Open Access Article
Open Access ArticlePreparation of a reusable and pore size controllable porous polymer monolith and its catalysis of biodiesel synthesis†
Weiqing Chen ,
Zhaoji Wu,
Zhengge Wang,
Changjiu Chen and
Zhigang Zhang*
,
Zhaoji Wu,
Zhengge Wang,
Changjiu Chen and
Zhigang Zhang*
College of Chemical Engineering, Hebei Normal University of Science and Technology, Qinhuangdao, 066600, China. E-mail: zgzhang333@163.com
First published on 25th April 2022
Abstract
A sulfonated porous polymer monolith (PPM-SO3H) has been prepared via the polymerisation of styrene (St) and divinyl benzene (DVB) with organic microspheres as pore-forming agents, followed by sulfonation with concentrated sulfuric acid. It was characterized by acid–base titration in order to determine its acid density, scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR) spectroscopy, mercury intrusion porosimetry (MIP) and thermogravimetric analysis (TG). The PPM-SO3H showed an acid density of 1.89 mmol g−1 and pore cavities with an average diameter of 870 nm. The catalytic activity of PPM-SO3H in practical biodiesel synthesis from waste fatty acids was investigated and the main reaction parameters were optimized through orthogonal experiment. The best reaction conditions obtained for the optimization of methanol to oil ratio, catalyst concentration, reaction temperature and reaction time were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20%, 80 °C and 8 h, respectively. PPM-SO3H showed excellent catalytic activity. In biodiesel synthesis, the esterification rate of PPM-SO3H is 96.9%, which is much higher than that of commercial poly(sodium-p-styrenesulfonate) (esterification rate 29.0%). The PPM-SO3H can be reused several times without significant loss of catalytic activity; the esterification rate was still 90.8% after 6 cycles. The pore size of this porous polymer monolith can be controlled. The dimension and shape of this porous polymer monolith were also adjustable by choosing a suitable polymerisation container.
1, 20%, 80 °C and 8 h, respectively. PPM-SO3H showed excellent catalytic activity. In biodiesel synthesis, the esterification rate of PPM-SO3H is 96.9%, which is much higher than that of commercial poly(sodium-p-styrenesulfonate) (esterification rate 29.0%). The PPM-SO3H can be reused several times without significant loss of catalytic activity; the esterification rate was still 90.8% after 6 cycles. The pore size of this porous polymer monolith can be controlled. The dimension and shape of this porous polymer monolith were also adjustable by choosing a suitable polymerisation container.
1. Introduction
Porous materials usually refer to materials with interconnected pores including micropores, mesopores and macropores. During the past decades, porous materials including organic, inorganic and organic–inorganic materials have been extensively studied1–10 due to their special physicochemical properties. Porous materials have been used in various fields, such as energy storage,11–14 energy conversion,15–18 adsorption,19–25 catalysis,26–28 sensing,29–31 CO2 capture,32–36 etc. In recent years, the investigation of porous organic polymer (a class of highly crosslinked amorphous polymers possessing nano-pores) based catalysts has attracted significant attention owing to their confined pore space and high surface area which is beneficial for improving catalytic performance and stability. For example, Roy et al. synthesised a pristine conjugated microporous polymer with semi-conduction and redox-activity and used it in a metal-free oxygen reduction reaction.37 Singh et al. synthesised Co2+ and Zn2+ phthalocyanine based redox active metal–organic conjugated microporous polymers for oxygen evolution reaction catalysis through a Schiff base condensation reaction.38 Alkordi et al. reported the synthesis of novel porous organic polymers using functionalized Cr and Co–salen complexes as molecular building blocks and the porous polymers were used as heterogeneous catalysts for CO2-epoxide insertion.39 Alavinia et al. synthesised a kind of porous alginate-g-poly(p-styrene sulfonamide-co-acrylamide) catalyst, which showed high catalytic activity for the one-step synthesis of 1,3,4-oxadiazoles from the reaction of hydrazides and aryl iodides through isocyanide insertion/cyclization.40 Therefore, we considered that functionalized porous organic polymers may exhibit desirable catalytic activity and stability in biodiesel preparation.Biodiesel (fatty acid alkyl esters), as a kind of renewable energy, has received considerable attention during the past decades because of its excellent features, such as nontoxic, low sulphur content, free of polycyclic aromatic hydrocarbons, biodegradable and environment-friendly.41,42 In industrial production process, biodiesel is typically prepared by esterification and transesterification of waste animal and vegetable oils with methanol in the presence of catalyst. Catalyst is one of the key roles to influence the biodiesel yield and sustainability of the industrial process.43 Industrial biodiesel production using homogeneous catalysts has its intrinsic drawbacks, such as equipment corrosion, separation difficulty and low reusability of catalyst. Furthermore, base-catalysed transesterification process are not suitable for feedstocks with high free fatty acids (FFAs), because FFAs cannot be converted into biodiesel through this process which will result in low yield of biodiesel.44 Immobilized enzyme as well as free enzyme also has problems on a large industrial scale, such as cost, reusability and deactivation of enzymes.45,46 As an alternative, heterogeneous acid catalyst may overcome some of these problems since it can be isolated through simple procedure and convert triacylglycerols by transesterification and free fatty acids by esterification simultaneously.47,48 Developing high efficient heterogeneous acid catalyst is essential for practical process.49 Among many kinds of heterogeneous acid catalysts, sulfonated-solid catalysts exhibit desirable catalytic activity owing to the presence of active –SO3H. Such as sulfonated carbon-based catalyst made from biomass resource,50–52 sulfonated graphene and graphene oxide53,54 and sulfonated resin55 and so on. However, many of these heterogeneous acid catalysts are mainly powdery small particles which is also inconvenient to operate in the industrial production of biodiesel. Some conjugated microporous polymer materials derived from π-conjugated aromatic building block have been reported as catalysts due to their unique properties.3,37,38 It is presumed that functionalized porous organic polymers may exhibit desirable catalytic activity in biodiesel preparation. Hence, we designed a sulfonated porous polymer monolith catalyst synthesised from aromatic monomers.
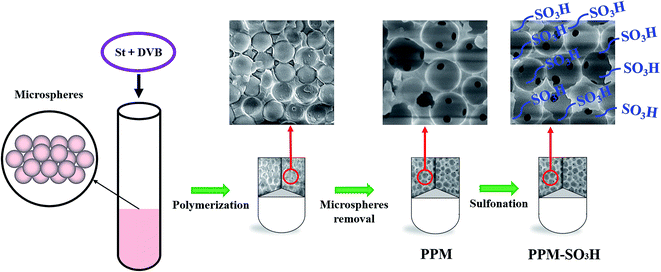
In this study, a porous polymer monolith catalyst was synthesised through the polymerisation of styrene (St) and divinyl benzene (DVB) with microspheres as pore-forming agent and followed by sulfonation (Scheme 1). As shown in Scheme 1, the mixture of St and DVB was added into the glass tube filled with microspheres (polymerization of maleic anhydride and vinyl acetate) for polymerisation. After polymerisation, the tube was broken and left a composite cylinder of crosslinked polystyrene/microspheres. Then, the porous polymer monolith (PPM) was available after removal of microspheres. At last, the PPM-SO3H was obtained through the sulfonation of PPM. The PPM-SO3H showed excellent catalytic activity in biodiesel synthesis, which is much higher than that of commercial poly(sodium-p-styrenesulfonate). The PPM-SO3H can be reused for several times without significant loss of catalytic activity. In addition, the pore size of this porous polymer monolith can be controlled by adjusting the diameter of microspheres. The dimension and shape of this porous polymer monolith were also adjustable by choosing suitable polymerisation container. The PPM-SO3H has the potential to be used as both catalyst and reactor in industrial biodiesel production.
2. Materials and methods
2.1. Materials
The following chemicals were purchased from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China): benzoyl peroxide (BPO, 99.0% purity), vinyl acetate (VAc, 99.0% purity), styrene (St, 99.0% purity, contains 10–15 ppm of 4-tert-butylcatechol) and divinyl benzene (DVB, 80% mixture of isomers, contains 1000 ppm of 4-tert-butylcatechol). Maleic anhydride (MAn, >99.0% purity), n-butyl acetate (99.0% purity), N,N-dimethylformamide (DMF, ≥99.9% purity), methanol (99.5% purity) and poly(sodium-p-styrenesulfonate)(PSS, average molecular weight 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, powder) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China). Sodium hydroxide (NaOH), potassium hydroxide (KOH), hydrochloric acid (HCl), chloroform, acetone and sulfuric acid (H2SO4, 98% purity) were purchased from Institute of Tianjin Chemical Reagent (Tianjin, China). Waste fatty acids was prepared from waste cooking oils. All other reagents (NaOH, KOH, HCl, chloroform, acetone, etc.) were of analytical grade. All reagents were used as received except VAc, St, DVB and BPO. VAc was refined by distillation. St and DVB were purified by sequential thoroudslfghly washing with NaOH aqueous solution and distilled water and reduced pressure distillation. BPO was purified through a typical procedure: 5 g benzoyl peroxide was dissolved in 20 mL chloroform and filtered, then the filtrate was poured into ca. 50 mL methanol for recrystallisation, the needle-like crystal was obtained by filtration.
000 Da, powder) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China). Sodium hydroxide (NaOH), potassium hydroxide (KOH), hydrochloric acid (HCl), chloroform, acetone and sulfuric acid (H2SO4, 98% purity) were purchased from Institute of Tianjin Chemical Reagent (Tianjin, China). Waste fatty acids was prepared from waste cooking oils. All other reagents (NaOH, KOH, HCl, chloroform, acetone, etc.) were of analytical grade. All reagents were used as received except VAc, St, DVB and BPO. VAc was refined by distillation. St and DVB were purified by sequential thoroudslfghly washing with NaOH aqueous solution and distilled water and reduced pressure distillation. BPO was purified through a typical procedure: 5 g benzoyl peroxide was dissolved in 20 mL chloroform and filtered, then the filtrate was poured into ca. 50 mL methanol for recrystallisation, the needle-like crystal was obtained by filtration.
2.2. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13) was added into the glass tube filled with polymer microspheres. The liquid level of the mixture added should properly higher than the height of the microspheres in the glass tube. Then, the tube was sealed with rubber plug and placed for several hours to make the solution penetrated into the gaps between the polymer microspheres. The state of the mixture in the glass tube would become translucent after full penetration. Next, the polymerisation reaction was performed by keeping the glass tube at 70 °C for 12 h. After polymerisation, the tube was broken carefully and left a composite cylinder of crosslinked polystyrene/microspheres. The cylinder was extracted by acetone for 72 h to obtain a porous polystyrene monolith, which was subsequently vacuum-dried at 40 °C for 24 h.
13) was added into the glass tube filled with polymer microspheres. The liquid level of the mixture added should properly higher than the height of the microspheres in the glass tube. Then, the tube was sealed with rubber plug and placed for several hours to make the solution penetrated into the gaps between the polymer microspheres. The state of the mixture in the glass tube would become translucent after full penetration. Next, the polymerisation reaction was performed by keeping the glass tube at 70 °C for 12 h. After polymerisation, the tube was broken carefully and left a composite cylinder of crosslinked polystyrene/microspheres. The cylinder was extracted by acetone for 72 h to obtain a porous polystyrene monolith, which was subsequently vacuum-dried at 40 °C for 24 h.
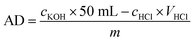 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.00 psia.
000.00 psia.
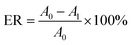 | (2) |
3. Results and discussion
3.1. Characterization
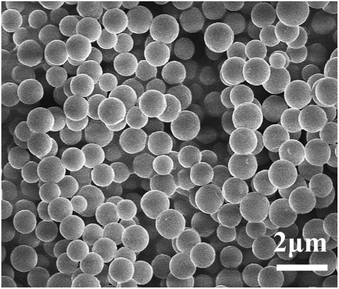
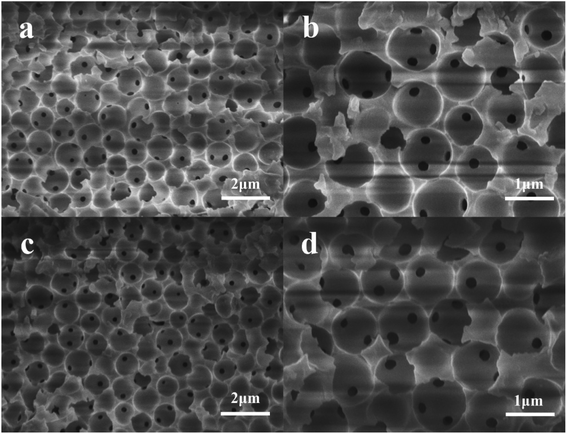
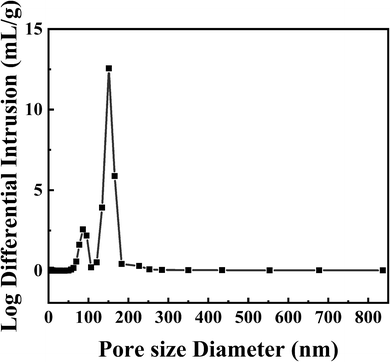
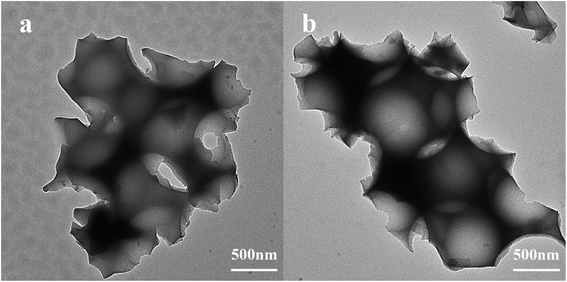
PPM and PPM-SO3H were successfully prepared. Fig. 2 shows scanning electron microscopy images for the morphology of the PPM and its sulfonated counterpart (PPM-SO3H). As observed from Fig. 2, the morphology of PPM and PPM-SO3H was similar, the pore cavities previously occupied by polymer microspheres in PPM and PPM-SO3H were spherical. The pore structure has no obvious deformation after sulfonation. Furthermore, there were some other small pores between the pore cavities connecting the pore cavities. The SEM images were analysed using Image J software for obtaining the size of pore cavities and small pores. The average diameter of pore cavities was about 870 nm, which was basically consistent with the polymer microspheres in size. In addition, the average diameter of small pores was about 145 nm.
3.2. Orthogonal experiment of biodiesel preparation from waste fatty acids
The esterification of waste fatty acids with methanol catalysed by PPM-SO3H was planned using a four-level, five factor (L16(45)) orthogonal design. Four important parameters (MeOH to oil ratio, catalyst concentration, reaction temperature and reaction time), which have been identified to have larger effects on the yield of biodiesel produced from other feedstocks,67 was investigated. The results are shown in Table S1 (see ESI†), it can be observed that the range of the esterification rate varies from 21.3% to 92.4%; these data were taken as the original data and used in range analysis and univariate analysis. The range analysis results for the orthogonal experiment are listed in Table S2 (see ESI†). It can be concluded that reaction time has significant effect on the esterification rate and the optimal condition is A2B4C4D4. Therefore, the optimum conditions were obtained as follows: MeOH to oil ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; catalyst concentration, 20.0%; reaction temperature, 80 °C; reaction time, 8 h. It reached an esterification rate of 96.9%. Furthermore, the univariate analysis results for the orthogonal experiment are shown Table S3 (see ESI†). For each factor, a higher F value or R indicates that the level has a larger effect on esterification rate. According to the F value, the factors influencing esterification rate were listed in a decreasing order as follows: reaction time > reaction temperature > catalyst concentration > MeOH to oil ratio. This result was consistent with the judgment according to the R in Table S2.†
1; catalyst concentration, 20.0%; reaction temperature, 80 °C; reaction time, 8 h. It reached an esterification rate of 96.9%. Furthermore, the univariate analysis results for the orthogonal experiment are shown Table S3 (see ESI†). For each factor, a higher F value or R indicates that the level has a larger effect on esterification rate. According to the F value, the factors influencing esterification rate were listed in a decreasing order as follows: reaction time > reaction temperature > catalyst concentration > MeOH to oil ratio. This result was consistent with the judgment according to the R in Table S2.†
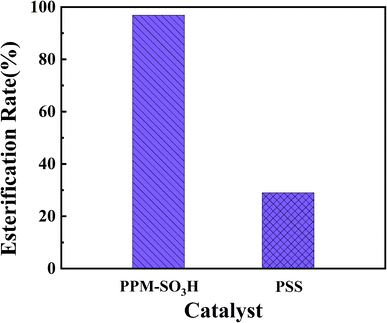
3.3. Comparison of the catalytic performance of PPM-SO3H with PSS
In order to further study the catalytic performance of PPM-SO3H, we compared the esterification rates in the synthesis of biodiesel from waste fatty acids using PPM-SO3H and PSS as catalysts respectively according to method Section 2.2.6. The results are presented in Table 1 and Fig. 7. Under the same reaction conditions, the esterification rate of PPM-SO3H is 96.9%, whereas commercial poly(sodium-p-styrenesulfonate) is 29.0%. It is quite clear that the PPM-SO3H showed much better catalytic performance than PSS.| Trial number | Catalyst | Esterification rate (%) |
|---|---|---|
| 1 | PPM-SO3H (1 g) | 96.9% |
| 2 | PSS (1 g) | 29.0% |
3.4. Catalyst reusability
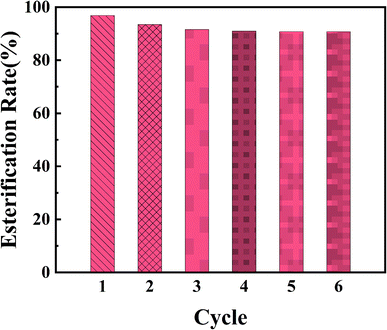
Reusability is one of the most important properties of heterogeneous catalyst. In order to investigate the reusability of PPM-SO3H, the catalyst was recovered by simple filtration, washing with methanol and drying in a vacuum oven at 40 °C. Then, PPM-SO3H was reused in a subsequent esterification reaction. The esterification reaction experiments were performed for six times under the optimal conditions. The results indicate that esterification rate was still 90.8% after 6 cycles (Fig. 8). Therefore, the PPM-SO3H we synthesised have great potential to be a stable and highly active solid acid catalyst used for biodiesel preparation.4. Conclusions
A novel pore size controllable sulfonated porous polymer monolith (PPM-SO3H) has been prepared via the polymerisation of St and DVB with polymer microspheres as pore-forming agent, followed by sulfonation. The prepared PPM-SO3H was successfully applied to the synthesis of biodiesel from waste fatty acids. The reaction parameters were optimized through orthogonal experiment. The best reaction conditions obtained in the optimization of methanol to oil ratio, catalyst concentration, reaction temperature and reaction time were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20%, 80 °C and 8 h, respectively. The sulfonated porous polymer monolith (esterification rate 96.9%) showed much better catalytic activity than that of commercial PSS (esterification rate 29.0%) in the synthesis of biodiesel. It can also be reused for several times without significant loss of activity, esterification rate still maintains 90.8% after 6 cycles. The PPM-SO3H has the potential to be used as both catalyst and reactor in industrial biodiesel production. In addition, the pore size, dimension and shape of this porous polymer monolith can be controlled. Consequently, according to process requirements and raw material characteristics, we can choose polymer monolith with different pore size, dimension and shape. This is of great significance from the perspective of industrial biodiesel production.
1, 20%, 80 °C and 8 h, respectively. The sulfonated porous polymer monolith (esterification rate 96.9%) showed much better catalytic activity than that of commercial PSS (esterification rate 29.0%) in the synthesis of biodiesel. It can also be reused for several times without significant loss of activity, esterification rate still maintains 90.8% after 6 cycles. The PPM-SO3H has the potential to be used as both catalyst and reactor in industrial biodiesel production. In addition, the pore size, dimension and shape of this porous polymer monolith can be controlled. Consequently, according to process requirements and raw material characteristics, we can choose polymer monolith with different pore size, dimension and shape. This is of great significance from the perspective of industrial biodiesel production.
Author contributions
Weiqing Chen: investigation, data curation, writing-original draft. Zhaoji Wu: visualization. Zhengge Wang: formal analysis. Changjiu Chen: validation. Zhigang Zhang: funding acquisition, supervision, writing-review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Department of Education of Hebei Province [No. ZD2020326].References
- J. Castro-Gutiérrez, A. Sanchez-Sanchez, J. Ghanbaja, N. Díez, M. Sevilla, A. Celzard and V. Fierro, Green Chem., 2018, 20, 5123–5132 RSC.
- D. I. Fried, F. J. Brieler and M. Fröba, ChemCatChem, 2013, 5, 862–884 CrossRef CAS.
- A. Singh, D. Samanta, M. Boro and T. K. Maji, Chem. Commun., 2019, 55, 2837–2840 RSC.
- J. Wang, G. Wang, Z. Zhang, G. Ouyang and Z. Hao, RSC Adv., 2021, 11, 36577–36586 RSC.
- S. Wang, L. Tan, C. Zhang, I. Hussain and B. Tan, J. Mater. Chem. A, 2015, 3, 6542–6548 RSC.
- Y. Wang, B. Wang, J. Wang, Y. Ren, C. Xuan, C. Liu and C. Shen, J. Hazard. Mater., 2018, 344, 849–856 CrossRef CAS PubMed.
- J. Zhang, J. Chen, S. Peng, S. Peng, Z. Zhang, Y. Tong, P. W. Miller and X.-P. Yan, Chem. Soc. Rev., 2019, 48, 2566–2595 RSC.
- H. Zheng, F. Gao and V. Valtchev, J. Mater. Chem. A, 2016, 4, 16756–16770 RSC.
- W. Zhou, Y. Zhang, J. Wang, H. Li, W. Xu, B. Li, L. Chen and Q. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 46767–46778 CrossRef CAS PubMed.
- B. Zheng, X. Lin, X. Zhang, D. Wu and K. Matyjaszewski, Adv. Funct. Mater., 2020, 30, 1907006 CrossRef CAS.
- D. G. Atinafu, W. Dong, C. Hou, R. S. Andriamitantsoa, J. Wang, X. Huang, H. Gao and G. Wang, Mater. Today Energy, 2019, 12, 239–249 CrossRef.
- J. Min, K. Kierzek, X. Chen, P. K. Chu, X. Zhao, R. J. Kaleńczuk, T. Tang and E. Mijowska, New J. Chem., 2017, 41, 13553–13559 RSC.
- B. Xue, Z. Wang, Y. Zhu, X. Wang and R. Xiao, Ind. Crop. Prod., 2021, 170, 113724 CrossRef CAS.
- J. Zhou and W. Bo, Chem. Soc. Rev., 2017, 46, 6927–6945 RSC.
- L. Kong, M. Zhong, W. Shuang, Y. Xu and X.-H. Bu, Chem. Soc. Rev., 2020, 49, 2378–2407 RSC.
- Y.-F. Shen, C. Zhang, C.-G. Yan, H.-Q. Chen and Y.-J. Zhang, Chin. Chem. Lett., 2017, 28, 1312–1317 CrossRef CAS.
- D. Wei, F. Xu, J. Xu, J. Fang, S. W. Koh, K. Li and Z. Sun, Ceram. Int., 2020, 46, 1396–1402 CrossRef CAS.
- H. Zhong, Y. Su, X. Chen, X. Li and R. Wang, ChemSusChem, 2017, 10, 4855–4863 CrossRef CAS PubMed.
- Y. He, W. Bao, Y. Hua, Z. Guo, X. Fu, B. Na, D. Yuan, C. Peng and H. Liu, RSC Adv., 2022, 12, 5587–5594 RSC.
- G. Li, C. Yao, J. Wang and Y. Xu, Sci. Rep., 2017, 7, 13972 CrossRef.
- Y. Li, H. Wang, W. Zhao, X. Wang, Y. Shi, H. Fan, H. Sun and L. Tan, J. Appl. Polym. Sci., 2019, 136, 47987 CrossRef.
- S. Lu, Q. Liu, R. Han, M. Guo, J. Shi, C. Song, N. Ji, X. Lu and D. Ma, J. Environ. Sci., 2021, 105, 184–203 CrossRef CAS PubMed.
- R. Matsuda, Bull. Chem. Soc. Jpn., 2013, 86, 1117–1131 CrossRef CAS.
- W. Peng, S. Liu, H. Sun, Y. Yao, L. Zhi and S. Wang, J. Mater. Chem. A, 2013, 1, 5854–5859 RSC.
- K. V. Rao, S. Mohapatra, C. Kulkarni, T. K. Maji and S. J. George, J. Mater. Chem., 2011, 21, 12958–12963 RSC.
- A. Munyentwali, C. Li, H. Li and Q. Yang, Chem. Asian J., 2021, 16, 2041–2047 CrossRef CAS PubMed.
- S.-S. Qin, Z.-K. Wang, L. Hu, X.-H. Du, Z. Wu, M. Strømme, Q.-F. Zhang and C. Xu, Nanoscale, 2021, 13, 3967–3973 RSC.
- W. Tong, W.-H. Li, Y. He, Z.-Y. Mo, H.-T. Tang, H.-S. Wang and Y.-M. Pan, Org. Lett., 2018, 20, 2494–2498 CrossRef CAS PubMed.
- C. Dai, H.-L. Qian and X.-P. Yan, J. Hazard. Mater., 2021, 416, 125860 CrossRef CAS PubMed.
- L. Guo and D. Cao, J. Mater. Chem. C, 2015, 3, 8490–8494 RSC.
- Z. Song, Y. Ma, A. Morrin, C. Ding and X. Luo, TrAC, Trends Anal. Chem., 2021, 135, 116155 CrossRef CAS.
- S. Shingdilwar, S. Dolui, D. Kumar and S. Banerjee, Mater. Adv., 2022, 3, 665–671 RSC.
- E. S. Sanz-Perez, C. R. Murdock, S. A. Didas and C. W. Jones, Chem. Rev., 2016, 116, 11840–11876 CrossRef CAS PubMed.
- M. Niu, H. Yang, X. Zhang, Y. Wang and A. Tang, ACS Appl. Mater. Interfaces, 2016, 8, 17312–17320 CrossRef CAS PubMed.
- H. He, W. Li, M. Zhong, D. Konkolewicz, D. Wu, K. Yaccato, T. Rappold, G. Sugar, N. E. David and K. Matyjaszewski, Energy Environ. Sci., 2013, 6, 488–493 RSC.
- C. Rosu, S. H. Pang, A. R. Sujan, M. A. Sakwa-Novak, E. W. Ping and C. W. Jones, ACS Appl. Mater. Interfaces, 2020, 12, 38085–38097 CrossRef CAS PubMed.
- S. Roy, A. Bandyopadhyay, M. Das, P. P. Ray, S. K. Pati and T. K. Maji, J. Mater. Chem. A, 2018, 6, 5587–5591 RSC.
- A. Singh, S. Roy, C. Das, D. Samanta and T. K. Maji, Chem. Commun., 2018, 54, 4465–4468 RSC.
- M. H. Alkordi, Ł. J. Weseliński, V. D'Elia, S. Barman, A. Cadiau, M. N. Hedhili, A. J. Cairns, R. G. AbdulHalim, J.-M. Basset and M. Eddaoudi, J. Mater. Chem. A, 2016, 4, 7453–7460 RSC.
- S. Alavinia and R. Ghorbani-Vaghei, RSC Adv., 2021, 11, 29728–29740 RSC.
- A. Demirbas, Energy Pol., 2007, 35, 4661–4670 CrossRef.
- X. Tang, S. Niu, S. Zhao, X. Zhang, X. Li, H. Yu, C. Lu and K. Han, J. Ind. Eng. Chem., 2019, 77, 432–440 CrossRef CAS.
- H. H. Mardhiah, H. C. Ong, H. H. Masjuki, S. Lim and H. V. Lee, Renewable Sustainable Energy Rev., 2017, 67, 1225–1236 CrossRef CAS.
- A. Rajalingam, S. P. Jani, A. S. Kumar and M. A. Khan, J. Chem. Pharm. Res., 2016, 8, 170–173 CAS.
- A. Bajaj, P. Lohan, P. N. Jha and R. Mehrotra, J. Mol. Catal. B: Enzym., 2010, 62, 9–14 CrossRef CAS.
- A. Guldhe, B. Singh, T. Mutanda, K. Permaul and F. Bux, Renewable Sustainable Energy Rev., 2015, 41, 1447–1464 CrossRef CAS.
- B.-X. Peng, Q. Shu, J.-F. Wang, G.-R. Wang, D.-Z. Wang and M.-H. Han, Process Saf. Environ. Prot., 2008, 86, 441–447 CrossRef CAS.
- W. Xie and H. Wang, Renew. Energy, 2020, 145, 1709–1719 CrossRef CAS.
- Q. Guan, Y. Li, Y. Chen, Y. Shi, J. Gu, B. Li, R. Miao, Q. Chen and P. Ning, RSC Adv., 2017, 7, 7250–7258 RSC.
- W. Y. Lou, Q. Guo, W. J. Chen, M. H. Zong, H. Wu and T. J. Smith, ChemSusChem, 2012, 5, 1533–1541 CrossRef CAS PubMed.
- H. H. Mardhiah, H. C. Ong, H. H. Masjuki, S. Lim and Y. L. Pang, Energy Convers. Manage., 2017, 144, 10–17 CrossRef CAS.
- Q. Shu, J. Gao, Z. Nawaz, Y. Liao, D. Wang and J. Wang, Appl. Energy, 2010, 87, 2589–2596 CrossRef CAS.
- J. Cheng, Y. Qiu, J. Zhang, R. Huang, W. Yang and Z. Fan, Bioresour. Technol., 2017, 244, 569–574 CrossRef CAS PubMed.
- M. C. Nongbe, T. Ekou, L. Ekou, K. B. Yao, E. Le Grognec and F.-X. Felpin, Renew. Energy, 2017, 106, 135–141 CrossRef CAS.
- V. Trombettoni, D. Lanari, P. Prinsen, R. Luque, A. Marrocchi and L. Vaccaro, Prog. Energy Combust. Sci., 2018, 65, 136–162 CrossRef.
- C. M. Xing and W. T. Yang, J. Polym. Sci., Polym. Chem. Ed., 2005, 43, 3760–3770 CrossRef CAS.
- R. R. C. Bastos, A. P. da Luz Corrêa, P. T. S. da Luz, G. N. da Rocha Filho, J. R. Zamian and L. R. V. da Conceição, Energy Convers. Manage., 2020, 205, 112457 CrossRef CAS.
- K. Dong, Q. Sun, X. Meng and F.-S. Xiao, Catal. Sci. Technol., 2017, 7, 1028–1039 RSC.
- Q. Sun, Z. Dai, X. Meng and F.-S. Xiao, Chem. Soc. Rev., 2015, 44, 6018–6034 RSC.
- H. Giesche, Part. Part. Syst. Charact., 2006, 23, 9–19 CrossRef.
- C. Voigt, J. Hubálková, H. Giesche and C. G. Aneziris, Microporous Mesoporous Mater., 2020, 299, 110125 CrossRef CAS.
- Y. Yang, Y. Chu, F. Yang and Y. Zhang, Mater. Chem. Phys., 2005, 92, 164–171 CrossRef CAS.
- D. Li, W. D. Yan, Z. Z. Yang and J. M. Zhang, Acta Polym. Sin., 2001, 561–564 CAS.
- J. C. Yang, M. J. Jablonsky and J. W. Mays, Polymer, 2002, 43, 5125–5132 CrossRef CAS.
- S. B. Brijmohan, S. Swier, R. A. Weiss and M. T. Shaw, Ind. Eng. Chem. Res., 2005, 44, 8039–8045 CrossRef CAS.
- S. Hosseini, J. Janaun and T. S. Y. Choong, Process Saf. Environ. Prot., 2015, 98, 285–295 CrossRef CAS.
- D. Y. C. Leung and Y. Guo, Fuel Process. Technol., 2006, 87, 883–890 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01610a |
| This journal is © The Royal Society of Chemistry 2022 |