Open Access Article
Open Access ArticleLight-responsive polyurethanes: classification of light-responsive moieties, light-responsive reactions, and their applications
Ki Yan Lam
a,
Choy Sin Lee
 *b,
Mallikarjuna Rao Pichika
bc,
Sit Foon Cheng
d and
Rachel Yie Hang Tan
a
*b,
Mallikarjuna Rao Pichika
bc,
Sit Foon Cheng
d and
Rachel Yie Hang Tan
a
aSchool of Postgraduate, International Medical University, No. 126, Jalan Jalil Perkasa 19, Bukit Jalil, 57000 Kuala Lumpur, Malaysia. E-mail: choysin_lee@imu.edu.my
bDepartment of Pharmaceutical Chemistry, School of Pharmacy, International Medical University, No. 126, Jalan Jalil Perkasa 19, Bukit Jalil, 57000 Kuala Lumpur, Malaysia
cCentre for Bioactive Molecules and Drug Delivery, Institute for Research, Development and Innovation, No. 126, Jalan Jalil Perkasa 19, Bukit Jalil, 57000 Kuala Lumpur, Malaysia
dUnit of Research on Lipids (URL), Department of Chemistry, Faculty of Science, University of Malaya, Kuala Lumpur 50603, Malaysia
First published on 19th May 2022
Abstract
Stimuli responsiveness has been an attractive feature of smart material design, wherein the chemical and physical properties of the material can be varied in response to small environmental change. Polyurethane (PU), a widely used synthetic polymer can be upgraded into a light-responsive smart polymer by introducing a light-sensitive moiety into the polymer matrix. For instance, azobenzene, spiropyran, and coumarin result in reversible light-induced reactions, while o-nitrobenzyl can result in irreversible light-induced reactions. These variations of light-stimulus properties endow PU with wide ranges of physical, mechanical, and chemical changes upon exposure to different wavelengths of light. PU responsiveness has rarely been reviewed even though it is known to be one of the most versatile polymers with diverse ranges of applications in household, automotive, electronic, construction, medical, and biomedical industries. This review focuses on the classes of light-responsive moieties used in PU systems, their synthesis, and the response mechanism of light-responsive PU-based materials, which also include dual- or multi-responsive light-responsive PU systems. The advantages and limitations of light-responsive PU are reviewed and challenges in the development of light-responsive PU are discussed.
1. Introduction
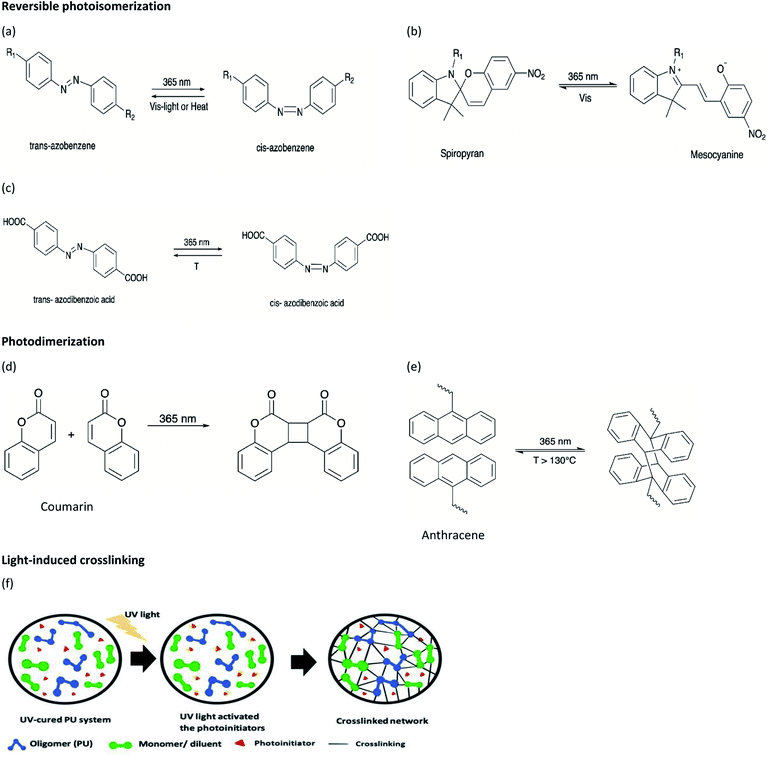
In recent years, a variety of stimulus-responsive polymers have been developed and their applications have been proposed. Stimulus-responsive polymers, also known as ‘smart’ polymers are gaining much attention from polymer industries due to their unique characteristics, demonstrating sensitive response to a slight change in stimulus factors such as light or ultraviolet (UV) light, temperature, pH variation, force, solvent, or reductant.1 The activated polymers produce observable or detectable micro or nanoscale changes, such as morphology, molecular bond rearrangement, and molecular motion, which can induce changes in their macroscopic properties such as color, shape, and functionality.2 Among the different stimuli that can be used for activating smart polymers, light has several outstanding attractive features as it can be applied without contact, remotely, and provide spatial resolution to realize precise controllability, without time and space limitations, and tunable parameters such as source, wavelength, and intensity. Therefore, light-responsive polymers have attracted significant attention in recent years and attained great interest in engineering, biotechnology, biomedicine, and pharmaceutical applications.3,4Light-responsive polymers can be enabled through the incorporation of light-sensitive moieties into the polymer networks. The light-sensitive moieties can respond to different wavelength ranges of light such as UV-C (100–280 nm), UV-B (280–315 nm), UV-A (315–400 nm), visible (400–780 nm), or near infrared (NIR) (780–2500 nm). Besides this, light-sensitive moieties undergo light-induced reversible and irreversible molecular structural changes, photothermal, crosslinking, as well as fluorescence up-conversion mechanisms. For instance, azobenzene and spiropyran perform reversible photoisomerization that can modify their polarity and/or conformation, while coumarin and anthracene undergo reversible photodimerization when exposed to light of an appropriate wavelength. The moieties with irreversible light-induced reactions include o-nitrobenzyl, acrylate monomer ion powder, and inorganic particles such as gold nanorods and carbon nanotubes. Polymers that respond to single stimulus, for instance, light responsiveness, may not satisfy certain requirements in practical application; thus, dual or multiple stimuli responsive polymers were studied as they respond to the respective stimuli, which could achieve the desired goals.
Table 1 summarizes the light-responsive groups used in polymers (excluded polyurethane) and their corresponding responsiveness and wavelength used for light irradiation. Due to the unique features of property changes in response to light, these polymer systems are emerging in wide ranges of applications in drug and gene delivery, photothermal therapy, photodynamic therapy, tissue engineering, and biosensors under UV or NIR light. Their applications are in engineering areas are such as logic gates, white light emitting diodes, and UV-curing technique for coating and paints. These polymers are responsive to UV and/or Vis and/or NIR light.
| Polymer | Light sensitive moiety | Light-responsive region [nm] | Responsiveness | Application | Ref. | ||
|---|---|---|---|---|---|---|---|
| UV | Vis | NIR | |||||
| (a) Medical and biomedical applications | |||||||
| Polyester (PE) | Graphene quantum dots | 365 | 400–700 | Light | Drug delivery system | 21 | |
| Poly[oligo(ethylene glycol)methylether methacrylate] | 1-Pyrenemethyl methacrylate | 365 | Light | Drug delivery system | 22 | ||
| Poly(ε-caprolactone) (PCL) | Indocyanine green | 808 | Light | Drug delivery system | 23 | ||
| Poly(ethyleneimine) | Cinnamic acid-UV gold nanoparticle-NIR | 365 | 808 | Light | Drug delivery system | 24 | |
| Poly(2-isopropyl-2-oxazoline) | 1,3,3-Trimethylindolino-6′-nitrobenzopyrylospiran | 365 | 440 | Thermo | Drug delivery system | 25 | |
| Light | |||||||
| Polyacrylamide | Azobenzene acrylamide | 365 | 450 | Thermo | Drug delivery system | 26 | |
| Light | Catalyst carriers | ||||||
| Host-molecule | |||||||
| Poly((7-(4-vinyl-benzyloxyl)-4-methylcoumarin)-co-acrylicacid)-b-poly((2-(dimethylamino)ethyl methacrylate)-co-styrene) (P(VBMC-co-AA)-b-P(DMAEMA-co-St)) | 7-(4-Vinyl-benzyloxyl)-4-methylcoumarin | 254, 365 | pH | Drug delivery system | 27 | ||
| Light | |||||||
| Polyethylene glycol (PEG)/PCL | Host-guest interaction between β-CD and azobenzene | 365 | Glutathione | Drug delivery system | 28 | ||
| Light | |||||||
| Poly(methyl methacrylate) (PMMA) | 9-Anthracenecarboxylic acid | 254![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365 |
CO2 | Drug delivery system | 29 | ||
| Light | |||||||
| 1,3-Dihydro-1,3,3-trimethylspiro[2H-indole-2,3′-[3H]naphth[2,1-b][1,4]oxazine] | 254 | Light | Smart sensor | 30 | |||
| Tetraphenylethylene-based covalent organic polymer | Phosphor | 370 | Fe3+ | Biosensors of Fe3+ | 31 | ||
| Light | White-light emitting diodes | ||||||
| Poly(N-isopropylacrylamide) (PNIPAM)/cysteamine | Gold | 405![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 462 520 462 520![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 635 635 |
785 | Thermo | Gas sensors | 32 | |
| Light | |||||||
| Polycarbonate | Photosensitizer 5,10,15,20-tetrakis(m-hydroxyphenyl)chlorin | 365 | Light | Photodynamic therapy | 33 | ||
| Poly(N-vinylpyrrolidone) | Crown- and phosphoryl-containing metal phthalocyanines | 670 | Light | Photodynamic therapy | 34 | ||
| Poly(D,L-lactic-co-glycolic acid) | Gold nanorods | 808 | Light | Photothermal therapy | 35 | ||
| PEG | Pd(II) | 820, 1064 | Light | Photoacoustic (PA) imaging-guided photothermal therapy (PTT) | 36 | ||
| Thiolated cyclo(Arg–Gly–Asp–D-Phe–Lys(mpa)) peptide | Conjugated polymers | 808 | Thermo | Photothermal therapy | 37 | ||
| Light | |||||||
| PEG–PPG copolymer | Photosensitive porphyrin zirconium metal | 488 | Redox | Chemo-photodynamic therapy | 38 | ||
| Light | |||||||
| Conjugated 1,2-distearoyl-sn-glycero-3 phosphoethanolamine-N-[carboxy(polyethylene-glycol)-2000]-lanreotide | Copper sulfide nanoparticles | 808 | Light | Chemo-photothermal therapy | 39 | ||
| Fluorinated polyethylenimine | Semiconducting polymer brush | 808 | Light | Genome-editing-based precise gene therapy | 40 | ||
| Polystyrene-b-poly(N-isopropylacrylamide-co-acrylic acid) | Diketopyrrolopyrrole-based semiconducting polymer poly[{2,5-bis(2-hexyldecyl)-2,3,5,6-tetrahydro-3,6-dioxopyrrolo[3,4-c]pyrrole-1,4-diyl}-alt-{[2,2′:5′,2′′-terthiophene]-5,5′′-diyl}] | 785 | pH | Chemo-photothermal therapy | 41 | ||
| Thermo | |||||||
| Light | |||||||
| Polyimide | 2,2′-Azobisisobutyronitrile | 375 | 530 | Thermo | — | 42 | |
| Light | |||||||
| Cross-linked polyethylene glycol diacrylate | Oligo(ethylene glycol)-modified W18O49 nanowires | 365 | 808![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 980 980 |
Light | Artificial muscles and minimally invasive surgery | 43 | |
| Poly(L-lactide)–poly(ε-caprolactone) copolymer | 7-Hydroxy-4-methylcoumarin | 254![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365 |
Thermo | Medicine | 44 | ||
| Light | Textile | ||||||
| Soft robots | |||||||
| PNIPAM/clay | Polydopamine nanoparticles | 808 | Thermo | Soft intelligent robots Wearable devices | 45 | ||
| Light | |||||||
| Poly(2-(2-methoxyethoxy)ethyl methacrylate) and poly(N-[4-[(4-nitrophenyl)azo]phenyl] acrylamide) mixed polymer brushes | (N-[4-[(4-Nitrophenyl)azo]phenyl]acrylamide) | 365 | 450 | Thermo | Cell sheet preparation and detachment state | 46 | |
| Light | |||||||
| L-Phenylalanine benzyl ester | 4-[(4-methacryloyloxy)phenylazo]benzoic acid | 365 | 400–700 | Light | Molecular imprinting | 47 | |
| Polyethylene/polylactic acid | Azobenzene | 360 | Light | Antimicrobial active packaging | 48 | ||
| Drug delivery | |||||||
| Agriculture | |||||||
| Household and cosmetics | |||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| (b) Engineering applications | |||||||
| Polyimide | 4-Amino-1,1′-azobenzene-3,4′-disulfonic acid monosodium salt, (C12H10N3NaO6S2) | 365 | Light | Coated optical fiber | 49 | ||
| UV curing agent (Trade name: LS-2211) | |||||||
| PEG | Benzophenone | 365 | Light | Waterborne UV coatings, inks and adhesives | 50 | ||
| Latexes | 2-(4-(4-Butylphenyl)diazenylphenoxy)ethyltrimethylammonium bromide | 365 | 460 | Thermo | Reversible destabilization | 51 | |
| Light | Paints | ||||||
| Polydimethylsiloxane | Zirconia nanoparticles | 365 | Thermo | Anti-reflective coatings | 52 | ||
| Light | Gas separation | ||||||
| PMMA | 1,3-Dihydro-1,3,3-trimethylspiro[2H-indole-2,3′-[3H]naphth[2,1-b][1,4]oxazine] | 254 | Light | Smart sensor | 30 | ||
| Poly(3-hexylthiophene)graphene | Poly(3-hexylthiophene)-graphene | 532 | Light | Smart sensors telecommunication | 53 | ||
| Cellulose acetate | Azobenzene | 254 | Light | Optical switches for liquid crystalline alignment | 54 | ||
| PE | 4,4-Dihydroxyazobenzene | 365 | 550 | Thermo | Actuators to electro-optical devices | 55 | |
| Light | |||||||
| Polystyrene | Carbon dots | 365 | Light | White-light-emitting devices | 56 | ||
| Poly(ethylene-co-vinyl acetate) | Graphene | — | Thermo | Shape memory actuator | 57 | ||
| Light | |||||||
| Liquid crystalline elastomers network | Azobenzene | 532 | Light | Shape memory polymer | 58 | ||
| Poly(allyl methacrylate) | Asymmetric divinyl monomers | 254 | Light | Crosslinked polymer | 59 | ||
| Benzene-1,4-diboronic acid with the 1,3-diol polymer | Azobenzene | 365 | 435 | Light | Crosslinked polymer | 60 | |
| Poly[(Z)-ω-(4-(1-cyano-2-(4-cyanophenyl)vinyl)phenoxy)alkyl methacrylate] | α-Cyanostilbene | 254![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365 |
Thermo | Luminescent liquid crystals | 61 | ||
| Light | |||||||
| α-CD-bearing telechelic poly(2-isopropyl-2-oxazoline) | Azobenzene-bearing penta(ethylene glycol) | 254![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365 |
Thermo | Multi-functional thermo- and photo-sensitive materials | 62 | ||
| Light | |||||||
| Polymeric O and N co-linked carbon nitride framework | Carbon dots | 450 | Light | Pollutant degradation environmental remediation | 63 | ||
| Poly(ethylene-co-butylene) | Isophthalic acid-pyridine | 320–390 | Thermo | Debonding-on-demand applications | 64 | ||
| Light | |||||||
| 1,4-(Bis-chlorotetrafluoro-λ6-sulfanyl) benzene with 1,4-diethynylbenzene | Hypervalent fluorinated sulfur | 200–450 | Light | Micro-electronic lithographic | 65 | ||
| Poly(dimethylsiloxane) | 1-(4-(Hex-5-enyloxy)phenyl)-2-phenyldiazene | 365 | Thermo | Logic gate | 66 | ||
| Light | |||||||
| Poly(3-hexylthiophene) | Poly(3-hexylthiophene) | 808 | Light | Liquid marble stabilizer | 67 | ||
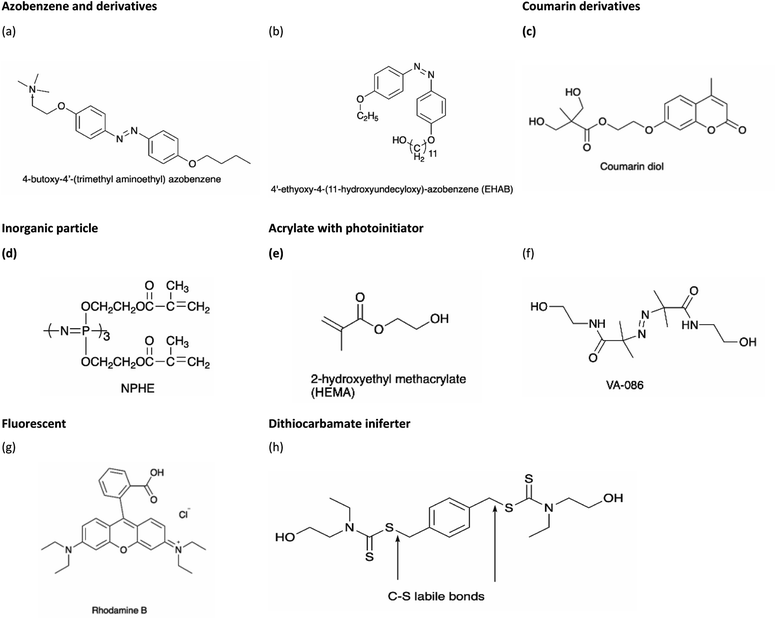
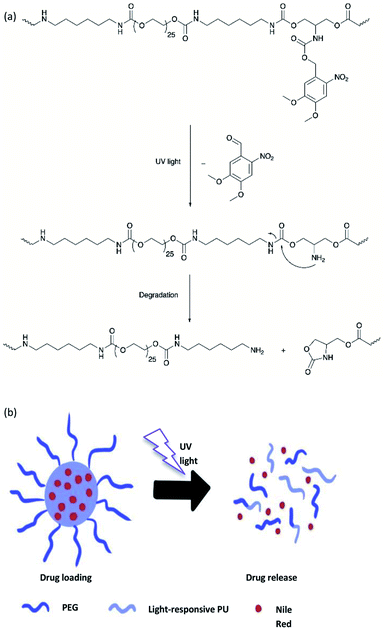
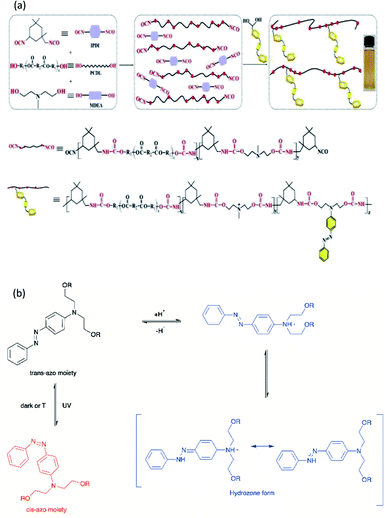
On the other hand, polyurethane (PU) is a synthetic polymer that is majorly produced by reacting polyisocyanates and polyhydroxyl compounds, also known as polyols. PUs with different macromolecular structures are specified for diverse applications in engineering, automotive, military, medical, and biomedical products. The reactions of different types and ratios of polyisocyanates and polyols are able to customize the physical, chemical, mechanical, and thermal properties of the final products. This is because of PU is formed by two different segments, i.e., hard and soft segments in the polymer matrices. The hard segment is mainly structured by the urethane linkages between the isocyanate and the chain extender, whereas the soft segment is confirmed by the polyol or diol groups,5 making it a versatile polymeric material with wide ranges of specifications and properties. The highly tunable properties of PU also enable its incorporation with different kind of photochromic groups without compromising its physical and mechanical properties, which is beneficial for stimuli responsiveness as a smart material (Fig. 1).
Light-responsive PUs are promising candidates for engineering applications such as smart coating for textiles and optical devices,6,7 UV-curable PU in flame retardant8 and coatings,9 piezoresistive10 or photo-thermal sensors,11 as well as robotic fingers with shape memory properties.12 The excellent physical and mechanical properties as well as biocompatibility of PUs13 with the additional advantages of light responsiveness have further widened the applications of PUs as a smart material for medical and biomedical applications. For instance, light-responsive PUs have been used as biomimetic actuators with self-healing properties,14 implantable devices,15 or skin care patches16 with shape memory effect, artificial skin for tissue engineering,17 and drug carriers for controlled drug delivery systems,18–20 which all arise from the tunable properties of PUs and the highly controllable properties during light-induced reactions. A summary of the currently available studies involving light-responsive moieties and responsiveness of these light-responsive PUs is presented in Table 2.
| Light-responsive group | Responsiveness | Wavelength used for light irradiation [nm] | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Classification | Light-responsive moiety | Light | Thermal | pH | Forces | Reduction | UV | Vis | NIR | |
| Azobenzene and derivatives | Dihydroxyazobenzene (DHAB) | — | — | 320–500 | 68 | |||||
| 4,4′-Diaminoazobenzene (DAB) | — | 385 | 440 | 69 | ||||||
| 4-Butoxy-4′-(trimethylaminoethoxy) azobenzene (BTAEAzo) | — | 360 | 450 | 70 | ||||||
| Azobenzene, 4-cyano-4′-pentyloxyazobenzene (5CAZ) and upconversion nanoparticles | — | — | 365 | 530 | 808 | 71 | ||||
| Pentaerythritol [SCLCPU(AZO)-N] | — | — | 450, 550 | 72 | ||||||
| 4-Octyldecyloxybenzoic acid (TABA) | — | — | 365 | 460 | 11 | |||||
| 4′-Ethyoxy-4-(11-hydroxyundecyloxy)-azobenzene (EHAB) and graphene oxide (GO) | — | — | — | — | 14 | |||||
| 4-(4-Oxyalkyl chain carbonyl)azodibenzoic acid (Azo11) | — | — | 365 | 73 | ||||||
| 4,4-Azodibenzoic acid (Azoa) | — | — | 365 | 74 | ||||||
| N,N-Dihydroxyethylazobenzene | — | — | 365 | 6 | ||||||
| 2,20-(4-((4-Ethylphenyl)diazenyl)phenylazanediyl)diethanol (EDPD) | — | — | 365 | 75 | ||||||
| Azobenzene and 3,3′-disulfanediyldipropane-1,2-diol (DSO) | — | — | 365 | 18 | ||||||
| Spiropyran | Di-hydroxylspiropyran | — | — | 365 | 7 | |||||
| Coumarin derivatives | 7-(Hydroxyethoxy)-4-methylcoumarin (HMEC) | — | 254, 365 | 76 | ||||||
| 7-Bis(2-hydroxyethoxy)-4-methylcoumarin (DHEMC) | — | 254, 365 | 77 | |||||||
| Coumarin diol | — | 254, 365 | 78 | |||||||
| 4-Methyl-7-((4-oxopentyl)oxy)-2H-chromen-2-one (OMC) and hydrazone bond | — | — | 365 | 19 | ||||||
| Anthracene | 3-(N,N-Bis (2-hydroxyethyl)amine)propionic acid-9-anthracenemethanol ester (BHPAE) | — | — | 365 | 79 | |||||
| Nitrobenzyl derivative | o-Nitrobenzyl (o-NB) | — | 365 | 20 | ||||||
| Inorganic particles | Multiwalled carbon nanotubes, NC7000™ | — | 395 | 10 | ||||||
| Phosphorus derivative (phosphazene) | — | 365 | 8 | |||||||
| Acrylate with photoinitiator | 2,2′-Azobis[2-methyl-N-(2-hydroxyethyl)propionamide] (tradename: VA-086) | — | — | 365 | 80 | |||||
| Irgacure 184 and Irgacure 819 | — | 365 | 81 | |||||||
| Darocur 1173 | — | 365 | 9 | |||||||
| Darocur 4265 | — | 365 | 82 | |||||||
| Negative ion | Ag3PO4@AgBr | — | > 420 | 83 | ||||||
| Fluorescent | Rhodamine B (RhB) | — | — | 365 | 84 | |||||
| Other | Dithiocarbamate iniferter | — | 365 | 85 | ||||||
To the best of our knowledge, there is no reported review article on the topic of light-responsive PUs, especially in medical and biomedical areas. The main aim of this review is to summarize the potential light-responsive polyurethane-based materials that have been used in medical and biomedical engineering and wastewater treatment applications. After the introduction of light responsiveness PUs, Section 2 will summarize the recent reports on the synthesis and design of PUs, which show potential for developing single or multifunctional stimuli responsiveness to light and other factors. The chemical, physical, mechanical, and thermal properties of the PUs triggered by light stimuli are also included in this review. Finally, the key points have been highlighted for development and challenges in future research.
2. Light-responsive polyurethane
Table 2 summarizes the light-responsive groups used in polyurethane and their corresponding responsiveness and wavelength used for light irradiation. The light-responsive PUs reported here have been discussed in the following section. Section 2 presents the reversible light-induced isomerization and dimerization reaction, irreversible light-induced cleavable reaction, and light-induced crosslinking reactions.2.1. Light-induced isomerization reaction
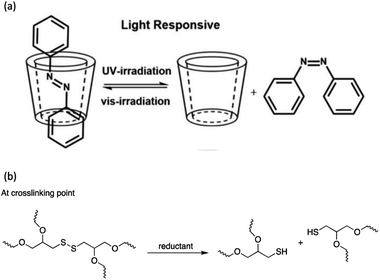
Light-induced isomerization or photoisomerization is a molecular behavior in which structural changes as the trans–cis isomerization of molecules with a double bond is caused by photoexcitation. For certain molecules, this process is reversible and repeatable, making these photoisomerization groups more attractive in designing polymers with a broad range of applications. Dichloroethylene is an example of a molecule for which a light-induced irreversible isomerization is known.86 By considering biomedical applications, the irreversibility of isomerization prevents a non-invasive subcutaneous application or the easy recovery of the cells when hydrogels are used as scaffolds for cell housing. In this review, only reversible photoisomerization will be discussed. The reversible photoisomerization groups that have been incorporated in PU are azobenzene (AZO) (Fig. 2a) and spiropyran (SP) (Fig. 2b). The light-induced transitions in azobenzene groups caused changes in both the isomerization structures as well as the polarity of the compound. The linear trans-isomer is non-polar, while the bent cis-isomer is more polar due to the change in the symmetry. For the cis-isomer, the benzene rings are on the same side of the molecule; thus, the atoms experience different polarity. Hence, the cis-isomer is a polar molecule. The AZO-based PUs can be transformed upon UV light irradiation (365 nm)6,18,73–75 from trans to cis-azobenzene and reversibly from cis to trans when exposed to visible light (450 nm to 530 nm)11,68–72 or heat for reversible photoisomerization. Besides, colorless SP is one of the mechanophores that can be ring-opened in response to UV at 365 nm or applied stress and thus convert to a purple merocyanine (MC) form. Photoisomerization is one of the simplest light-induced reactions that can be applied in polymers and include trans–cis isomerization and heterocyclic cleavage.The single and dual responsiveness of AZO-based PU with light-induced trans–cis isomerization and SP-based PU with heterocyclic cleavage reaction are discussed in the following sections.
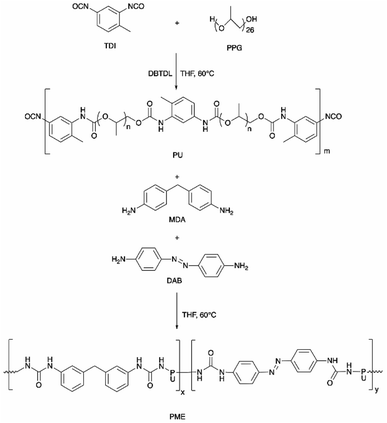
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, while the urethane groups were obtained by the reaction of polyol, PEG, and isocyanates that provide one site of weak monodentate hydrogen bonding linkage (Fig. 3b). In addition, hydrogen bonding can be established between the bidentate urea groups and the monodentate urethane groups.69,89 The strong hydrogen bonding gives elasticity that ensures full recovery from large strains, while the weak hydrogen bonding leads to energy dissipation after breaking and reforming.69,90 The PU system was proposed to serve for the construction of light-responsive soft robotic gripper.
O, while the urethane groups were obtained by the reaction of polyol, PEG, and isocyanates that provide one site of weak monodentate hydrogen bonding linkage (Fig. 3b). In addition, hydrogen bonding can be established between the bidentate urea groups and the monodentate urethane groups.69,89 The strong hydrogen bonding gives elasticity that ensures full recovery from large strains, while the weak hydrogen bonding leads to energy dissipation after breaking and reforming.69,90 The PU system was proposed to serve for the construction of light-responsive soft robotic gripper.
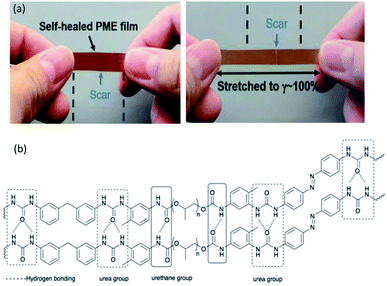 | ||
| Fig. 3 (a) The PME film was cut into two pieces and held together for 5 min at 23 °C. The film can then be stretched to 100% strain without breakage. Reproduced with permission.69 Copyright 2019, John Wiley and Sons, and (b) the H-bonding in the PU structure of the thermoplastic PU-based PME with light-responsive and self-healing properties. The hashed bonds show the H-bonding. | ||
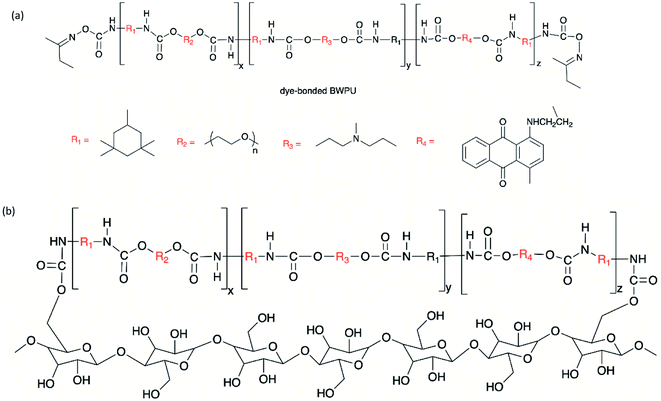
Chen et al. developed a dye-bonded blocked waterborne polyurethane (BWPU) as a coloring agent and light-responsive cationic surfactant BTAEAzo in a green cotton coloring process for cotton fabrics.70 The PU foam was synthesized by reacting isophorone diisocyanate (IPDI) and PEG, and subsequently coated with a BTAEAzo containing dye liquid as the surfactant to prepare the dye-bonded BWPU (Fig. 4). The synthesized BTAEAzo showed light sensitivity photoisomerization capability under UV or visible light irradiation. The dye-bonded BWPU foam was applied onto cotton fabrics using a control coater to dye the fabrics. The residual foam collected after the dying process was exposed to UV light (λ = 360 nm) so that the BWPU foam ruptured and was recycled in the liquid form. The recycled coating liquid was then irradiated with visible light (λ = 450 nm) to regain the foam stabilized capability to be reused in the dying processes. BTAEAzo-PU was reported to be light-sensitive after 20 cycles of repetitive photoisomerization, which indicated that the addition of the dye did not affect the foamability of BTAEAzo in the foaming process under light exposure.
Meanwhile, dual light- and pH-responsive PUs containing AZO have potential application in sensors for the purpose of monitoring the environmental changes.6,74,91 Considering the biomedical field as an example, the intracellular pH plays an important role in cell, tissue, and enzymatic activities. Monitoring the pH changes and gradients are crucial to investigate cellular internalization pathways and diagnose certain diseases. With the addition of pH response, the light-responsive PU could be used as a biosensor to specifically monitor pathophysiological conditions of the targets.
When azobenzene is incorporated with other functional groups such as disulfide with reductant properties, the azobenzene PU is used in the drug delivery system.18 The synergistic effect of the light-responsive azobenzene moiety and the reductant-responsive disulfide in the PU hydrogel, which improved the drug release profile of the hydrophobic drug.
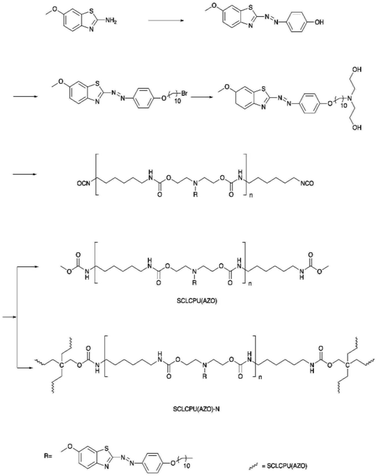
Wen et al. reported a dual light- and thermo-responsive PU, side-chain liquid crystalline polyurethane networks based on the pendant azobenzene group [SCLCPU(AZO)-N]. SCLCPU(AZO)-N was synthesized by reacting the functional azobenzene diol monomer (derived from 2-amino-6-methoxybenzothiazole) with 1,6-hexamethylene diisocyanate (HDI) to form the linear SCLCPU(AZO) precursor and subsequently crosslinked the SCLCPU(AZO) precursor with pentaerythritol (Scheme 2).72 The bending and unbending of the SCLCPU(AZO)-N films were due to the trans–cis photoisomerization of azobenzene by irradiation with visible light at 450 nm and 550 nm. When the SCLCPU(AZO)-N film was exposed to visible light (λ = 450 nm), the sample underwent trans to cis isomerization and SCLCPU(AZO)-N returned to the initial state from cis to trans at an irradiation wavelength of 550 nm; SCLCPU(AZO)-N displayed a triple shape memory effect by demonstrating two independent glass transition temperature (Tg) and a clearing point (Tcl) with a temperature difference of more than 40 °C, making it a thermo-responsive PU material. The architecture of the SCLCPU(AZO)-N networks giving the triple shape memory effect could be adjusted by the molecular weight of the SCLCPU(AZO) precursors used in the reactions.
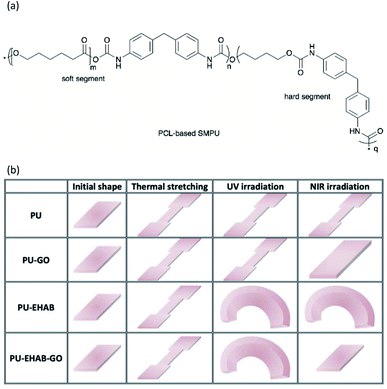
Zhou et al. reported on the fabrication of PU-GO, PU-EHAB film, and PU-EHAB-GO films.14 The PU employed was a PCL-based commercial shape memory PU (SMPU) (KH330, Yuntaihaina Environmental Protection Technology Co., Ltd) with thermal responsive properties. The PU composite film was fabricated by casting the SMPU, GO, and/or EHAB solution in DMF on a glass slide and left to dry to obtain the PU-GO, PU-EHAB, and PU-EHAB-GO films. EHAB and GO acted as photo-harvesters for UV and NIR light, and their light-responsiveness was transferred to the host SMP films. EHAB was proven for its trans–cis photoisomerization that was triggered by UV light. Upon exposure to UV light (source: HTLD-4 II Shenzhen Height-LED Opto-electronic Tech Co., Ltd, wavelength: not reported), the mechanically stretched PU-EHAB-GO films bent toward the light sources as the light-induced photoisomerization occurred on the film surface. On the other hand, GO with photothermal effect with good absorption toward NIR light (source: MDL-H Changchun New Industries Optoelectronics Technology Co., Ltd, wavelength: not reported) and the bent PU-EHAB-GO film also recovered its initial shape after NIR light irradiation (Fig. 6b). Besides, the stretched PU-GO-EHAB film was also nearly recovered to its original shape by heating at 85 °C. The PU-EHAB-GO composites were reported for their potential application in biomimetic actuation.
Zhang et al. developed a UV–Vis–NIR photo-activated PU composites via the incorporation of 5CAZ and up-conversion nanoparticles (UCNPs) into a commercial thermo-responsive shape memory polyurethane (SMPU) (Mn = 1.2 × 105 g mol−1, PDI = 2.25).71 The UCNPs were able to convert low energy light such as NIR light into a high energy light; thus, a long wavelength (NIR light) light-responsive polymer also can be obtained by introducing UCNP into the polymer matrix. 5CAZ served as a light sensitive group and underwent trans–cis photoisomerization under UV light (365 nm) and visible light (530 nm) exposure. The synthesized UNCP was used to convert NIR light at 808 nm into green fluorescence by continuous photon absorption. The 5CAZ/UCNP/SMPU film formed a bilayer structure and the concentration of 5CAZ was higher at the upper layer due to small surface energy. The authors applied mechanical stretching on the 5CAZ/UCNP/SMPU film before performing light irradiation in order to get a more ordered distribution of the 5CAZ content. When the surface of the stretched 5CAZ/UCNP/SMPU film exposed to UV/Vis/NIR light, the volume contraction of the upper layer was higher than the lower layer, which results in film bending toward the light source. The 5CAZ/UCNP/SMPU film exhibited triple shape memory effects as the stretched film was bent upward upon UV/visible light/NIR light irradiation. The film was able to recover to its initial shape (before stretching) upon heating at 90 °C. It was reported that the NIR light could not induce the azo-moieties of 5CAZ to isomerize from trans to cis and up-conversion fluorescence without the presence of UCNPs (Fig. 7). The assembled PU film was prepared as a biomimetic finger-like product, which demonstrated a larger bending degree upon UV irradiation as compared to NIR irradiation.
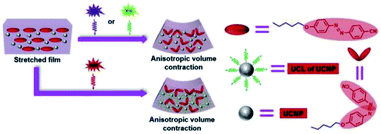 | ||
| Fig. 7 Schematic illustration of the bending mechanisms for 5CAZ/UCNP/SMPU films upon UV/vis/NIR irradiation. Reprinted with permission.71 Copyright 2019, Elsevier. | ||
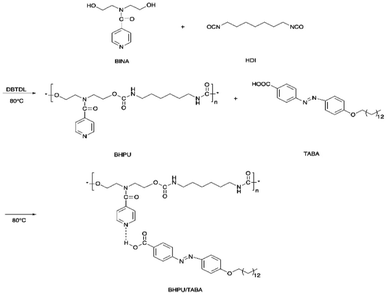
Li et al. also reported a dual light-and thermo-responsive staging supramolecular shape memory PU complexes formed by the pyridine-based PUs and azobenzene-based mesogens.11 The pyridine-based SMPU (BHPU) complexes obtained by reacting HDI and N,N-bis(2-hydroxyethyl) isonicotinamide (BINA) were first synthesized by reacting N,N-bis(2-hydroxyethyl) isonicotinamide (BINA) with HDI. Subsequently, BHPUs were added with azobenzene mesogens TABA to form the supramolecular BHPU/TABA complex via hydrogen bonds between the pyridine rings and carboxyl groups of TABA mesogens (Scheme 3). The addition of TABA increased the glass transition temperature, thermal stability, and storage modulus while promoting the microphase separation in the new complexes. These physical, thermal, and structural changes (microphase separation) contributed to the multi-shape memory and thermal responsive properties of the PU. Under the thermal-induced shape memory effect, the TABA formed intermolecular hydrogen bonds with the pyridine of the PU networks while breaking the intramolecular H-bonding in the PU network. In terms of light-responsive properties, the stretched BHPU/TABA film was curled under UV irradiation (λ = 365 nm) and the cis-TABA/BHPU film remained stable under visible light irradiation (λ = 460 nm). After UV exposure, the cis-TABA was fixed at lower temperature due to the vitrification of polymer chains and insufficient visible light, under which the cis-isomer could not o the steric hindrance.11,74 However, the curled BHPU/TABA film was able to recover to the original shape by reheating to 80 °C (Fig. 8). As compared to the normal thermal-induced shape memory effect, the light-responsive shape memory does not require a certain temperature field and avoided the uncontrollable continuous heating during the application. This has shown the exceptional properties of the light-responsive BHPU/TABA complexes.
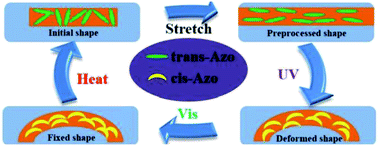 | ||
| Fig. 8 Schematic illustration of the mechanism of photo-thermal staged-responsive shape memory properties in the molecular structure. Reprinted with permission.11 Copyright 2019, Elsevier. | ||
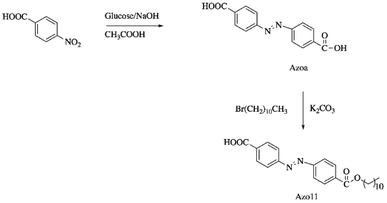
Chen et al. synthesized a dual light- and thermo-responsive SMPU by reacting polycaprolactone (PCL, Mn = 4000 g mol−1) and diphenylmethane diisocyanate (MDI), followed by the reaction with 1,4-butanediol.73 4-(4-Oxyalkyl chain carbonyl) azodibenzoic acid (Azo11) was incorporated as a filler in the SMPU matrix by casting the SMPU and Azo11 in DMF solution onto a Teflon plate and left to dry at 80 °C. Azo11 (Scheme 4) was reported to enhance the crystallization capacity of the soft segment of SMPU and resulted in the curled Azo11-SMPU film that did not recover to the initial shape but fixed temporarily under visible light irradiation. The recovery of the SMPU was aided by heat. This unique characteristic is known as staging-responsive shape memory properties.74 The Azo11-SMPU film first underwent UV light-triggered deformation, followed by fixation under visible light irradiation (that remained in a deformed shape), and thirdly, thermal-induced recovery into its original shape. These three stages are independently responsive to external UV, visible light, and heat stimuli. Upon exposure to UV light (365 nm), the Azo11 moieties isomerized from the trans-isomers to the cis-isomers, demonstrating the shrinkage of the azo11-PSMPU chain specifically on the film surface (film curling). The curling of the film was maintained for an extended time (>90 min) even though the film was irradiated by visible light (source and wavelength: not reported) due to the weak forces arising from the photoisomerization triggered by visible light, which was insufficient to destroy the intermolecular forces formed. The curled shape was able to recover to its original shape upon heating at 80 °C due to the crystal melting phase transition occurring at a higher temperature; thus, the increased energy aided the photoisomerization. The azo11-PSMPU has good potential applications in smart electronics, smart sensors, smart optical devices, and robotic fingers.
Yang et al. synthesized a dual light- and thermo-responsive staging SMPU by reacting HDI, PCL-4000, and Azoa, followed by the addition of glycerol as the crosslinker.12 Scanning electron microscopy (SEM), atomic force microscopy (AFM), wide-angle X-ray diffraction (WAZRD), differential scanning calorimetry (DSC), and thermogravimetric (TG) results demonstrated that Azoa-SMPU formed microphase separation structures containing semi-crystallized PCL soft phase and an Azoa amorphous hard phase that could influence the crystallinity of the PCL soft phases. Upon UV irradiation, the stretched Azoa-SMPU film underwent deformation (bending) due to the isomerization of trans-Azoa to cis-Azoa. The deformation of Azoa-SMPU was reported to be due to the decreased distance between the PU chains in the cis-isomer form, which increased the hydrogen bonding of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N
O and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H. After UV exposure, the cis-Azoa isomers were fixed by strong intermolecular forces and the PCL crystalline soft phase; therefore, no recovery occurred under visible light. The author reported that the increased temperature caused the melting of the PCL soft segments, thus destroying the hydrogen bonding of the hard segments and weakening the intermolecular forces of the polymer chains. Therefore, the deformation of shape was recovered at above 50 °C. With similar results of staging responsive SMPU, Ban et al. also investigated the same light-responsive group Azoa but the final SMPU was recovered at 80 °C.74
H. After UV exposure, the cis-Azoa isomers were fixed by strong intermolecular forces and the PCL crystalline soft phase; therefore, no recovery occurred under visible light. The author reported that the increased temperature caused the melting of the PCL soft segments, thus destroying the hydrogen bonding of the hard segments and weakening the intermolecular forces of the polymer chains. Therefore, the deformation of shape was recovered at above 50 °C. With similar results of staging responsive SMPU, Ban et al. also investigated the same light-responsive group Azoa but the final SMPU was recovered at 80 °C.74
 | ||
| Fig. 9 (a) Synthesis route of the AZO-PU polymeric dye. Reprinted with permission.6 Copyright 2018, Elsevier and (b) reaction scheme of the AZO moiety under UV light irradiation and acid condition. | ||
Li et al. reported on a dual light- and pH-responsive cationic waterborne PU (CWPU) with the side chain aromatic azobenzene group (AZO-CWPU) by reacting IPDI, polyether polyol, N-methyl diethanolamine, and EDPD via step growth polymerization.75 The author reported that the incorporation of azobenzene increased the dipole moment of WPU, resulting in decreased static water contact angles and increased surface free energy coating as compared to the ordinary WPU. Upon UV irradiation at 365 nm, the static water contact angle was reduced from 61° to 55° due to the photoisomerization of azobenzene from the trans- to cis-form. The AZO-CWPU dispersion showed color change from red to yellow on increasing the pH values, which indicated the responsiveness of AZO-CWPU toward the change in the pH values. The AZO-PU dye existed in two equilibrium forms, i.e., the hydrazone form and the azoic form, in acidic environment due to the formation of two symmetry centers and protonated nitrogen.75,96 When the environment is more acidic, the hydrogen ions are attached to the nitrogen atoms of AZO-moieties and the diethylaminoethyl groups in AZO-PU; hence, the molecules became protonated. Under a weak acidic environment, the equilibrium of the hydrazone form and the azoic form was reached, and the mixture displayed an orange color. AZO-CWPU was also reported to be thermally more stable due to the good conjugation of the AZO moieties. The dual responsive AZO-CWPU was proposed to be potentially applied in optics and bioengineering areas.
 | ||
| Scheme 5 (a) Host-guest interaction of AZO. Reprinted with permission from L. Peng, S. Liu, A. Feng and J. Yuan, Mol. Pharm., 2017, 14, 2475–2486.151 Copyright 2017, American Chemical Society and CD under light irradiation and (b) the reductant breaks the disulfide bond and the crosslinkers are dissociated. | ||
2.2. Spiropyran-based polyurethane
Spiropyran (SP) is one of the unique organic chemical groups that contains dynamic covalent bond, in which the ring will be opened in response to UV and be converted to the colored merocyanine (MC) form.97 The MC form reverts to stable SP under visible light or in dark situation (Fig. 2b). Different SP-based PU (SP-PU) have been reported for their UV activation and/or ultrasonic sensitive properties. SP is commonly involved in the preparation of optical devices,98 biological imaging,99 and chemical sensors.100,101Zhang et al. presented an ionic WPU with dual light- and force-responsiveness.7 Ionic WPU was synthesized by reacting IPDI and polytetramethylene glycol as well as the synthesized di-hydroxyl spiropyran (SP)7,102,103 as the chain extender to produce an SP-WPU prepolymer, followed by reacting the SP-WPU prepolymer with dimethylolpropionic acid (DMPA) as the ionized segment and triethylamine (TEA) as the neutralizer. The ionic SP-WPU produced exhibited light-sensitive properties in both the emulsion and polymer film. The yellowish emulsion was turned to purple instantly upon exposure to UV light (λ = 365 nm), which indicated the ring opening of SP (yellow) to form MC (purple) in the PU chain. The recovery process of the MC to the thermodynamically more stable SP form was induced by visible light or placing the WPU in dark. The author reported that the SP-WPU film had higher UV activation rate than the SP-WPU emulsion, which was due to the equilibrium reaction between SP and MC. As for the recovery rate, it was reported that the MC in the WPU emulsion form had a higher recovery rate than the WPU film because the light-sensitive molecule in the emulsion state could move more freely, thus taking lesser time for molecular conversion to a thermodynamically stable SP.
The author also investigated the mechanical responsiveness of SP-WPU by sonication and tensile test. The ultrasonication responsiveness properties of the SP-WPU dispersion relied on the water content due to the synergetic effect of the polarity of the mixed solvents. In terms of stretch responsiveness, the SP-WPU film showed blue color when stretching and changed to purple and pink when the film was unloaded. This secondary color change is due to the isomerization of the methane bridge in the activated MC.7,104 SP-WPU was proposed for its potential application as a light sensitive coating material in sunglasses (Fig. 10).
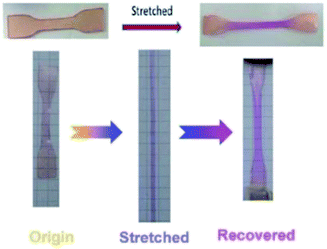 | ||
| Fig. 10 The digital photos of stretch sensitive SP-WPU film. Reprinted under terms of the CC-BY license.7 Copyright 2017, Royal Society of Chemistry. | ||
2.3. Reversible light-induced dimerization reaction
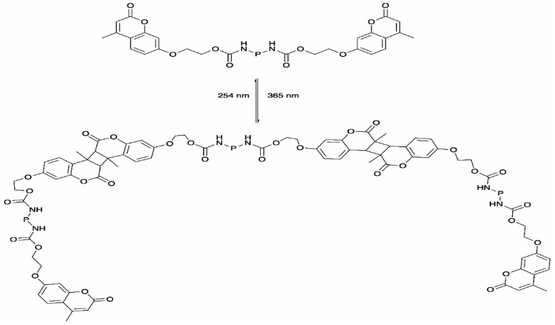
The coumarin moieties and anthracene can perform photodimerization that were triggered by irradiating the PU under UV light (Fig. 2d). The UV-irradiated (λ = 365 nm) coumarin dimers convert back to the original structure on irradiation at a wavelength of 254 nm.76 The light-responsive coumarin can be crosslinked with other responsive functional groups (e.g., pH-responsive groups) to produce PU material with greater targetability in drug delivery systems in order to improve the performance of the smart polymer with large variation of the physiological microenvironment.19 This coumarin-based PU offers the possibility of using environment friendly materials with healing properties in smart coating applications.77,78 On the other hand, the anthracene light-induced dimerization does not have a strict requirement of the wavelength of the UV-light source.44,79,105–107 It has been reported that the anthracene groups undergo [4 + 4] cycloaddition reactions under UV light exposure (λ > 300 nm) and the anthracene groups are regenerated by the scission of the dimers when they are irradiated with short wavelength UV light (λ < 300 nm) or stimulated at a temperature above 120 °C.79 The coumarin groups and anthracene groups were also reported to endow the PU with self-healing properties.77,79The single and dual light- and pH-response of PU-containing coumarin as well as dual light- and thermo-responsive PU containing anthracene with light-induced dimerization are discussed in the following paragraphs.
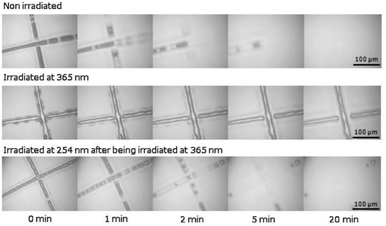 | ||
| Fig. 11 Optical microscopy of coumarin-PU during the filling process. Reprinted with permission.77 Copyright 2016, Elsevier. | ||
Salgado et al. reported the production of a light-responsive PU containing coumarin by reacting a coumarin diol-HDI pre-polymer with PCL.78,109 Silica nanoparticles (mSiNPs) were dispersed into the coumarin-based matrix. The mechanical and thermal properties of the PU were reported to be improved by the incorporation of the coumarin diol as the chain extender, while the coumarin offered its light-responsive properties to the final PU product. The coumarin moieties underwent reversible light-induced dimerization and photocleavage at 365 nm and 254 nm, respectively. The coumarin-based PU dispersed with mSiNP showed higher photodimerization behavior due to the good interaction between the mSiNPs and the hard segments of the PU matrix, as is evident by FTIR. The photodimerization yield was also higher than that of the photocleavage yield due to the equilibrium between the dimers and the cleaved coumarins after irradiation with UV light.110 Photodimerization between the hard segment and mSiNPs were agglomerated, which are less likely to perform photocleavage. The drawback of the addition of coumarin diol into the PU matrix was the detrimental effect on the transparency of the PU matrices, causing a complete blocking effect in the region of the UV spectrum from 245 to 355 nm.
Long et al. utilized light-responsive coumarin derivatives and pH-responsive hydrazone groups in PU production for targeted cancer chemotherapy.19 PU was produced by reacting L-lysine ethyl ester diisocyanate (LDI) and hydrophilic PEG-600 for a biocompatible micelle drug delivery PU system.19,111 PU was further reacted with the newly synthesized coumarin derivative OMC19 as the crosslinker via hydrazone bonding, followed by the introduction of folic acid (FA) into the PU network via amidation. The hydrophobic anticancer drug doxorubicin was encapsulated into the micelles by the dialysis method. The release profiles showed that the drug release rate at pH 5.8 was faster than at pH 7.4. The fast release of the drug was postulated to be related to the repulsion between the ionized amino groups at lower pH and led to looser nanoparticles, which caused the drugs to be more likely to diffuse out from the micelles. Upon UV irradiation at 365 nm, coumarin dimers were formed via the [2 + 2] cycloaddition formation of the cyclobutene ring19,112 and the crosslinking of the micelles, as proved using UV-Vis spectroscopy. At wavelength 320 nm, the intensity of the peak of the OMC groups was reduced with the irradiation time, which showed the dimerization of the OMC groups and the crosslinking of the micelles. The crosslinking process reached the maximum yield with the increase in the irradiation time (∼130 min), hence resulting in the rigidity of the polymer chain inside the micelle cores, which prevented the further dimerization of the OMC groups. The studies also reported that light-induced crosslinking did not exhibit toxicity and interrupt drug release in in vitro studies. The dual-responsive PU-containing coumarin micelles are potential drug carriers due to excellent stability in physiological environments after UV light-induced crosslinking and can release loaded drugs under low pH condition (e.g., tumor cell) (Fig. 12). To date, dual light- and pH-responsive PU-containing coumarin that responded to visible or NIR light and meant for drug delivery have not been reported. The dual light- and pH-responsive PU-containing coumarin that responded to UV light also has limited applications in drug delivery due to UV light being unable to penetrate deeply into the body. Hence, research on smart PU with visible light- or NIR light-responsive and pH-responsive is worth exploring for the emerging chemo-phototherapy for cancer treatment.
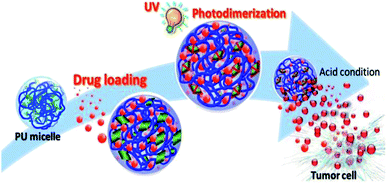 | ||
| Fig. 12 Illustration of the core crosslinked polyurethane micelles for the intracellular release of anticancer drugs triggered by the acidic microenvironment inside the tumor cell. Adaptation under terms of the CC-BY license.152 Copyright 2020, International Journal of Molecular Sciences. | ||
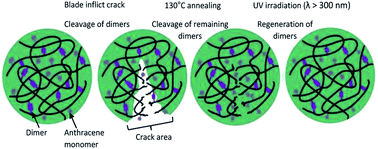
From the study conducted by Fang et al., a novel dual light- and thermo-responsive PU film was produced by reacting IPDI and PPG-2000, followed by incorporating the anthracene diol derivative BHPAE, which endowed the PU films with dual responsiveness as well as self-healing properties.79 The pendent anthracene groups underwent reversible [4 + 4] cycloaddition and formed a crosslinked structure when exposed to UV light (λ > 300 nm) and reverted back when stimulated at a temperature above 130 °C. When the [4 + 4] cycloaddition reaction occurred, the carbon positions 9 and 10 of anthracene were dimerized as a result of the cleavage of the conjugated π–π system of the anthracene groups. The rate of dimerization was fast, which reached the equilibrium state after 10 min of UV irradiation. The dimerization rate was reported to decrease when the number of photodimerization cycles increased due to the amount of restored anthracene groups that decreased with increasing cycles. The light-induced crosslinked structure reduced the mobility of the BHPAE-PU chains. The increased BHPAE contents also resulted in the rising of Tg. This means that the presence of anthracene increased the crosslinking density and further enhanced the thermal stability. The dual responsiveness reversible bonds located in the PU chain between the crosslinking points provided the BHPAE-PU with excellent self-healing ability. The mechanism of the healing process is as illustrated in Fig. 13. At the crack area, a majority of the dimers reverted back to anthracene monomers due to physical damage. The subsequent heating process guaranteed that the remaining dimers around the injured areas were cleaved and reverted to anthracene monomers. Meanwhile, the reduced crosslinking density permits the mobility of the PU chains at elevated temperature, which contributed to the healing of cracks. The healing process completed after the dimers were regenerated under UV light illumination (λ > 300 nm).
2.4. Irreversible light-induced photocleavage reaction
The irreversible photocleavage reaction normally occurs in o-nitrobenzyl (o-NB)-incorporated polymers. The o-NB photocleavage groups can be located in the side chain, main chain, or as a cross-linker of copolymers.20 After UV irradiation, the by-product of o-NB such as o-nitrosobenzaldehyde could act as an internal filter and hence reduced the o-NB cleavage efficiency.114 However, the by-product could possibly react with amine to form an imine compound, which would cause problems to the nearby proteins (Scheme 7).115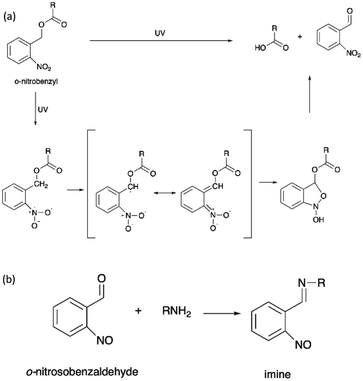 | ||
| Scheme 7 (a) The photocleavage reaction of o-nitrobenzyl and (b) the reaction of o-nitrosobenzaldehyde and primary amine. | ||
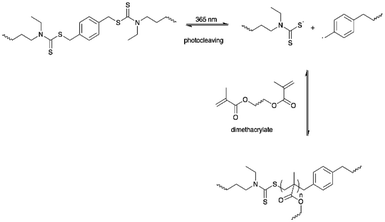 | ||
| Scheme 8 The LBCL was photocleaved under 365 nm and further initiated light-induced methacrylate polymerization. | ||
A light-responsive PU system from a 2-amino-1,3-propanediol (serinol)-based monomer in which the amino group is protected by the o-NB group via a carbamate linker was developed by Sun and et al.20 Upon UV light exposure, the o-NB groups underwent irreversible photocleavage reaction to release the functional amine groups, which further induced the partial degradation of the PU backbone via intramolecular cyclization (Fig. 14a). The author proposed that the degradation mechanism indicated that the addition of the base would speed up the degradation rate because the deprotonated amino groups are more nucleophilic. The light-responsive PU was formulated into nanoparticles, which was employed as the drug carrier for the encapsulation of the hydrophobic model drug Nile Red. On irradiation with UV light (320–480 nm), the light-responsive PU nanoparticles possessed a structural change, which resulted in the burst release of the hydrophobic Nile Red payload in the aqueous environment (pH 7.0) and reached maximum release within 20 seconds of UV light irradiation. Nevertheless, a NIR-responsive moiety can be used for developing polymeric materials instead of UV-responsive moieties to obtain deeper tissue penetration.116
2.5. Light-induced crosslinking reaction
Light-induced crosslinking reaction is known as photopolymerization, such as the UV curing of multifunctional monomers to transform a liquid resin into a solid polymer almost instantly and selectively at the illuminated areas.117 UV light curable materials mainly include oligomers, diluents/monomers, and a photoinitiator. The photoinitiator is an important reactant for the preparation of high-performance photopolymers. Photoinitiator can be used in free radical, cationic, and anionic polymerization and also helps to increase the photosensitivity in UV-curable PU. In the following section, the PUs will be crosslinked with acrylate or inorganic oligomers and further reinforced the physical properties of PU under the exposure of UV light80 or visible light.118Hsiao et al. reported dual light- and thermo-responsive PU dispersions synthesized from PCL diol, oligodiol, and IPDI via the waterborne process.80 2-hydroxyethyl methacrylate (HEMA) groups were introduced at the end of the thermo-sensitive PU main chains as single hydroxyl acrylate to generate vinyl-terminated PU, where the acrylic functionality served as the UV-responsive curing site.80,119 Photoinitiator 2,2′-azobis[2-methyl-N-(2-hydroxyethyl)propionamide] (VA-086) was incorporated to increase the cell viability80,120–122 and initiate the light-induced crosslinking process. The UV light source (λ = 365 nm) transmitted through the PU dispersion to excite the photoinitiator and initiate the UV-curing process. The PU nanoparticles (NP) showed light responsiveness, which gave rise to an increase in the molecular weight, particle size, and crosslinked network after UV irradiation. Furthermore, the cured PU NP became more rod-shaped and formed a compact structure after further heating. The UV-cured PU dispersion underwent a thermal-induced sol–gel transition at 37 °C and was able to recover the solid-like structure to obtain a stable construct. The UV-curable crosslinking network may limit the mobility of the polymer chain and reduce the free volume in a PU NP by decreasing the degree of swelling of the cured NP after further heating. Therefore, a shorter distance among the cured PU NPs may stimulate the formation of hydrogen bonding and undergo phase transition at higher temperatures, therefore enhancing the mechanical properties of the PU. There is no report on NIR light-induced crosslinking in PU.
Urethane oligomers are commonly used for high performance coating materials. In addition, PU is known for excellent weathering and chemical resistance of the coating and is usually used in a wide range of UV-curable coating materials.81,123,124 Using different forms and concentrations of the reactive diluents, the adhesion, mechanical, and physical properties of the PU are possibly modified without altering the composition of the PU coating system. Zvonkina et al. reported on a UV-curable PU coating from the aliphatic difunctional urethane oligomer, functional acrylic reactive diluent, and a mixture of photoinitiators, i.e., Irgacure 184 and Irgacure 819.81 The photopolymerization mechanism was employed in the film form of PU. The polymerization process was examined by measuring the heat flow under UV light irradiation (light source: Omnicure Series 2000 device, 160 W, wavelength: not reported) and was assessed by exothermic enthalpy during the photopolymerization process. The improvement of the PU coating adhesion while enhancing the coating flexibility in response to UV light was reported due to the crosslinking process during photopolymerization. The physical properties of the UV-curable coating can be modified according to the desired requirements by selecting the composition of the monomers.
Hu et al. reported the synthesis of a biobased cardanyl acrylate (CA) as a reactive diluent in an UV-curable castor oil-based polyurethane-acrylate (COPUA) by reacting cardanol and acryloyl chloride,9,125 followed by the incorporation of Darocur1173 as the photoinitiator.9 The modified PUA showed an improvement in the volumetric shrinkage and biobased carbon contents as compared to petroleum-based PU. Volumetric shrinkage is one of the major challenges for UV-curable acrylic, which may deform the final cured materials and affect the molding efficiency.9,126 By incorporating CA, the hydrophobicity was increased due to the long hydrophobic alkyl chains scattered into the castor oil-based PUA matrix and prevented the penetration of water molecules.9,127 The improvement in the physical properties such as hardness, adhesion, and thermal stability of PUA were related to the flexible long C15 alkyl chains and rigid benzene ring from cardanol. Nevertheless, the castor oil-based PUA coating system was rapidly cured (40 seconds) upon UV light irradiation. Higher CA content resulted in higher carbon–carbon double bonds conversion into single bonds (–C–C–). The higher CA content caused lower viscosity and thus increased the binding opportunities between the radicals and enhanced the carbon–carbon double bond conversion.9,128,129
Wang et al. reported a series of environment-friendly UV-curable PU coatings synthesized using acrylate polyester (APE) as oligomers with different non-isocyanate urethane dimethacrylate as reactive diluents.82 The reactive diluents were 1-(methacryloyloxy)propan-2-yl 3-(methacryloyloxy)propylcarbamate (POAPD), 2-(methacryloyloxy)ethyl 3-(methacryloyloxy)propylcarbamate (POAED), and 2-(methacryloyloxy)ethyl 2-(methacryloyloxy)ethylcarbamate (EOAED). Darocur 4265 was selected as the free radical photoinitiator. The introduction of urethane dimethacrylate could decrease the viscosity of the APE oligomer for ease of processing. Upon UVB irradiation at a wavelength of 257 nm, the photoinitiator was activated and initiated photopolymerization to crosslink the PU network. The toughness of the PU coating was enhanced due to the formation of hydrogen bonds from N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in the urethane groups as a result of the crosslinking in the PU matrix, which was triggered by UVB irradiation. Besides, the Tg of PU increased with the increase in the crosslinking density of the PU system, which contributed to better thermal stability of the UV-cured PU.82,130
O groups in the urethane groups as a result of the crosslinking in the PU matrix, which was triggered by UVB irradiation. Besides, the Tg of PU increased with the increase in the crosslinking density of the PU system, which contributed to better thermal stability of the UV-cured PU.82,130
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond conversion and photopolymerization rate due to the competition of light adsorption with the photoinitiator10,131 and high conversion at the immobilization of the polymer chains caused by the UV curing reaction.10,132 The decrease in the Tg was due to the nanofillers' plasticizing effect that led to a larger free volume within the polymer with increasing MWCNT content.10,133 Nevertheless, the uniform dispersion of the MWCNT enhanced the electrical conductivity due to the low electrical percolation thresholds,10,134 while it still maintained the mechanical properties of the PUA matrix. MWCNT showed increased mechanical strength than the PUA matrix. PUA/MWCNT was also reported for good wettability due to the fillers and its prospect in sensor applications.10,135–137
C double bond conversion and photopolymerization rate due to the competition of light adsorption with the photoinitiator10,131 and high conversion at the immobilization of the polymer chains caused by the UV curing reaction.10,132 The decrease in the Tg was due to the nanofillers' plasticizing effect that led to a larger free volume within the polymer with increasing MWCNT content.10,133 Nevertheless, the uniform dispersion of the MWCNT enhanced the electrical conductivity due to the low electrical percolation thresholds,10,134 while it still maintained the mechanical properties of the PUA matrix. MWCNT showed increased mechanical strength than the PUA matrix. PUA/MWCNT was also reported for good wettability due to the fillers and its prospect in sensor applications.10,135–137Huang et al. reported on a UV-curable PU system consisting of the UV-sensitive phosphazene acrylate (NPHE) monomer, PUA oligomer, and a photoemitter.8 The PU system was first prepared by reacting PPG-2000 and IPDI to obtain NCO-terminated PU prepolymer, followed by reaction with HEMA to form the PUA polymer. The UV-sensitive phosphazene monomer was derived from the HEMA substitution reaction to hexachlorocyclotriphosphazene (NPCl2)3 and incorporated into the PUA polymer. The thermal stability of NPHE-PU was slightly increased due to crosslinking between the PU polymer main chains and the NPHE monomer stimulated by the UV irradiation. In addition, the final UV-curable NPHE-PU film also reported improved thermal, physical, and mechanical properties. On the other hand, NHPE-PU also demonstrated the flame-inhibition properties because the presence of phosphorus and nitrogen compositions of NPHE producing non-flammable nitrogen oxides and phosphorus oxides during combustion prevented air supply on the material surface and thus inhibited combustion at elevated temperature. The multifunctional UV-sensitive NPHE was proposed to be used as a flame retardant, diluent, and crosslinker in the UV-curable PU coating system.
A polyester PU particle was prepared by the reaction of PEG with PU particles, followed by the addition of nanosized negative ion powder to obtain the PU negative ion (PU/NI) film via a nonsolvent-induced phase separation method.83 The negative ion powder (NI) is a kind of complex inorganic material used to accelerate the adsorption of the silver precursor due to its strong electronegativity.83,138 Subsequently, the Ag3PO4@AgBr nanoparticles, Ag3PO4 and AgBr were loaded in situ onto the PU/NI film by the impregnation–precipitation-ion exchange method.83,139 In order to compare the results from individual photocatalysts and combine them, the authors prepared three films for comparison, i.e., Ag3PO4-PU/NI, AgBr-PU/NI, and Ag3PO4@AgBr-PU/NI. The recyclability and photocatalytic activity of the films were evaluated by the degradation of methyl orange in the recycling process under visible light exposure (λ > 420 nm). At the beginning, the partial transformation of Ag+ ions into metallic Ag NPs occurred via the reduction effect of photogenerated electrons.83,140–142 Hence, strong surface plasmon resonance (SPR) effect enhanced the photocatalytic activity of the films. Furthermore, the energy band structure of Ag3PO4 and AgBr facilitated the electron–hole separation. Upon visible light exposure, Ag3PO4 and AgBr within the PU/NI films were excited to photogenerate electrons and holes in their conduction and valence bands. The holes that were generated by the surface plans due to the resonance (SPR) effect on the Ag NP could directly oxidize the organic dye. The introduction of AgBr into the Ag3PO4-PU/NI film was also reported to synergistically enhance the photocatalytic activity of the film in degrading organic pollutants due to the reduced recombination rate of the electron–hole pairs, resulting from the heterojunction between AgBr and Ag3PO4 that formed on the Ag3PO4@AgBr-PU/NI film.83,143 The Ag+ ions from the Ag3PO4PU/NI, AgBr-PU/NI, and Ag3PO4@AgBr-PU/NI films damaged the bacterial protein and intracellular organelles.83,144,145
2.6. Fluorescent dye
Rhodamine (Rh) is a widely used fluorescent chromophore with excellent optical properties of good stability and quantum efficiency. The derivatives of rhodamine have been used widely in industrial coloration, fluorometric probes, and fluorescent labelling of biomolecules for a new photochromic system.146,147 Rhodamine B can be incorporated in SMPU to increase the sensitivity of SMPU toward UV light with reversible photochromic behavior.84Cai et al. reported that a dual light- and thermo-responsive SMPU composite was prepared from a commercial available poly(ester urethane) (Desmopan DP 2795A SMP) as a multifunctional PU composite that was composed of MDI and 1,4-butanediol as a chain extender, whereas soft segments were based on poly(1,4-butylene adipate),1,84 followed by the reaction with functionalized microcrystal cellulose (20 μm) as a dual functional modifier that acts as a crosslinker and a reinforcement agent.84 Meanwhile, RhB was incorporated as a toughening modifier to enhance the UV sensitivity and generated a light-responsive SMPU. The RhB-based PU films showed thermally induced shape memory effect, which can be addressed by the H-bonding and chemical crosslinking. The deformed PU film was fixed at 0 °C and recovered to its initial shape after reheating to 60 °C. The flexibly chemically crosslinked RhB and hard segment of PU contributed to the toughness and shape memory effect. RhB also demonstrated reversible photochromic behavior as evident by the clear PU solution that turned pale pink under UV light exposure at 365 nm wavelength, while the PU solution was gradually changed back to a clear solution after heating at 80 °C for 30 min. This is because the RhB surface spiroamide groups were switched reversibly from non-fluorescent (ring-closed structure) to fluorescent (ring-opened structure) in response to UV illumination or heating.84,148
2.7. Other photochromic groups
Gordon et al. developed a light-induced PU with simultaneous healing and strengthening properties by reacting HDI with triethanolamine.85 The authors introduced a photoactive dithiocarbamate iniferter unit containing carbon-sulfur (C–S) bonds as a labile bond crosslinker (LBCL) and ethylene glycol dimethacrylate in the PU network. The C–S bonds underwent photocleaving under UV light (λ = 365 nm) and generated a radical to initiate the first free radical polymerization on the dimethacrylate monomers. Thus, secondary polymerization occurred to give greater crosslinking density, which further enhanced the network modulus and strengthened the PU. LBCL differed from photoinitiators since the photoinitiators normally terminate after light irradiation but the radicals produced from dithiocarbamate iniferter in this PU system reversibly recombined and allowed radical generation within the PU network and be constantly induced using light.85,149,150 The iniferter acted as a macroinitiator triggered by UV light and initiated free radical polymerization that increases the network modulus and at the same time aids in healing the PU materials after damage. The authors proposed the development of a formulation for other applications that may require secondary polymerization such as wrinkling and surface functionalization (Scheme 8).85,1503. Conclusion and future aspects
In this review, different light-responsive groups are incorporated into the PU matrix to endow responsiveness toward light. These light-responsive PUs have been designed into biosensors, drug carriers, shape memory polymer for biomimetic robot, coating for optical device, and fabric. The light-responsive regions of the respective PUs have also been highlighted. For better understanding, schematic illustration and reaction scheme are also included to illustrate the chemical light-induced reactions. Light as a stimulus is particularly attractive due to its availability. However, the essential element of light-responsive polyurethane is a light-sensitive moiety and its requirement for a specific wavelength of light, which may limit its practical applications. At present, light-responsive PU is commonly developed for mechanical applications such as coating, shape memory polymers, or electronic sensors. Although PUs have been known for their biocompatibility, the use of light-responsive PU for medical, biomedical, and pharmaceutical applications remains largely unexplored. This is due to the toxicity, biocompatibility, and biodegradability of the light-responsive group and its photo-reaction products are still not fully understood. Moreover, the restriction of safety measurement in medical and biomedical applications is the current challenge for light-responsive polymers. Further investigation is also needed to explore the feature of light-responsive PU and broaden its application in different areas.Abbreviations
| 5CAZ | 4-Cyano-4′-pentyloxyazobenzene |
| EHAB | 4′-Ethyoxy-4-(11-hydroxyundecyloxy)-azobenzene |
| PE | Polyester |
| AZO | Azobenzene |
| EVA | Poly(ethylene-co-vinyl acetate) |
| PEG | Polyethylene glycol |
| Azo11 | 4-(4-Oxyalkyl chain carbonyl)azodibenzoic acid |
| GO | Graphene oxide |
| PNIPAM | Poly(N-isopropylacrylamide) |
| Azoa | 4,4-Azodibenzoic acid |
| HDI | Hexamethylene diisocyanate |
| PPG | Polypropylene glycol |
| BHPAE | 3-(N,N-Bis(2-hydroxyethyl)amine)propionic acid-9-anthracenemethanol ester |
| HMEC | 7-(Hydroxyethoxy)-4-methylcoumarin |
| PU | Polyurethane |
| BTAEAzo | 4-Butoxy-4′-(trimethylaminoethoxy)azobenzene |
| IPDI | Isophorone diisocyanate |
| RhB | Rhodamine B |
| BWPU | Dye-bonded blocked waterborne polyurethane |
| LDI | L-Lysine ethyl ester diisocyanate |
| SCLCPU (AZO)-N | Pentaerythritol |
| CNT | Carbon nanotube |
| MC | Merocyanine |
| SMPU | Shape memory polyurethane |
| DAB | 4,4′-Diaminoazobenzene |
| MDI | Diphenylmethane diisocyanate |
| SP | Spiropyran |
| DHAB | Dihydroxyazobenzene |
| o-NB | o-Nitrobenzyl |
| TABA | 4-Octyldecyloxybenzoic acid |
| DHEMC | 7-Bis(2-hydroxyethoxy)-4-methylcoumarin |
| TDI | Toluenediisocyanate |
| DSO | 3,3′-Disulfanediyldipropane-1,2-diol |
| OMC | 4-Methyl-7-((4-oxopentyl)oxy)-2H-chromen-2-one |
| Tg | Glass transition temperature |
| EDPD | 2,20-(4-((4-Ethylphenyl)diazenyl)phenylazanediyl)diethanol |
| PCDL | Polycarbonate diols |
| WPU | Waterborne polyurethane |
Author contributions
Ki Yan, Lam: investigation, methodology, visualization, writing – original draft & editing. Choy Sin, Lee: conceptualization, methodology, visualization, supervision, investigation, writing – review & editing. Mallikarjuna Rao, Pichika: supervision, methodology, writing – review & editing. Sit Foon, Cheng: supervision, methodology, writing – review. Rachel Yie Hang, Tan: investigation, methodology, writing – original draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the Fundamental Research Grant Scheme (FRGS) for financial support (Grant No. FRGS/1/2019/STG07/IMU/02/1)Notes and references
- L. Peponi, M. P. Arrieta, A. Mujica-Garcia and D. López, Modif. Polym. Prop., 2017, 131–154 Search PubMed.
- D. Wang, M. D. Green, K. Chen, C. Daengngam and Y. Kotsuchibashi, Int. J. Polym. Sci., 2016, 2016, 2–4 Search PubMed.
- C. Hazra, D. Kundu and A. Chatterjee, Stimuli-responsive nanocomposites for drug delivery, Elsevier Inc., 2018 Search PubMed.
- B. Jeong, S. W. Kim and Y. H. Bae, Adv. Drug Delivery Rev., 2002, 54, 37–51 CrossRef CAS PubMed.
- K. Petcharoen and A. Sirivat, Curr. Appl. Phys., 2013, 13, 1119–1127 CrossRef.
- H. Mao, L. Lin, Z. Ma and C. Wang, Sens. Actuators, B, 2018, 266, 195–203 CrossRef CAS.
- Q. Zhang, Y. Wang, C. Xing, Y. Cai, K. Xi and X. Jia, RSC Adv., 2017, 7, 12682–12689 RSC.
- W. K. Huang, K. J. Chen, J. T. Yeh and K. N. Chen, J. Appl. Polym. Sci., 2002, 85, 1980–1991 CrossRef CAS.
- Y. Hu, Q. Shang, J. Tang, C. Wang, F. Zhang, P. Jia, G. Feng, Q. Wu, C. Liu, L. Hu, W. Lei and Y. Zhou, Ind. Crops Prod., 2018, 117, 295–302 CrossRef CAS.
- C. Mendes-Felipe, J. Oliveira, P. Costa, L. Ruiz-Rubio, A. Iregui, A. González, J. L. J. L. J. L. Vilas and S. Lanceros-Mendez, Eur. Polym. J., 2019, 120, 109226 CrossRef.
- Y. Li, H. Zhuo, H. Chen and S. Chen, Polymer, 2019, 179, 121671 CrossRef CAS.
- J. Yang, H. Wen, H. Zhuo, S. Chen and J. Ban, Polymers, 2017, 9, 287 CrossRef PubMed.
- N. Shelke, R. Nagarale and S. Kumbar, Natural and Synthetic Biomedical Polymers, 2014, pp. 123–144 Search PubMed.
- L. Zhou, Q. Liu, X. Lv, L. Gao, S. Fang and H. Yu, J. Mater. Chem. C, 2016, 4, 9993–9997 RSC.
- H. Xie, J. Shao, Y. Ma, J. Wang, H. Huang, N. Yang, H. Wang, C. Ruan, Y. Luo, Q.-Q. Wang, P. K. Chu and X.-F. Yu, Biomaterials, 2018, 164, 11–21 CrossRef CAS PubMed.
- L. Yang, R. Tong, Z. Wang and H. Xia, ChemPhysChem, 2018, 19, 2052–2057 CrossRef CAS PubMed.
- Q. Zheng, Z. Ma and S. Gong, J. Mater. Chem. A, 2016, 4, 3324–3334 RSC.
- J. Li, L. Ma, G. Chen, Z. Zhou and Q. Li, J. Mater. Chem. B, 2015, 3, 8401–8409 RSC.
- Y.-B. Long, W.-X. Gu, C. Pang, J. Ma and H. Gao, J. Mater. Chem. B, 2016, 4, 1480–1488 RSC.
- J. Sun, T. Rust and D. Kuckling, Macromol. Rapid Commun., 2019, 40, 5–9 Search PubMed.
- C. Yang, K. K. Chan, G. Xu, M. Yin, G. Lin, X. Wang, W. J. Lin, M. D. Birowosuto, S. Zeng, T. Ogi, K. Okuyama, F. A. Permatasari, F. Iskandar, C. K. Chen and K. T. Yong, ACS Appl. Mater. Interfaces, 2019, 11, 2768–2781 CrossRef CAS.
- A. Bagheri, C. Boyer and M. Lim, Macromol. Rapid Commun., 2019, 40, 1–7 CrossRef.
- J. H. Park, D. I. Kim, S. G. Hong, H. Seo, J. Kim, G. D. Moon and D. C. Hyun, Pharmaceutics, 2019, 11, 528 CrossRef CAS PubMed.
- D. Park and J. C. Kim, Int. J. Pharm., 2019, 554, 420–428 CrossRef CAS PubMed.
- S. Machida, Y. Nakamura, A. Itaya and N. Ikeda, Macromol. Chem. Phys., 2019, 1900240, 1–7 Search PubMed.
- C. Zhao, J. Lu and X. X. Zhu, ACS Appl. Polym. Mater., 2020, 2, 256–262 CrossRef CAS.
- M. Wang, K. He, J. Li, T. Shen, Y. Li, Y. Xu, C. Yuan and L. Dai, J. Biomater. Sci., Polym. Ed., 2020, 31, 849–868 CrossRef CAS PubMed.
- J. Li, X. Li, H. Liu, T. Ren, L. Huang, Z. Deng, Y. Yang and S. Zhong, Polym. Degrad. Stab., 2019, 168, 108956 CrossRef CAS.
- J. Tian, B. Huang, C. Xiao and P. Vana, Polym. Chem., 2019, 10, 1610–1618 RSC.
- M. Kang, A. N. Cha, S. A. Lee, S. K. Lee, S. Bae, D. Y. Jeon, J. M. Hong, S. Fabiano, M. Berggren and T. W. Kim, Org. Electron., 2019, 105554 Search PubMed.
- H. Zhang, J. Zhou, G. G. Shan, G. F. Li, C. Y. Sun, D. X. Cui, X. L. Wang and Z. M. Su, Chem. Commun., 2019, 55, 12328–12331 RSC.
- Y. Cozzens, D. M. Steeves, J. W. Soares and J. E. Whitten, Macromolecules, 2019, 52, 2900–2910 CrossRef CAS.
- J. Anderski, L. Mahlert, J. Sun, W. Birnbaum, D. Mulac, S. Schreiber, F. Herrmann, D. Kuckling and K. Langer, Int. J. Pharm., 2019, 557, 182–191 CrossRef CAS PubMed.
- M. Lapshina, A. Ustyugov, V. Baulin, A. Terentiev, A. Tsivadze and N. Goldshleger, J. Photochem. Photobiol., B, 2020, 202, 111722 CrossRef CAS.
- J. H. Choi, H. Seo, J. H. Park, J. H. Son, D. I. Kim, J. Kim, G. D. Moon and D. C. Hyun, Colloids Surf., B, 2019, 173, 258–265 CrossRef CAS PubMed.
- C. Yin, X. Li, G. Wen, B. Yang, Y. Zhang, X. Chen, P. Zhao, S. Li, R. Li, L. Wang, C. S. Lee and L. Bian, Biomaterials, 2020, 232, 119684 CrossRef CAS PubMed.
- Z. Wang, B. Guo, E. Middha, Z. Huang, Q. Hu, Z. Fu and B. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 11167–11176 CrossRef CAS PubMed.
- Z. Luo, L. Jiang, S. Yang, Z. Li, W. M. W. Soh, L. Zheng, X. J. Loh and Y. L. Wu, Adv. Healthcare Mater., 2019, 8, 1–13 Search PubMed.
- K. Poudel, R. K. Thapa, M. Gautam, W. Ou, Z. C. Soe, B. Gupta, H. B. Ruttala, H. N. Thuy, P. C. Dai, J. H. Jeong, S. K. Ku, H. G. Choi, C. S. Yong and J. O. Kim, Nanomed. Nanotechnol. Biol. Med., 2019, 21, 102042 CrossRef CAS PubMed.
- L. Li, Z. Yang, S. Zhu, L. He, W. Fan, W. Tang, J. Zou, Z. Shen, M. Zhang, L. Tang, Y. Dai, G. Niu, S. Hu and X. Chen, Adv. Mater., 2019, 31, 1–9 Search PubMed.
- Y. Xu, J. Chen, L. Tong, P. Su, Y. Liu, B. Gu, B. Bao and L. Wang, J. Controlled Release, 2019, 293, 94–103 CrossRef CAS PubMed.
- J. Shin, J. Sung, M. Kang, X. Xie, B. Lee, K. M. Lee, T. J. White, C. Leal, N. R. Sottos, P. V. Braun and D. G. Cahill, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 5973–5978 CrossRef CAS PubMed.
- Y. Zhou, J. Tan, D. Chong, X. Wan and J. Zhang, Adv. Funct. Mater., 2019, 29, 1–8 Search PubMed.
- Y. Wu, Z. Hu, H. Huang and Y. Chen, Polym. Chem., 2019, 10, 1537–1543 RSC.
- X. Di, Y. Kang, F. Li, R. Yao, Q. Chen, C. Hang, Y. Xu, Y. Wang, P. Sun and G. Wu, Colloids Surf., B, 2019, 177, 149–159 CrossRef CAS PubMed.
- M. Evci, A. Tevlek, H. M. Aydin and T. Caykara, Appl. Surf. Sci., 2020, 511, 145572 CrossRef.
- T. Sajini, R. Thomas and B. Mathew, Polymer, 2019, 173, 127–140 CrossRef CAS.
- V. Marturano, V. Bizzarro, V. Ambrogi, A. Cutignano, G. Tommonaro, G. R. Abbamondi, M. Giamberini, B. Tylkowski, C. Carfagna and P. Cerruti, Polymers, 2019, 11, 1–10 CrossRef PubMed.
- T. J. Ahn, G. S. Seo and O. R. Lim, IEEE Photonics Technol. Lett., 2019, 31, 987–989 CAS.
- T. Guan, Z. Du, X. Chang, D. Zhao, S. Yang, N. Sun and B. Ren, Polymer, 2019, 178, 121552 CrossRef CAS.
- F. Jasinski, T. R. Guimarães, S. David, C. Suniary, T. Funston, Y. Takahashi, Y. Kondo and P. B. Zetterlund, Macromol. Rapid Commun., 2019, 40, 1900355 CrossRef CAS PubMed.
- R. D. Corder, J. C. Tilly, W. F. Ingram, S. Roh, R. J. Spontak and S. A. Khan, ACS Appl. Polym. Mater., 2020, 2, 394–403 CrossRef CAS.
- C. Yongli, C. Xiaolong, Z. Yating and Y. Jianquan, J. Appl. Phys., 2019, 125, 193102 CrossRef.
- B. Sivaranjini, K. Mohana, S. Esakkimuthu, V. Ganesh and S. Umadevi, Liq. Cryst., 2020, 1–12 Search PubMed.
- J. Wang, Q. Jiang, X. Hao, H. Yan, H. Peng, B. Xiong, Y. Liao and X. Xie, RSC Adv., 2020, 10, 3726–3733 RSC.
- C. Wang, T. Hu, Y. Chen, Y. Xu and Q. Song, ACS Appl. Mater. Interfaces, 2019, 11, 22332–22338 CrossRef CAS PubMed.
- H. Xie, L. Li, C. Y. Cheng, K. K. Yang and Y. Z. Wang, Compos. Sci. Technol., 2019, 173, 41–46 CrossRef CAS.
- D. Martella, S. Nocentini, F. Micheletti, D. S. Wiersma and C. Parmeggiani, Soft Matter, 2019, 15, 1312–1318 RSC.
- H. Yang, H. Wang, J. Feng, Y. Ye and W. Liu, Macromol. Chem. Phys., 2019, 220, 1–5 Search PubMed.
- J. Yu, K. Li, L. Li, L. Liu, Y. Zhou, Z. Zhang, M. Guo, N. Zhou and X. Zhu, Polym. Chem., 2019, 10, 2872–2880 RSC.
- Y. Yuan, L. He, J. Li and H. Zhang, Polym. Chem., 2019, 10, 2706–2715 RSC.
- J. Lee, J. M. Park and W. D. Jang, Carbohydr. Polym., 2019, 221, 48–54 CrossRef CAS PubMed.
- Q. Zhang, C. Tan, X. Zheng, P. Chen, M. Zhuo, T. Chen, Z. Xie, F. Wang, H. Liu, Y. Liu, X. Zhang, W. Lv and G. Liu, Environ. Sci.: Nano, 2019, 6, 2577–2590 RSC.
- A. C. Ferahian, D. K. Hohl, C. Weder and L. Montero de Espinosa, Macromol. Mater. Eng., 2019, 304, 1–10 CrossRef.
- K. A. Bonetti, M. Murphy, R. L. Brainard, L. Zhong and J. T. Welch, J. Polym. Sci., 2020, 1–5 Search PubMed.
- J. Lee, W. Lee, D. Kim, M. Kim and J. Kim, Sci. Rep., 2019, 9, 1–7 Search PubMed.
- H. Inoue, T. Hirai, H. Hanochi, K. Oyama, H. Mayama, Y. Nakamura and S. Fujii, Macromolecules, 2019, 52, 708–717 CrossRef CAS.
- T. Fang, L. Cao, S. Chen, J. Fang, J. Zhou, L. Fang, C. Lu and Z. Xu, Mater. Des., 2018, 144, 129–139 CrossRef CAS.
- S. Li, Y. Tu, H. Bai, Y. Hibi, L. W. Wiesner, W. Pan, K. Wang, E. P. Giannelis and R. F. Shepherd, Macromol. Rapid Commun., 2019, 40, 1800815 CrossRef PubMed.
- S. Chen, W. Zhang, C. Wang and S. Sun, Green Chem., 2016, 18, 3972–3980 RSC.
- P. Zhang, B. Wu, S. Huang, F. Cai, G. Wang and H. Yu, Polymer, 2019, 178, 121644 CrossRef CAS.
- Z.-B. Wen, D. Liu, X.-Y. Li, C.-H. Zhu, R.-F. Shao, R. Visvanathan, N. A. Clark, K.-K. Yang and Y.-Z. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 24947–24954 CrossRef CAS PubMed.
- S. Chen, J. Ban, L. Mu and H. Zhuo, Polym. Chem., 2018, 9, 576–583 RSC.
- J. Ban, L. Mu, J. Yang, S. Chen and H. Zhuo, J. Mater. Chem. A, 2017, 5, 14514–14518 RSC.
- J. Li, X. Zhang, J. Gooch, W. Sun, H. Wang and K. Wang, Polym. Bull., 2015, 72, 881–895 CrossRef CAS.
- R. H. H. Aguirresarobe, L. Irusta, M. J. Fernández-Berridi, M. J. F.-B. L Irusta, L. Irusta and M. J. Fernández-Berridi, J. Polym. Res., 2014, 21, 505 CrossRef.
- R. H. Aguirresarobe, L. Martin, N. Aramburu, L. Irusta and M. J. Fernandez-Berridi, Prog. Org. Coat., 2016, 99, 314–321 CrossRef CAS.
- C. Salgado, M. P. Arrieta, L. Peponi, D. López and M. Fernández-García, Prog. Org. Coat., 2018, 123, 63–74 CrossRef CAS.
- Y. Fang, X. Du, Z. Du, H. Wang and X. Cheng, J. Mater. Chem. A, 2017, 5, 8010–8017 RSC.
- S.-H. Hsiao and S.-H. Hsu, ACS Appl. Mater. Interfaces, 2018, 10, 29273–29287 CrossRef CAS PubMed.
- I. J. Zvonkina and M. Hilt, Prog. Org. Coat., 2015, 89, 288–296 CrossRef CAS.
- X. Wang and M. D. Soucek, Prog. Org. Coat., 2013, 76, 1057–1067 CrossRef CAS.
- X. Wang, J. Jian, Z. Yuan, J. Zeng, L. Zhang, T. Wang and H. H. Zhou, Eur. Polym. J., 2020, 125, 109515 CrossRef CAS.
- C. Cai, Z. Wei, X. Wang, C. Mei, Y. Fu and W. H. Zhong, J. Mater. Chem. A, 2018, 6, 17457–17472 RSC.
- M. B. Gordon, J. M. French, N. J. Wagner and C. J. Kloxin, Adv. Mater., 2015, 27, 8007–8010 CrossRef CAS PubMed.
- K. Nagai and M. Katayama, Chem. Phys. Lett., 1977, 51, 329–332 CrossRef CAS.
- Y. Chen, A. M. Kushner, G. A. Williams and Z. Guan, Nat. Chem., 2012, 4, 467–472 CrossRef CAS PubMed.
- P. Cordier, F. Tournilhac, C. Soulié-Ziakovic and L. Leibler, Nature, 2008, 451, 977–980 CrossRef CAS PubMed.
- A. Sánchez-Ferrer, D. Rogez and P. Martinoty, Macromol. Chem. Phys., 2010, 211, 1712–1721 CrossRef.
- Y. Peng, L. Zhao, C. Yang, Y. Yang, C. Song, Q. Wu, G. Huang and J. Wu, J. Mater. Chem. A, 2018, 6, 19066–19074 RSC.
- X. Liu, M. Li, X. Zheng, E. Retulainen and S. Fu, Materials, 2018, 11, 1725 CrossRef PubMed.
- A. Matsumoto, M. Kawaharazuka, Y. Takahashi, N. Yoshino, T. Kawai and Y. Kondo, J. Oleo Sci., 2010, 59, 151–156 CrossRef CAS PubMed.
- T. Carofiglio, C. Fregonese, G. J. Mohr, F. Rastrelli and U. Tonellato, Tetrahedron, 2006, 62, 1502–1507 CrossRef CAS.
- Y. Yu, M. Nakano and T. Ikeda, Nature, 2003, 425, 145 CrossRef CAS PubMed.
- H. Gan and C. Yi, Fibers Polym., 2015, 16, 17–22 CrossRef CAS.
- R. Christie, Colour Chemistry, The Royal Society of Chemistry, 2001 Search PubMed.
- C. Xing, L. Wang, L. Xian, Y. Wang, L. Zhang, K. Xi, Q. Zhang and X. Jia, Macromol. Chem. Phys., 2018, 219, 1800042 CrossRef.
- M.-Q. Zhu, L. Zhu, J. J. Han, W. Wu, J. K. Hurst and A. D. Q. Li, J. Am. Chem. Soc., 2006, 128, 4303–4309 CrossRef CAS PubMed.
- C. Li and S. Liu, Chem. Commun., 2012, 48, 3262–3278 RSC.
- Q. Chen, Y. Feng, D. Zhang, G. Zhang, Q. Fan, S. Sun and D. Zhu, Adv. Funct. Mater., 2010, 20, 36–42 CrossRef CAS.
- C. Li, Y. Zhang, J. Hu, J. Cheng and S. Liu, Angew. Chem., 2010, 122, 5246–5250 CrossRef.
- G. O'Bryan, B. M. Wong and J. R. McElhanon, ACS Appl. Mater. Interfaces, 2010, 2, 1594–1600 CrossRef PubMed.
- F. M. Raymo and S. Giordani, J. Am. Chem. Soc., 2001, 123, 4651–4652 CrossRef CAS PubMed.
- G. R. Gossweiler, G. B. Hewage, G. Soriano, Q. Wang, G. W. Welshofer, X. Zhao and S. L. Craig, ACS Macro Lett., 2014, 3, 216–219 CrossRef CAS.
- P. Froimowicz, H. Frey and K. Landfester, Macromol. Rapid Commun., 2011, 32, 468–473 CrossRef CAS PubMed.
- J.-F. Xu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Org. Lett., 2013, 15, 6148–6151 CrossRef CAS PubMed.
- H. Xie, M. He, X.-Y. Deng, L. Du, C.-J. Fan, K.-K. Yang and Y.-Z. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 9431–9439 CrossRef CAS PubMed.
- J. Ling, M. Z. Rong and M. Q. Zhang, J. Mater. Chem., 2011, 21, 18373–18380 RSC.
- C. Salgado, M. P. Arrieta, L. Peponi, M. Fernández-García and D. López, Macromol. Mater. Eng., 2017, 302, 1600515 CrossRef.
- C. Salgado, M. P. Arrieta, L. Peponi, M. Fernández-García and D. López, Eur. Polym. J., 2017, 93, 21–32 CrossRef CAS.
- K. Bouchemal, S. Briançon, E. Perrier, H. Fessi, I. Bonnet and N. Zydowicz, Int. J. Pharm., 2004, 269, 89–100 CrossRef CAS PubMed.
- L. Korchia, C. Bouilhac, V. Lapinte, C. Travelet, R. Borsali and J.-J. Robin, Polym. Chem., 2015, 6, 6029–6039 RSC.
- H. Xie, K.-K. Yang and Y.-Z. Wang, Mater. Today: Proc., 2019, 16, 1524–1530 CAS.
- M. Rubinstein, B. Amit and A. Patchornik, Tetrahedron Lett., 1975, 16, 1445–1448 CrossRef.
- H. Zhao, E. S. Sterner, E. B. Coughlin and P. Theato, Macromolecules, 2012, 45, 1723–1736 CrossRef CAS.
- S. Wu and H.-J. Butt, Phys. Chem. Chem. Phys., 2017, 19, 23585–23596 RSC.
- C. Decker, Polym. Int., 2002, 51, 1141–1150 CrossRef CAS.
- A. Ravve, Light-Associated Reactions of Synthetic Polymers, 2006, pp. 23–122 Search PubMed.
- F. Wang, J. Q. Hu and W. P. Tu, Prog. Org. Coat., 2008, 62, 245–250 CrossRef CAS.
- P. Occhetta, R. Visone, L. Russo, L. Cipolla, M. Moretti and M. Rasponi, J. Biomed. Mater. Res., Part A, 2015, 103, 2109–2117 CrossRef CAS PubMed.
- T. Billiet, E. Gevaert, T. De Schryver, M. Cornelissen and P. Dubruel, Biomaterials, 2014, 35, 49–62 CrossRef CAS PubMed.
- A. D. Rouillard, C. M. Berglund, J. Y. Lee, W. J. Polacheck, Y. Tsui, L. J. Bonassar and B. J. Kirby, Tissue Eng., Part C, 2010, 17, 173–179 CrossRef PubMed.
- E. A. Papaj, D. J. Mills and S. S. Jamali, Prog. Org. Coat., 2014, 77, 2086–2090 CrossRef CAS.
- E. S. Džunuzović, S. V. Tasić, B. R. Božić, J. V. Džunuzović, B. M. Dunjić and K. B. Jeremić, Prog. Org. Coat., 2012, 74, 158–164 CrossRef.
- T. Vermonden, N. A. M. Besseling, M. J. van Steenbergen and W. E. Hennink, Langmuir, 2006, 22, 10180–10184 CrossRef CAS PubMed.
- D. Liu, F. Liu, J. He, L. V. J. Lassila and P. K. Vallittu, J. Mater. Sci.: Mater. Med., 2013, 24, 1595–1603 CrossRef CAS PubMed.
- S. Li, J. Xia, Y. Xu, X. Yang, W. Mao and K. Huang, Carbohydr. Polym., 2016, 142, 250–258 CrossRef CAS PubMed.
- Y. Huang, L. Pang, H. Wang, R. Zhong, Z. Zeng and J. Yang, Prog. Org. Coat., 2013, 76, 654–661 CrossRef CAS.
- Y. Hu, C. Liu, Q. Shang and Y. Zhou, J. Coat. Technol. Res., 2018, 15, 77–85 CrossRef CAS.
- G. ten Brinke, F. E. Karasz and T. S. Ellis, Macromolecules, 1983, 16, 244–249 CrossRef CAS.
- J. Hector Sandoval and R. B. Wicker, Rapid Prototyp. J., 2006, 12, 292–303 CrossRef.
- O. Llorente, M. J. Fernández-Berridi, A. González and L. Irusta, Prog. Org. Coat., 2016, 99, 437–442 CrossRef CAS.
- B. Fernández-d’Arlas and A. Eceiza, J. Nanopart. Res., 2013, 16, 2166 CrossRef.
- W. Yi, Y. Wang, G. Wang and X. Tao, Polym. Test., 2012, 31, 677–684 CrossRef CAS.
- J. R. Dios, C. Garcia-Astrain, S. Gonçalves, P. Costa and S. Lanceros-Méndez, Compos. Sci. Technol., 2019, 181, 107678 CrossRef CAS.
- K. Ke, V. Solouki Bonab, D. Yuan and I. Manas-Zloczower, Carbon, 2018, 139, 52–58 CrossRef CAS.
- Y. Zheng, Y. Li, K. Dai, M. Liu, K. Zhou, G. Zheng, C. Liu and C. Shen, Composites, Part A, 2017, 101, 41–49 CrossRef CAS.
- G. Liao, W. Zhao, Q. Li, Q. Pang and Z. Xu, Chem. Lett., 2017, 46, 1631–1634 CrossRef CAS.
- H. Zhou, X. Wang, T. Wang, J. Zeng, Z. Yuan, J. Jian, Z. Zhou, L. Zeng and H. Yang, Eur. Polym. J., 2019, 118, 153–162 CrossRef CAS.
- Z. Yi, J. Ye, N. Kikugawa, T. Kako, S. Ouyang, H. Stuart-Williams, H. Yang, J. Cao, W. Luo, Z. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559–564 CrossRef CAS PubMed.
- S. Wang, D. Li, C. Sun, S. Yang, Y. Guan and H. He, J. Mol. Catal. A: Chem., 2014, 383–384, 128–136 CAS.
- B. Wang, X. Gu, Y. Zhao and Y. Qiang, Appl. Surf. Sci., 2013, 283, 396–401 CrossRef CAS.
- J. Cao, B. Luo, H. Lin, B. Xu and S. Chen, J. Hazard. Mater., 2012, 217–218, 107–115 CrossRef CAS PubMed.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- Q. L. Feng, J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim and J. O. Kim, J. Biomed. Mater. Res., 2000, 52, 662–668 CrossRef CAS PubMed.
- R. R. Hardy, Encycl. Immunol., 1998, 943–947 Search PubMed.
- J. B. Grimm, L. M. Heckman and L. D. Lavis, Prog. Mol. Biol. Transl. Sci., 2013, 113, 1–34 CrossRef CAS PubMed.
- L. Zhao, W. Li, A. Plog, Y. Xu, G. Buntkowsky, T. Gutmann and K. Zhang, Phys. Chem. Chem. Phys., 2014, 16, 26322–26329 RSC.
- D. P. Nair, N. B. Cramer, M. K. McBride, J. C. Gaipa, R. Shandas and C. N. Bowman, Polymer, 2012, 53, 2429–2434 CrossRef CAS PubMed.
- S. J. Ma, S. J. Mannino, N. J. Wagner and C. J. Kloxin, ACS Macro Lett., 2013, 2, 474–477 CrossRef CAS PubMed.
- L. Peng, S. Liu, A. Feng and J. Yuan, Mol. Pharm., 2017, 14, 2475–2486 CrossRef CAS PubMed.
- Y. A. Alemayehu, W.-L. Fan, F. B. Ilhami, C.-W. Chiu, D.-J. Lee and C.-C. Cheng, Int. J. Mol. Sci., 2020, 21, 4677 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |