Open Access Article
Open Access ArticleInvestigation on the thermo-electric-electrochemical characteristics of retired LFP batteries for echelon applications
Youfu Lv*ab,
Weiming Luoab,
Ya Moab and
Guoqing Zhangc
aSchool of Energy and Power Engineering, Changsha University of Science and Technology, Changsha, 410114, China. E-mail: lvyoufufighting@163.com
bGuangdong Provincial Key Laboratory of Distributed Energy Systems, Dongguan University of Technology, Dongguan, 523808, China
cSchool of Energy and Materials, Guangdong University of Technology, Guangzhou, 510006, China
First published on 11th May 2022
Abstract
Electric vehicles (EVs) have been developed to alleviate environmental pollution and climate change, but they leave behind a large amount of retired lithium-ion batteries (LIBs). Since the replacement of LIBs from EVs will lead to considerable waste generation, improving the echelon utilization of retired LIBs is becoming increasingly critical. In this paper, we studied the thermo-electric-electrochemical performance of retired LiFePO4 (LFP) batteries using traditional methods, and found that the remaining capacity of retired LFP batteries has a strong correlation with their internal resistance. This result helped us to propose a rapid and elementary classification method for the calibration of the remaining capacity, and to then formulate a test protocol seeking to balance the time spent and the test cost. Besides, the cut-off voltage and charge–discharge current density have a significant impact on the calibration of the remaining capacity, especially for retired LFP batteries with low residual capacity. In the cycle life test and temperature reliability evaluation process, the results demonstrate that the retired LFP batteries have a good service life when under a lower current of charge/discharge, and the capacity reductions were 2.3%, 11.2% and 4.8% for retired LFP batteries with 80% state of health (SOH), 70% SOH and 60% SOH, respectively, after 500 cycles. Finally, considering the temperature reliability, voltage consistency and large current cycling performance of retired LFP batteries, there are still many challenges in their future echelon utilization.
1 Introduction
In order to alleviate the fossil fuel and environmental pressures and promote the sustainable development of the transportation sector, EVs offer an attractive, environmental friendly and high-energy-efficient solution.1,2 Moreover, in order to address the challenge of global warming, the sale of fossil fuel vehicles will be stopped in countries around the world in the coming years; for instance, in the next 5 to 10 years in certain developed countries.3,4 LIBs are the best energy storage and power supply system of choice for EVs because of their long cycle life, high energy density, high voltage and low self-discharge.5–7The service life of LIBs is approximately 5 to 8 years due to the degradation of their capacity and cycle life.8 Therefore, LIBs in EVs need to be replaced before the remaining capacity fades to 70–80% of the original level9 to avoid unexpected driving malfunctions or safety risks.10 In other words, the first batch of LIBs from EVs are quickly reaching the end of their lives, and it is inevitable that more and more retired LIBs will be generated in the near future.11 Retired LFP batteries will particularly be obtained in large amounts since they are so widely used due to their low cost and high safety. Furthermore, the amount of retired LIBs could reach 70.8 × 104 tons in China by 2030,12 which will create some serious challenges for reducing battery costs and avoiding environmental pollution. If retired LIBs are not properly disposed, the toxic electrolytes, acid and alkali of the electrode material can lead to soil and water pollution as well as threats to human health.13 Therefore, a high efficiency and sustainable utilization strategy has been sought by numerous researchers, in which the echelon application of retired LIBs is considered as one of the most promising ways to reduce the manufacturing cost and extend the service life.14 Retired LIBs still have at least 70% capacity, sufficient to meet the demands of low energy storage devices, e.g., in home energy storage, portable power sources, and distributed energy systems.
In prior investigations, many research groups have aimed to develop recycling technology with high efficiency and economics for retired LIBs, which can be divided into two main technical routes.15,16 Firstly, retired LIBs can be used to recycle precious metal materials and relieve the shortage of valuable metals, e.g., Li, Co, Ni, Cu, and Al metals. According to the U.S. Geological Survey, Co reserves are limited to approximately 7 million tons,17 which has led to a sharp increase in the price of Co in recent years. However, these recycling processes have some disadvantages, such as (i) large energy consumption, (ii) large investments and costs, and (iii) environmental impact and pollutant emissions. Therefore, a second technical route is considered the most feasible and cost-effective by battery manufacturers and governments, which is involves recycling the valuable materials of retired LIBs when its completed the gradient utilization after.18,19 Although it is generally viewed that the application of retired batteries for energy storage is a good idea, there are a lot of key technical issues that need to be resolved, such as estimating the remaining capacity,20,21 predicting the state of health (SOH) of the battery,22 cycle life, and the safety.23 Different user behaviors and operating conditions can lead to different battery aging degrees, resulting in battery inconsistency and more sensitivity. To explore the feasibility of the echelon utilization of retired batteries, Tong et al.24 reported the application of retired batteries in an off-grid photovoltaic vehicle charging system, and the results showed that the cycle life reached 1400 cycles at 1C charge/discharge and 80% discharge of depth; Omar et al.25 studied the cycle life of batteries with 80% SOH under a test protocol of charging at 1C and discharging at 2C, and the results displayed that the remaining life of the retired battery was still more than 1000 cycle times. Although the retired battery showed a good cycle life, the remaining capacity and consistency evaluation is more important for potential applications, especially in energy storage. For assessing the remaining capacity, the most commonly used modeling methods include semi-empirical, equivalent circuit, mechanism, and data-driven models, which focus on accuracy, speed, and adaptability. Lai et al.20 reported a novel simple and efficient battery testing strategy, which was based on a support vector machine (SVM) algorithm to realize the large-scale rapid capacity estimation of retired LIBs. Ni et al.21 reported that using mechanism and data-driven models can offer a fast and accurate evaluation of the remaining capacity of retired batteries. Zhou et al.26 proposed a rapid classification method based on the internal resistance and voltage curves in charge/discharge, in which this method only needed 5 min compared with the traditional method and it had a maximum error within 4.2%. Lai et al.27 studied a rapid sorting and reasonable regrouping method using a Gaussian mixture model based on electrochemical impedance spectroscopy, in which this method only took about 10 min to obtain the capacity of each cell, and the error was within 4%. Despite all that, there are many studies that have reported various technologies, from the sorting technique, capacity evaluation, SOH prediction and cycle life tests for assessing retired batteries.28–30 However, there are few reports investigating the thermo-electrochemical performance of retired batteries, and this is important for assessing how to utilize them in echelon applications.
To address the knowledge gaps, this paper focuses on a study of the thermo-electric-electrochemical performance of retired LFP batteries, and provides a basic and comprehensive understanding of retired batteries. A test protocol for the remaining capacity assessment was systematically investigated, and the results implied that the influences of the current density and cut-off voltage on the capacity calibration were significant. Furthermore, the remaining capacity of retired LFP batteries was strongly related to the internal resistance, and this result suggested that examining the internal resistance is a rapid and elementary classification method for the remaining capacity assessment and calibration, and it can reduce unnecessary testing for retired LFP batteries. Additionally, the voltage consistency, cycle life, and temperature reliability of retired LFP batteries were also studied, and the results contributed to the development of a helpful strategy for the echelon utilization of retired LFP batteries. Finally, the micro-characteristics analysis of the electrode and separators materials showed that the degeneration of the battery capacity could be caused by the collapse of the material structure and the disappearance of holes.
2 Experimental
2.1 Retired LFP batteries
The retired batteries used in this work were donated by Shenzhen OptimumNano Energy Co., Ltd, amounting to a total of 800 batteries. These were normal commercially available 32650 LiFePO4 batteries with a capacity of 5.5 A h and internal resistance of approximately 7 mΩ (more detailed parameters are shown in Table 1). In the normal charging and discharging process, the cut-off voltages were 3.65 and 2.0 V, respectively.| Parameter | Value |
|---|---|
| Rated voltage (V) | 3.2 |
| Rated capacity (A h) | 5.5 |
| Diameter (mm) | 32.5 |
| High (mm) | 65.0 |
| Copper connector high (mm) | 5.0 |
| Rated resistance (mΩ) | 7.0 |
| Mass (g) | 145 ± 2 |
| Cut-off voltage (V) | 2.0–3.65 |
2.2 Screening methods
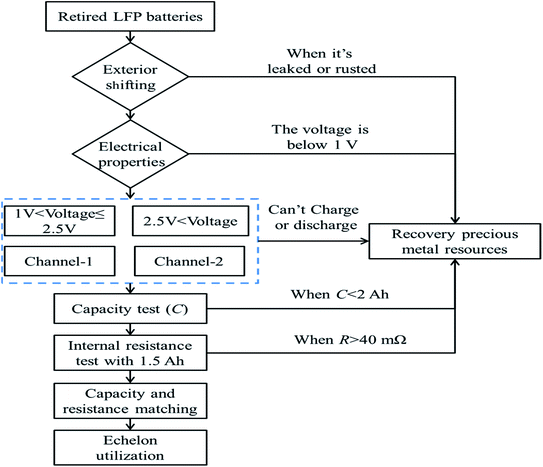
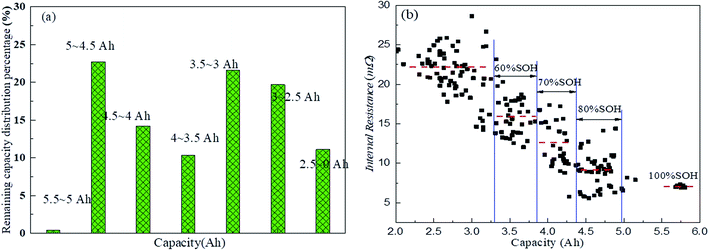
In order to explore the availability of the retired LFP batteries, all the batteries needed to be screened, as shown in Fig. 1 and as described in the following. First, if a retired battery showed rust, leakage, or the voltage was below 1.0 V, then it was directly recycled. Second, if the voltage of a retired battery was between 1.0 and 2.5 V, it was tested by adopting a constant-current and constant-voltage (CC–CV) protocol charge at 2.5 A, until the cut-off current was below 0.2 A. Subsequently, the retired battery was discharged at 2.5 A until the voltage was below 2.5 V. After that, the battery was tested again using the same test protocol. Furthermore, when the voltage of the battery was greater than 2.5 V, the battery was discharged at 2.5 A until the voltage was below 2.5 V, and then the battery was tested once again according to the above-mentioned charge–discharge test protocol. At the same time, any batteries without a charge–discharge capability were entered into resources recycling treatment. Next, the average of three discharge capacities was considered as the remaining capacity and the same test protocol as above was applied. If the capacity was below 2 A h, the retired battery also entered into resources recycling treatment. Finally, all the retired batteries underwent internal resistance testing after completing the remaining capacity assessment, and if the internal resistance was greater than 40 mΩ, the retired battery entered into resources recycling treatment. However, before echelon application, all retired LFP batteries must be graded and matched according to the remaining capacity and internal resistance, and to ensure they have a good consistency and safe reliability. After completing all the above-mentioned screening processes, only 57.3% of the retired LFP batteries were considered feasible for echelon utilization. These results implied that the batteries suffered different aging progresses before retiring, and this inconsistent aging was further aggravated when the retired LFP batteries were stored.2.3 Experimental platform
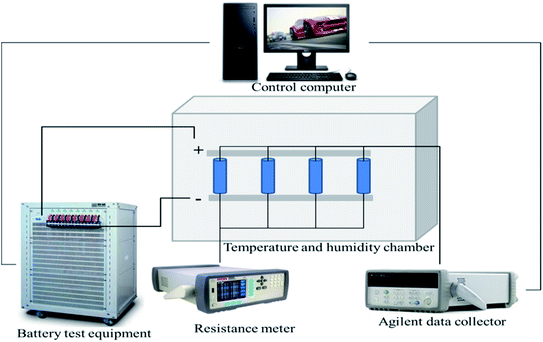
The thermo-electric-electrochemical performance of the retired LFP batteries was evaluated, as shown in Fig. 2. All the batteries were placed in an oven (Dongguan Sailham Instrument Co., Ltd.) at a constant temperature of 25 ± 0.5 °C, and the battery performance tests equipment was employed with 8 channels (BTS-4008T-5V30A, Shenzhen Neware Technology Co., Ltd.) to test the remaining capacity and cycle life. To evaluate the internal resistance (R) of the retired LFP batteries, resistance test equipment (AT5210, Changzhou Anbai Precision Instrument Co., Ltd.) was used. A T-type thermocouple wire (TC-TFF, 2 × 0.25 × 1000 mm, accuracy of ±0.1C, USA) was mounted on the middle of battery surface, and an Agilent data collector (USA, 34970A) was used to collect the data.3 Results and discussion
3.1 Electrochemical characteristics tests
Basis on Section 2.2, the remaining capacity of all the retired LFP batteries that have a feasibility for echelon utilization needed to be recalibrated. The average of three discharge capacities was used as the calibration for the remaining capacity, and the standard test protocol used is shown in Table 2.| Steps | Projects | Values |
|---|---|---|
| 1 | CC–CV | 2.5 A |
| Upper cut-off voltage | 3.65 V | |
| Cut-off current | 0.2 A | |
| 2 | Rest time | 10 min |
| 3 | Constant-current discharge | 2.5 A |
| Cut-off voltage | 2.5 V | |
| 4 | Rest time | 10 min |
| 5 | Cycle three times from 1 to 4 | |
| 6 | Constant-current charge | 2.5 A |
| Cut-off capacity | 1.5 A h |
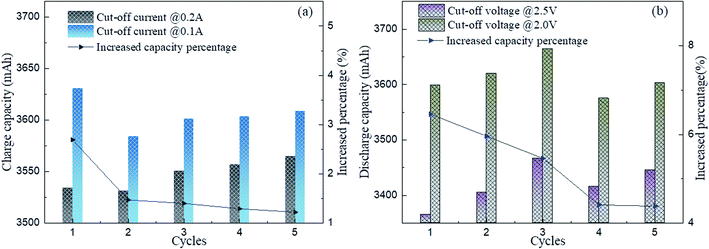 | ||
| Fig. 3 Influence of the different cut-off current and the voltage test protocol for the test capacity: (a) cut-off current and (b) cut-off voltage. | ||
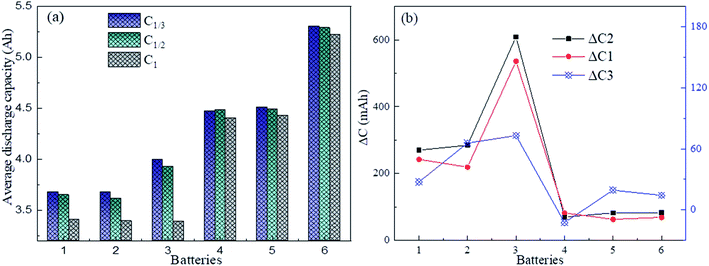
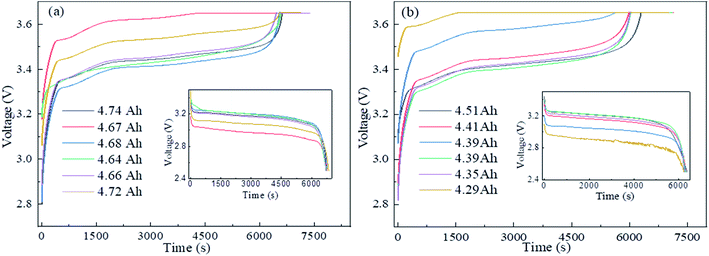
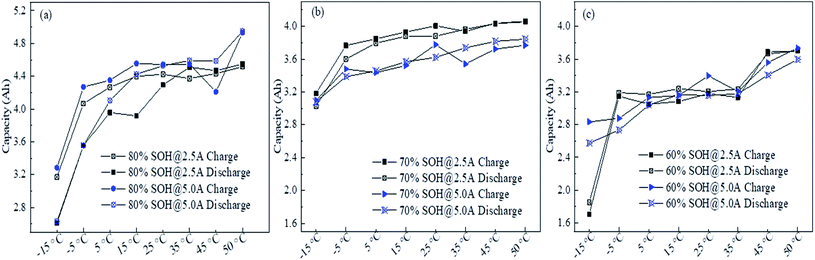
Fig. 4 shows the influence of different charge–discharge rates on the capacity, where it can be seen that the retired LFP batteries with lower remaining capacity were most significantly influenced by the charge–discharge rate. Here, ΔC1, ΔC2, and ΔC3 are defined as the average difference in the remaining capacity of the discharge between C1 and C1/2, C1 and C1/3 and C1/2 and C1/3, respectively. Here, C1, C1/2, and C1/3 represent the values the retired LFP batteries will adopt when the charge–discharge current is 5.5, 2.5, and 1.83 A, respectively. In Fig. 4b, ΔC1 and ΔC2 were over 220 mA h when the SOH of retired LFP batteries was less than 80% and the max peak values approximated to 600 mA h. In contrast, ΔC1 and ΔC2 were only 80 mA h when the SOH exceeded 80%, which shows a decline by about 2 times. Furthermore, ΔC3 showed similar trends, whereby it approached 60 mA h when the SOH was lower than 80%, and the difference fell below 30 mA h when the SOH was higher than 80%. Therefore, the results implied that the capacity calibration of retired LFP batteries sensitively undulated for the large charge–discharge current density, especially in retired LFP batteries with a lower SOH. In addition, the values of ΔC3 demonstrated that the remaining capacity did not significantly increase with the lower current density test protocol, and the test time was prolonged.
3.2 Electrical characteristics
3.3 Micro-characteristic analyses of the retired LFP batteries
4 Conclusions
The utilization of these retired batteries is highly desirable from an economic and environmental viewpoint. However, the biggest challenge to the echelon application of retired power batteries is how to quickly and efficiently detect their remaining capacity, cycle life, charge–discharge performance, and temperature reliability. Herein, we systematically investigated the thermo-electric-electrochemical performance of retired LFP batteries, and the results that need to be considered are summarized in the following:(1) The internal resistance of the retired LFP batteries showed a strong correlation with the remaining capacity. Therefore, we could quickly detect the internal resistance of the retired LFP batteries to roughly classify them, so as to develop different test processes to save testing time and cost.
(2) The evaluation of the remaining capacity of the retired LFP batteries was significantly affected by the test protocol, which implied that the test protocol should adopt a small test current and cut-off voltage.
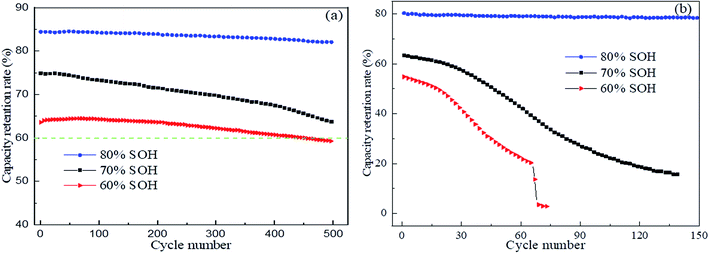
(3) The retired LFP batteries showed a good cycle life under a lower charge/discharge rate, implying that they could feasibly meet the low power requirement for energy storage. Nevertheless, according to the effects of ambient temperature and voltage inconsistency, echelon utilization may potentially require more costs.
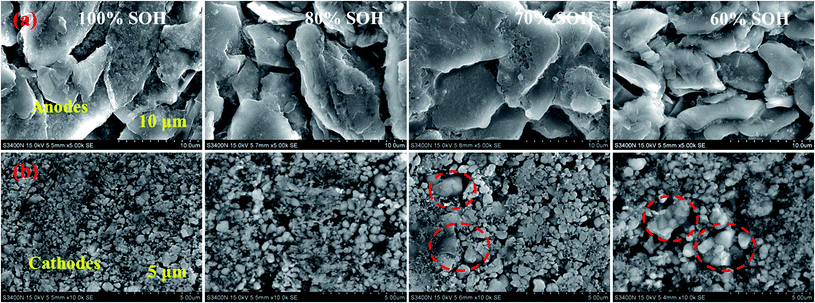
(4) The capacity fade and internal resistance increase of the retired LFP batteries may be attributed to the electrode material structure failure and separator dissolution. Eventually, the Li+-ion diffusion and de-intercalation rate rapidly declined during the charging/discharging process within the battery.
Author contributions
Youfu Lv: writing-review & editing, visualization, investigation, supervision, project administration. Weiming Luo: writing-original draft, experimental, data-processing, software. Ya Mo: experimental, data-processing. Guoqing Zhang: project administration, visualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Science and Technology Project of Education Department of Hunan Province (20C0044), Guangdong Provincial Key Laboratory of Distributed Energy Systems (No. 2020B1212060075) and National Natural Science Foundation of China (NNSFC, 21875046).References
- H. Byun, J. Shin and C.-Y. Lee, Energy, 2018, 144, 312–321 CrossRef.
- C. M. Costa, J. C. Barbosa, H. Castro, R. Gonçalves and S. Lanceros-Méndez, Renewable Sustainable Energy Rev., 2021, 151, 111548 CrossRef.
- K. Osieczko, D. Zimon, E. Placzek and I. Prokopiuk, J. Environ. Manage., 2021, 296, 113177 CrossRef PubMed.
- D. Burchart-Korol, S. Jursova, P. Folęga and P. Pustejovska, J. Cleaner Prod., 2020, 257, 120476 CrossRef.
- G. Zhong, K. Qu, C. Ren, Y. Su, B. Fu, M. Zi, L. Dai, Q. Xiao, J. Xu, X. Zhong, F. An, M. Ye, S. Ke, S. Xie, J. Wang, P. Gao and J. Li, Nano Energy, 2020, 74, 104876 CrossRef CAS.
- X. Wang, Y. Li, P. Das, S. Zheng, F. Zhou and Z.-S. Wu, Energy Storage Mater., 2020, 31, 156–163 CrossRef.
- Z. Su, C. Zhou, X. Gao, R. Cao, S. Yang and X. Liu, Rare Met., 2022, 41, 14–20 CrossRef.
- Y. Tang, Q. Zhang, Y. Li, H. Li, X. Pan and B. McLellan, Appl. Energy, 2019, 251, 113313 CrossRef.
- S. Saxena, C. Le Floch, J. MacDonald and S. Moura, J. Power Sources, 2015, 282, 265–276 CrossRef CAS.
- R. Planteu, A. W. Golubkov, P. Krohn, B. Rasch, B. Brunnsteiner, A. Thalera and V. Hacker, RSC Adv., 2018, 8, 40172–40186 RSC.
- X. Lai, Y. Huang, C. Deng, H. Gu, X. Han, Y. Zheng and M. Ouyang, Renewable Sustainable Energy Rev., 2021, 146, 111162 CrossRef CAS.
- R. Jing, J. Wang, N. Shah and M. Guo, Advances in Applied Energy, 2021, 1, 100002 CrossRef.
- Y. Hua, S. Zhou, Y. Huang, X. Liu, H. Ling, X. Zhou, C. Zhang and S. Yang, J. Power Sources, 2020, 478, 228753 CrossRef CAS.
- S. Chen, A. Ran, S. Zhang, S. Liu, Z. Zhou, P. Nie, K. Qian, L. Fang, S. Zhao, B. Li, F. Kang, X. Zhou, H. Sun, X. Zhang and G. Wei, RSC Adv., 2020, 10, 19117–19123 RSC.
- F. A. Kayakool, B. Gangaja, S. Nair and D. Santhanagopalan, Sustainable Mater. Technol., 2021, 28, e00262 CrossRef CAS.
- S. Windisch-Kern, E. Gerold, T. Nigl, A. Jandric, M. Altendorfer, B. Rutrecht, S. Scherhaufer, H. Raupenstrauch, R. Pomberger, H. Antrekowitsch and F. Part, Waste Manage., 2022, 138, 125–139 CrossRef CAS PubMed.
- E. Fan, L. Li, Z. Wang, J. Lin, Y. Huang, Y. Yao, R. Chen and F. Wu, Chem. Rev., 2020, 120, 7020–7063 CrossRef CAS PubMed.
- T. Yang, L. Li and Z. Li, RSC Adv., 2021, 11, 24132–24136 RSC.
- Y. Zhang, Y. Li, Y. Tao, J. Ye, A. Pan, X. Li, Q. Liao and Z. Wang, Energy, 2020, 193, 116555 CrossRef.
- X. Lai, C. Deng, J. Li, Z. Zhu, X. Han and Y. Zheng, IEEE Trans. Veh. Technol., 2021, 70, 1246–1254 Search PubMed.
- Y. Ni, J. Xu, C. Zhu and L. Pei, Appl. Energy, 2022, 305, 117922 CrossRef CAS.
- N. Yan, H. Zhao, T. Yan and S. Ma, Energy Rep., 2020, 6, 1106–1113 CrossRef.
- Y. Jiang, J. Jiang, C. Zhang, W. Zhang, Y. Gao and N. Li, J. Cleaner Prod., 2018, 205, 754–762 CrossRef CAS.
- S. J. Tong, A. Same, M. A. Kootstra and J. W. Park, Appl. Energy, 2013, 104, 740–750 CrossRef CAS.
- N. Omar, M. A. Monem, Y. Firouz, J. Salminen, J. Smekens, O. Hegazy, H. Gaulous, G. Mulder, P. Van den Bossche, T. Coosemans and J. Van Mierlo, Appl. Energy, 2014, 113, 1575–1585 CrossRef CAS.
- P. Zhou, Z. He, T. Han, X. Li, X. Lai, L. Yan, T. Lv, J. Xie and Y. Zheng, Energy Rep., 2020, 6, 672–683 CrossRef.
- X. Lai, C. Deng, X. Tang, F. Gao, X. Han and Y. Zheng, J. Cleaner Prod., 2022, 339, 130786 CrossRef CAS.
- Z. Xu, J. Wang, P. D. Lund, Q. Fan, T. Dong, Y. Liang and J. Hong, J. Energy Storage, 2020, 29, 101303 CrossRef.
- Q. Yu, R. Xiong, R. Yang and M. G. Pecht, Appl. Energy, 2019, 255, 113817 CrossRef.
- Z. Zhou, A. Ran, S. Chen, X. Zhang, G. Wei, B. Li, F. Kang, X. Zhou and H. Sun, J. Energy Storage, 2020, 31, 101739 CrossRef.
- S. Yang, R. He, Z. Zhang, Y. Cao, X. Gao and X. Liu, Matter, 2020, 3, 27–41 CrossRef.
- W. Wu, S. Wang, W. Wu, K. Chen, S. Hong and Y. Lai, Energy Convers. Manage., 2019, 182, 262–281 CrossRef.
- C. Wu, Z. Wang, Y. Bao, J. Zhao and Z. Rao, J. Energy Storage, 2021, 41, 102882 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |