures through the evolution of terpene synthases that mediate deprenylation, cyclization, folding, and (sometimes) hydroxylation.8,9 Base terpenes can be further chemically decorated by the action of cytochrome P450s, which add functional groups to increase their chemical diversity.10,11 Terpene synthases have evolved in bacteria,12 fungi,13 plants,9 insects,14,15 and some algae13 as a means to generate specialty chemicals from base isoprenoids IPP and DMAPP to be used in signaling, defense, and attraction/repulsion.Many isoprenoids are naturally produced in small quantities and may be found on slow-growing, non-farmable, or environmentally sensitive organisms.8 The biological universality of isoprenoid precursors IPP and DMAPP enables the transfer of modular terpene pathways from progenitors to microbial hosts by the heterologous expression of their terpene synthases and P450s.9 Engineered microbes serve this role well and can be used to scale the production of isoprenoid(s) from their biomass in fermenters and bioreactors.16 At lab-scale, microbial isoprenoid productivity can be quantified using hydrophobic biocompatible solvent overlays as a two-phase constant extraction method on living cultures. These solvents act as a physical sink to simultaneously remove and capture heterologous isoprenoids from microbial cells; a process sometimes referred to as ‘milking’. Some commonly used solvents include decane,17 dodecane,18 or isopropyl myristate.19,20 These act similarly to the trichomes of plants by accumulating the hydrophobic isoprenoid products, alleviating product inhibition, and promoting forward reactions while serving as a simple-to-separate phase for easy product analysis.
Hydrocarbon solvent-medium dual-phase concepts are convenient at small scales, however, they have significant drawbacks that prevent their use at volumes greater than a few hundred milliliters. Alkane solvents are flammable and require specialized infrastructure for pumping, as they are incompatible with silicone-based tubing. These solvents are also distillates of petroleum, which can contain many contaminants depending on the supplier. The most notable issue with using hydrophobic solvent overlays is the difficulty in scaling their application to larger culture volumes in turbid and sparged bioreactor concepts.3 As an overlay, any turbidity at the culture–solvent interface creates an emulsion of hydrophobic cellular components and the solvent that can blow out of reactors or lyse cells, hindering the processibility and productivity of extracted isoprenoids.
The lack of translatability of these lab processes to scale leaves significant room for improvement. Here, we show that long-chain hydrophobic perfluorocarbons that are liquid in operational temperature ranges can act as alternatives to hydrocarbon solvents for microbial culture milking. These liquids have current application as heat-transfer fluids and are used in cooling systems for electronics.21,22 As they are more dense than water, perfluorocarbon liquids settle as a lower-phase or ‘underlay’ below the culture. Perfluorocarbon liquids are inert, available in a range of chemical conformations with high heat stability, and significantly easier to handle than hydrocarbon solvents. We show that perfluorocarbon liquids provide cleaner extraction of isoprenoid products from microbial culture than dodecane, fluorocarbon type can be tailored to target isoprenoid properties, and their extreme densities enable subsequent liquid–liquid extraction of collected terpenoids with alcohols. Hydrophobic underlays also provide unique opportunities for the direct cultivation and milking of biofilms at the medium–fluorocarbon interface.
Materials and methods
Chemicals
Three perfluoro liquids, hereafter referred to as FCs, were used in this work: the perfluorinated amine Fluorinert™ FC-40 was purchased from Sigma-Aldrich (Overijse, Belgium), while the perfluorinated ether FC-770 and the perfluorinated amine FC-3283 were obtained from Acros Organics (Geel, Belgium). Ethanol (96% vol.) and n-dodecane (≥99%) were purchased from VWR International (Fontenay-sous-Bois, France). Prior to its use, dodecane was passed through a Supelclean™ LC-Si solid-phase extraction (SPE) column (Product no. 505374; Sigma-Aldrich, Taufkirchen, Germany) to remove impurities.Microalgal cultures and growth conditions
Four strains of the green model microalga Chlamydomonas reinhardtii that had been previously engineered to produce the sesquiterpenoid patchoulol,23 the diterpenoids taxadiene, casbene, or 13R(+) manoyl oxide,24 as well as their parental strain (‘UVM4’ hereafter termed wild type),25 were used in this work to investigate the terpenoid-extraction potential of perfluoro liquids from two-phase solvent-medium living microbial cultures. All algal cultures were maintained routinely on Tris acetate phosphate (TAP)26 agar plates under ∼50 μmol m−2 s−1 light intensity before transfer into 24-well plates containing 1 mL TAP medium, and shaken at 190 rpm on a 12 h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h dark: light (∼150 μmol m−2 s−1) cycle. After 2 d, an additional 500 μL of medium was added to each well to replenish nutrients. At 4 d, 400 μL of each culture was inoculated in 40 mL TAP in Erlenmeyer flasks at 120 rpm under the light conditions indicated above and used for subsequent experiments.
12 h dark: light (∼150 μmol m−2 s−1) cycle. After 2 d, an additional 500 μL of medium was added to each well to replenish nutrients. At 4 d, 400 μL of each culture was inoculated in 40 mL TAP in Erlenmeyer flasks at 120 rpm under the light conditions indicated above and used for subsequent experiments.
Culture growth measurements
Culture growth was measured by recording cell densities using flow-cytometry with an Invitrogen Attune NxT flow cytometer (Thermo Fisher Scientific, UK) equipped with a Cytkick microtiter plate autosampler unit. Prior to analysis, each biological triplicate sample was diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 with 0.9% NaCl solution. Of each diluted sample, 250 μL was measured in technical duplicates (n = 2) using a 96-well microtiter plate loaded into the autosampler. Samples were mixed three times each immediately before analysis, and the first 25 μL of sample was discarded to ensure stable cell flow rate during measurement. Data acquisition stopped when 50 μL from each well was analyzed. All post-acquisition analyses and population clustering were performed using Attune NxT Software v3.2.1 (Life Technologies, USA). Mean cell concentrations of shaken and static cultures were compared against each other by performing Student's t-tests (one test per sampling point) using the software JMP Pro 16.2 (SAS Institute Inc., Cary, NC).
100 with 0.9% NaCl solution. Of each diluted sample, 250 μL was measured in technical duplicates (n = 2) using a 96-well microtiter plate loaded into the autosampler. Samples were mixed three times each immediately before analysis, and the first 25 μL of sample was discarded to ensure stable cell flow rate during measurement. Data acquisition stopped when 50 μL from each well was analyzed. All post-acquisition analyses and population clustering were performed using Attune NxT Software v3.2.1 (Life Technologies, USA). Mean cell concentrations of shaken and static cultures were compared against each other by performing Student's t-tests (one test per sampling point) using the software JMP Pro 16.2 (SAS Institute Inc., Cary, NC).
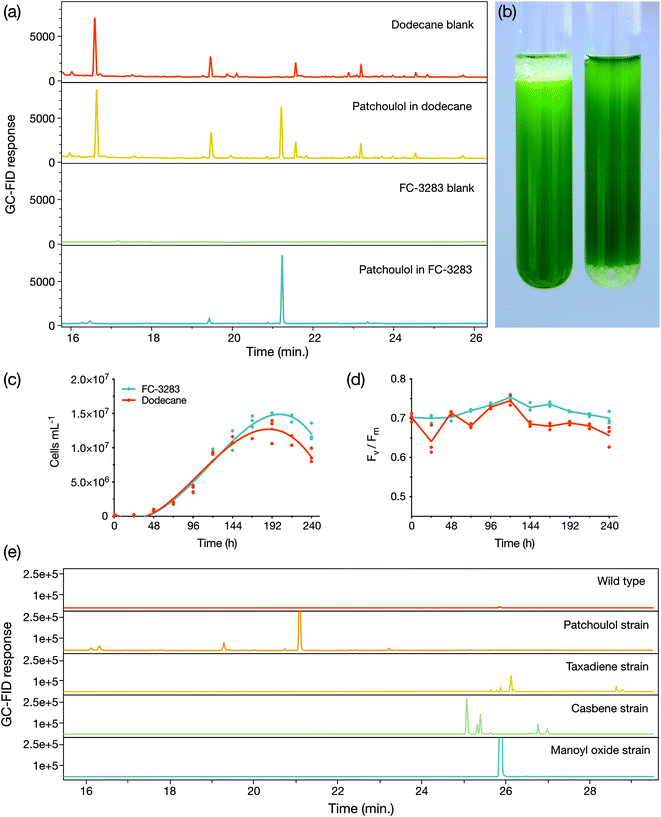
Algal culture health in contact with dodecane or perfluoro liquids was measured by the variable chlorophyll fluorescence of photosystem II (PSII) with a Pulse Amplitude Modulation (PAM) fluorometer (Mini-PAM-II; Heinz Walz GmbH, Germany). Before each measurement, microtiter plates containing cultures were dark-adapted at room temperature for 15 min to ensure all PSII reaction centers were open. For each sample, the signal amplitude was adjusted before one single-turnover measurement was recorded per sample. The maximum photochemical efficiency (Fv/Fm) was determined using the following relationship (eqn 1), where Fm and F0 are the maximal and minimal PSII fluorescence of dark-acclimated C. reinhardtii cells, respectively:27,28
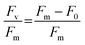 | (1) |
Terpenoid capture from microalgal cultures
Two-phase living extractions were performed with dodecane, FC-40, FC-3283, and FC-770 by gently pipetting 500 μL of each compound onto 4.5 mL liquid cultures of C. reinhardtii immediately after inoculation. Dodecane instantly formed an upper ‘overlay’ phase as reported in several previous studies,18,23 while FC compounds sank under cultures to form ‘underlays’. FC compounds, however, resembled large bubbles under growing cultures and did not spread as evenly as dodecane on the surface, likely due to the liquid dynamics of water pressing on top of the FC liquids. The two-phase cultures were grown for 10 d in triplicates in 6-well microtiter plates on laboratory shakers as described above.Liquid–liquid extractions
To test whether the extracted patchoulol could be readily isolated from FC-3283 and enable fluorocarbon recycling, we performed liquid–liquid extractions with ethanol. Specifically, after the terpenoid capture experiments, the FC-3283 underlay from each microtiter well (∼500 μL) was recovered and transferred into a separate 1.5 mL microcentrifuge tube. The tubes were centrifuged at 3500×g for 5 min to separate the FC-3283 from algal cell debris. From each tube, 250 μL of the clean FC-3283 fraction was pipetted into a clean 2 mL microcentrifuge tube to which 250 μL of ethanol (96% vol.) was added. The fluorocarbon/ethanol mix was shaken at room temperature for 16 h at 1000 rpm using an Eppendorf ThermoMixer C (Eppendorf AG, Germany) to perform the liquid–liquid extraction. After the extraction, each sample was centrifuged at 3500×g for 5 min to separate the two liquid phases. From each sample, 90 μL of both fractions were pipetted into separate gas chromatography (GC) vials and stored at −20 °C until further analysis as described below.Gas chromatography
Dodecane and perfluoro liquids fractions were analyzed using a gas chromatograph equipped with a mass spectrometer and a flame ionization detector (GC-MS-FID) with minor modifications to a previously described protocol.23 Briefly, the GC-MS-FID analyses were performed using an Agilent 7890A gas chromatograph connected to a 5975C inert MSD with triple-axis detector. The system was equipped with a DB-5MS column (30 m × 0.25 mm i.d., 0.25 μm film thickness) (Agilent J&W, USA). The temperature profile was set to: injector (250 °C), interface (250 °C), and ion source (220 °C). 1 μL of sample was injected in splitless mode with an autosampler (Model G4513A, Agilent). Column flow was kept constant at 1 mL min−1 with helium as a carrier gas. The initial GC oven temperature of 80 °C was held for 1 min, then raised to 120 °C at a rate of 10 °C min−1, followed by 3 °C min−1 to 160 °C, and further to 240 °C at 10 °C min−1, which was held for 3 min. Mass spectra were recorded after a 13.2 min solvent delay using a scanning range of 50–750 m/z at 20 scans per s.Gas chromatograms were evaluated with MassHunter Workstation software version B.08.00 (Agilent Technologies, USA). The NIST library (National Institute of Standards and Technology, Gaithersburg, MD, USA) was used to identify patchoulol, along with verification using a purified patchoulol standard (Item: 18450, Cayman Chemical Company, MI, USA). Standard calibration curves in the range of 1–200 μM patchoulol in dodecane and FC-3283 were used for quantification. In dodecane samples only, 250 μM of α-humulene was additionally applied as internal standard. Extracted-ion chromatograms (XIC) with mass ranges of 91.00, 138.50, and 223.00 were used for samples with internal standard, and 138.00 and 222.00 for samples without internal standards. All GC-MS-FID measurements were performed in duplicate, and chromatograms were manually reviewed for quality control.
Results and discussion
Microbial engineering requires design-build-test-learn cycles as an iterative process of genetic engineering steps to identify optimal combinations of elements to drive cellular flux towards a desired product. These iterations require lengthy transformation and phenotypic screening that can be resource-heavy and laborious. Our engineering efforts in C. reinhardtii have relied on the use of dodecane for solvent-culture two-phase extraction of these products, which has served as a bio-compatible compound that can be readily separated and used directly in chromatography.3 Recently, it was shown that individual cells or groups of cells can be handled through encapsulation in microfluidic droplets to enable phenotyping without the need for selection agents.29 We were surprised to note that the perfluoro liquids used to make these droplets form a dense under-layer to the culture medium in large quantities. When incubated with our engineered C. reinhardtii cultures, we could observe the accumulation of heterologous isoprenoids in these perfluorocarbons. Here, we report on and characterize the suitability of perfluoro liquids (FCs) as alternatives to currently used petroleum solvents for microbial cell isoprenoid milking.Potential of FC-3283 to extract terpenoids from growing microbial cultures
We first cultivated a C. reinhardtii strain previously engineered to produce the heterologous sesquiterpenoid alcohol patchoulol23 with either a dodecane overlay or a commercially available perfluorocarbon liquid FC-3283 underlay (Fig. 1). The suitability of FC-3283 for patchoulol extraction for this culture is evident in the clear appearance of the product peak in GC-FID chromatograms in both solvents (Fig. 1a). Patchoulol was confirmed as the appropriate product by mass fractions. FC-3283 is more dense than water and was found underneath the culture medium in contrast to the lighter dodecane, which floats on top (Fig. 1b). Both solvents enabled the algal culture to grow, with cell densities increasing throughout cultivation in standard conditions and consistent linear electron flow in both, indicating healthy cultures (Fig. 1c and d). Healthy green culture is also noticeable in Fig. 1b. We then checked the suitability of FC-3283 for extraction of other heterologous isoprenoid products using strains previously engineered to produce taxadiene, casbene, and 13R(+) manoyl oxide.24 Each strain generated unique products in chromatograms (Fig. 1e), as previously observed for living extraction with dodecane.24 The results indicated that FC-3283 is bio-compatible and amenable to accumulating heterologous isoprenoids from our engineered algal cells.New options for two-phase cultivation with underlays
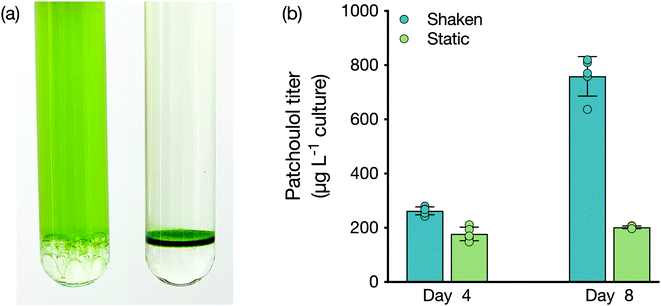
As a lower phase to growing liquid microbial culture, FCs present a new avenue for bio-process designs aimed at milking heterologous products. We investigated whether the lower phase could be used as a support for microbes to grow while concomitantly enabling extraction from the bio-film. We compared a shaken culture to a static culture for their abilities to produce patchoulol (Fig. 2). Both cultures grew, with the non-shaken culture accumulating a layer of cells at the medium–FC interface. After four days of cultivation, patchoulol concentration in the FC-3283 phase of the static culture was already significantly lower (177 ± 25 μg L−1 culture; mean ± SD) compared to the shaken culture (263 ± 14 μg L−1 culture) (t(8) = 6.56, p < 0.001). By day eight, overall patchoulol titers were four-fold lower in the static (202 ± 5 μg L−1 culture) than in the mixed culture (759 ± 73 μg L−1 culture) (t(8) = 17.05, p < 0.0001) (Fig. 2b). It seems logical that static cultures would have lower overall production rates, as only the cells close to the interface would interact with the FC. Future concepts may involve some amount of mixing and settling, or agitation of the FC liquid below the culture to increase this interaction. The ability to grow organisms at the solvent interface could enable the cultivation of mat-forming organisms and the extraction of their natural products. For example, the members of the genus Botryococcus, often found on the surface of stagnant waters, are slow-growing algae that can secrete alkadienes and alkatrienes or botryococene isoprenoids.30–32 Cultures set up with larger surface area interaction between medium and FC may enable interesting process designs for milking secreted products.Liquid–liquid extraction of isoprenoids from FC-3283
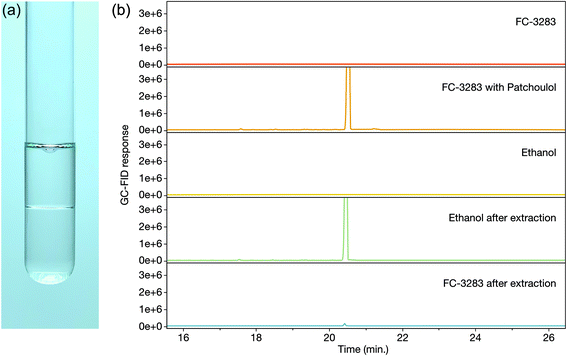
Once accumulated in a solvent, it is desired to extract and isolate the target chemicals and recycle the solvent for further use. With dodecane, silica-based solid phase extraction (SPE) allows patchoulol to be isolated from the solvent due to its hydroxyl group, however, separation is more challenging with non-functionalized isoprenoids. This challenge is different for every target compound, but many sesqui- and diterpenoids have similar chemical properties to the C12 dodecane, making processes like distillation or molecular separation more challenging. We sought to determine how readily we could isolate target isoprenoids from FC-3283 while regenerating the FC (Fig. 3). As it is immiscible with ethanol (Fig. 3a), we performed liquid–liquid extraction on FC-3283 which had accumulated patchoulol from a C. reinhardtii cultivation. Surprisingly, patchoulol partitioned readily into the ethanol phase (Fig. 3b), which phase-separated from FC-3283 after extraction. We found that the process can be completed in as fast as 2 hours of incubation (data not shown), indicating the potential to obtain formulated isoprenoid product from microbial cultures in as little as two downstream process steps.Extraction potential of other fluorinated liquids
Encouraged by the results of FC-3283 microbial milking, we cultivated C. reinhardtii strains producing multiple heterologous isoprenoids with other perfluorocarbon heat-transfer liquids FC-40 and FC-770 (Fig. 4). The different FCs have unique physical properties and exhibited variable performance in extracting different isoprenoids from the algal cells (Fig. 4). Each fluorocarbon resulted in varying extraction rates for each isoprenoid (Fig. 4), and these behaviors will enable tailoring of bio-processes to specific product chemistries giving the operator several choices in two-phase synthetic solvents. FC-770, for example, routinely exhibited product peaks in GC chromatograms which were absent or in reduced quantities for the other FCs (ESI Fig. 1†). Perhaps this represents a more accurate profile of side-product isoprenoids produced by these strains. Indeed, chromatograms of FCs contain much fewer background peaks and contaminants compared to n-dodecane samples which also makes characterization more straightforward (Fig. 1a). We also found that the liquid–liquid extraction with ethanol was highly effective for all analyzed isoprenoids, regardless of the FC compound used or the chemical nature of the isoprenoid. We were able to recover patchoulol into ethanol at efficiencies >99%, while for other terpenoids the efficiency ranged from 95–98% (ESI Fig. 2†), indicating a greater practical value than traditional solvents.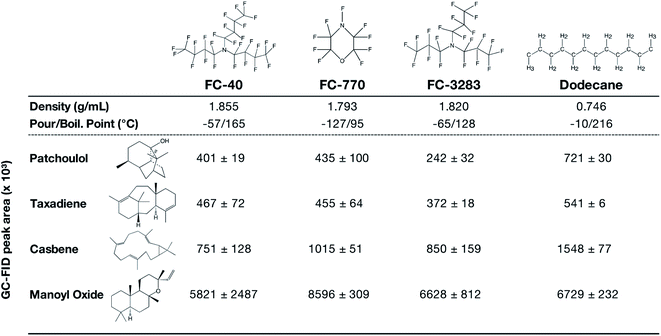 | ||
| Fig. 4 Chemical structures and properties of commercial FC liquids and dodecane that were investigated in this work. Indicated underneath are GC-FID peak areas of different terpenoids after 10 d of 2-phase living C. reinhardtii extraction cultures with each solvent. Peak areas represent mean ± SD (n = 5). The chemical structures of FC liquids were obtained from published literature (FC-40;33 FC-770 & FC-328334). All Chemical structures were drawn using ChemDraw v20.1. | ||
Compared to dodecane, most FCs were less efficient in isoprenoid extraction from the algal cells in batch culture (Fig. 4). This can likely be an acceptable compromise in bio-process designs when considering the efficiencies of liquid–liquid extractability of the isoprenoid products from the FCs (Fig. 3). Cultures with FC-3283 overlays also exhibited higher linear electron flow rates than those with dodecane throughout batch cultivation (Fig. 1d), indicating FC-3283 is less aggressive as a two-phase culture system than dodecane. It could be speculated that higher efficiencies of extraction with dodecane are due to microbial lysis rather than efficient partitioning, although this aspect was not investigated here.
Conclusions
Here, we have presented evidence that commercially available heat-transfer perfluorocarbons can be used as bio-compatible liquids for microbial cell milking of heterologous isoprenoids. The FCs tested here showed capacities for easier handling, consistent product accumulation, and ease of product isolation compared to petroleum-based solvent counterparts. As dense liquids, FCs form an underlay with microbial cultures, which opens new avenues for bio-process designs, especially for slow-growing or mat-forming microbes. The use of FC liquids in microbial metabolite milking permits inert, room temperature extraction conditions and likely provides a route to well-preserved natural product extraction from microbial cells. Here, we have used engineered microalgae as a model organism, however, these results could have applicability in the synthetic biology and metabolic engineering fields for a broad spectrum of microbes.Author contributions
SO performed the experiments, and contributed to the research planning and design. The research was conducted in the laboratory of KJL, who conceived the study and contributed to experiment planning. Both authors contributed to the writing of this manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to express special thanks to Najeh Kharbatia of the KAUST Analytical Core Labs for helpful early discussions, Chandrasekaren Lakshmipathy, Abdulkhalik Khalifa, and Abdullah Alabdullatif of KAUST Lab Equipment Maintenance (LEM) team for assistance in upgrading and initializing the GC-FID-MS unit. The authors acknowledge Prof. Dr Ralph Bock and Dr Juliane Neupert for providing C. reinhardtii UVM4, obtained under material transfer agreement between KAUST and the Max-Planck-Institut für Molekulare Pflanzenphysiologie Potsdam. Diterpenoid production strains of C. reinhardtii used in this work were developed by KJL in the lab of Prof. Dr Olaf Kruse at the Center for Biotechnology, Universität Bielefeld as referenced in the manuscript. The research reported in this publication was supported by the KAUST Impact Acceleration Funds program (grant 4238) and KAUST baseline funding awarded to Kyle Lauersen.References
- G. Daletos, C. Katsimpouras and G. Stephanopoulos, Trends Biotechnol., 2020, 38, 811–822 CrossRef CAS PubMed
.
- S. A. E. Heider, P. Peters-Wendisch and V. F. Wendisch, BMC Microbiol., 2012, 12 Search PubMed
.
- K. J. Lauersen, Planta, 2019, 249, 155–180 CrossRef CAS PubMed
.
- G. R. Navale, M. S. Dharne and S. S. Shinde, Appl. Microbiol. Biotechnol., 2021, 105, 457–475 CrossRef CAS PubMed
.
- S. Wolf, J. Becker, Y. Tsuge, H. Kawaguchi, A. Kondo, J. Marienhagen, M. Bott, V. F. Wendisch and C. Wittmann, Essays Biochem., 2021, 65, 197–212 CrossRef CAS PubMed
.
- X. Zhan, Y. H. Zhang, D. F. Chen and H. T. Simonsen, Front. Plant Sci., 2014, 5, 636 Search PubMed
.
- J. Wichmann, K. J. Lauersen and O. Kruse, Curr. Opin. Biotechnol., 2020, 61, 28–37 CrossRef CAS PubMed
.
- J. Gershenzon and N. Dudareva, Nat. Chem. Biol., 2007, 3, 408–414 CrossRef CAS PubMed
.
- J. Kirby and J. D. Keasling, Annu. Rev. Plant Biol., 2009, 60, 335–355 CrossRef CAS PubMed
.
- S. B. Jensen, S. Thodberg, S. Parween, M. E. Moses, C. C. Hansen, J. Thomsen, M. B. Sletfjerding, C. Knudsen, R. Del Giudice, P. M. Lund, P. R. Castano, Y. G. Bustamante, M. N. R. Velazquez, F. S. Jorgensen, A. V. Pandey, T. Laursen, B. L. Moller and N. S. Hatzakis, Nat. Commun., 2021, 12, 2260 CrossRef CAS PubMed
.
- A. Wlodarczyk, T. Gnanasekaran, A. Z. Nielsen, N. N. Zulu, S. B. Mellor, M. Luckner, J. F. B. Thofner, C. E. Olsen, M. S. Mottawie, M. Burow, M. Pribil, I. Feussner, B. L. Moller and P. E. Jensen, Metab. Eng., 2016, 33, 1–11 CrossRef CAS PubMed
.
- Y. Yamada, T. Kuzuyama, M. Komatsu, K. Shin-Ya, S. Omura, D. E. Cane and H. Ikeda, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 857–862 CrossRef CAS PubMed
.
- G. Wei, F. Eberl, X. Chen, C. Zhang, S. B. Unsicker, T. G. Kollner, J. Gershenzon and F. Chen, Sci. Rep., 2020, 10, 14944 CrossRef CAS PubMed
.
- F. Beran, P. Rahfeld, K. Luck, R. Nagel, H. Vogel, N. Wielsch, S. Irmisch, S. Ramasamy, J. Gershenzon, D. G. Heckel and T. G. Kollner, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 2922–2927 CrossRef CAS PubMed
.
- A. B. Gilg, C. Tittiger and G. J. Blomquist, Naturwissenschaften, 2009, 96, 731–735 CrossRef CAS PubMed
.
- M. Li, F. Hou, T. Wu, X. Jiang, F. Li, H. Liu, M. Xian and H. Zhang, Nat. Prod. Rep., 2020, 37, 80–99 RSC
.
- P. P. Peralta-Yahya, M. Ouellet, R. Chan, A. Mukhopadhyay, J. D. Keasling and T. S. Lee, Nat. Commun., 2011, 2, 483 CrossRef PubMed
.
- J. Beekwilder, A. van Houwelingen, K. Cankar, A. D. J. van Dijk, R. M. de Jong, G. Stoopen, H. Bouwmeester, J. Achkar, T. Sonke and D. Bosch, Plant Biotechnol. J., 2014, 12, 174–182 CrossRef CAS PubMed
.
- W. Liu, X. Xu, R. Zhang, T. Cheng, Y. Cao, X. Li, J. Guo, H. Liu and M. Xian, Biotechnol. Biofuels, 2016, 9, 131 CrossRef PubMed
.
- I. S. Yunus and P. R. Jones, Metab. Eng., 2018, 49, 59–68 CrossRef CAS PubMed
.
- M. Choi and K. Cho, Int. J. Heat Mass Transfer, 2000, 43, 209–218 CrossRef CAS
.
- H. Jouhara and A. J. Robinson, Appl. Therm. Eng., 2010, 30, 201–211 CrossRef CAS
.
- K. J. Lauersen, T. Baier, J. Wichmann, R. Wordenweber, J. H. Mussgnug, W. Hubner, T. Huser and O. Kruse, Metab. Eng., 2016, 38, 331–343 CrossRef CAS PubMed
.
- K. J. Lauersen, J. Wichmann, T. Baier, S. C. Kampranis, I. Pateraki, B. L. Moller and O. Kruse, Metab. Eng., 2018, 49, 116–127 CrossRef CAS PubMed
.
- J. Neupert, D. Karcher and R. Bock, Plant J., 2009, 57, 1140–1150 CrossRef CAS PubMed
.
- D. S. Gorman and R. Levine, Proc. Natl. Acad. Sci. U. S. A., 1965, 54, 1665 CrossRef CAS PubMed
.
- B. Genty, J. M. Briantais and N. R. Baker, Biochim. Biophys. Acta, 1989, 990, 87–92 CrossRef CAS
.
- N. R. Baker, Annu. Rev. Plant Biol., 2008, 59, 89–113 CrossRef CAS PubMed
.
- Z. Y. Yu, K. Geisler, T. Leontidou, R. E. B. Young, S. E. Vonlanthen, S. Purton, C. Abell and A. G. Smith, Algal Res., 2021, 56 Search PubMed
.
- E. Eroglu and A. Melis, Bioresour. Technol., 2010, 101, 2359–2366 CrossRef CAS PubMed
.
- J. Templier, C. Largeau and E. Casadevall, Phytochemistry, 1991, 30, 2209–2215 CrossRef CAS
.
- E. Lozoya-Gloria, X. Morales-de la Cruz and T. A. Ozawa-Uyeda, in Microalgae: From Physiology to Application, ed. M. Vítová, IntechOpen, London, UK, 2019, pp. 123–144 Search PubMed
.
- W. L. Hasi, Z. W. Lu, S. Gong, S. J. Liu, Q. Li and W. M. He, Appl. Opt., 2008, 47, 1010–1014 CrossRef CAS PubMed
.
- W. L. J. Hasi, X. Y. Wang, S. X. Cheng, Z. M. Zhong, Z. Qiao, Z. X. Zheng, D. Y. Lin, W. M. He and Z. W. Lu, Laser Part. Beams, 2013, 31, 301–305 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01112c |
| This journal is © The Royal Society of Chemistry 2022 |