Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pd-catalyzed C–C and C–N cross-coupling reactions in 2-aminothieno[3,2-d]pyrimidin-4(3H)-one series for antiplasmodial pharmacomodulation†
Romain Mustière a,
Prisca Lagardèreb,
Sébastien Hutterc,
Céline Deraeved,
Florian Schwalend,
Dyhia Amranea,
Nicolas Masurier
a,
Prisca Lagardèreb,
Sébastien Hutterc,
Céline Deraeved,
Florian Schwalend,
Dyhia Amranea,
Nicolas Masurier b,
Nadine Azasc,
Vincent Lisowskib,
Pierre Verhaeghede,
Dominique Mazierf,
Patrice Vanelleag and
Nicolas Primas
b,
Nadine Azasc,
Vincent Lisowskib,
Pierre Verhaeghede,
Dominique Mazierf,
Patrice Vanelleag and
Nicolas Primas *ag
*ag
aAix Marseille Université, CNRS, ICR UMR 7273, Equipe Pharmaco-Chimie Radicalaire, Faculté de Pharmacie, Marseille, France. E-mail: nicolas.primas@univ-amu.fr
bInstitut des Biomolécules Max Mousseron, UMR 5247, CNRS, Université de Montpellier, ENSCM, UFR des Sciences Pharmaceutiques et Biologiques, Montpellier, France
cAix Marseille Université, IRD, AP-HM, SSA, VITROME, Marseille, France
dLCC-CNRS, Université de Toulouse, CNRS UPR 8241, UPS, Toulouse, France
eCHU de Nîmes, service de pharmacie, Nimes, France
fCentre d’Immunologie et des Maladies Infectieuses (CIMI), INSERM, CNRS, Sorbonne Université, Paris, France
gService Central de la Qualité et de l'Information Pharmaceutiques, AP-HM, Hôpital Conception, Marseille, France
First published on 8th July 2022
Abstract
In 2015, we identified gamhepathiopine (M1), a 2-tert-butylaminothieno[3,2-d]pyrimidin-4(3H)-one antiplasmodial hit targeting all development stages of the human malarial parasite P. falciparum. However, this hit compound suffers from sensitivity to hepatic oxidative metabolism. Herein, we describe the synthesis of 33 new compounds in the 2-aminothieno[3,2-d]pyrimidin-4(3H)-one series modulated at position 6 of this scaffold. The modulations were performed using three palladium-catalyzed cross coupling reactions, namely Suzuki–Miyaura, Sonogashira, and Buchwald–Hartwig. For the latter, we developed the reaction conditions. Then, we evaluated the synthesized compounds for their antiplasmodial activity on the K1 P. falciparum strain and their cytotoxicity on the human HepG2 cell line. Although we did not obtain a compound better than M1 in terms of the antiplasmodial activity, we identified compound 1g bearing a piperidine at position 6 of the thieno[3,2-d]pyrimidin-4(3H)-one ring with an improved cytotoxicity and metabolic stability. 1g is an interesting new starting point for further pharmacomodulation studies. This study also provides valuable antiplasmodial SAR data regarding the nature of the ring at position 6, the possible substituent on this ring, and the introduction of a spacer between this ring and the thienopyrimidinone moiety.
1. Introduction
Malaria is a heavy burden for endemic countries, mainly in Africa, with an estimated 241 million cases and 627![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths in 2020, according to the World Health Organization.1 Seventy seven percent of the victims were children under 5 years old. The disease is caused by parasites of the Plasmodium genus transmitted by the bite of an infected female mosquito belonging to the Anopheles genus. Of the five species causing malaria in humans, P. falciparum is responsible for the most deaths. Along with the spread of long-lasting insecticide-treated bed nets, the introduction of artemisinin-based combined therapies (ACT) as first line treatments against severe P. falciparum infections were key to the fight against malaria.2
000 deaths in 2020, according to the World Health Organization.1 Seventy seven percent of the victims were children under 5 years old. The disease is caused by parasites of the Plasmodium genus transmitted by the bite of an infected female mosquito belonging to the Anopheles genus. Of the five species causing malaria in humans, P. falciparum is responsible for the most deaths. Along with the spread of long-lasting insecticide-treated bed nets, the introduction of artemisinin-based combined therapies (ACT) as first line treatments against severe P. falciparum infections were key to the fight against malaria.2
However, the emergence of artemisinin-resistant P. falciparum in South-East Asia first reported in 2009,3 mediated by Pfkelch13 mutations,4 and its spread in the greater Mekong subregion is currently limiting the clinical effectiveness of ACT in this area.5 While the combination of ganaplacide (Fig. 1) and lumefantrine is the most promising drug combination currently being investigated by Medicines for Malaria Venture (MMV),6 the recent withdrawal of MMV048, P218, and DSM265 in clinical trials (for teratogenicity, low half-time in vivo, and high selection of resistances, respectively; Fig. 1)7,8 increases the urgent need for new antimalarial compounds with novel mechanisms of action.
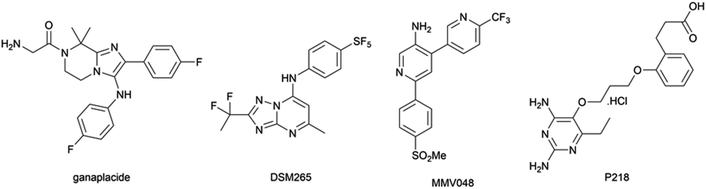 | ||
| Fig. 1 Chemical structures of antimalarial compounds in clinical trial or recently withdrawn from them. | ||
In 2015, we described the synthesis and activity of a new molecule called gamhepathiopine (also M1, Scheme 1) that is active on all stages of P. falciparum, including quiescent parasites of the artemisinin-resistant strain F32-ART5, with an unknown mechanism of action.9 We later discovered that the in vivo activity of M1 was limited by its sensitivity to mouse hepatic metabolism. Indeed, the in vivo activity in P. berghei infected mice was only achieved with the addition of 1-aminobenzotriazole, a pan-CYP450 inhibitor.10 Therefore, the synthesis of new thieno[3,2-d]pyrimidines to afford a new hit compound with improved metabolic stability and similar activity profile was initiated. We also expected to obtain new antiplasmodial SAR data on the thieno[3,2-d]pyrimidine series. Indeed, we observed important modifications to the biological values with minor modifications on the molecular scaffold during the first round of pharmacomodulations, leading to compound M1.9 Previously explored substituents on the phenyl ring at position 6 of the thienopyrimidinone scaffold were simple, i.e., halogen atoms or methoxy group, but some of them maintained good antiplasmodial potencies. Moreover, on M1, position 6 (and position 2) is sensitive to oxidative metabolism because of the methyl group.10 Thus, we decided to explore more diverse substituents at this position with the help of palladium-catalyzed cross-coupling reactions. Herein, we describe the synthesis of 2-aminothieno[3,2-d]pyrimidin4(3H)-ones, structurally modulated on the west part of the scaffold at position 6 (Scheme 1) by Buchwald–Hartwig, Sonogashira, or Suzuki–Miyaura cross-coupling reactions, along with their antiplasmodial and cytotoxicity evaluations.
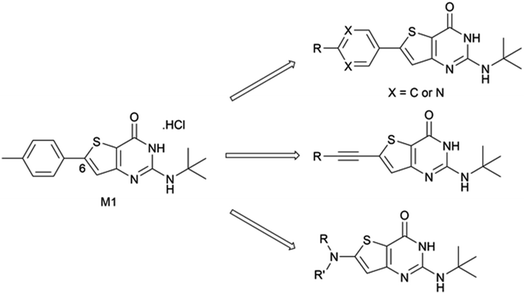 | ||
| Scheme 1 Chemical structure of compound M1 and general structures for the compounds presented in this work. | ||
2. Results and discussion
2.1. Buchwald–Hartwig coupling
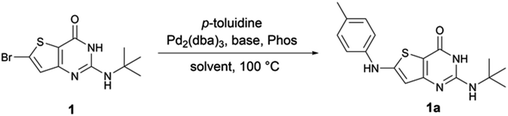
To our knowledge, no examples of Buchwald–Hartwig coupling on a thienopyrimidine cycle are reported in the literature to date and there are only a few examples of this coupling on a thiophene ring.11–13 Therefore, we first explored the reaction conditions between 6-bromo-2-tertbutylaminothieno[3,2-d]pyrimidin-4(3H)-one 1 and para-toluidine in order to determine the best reaction conditions (Table 1). During different experiments, we used LC-MS to estimate the yield of product 1a.| Entry | Eq. p-toluidine | mol% Pd | Phosphine | Mol% Phos | Base | Eq. Base | Solvent | Estimated yielda of 1a (%) |
|---|---|---|---|---|---|---|---|---|
| a Using LCMS.b Pd(dba)2.c Dehalogenation of the starting material mainly observed. | ||||||||
| 1 | 1.2 | 2 | Rac-BINAP | 4 | Cs2CO3 | 3 | Dioxane | 0 |
| 2 | 1.2 | 10b | Rac-BINAP | 10 | Cs2CO3 | 1.4 | Toluene | 0 |
| 3 | 1.2 | 10b | Rac-BINAP | 10 | NaOtBu | 1.4 | Toluene | 0c |
| 4 | 1.2 | 2 | RuPhos | 8 | NaOtBu | 1.4 | Toluene | 0 |
| 5 | 1.2 | 5 | RuPhos | 10 | NaOtBu | 3 | Toluene | 0 |
| 6 | 1.2 | 5 | SPhos | 10 | NaOtBu | 3 | Toluene | Tracesc |
| 7 | 1.2 | 5 | RuPhos | 10 | NaOtBu | 3 | Dioxane | 4 |
| 8 | 1.2 | 5 | SPhos | 10 | NaOtBu | 3 | Dioxane | 46 |
| 9 | 1.2 | 5 | XPhos | 10 | NaOtBu | 3 | Dioxane | 47 |
| 10 | 1.2 | 5 | tBuXPhos | 10 | NaOtBu | 3 | Dioxane | 0 |
| 11 | 1.2 | 5 | SPhos | 10 | LiHMDS | 3 | Dioxane | 0 |
| 12 | 1.2 | 5 | SPhos | 10 | NaOtBu | 3 | DME | Tracesc |
| 13 | 5 | 5 | SPhos | 10 | NaOtBu | 3 | Dioxane | 66 |
| 14 | 5 | 5 | XPhos | 10 | NaOtBu | 3 | Dioxane | 100 (1 h) |
| 15 | 3 | 5 | XPhos | 10 | NaOtBu | 3 | Dioxane | 100 (2 h) |
| 16 | 5 | 4 | XPhos | 8 | NaOtBu | 3 | Dioxane | 100 (2 h) |
First, we used the conditions described in the literature involving a thiophene ring (entry 1,11 entries 2–3,12 and entries 4–5,13 Table 1). We did not observe any reaction with these conditions. From the conditions of entry 5, we then decided to study the influence of the phosphine ligand using SPhos instead of RuPhos (entry 6, Table 1). While we observed 1a for the first time, the main product of the reaction resulted from the dehalogenation of the starting material. Also, from entry 5, we decided to improve the solubility of starting material 1 by changing the reaction solvent from toluene to dioxane (entry 6, Table 1). We observed the formation of 1a in an estimated 4% yield. By combining the conditions of entries 6 and 7, the estimated yield increased to 46% (entry 8, Table 1). Using the closely related XPhos phosphine, instead of SPhos, the same estimated yield was maintained (entry 9, Table 1). However, tBuXPhos, a more hindered version of XPhos, did not allow the formation of 1a. From entry 8, we tried to explore the changes in the base and the solvent using LiHMDS instead of NaOtBu (entry 11, Table 1) and using DME instead of dioxane (entry 12, Table 1). The use of LiHMDS was unsuccessful in affording 1a, while DME caused a significant dehalogenation of 1. From entries 8 and 9, we decided to increase the load of p-toluidine in the reaction (entries 13 and 14, Table 1). We observed an increase in the estimated yield with full conversion in 1 h when XPhos was used. From these conditions, we reduced the load of p-toluidine or the phosphine/palladium duo (entries 15 and 16, Table 1), and we observed only an increase in the reaction time from 1 to 2 h. We finally decided to use the conditions of entry 16 thereafter since amines are cheap starting materials compared to palladium and phosphine.
The Ullman reaction, a possible alternative to the Buchwald–Hartwig coupling reaction, was also tried on compound 1 or its precursor 2. In both cases, no reaction was observed after 24 h at 100 °C with the following tested conditions: para-toluidine (3 eq.), dimethylglycine (20 mol%), CuI (10 mol%), and Cs2CO3 (3 eq.).
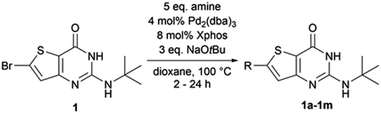
Therefore, the conditions of entry 16 (Table 1) were then applied on various substrates to explore the scope of the operating procedure and obtain a new series of functionalized 2-tertbutylaminothieno[3,2-d]pyrimidin-4(3H)-one (Table 2).
| Compound | R | Isolated yielda (%) or observed outcome |
|---|---|---|
| a Conversion rate (determined by LCMS) indicated between brackets if the conversion was not complete. | ||
| 1a | 4-Me–Ph–NH– | 55 |
| 1b | 3-Me–Ph–NH– | 31 |
| 1c | 2-Me–Ph–NH– | 15 (53) |
| 1d | 4-MeO–Ph–NH– | 14 (63) |
| 1e | 4 F–Ph–NH– | 23 (65) |
| 1f | Morpholinyl– | 35 (74) |
| 1g | Piperidinyl– | 15 (51) |
| 1h | N-Methylpiperazinyl– | Complete dehalogenation of 1 |
| 1i | Pyrazole | No reaction |
| 1j | Imidazole | No reaction |
| 1k | Pyrimidinyl-3–amine | Traces of 1k and dehalogenated byproduct |
| 1l | Cyclopropylamine | Complete dehalogenation of 1 |
| 1m | Ethanolamine | Complete dehalogenation of 1 |
Purifications, using flash chromatography, were challenging for the whole series, thus leading to a moderate isolated yield of 55% for compound 1a. The isolated yields for the other compounds were decreased by two additional factors–incomplete conversion and dehalogenation of 1. When moving the methyl substituent from the para position to the ortho (1c) position, the conversion was not complete after 24 h. Conversion was also incomplete after 24 h of heating when the methyl group was replaced by an electron-donating (1d) or electron-withdrawing (1e) group. When performing the reaction with morpholine (1f) or piperidine (1g), we also observed moderate dehalogenation of the starting material in addition to incomplete conversion after 24 h, both reasons being responsible for the low reaction yields. For N-methylpiperazine (1h), we observed a complete and rapid (less than 2 h) dehalogenation of the starting material. The same result was observed with alkyl amines such as cyclopropylamine (1l) and ethanolamine (1m). This dehalogenation could be caused by the choice of our catalyst ligand.14 Finally, heteroaromatic amines, such as pyrazole, imidazole, and 3-aminopyridine, did not react using the same conditions. Although our conditions seemed to work for a simple substrate such as para-toluidine, they are strongly impacted by the modification of the para-substituent and the nature of the amine used.
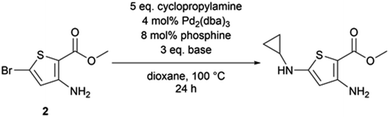
We applied these Buchwald–Hartwig conditions to compound 2, the synthetic precursor of compound 1, to possibly overcome the dehalogenation problem encountered with aliphatic amines and N-methylpiperazine (Table 3). While our conditions properly functionalized position 5 of the thiophene aminoester, we also observed a partial saponification of the methyl ester group. The corresponding carboxylic acid derivative could not be isolated because of its high polarity. We hypothesized that sodium tertbutoxyde could saponify the ester at position 2 of thiophene. We decided to explore other bases to observe the behavior of the ester; caesium carbonate promoted the homo-coupling of compound 2, while LiHMDS led to the formation of a cyclopropylamide derivative. The replacement of the phosphine ligand with caesium carbonate did not cause any change and the homo-coupling product was still formed.
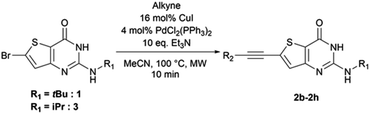
2.2. Sonogashira coupling
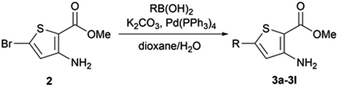
From the reaction conditions found in the literature,15 we performed a conclusive test on compound 2 (Scheme 2). We then used these reaction conditions on compound 1 and its isopropylamine analog 3, leading to seven 2-aminothieno[3,2-d]pyrimidin-4(3H)-ones bearing an alkyne substituent at position 6 in yields ranging from 23 to 85% (Table 4). The poor yields are due either to purification difficulties (compound 2c and 2g) or the volatile behavior of some alkynes (compound 2e). Depending on the boiling point of the alkyne, we tried to improve the low yields by increasing the load of the alkyne in the reaction, but still observed low yields. Compound 2f was obtained after the protodesilylation of the trimethylsilyl alkyne using potassium carbonate in a mixture of methanol and dichloromethane.2.3. Suzuki–Miyaura coupling
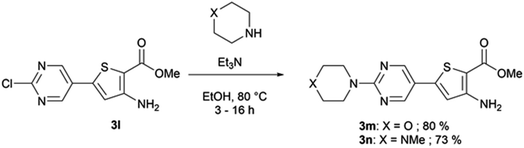
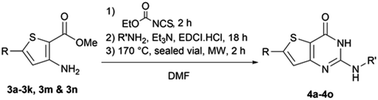
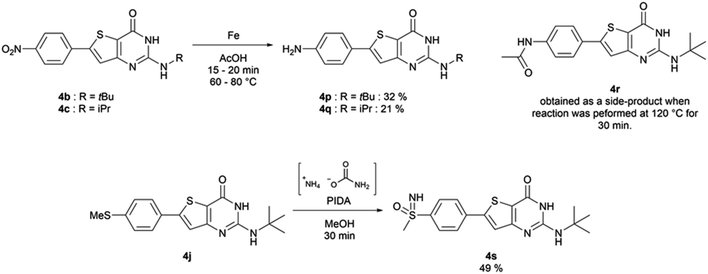
When Suzuki–Miyaura coupling was performed on compound 1 or 3, applying the reaction conditions used for the synthesis of M1 metabolites,10 we observed the dehalogenation of the starting material, which complicated the purification. Therefore, we decided to perform Suzuki–Miyaura coupling on 2 with conditions from the literature and previously used in our lab.16 Eleven thiophene aminoesters functionalized at position 5 were synthesized in yields above 50%, except for compound 3l (Table 5). In this specific case, we removed water from the reaction medium to avoid the substitution of the chlorine atom contained in the chloroimine moiety. The chlorine atom of compound 3l was then engaged in a SNAr reaction with morpholine or N-methylpiperazine (Scheme 3).The functionalized thiophene aminoesters (3a–3k, 3m, and 3n) were then engaged in a cyclization reaction to form the thieno[3,2-d]pyrimidin-4(3H)-one core. This three step one-pot reaction afforded 15 compounds, 13 including a tertbutylamine group and 2 bearing an isopropyl group at position 2 (Table 6). The 4-nitrophenyl-containing compounds 4b and 4c were then engaged in a reduction reaction to afford the 4-aminophenyl compounds 4p and 4q in poor yields (Scheme 4). We initially performed this reduction at 120 °C for 30 min, but this led to the formation of compound 4r, resulting from the acylation of compound 4p, which was isolated. Compound 4j was used to synthesize the sulfoximine derivative 4s (Scheme 4) using a one-step reaction with ammonium carbamate and (diacetoxyiodo)benzene (PIDA).
| Compound | R | R′ | Isolated yield (%) |
|---|---|---|---|
| 4a | 4-CO2Me–Ph– | tBu | 55 |
| 4b | 4-NO2–Ph– | tBu | 55 |
| 4c | iPr | 33 | |
| 4d | 4-HO–Ph– | tBu | 13 |
| 4e | 3-Cl-4-F–Ph– | tBu | 49 |
| 4f | iPr | 37 | |
| 4g | 4-Morpholinyl–Ph– | tBu | 69 |
| 4h | 4-(N-Methylpiperazinyl)–Ph– | tBu | 23 |
| 4i | 4-Piperidinyl–Ph– | tBu | 51 |
| 4j | 4-MeS–Ph– | tBu | 65 |
| 4k | 4-MeSO2–Ph– | tBu | 44 |
| 4l | 4-(H2N–SO2)-Ph- | tBu | 56 |
| 4m | 4-(Morpholinyl–SO2)–Ph– | tBu | 33 |
| 4n | 2-Morpholinopyrimidin-5-yl– | tBu | 57 |
| 4o | 2-(N-Methylpyperazin-1-yl)pyrimidin-5-yl– | tBu | 19 |
2.4. Biological evaluation
The synthesized compounds were evaluated for their antiplasmodial activity (EC50) on the multi-resistant K1 strain of P. falciparum (resistant to chloroquine, sulfadoxine, and pyrimethamine) and their cytotoxicity (CC50) on the human HepG2 cell-line in order to determine their selectivity index (SI, EC50/CC50) and to compare them to reference drugs (chloroquine, atovaquone, and doxycycline for activity and doxorubicine for cytotoxicity; Table 7). Of the 33 tested compounds, 16 displayed aqueous solubility problems in the test medium, limiting the determination of precise values for EC50, CC50, or both. Such behavior for the thieno[3,2-d]pyrimidin-4(3H)-one series was already seen during the pharmacomodulation, leading to compound M1.9 The thienopyrimidinone scaffold is found in cytotoxic compounds,17 but apart from compound 4r (CC50 = 0.16 μM, more toxic than doxorubicine), the CC50 of the compounds ranged widely from 2.1 to 89.1 μM, while the EC50 ranged from 0.77 to 43.2; 18 compounds showed an antiplasmodial activity below 5 μM. Compound 1g was the best among all the synthesized compounds with submicromolar antiplasmodial activity (0.77 μM), which is still far from the M1 potency (0.045 μM) but with improved cytotoxicity (33.9 μM) compared to M1 (24 μM).9 These results provided valuable data regarding the SAR of the thieno[3,2-d]pyrimidin-4(3H)-one core.| Entry | R1 | R2 | EC50 PfK1 (μM) | CC50 HepG2 (μM) | SI | c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4b D7.4b |
|---|---|---|---|---|---|---|
| a Values from ref. 9.b See experimental part for details. | ||||||
| 1 | Br | tBu | 43.2 ± 2.6 | 38.2 ± 3.5 | 0.9 | 3.19 |
| 3 | iPr | >50 | 89.1 ± 9.7 | <1.8 | 2.91 | |
| 1a | 4-Me–Ph–NH– | tBu | 10.3 ± 0.2 | 21.7 ± 2.1 | 2.1 | 4.38 |
| 1b | 3-Me–Ph–NH– | tBu | 1.1 ± 0.3 | 5.3 ± 0.4 | 4.8 | 4.38 |
| 1c | 2-Me–Ph–NH– | tBu | 4.3 ± 0.2 | 14.1 ± 0.3 | 3.3 | 4.38 |
| 1d | 4-MeO–Ph–NH– | tBu | 7.2 ± 2.0 | 24.0 ± 3.1 | 3.3 | 3.71 |
| 1e | 4 F–Ph–NH– | tBu | 7.1 ± 1.4 | 21.3 ± 1.6 | 3.0 | 4.01 |
| 1f | Morpholinyl– | tBu | 4.5 ± 1.1 | >50 | >11.1 | 2.31 |
| 1g | Piperidinyl– | tBu | 0.77 ± 0.13 | 33.9 ± 1.4 | 44.0 | 3.38 |
| 2b | 4-Me–Ph– | tBu | 2.35 ± 0.38 | >12.5 | >5.3 | 5.03 |
| 2c | iPr | 4.6 ± 1.0 | 3.1 ± 0.6 | 0.7 | 4.75 | |
| 2d | HO(CH2)2– | tBu | >10 | >50 | — | 2.39 |
| 2e | Cyclopropyl– | tBu | 1.9 ± 0.6 | 20.8 ± 1.4 | 10.9 | 3.65 |
| 2f | H– | tBu | >12.5 | 4.7 ± 1.0 | <0.4 | 2.54 |
| 2g | N-Methylpiperazine–CH2– | tBu | >5 | 41.6 ± 5.2 | <8.3 | 2.20 |
| 2h | Ph– | iPr | 2.8 ± 1.0 | >12.5 | >4.5 | 4.23 |
| 4a | 4-CO2Me–Ph– | tBu | 2.5 ± 0.9 | >12.5 | >5.0 | 3.91 |
| 4d | 4-HO–Ph– | tBu | 7.5 ± 1.0 | 12.2 ± 0.3 | 1.6 | 3.60 |
| 4e | 3-Cl-4-F–Ph– | tBu | 2.0 ± 0.9 | >12.5 | >6.3 | 4.65 |
| 4f | iPr | 3.6 ± 1.2 | >6.25 | >1.7 | 4.37 | |
| 4g | 4-Morpholinyl–Ph– | tBu | 2.4 ± 0.9 | 7.9 ± 1.3 | 3.3 | 3.79 |
| 4h | 4-(N-Methylpiperazinyl)–Ph– | tBu | 4.1 ± 0.7 | 5.8 ± 0.5 | 1.4 | 3.29 |
| 4i | 4-Piperidinyl–Ph- | tBu | 4.1 ± 1.2 | >6.25 | >1.5 | 4.86 |
| 4j | 4-MeS–Ph– | tBu | 1.0 ± 0.3 | >12.5 | >12.5 | 4.53 |
| 4k | 4-MeSO2–Ph– | tBu | 3.0 ± 0.6 | >25 | >8.3 | 2.74 |
| 4l | 4-(H2N–SO2)–Ph– | tBu | >12.5 | >6.25 | — | 2.51 |
| 4m | 4-(Morpholinyl–SO2)–Ph– | tBu | 5.1 ± 2.6 | >12.5 | >2.5 | 2.74 |
| 4n | 2-Morpholinopyrimidin-5-yl– | tBu | 2.1 ± 0.6 | 26.4 ± 4.6 | 12.6 | 2.55 |
| 4o | 2-(N-Methylpyperazin-1-yl)pyrimidin-5-yl– | tBu | 4.4 ± 1.0 | 11.2 ± 1.3 | 2.5 | 2.40 |
| 4p | 4-NH2-Ph– | tBu | 3.5 ± 1.2 | 2.5 ± 1.0 | 0.7 | 3.07 |
| 4q | iPr | 6.0 ± 1.1 | 11.3 ± 3.5 | 1.9 | 2.79 | |
| 4r | 4-(CH3–CO–NH)-Ph– | tBu | 3.9 ± 1.1 | 0.16 ± 0.04 | <0.1 | 3.14 |
| 4s | 4-(CH3–SONH)-Ph– | tBu | >12.5 | 63.3 ± 14.6 | <5.1 | 2.76 |
| M1a | 0.045 | 24 | 533 | 4.42 | ||
| M1 (free base)a | 0.2 | 25.6 | 128 | 4.42 | ||
| Chloroquinea | 0.5 | 30 | 60 | 0.88 | ||
| Atovaquonea | 0.0013 | >15.6 | >12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000 |
4.37 | ||
| Doxycyclinea | 5 | 20 | 4 | −0.80 | ||
| Doxorubicinea | — | 0.2 | — | 0.04 | ||
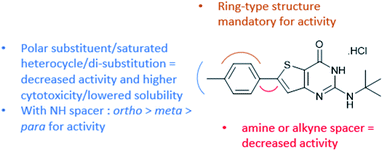
The introduction of an amine spacer between the thienopyrimidinone core and the C6-substituent (1a) significantly reduced the antiplasmodial activity. The cytotoxicity value was worsened when the methyl was moved on the phenyl ring (para 1a < ortho 1c < meta 1b). The replacement of the methyl group by an electron-donating (1d) or withdrawing (1e) group moderately improved the antiplasmodial activity. The introduction of an alkyne spacer in the M1 structure (2b) reduced the antiplasmodial activity and aqueous solubility in the test medium of the compound. Among the different alkyne substituents, only cyclopropylalkyne 2e showed a decent combination of the biological values. Therefore, the conformational changes of the C6-sidechain induced by the amine spacer or sidechain elongation following the introduction of an alkyne are not effective strategies to obtain an antiplasmodial hit compound in the thienopyrimidinone series.
We also investigated the para-substituent on the phenyl ring at position 6 of the thienopyrimidinone core. The replacement of the methyl substituent by a more polar group to potentially improve the aqueous solubility (methyl ester 4a, phenol 4d, anilines 4p and 4q and acetamide 4r, and sulphur-containing functions 4j–4m and 4s) was favorable for neither the antiplasmodial activity nor the cytotoxicity, while the replacement of the methyl by a fluorine atom was interesting in our previous work;9 the addition of a chlorine atom at position 3 of the 4-fluorophenyl cycle induced a loss of activity and aqueous solubility in the test medium (4e and 4f) compared to M1. We also replaced the methyl substituent by different saturated heterocycles (4g, 4h and 4i), but this was associated with an increased cytotoxicity and reduced antiplasmodial activity. The cytotoxicity values were improved when the phenyl ring was replaced by a pyrimidine (4n and 4o).
Finally, Buchwald–Hartwig cross-couplings gave us the opportunity to replace the phenyl ring at position 6 by saturated heterocycles. When the phenyl ring is replaced by a piperidine (1g), we observed a moderate loss of activity and improved cytotoxicity. The addition of an oxygen atom in the cyclic amine, leading to morpholine 1f, significantly decreased the antiplasmodial activity.
With this observation and the biological results of compounds bearing a polar substituent (4a, 4d, 4g, 4h, and 4k–4s), we concluded that a polar head (linked to c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 decrease) at this position is detrimental to the antiplasmodial activity (Fig. 2). We also concluded that a ring-type structure (aromatic or saturated) is required at position 6 since compounds lacking one of these structures (1, 3, 2d, and 2f) displayed a lack of antiplasmodial activity.
D7.4 decrease) at this position is detrimental to the antiplasmodial activity (Fig. 2). We also concluded that a ring-type structure (aromatic or saturated) is required at position 6 since compounds lacking one of these structures (1, 3, 2d, and 2f) displayed a lack of antiplasmodial activity.
Considering 1g gave good biological results, it was evaluated in vitro for its mouse microsomal stability. We observed a more than 3-fold improvement in the half-time between the tolyl moiety (M1, t1/2 = 11 min, Clint = 240 μLmin−1mg) and the piperidine moiety (1g, t1/2 = 40 min, Clint = 42 μLmin−1mg). This important improvement shows that the piperidine moiety is stable.
3. Conclusion
We synthesized 33 original compounds in the thieno[3,2-d]pyrimidin-4(3H)-one series using three different palladium-catalyzed cross-coupling reactions, namely Buchwald–Hartwig, Sonogashira, and Suzuki–Miyaura. For the first time, we developed reaction conditions to directly functionalize the position 6 of the thienopyrimidinone core with anilines and saturated heterocycles. However, additional work on this reaction is required to extend the scope of useable substrates and to improve the reaction yields. Sonogashira coupling provided compounds with the side chains of various natures. However, the introduction of the alkyne spacer that reduced the antiplasmodial activity led us to stop the further synthesis of 6-alkynyl-thieno[3,2-d]pyrimidin-4(3H)-ones. Seventeen compounds were obtained using Suzuki–Miyaura coupling with various substituents on the phenyl ring, and there are still a lot of possibilities to explore with this coupling. The synthesized compounds were tested on the blood-stage of PfK1 and the human HepG2 cell-line to measure their antiplasmodial activity and cytotoxicity. This work led us to the discovery of compound 1g, which has submicromolar antiplasmodial activity that is associated with a better cytotoxicity value than compound M1 and an improved in vitro metabolic stability compared to M1. Moreover, this work provided valuable RSA data on the thienopyrimidinone series (Fig. 2). Considering the microsomal stability of the piperidine moiety, 1g is an interesting starting point to synthesize new thieno[3,2-d]pyrimidines bearing, at position 6, a substituted piperidine. The substitution on this piperidine could provide us compounds with biological values at par with the M1 antiplasmodial profile and further improve the metabolic stability of the compounds.4. Experimental part
4.1. Chemistry
2-tert-Butylamino-6-[(4-methylphenyl)amino]thieno[3,2-d]pyrimidin-4(3H)-one (1a). Following procedure A with 4-methylaniline (0.532 g, 5 mmol), the obtained crude was purified via flash chromatography (using dichloromethane/methanol). Fractions of interest were triturated in diethyl ether, affording 2-tert-butylamino-6-[(4-methylphenyl)amino]thieno[3,2-d]pyrimidin-4(3H)-one as a brown powder (173 mg, 55% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.06 (s, 1H), 9.47 (s, 1H), 7.17–7.07 (m, 4H), 6.22 (s, 1H), 5.94 (s, 1H), 2.25 (s, 3H), 1.38 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.5, 156.8, 155.4, 152.3, 139.4, 130.7, 129.8 (2C), 117.5 (2C), 101.5, 98.5, 50.8, 28.7 (3C), 20.3. Mp = 204–206 °C. HRMS (ESI) m/z calculated for C17H21N4OS [M + H]+ 329.1431, found 329.1429.
2-tert-Butylamino-6-[(3-methylphenyl)amino]thieno[3,2-d]pyrimidin-4(3H)-one (1b). Following procedure A with 3-methylaniline (0.532 g, 5 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol), affording 2-tert-butylamino-6-[(3-methylphenyl)amino]thieno[3,2-d]pyrimidin-4(3H)-one as a beige powder (0.1 g, 31% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.09 (s, 1H), 9.52 (s, 1H), 7.24–7.17 (m, 1H), 7.04–6.99 (m, 2H), 6.78 (d, 3J = 7.5 Hz, 1H), 6.28 (s, 1H), 5.98 (bs, 1H), 2.29 (s, 3H), 1.39 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.3, 156.9, 154.9, 152.3, 141.9, 138.8, 129.3, 122.4, 117.6, 114.3, 102.3, 98.9, 50.9, 28.7 (3C), 21.1. Mp = 225–227 °C. HRMS (ESI) m/z calculated for C17H21N4OS [M + H]+ 329.1431, found 329.1428.
2-tert-Butylamino-6-[(2-methylphenyl)amino]thieno[3,2-d]pyrimidin-4(3H)-one (1c). Following procedure A with 2-methylaniline (0.532 mL, 5 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol). Fractions of interest were triturated in cyclohexane affording 2-tert-butylamino-6-[(2-methylphenyl)amino]thieno[3,2-d]pyrimidin-4(3H)-one as a brown powder (50 mg, 15% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.02 (s, 1H), 8.79 (s, 1H), 7.36 (d, 3J = 7.9 Hz, 1H), 7.27–7.17 (m, 2H), 7.04 (t, 3J = 7.4 Hz, 1H), 5.97 (s, 1H), 5.91 (s, 1H), 2.23 (s, 3H), 1.37 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.5, 157.8, 156.8, 152.2, 140.2, 131.1, 130.4, 126.8, 124.1, 121.4, 100.9, 98.6, 50.8, 28.7 (3C), 17.7. Mp = 159–161 °C. HRMS (ESI) m/z calculated for C17H21N4OS [M + H]+ 329.1431, found 329.1429.
2-tert-Butylamino-6-[(4-methoxyphenyl)amino]thieno[3,2-d]pyrimidin-4(3H)-one (1d). Following procedure A with 4-methoxyaniline (0.611 g, 5 mmol), the obtained crude was purified via two successive flash chromatography runs (using dichloromethane and methanol). Fractions of interest were triturated in diethyl ether affording 2-tert-butylamino-6-[(4-methoxyphenyl)amino]thieno[3,2-d]pyrimidin-4(3H)-one as a beige powder (49 mg, 14% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.02 (s, 1H), 9.33 (s, 1H), 7.20–7.13 (m, 2H), 6.96–6.90 (m, 2H), 6.11 (s, 1H), 5.92 (s, 1H), 3.73 (s, 3H), 1.38 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.6, 156.7, 156.7, 154.8, 152.3, 135.1, 120.1 (2C), 114.7 (2C), 100.2, 97.9, 55.3, 50.8, 28.7 (3C). Mp = 205–207 °C. HRMS (ESI) m/z calculated for C17H21N4O2S [M + H]+ 345.1380, found 345.1381.
2-tert-Butylamino-6-[(4-fluorophenyl)amino]thieno[3,2-d]pyrimidin-4(3H)-one (1e). Following procedure A with 4-fluoroaniline (0.470 mL, 5 mmol), the obtained crude was purified via two successive flash chromatography runs (using dichloromethane and methanol). Fractions of interest were triturated in diethyl ether, affording 2-tert-butylamino-6-[(4-fluorophenyl)amino]thieno[3,2-d]pyrimidin-4(3H)-one as a beige powder (77 mg, 23% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.10 (s, 1H), 9.53 (s, 1H), 7.26–7.20 (m, 2H), 7.20–7.13 (m, 2H), 6.24 (s, 1H), 5.96 (s, 1H), 1.38 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.4, 157.2 (d, 1J = 238 Hz, 1C), 156.8, 155.3, 152.3, 138.4 (d, 4J = 2.8 Hz, 1C), 119.2 (d, 3J = 8.0 Hz, 2C), 116.0 (d, 2J = 22.5 Hz, 2C), 101.8, 98.8, 50.8, 28.7 (3C). Mp = 195–197 °C. HRMS (ESI) m/z calculated for C16H18FN4OS [M + H]+ 333.1180, found 333.1182.
2-tert-Butylamino-6-(morpholin-4-yl)thieno[3,2-d]pyrimidin-4(3H)-one (1f). Following procedure A with morpholine (0.434 mL, 5 mmol), the obtained crude was purified via two successive flash chromatography runs (using dichloromethane and methanol), affording 2-tert-butylamino-6-(morpholin-4-yl)thieno[3,2-d]pyrimidin-4(3H)-one as a yellow solid (106 mg, 35% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.04 (bs, 1H), 6.07 (s, 1H), 5.94 (s, 1H), 3.75–3.68 (m, 4H), 3.25–3.17 (m, 4H), 1.38 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 163.6, 160.8, 156.8, 152.3, 99.5, 99.0, 65.3 (2C), 50.8, 49.1 (2C), 28.6 (3C). Mp = 264–266 °C. HRMS (ESI) m/z calculated for C14H21N4O2S [M + H]+ 309.1380, found 309.1379.
2-tert-Butylamino-6-(piperidin-1-yl)thieno[3,2-d]pyrimidin-4(3H)-one (1g). Following procedure A with piperidine (0.490 mL, 5 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol), affording 2-tert-butylamino-6-(piperidin-1-yl)thieno[3,2-d]pyrimidin-4(3H)-one as a pale-yellow powder (45 mg, 15% yield). 1H NMR (DMSO-d6, 400 MHz) δ 9.95 (s, 1H), 5.95 (s, 1H), 5.90 (s, 1H), 3.28–3.20 (m, 4H), 1.65–1.51 (m, 6H), 1.37 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 163.7, 161.3, 156.8, 152.2, 98.5, 98.2, 50.8, 50.2 (2C), 28.7 (3C), 24.5 (2C), 23.2. Mp = 171–173 °C. HRMS (ESI) m/z calculated for C15H23N4OS [M + H]+ 307.1587, found 307.1590.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v with triethylamine). The obtained suspension was stirred for 10 min under microwave irradiation at 100 °C. Water (20 times the quantity of acetonitrile) was added to the reaction mixture, which was then extracted with dichloromethane. The organic layer was washed with water and the excess was removed in vacuo. The obtained crude was purified via the appropriate method.
1 v/v with triethylamine). The obtained suspension was stirred for 10 min under microwave irradiation at 100 °C. Water (20 times the quantity of acetonitrile) was added to the reaction mixture, which was then extracted with dichloromethane. The organic layer was washed with water and the excess was removed in vacuo. The obtained crude was purified via the appropriate method.
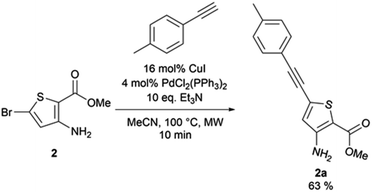
3-Amino-5-[(4-methylphenyl)ethynyl]thiophene-2-carboxylate (2a). Methyl-3-amino-5-bromothiophene-2-carboxylate (0.3 g, 1.27 mmol), 1-ethynyl-4-methylbenzene (0.155 g, 1.33 mmol), copper(I) iodide (39 mg, 0.2 mmol), triethylamine (1.76 mL, 12.7 mmol), tetrakis(triphenylphosphine)palladium(0) (4 mol%), and dried acetonitrile (0.5 mL) were put under nitrogen atmosphere. The obtained reaction mixture was stirred 10 min under microwave irradiation at 100 °C. Water (10 mL) was added and the mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with brine (3 × 30 mL), dried over anhydrous sodium sulphate, and the excess solvent was removed in vacuo. The obtained residue was purified via column chromatography on silica gel (90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 cyclohexane/ethyl acetate). The desired fractions were combined, the excess solvent removed in vacuo, and the obtained solid was triturated in cyclohexane, affording ethyl-3-amino-5-[(4-methylphenyl)ethynyl]thiophene-2-carboxylate as a brown powder (217 mg, 63% yield). 1H NMR (CDCl3, 400 MHz) δ 7.40 (d, 3J = 8.2 Hz, 2H), 7.16 (d, 3J = 8.2 Hz, 2H), 6.65 (s, 1H), 5.42 (bs, 2H), 3.83 (s, 3H), 2.37 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ 164.5, 153.2, 139.5, 131.7 (2C), 129.4 (2C), 128.7, 123.3, 119.3, 102.1, 95.7, 81.9, 51.1, 21.7. Mp = 156–158 °C. HRMS (ESI) m/z calculated for C15H14NO2S [M + H]+ 272.0740, found 272.0743.
10 cyclohexane/ethyl acetate). The desired fractions were combined, the excess solvent removed in vacuo, and the obtained solid was triturated in cyclohexane, affording ethyl-3-amino-5-[(4-methylphenyl)ethynyl]thiophene-2-carboxylate as a brown powder (217 mg, 63% yield). 1H NMR (CDCl3, 400 MHz) δ 7.40 (d, 3J = 8.2 Hz, 2H), 7.16 (d, 3J = 8.2 Hz, 2H), 6.65 (s, 1H), 5.42 (bs, 2H), 3.83 (s, 3H), 2.37 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ 164.5, 153.2, 139.5, 131.7 (2C), 129.4 (2C), 128.7, 123.3, 119.3, 102.1, 95.7, 81.9, 51.1, 21.7. Mp = 156–158 °C. HRMS (ESI) m/z calculated for C15H14NO2S [M + H]+ 272.0740, found 272.0743.
2-tert-Butylamino-6-[(4-methylphenyl)ethynyl]thieno[3,2-d]pyrimidin-4(3H)-one (2b). Following procedure B with 6-bromo-2-(tert-butylamino)thieno[3,2-d]pyrimidin-4(3H)-one (0.3 g, 0.99 mmol) and 1-ethynyl-4-methylbenzene (151 μL, 1.19 mmol), the obtained crude was purified via flash chromatography (using cyclohexane/ethyl acetate). Fractions of interest were combined, the excess solvent was removed in vacuo, and the solid triturated in diethyl ether, affording 2-tert-butylamino-6-[(4-methylphenyl)ethynyl]thieno[3,2-d]pyrimidin-4(3H)-one as a yellow/brown powder (200 mg, 60% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.59 (s, 1H), 7.49 (d, 3J = 10.8 Hz, 2H), 7.30–7.24 (m, 3H), 6.13 (s, 1H), 2.35 (s, 3H), 1.41 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 159.4, 157.7, 152.8, 140.2, 131.9 (2C), 130.0 (2C), 129.2, 129.1, 118.5, 113.7, 97.0, 82.3, 51.6, 29.0 (3C), 21.6. Mp = 235–237 °C. HRMS (ESI) m/z calculated for C19H20N3OS [M + H]+ 338.1322, found 338.1319.
6-[(4-methylphenyl)ethynyl]-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one (2c). Following procedure B with 6-bromo-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one (0.172 g, 0.60 mmol) and 1-ethynyl-4-methylbenzene (91 μL, 0.72 mmol), the obtained crude was purified via flash chromatography (cyclohexane and ethanol), affording 6-[(4-methylphenyl)ethynyl]-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one as a yellow solid (44 mg, 23% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.78 (s, 1H), 7.48 (d, 3J = 8.1 Hz, 2H), 7.30–7.23 (m, 3H), 6.22 (d, 3J = 7.7 Hz, 1H), 4.08–3.94 (m, 1H), 2.35 (s, 3H), 1.17 (d, 3J = 6.5 Hz, 6H). 13C NMR (DMSO-d6, 100 MHz) δ 159.2, 157.3, 152.8, 139.7, 131.4 (2C), 129.5 (2C), 128.7, 128.5, 118.0, 113.2, 96.4, 81.9, 42.2, 22.4 (2C), 21.1. Mp = 277–279 °C. HRMS (ESI) m/z calculated for C18H17N3OS [M + H]+ 324.1165, found 324.1163.
2-tert-Butylamino-6-(4-hydroxybut-1-yn-1-yl)thieno[3,2-d]pyrimidin-4(3H)-one (2d). Following procedure B with 6-bromo-2-(tert-butylamino)thieno[3,2-d]pyrimidin-4(3H)-one (0.3 g, 0.99 mmol) and but-3-yn-1-ol (90 μL, 1.19 mmol), the obtained crude was triturated in acetonitrile. The precipitate was filtered and isolated, while the filtrate was purified via column chromatography over silica gel (starting from 100% dichloromethane to 97
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dichloromethane/methanol), affording 2-tert-butylamino-6-(4-hydroxybut-1-yn-1-yl)thieno[3,2-d]pyrimidin-4(3H)-one as a brown powder (137 mg, 47% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.53 (bs, 1H), 7.09 (s, 1H), 6.09 (s, 1H), 4.95 (t, 3J = 5.6 Hz, 1H), 3.58 (q, 3J = 6.4 Hz, 2H), 2.63 (t, 3J = 6.4 Hz, 2H), 1.39 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 158.9, 157.1, 152.3, 129.6, 128.0, 112.4, 97.2, 74.2, 59.2, 51.0, 28.5 (3C), 23.6. Mp = 215–217 °C. HRMS (ESI) m/z calculated for C14H18N3O2S [M + H]+ 292.1114, found 292.1115.
3 dichloromethane/methanol), affording 2-tert-butylamino-6-(4-hydroxybut-1-yn-1-yl)thieno[3,2-d]pyrimidin-4(3H)-one as a brown powder (137 mg, 47% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.53 (bs, 1H), 7.09 (s, 1H), 6.09 (s, 1H), 4.95 (t, 3J = 5.6 Hz, 1H), 3.58 (q, 3J = 6.4 Hz, 2H), 2.63 (t, 3J = 6.4 Hz, 2H), 1.39 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 158.9, 157.1, 152.3, 129.6, 128.0, 112.4, 97.2, 74.2, 59.2, 51.0, 28.5 (3C), 23.6. Mp = 215–217 °C. HRMS (ESI) m/z calculated for C14H18N3O2S [M + H]+ 292.1114, found 292.1115.
2-tert-Butylamino-6-(cyclopropylethynyl)thieno[3,2-d]pyrimidin-4(3H)-one (2e). Following procedure B with 6-bromo-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one (0.3 g, 0.99 mmol) and ethynylcyclopropane (336 μL, 3.97 mmol), the obtained crude was purified via flash chromatography (using cyclohexane and ethanol). Fractions of interest were triturated in cyclohexane, affording 2-tert-butylamino-6-(cyclopropylethynyl)thieno[3,2-d]pyrimidin-4(3H)-one as a beige powder (66 mg, 23% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.53 (s, 1H), 7.07 (s, 1H), 6.08 (s, 1H), 1.68–1.59 (m, 1H), 1.38 (s, 9H), 0.98–0.91 (m, 2H), 0.83–0.77 (m, 2H). 13C NMR (DMSO-d6, 100 MHz) δ 158.9, 157.1, 152.3, 129.7, 128.0, 112.3, 102.0, 68.9, 51.0, 28.5 (3C), 8.8 (2C), 0.0. Mp = 158–160 °C. HRMS (ESI) m/z calculated for C15H17N3OS [M + H]+ 288.1165, found 288.1166.
2-tert-Butylamino-6-ethynylthieno[3,2-d]pyrimidin-4(3H)-one (2f). Following procedure B with 6-bromo-2-(tert-butylamino)thieno[3,2-d]pyrimidin-4(3H)-one (0.3 g, 0.99 mmol) and ethynyltrimethylsilane (550 μL, 3.97 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol), affording 2-tert-butylamino-6-[(trimethylsilyl)ethynyl]thieno[3,2-d]pyrimidin-4(3H)-one as a yellow solid (198 mg), which was used without further purification. 1H NMR (DMSO-d6, 400 MHz) δ 10.59 (s, 1H), 7.22 (s, 1H), 6.11 (s, 1H), 1.39 (s, 9H), 0.25 (s, 9H).
To a solution of 2-(tert-butylamino)-6-((trimethylsilyl)ethynyl)thieno[3,2-d]pyrimidin-4(3H)-one (0.183 g, 0.57 mmol) in a mixture of dichloromethane and methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 0.2 M), potassium carbonate (0.396 g, 2.86 mmol) was added and the reaction mixture was stirred for 16 h at room temperature. The reaction mixture was poured into water and extracted with dichloromethane. The combined organic layers were dried over sodium sulphate and the excess solvent was removed in vacuo. The obtained crude was purified via column chromatography on silica gel (starting from 100% dichloromethane to 97
1 v/v, 0.2 M), potassium carbonate (0.396 g, 2.86 mmol) was added and the reaction mixture was stirred for 16 h at room temperature. The reaction mixture was poured into water and extracted with dichloromethane. The combined organic layers were dried over sodium sulphate and the excess solvent was removed in vacuo. The obtained crude was purified via column chromatography on silica gel (starting from 100% dichloromethane to 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dichloromethane/methanol), affording 2-tert-butylamino-6-ethynylthieno[3,2-d]pyrimidin-4(3H)-one as a grey powder (150 mg, 92% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.60 (s, 1H), 7.27 (s, 1H), 6.13 (s, 1H), 4.87 (s, 1H), 1.39 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 158.6, 157.1, 152.4, 129.7, 127.7, 113.2, 88.5, 76.6, 51.1, 28.5 (3C). Mp = 195–197 °C. HRMS (ESI) m/z calculated for C12H14N3OS [M + H]+ 248.0852, found 248.0854.
3 dichloromethane/methanol), affording 2-tert-butylamino-6-ethynylthieno[3,2-d]pyrimidin-4(3H)-one as a grey powder (150 mg, 92% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.60 (s, 1H), 7.27 (s, 1H), 6.13 (s, 1H), 4.87 (s, 1H), 1.39 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 158.6, 157.1, 152.4, 129.7, 127.7, 113.2, 88.5, 76.6, 51.1, 28.5 (3C). Mp = 195–197 °C. HRMS (ESI) m/z calculated for C12H14N3OS [M + H]+ 248.0852, found 248.0854.
2-tert-Butylamino-6-[3-(4-methylpiperazin-1-yl)prop-1-yn-1-yl]thieno[3,2-d]pyrimidin-4(3H)-one (2g). Following procedure B with 6-bromo-2-(tert-butylamino)thieno[3,2-d]pyrimidin-4(3H)-one (0.3 g, 0.99 mmol) and 1-methyl-4-(prop-2-yn-1-yl)piperazine (0.36 g, 2.60 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol), affording 2-tert-butylamino-6-[3-(4-methylpiperazin-1-yl)prop-1-yn-1-yl]thieno[3,2-d]pyrimidin-4(3H)-one as an orange solid (135 mg, 35% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.58 (bs, 1H), 7.18 (s, 1H), 6.13 (s, 1H), 3.57 (s, 2H), 2.34 (bs, 4H), 2.16 (s, 3H), 1.39 (s, 9H), 4H presumably situated below DMSO signal. 13C NMR (DMSO-d6, 100 MHz) δ 158.8, 157.1, 152.4, 128.7, 128.6, 112.7, 93.7, 77.9, 54.5, 51.2 (2C), 51.1 (2C), 46.8, 45.6, 28.5 (3C). Mp = 109–111 °C. HRMS (ESI) m/z calculated for C18H26N5OS [M + H]+ 360.1853, found 360.1853.
6-(phenylethynyl)-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one (2h). Following procedure B with 6-bromo-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one (0.3 g, 1.04 mmol) and ethynylbenzene (137.2 μL, 1.25 mmol), the obtained crude was triturated in diethyl ether, affording 6-(phenylethynyl)-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one as a brown solid (285 mg, 85% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.94 (bs, 1H), 7.64–7.56 (m, 2H), 7.51–7.42 (m, 3H), 7.32 (s, 1H), 6.41 (bs, 1H), 4.08–3.95 (m, 1H), 1.17 (d, 3J = 6.4 Hz, 6H). 13C NMR (DMSO-d6, 100 MHz) δ 159.1, 157.3, 153.1, 131.5 (2C), 129.7, 128.9 (2C), 128.7, 128.2, 121.0, 113.4, 96.0, 82.4, 42.1, 20.8 (2C). Mp = 276–278 °C. HRMS (ESI) m/z calculated for C17H16N3OS [M + H]+ 310.1009, found 310.1003.
•If a precipitate was formed, this precipitate was filtered, washed with water, and dried in vacuo. If needed, the precipitate was then purified with the appropriate method.
•If no precipitate was formed, the mixture was extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulphate, and the excess solvent was removed in vacuo. Then, the obtained crude was purified with the appropriate method.
Methyl-3-amino-5-[4-(methoxycarbonyl)phenyl]thiophene-2-carboxylate (3a). Following procedure C starting from methyl-3-aminothiophene-2-carboxylate (0.5 g, 2.12 mmol) and 4-methoxycarbonylphenylboronic acid (0.610, 3.39 mmol) with a reaction time of 6 h, the obtained crude was purified via column chromatography on silica gel (deactivated with 3% triethylamine using 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 cyclohexane/dichloromethane), affording methyl 3-amino-5-[4-(methoxycarbonyl)phenyl]thiophene-2-carboxylate as a yellow solid (340 mg, 55% yield). 1H NMR (CDCl3, 250 MHz) δ 8.05 (d, 3J = 8.5 Hz, 2H), 7.64 (d, 3J = 8.5 Hz, 2H), 6.86 (s, 1H), 5.49 (bs, 2H), 3.93 (s, 3H), 3.85 (s, 3H). 13C NMR (CDCl3, 62.5 MHz) δ 166.6, 164.9, 154.3, 147.5, 137.6, 130.4 (2C), 130.3, 125.9 (2C), 116.7, 101.7, 52.4, 51.6. Mp = 190–192 °C. HRMS (ESI) m/z calculated for C14H14NO4S [M + H]+ 292.0638, found 292.0637.
20 cyclohexane/dichloromethane), affording methyl 3-amino-5-[4-(methoxycarbonyl)phenyl]thiophene-2-carboxylate as a yellow solid (340 mg, 55% yield). 1H NMR (CDCl3, 250 MHz) δ 8.05 (d, 3J = 8.5 Hz, 2H), 7.64 (d, 3J = 8.5 Hz, 2H), 6.86 (s, 1H), 5.49 (bs, 2H), 3.93 (s, 3H), 3.85 (s, 3H). 13C NMR (CDCl3, 62.5 MHz) δ 166.6, 164.9, 154.3, 147.5, 137.6, 130.4 (2C), 130.3, 125.9 (2C), 116.7, 101.7, 52.4, 51.6. Mp = 190–192 °C. HRMS (ESI) m/z calculated for C14H14NO4S [M + H]+ 292.0638, found 292.0637.
Methyl-3-amino-5-(4-nitrophenyl)thiophene-2-carboxylate (3b). Following procedure C starting from methyl-3-aminothiophene-2-carboxylate (0.4 g, 1.69 mmol) and 4-nitrophenylboronic acid (0.453 g, 2.71 mmol) with a reaction time of 18 h, the obtained crude was purified via column chromatography on silica gel (starting from 75
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 cyclohexane/ethyl acetate to 50
25 cyclohexane/ethyl acetate to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 cyclohexane/ethyl acetate), affording methyl-3-amino-5-(4-nitrophenyl)thiophene-2-carboxylate as an orange solid (316 mg, 65% yield), which was used without further purification. 1H NMR (DMSO-d6, 250 MHz) δ 8.32–8.23 (m, 2H), 7.95–7.87 (m, 2H), 7.18 (s, 1H), 6.68 (s, 2H), 3.76 (s, 3H). 13C NMR (DMSO-d6, 75 MHz) δ 163.8, 155.3, 147.2, 144.3, 138.8, 126.6 (2C), 124.5 (2C), 118.7, 98.8, 51.2. Mp = 212–214 °C.
50 cyclohexane/ethyl acetate), affording methyl-3-amino-5-(4-nitrophenyl)thiophene-2-carboxylate as an orange solid (316 mg, 65% yield), which was used without further purification. 1H NMR (DMSO-d6, 250 MHz) δ 8.32–8.23 (m, 2H), 7.95–7.87 (m, 2H), 7.18 (s, 1H), 6.68 (s, 2H), 3.76 (s, 3H). 13C NMR (DMSO-d6, 75 MHz) δ 163.8, 155.3, 147.2, 144.3, 138.8, 126.6 (2C), 124.5 (2C), 118.7, 98.8, 51.2. Mp = 212–214 °C.
Methyl-3-amino-5-(4-hydroxyphenyl)thiophene-2-carboxylate (3c). Following procedure C starting from methyl-3-aminothiophene-2-carboxylate (0.8 g, 3.39 mmol) and 4-hydroxyphenylboronic acid (0.748 g, 5.42 mmol) with a reaction time of 24 h, the obtained crude was purified via flash chromatography (using cyclohexane and ethyl acetate), affording methyl-3-amino-5-(4-hydroxyphenyl)thiophene-2-carboxylate as a light brown solid (540 mg, 64% yield). 1H NMR (DMSO-d6, 400 MHz) δ 9.86 (s, 1H), 7.45 (d, 3J = 8.6 Hz, 2H), 6.82 (d, 3J = 8.6 Hz, 2H), 6.79 (s, 1H), 6.54 (bs, 2H), 3.71 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ 164.0, 158.6, 155.8, 148.4, 127.1 (2C), 123.8, 115.9 (2C), 114.3, 95.3, 50.8. Mp = 175–177 °C. HRMS (ESI) m/z calculated for C12H11NO3SNa [M + Na]+ 272.0352, found 272.0352.
Methyl-3-amino-5-(3-chloro-4-fluorophenyl)thiophene-2-carboxylate (3d). Following procedure C starting from methyl-3-aminothiophene-2-carboxylate (0.8 g, 3.39 mmol) and (3-chloro-4-fluorophenyl)boronic acid (0.945 g, 5.42 mmol) with a reaction time of 24 h, the obtained crude was purified via column chromatography on silica gel (starting from 100% cyclohexane to 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 cyclohexane/ethyl acetate), affording methyl-3-amino-5-(3-chloro-4-fluorophenyl)thiophene-2-carboxylate as a beige solid (790 mg, 82% yield). 1H NMR (DMSO-d6, 400 MHz) δ 7.61 (dd, 3J = 6.8 Hz, 4J = 2.3 Hz, 1H), 7.42 (ddd, 3J = 8.6 Hz, 4J = 4.4 Hz, 4J = 2.3 Hz, 1H), 7.15 (t, 3J = 8.6 Hz, 1H), 6.71 (s, 1H), 3.85 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ 164.9, 158.5 (d, 1J = 252 Hz, 1C), 154.3, 146.4, 130.9 (d, 3J = 4.3 Hz, 1C), 128.6, 128.3, 125.9 (d, 3J = 7.3 Hz, 1C), 121.9 (d, 2J = 18.2 Hz, 1C), 117.3 (d, 2J = 21.7 Hz, 1C), 116.2, 51.5. Mp = 161–163 °C. HRMS (ESI) m/z calculated for C12H10ClFNO2S [M + H]+ 286.0099, found 286.0102.
20 cyclohexane/ethyl acetate), affording methyl-3-amino-5-(3-chloro-4-fluorophenyl)thiophene-2-carboxylate as a beige solid (790 mg, 82% yield). 1H NMR (DMSO-d6, 400 MHz) δ 7.61 (dd, 3J = 6.8 Hz, 4J = 2.3 Hz, 1H), 7.42 (ddd, 3J = 8.6 Hz, 4J = 4.4 Hz, 4J = 2.3 Hz, 1H), 7.15 (t, 3J = 8.6 Hz, 1H), 6.71 (s, 1H), 3.85 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ 164.9, 158.5 (d, 1J = 252 Hz, 1C), 154.3, 146.4, 130.9 (d, 3J = 4.3 Hz, 1C), 128.6, 128.3, 125.9 (d, 3J = 7.3 Hz, 1C), 121.9 (d, 2J = 18.2 Hz, 1C), 117.3 (d, 2J = 21.7 Hz, 1C), 116.2, 51.5. Mp = 161–163 °C. HRMS (ESI) m/z calculated for C12H10ClFNO2S [M + H]+ 286.0099, found 286.0102.
Methyl-3-amino-5-[4-(morpholin-4-yl)phenyl]thiophene-2-carboxylate (3e). Following procedure C starting from methyl 3-aminothiophene-2-carboxylate (0.4 g, 1.69 mmol) and (4-(morpholin-4-yl)phenyl)boronic acid (0.561 g, 2.71 mmol) with a reaction time of 16 h, the obtained crude was purified via flash chromatography (using cyclohexane/ethyl acetate). Fractions of interest were combined and triturated in diethyl ether, affording methyl-3-amino-5-[4-(morpholin-4-yl)phenyl]thiophene-2-carboxylate as a yellow powder (0.395 g, 73% yield). 1H NMR (CDCl3, 400 MHz) δ 7.50 (d, 3J = 8.8 Hz, 2H), 6.89 (d, 3J = 8.8 Hz, 2H), 6.56 (s, 1H), 5.46 (bs, 2H), 3.90–3.85 (m, 4H), 3.83 (s, 3H), 3.25–3.17 (m, 4H). 13C NMR (CDCl3, 100 MHz) δ 165.1, 154.7, 151.7, 149.6, 127.1 (2C), 124.9, 115.4 (2C), 114.1, 99.4, 66.8, 51.3 (2C), 48.8 (2C). Mp = 208–210 °C. HRMS (ESI) m/z calculated for C16H19NO3S [M + H]+ 319.1111, found 319.1110.
Methyl-3-amino-5-[4-(4-methylpiperazin-1-yl)phenyl]thiophene-2-carboxylate (3f). Following procedure C starting from methyl-3-aminothiophene-2-carboxylate (0.4 g, 1.69 mmol) and (4-(4-methylpiperazin-1-yl)phenyl)boronic acid (0.597 g, 2.71 mmol) with a reaction time of 16 h, the obtained crude was purified via flash chromatography (using dichloromethane and methanol). Fractions of interest were combined and triturated in diethyl ether affording methyl-3-amino-5-[4-(4-methylpiperazin-1-yl)phenyl]thiophene-2-carboxylate as a yellow powder (0.4 g, 71% yield). 1H NMR (CDCl3, 400 MHz) δ 7.47 (d, 3J = 8.8 Hz, 2H), 6.89 (d, 3J = 8.8 Hz, 2H), 6.64 (s, 1H), 5.46 (s, 2H), 3.83 (s, 3H), 3.30–3.23 (m, 4H), 2.60–2.53 (m, 4H), 2.35 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ 165.1, 154.7, 151.7, 149.8, 127.0 (2C), 124.3, 115.5 (2C), 113.9, 99.1, 55.0 (2C), 51.3, 48.4 (2C), 46.3. Mp = 191–193 °C. HRMS (ESI) m/z calculated for C17H22N3O2S [M + H]+ 332.1427, found 332.1427.
Methyl-3-amino-5-[4-(piperidin-1-yl)phenyl]thiophene-2-carboxylate (3g). Following procedure C starting from methyl 3-aminothiophene-2-carboxylate (0.4 g, 1.69 mmol) and (4-(piperidin-1-yl)phenyl)boronic acid (0.556 g, 2.71 mmol) with a reaction time of 16 h, the obtained crude was purified via flash chromatography (using cyclohexane/ethyl acetate). Fractions of interest were combined and triturated in diethyl ether affording methyl-3-amino-5-[4-(piperidin-1-yl)phenyl]thiophene-2-carboxylate as a yellow powder (0.38 g, 71% yield). 1H NMR (CDCl3, 400 MHz) δ 7.46 (d, 3J = 8.8 Hz, 2H), 6.89 (d, 3J = 8.8 Hz, 2H), 6.63 (s, 1H), 5.45 (s, 2H), 3.83 (s, 3H), 3.29–3.18 (m, 4H), 1.76–1.53 (m, 6H). 13C NMR (CDCl3, 100 MHz) δ 165.2, 154.8, 152.4, 150.1, 127.0 (2C), 123.5, 115.7 (2C), 113.6, 98.9, 51.3 (2C), 49.8, 25.7 (2C), 24.4. Mp = 191–193 °C. HRMS (ESI) m/z calculated for C17H21N2O2S [M + H]+ 317.1318, found 317.1319.
Methyl-3-amino-5-[4-(methylsulfanyl)phenyl]thiophene-2-carboxylate (3h). Following procedure C starting from methyl-3-aminothiophene-2-carboxylate (0.8 g, 3.39 mmol) and [4-(methylsulfanyl)phenyl]boronic acid (0.911 g, 5.42 mmol) with a reaction time of 24 h, the obtained crude was purified via flash chromatography (using cyclohexane/ethyl acetate), affording methyl-3-amino-5-[4-(methylsulfanyl)phenyl]thiophene-2-carboxylate as a pale-yellow solid (0.678 g, 72% yield). 1H NMR (CDCl3, 400 MHz) δ 7.52–7.46 (m, 2H), 7.26–7.21 (m, 2H), 6.73 (s, 1H), 3.84 (s, 3H), 2.50 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ 165.1, 154.5, 148.8, 140.2, 130.1, 126.6 (2C), 126.3 (2C), 115.2, 100.2, 51.4, 15.6. Mp = 173–175 °C. HRMS (ESI) m/z calculated for C13H14NO2S2 [M + H]+ 280.0460, found 280.0460.
Methyl-3-amino-5-[4-(methylsulfonyl)phenyl]thiophene-2-carboxylate (3i). Following procedure C starting from methyl 3-aminothiophene-2-carboxylate (0.4 g, 1.69 mmol) and [4-(methylsulfonyl)phenyl]boronic acid (0.542 g, 2.71 mmol) with a reaction time of 4 h, the obtained crude was purified via flash chromatography (using cyclohexane/ethyl acetate), affording methyl-3-amino-5-[4-(methylsulfonyl)phenyl]thiophene-2-carboxylate as a yellow solid (0.325 g, 62% yield). 1H NMR (CDCl3, 400 MHz) δ 7.96 (d, 3J = 8.5 Hz, 2H), 7.75 (d, 3J = 8.5 Hz, 2H), 6.88 (s, 1H), 3.86 (s, 3H), 3.07 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ 164.8, 154.2, 146.1, 140.4, 138.8, 128.4 (2C), 126.7 (2C), 117.4, 102.4, 51.6, 44.7. Mp = 213–214 °C. HRMS (ESI) m/z calculated for C14H14NO4S2 [M + H]+ 312.0359, found 312.0359.
Methyl-3-amino-5-(4-sulfamoylphenyl)thiophene-2-carboxylate (3j). Following procedure C starting from methyl 3-aminothiophene-2-carboxylate (0.4 g, 1.69 mmol) and (4-sulfamoylphenyl)boronic acid (0.545, 2.71 mmol) with a reaction time of 16 h, the obtained crude was purified via flash chromatography (using cyclohexane/ethyl acetate), affording methyl-3-amino-5-(4-sulfamoylphenyl)thiophene-2-carboxylate as a yellow powder (0.249 g, 47%), which was used without further purification. 1H NMR (DMSO-d6, 400 MHz) δ 7.90–7.79 (m, 4H), 7.44 (s, 2H), 7.09 (s, 1H), 6.64 (s, 2H), 3.75 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ 163.8, 155.4, 145.5, 144.1, 135.7, 126.6 (2C), 126.0 (2C), 117.6, 97.7, 51.1. Mp = 225–227 °C. HRMS (ESI) m/z calculated for C12H13N2O4S2 [M + H]+ 313.0311, found 313.0312.
Methyl-3-amino-5-[4-(morpholin-4-ylsulfonyl)phenyl]thiophene-2-carboxylate (3k). Following procedure C starting from methyl 3-aminothiophene-2-carboxylate (0.4 g, 1.69 mmol) and [4-(morpholinosulfonyl)phenyl]boronic acid (0.734 g, 2.71 mmol) with a reaction time of 24 h, the obtained crude was purified via column chromatography on silica gel (starting from 50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) (cyclohexane/ethyl acetate to 100% ethyl acetate), affording methyl-3-amino-5-[4-(morpholin-4-ylsulfonyl)phenyl]thiophene-2-carboxylate as an orange powder (570 mg, 88% yield). 1H NMR (DMSO-d6, 400 MHz) δ 7.90 (d, 3J = 8.5 Hz, 2H), 7.78 (d, 3J = 8.5 Hz, 2H), 7.15 (s, 1H), 6.65 (s, 2H), 3.75 (s, 3H), 3.67–3.60 (m, 4H), 2.94–2.86 (m, 4H). 13C NMR (DMSO-d6, 100 MHz) δ 163.8, 155.3, 145.0, 137.2, 134.4, 128.6 (2C), 126.4 (2C), 118.1, 98.2, 65.3 (2C), 51.2, 45.9 (2C). Mp = 272–274 °C. HRMS (ESI) m/z calculated for C16H19N2O5S2 [M + H]+ 405.0549, found 405.0546.
50) (cyclohexane/ethyl acetate to 100% ethyl acetate), affording methyl-3-amino-5-[4-(morpholin-4-ylsulfonyl)phenyl]thiophene-2-carboxylate as an orange powder (570 mg, 88% yield). 1H NMR (DMSO-d6, 400 MHz) δ 7.90 (d, 3J = 8.5 Hz, 2H), 7.78 (d, 3J = 8.5 Hz, 2H), 7.15 (s, 1H), 6.65 (s, 2H), 3.75 (s, 3H), 3.67–3.60 (m, 4H), 2.94–2.86 (m, 4H). 13C NMR (DMSO-d6, 100 MHz) δ 163.8, 155.3, 145.0, 137.2, 134.4, 128.6 (2C), 126.4 (2C), 118.1, 98.2, 65.3 (2C), 51.2, 45.9 (2C). Mp = 272–274 °C. HRMS (ESI) m/z calculated for C16H19N2O5S2 [M + H]+ 405.0549, found 405.0546.
Methyl-3-amino-5-(2-chloropyrimidin-5-yl)thiophene-2-carboxylate (3l). To a solution of methyl-3-aminothiophene-2-carboxylate (0.5 g, 2.12 mmol) in dioxane (3.75 mL), (2-chloropyrimidin-5-yl)boronic acid (0.537 g, 3.39 mmol) and potassium carbonate (0.585 g, 4.24 mmol) were added and the solution was deoxygenated with nitrogen during 5 min. Tetrakis(triphenylphosphine)palladium(0) (0.05% mol) was added, the mixture was put under nitrogen atmosphere, and stirred at 85 °C for 24 h. The reaction mixture was poured into water (75 mL) and extracted with ethyl acetate (3 × 75 mL). The combined organic phases were washed with brine (3 × 150 mL), dried over sodium sulphate, and the excess solvent was removed in vacuo. The obtained crude was purified via column chromatography on silica gel (using 100% dichloromethane), affording methyl 3-amino-5-(2-chloropyrimidin-5-yl)thiophene-2-carboxylate as a yellow powder (0.25 g, 44% yield). 1H NMR (CDCl3, 400 MHz) δ 8.79 (s, 2H), 6.84 (s, 1H), 5.55 (bs, 2H), 3.86 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ 164.6, 161.0, 156.2 (2C), 154.1, 139.2, 126.7, 117.7, 102.6, 51.8. Mp = 176–178 °C. HRMS (ESI) m/z calculated for C10H9ClN3O2S [M + H]+ 270.0099, found 270.0097.
Methyl-3-amino-5-[2-(morpholin-4-yl)pyrimidin-5-yl]thiophene-2-carboxylate (3m). To a solution of methyl-3-amino-5-(2-chloropyrimidin-5-yl)thiophene-2-carboxylate (0.25 g, 0.93 mmol) in ethanol (10 mL), morpholine (77.3 μL, 0.88 mmol) and triethylamine (0.33 mL, 2.38 mmol) were added. The reaction mixture was stirred for 3 h at 80 °C. After being allowed to cool to room temperature, the reaction mixture was filtered and the obtained precipitate was washed with ethanol, brine, and water, affording methyl 3-amino-5-[2-(morpholin-4-yl)pyrimidin-5-yl]thiophene-2-carboxylate as a white solid (0.225 g, 80% yield). 1H NMR (DMSO-d6, 400 MHz) δ 8.66 (s, 2H), 6.88 (s, 1H), 6.60 (s, 2H), 3.79–3.74 (m, 4H), 3.72 (s, 3H), 3.69–3.64 (m, 4H). 13C NMR (DMSO-d6, 100 MHz) δ 163.8, 160.7, 155.6, 155.0 (2C), 142.4, 116.2, 114.9, 95.6, 65.9 (2C), 50.9, 44.0 (2C). Mp = 170–172 °C. HRMS (ESI) m/z calculated for C14H17N4O3S [M + H]+ 321.1016, found 321.1017.
Methyl-3-amino-5-[2-(4-methylpiperazin-1-yl)pyrimidin-5-yl]thiophene-2-carboxylate (3n). To a solution of methyl-3-amino-5-(2-chloropyrimidin-5-yl)thiophene-2-carboxylate (0.25 g, 0.93 mmol) in ethanol (10 mL), N-methylpyperazine (97.4 μL, 0.88 mmol) and triethylamine (0.33 mL, 2.38 mmol) were added. The reaction mixture was stirred for 16 h at 80 °C. The reaction mixture was poured into water (100 mL) and extracted with ethyl acetate (3 × 100 mL). The combined organic layers were washed with water (2 × 200 mL), dried over sodium sulphate, and the excess solvent was removed in vacuo, affording methyl-3-amino-5-[2-(4-methylpiperazin-1-yl)pyrimidin-5-yl]thiophene-2-carboxylate as a yellow powder (215 mg, 73% yield), which was used without further purification. 1H NMR (DMSO-d6, 400 MHz) δ 8.63 (s, 2H), 6.87 (s, 1H), 6.60 (s, 2H), 3.82–3.75 (m, 4H), 3.72 (s, 3H), 2.41–2.34 (m, 4H), 2.22 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ 164.3, 161.1, 156.1, 155.5 (2C), 143.0, 116.4, 115.2, 95.9, 54.7 (2C), 51.4, 46.2, 43.8 (2C). Mp = 148–150 °C. HRMS (ESI) m/z calculated for C15H20N5O2S [M + H]+ 334.1332, found 334.1331.
•If a precipitate was formed, this precipitate was filtered, washed with water, and dried in vacuo. If needed, the precipitate was then purified with the appropriate method.
•If no precipitate was obtained, the mixture was extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulphate, and the excess solvent was removed in vacuo. Then, the obtained crude was purified with the appropriate method.
6-Bromo-2-(tert-butylamino)thieno[3,2-d]pyrimidin-4(3H)-one (1). Following procedure D with methyl-3-amino-5-bromothiophene-2-carboxylate (2 g, 8.47 mmol) and tert-butylamine (1.78 mL, 16.9 mmol), the obtained crude was triturated in acetonitrile, affording 6-Bromo-2-(tert-butylamino)thieno[3,2-d]pyrimidin-4(3H)-one as a beige powder (1.3 g, 51% yield). 1H NMR (DMSO-d6, 250 MHz) δ 10.53 (bs, 1H), 7.23 (s, 1H), 6.12 (s, 1H), 1.39 (s, 9H). 13C NMR (DMSO-d6, 62.5 MHz) δ 159.3, 156.5, 152.4, 127.8, 121.9, 113.4, 51.1, 28.5 (3C). Mp: degradation observed at 162–164 °C. HRMS (ESI) m/z calculated for C10H13BrN3OS [M + H]+ 301.9957, found 301.9954.
6-Bromo-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one (3). Following procedure D with methyl 3-amino-5-bromothiophene-2-carboxylate (1 g, 4.24 mmol) and with isopropylamine (0.728 mL, 8.47 mmol), the obtained crude was triturated in acetonitrile. The precipitate was isolated and the filtrate was further purified via flash chromatography (using dichloromethane and methanol), affording 6-bromo-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one as a beige powder (0.695 g, 57% yield). 1H NMR (DMSO-d6, 250 MHz) δ 10.72 (bs, 1H), 7.25 (s, 1H), 6.21 (d, 3J = 7.6 Hz, 1H), 3.99 (m, 1H), 1.16 (d, 3J = 6.5 Hz, 6H). 13C NMR (DMSO-d6, 62.5 MHz) δ 161.2, 156.7, 152.9, 127.5, 122.0, 113.3, 42.2, 22.3 (2C). Mp = 240–242 °C. HRMS (ESI) m/z calculated for C9H11BrN3OS [M + H]+ 287.9801, found 287.9801.
Methyl-4-(2-tert-butylamino-4-oxo-3,4-dihydrothieno[3,2-d]pyrimidin-6-yl)benzoate (4a). Following procedure D with methyl-3-amino-5-(4-(methoxycarbonyl)phenyl)thiophene-2-carboxylate (0.2 g, 0.69 mmol) and with tert-butylamine (144 μL, 1.37 mmol), the obtained crude was triturated in methanol, affording methyl-4-(2-tert-butylamino-4-oxo-3,4-dihydrothieno[3,2-d]pyrimidin-6-yl)benzoate as a beige powder (0.134 mg, 55% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.54 (bs, 1H), 8.01 (d, 3J = 8.5 Hz, 2H), 7.94 (d, 3J = 8.5 Hz, 2H), 7.66 (s, 1H), 6.12 (s, 1H), 3.87 (s, 3H), 1.42 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 165.7, 160.0, 157.6, 152.3, 148.1, 137.2, 130.0 (2C), 129.7, 126.1 (2C), 122.3, 112.6, 52.3, 51.0, 28.6 (3C). Mp > 300 °C. HRMS (ESI) m/z calculated for C18H20N3O3S [M + H]+ 358.1220, found 358.1216.
2-tert-Butylamino-6-(4-nitrophenyl)thieno[3,2-d]pyrimidin-4(3H)-one (4b). Following procedure D with methyl 3-amino-5-(4-nitrophenyl)thiophene-2-carboxylate (0.41 g, 1.47 mmol) and with tert-butylamine (313 μL, 2.95 mmol), the obtained crude was purified via column chromatography on silica gel (starting from 60
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to 50
40 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 cyclohexane/ethyl acetate), affording 2-tert-butylamino-6-(4-nitrophenyl)thieno[3,2-d]pyrimidin-4(3H)-one as an orange solid (0.277 g, 55% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.56 (s, 1H), 8.26 (d, 3J = 8.8 Hz, 2H), 8.06 (d, 3J = 9.2 Hz, 2H), 7.76 (s, 1H), 6.13 (s, 1H), 1.43 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 159.9, 157.5, 152.3, 147.2, 146.7, 139.1, 126.8 (2C), 124.3 (2C), 123.5, 113.5, 51.0, 28.5 (3C). Mp > 300 °C. HRMS (ESI) m/z calculated for C16H17N4O3S [M + H]+ 345.1016, found 345.1016.
50 cyclohexane/ethyl acetate), affording 2-tert-butylamino-6-(4-nitrophenyl)thieno[3,2-d]pyrimidin-4(3H)-one as an orange solid (0.277 g, 55% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.56 (s, 1H), 8.26 (d, 3J = 8.8 Hz, 2H), 8.06 (d, 3J = 9.2 Hz, 2H), 7.76 (s, 1H), 6.13 (s, 1H), 1.43 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 159.9, 157.5, 152.3, 147.2, 146.7, 139.1, 126.8 (2C), 124.3 (2C), 123.5, 113.5, 51.0, 28.5 (3C). Mp > 300 °C. HRMS (ESI) m/z calculated for C16H17N4O3S [M + H]+ 345.1016, found 345.1016.
6-(4-nitrophenyl)-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one (4c). Following procedure D with methyl 3-amino-5-(4-nitrophenyl)thiophene-2-carboxylate (0.4 g, 1.44 mmol) and with isopropylamine (244 μL, 2.88 mmol), the obtained crude was purified via column chromatography on silica gel (staring from 70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 to 50
30 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 dichloromethane/ethyl acetate), affording 6-(4-nitrophenyl)-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one as an orange solid (0.156 g, 33% crude yield), which was used without further purifications. 1H NMR (DMSO-d6, 400 MHz) δ 10.79 (s, 1H), 8.31–8.25 (m, 2H), 8.09–8.02 (m, 2H), 7.79 (s, 1H), 6.26 (d, 3J = 7.1 Hz, 1H), 4.08–3.98 (m, 1H), 1.19 (d, 3J = 6.5 Hz, 6H).
50 dichloromethane/ethyl acetate), affording 6-(4-nitrophenyl)-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one as an orange solid (0.156 g, 33% crude yield), which was used without further purifications. 1H NMR (DMSO-d6, 400 MHz) δ 10.79 (s, 1H), 8.31–8.25 (m, 2H), 8.09–8.02 (m, 2H), 7.79 (s, 1H), 6.26 (d, 3J = 7.1 Hz, 1H), 4.08–3.98 (m, 1H), 1.19 (d, 3J = 6.5 Hz, 6H).
2-(tert-butylamino)-6-(4-hydroxyphenyl)thieno[3,2-d]pyrimidin-4(3H)-one (4d). Following procedure D with methyl-3-amino-5-(4-hydroxyphenyl)thiophene-2-carboxylate (0.44 g, 1.77 mmol) and with tert-butylamine (371 μL, 3.53 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol), affording 2-(tert-butylamino)-6-(4-hydroxyphenyl)thieno[3,2-d]pyrimidin-4(3H)-one as a light brown solid (70 mg, 13%). 1H NMR (DMSO-d6, 400 MHz) δ δ 10.39 (bs, 1H), 9.89 (bs, 1H), 7.61 (d, 3J = 8.7 Hz, 2H), 7.30 (s, 1H), 6.83 (d, 3J = 8.7 Hz, 2H), 6.05 (s, 1H), 1.42 (s, 3H). 13C NMR (DMSO-d6, 100 MHz) δ 160.9, 159.1, 157.9, 152.6, 151.2, 127.9 (2C), 124.5, 118.9, 116.4 (2C), 110.6, 51.4, 29.1. Mp = 276–278 °C. HRMS (ESI) m/z calculated for C16H18N3O2S [M + H]+ 316.1120, found 316.1123.
2-tert-Butylamino-6-(3-chloro-4-fluorophenyl)thieno[3,2-d]pyrimidin-4(3H)-one (4e). Following procedure D with methyl-3-amino-5-(3-chloro-4-fluorophenyl)thiophene-2-carboxylate (0.3 g, 1.05 mmol) and tert-butylamine (220 μL, 2.1 mmol), the obtained crude was triturated in diethyl ether, then purified via flash chromatography (using dichloromethane and methanol), affording 2-tert-butylamino-6-(3-chloro-4-fluorophenyl)thieno[3,2-d]pyrimidin-4(3H)-one as a white powder (180 mg, 49% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.51 (s, 1H), 8.07 (dd, 4J = 7 Hz, 4J = 2.4 Hz, 1H), 7.79 (ddd, 3J = 8.6 Hz, 4J = 4.6 Hz, 4J = 2.4 Hz, 1H), 7.61 (s, 1H), 7.50 (t, 3J = 9 Hz, 1H), 6.11 (s, 1H), 1.42 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.1, 157.5 (d, 1J = 250 Hz, 1C), 157.5, 152.3, 147.0, 130.9 (d, 3J = 3.7 Hz, 1C), 127.9, 126.7 (d, 3J = 7.5 Hz, 1C), 121.9, 120.6 (d, 2J = 18.1 Hz, 1C), 117.7 (d, 3J = 21.6, 1C), 112.1, 51.0, 28.6 (3C). Mp = 259–261 °C. HRMS (ESI) m/z calculated for C16H16ClFN3OS [M + H]+ 352.0681, found 352.0681.
2-(Propan-2-ylamino)-6-(3-chloro-4-fluorophenyl)thieno[3,2-d]pyrimidin-4(3H)-one (4f). Following procedure D with methyl-3-amino-5-(3-chloro-4-fluorophenyl)thiophene-2-carboxylate (0.3 g, 1.05 mmol) and isopropylamine (180 μL, 2.1 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol). Fractions of interest were triturated in diethyl ether, affording 2-(propan-2-ylamino)-6-(3-chloro-4-fluorophenyl)thieno[3,2-d]pyrimidin-4(3H)-one as a grey solid (130 mg, 37% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.67 (s, 1H), 8.04 (dd, 4J = 7 Hz, 4J = 2.4 Hz, 1H), 7.78 (ddd, 3J = 8.7 Hz, 4J = 4.6 Hz, 4J = 2.4 Hz, 1H), 7.63 (s, 1H), 7.51 (t, 3J = 8.9 Hz, 1H), 6.15 (d, 3J = 7.5 Hz, 1H), 4.09–3.94 (m, 1H), 1.18 (d, 3J = 6.5 Hz, 6H). 13C NMR (DMSO-d6, 100 MHz) δ 160.4, 157.6, 157.5 (d, 1J = 250 Hz, 1C), 152.7, 147.1, 131.0 (d, 3J = 3.9 Hz, 1C), 127.9, 126.7 (d, 3J = 7.5 Hz, 1C), 121.6, 120.6 (d, 2J = 18.1 Hz, 1C), 117.7 (d, 2J = 21.5 Hz, 1C), 112.0, 42.2, 22.4 (2C). Mp > 300 °C. HRMS (ESI) m/z calculated for C15H14ClFN3OS [M + H]+ 338.0525, found 338.0522.
2-tert-Butylamino-6-[4-(morpholin-4-yl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one (4g). Following procedure D with methyl 3-amino-5-(4-(morpholin-4-yl)phenyl)thiophene-2-carboxylate (0.36 g, 1.13 mmol) and tert-butylamine (238 μL, 2.26 mmol), the obtained crude was triturated in acetonitrile, affording 2-tert-butylamino-6-[4-(morpholin-4-yl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one as a pale-yellow powder (0.3 g, 69% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.41 (s, 1H), 7.64 (d, 3J = 8.4 Hz, 2H), 7.33 (s, 1H), 6.99 (d, 3J = 8.4 Hz, 2H), 6.07 (s, 1H), 3.74 (s, 4H), 3.18 (s, 4H), 1.42 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.5, 157.5, 152.2, 151.6, 150.7, 126.8 (2C), 123.4, 118.2, 114.8 (2C), 110.0, 65.9 (2C), 50.9, 47.6 (2C), 28.6 (3C). Mp > 300 °C. HRMS (ESI) m/z calculated for C20H25N4O2S [M + H]+ 385.1683, found 385.1689.
2-tert-Butylamino-6-[4-(4-methylpiperazin-1-yl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one (4h). Following procedure D with methyl-3-amino-5-(4-(4-methylpiperazin-1-yl)phenyl)thiophene-2-carboxylate (0.325 g, 0.98 mmol) and tert-butylamine (200 μL, 1.96 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol), affording 2-tert-butylamino-6-[4-(4-methylpiperazin-1-yl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one as a yellow powder (90 mg, 23% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.39 (s, 1H), 7.62 (d, 3J = 8.8 Hz, 2H), 7.31 (s, 1H), 6.97 (d, 3J = 9.2 Hz, 2H), 6.07 (s, 1H), 3.27–3.18 (m, 4H), 2.47–2.41 (m, 4H), 2.22 (s, 3H), 1.41 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.5, 157.5, 152.2, 151.4, 150.8, 126.8 (2C), 122.9, 118.1, 114.9 (2C), 109.9, 54.4 (2C), 50.9, 47.2 (2C), 45.7, 28.6 (3C). Mp = 282–284 °C. HRMS (ESI) m/z calculated for C21H28N5OS [M + H]+ 398.2009, found 398.2006.
2-tert-Butylamino-6-[4-(piperidin-1-yl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one (4i). Following procedure D with methyl-3-amino-5-(4-(piperidin-1-yl)phenyl)thiophene-2-carboxylate (0.350 g, 1.11 mmol) and tert-butylamine (232 μL, 2.21 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol), affording 2-tert-butylamino-6-[4-(piperidin-1-yl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one as a yellow powder (216 mg, 51% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.37 (s, 1H), 7.60 (d, 3J = 8.8 Hz, 2H), 7.28 (s, 1H), 6.95 (d, 3J = 8.8 Hz, 2H), 6.04 (s, 1H), 3.29–3.20 (m, 4H), 1.64–1.51 (m, 6H), 1.45–1.36 (m, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.5, 157.5, 152.2, 151.7, 150.9, 126.8 (2C), 122.2, 117.8, 115.0 (2C), 109.7, 50.9, 48.5 (2C), 28.6 (3C), 24.9 (2C), 23.9. Mp = 289–291 °C. HRMS (ESI) m/z calculated for C21H27N4OS [M + H]+ 383.1900, found 383.1898.
2-tert-Butylamino-6-[4-(methylsulfanyl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one (4j). Following procedure D with methyl-3-amino-5-[4-(methylsulfanyl)phenyl]thiophene-2-carboxylate (0.65 g, 2.45 mmol) and tert-butylamine (515 μL, 4.9 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol). Fractions of interest were triturated in diethyl ether, affording 2-tert-butylamino-6-[4-(methylsulfanyl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one as an off-white solid (547 mg, 65% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.46 (s, 1H), 7.73 (d, 3J = 8.5 Hz, 2H), 7.48 (s, 1H), 7.32 (d, 3J = 8.5 Hz, 2H), 6.09 (s, 1H), 2.52 (s, 3H), 1.42 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.3, 157.6, 152.3, 149.6, 140.0, 129.4, 126.3 (2C), 126.1 (2C), 120.0, 111.1, 51.0, 28.6 (3C), 14.4. Mp = 272–274 °C. HRMS (ESI) m/z calculated for C17H20N3OS2 [M + H]+ 346.1042, found 346.1039.
2-tert-Butylamino-6-[4-(methylsulfonyl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one (4k). Following general procedure D with methyl-3-amino-5-[4-(methylsulfonyl)phenyl]thiophene-2-carboxylate (0.3 g, 0.96 mmol) and tert-butylamine (200 μL, 1.93 mmol), the obtained crude was triturated in acetonitrile, affording 2-tert-butylamino-6-[4-(methylsulfonyl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one as a beige powder (160 mg, 44% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.56 (s, 1H), 8.08 (d, 3J = 8.5 Hz, 2H), 7.98 (d, 3J = 8.5 Hz, 2H), 6.13 (s, 1H), 3.26 (s, 3H), 1.43 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.0, 157.6, 152.3, 147.5, 140.8, 137.6, 127.9 (2C), 126.6 (2C), 122.9, 112.9, 51.1, 43.4, 28.6 (3C). Mp = 294–296 °C. HRMS (ESI) m/z calculated for C17H20N3O3S2 [M + H]+ 378.0941, found 378.0934.
4-(2-tert-Butylamino-4-oxo-3,4-dihydrothieno[3,2-d]pyrimidin-6-yl)benzenesulfonamide (4l). Following general procedure D with methyl-3-amino-5-(4-sulfamoylphenyl)thiophene-2-carboxylate (0.2 g, 0.64 mmol) and tert-butylamine (135 μL, 1.28 mmol), the obtained crude was purified via column chromatography on silica gel (from 100% dichloromethane to 97
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dichloromethane/methanol), affording 4-(2-tert-butylamino-4-oxo-3,4-dihydrothieno[3,2-d]pyrimidin-6-yl)benzenesulfonamide as a yellow powder (135 mg, 56% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.55 (s, 1H), 8.00 (d, 3J = 8.5 Hz, 2H), 7.87 (d, 3J = 8.5 Hz, 2H), 7.67 (s, 1H), 7.44 (s, 2H), 6.14 (s, 1H), 1.42 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.0, 157.6, 152.3, 147.9, 144.2, 136.0126.5 (2C), 126.3 (2C), 122.3, 112.5, 51.0, 28.6 (3C). Mp > 300 °C. HRMS (ESI) m/z calculated for C16H19N4O3S2 [M + H]+ 379.0893, found 379.0893.
3 dichloromethane/methanol), affording 4-(2-tert-butylamino-4-oxo-3,4-dihydrothieno[3,2-d]pyrimidin-6-yl)benzenesulfonamide as a yellow powder (135 mg, 56% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.55 (s, 1H), 8.00 (d, 3J = 8.5 Hz, 2H), 7.87 (d, 3J = 8.5 Hz, 2H), 7.67 (s, 1H), 7.44 (s, 2H), 6.14 (s, 1H), 1.42 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.0, 157.6, 152.3, 147.9, 144.2, 136.0126.5 (2C), 126.3 (2C), 122.3, 112.5, 51.0, 28.6 (3C). Mp > 300 °C. HRMS (ESI) m/z calculated for C16H19N4O3S2 [M + H]+ 379.0893, found 379.0893.
2-tert-Butylamino-6-[4-(morpholin-4-ylsulfonyl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one (4m). Following procedure D with methyl-3-amino-5-[4-(morpholin-4-ylsulfonyl)phenyl]thiophene-2-carboxylate (0.3 g, 0.78 mmol) and tert-butylamine (170 μL, 1.57 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol). Fractions of interest were triturated in diethyl ether, affording 2-tert-butylamino-6-[4-(morpholin-4-ylsulfonyl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one as a yellow powder (117 mg, 33% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.57 (s, 1H), 8.09 (d, 3J = 8.5 Hz, 2H), 7.80 (d, 3J = 8.5 Hz, 2H), 7.74 (s, 1H), 6.14 (s, 1H), 3.68–3.61 (m, 4H), 2.96–2.87 (m, 4H), 1.43 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.0, 157.6, 152.3, 147.4, 137.4, 134.5, 128.6 (2C), 126.7 (2C), 122.8, 112.9, 62.3 (2C), 51.1, 45.9 (2C), 28.6 (3C). Mp > 300 °C. HRMS (ESI) m/z calculated for C20H25N4O4S2 [M + H]+ 449.1312, found 449.1305.
2-tert-Butylamino-6-[2-(morpholin-4-yl)pyrimidin-5-yl]thieno[3,2-d]pyrimidin-4(3H)-one (4n). Following procedure D with methyl-3-amino-5-(2-(morpholin-4-yl)pyrimidin-5-yl)thiophene-2-carboxylate (0.2 g, 0.62 mmol) and tert-butylamine (131 μL, 1.25 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol), affording 2-tert-butylamino-6-[2-(morpholin-4-yl)pyrimidin-5-yl]thieno[3,2-d]pyrimidin-4(3H)-one as a yellow powder (138 mg, 57% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.43 (s, 1H), 8.80 (s, 2H), 6.09 (s, 1H), 3.81–3.74 (m, 4H), 3.70–3.64 (m, 4H), 1.41 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.7, 160.2, 157.4, 155.2 (2C), 152.3, 144.7, 119.1, 116.5, 110.4, 65.9 (2C), 51.0, 44.0 (2C), 28.6 (3C). Mp > 300 °C. HRMS (ESI) m/z calculated for C18H23N6O2S [M + H]+ 387.1598, found 387.1597.
2-tert-Butylamino-6-[2-(4-methylpiperazin-1-yl)pyrimidin-5-yl]thieno[3,2-d]pyrimidin-4(3H)-one (4o). Following procedure D with methyl-3-amino-5-(2-(4-methylpiperazin-1-yl)pyrimidin-5-yl)thiophene-2-carboxylate (0.195 g, 0.58 mmol) and tert-butylamine (122 μL, 1.17 mmol), the obtained crude was purified via flash chromatography (using dichloromethane and methanol), affording 2-tert-butylamino-6-[2-(4-methylpiperazin-1-yl)pyrimidin-5-yl]thieno[3,2-d]pyrimidin-4(3H)-one as a beige powder (45 mg, 19% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.43 (s, 1H), 8.77 (s, 2H), 7.43 (s, 1H), 6.09 (s, 1H), 3.84–3.75 (m, 4H), 2.41–2.32 (m, 4H), 2.21 (s, 3H), 1.41 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.6, 160.3, 157.4, 155.2 (2C), 152.3, 144.8, 119.0, 116.1, 110.3, 54.3 (2C), 51.0, 45.8, 43.4 (2C), 28.6 (3C). Mp = 289–291. HRMS (ESI) m/z calculated for C19H26N7OS [M + H]+ 400.1914, found 400.1914.
6-(4-aminophenyl)-2-tert-butylaminothieno[3,2-d]pyrimidin-4(3H)-one (4p). To a solution of 2-(tert-butylamino)-6-(4-nitrophenyl)thieno[3,2-d]pyrimidin-4(3H)-one (0.227 g, 0.66 mmol) in acetic acid (6.9 mL), iron powder (0.368 g, 6.59 mmol) was added and the mixture was stirred at 80 °C for 15 min. Excess solvent was removed in vacuo and the crude product was purified via column chromatography on silica gel (starting from 100% dichloromethane to 95
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dichloromethane/methanol). Fractions of interest were purified via flash chromatography (using dichloromethane/methanol), affording 6-(4-aminophenyl)-2-tert-butylaminothieno[3,2-d]pyrimidin-4(3H)-one as a beige solid (66 mg, 32% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.31 (s, 1H), 7.45 (d, 3J = 8.4 Hz, 2H), 7.16 (s, 1H), 6.60 (d, 3J = 8.4 Hz, 2H), 6.01 (s, 1H), 5.56 (s, 2H), 1.41 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.5, 157.4, 152.1, 151.9, 150.2, 126.9 (2C), 120.3, 116.7, 113.8 (2C), 109.0, 50.9, 28.6 (3C). Mp = 259–261 °C. HRMS (ESI) m/z calculated for C16H19N4OS [M + H]+ 315.1274, found 315.1274.
5 dichloromethane/methanol). Fractions of interest were purified via flash chromatography (using dichloromethane/methanol), affording 6-(4-aminophenyl)-2-tert-butylaminothieno[3,2-d]pyrimidin-4(3H)-one as a beige solid (66 mg, 32% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.31 (s, 1H), 7.45 (d, 3J = 8.4 Hz, 2H), 7.16 (s, 1H), 6.60 (d, 3J = 8.4 Hz, 2H), 6.01 (s, 1H), 5.56 (s, 2H), 1.41 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.5, 157.4, 152.1, 151.9, 150.2, 126.9 (2C), 120.3, 116.7, 113.8 (2C), 109.0, 50.9, 28.6 (3C). Mp = 259–261 °C. HRMS (ESI) m/z calculated for C16H19N4OS [M + H]+ 315.1274, found 315.1274.
6-(4-aminophenyl)-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one (4q). To a solution of 6-(4-nitrophenyl)-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one (0.156 g, 0.47 mmol) in acetic acid (4.7 mL), iron powder (0.264 g, 4.72 mmol) was added and the mixture was stirred at 60 °C for 20 min. The reaction mixture was filtered through celite, the filtrate was isolated, and the excess solvent was removed in vacuo. The obtained crude was purified via column chromatography on silica gel (starting from 100% dichloromethane to 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dichloromethane/isopropanol). Fractions of interest were recrystallized in isopropanol, affording 6-(4-aminophenyl)-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one as light brown powder (30 mg, 21% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.47 (s, 1H), 7.44 (d, 3J = 8.6 Hz, 2H), 7.18 (s, 1H), 6.60 (d, 3J = 8.6 Hz, 2H), 6.07 (d, 3J = 7.3 Hz, 1H), 5.56 (s, 2H), 4.08–3.94 (m, 1H), 1.17 (d, 3J = 6.55 Hz, 6H). 13C NMR (DMSO-d6, 100 MHz) δ 160.8, 157.5, 152.6, 152.0, 150.2, 126.9 (2C), 120.3, 116.4, 113.8 (2C), 109.0, 42.0, 22.4 (2C). Mp = 296–298 °C. HRMS (ESI) m/z calculated for C15H17N4OS [M + H]+ 301.1118, found 301.1117.
10 dichloromethane/isopropanol). Fractions of interest were recrystallized in isopropanol, affording 6-(4-aminophenyl)-2-(propan-2-ylamino)thieno[3,2-d]pyrimidin-4(3H)-one as light brown powder (30 mg, 21% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.47 (s, 1H), 7.44 (d, 3J = 8.6 Hz, 2H), 7.18 (s, 1H), 6.60 (d, 3J = 8.6 Hz, 2H), 6.07 (d, 3J = 7.3 Hz, 1H), 5.56 (s, 2H), 4.08–3.94 (m, 1H), 1.17 (d, 3J = 6.55 Hz, 6H). 13C NMR (DMSO-d6, 100 MHz) δ 160.8, 157.5, 152.6, 152.0, 150.2, 126.9 (2C), 120.3, 116.4, 113.8 (2C), 109.0, 42.0, 22.4 (2C). Mp = 296–298 °C. HRMS (ESI) m/z calculated for C15H17N4OS [M + H]+ 301.1118, found 301.1117.
N-[4-(2-tert-butylamino-4-oxo-3,4-dihydrothieno[3,2-d]pyrimidin-6-yl)phenyl]acetamide (4r). Isolated as a side product from the purification of 6-(4-aminophenyl)-2-(tert-butylamino)thieno[3,2-d]pyrimidin-4(3H)-one when the reaction was stirred at 120 °C for 30 min (brown powder). 1H NMR (DMSO-d6, 400 MHz) δ 10.43 (bs, 1H), 10.12 (s, 1H), 7.73 (d, 3J = 11.6 Hz, 2H), 7.66 (d, 3J = 12 Hz, 2H), 7.41 (s, 1H), 6.07 (s, 1H), 2.07 (s, 3H), 1.42 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 168.5, 160.3, 157.5, 152.2, 150.0, 140.3, 127.5, 126.4 (2C), 119.5, 119.2 (2C), 110.8, 51.0, 28.6 (3C), 24.1. Mp > 300 °C HRMS (ESI) m/z calculated for C18H21N4O2S [M + H]+ 357.1380, found 357.1377.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dichloromethane/methanol), affording 2-tert-butylamino-6-[4-(methylsulfonimidoyl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one as a yellow powder (80 mg, 49% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.55 (bs, 1H), 8.05–7.94 (m, 4H), 7.69 (s, 1H), 6.13 (s, 1H), 4.30 (s, 1H), 3.10 (s, 3H), 1.43 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.1, 157.6, 152.3, 147.9, 144.3, 136.6, 128.2 (2C), 126.3 (2C), 122.4, 112.7, 51.0, 45.6, 28.6 (3C). Mp = 251–253 °C. HRMS (ESI) m/z calculated for C17H21N4O2S2 [M + H]+ 377.1100, found 377.1094.
5 dichloromethane/methanol), affording 2-tert-butylamino-6-[4-(methylsulfonimidoyl)phenyl]thieno[3,2-d]pyrimidin-4(3H)-one as a yellow powder (80 mg, 49% yield). 1H NMR (DMSO-d6, 400 MHz) δ 10.55 (bs, 1H), 8.05–7.94 (m, 4H), 7.69 (s, 1H), 6.13 (s, 1H), 4.30 (s, 1H), 3.10 (s, 3H), 1.43 (s, 9H). 13C NMR (DMSO-d6, 100 MHz) δ 160.1, 157.6, 152.3, 147.9, 144.3, 136.6, 128.2 (2C), 126.3 (2C), 122.4, 112.7, 51.0, 45.6, 28.6 (3C). Mp = 251–253 °C. HRMS (ESI) m/z calculated for C17H21N4O2S2 [M + H]+ 377.1100, found 377.1094.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) D7.4 calculations. c
D7.4 calculations. c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 were calculated for all compounds using MarvinSketch software (developed by ChemAxon). The following calculations options were used: consensus (log
D7.4 were calculated for all compounds using MarvinSketch software (developed by ChemAxon). The following calculations options were used: consensus (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P method), 0.1 mol dm−13 Cl− concentration, and 0.1 mol dm−13 Na+ K+ concentration.
P method), 0.1 mol dm−13 Cl− concentration, and 0.1 mol dm−13 Na+ K+ concentration.4.2. Biology
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 10 min at 4 °C, the supernatants were conserved at 4 °C for immediate analysis or placed at −80 °C in case of postponement of the analysis. Controls (time zero and final time point) in triplicate were prepared by the incubation of the internal standard with microsomes denatured by acetonitrile. The LC-MS system used for this study was a Waters Acquity I-Class/Xevo TQD equipped with a Waters Acquity BEH C18 column, 50 by 2.1 mm, 1.7 μm. The mobile phases were 10 mM ammonium acetate (mobile phase A) and acetonitrile with 0.1% formic acid (mobile phase B). The injection volume was 1 μL, and the flow rate was 600 μL min−1. Chromatographic analysis, with a total duration of 4 min, was made with the following gradient: 0 < t < 0.2 min, 2% mobile phase B; 0.2 < t < 2 min, linear increase to 98% mobile phase B; 2 < t < 2.5 min, 98% mobile phase B; 2.5 < t < 2.6 min, linear decrease to 2% mobile phase B; 2.6 < t < 4 min, 2% mobile phase B. The quantification of each compound was obtained by converting the average of the ratios of the analyte/internal standard surfaces to the percentage of consumed product. The ratio of the control at t0 corresponded to 0% of the product consumed. The calculation of the half-life (t1/2) of each compound in the presence of microsomes was carried out according to the equation t1/2 = (ln2)/k, where k is the first-order degradation constant (the slope of the logarithm of the compound concentration versus incubation time). The intrinsic clearance in vitro (Clint, expressed in mlmin−1mg) was calculated according to the equation Clint = (dose/AUC∞)/[microsomes], where dose is the initial concentration of the product in the sample, AUC∞ is the area under the concentration–time curve extrapolated to infinity, and [microsomes] is the microsome concentration expressed in mg μL−1.
000×g for 10 min at 4 °C, the supernatants were conserved at 4 °C for immediate analysis or placed at −80 °C in case of postponement of the analysis. Controls (time zero and final time point) in triplicate were prepared by the incubation of the internal standard with microsomes denatured by acetonitrile. The LC-MS system used for this study was a Waters Acquity I-Class/Xevo TQD equipped with a Waters Acquity BEH C18 column, 50 by 2.1 mm, 1.7 μm. The mobile phases were 10 mM ammonium acetate (mobile phase A) and acetonitrile with 0.1% formic acid (mobile phase B). The injection volume was 1 μL, and the flow rate was 600 μL min−1. Chromatographic analysis, with a total duration of 4 min, was made with the following gradient: 0 < t < 0.2 min, 2% mobile phase B; 0.2 < t < 2 min, linear increase to 98% mobile phase B; 2 < t < 2.5 min, 98% mobile phase B; 2.5 < t < 2.6 min, linear decrease to 2% mobile phase B; 2.6 < t < 4 min, 2% mobile phase B. The quantification of each compound was obtained by converting the average of the ratios of the analyte/internal standard surfaces to the percentage of consumed product. The ratio of the control at t0 corresponded to 0% of the product consumed. The calculation of the half-life (t1/2) of each compound in the presence of microsomes was carried out according to the equation t1/2 = (ln2)/k, where k is the first-order degradation constant (the slope of the logarithm of the compound concentration versus incubation time). The intrinsic clearance in vitro (Clint, expressed in mlmin−1mg) was calculated according to the equation Clint = (dose/AUC∞)/[microsomes], where dose is the initial concentration of the product in the sample, AUC∞ is the area under the concentration–time curve extrapolated to infinity, and [microsomes] is the microsome concentration expressed in mg μL−1.Author contributions
R. M.: conceptualization, investigation, visualization, writing – original draft, writing – review and editing; P. L.: investigation, writing – review and editing; S. H.: formal analysis, investigation, validation; C. D.: investigation, writing – review and editing; F. S.: investigation; D. A.: investigation; N. M.: supervision, writing – review and editing; N. A.: resources, supervision, validation; V. L.: resources, supervision, funding acquisition; P. V.: supervision, writing – review and editing; D. M.: funding acquisition; P. V.: resources, supervision, writing – review and editing; N. P.: conceptualization, funding acquisition, project administration, resources, supervision, validation, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financed by the grants of the Fondation pour la Recherche Médicale (FRM Palukill DMC20181039565) and the Agence Nationale de la Recherche (ANR Plasmodrug 18-CE18-0009-01).We want to thank Dr Vincent Remusat (Institut de Chimie Radicalaire, Marseille) for his help with NMR analysis, Dr Valérie Monnier and Ms Gaëlle Hisler (Spectropole, Marseille) for performing HRMS analysis and Ms Catherine Piveteau (Institut Pasteur de Lille) for performing microsomal stability studies.Notes and references
- World malaria report 2021. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO Search PubMed.
- S. Bhatt, D. J. Weiss, E. Cameron, D. Bisanzio, B. Mappin, U. Dalrymple, K. E. Battle, C. L. Moyes, A. Henry, P. A. Eckhoff, E. A. Wenger, O. Briët, M. A. Penny, T. A. Smith, A. Bennett, J. Yukich, T. P. Eisele, J. T. Griffin, C. A. Fergus, M. Lynch, F. Lindgren, J. M. Cohen, C. L. J. Murray, D. L. Smith, S. I. Hay, R. E. Cibulskis and P. W. Gething, Nature, 2015, 526, 207–211, DOI:10.1038/nature15535.
- A. M. Dondorp, F. Nosten, P. Yi, D. Das, A. P. Phyo, J. Tarning, K. M. Lwin, F. Ariey, W. Hanpithakpong, S. J. Lee, P. Ringwald, K. Silamut, M. Imwong, K. Chotivanich, P. Lim, T. Herdman, S. S. An, S. Yeung, P. Singhasivanon, N. P. J. Day, N. Lindegardh, D. Socheat and N. J. White, N. Engl. J. Med., 2009, 361, 455–467, DOI:10.1056/NEJMoa0808859.
- F. Ariey, B. Witkowski, C. Amaratunga, J. Beghain, A.-C. Langlois, N. Khim, S. Kim, V. Duru, C. Bouchier, L. Ma, P. Lim, R. Leang, S. Duong, S. Sreng, S. Suon, C. M. Chuor, D. M. Bout, S. Ménard, W. O. Rogers, B. Genton, T. Fandeur, O. Miotto, P. Ringwald, J. Le Bras, A. Berry, J.-C. Barale, R. M. Fairhurst, F. Benoit-Vical, O. Mercereau-Puijalon and D. Ménard, Nature, 2014, 505, 50–55, DOI:10.1038/nature12876.
- R. W. van der Pluijm, M. Imwong, N. H. Chau, N. T. Hoa, N. T. Thuy-Nhien, N. V. Thanh, P. Jittamala, B. Hanboonkunupakarn, K. Chutasmit, C. Saelow, R. Runjarern, W. Kaewmok, R. Tripura, T. J. Peto, S. Yok, S. Suon, S. Sreng, S. Mao, S. Oun, S. Yen, C. Amaratunga, D. Lek, R. Huy, M. Dhorda, K. Chotivanich, E. A. Ashley, M. Mukaka, N. Waithira, P. Y. Cheah, R. J. Maude, R. Amato, R. D. Pearson, S. Gonçalves, C. G. Jacob, W. L. Hamilton, R. M. Fairhurst, J. Tarning, M. Winterberg, D. P. Kwiatkowski, S. Pukrittayakamee, T. T. Hien, N. P. Day, O. Miotto, N. J. White and A. M. Dondorp, Lancet Infect. Dis., 2019, 19, 952–961, DOI:10.1016/S1473-3099(19)30391-3.
- Medicines for Malaria Venture. https://www.mmv.org/research-development/mmv-supported-projects (accessed on 04/01/22) Search PubMed.
- Medicines for Malaria Venture. Annual report 2020, 2021. https://www.mmv-annualreport2020.org/(accessed on 04/01/22) Search PubMed.
- M. Duffey, B. Blasco, J. N. Burrows, T. N. C. Wells, D. A. Fidock and D. Leroy, Trends Parasitol., 2021, 37, 709–721, DOI:10.1016/j.pt.2021.04.006.
- A. Cohen, P. Suzanne, J.-C. Lancelot, P. Verhaeghe, A. Lesnard, L. Basmaciyan, S. Hutter, M. Laget, A. Dumètre, L. Paloque, E. Deharo, M. D. Crozet, P. Rathelot, P. Dallemagne, A. Lorthiois, C. H. Sibley, P. Vanelle, A. Valentin, D. Mazier, S. Rault and N. Azas, Eur. J. Med. Chem., 2015, 95, 16–28, DOI:10.1016/j.ejmech.2015.03.011.
- H. Bosson-Vanga, N. Primas, J.-F. Franetich, C. Lavazec, L. Gomez, K. Ashraf, M. Tefit, V. Soulard, N. Dereuddre-Bosquet, R. Le Grand, M. Donnette, R. Mustière, N. Amanzougaghene, S. Tajeri, P. Suzanne, A. Malzert-Fréon, S. Rault, P. Vanelle, S. Hutter, A. Cohen, G. Snounou, P. Roques, N. Azas, P. Lagardère, V. Lisowski, N. Masurier, M. Nguyen, L. Paloque, F. Benoit-Vical, P. Verhaeghe and D. Mazier, Microbiol. Spectrum, 2021, 9, e00274, DOI:10.1128/Spectrum.00274-21.
- M. M. Heravi, Z. Kheilkordi, V. Zadsirjan, M. Heydari and M. Malmir, J. Organomet. Chem., 2018, 861, 17–104, DOI:10.1016/j.jorganchem.2018.02.023.
- M. D. Charles, P. Schultz and S. L. Buchwald, Org. Lett., 2005, 7, 3965–3968, DOI:10.1021/ol0514754.
- A. Begouin, S. Hesse, M.-J. R. Queiroz and G. Kirsch, Synthesis, 2005, 2373–2378, DOI:10.1055/s-2005-870014.
- D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27–50, 10.1039/C0SC00331J.
- L. Wang, S. K. Desmoulin, C. Cherian, L. Polin, K. White, J. Kushner, A. Fulterer, M.-H. Chang, S. Mitchell-Ryan, M. Stout, M. F. Romero, Z. Hou, L. H. Matherly and A. Gangjee, J. Med. Chem., 2011, 54, 7150–7164, DOI:10.1021/jm200739e.
- Synthesis med (Shanghaï) Co Ltd., Method for preparing Chk protein kinase antagonist AZD-7762. CN101967141A, 2011 Search PubMed.
- E. M. H. Ali, M. S. Abdel-Maksoud and C.-H. Oh, Bioorg. Med. Chem., 2019, 27, 1159–1194, DOI:10.1016/j.bmc.2019.02.044.
- W. Trager and J. B. Jensen, Science, 1976, 193, 673–675, DOI:10.1126/science.781840.
- W. A. Guiguemde, A. A. Shelat, D. Bouck, S. Duffy, G. J. Crowther, P. H. Davis, D. C. Smithson, M. Connelly, J. Clark, F. Zhu, M. B. Jimenez-Diaz, M. S. Martinez, E. B. Wilson, A. K. Tripathi, J. Gut, E. R. Sharlow, I. Bathurst, F. El Mazouni, J. W. Fowble, I. Forquer, P. L. McGinley, S. Castro, I. Angulo-Barturen, S. Ferrer, P. J. Rosenthal, J. L. DeRisi, D. J. Sullivan, J. S. Lazo, D. S. Roos, M. K. Riscoe, M. A. Phillips, P. K. Rathod, W. C. Van Voorhis, V. M. Avery and R. K. Guy, Nature, 2010, 465, 311–315, DOI:10.1038/nature09099.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63, DOI:10.1016/0022-1759(83)90303-4.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01687g |
| This journal is © The Royal Society of Chemistry 2022 |