Open Access Article
Open Access ArticleReview on the preparation and application of lignin-based carbon aerogels
Cai-Wen Wu
 ab,
Peng-Hui Li
ab,
Peng-Hui Li
 ab,
Yu-Meng Wei
b,
Chi Yang
b and
Wen-Juan Wu
*ab
ab,
Yu-Meng Wei
b,
Chi Yang
b and
Wen-Juan Wu
*ab
aJiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Nanjing Forestry University, Nanjing, 210037, P. R. China. E-mail: wenjuanwu@njfu.edu.cn
bCollege of Light Industry and Food Engineering, Nanjing Forestry University, Nanjing, 210037, P. R. China
First published on 7th April 2022
Abstract
Carbon aerogels (CAs) are excellent carrier materials with a large surface area and high porosity. In addition to the above-mentioned wonderful characteristics, aerogel with lignin as a precursor is also a material with high bioactivity and degradability. Lignin carbon aerogels (LCAs) have a wide range of applications, and can be used in supercapacitors, adsorbents and catalysts, etc., but their preparation process is more complex. In this paper, we review the preparation and influencing factors of LCAs, analyze their properties and structural characterization, and aim to provide references for the optimal preparation, effective characterization, and expansion of applications of LCAs.
1 Introduction
The production of value-added chemicals and materials from green and renewable resources is in line with the concept of the circular economy while stimulating global economic growth and being quite friendly to the environment.1,2 Lignocellulosic biomass is abundant, widely distributed and readily available and is considered to be a unique sustainable organic carbon source for the natural world.3 In the pulp and paper industry, lignin is often treated as waste material and is mostly burned to produce steam energy for the pulping process. Along with cellulose and hemicellulose, lignin is described as an extremely complex three-dimensional reticulated chemical structure of a macromolecule called among the three main components of lignocellulosic biomass.4 Given the complicated structure, the reactive regime of lignin conversion has been a hot topic for scientists to explore.5 Lignin mainly consists of three basic components: guaiacyl unit (G), syringl unit (S), and p-hydroxyphenyl unit (H). They are linked by strong C–C bonds and unstable C–O–C bonds.6 Lignin has a high carbon content (40–60%) and can be used as an ideal precursor for activated carbon.7 Lignin activated carbon materials have many properties, such as high specific surface area, graded pore structure, high porosity and high electrical conductivity. Due to the demand for stable and lightweight carbon electrodes for energy storage applications, and a more efficient adsorption effect with catalytic activity, lignin carbon aerogels (LCAs) were created.8Carbon aerogels (CAs) were first reported in 19929 and a new class of perforated carbon materials with huge specific surface area, large porosity, ultra-low density (0.003 g cm−3) and good electrical conductivity. CAs are usually obtained by carbonization of organic aerogels under an inert atmosphere, and their porous properties lead to applications in adsorption,10–13 energy storage,8,14,15 catalysis,16,17 etc. There are still many challenges in the preparation of CAs, e.g., expensive fabrication costs, redundant preparation processes and noxious precursors, which limit the commercialization of CAs.18 Lignin is phenolic in nature and extremely cheap, and its high reactivity is attributed to the presence of various reactive functional groups, such as carboxyl, carbonyl and hydroxyl groups. Due to the above advantages of lignin, inexpensive and green biomass CAs can be prepared.19 This review will be based on the preparation and application of lignin-based carbon aerogels, firstly introducing this viewpoint through the preparation of organic carbon aerogels in general, declaring that the raw material price of lignin is much lower than that of m-diphenol, as well as elaborating the structural and compositional characteristics of lignin to provide a strong viewpoint for the preparation of carbon aerogels from lignin. After that, it will focus on the applications of lignin-based carbon aerogels in supercapacitors, adsorbents, and catalysts, respectively, to dissect its functional properties and provide an outlook on future applications.
2 Preparation of LCAs
At present, the main industrial lignin produced in large quantities in the paper industry are kraft lignin, lignin sulfonate, soda lignin, organosolv lignin, which is obtained by separation and purification and other steps, and then used for high quality and high-value applications. The structure of lignin often varies depending on the plant species and its environment.20Lignin is a complex three-dimensional phenolic polymer formed by phenyl propane units linked by C–C or C–O–C bonds, which contains a variety of reactive functional groups, such as carbonyl, carboxyl and hydroxyl groups so that lignin has phenolic properties. By density flooding theory (DFT) analysis, the magnitude of the bond dissociation energy in lignin is ranked as Cα–O < Cβ–O < Cα–Cβ < other connecting bonds.21 During lignin treatment, the bonds in which the chemical bonds require the least energy to break are the α-O-4 and β-O-4 bonds, respectively, which break easily and produce hydroxyl groups.4,22
Conventional CAs are prepared according to the chemical reaction steps of phenolic resin formation: resorcinol and its derivatives, formaldehyde and its derivatives are used as precursors, and then prepared by sol–gel process, solvent replacement and drying, carbonization, activation treatment and other steps.23 The formation mechanism of organic aerogels reported in the literature is shown in Fig. 1.
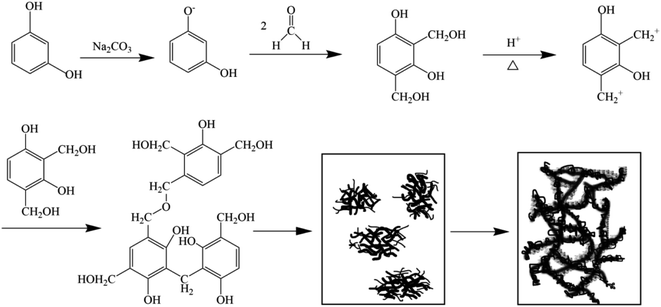 | ||
| Fig. 1 Diagram of the formation mechanism of organic aerogel.23 | ||
Hydroxymethyl derivatives of resorcinol are obtained by using resorcinol or its derivatives to add them with formaldehyde or its derivatives, supplemented with an acid condition. The hydroxymethyl derivatives undergo a polycondensation reaction with each other to produce matrix particles linked by methylene bonds (–CH2–) or methylene ether bonds (–CH2–O–CH2–). The matrix particles are then further wrapped to form clusters, which are further condensed into network-like polymers to form the three-dimensional structure of the CAs.24 The structure and composition of lignin have a large influence on the gelation process, compared to the molecular weight of lignin, which has a very small effect (These will be specifically mentioned below). The phenolic hydroxyl content of lignin is related to the reactivity of formaldehyde, and the phenolic hydroxyl position is related to the pore structure. For the stability of the network structure in the gel during the subsequent drying process, methanol, ethanol, and isopropanol solvents are generally used to replace the solvent (Inorganic solvent). After the replacement, the organogel is dried, usually supercritically, after which the aerogel is formed.25 The most important step is the charring of the aerogel, which is placed under inert gas or vacuum conditions for calcination, forming a carbon aerogel. Some specific charring conditions can vary from one material to another with different pre-treatment works, so the conditions (e.g., charring temperature, heating rate, charring time) need to be optimized.26 The composition of lignin is related to the specific surface area, mesopores and micropores of CAs. The next step can be physical activation (generally CO2 activation), which can increase the microporosity of CAs and enhance it with the increase of activation time;27 or, chemical activation (generally KOH activation) is more likely to facilitate the formation of CAs microporosity and produce CAs with high surface area.28 Both of the above activation methods can effectively enhance the performance of CAs and optimize their pore structure and surface topography.
As the conditions described above, resorcinol can be well prepared for organic carbon aerogels, but resorcinol is expensive and polluting relative to lignin. Studies have reported that approximately 80% of the total cost of preparing organic carbon aerogels is spent on obtaining raw materials.29 Therefore, we believe that the costs incurred by conventional materials in the production cycle are much higher than those expected for lignin as a raw material. Lignin is an inexpensive byproduct of pulp and biorefinery processes and is considered as a substitute for resorcinol carbon feedstock.
Different sources and processing methods result in significant differences in the chemical structure of lignin, and thus the effect of different species of lignin on LCAs products. Softwoods contain more lignin than hardwoods, while grasses contain the least amount of lignin. Compared to lignin in broadleaf and grasses, softwood lignin has a high ratio of G-units (nearly 95%) and only one methoxy in the phenolic hydroxyl neighbor of the G-unit, which readily forms a 5–5′ bond, resulting in a high degree of carbon chain branching.30 Softwood lignin is a network polymer with one branching unit in every 2–3 C9 units. Harwood lignin has a less biphenyl structure compared to softwood lignin, with one branching point in every four C9 units, and hardwood lignin is a highly branched polymer.31 Different lignin S/G units from different sources, hardwood (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), softwood (1
1), softwood (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–1
2–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), and grasses (1
3), and grasses (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The lignin of softwoods and grasses also contain a proportion of H-units. Two methoxy groups next to the phenolic group on the aromatic ring of the S-unit to avoid the formation of 5–5 or dibenzodioxins bonds.32 Hu et al.33 compared a variety of lignins isolated from softwoods, hardwoods, and graminoids and found that hardwood lignins were mainly composed of lilac and guaiacyl units connected by β-O-4, β-5, and β–β bonds. A large number of β-O-4 bonds were broken during the pulping process and there was a large number of condensed structures. Softwood lignans have stronger C–C bonds at the C5 position of the guaiacyl subunit compared to hardwood lignans. Grass-like lignin consists of zwitterionic, guaiacyl and p-hydroxyphenyl units as well as p-coumarate and ferulic acid salt structures. Due to different preparation processes, the cellulolytic enzyme corn stover lignin has 30 ferulate structures and 35 p-coumarate structures per 100 C9 units. p-coumarate structures are more susceptible to cleavage than ferulate structures. Therefore, the degradation of softwood lignin should produce more phenolic hydroxyl groups than hardwood lignin, which is easier to react with formaldehyde. At present, with the advancement of social processes, industrial lignin production should not be underestimated. In the pulping process, the β-O-4 bond is easily broken due to the reagents, and the obtained lignin contains more phenolic hydroxyl groups than natural lignin.34
2). The lignin of softwoods and grasses also contain a proportion of H-units. Two methoxy groups next to the phenolic group on the aromatic ring of the S-unit to avoid the formation of 5–5 or dibenzodioxins bonds.32 Hu et al.33 compared a variety of lignins isolated from softwoods, hardwoods, and graminoids and found that hardwood lignins were mainly composed of lilac and guaiacyl units connected by β-O-4, β-5, and β–β bonds. A large number of β-O-4 bonds were broken during the pulping process and there was a large number of condensed structures. Softwood lignans have stronger C–C bonds at the C5 position of the guaiacyl subunit compared to hardwood lignans. Grass-like lignin consists of zwitterionic, guaiacyl and p-hydroxyphenyl units as well as p-coumarate and ferulic acid salt structures. Due to different preparation processes, the cellulolytic enzyme corn stover lignin has 30 ferulate structures and 35 p-coumarate structures per 100 C9 units. p-coumarate structures are more susceptible to cleavage than ferulate structures. Therefore, the degradation of softwood lignin should produce more phenolic hydroxyl groups than hardwood lignin, which is easier to react with formaldehyde. At present, with the advancement of social processes, industrial lignin production should not be underestimated. In the pulping process, the β-O-4 bond is easily broken due to the reagents, and the obtained lignin contains more phenolic hydroxyl groups than natural lignin.34
In the pulping process, industrial lignin has different molecular weights, functional groups and physicochemical properties, classified as: alkaline lignin (AL), lignin sulfonate (LS), kraft lignin (KL), enzymatic hydrolysis lignin (EHL). AL has a molecular weight roughly between 1000 and 2000, and is produced by the soda ash or soda anthraquinone pulping process at a certain temperature. AL does not contain elemental sulfur. LS usually has a high molecular weight and is a by-product of the sulfite method of papermaking wood pulp with sulfonate groups on the lignin side chains that are readily soluble in water. KL also contains a small amount of sulfur and is obtained using the kraft pulping process. EHL is natural lignin that can be extracted from crop wastes used in the preparation of bioethanol. It retains to a large extent its original structure and has a higher reactivity. Although the pyrolysis chemistry of various lignin derivatives is the same, the type of lignin has a significant effect on the structure and morphology of the resulting carbon aerogels.35 It has been shown that compared to Ethanol Organosolv Lignin (EL) and Formic Acid/Acetic Acid Organosolv Lignin (FAL), AL has a better macromolecular chain orientation and is more suitable for the preparation of carbon materials, due to the fact that EL has the smallest molecular weight and is The molecular structure of FAL contains more side chains and the product has poor mechanical properties. AL has fewer side chains in its molecular structure and has the highest degree of graphitization.36 Sulfate alkaline lignin comes from the sulfate method of pulping containing a large amount of ash as well as the presence of NaOH. bioethanol lignin has little ash, and when KOH is used as a porogenic agent, the latter requires the addition of NaOH to obtain a graded porous structure.37
The polydispersity of KL (3.38) was greater than that of organic solvent lignin (2.04), so the polydispersity of organic solvent lignin was easier to react with formaldehyde. Moreover, the time of gel formation of organosolvent lignin was 14 hours less than that of KL. Lignin was involved in the step-growth polymerization reaction (Fig. 2) during the formation of carbon aerogels, similar to the reaction of resorcinol with formaldehyde mentioned above .29
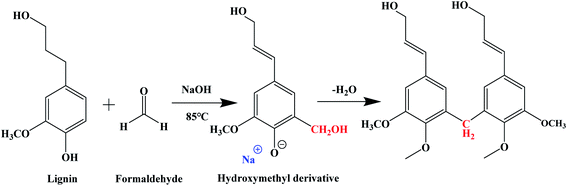 | ||
| Fig. 2 Chemical reaction scheme of lignin and formaldehyde under NaOH catalyst.29 | ||
The polymerization time of lignin depends on the lignin structure related to the ratio of G and H units and the molecular weight size. Since coniferous and broad-leaved woods have mainly G and S units, there are 35 phenolic hydroxyl groups in 100 phenol propane on average, and these are the active sites that can be directly polymerized.38
However, the active sites are still few, the reactivity of lignin with formaldehyde is low, and the lignin space-site resistance is high, so lignin does not gel completely. In Yang's study,29 it was found that organic solvent lignin contains more methoxy than KL, and the cross-linked structure affects the reaction with formaldehyde.39 The conclusion that the structure within the lignin is more important than the molecular weight and polydispersity was obtained. Therefore, we can use the different structural and physicochemical properties of lignin and its lignin derivatives to design specific methods to synthesize carbon aerogels for supercapacitors, dyes, adsorption and other applications.
The following figure shows SEM images of carbon aerogels prepared from different lignin sources. The lignin-based carbon aerogels are porous as seen from SEM images. Hydrogels of sulfate with organic solvents lignin and formaldehyde were prepared by sol–gel condensation method, solvent exchange, freeze-drying and carbonization. The sulfate lignin-based carbon aerogels formed mesopores (Fig. 3a). The pore structure of organosolvent lignin-based carbon aerogel shrinks unevenly (Fig. 3b), and in combination, the specific surface area of sulfate lignin-based carbon aerogel should be larger than that of organosolvent lignin-based carbon aerogel. A mixture of enzymatically hydrolyzed lignin, resorcinol, and formaldehyde was subjected to gelation, aging, and environmental drying. The carbon aerogel particles are spherical in shape and there is partial adhesion between the particles, which helps the aerogel to form an interconnected porous network structure (Fig. 3c) and the carbon aerogel is used for supercapacitor electrodes. Fig. 3d shows the honeycomb microstructure of carbon aerogel cross-section formed by lignin (80%) and cellulose nanofibers (20%) in a unidirectional ice template process with 3D macroporous, honeycomb interconnected structure, which helps to form a more effective double electric layer for energy storage applications. Fig. 3e shows the oriented synthetic lignosulfonate-chitosan highly dispersed carbon aerogel, which forms carbon “fullerene-like” with LS at 600 °C. The carbon gel has low crystallinity and high enough specific surface area to be used as a material for conductive properties. Fig. 3f shows the cross section of lignin/TOCNF carbon aerogel (TOCNF concentration is 8% of total dry weight), lignin and TOCNF will be suspended ice template, freeze-dried, carbonized, the figure has obvious anisotropic macroporous structure, conducive to gas–liquid adsorption and thermal insulation, etc., can be used for carbon dioxide capture and energy storage.
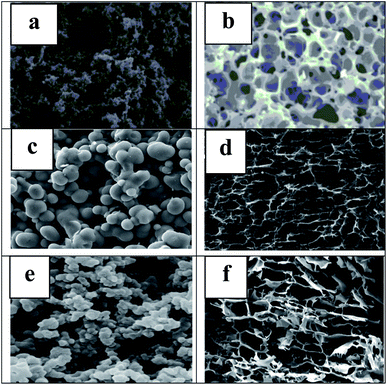 | ||
| Fig. 3 Prepared SEM images: sulfate lignin-based carbon aerogel (a), organic solvent lignin-based carbon aerogel (b),29 enzymatic hydrolysis lignin-based carbon aerogel (c),41 soda lignin-based carbon aerogel (d),8 lignosulfonates based carbon aerogel (e)56 Kraft lignin & TEMPO-oxidized cellulose nanofibers carbon aerogel (f).43 | ||
If lignin or degraded lignin (with many phenolic hydroxyl groups) is used to mostly or completely replace resorcinol and its derivatives in the preparation of CAs (see Fig. 4). Firstly, the cost of reagents can be saved because lignin itself is a waste from the papermaking process, and secondly, it is quite friendly to the environment due to the biodegradability of lignin-based carbon materials. Finally, the inherent three-dimensional structure of lignin would allow the prepared CAs to possess more remarkable properties. In addition, chemical modification of lignin to change its spatial network structure produces high-performance CAs that can be substituted for more expensive carbon materials with resin-based products, etc.
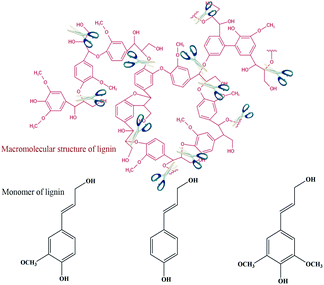 | ||
| Fig. 4 Mechanism of ether bonding for lignin degradation and bond breaking and lignin-forming monomers. | ||
3 Application of LCAs
With unique three-dimensional network structure and outstanding characteristics such as suitable pore size and large specific surface area, LCAs have wonderful applications in supercapacitors,40,41 adsorbents (dyes,42 carbon dioxide,43 oil/organic compound,44 heavy metal ion (graphene hydrogel)45), and catalyst/catalyst carriers (Fig. 5).46,47 The application of LCAs has also attracted the attention of many scholars,48,49 but they do not summarize the specific aspects of lignin in-depth, and many literature reviews focus on the general overview of biomass precursors.The following table provides an overview of recent advances in CAs using lignin as a precursor(Table 1).
| Raw materials | Preparation methods | Applications | Ref. |
|---|---|---|---|
| Alkali lignin | Lignin + KOH | Supercapacitor | 40 |
| Ultra-fast freeze drying | |||
| Carbonization | |||
| Enzymatic hydrolysis lignin | Sol–gel polymerization | Supercapacitor | 41 |
| Aging | |||
| Solvent exchange | |||
| Dry environment (50 °C) | |||
| Carbonization | |||
| KOH revitalization | |||
| Alkali lignin | Sol–gel polymerization (adding Cu ions) | Supercapacitor | 52 |
| Microwave reaction | |||
| Freeze-drying | |||
| Carbonization | |||
| Alkali lignin | Sol–gel polymerization (adding Mn ions) | Supercapacitor | 53 |
| Microwave reaction | |||
| Freeze-drying | |||
| Carbonization | |||
| Sodium lignosulfonate | Homogeneous dispersion in the “double helix” structure of carrageenan | Supercapacitor | 54 |
| Carbonization | |||
| Revitalization | |||
| Technical soda lignin | Polymerization on nickel foam under ZnCl2 hypersaline conditions | Supercapacitor electrodes | 55 |
| Vacuum drying | |||
| Carbonization | |||
| Lignosulphonate | Aqueous solutions of anionic polyelectrolyte (PE) lignosulphonate and acetic acid cationic PE chitosan were mixed | Conductive dispersed particles | 56 |
| Water was displaced with acetone | |||
| Dried in a stream of carbon dioxide (CO2) under supercritical conditions | |||
| Carbonization | |||
| Sodium lignin sulfonate | Sol–gel polymerization | Methylene-blue removal | 42 |
| Gelling and aging | |||
| Freeze-drying | |||
| Carbonization | |||
| Revitalization | |||
| Kraft lignin | Ice-templating | CO2 capture and energy storage | 43 |
| Mechanical mixing | |||
| Freeze-drying | |||
| Carbonization | |||
| Alkali lignin | Gel polymerization | Esterification catalyst | 47 |
| Freeze-drying | |||
| Carbonization | |||
| Purchased lignin | Lignin, furfural and water mixtures were mixed | Esterification catalyst | 64 |
| Solvent exchange | |||
| Freeze-drying | |||
| Carbonization | |||
| Kraft lignin | Lignin/CNF suspension | CO2 capture | 59 |
| Ice-templating | |||
| Freeze-drying | |||
| Carbonization | |||
| Alkali lignin | Graphene oxide was prepared by copolymerization | Oil/water separation | 44 |
| Lyophilized and vacuum dried | |||
| Annealing | |||
| Lignin from corncob | Lignin-modified graphene aerogel | Absorbents for oil and organic solvents | 60 |
| Oscillating ultrasound | |||
| Freeze-drying | |||
| Carbonization | |||
| Alkali lignin | Magnetic stirring | Ultrahigh electromagnetic interference shielding | 65 |
| Freeze-drying | |||
| Carbonization | |||
| Alkali lignin | Mixing alkali lignin and CNF | Pressure sensing and energy storage | 66 |
| Directional freezing-casting | |||
| Vacuum freeze-drying | |||
| Carbonization | |||
| Purchased lignin | Lignin–CNC–GO suspension | Detect bio-signals | 67 |
| Bidirectional freezing | |||
| Freeze-drying | |||
| Carbonization pyrolysis | |||
| Sulfonomethylated lignin | Ultrasonic treatment, mechanical stirring | Capture and recovery of phosphate | 70 |
| Freeze-drying | |||
| Calcination |
3.1 Supercapacitor
CAs has very good properties such as low density, strong electrical conductivity, large surface area and low thermal conductivity, and these excellent properties make them promising as electrode materials for rechargeable batteries or supercapacitors. (For example, activated carbon is used as the active electrode material of supercapacitor, and its SSA is about 1000–2000 m2 g−1.50 Graphene material used as the active electrode material of supercapacitor can produce a volume capacitance of 92 F cm−3, a mass specific capacitance of 231 F g−1 and an energy density of 98 wh kg−1.51 The application of these materials provides an important reference significance for the development of carbon electrode materials for supercapacitors).LCAs have many applications in electrical double layer capacitors and are a promising material for energy storage electrodes. Zhang et al.40 prepared CAs quickly and easily by ultrafast freezing of lignin with the KOH solution droplet technique. This simple method is more beneficial to reduce the amount of KOH compared to the conventional method. The prepared LCAs have a rewarding specific surface area (1681.6 m2 g−1) and a large number of large mesopores, which facilitate the free movement of electrolytes and facilitate charge transport. The specific capacitance of the tested LCAs exceeds 189 F g−1. The oxygen content in LCAs is also high (12.92 at%), allowing a large range of Faraday redox reactions, which makes the CAs possess superb electrochemical performance as supercapacitor electrodes.
Enzymatic hydrolysis lignin is natural lignin obtained by enzymatic hydrolysis of biomass raw materials to remove sugars, which has most of the structure of the original lignin and therefore has high reactivity. Xu et al.41 prepared LCAs with a specific surface area of 779 m2 g−1 from enzymatic hydrolysis lignin, resorcinol and formaldehyde (see Fig. 6). The specific capacitance reached 142.8 F g−1 at a current density of 0.5 A g−1. Incredibly, at a high current density of 10 A g−1, the specific capacitance of LCAs electrodes was 112.5 F g−1. After 2000 charge/discharge cycles, the specific capacitance remained 96% of its original value, showing outstanding endurance. Many of the above conditions show that LCAs will play a great advantage as supercapacitor electrodes. In addition, their group also improved the pore structure of LCAs by introducing metal ions with alkali lignin as precursors. (i) The specific surface area of LCAs obtained after adding Cu ions (CuCl2·6H2O) and CO2 activation is 899 m2 g−1, and the high specific capacitance is 257.65 F g−1. The microporosity of LCAs reaches 66.7%. At a current density of 20 A g−1, the specific capacitance is always maintained at 95% after 2000 cycles.52 (ii) With the addition of Mn ions (MnCl2·6H2O), the resulting LCAs have a specific surface area of 582 m2 g−1, a total pore volume of 0.35 cm3 g−1, and a high specific capacitance of 186 F g−1. The electrochemical performance of the LCAs is better, and the specific capacitance is maintained at 96% after 2000 cycles.53 In summary, it can be seen that lignin composite CAs with improved pore structure show great application in supercapacitors.
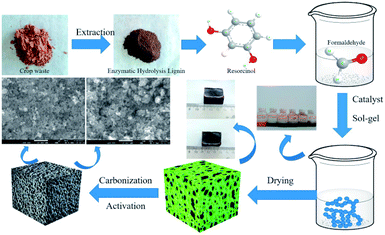 | ||
| Fig. 6 Schematic diagram of the preparation of layered porous LCAs.41 | ||
In addition, doping nitrogen in LCAs enhances the conductivity, which is helpful for the performance of supercapacitors. Nitrogen groups improve permeability and conductivity, enhance ion transport and further expand specific surface area. Lv et al.54 used sodium lignosulfonate as the carbon source and embedded carrageenan gum to prepare high nitrogen-containing LCAs. The specific capacitance of LCAs was as high as 231.8 F g−1 at a current density of 0.5 A g−1, and the capacitance was maintained at 85.7% after 5000 cycles.
An important factor affecting the electrochemical performance of supercapacitor electrodes is the electrode thickness. Guo et al.55 prepared lignin carbon aerogel/nickel (LCAN) bulk ultra-thick cubic electrodes by polymerization under ZnCl2 high salt conditions. The combination of LCAN networks prepared 4.2 mm thick electrodes with an ultra-high area capacitance of 26.6 F cm−2.
Brovko et al.56 designed a polyelectrolyte composite sodium lignosulfonate-chitosan carbon aerogel with a porous “fullerene-like” carbon structure. This approach to the synthesis of new carbon materials allows for the immobilization of many metals in the aerogel matrix during the gel formation phase. The metal ions (K+, Na+, Li+) inlaid in the CAs structure increase the pore volume and average pore width, leading to an enhancement in the specific surface area of the carbon gel. The obtained carbon gels have a well-developed porous structure with a specific surface area of 465 m2 g−1, a total pore volume of up to 0.5 cm3 g−1, and an average pore size of up to 4.6 nm. The resulting new carbon nanomaterials can develop porosity and improve the electrophysical properties of carbon gel materials, which can be used as conductive dispersed particles in nanoelectronic devices. Xu et al.57 developed a new type of LCAs, and the obtained CAs exhibits a unique blackberry-like nanostructure, showing a moderate surface area (2130 m2 g−1) but a high area capacitance (100 F g−1, 4.7 μF cm−2). This high value is attributed to the complicated conductive carbon fiber network and the porous “blackberry” surface suitable for ion storage.
3.2 Adsorbent
LCAs not only play an important role in energy storage but also can be applied to adsorption. LCAs have a layered porous structure with isotropic or non-directional macropores and micropores. These interesting structural properties will contribute to fast adsorption and desorption kinetics will achieve high adsorption capacity, and have good applications in dye adsorption, carbon dioxide adsorption, oil-water separation and heavy metal adsorption (Fig. 7).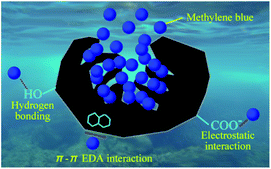 | ||
| Fig. 8 Mechanism of LCAs adsorption of methylene blue.42 | ||
Graphene oxide (GO) is an oxide of graphene, which is often complexed with polymers as adsorbent materials. Yan et al.58 environmentally friendly prepared a novel GO/lignosulfonate aerogel (GLCAs) interlinked by chitosan. The obtained GLCAs can effectively capture MB molecules and can remove more than 99% of methylene blue (MB) from MB solution (100 mg L−1). Because of π–π interaction, electrostatic attraction and hydrogen bonding interaction between GLCAs and MB, the largest adsorption capacity of MB on GLCA is 1023.9 mg g−1. Such a strong adsorption power is wonderful for the removal of dyes from water.
Geng et al.43 produced LTCAs with specific structure and surface area by combining lignin with TEMPO-oxidized CNFs (TOCNFs). LTCAs can have a good performance by washing to a specific surface area of 1101 m2 g−1 and CO2 adsorption power of 5.2 mmol g−1 at 273 K and 100 kPa. Such materials have good adsorption performance of CO2. Geng et al.10 evaluated the CO2 adsorption properties of lignin/CNF-based CAs, where most conventional adsorbent materials are in powder form and its performance is degraded by binder blocking effects when used with binders. LTCAs adsorbents exhibited high CO2 adsorption capacity, better than many activated carbon adsorbents reported in the literature using more complex steps for synthesis.
CAs obtained by lignin derivatives with hydrophobic and porous backbone and GO composite have superhydrophobicity and good mechanical properties, leading to their full adaptation to oil-water separation. Meng et al.44 fabricated a LCAs compounded by graphene oxide. Due to the strong reaction between the amine group of the lignin derivative and the carboxyl group of GO and acrylic acid, the denseness and cross-linkage of the gel were substantially enhanced. The CAs can reach a water contact angle of 150° after high temperature with an oil adsorption ratio of about 32–34 g g−1. This also fully demonstrates that it is an efficient and practical water purification material that can be applied for impurity separation and oil enrichment in industrial waste streams. Similarly, Chen et al.60 modified lignin and compounded it with graphene to obtain CAs, which was used to adsorb toxic solvents such as petroleum, toluene, chloroform and carbon tetrachloride all showed efficient adsorption (the mass after adsorption can reach 522 times of the original one), and the adsorption effect was much higher than that of similar adsorbents (lignin-based xerogel (19–47 times), lignin-based polyurethane foam (26–68 times)). This material can be recycled by repeated heat treatment and extrusion methods without destroying its own structure.
Unlike LCAs, Rao et al.61 innovatively propose a carbon foam made of lignin-resorcinol-glycolaldehyde resin for powerful absorption of organic agents from water. The prepared lignin carbon foam has a greater surface area and higher porosity, which can fully absorb various oils or organic solvents from aqueous media. In addition, it can be regenerated by heat treatment and its absorption capacity is not significantly reduced.
Compared with LCAs, lignin sulfonate-graphene hydrogels (LS-GH) lack a one-step carbonization process. High-capacity LS-GH adsorbents are commercially available, environmentally friendly and reusable, and are wonderful adsorbents for the adsorption of heavy metal ions on wastewater. Li et al.45 synthesized an LS-GH for the adsorption of Pb(II) with a large specific surface area, high porosity and ample active sites for Pb(II) removal exhibited an extremely strong adsorption capacity (1210 mg g−1). Guo et al.62 extracted lignin from black liquor and found that the number of phenolic acids on the surface was twice as much as that of carboxylic acids. The acid groups can adsorb metal ions by interacting with them, but the affinity varies for various divalent metal ions. For carboxylic acids, the connection force was ranked as Pb(II) > Cu(II) > Zn(II) > Cd(II) > Ni(II), and for phenols, it was ranked as Pb(II) > Cu(II) > Cd(II) > Zn(II) > Ni(II). Correspondingly, Brdar et al.63 also examined the ability of Kraft lignin to remove Cr(VI) ions from water. If a large number of studies laid stress on the adsorption capacity or selectivity by modification and carbonation, then the adsorption potential or selectivity must be substantially increased.
3.3 Catalyst
It has been reported that LCAs are a catalyst for promoting esterification reactions with large total surface area and high acidity, and the large-scale porous network including mesopores and macropores is beneficial to the loading of active materials without causing pore blockage, products and by-products are easily removed from the reaction system by the high porosity.645-Hydroxymethylfurfural is a valuable bio-derived chemical, Li et al.65 prepared lignin-based activated carbon solid by carbonization and activation, followed by sulfuric acid sulfonation to synthesize lignin-based carbon solid acid, which will be applied to fructose conversion. It is a catalyst for 5-hydroxymethyl furfural. Zainol et al.46 prepared lignin and furfural carbon cryogels by sol–gel with 98.1% (w/w) oleic acid conversion. It can be seen that carbon cryogels have great potential for the esterification of fatty acids to biodiesel. Meanwhile, Zainol et al.47 synthesized ethyl levulinate, a flow improver, by catalytic esterification of levulinic acid with ethanol using a carbon cryogel catalyst, which was determined to be a superior performance carbon-based catalyst to obtain a high yield of 86.5 mol% ethyl levulinate. In a deeper way, Zainol et al.66 still used lignin-furfural to prepare carbon cryogels for the esterification reaction, and the total surface area and acidity of the carbon cryogels after calcination were 426.5 m2 g−1 and 16.1 mmol g−1, respectively. Under optimal conditions, the conversion of levulinic acid and ethyl levulinate yields of the carbon cryogels. Under the optimal conditions, the conversion of levulinic acid and the yield of ethyl levulinate of carbon cryogels were 87.2% and 86.5 mol%, respectively.
3.4 Other applications of LCAs
LCAs have a unique three-dimensional network structure and excellent properties that make them attractive materials for applications such as electromagnetic interference shielding, pressure sensors, and flexible electrodes.Zeng et al.67 prepared reduced graphene oxide (RGO)/lignin-derived carbon (LDC) composite aerogels. The incorporation of LDC and honeycomb microstructures significantly improved the mechanical properties and comparatively strong elasticity of RGO/LDC aerogels, and improved the wave absorption capacity of the cell walls as well as the multiple reflectivities. The RGO/LDC aerogels could show considerable EMI SE of 80.0 to 23.2 dB with SSE/d values up to 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 dB cm2 g−1, higher than those reported for other carbon-based shields. This demonstrates the critical role it plays in determining EMI shielding performance.
250 dB cm2 g−1, higher than those reported for other carbon-based shields. This demonstrates the critical role it plays in determining EMI shielding performance.
LCAs is also a compressible carbon material that has potential applications in a variety of wearable devices. Chen et al.68 used cellulose nanofibers and lignin to design an elastic CAs. This CAs exhibits better mechanical flexibility, such as high compressibility (up to 95% strain), and it can also accurately detect human biosignals. These properties make CAs highly competitive for pressure sensors and plenty of electrode applications. Zhou et al.69 obtained compressible conductive CAs with ordered wavy laminar structure by cellulose nanocrystals (CNC), lignin, and reduced graphene oxide. The prepared CAs exhibit exceptional compressibility (up to 99% ultimate strain), extraordinary fatigue resistance and elasticity (high retention rate of 91.3% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 30% strain condition). Based on these properties, this material can be well used in wearable devices.
000 cycles at 30% strain condition). Based on these properties, this material can be well used in wearable devices.
4 Conclusion and outlook
LCAs are three-dimensional layered materials, a new type of CAs with large specific surface area, high porosity, low density, fine chemical stability and excellent electrical conductivity. At present, the research of LCAs is in the basic research stage, its large number of applications have not been discovered, and the preparation process needs further exploration and conclusion. In this paper, based on the existing preparation methods and known properties, we found some shortcomings: (i) the preparation process of LCAs is complicated, the preparation cost is high, and the gelation stage takes forever; (ii) for the sake of stabilizing the pore structure of LCAs, freeze-drying or supercritical CO2 drying is used, which increases the operational difficulty and cost; (iii) the application range of LCAs is narrow.At the same time, we also put forward some suggestions and prospects: (i) in the selection and treatment of raw materials, based on green and low cost, which has been achieved by lignin, followed by modification of lignin, access to phenolic hydroxyl groups and other polymerization-friendly groups or degradation of lignin to generate phenolic hydroxyl groups; strengthening the characterization of lignin, selecting the source of lignin (composition and structure), producing cross-linked; (ii) in the preparation of LCAs, the first step is to simplify and optimize the preparation process as much as possible to reduce the number of steps (mainly gelation, solvent exchange, freeze-drying and carbonization); lignin can be directly cross-linked and carbonized or compounded with GO; (iii) in the application of the designed products, the current applications are mainly focused on energy storage, adsorption, etc., but we should also expand the application of LCAs in catalysts/catalyst carriers, battery electrodes, sensors, etc., and strengthen the target design of supercapacitors and LCAs for adsorption of heavy metal ions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors appreciate the financial support for this work by the National Natural Science Foundation of China (No. 31730106; No. 21704045).Notes and references
- J. Y. Yang, W. J. Zhang, Y. Wang, M. F. Li, F. Peng and J. Bian, Carbohydr. Polym., 2022, 278, 118992 CrossRef CAS PubMed.
- B. L. Ai, W. Q. Li, J. Woomer, M. Li, Y. Q. Pu, Z. W. Sheng, L. L. Zheng, A. Adedeji, A. J. Ragauskas and J. Shi, Green Chem., 2020, 22, 6372 RSC.
- W. Schutyser, T. Renders, S. Van den Bosch, S. F. Koelewijn, G. T. Beckham and B. F. Sels, Chem. Soc. Rev., 2018, 47, 852 RSC.
- N. Ding, H. Liu, Y. Sun, X. Tang, T. Z. Lei, F. Xu, X. H. Zeng and L. Lin, J. Cleaner Prod., 2021, 278, 123984 CrossRef CAS.
- Z. Sun, B. Fridrich, A. de Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614 CrossRef CAS PubMed.
- L. P. Hou, D. D. Ji, W. F. Dong, L. Yuan, F. S. Zhang, Y. Li and L. H. Zang, Front. Bioeng. Biotechnol., 2020, 8, 99 CrossRef PubMed.
- C. Jiang, Z. H. Wang, J. X. Li, Z. J. Sun, Y. F. Zhang, L. Li, K. S. Moon and C. P. Wong, Electrochim. Acta, 2020, 353, 136482 CrossRef CAS.
- B. Thomas, S. Y. Geng, M. Sain and K. Oksman, Nanomaterials, 2021, 11, 653 CrossRef CAS PubMed.
- R. W. Pekala, C. T. Alviso, F. M. Kong and S. S. Hulsey, J. Non-Cryst. Solids, 1992, 145, 90 CrossRef CAS.
- S. Y. Geng, A. Maennlein, L. Yu, J. Hedlund and K. Oksman, Microporous Mesoporous Mater., 2021, 323, 111236 CrossRef CAS.
- T. Chen, M. X. Li, L. Zhou, X. B. Ding, D. J. Lin, T. Duan, G. C. Yang, R. He and W. K. Zhu, ACS Sustainable Chem. Eng., 2020, 8, 6458 CrossRef CAS.
- S. X. Wang, M. R. Shen, J. F. Qu, X. M. Zhuang, S. Q. Ni and X. R. Wu, J. Non-Cryst. Solids, 2020, 536, 120008 CrossRef CAS.
- Z. C. Yu, C. S. Hu, A. B. Dichiara, W. H. Jiang and J. Gu, Nanomaterials, 2020, 10, 169 CrossRef CAS PubMed.
- Q. H. Wang, T. Xia, X. W. Jia, J. Q. Zhao, Q. Y. Li, C. H. Ao, X. Y. Deng, X. M. Zhang, W. Zhang and C. H. Lu, Carbohydr. Polym., 2020, 245, 116554 CrossRef CAS PubMed.
- Y. L. Pan, X. D. Cheng, M. Y. Gao, Y. B. Fu, J. Feng, L. L. Gong, H. Ahmed, H. P. Zhang and V. S. Battaglia, ACS Appl. Mater. Interfaces, 2020, 12, 33621 CrossRef CAS PubMed.
- E. Garcia-Bordejé, A. M. Benito and W. K. Maser, Adv. Colloid Interface, 2021, 292, 102420 CrossRef PubMed.
- Z. Chen, S. Zhang, J. Yang, C. Chen, Y. C. Song, C. L. Xu, M. Q. Wu and J. X. Liao, Sustainable Energy Fuels, 2021, 5, 4973 RSC.
- C. C. Wan, Y. Jiao, S. Wei, L. Y. Zhang, Y. Q. Wu and J. Li, Chem. Eng. J., 2019, 359, 459 CrossRef CAS.
- D. Lv, Y. Li and L. J. Wang, Int. J. Biol. Macromol., 2020, 148, 979 CrossRef CAS PubMed.
- P. S. Roy, G. Garnier, F. Allais and K. Saito, Chem. Lett., 2021, 50, 1123 CrossRef CAS.
- Y. Jiang, N. Ding, B. Luo, Z. Li, X. Tang, X. Zeng, Y. Sun, S. Liu, T. Lei and L. Lin, ChemCatChem, 2017, 9, 2544 CrossRef CAS.
- C. Xu, R. A. Arancon, J. Labidi and R. Luque, Chem. Soc. Rev., 2014, 43, 7485 RSC.
- J. Xu, M. Z. Chen and X. Y. Zhou, Chem. Ind. For. Prod., 2017, 37, 5, DOI:10.3969/j.issn.0253-2417.2017.05.001.
- R. W. Pekala, C. T. Alviso and J. D. LeMay, Chemical Processing of Advanced Materials, New York: Wiley, 1992, p. 671 Search PubMed.
- C. E. I. Torres, T. E. S. Quezada, O. V. Kharissova, B. I. Kharisov and M. I. G. de la Fuente, J. Environ. Chem. Eng., 2021, 9, 104886 CrossRef.
- I. V. Elmanovich, M. S. Rubina and S. S. Abramchuk, Dokl. Phys. Chem., 2020, 493, 123 CrossRef CAS.
- W. J. Yang, D. C. Wu and R. W. Fu, J. Appl. Polym. Sci., 2007, 106, 2775 CrossRef CAS.
- M. Yu, J. Li and L. J. Wang, Chem. Eng. J., 2017, 310, 300 CrossRef CAS.
- B. S. Yang, K. Y. Kang and M. J. Jeong, J. Korean Phys. Soc., 2017, 71, 478 CrossRef CAS.
- H. Wang, H. Ben, H. Ruan, L. Zhang, Y. Pu, M. Feng, A. J. Ragauskas and B. Yang, ACS Sustainable Chem. Eng., 2017, 5, 1824–1830 CrossRef CAS.
- L. Zhang, G. Henrikssona and G. Gellerstedt, Org. Biomol. Chem., 2003, 1, 3621–3624, 10.1039/B306434D.
- J. Ralph, C. Lapierre and W. Boerjan, Curr. Opin. Biotechnol., 2019, 56, 240–249, DOI:10.1016/j.copbio.2019.02.019.
- J. Hu and X. Jiang, Energy Sources, Part A, 2020, 1–13, DOI:10.1080/15567036.2020.1860160.
- T. M. Liitiä, S. L. Maunu, B. Hortling, M. Toikka and I. Kilpeläinen, J. Agric. Food Chem., 2003, 51, 2136–2143, DOI:10.1021/jf0204349.
- W. Zhang, J. Yin, C. Wang, L. Zhao, W. Jian, K. Lu, H. Lin, X. Qiu and H. N. Alshareef, Small Methods, 2021, 5, 2100896, DOI:10.1002/smtd.202100896.
- X. Shi, X. Wang, B. Tang, Z. Dai, K. Chenand and J. Zhou, J. Appl. Polym. Sci., 2018, 135, 45580 CrossRef.
- D. W. Lee, M. H. Jin, J. H. Park, Y. J. Lee and Y. C. Choi, ACS Sustainable Chem. Eng., 2018, 6, 10454–10462, DOI:10.1021/acssuschemeng.8b01811.
- L. I. Grishechko, G. Amaral-Labat, A. Szczureck, V. Fierro, B. N. Kuznetsov and A. Celzard, Microporous Mesoporous Mater., 2013, 168, 19 CrossRef CAS.
- C. A. Nunes, C. F. Lima, L. C. A. Barbosa, J. L. Colodette, A. F. G. Gouveia and F. O. Silverio, Bioresour. Technol., 2010, 101, 4056 CrossRef CAS PubMed.
- Y. F. Zhang, C. Y. Zhao, W. K. Ong and X. H. Lu, ACS Sustainable Chem. Eng., 2019, 7, 403–411 CrossRef CAS.
- J. Xu, X. Y. Zhou, M. Z. Chen, S. K. Shi and Y. Z. Cao, Microporous Mesoporous Mater., 2018, 265, 258–265 CrossRef CAS.
- D. Lv, Y. Li and L. J. Wang, Int. J. Biol. Macromol., 2020, 148, 979–987 CrossRef CAS PubMed.
- S. Y. Geng, J. Y. Wei, S. Jonasson, J. Hedlund and K. Oksman, ACS Appl. Mater. Interfaces, 2020, 12, 7432–7441 CrossRef CAS PubMed.
- Y. Meng, T. L. Liu, S. S. Yu, Y. Cheng, J. Lu and H. S. Wang, Fuel, 2020, 278, 118376 CrossRef CAS.
- F. F. Li, X. l. Wang, T. q. Yuan and R. c. Sun, J. Mater. Chem. A, 2016, 4, 11888 RSC.
- M. M. Zainol, N. A. S. Amin and M. Asmadi, Bioresour. Technol., 2015, 190, 44 CrossRef CAS PubMed.
- M. M. Zainol, N. A. S. Amin and M. Asmadi, Renewable Energy, 2019, 130, 547 CrossRef CAS.
- D. K. Sam, E. K. Sam, A. Durairaj, X. M. Lv, Z. J. Zhou and J. Liu, Carbohydr. Res., 2020, 491, 107986 CrossRef CAS PubMed.
- O. A. T. Dias, D. R. Negrão, D. F. C. Gonçalves, I. Cesarino and A. L. Leão, 14th International Conference on Frontiers of Polymers and Advanced Materials, Mol. Cryst. Liq. Cryst., 2017, 655, 204 CrossRef CAS.
- D. Yu, K. Goh, H. Wang, L. Wei, W. Jiang, Q. Zhang, L. Dai and Y. Chen, Nat. Nanotechnol., 2014, 9, 555–562 CrossRef CAS PubMed.
- T. Kuilla, S. Bhadra, D. Yao, N. H. Kim, S. Bose and J. H. Lee, Prog. Polym. Sci., 2010, 35, 1350–1375 CrossRef CAS.
- J. Xu, X. Y. Zhou and M. Z. Chen, Mater. Res. Express, 2018, 5, 095002 CrossRef.
- J. Xu, X. Y. Zhou and M. Z. Chen, Mater. Res. Express, 2019, 6, 065036 CrossRef CAS.
- D. Lv, T. C. Zhang, D. Y. Wang, J. Li and L. J. Wang, Ind. Crops Prod., 2021, 170, 113750 CrossRef CAS.
- S. Q. Guo, H. C. Li, X. Zhang, H. Nawaz, S. Chen, X. M. Zhang and F. Xu, Carbon, 2021, 174, 500 CrossRef CAS.
- O. Brovko, I. Palamarchuk, N. Bogdanovich, A. Ivakhnov, D. Chukhchin, A. Malkov, A. Volkov, M. Arkhilin and N. Gorshkova, Microporous Mesoporous Mater., 2019, 282, 211 CrossRef CAS.
- X. Z. Xu, J. Zhou, D. H. Nagaraju, L. Jiang, V. R. Marinov, G. Lubineau, H. N. Alshareef and M. Oh, Adv. Funct. Mater., 2015, 25, 3193–3202 CrossRef CAS.
- M. F. Yan, W. X. Huang and Z. L. Li, Int. J. Biol. Macromol., 2019, 136, 927 CrossRef CAS PubMed.
- Q. B. Meng and J. Weber, Chemsuschem, 2014, 7, 3312 CrossRef CAS PubMed.
- C. Z. Chen, F. F. Li, Y. R. Zhang, B. X. Wang, Y. M. Fan, X. L. Wang and R. C. Sun, Chem. Eng. J., 2018, 350, 173 CrossRef CAS.
- G. S. Rao, H. Nabipour, P. Zhang, X. Wang, W. Y. Xing, L. Song and Y. Hu, J. Mater. Res. Technol., 2020, 9, 4655 CrossRef CAS.
- X. Guo, S. Zhang and X. Q. Shan, J. Hazard. Mater., 2008, 151, 134 CrossRef CAS PubMed.
- M. Brdar, M. Šćiban, A. Takači and T. Došenović, Chem. Eng. J., 2012, 183, 108 CrossRef CAS.
- J. H. Lee and S. J. Park, Carbon, 2020, 163, 1–18 CrossRef CAS.
- M. Li, Q. Zhang, B. Luo, C. Chen, S. Wang and D. Min, Ind. Crops Prod., 2020, 145, 111920 CrossRef CAS.
- M. M. Zainol, N. A. S. Amin and M. Asmadi, Fuel Process. Technol., 2017, 167, 431 CrossRef CAS.
- Z. H. Zeng, C. X. Wang, Y. F. Zhang, P. Y. Wang, S. I. S. Shahabadi, Y. M. Pei, M. J. Chen and X. H. Lu, ACS Appl. Mater. Interfaces, 2018, 10, 8205 CrossRef CAS PubMed.
- Z. H. Chen, H. Zhuo, Y. J. Hu, H. h. Lai, L. X. Liu, L. X. Zhong and X. W. Peng, Adv. Funct. Mater., 2020, 30, 1910292 CrossRef CAS.
- K. M. Zhou, C. Z. Chen, M. Lei, Q. Gao, S. X. Nie, X. L. Liu and S. F. Wang, RSC Adv., 2020, 10, 2150 RSC.
- G. J Jiao, J. Ma, J. Zhang, J. Zhou and R. Sun, Sci. Total Environ., 2022, 827, 154343 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |