DOI:
10.1039/D2RA02459D
(Paper)
RSC Adv., 2022,
12, 18736-18745
Study on the coordination of cyclopentanocucurbit[5,6]uril with Fe3+, Co2+ and Ni2+ ions†
Received
17th April 2022
, Accepted 16th June 2022
First published on 28th June 2022
Abstract
This paper reports the coordination of cyclopentanocucurbit[5]uril (CyP5Q[5]) and cyclopentanocucurbit[6]uril (CyP6Q[6]) with Fe(ClO4)3, Co(ClO4)2 and Ni(ClO4)2. Single crystal X-ray diffraction analysis shows the metal ions are directly coordinated with the portal of the cucurbit[n]uril to form a one-dimensional supramolecular chain or independent systems in the CyP5Q[5]@Fe(ClO4)3, CyP5Q[5]@Co(ClO4)2, CyP6Q[6]@Co(ClO4)2 and CyP5Q[5]@Ni(ClO4)2 complexes. In CyP6Q[6]@Fe(ClO4)3, the metal ion is not directly coordinated with the cucurbit[n]uril portal, but after forming Fe(H2O)6, it interacts with the cucurbit[n]uril portal via a hydrogen bond. The CyP6Q[6]@Ni(ClO4)2 complex is quite special; in this system, there are both metal ions directly coordinated with the cucurbit[n]uril portal and free on the outer surface of the cucurbit[n]uril.
1. Introduction
Cucurbit[n]uril (Q[n] or CB[n]),1–13 a macrocyclic compound with an inner surface cavity and rich carbonyl portals, is a good electron donor due to the presence of a large number of carbonyl groups and therefore has a high density of electron clouds at its portal. Numerous experimental facts prove that Q[n] can coordinate with metal ions with different ionic radii such as alkali metals, alkaline earth metals, transition metals, lanthanide metals, etc. to form supramolecular assemblies in the presence of structural inducers through intermolecular interactions between water and amino groups, etc. and portal carbonyl oxygen of Q[n], which, however, is slightly soluble in water and organic solvents, limiting its scope of study. Alkyl-substituted Q[n]s are new Q[n]s prepared by reacting formaldehyde with alkyl-substituted glycoluril under acidic conditions; their solubility greatly improves compared with that of ordinary Q[n]s due to the electron-donating effect of alkyl groups, which increases the electron density of Q[n] portals and makes it relatively easy to coordinate with metal ions. So far, the main alkyl-modified Q[n]s reported are methyl-substituted Q[n]s,14–16 cyclopentyl-substituted Q[n]s,9,10,17–19 cyclohexyl-substituted Q[n]s20–22 and cyclobutyl-substituted Q[n]s.23
In previous work, the coordination of CyP5Q[5] with alkali metal cations (A+)24 and alkaline earth metal cations (AE2+),25 CyP6Q[6] with alkaline earth metal cations (AE2+)26 and lanthanides (Ln3+)27 with [ZnCl4]2− as an inducer are investigated. In 2019, Zhao et al. reported the coordination of cyclopentyl-substituted Q[n]s with Cu and Zn ions,28 and in 2021, Ma et al. reported the supramolecular self-assembly of CyP5Q [5] with heavy metal ionic compounds (CoCl2, CrCl3, HgCl2, Pb(ClO4)2) under acidic conditions,29 extending the coordination studies of cyclopentyl-substituted Q[n]s with metal ions. In this paper, the cyclopentyl-substituted Q[n]s CyP5Q [5] and CyP6Q [6] are selected as organic ligands and the coordination modes with iron, cobalt and nickel ions are investigated, respectively, improving the model of the interaction of cyclopentyl-substituted Q[n]s with transition metals, from which the formed supramolecular framework structures are expected to have better application prospects in the fields of gas molecule adsorption, drug delivery, supramolecular chemistry and coordination chemistry.
2. Experimental section
2.1 General materials
All materials were reagent grade and used without any further purification. Cyclopentanocucurbit[5]uril and cyclopentanocucurbit[6]uril was prepared and purified in accordance with a literature method9,10 (as shown in Scheme 1) and its structure is shown in Fig. 1.
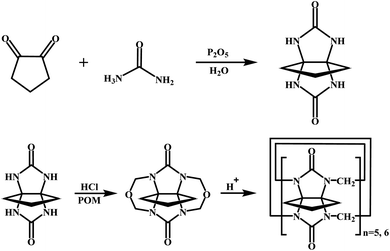 |
| | Scheme 1 The synthesis process of cyclopentanocucurbit[5,6]uril. | |
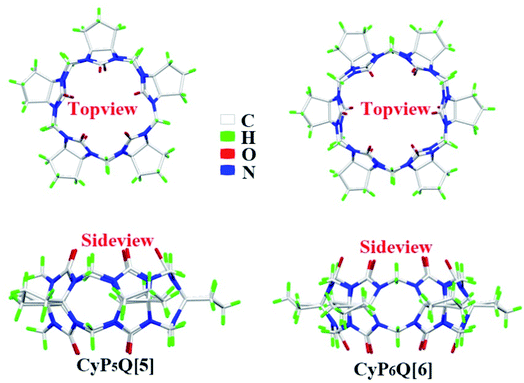 |
| | Fig. 1 The structures of CyP5Q[5] and CyP6Q[6]. | |
2.2 Synthesis of the complexes 1–6
Preparation of complex 1: a mixture of CyP5Q[5] (10 mg, 9.73 μmol) and Fe(ClO4)3·H2O(15.5 mg, 41.6 μmol) in 3 mL of 70% (v/v) formic acid aqueous solution was heated until dissolution. The solution was heated and stirred for 5–10 min in a water bath at 35 °C. The resulting solution was allowed to stand at room temperature for 7–10 d to obtain single crystals of C90H108Cl4Fe2N40O40 ref. 1 (32.5% yield), using a similar synthetic procedure gave single crystals of C90H108Cl3Co2N40O36 ref. 2 (38.4% yield), C45H60Cl2NiN20O23 ref.3 (42.3% yield); preparation of complex 4: a mixture of CyP6Q[6] (10 mg, 8.08 μmol) and Fe(ClO4)3·H2O (15.5 mg, 41.6 μmol) in 3 mL of 70% (v/v) formic acid aqueous solution was heated until dissolution. The solution was heated and stirred for 5–10 min in a water bath at 35 °C. The resulting solution was allowed to stand at room temperature for 7–10 d to obtain single crystals of C54H84Cl6Fe2N24O48 ref. 4 (35.8% yield), using a similar synthetic procedure gave single crystals of C54H68Cl2CoN24O24 ref. 5 (40.4% yield) and C54H82Cl4Ni2N24O39 ref. 6 (36.2% yield), respectively.
2.3 Crystal structure determination
A crystal with the appropriate size and transparency was selected and fixed on a glass probe with using petroleum jelly for X-ray single crystal diffraction analysis on a Bruker D8 Venture X-ray single crystal diffractometer in ω-scan mode using graphite to monochromatize the Mo-Kα rays (λ = 0.71073 Å, μ = 0.828 mm−1). The crystal data were collected and Lorentz polarization and absorption correction carried out. SHELXT-14 and SHELXL-14 program packages were used for structural analysis and full matrix least squares refinement. All non-hydrogen atoms atoms were anisotropically refined using the analytical expression of the neutral atom scattering factor and combined with anomalous dispersion correction. The SQUEEZE program in the PLATON package was used to delete some of the disordered solvent molecules. The X-ray crystallographic data for structures reportaled in this study have been deposited in the Cambridge Crystallographic Data Center under accession numbers CCDC: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 692,1 2
692,1 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 694,2 2
694,2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 707,3 2
707,3 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 712,4 20
712,4 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713 ref. 5 and 2
713 ref. 5 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 724.6 These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/datarequest/cif. The crystal parameters, data acquisition conditions, and parameters of the complexes 1–3 are listed in Table 1 and 4–6 are listed in Table 2.
724.6 These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/datarequest/cif. The crystal parameters, data acquisition conditions, and parameters of the complexes 1–3 are listed in Table 1 and 4–6 are listed in Table 2.
Table 1 The crystal structure parameters of complex 1–3
| Complex |
1 |
2 |
3 |
| Conventional R on Fhkl: ∑||Fo| − |Fc||/∑|Fo|. Weighted R on |Fhkl|2: ∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]1/2. |
| Empirical formula |
C90H108Cl4Fe2N40O40 |
C90H108Cl3Co2N40O36 |
C45H60Cl2NiN20O23 |
| Formula weight |
2643.66 |
2550.37 |
1378.74 |
| Crystal system |
Triclinic |
Triclinic |
Monoclinic |
| Space group |
P1 |
P1 |
P21/n |
| a [Å] |
15.734(6) |
15.667(4) |
11.981(4) |
| b [Å] |
19.695(7) |
19.553(5) |
32.393(10) |
| c [Å] |
23.581(9) |
23.434(6) |
16.243(5) |
| α [°] |
65.774(9) |
66.076(7) |
90 |
| β [°] |
81.704(9) |
81.367(7) |
98.681(10) |
| γ [°] |
84.656(9) |
84.437(7) |
90 |
| V [Å3 ] |
6590(4) |
6482(3) |
6232(3) |
| Z |
2 |
2 |
4 |
| Dc (g cm −3) |
1.332 |
1.307 |
1.470 |
| T [K] |
296.15 |
296.15 |
296.15 |
| F(000) |
2736 |
2642 |
2864 |
| μ [mm −1 ] |
0.391 |
0.404 |
0.489 |
| Data/params |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 242/1737 242/1737 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 835/1592 835/1592 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 988/937 988/937 |
| Rint |
0.0903 |
0.0801 |
0.0895 |
| R [I > 2σ(I)]a |
0.0779 |
0.0780 |
0.0884 |
| wR [I > 2σ(I)]b |
0.2147 |
0.2206 |
0.2253 |
| R (all data) |
0.1460 |
0.1297 |
0.1287 |
| wR (all data) |
0.2465 |
0.2499 |
0.2496 |
| GOF on F2 |
1.042 |
1.031 |
1.031 |
Table 2 The crystal structure parameters of complex 4–6
| Complex |
4 |
5 |
6 |
| Conventional R on Fhkl: ∑||Fo| − |Fc||/∑|Fo|. Weighted R on |Fhkl|2: ∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]1/2. |
| Empirical formula |
C54H84Cl6Fe2N24O48 |
C54H68Cl2CoN24O24 |
C54H82Cl4Ni2N24O39 |
| Formula weight |
2161.80 |
1567.15 |
1950.65 |
| Crystal system |
Trigonal |
Triclinic |
Triclinic |
| Space group |
R3 |
P1 |
P1 |
| a [Å] |
19.654(4) |
14.193(2) |
21.326(5) |
| b [Å] |
19.654(4) |
16.298(2) |
22.267(6) |
| c [Å] |
20.369(7) |
17.110(3) |
22.325(6) |
| α [°] |
90 |
79.312(5) |
92.482(7) |
| β [°] |
90 |
74.581(6) |
97.727(8) |
| γ [°] |
120 |
73.497(5) |
97.905(6) |
| V [Å3 ] |
6814(4) |
3632.2(10) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 385(5) 385(5) |
| Z |
3 |
2 |
4 |
| Dc (g cm−3) |
1.581 |
1.433 |
1.248 |
| T [K] |
296.0 |
273.15 |
273.15 |
| F(000) |
3342 |
1626 |
4040 |
| μ [mm −1 ] |
0.605 |
0.401 |
0.549 |
| Data/params |
2680/217 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022/969 022/969 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 444/2245 444/2245 |
| Rint |
0.1299 |
0.0526 |
0.1067 |
| R [I > 2σ(I)]a |
0.1067 |
0.0792 |
0.1370 |
| wR [I > 2σ(I)]b |
0.3438 |
0.2432 |
0.3692 |
| R (all data) |
0.1252 |
0.1044 |
0.2432 |
| wR (all data) |
0.3616 |
0.2678 |
0.4286 |
| GOF on F2 |
1.704 |
1.033 |
1.165 |
3. Results and discussion
3.1 Description of the crystal structure of complexes 1-6
The asymmetric unit of complex 1 contains two CyP5Q[5], three Fe3+ ions, four coordination water molecules and four free perchlorate ions, as shown in Fig. 2a. The free perchlorate oxygen atoms and methylene hydrogen atoms atoms on the outer surface of CyP5Q[5] were connected via ion–dipole interactions (Fig. 2b), the ion–dipole interactions distances range from 2.272 Å to 2.689 Å. The Fe3+ ion of the complex has a distorted octahedral coordination configuration; each Fe3+ ion is coordinated to four carbonyl oxygen atoms and two water molecules in CyP5Q[5]. The length of the coordination bond between Fe3+ and the CyP5Q[5] carbonyl oxygen atom is in the range of 2.105–2.134 Å, the coordination bond length between Fe3+ and oxygen atom of the coordinated water molecule is in the range of 2.057–2.093 Å, the bond angle around Fe3+ ion ranges from 83.79 to 93.72°. The CyP5Q[5] was coordinated and bridged by two Fe3+ ions through the oxygen atoms of the two portal carbonyl groups to form a one-dimensional supramolecular chain with alternating V- and Z-types (Fig. 2d). The distances between Fe1–Fe2, Fe2–Fe3 and Fe1–Fe3 in the one-dimensional supramolecular chain are 9.157, 9.680 and 18.761 Å, respectively. The angles between the two CyP5Q[5] molecules connected to Fe1, Fe2 and Fe3 are 0, 29.21 and 0°, respectively. There is also a hydrogen atoms bond formed between the water molecules participating in the coordination bond and the carbonyl oxygen atom at the end of the cucurbit[n]uril. Fig. 2c is a three-dimensional stacking diagram of complex 1.
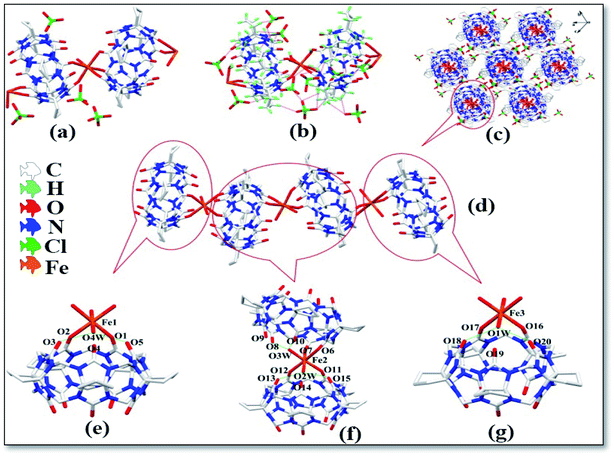 |
| | Fig. 2 Structure of complex 1: (a) asymmetric unit, (b) ion–dipole interactions, (c) stacking diagram, (d) one-dimensional supramolecular chain and (e–g) hydrogen atoms bond interactions. | |
The asymmetric unit of complex 2 is shown in Fig. 3a, which contains two CyP5Q[5], three Co2+ ions, four coordinated water molecules and three free perchlorate counter ions. The oxygen atoms of the free perchlorate and methylene hydrogen atoms atom on the outer surface of CyP5Q[5] are connected via ion–dipole interactions (Fig. 3b), the ion–dipole interactions distances range from 2.405 Å to 2.689 Å. The Co2+ ion of the complex has a distorted octahedral coordination configuration, each Co2+ ion is coordinated with the four carbonyl oxygen atoms and two water molecules of the cucurbit[n]uril. The coordinate bond length between Co2+ and the CyP5Q[5] carbonyl oxygen atom is in the range of 2.077–2.102 Å, the bond angle around Co2+ ion ranges from 85.06 to 95.19°, and the coordinate bond length to the coordinated water molecule oxygen is in the range of 2.017–2.059 Å. The cucurbit[n]uril is coordinated and bridged by two Co2+ ions via the oxygen atoms of two portal carbonyl groups to form a one-dimensional supramolecular chain with alternating V- and Z-types (Fig. 3d). The distances between Co1–Co2, Co2–Co3 and Co1–Co3 in the one-dimensional supramolecular chain are 9.588, 9.053 and 18.567 Å, respectively. The angles between the two CyP5Q[5] connected to Co1, Co2 and Co3 are 0, 29.23 and 0°, respectively. There is also a hydrogen atoms bond between the water molecules participating in the coordination bond and the carbonyl oxygen atom at the end of CyP5Q[5]. Fig. 3c is a three-dimensional stacking diagram of complex 2.
 |
| | Fig. 3 Crystal structure of complex 2: (a) asymmetric unit, (b) ion–dipole interactions, (c) stacking diagram, (d) one-dimensional supramolecular chain and (e–g) hydrogen atoms bond interactions. | |
The asymmetric unit structure of complex 3 is shown in Fig. 4a, which contains one CyP5Q[5], one Ni2+ ion, four coordinated water molecules, one free water molecule and two free perchlorate ions. Each CyP5Q[5] is surrounded by five free perchlorate counter ions, which are connected via ion–dipole interactions; the mode of action is shown in Fig. 4b, the ion–dipole interactions distances range from 2.333 Å to 2.698 Å. The Ni2+ ion of the complex has a twisted octahedral coordination configuration and each Ni2+ ion is coordinated to two carbonyl oxygen atoms and four water molecules at one portal carbonyl group of the cucurbit[n]uril. The length of the coordination bond between Ni2+ ion and CyP5Q[5] carbonyl oxygen atom is 2.085–2.091 Å, the length of coordination bond with the coordinated water molecule oxygen atom is 2.018–2.067 Å and the bond angle around Ni2+ ranges from 86.20 to 95.49°. There is a hydrogen atoms bond formed between the water molecules participating in the coordination bond and the carbonyl oxygen atom at the portal of CyP5Q[5], the carbonyl oxygen atom at the lower portal of the other CyP5Q[5] is connected via hydrogen atoms bonds, and the free water molecules are bridged and coordinated via hydrogen atoms bonding between the water molecule and carbonyl oxygen atom of the other CyP5Q[5] portal (Fig. 4c). Fig. 4d is a stacking diagram of the complex 3 viewed along the a-axis.
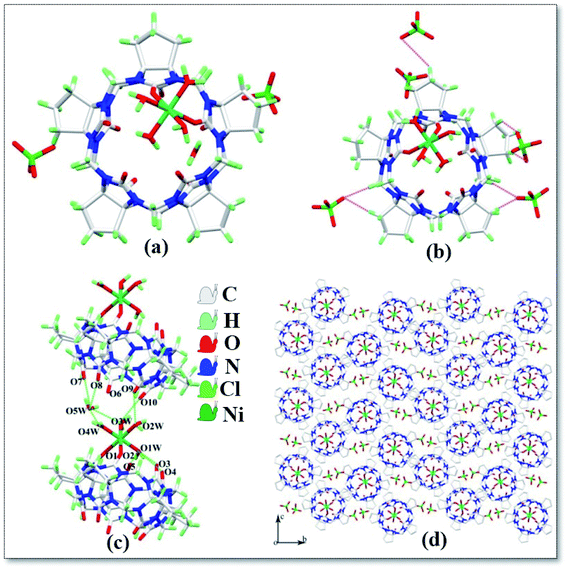 |
| | Fig. 4 Crystal structure of complex 3: (a) asymmetric unit; (b) ion–dipole interactions, (c) hydrogen atoms bond interactions and (d) stacking diagram viewed along the a-axis. | |
The asymmetric unit of complex 4 is shown in Fig. 5a, which contains one CyP6Q[6], one Fe3+ ion, six coordinated water molecules, and one free perchlorate ion. Each CyP6Q[6] is surrounded by ten free perchlorate counter ions, which are connected via ion–dipole interactions; the mode of action is shown in Fig. 5b, the ion–dipole interactions distances range from 2.641 Å to 2.719 Å. The Fe3+ ion of the complex has an octahedral coordination configuration; each Fe3+ ion is coordinated to six water molecules and the coordination bond length with the coordinated water molecule oxygen atom is 1.968–1.999 Å, and the bond angle around the Fe3+ ion ranges from 89.31 to 92.74°. There is a hydrogen atoms bond formed between the water molecule participating in the coordination bond and the carbonyl oxygen atom at the portal of the cucurbit[n]uril, which is connected with the carbonyl oxygen atom at the lower port of the other cucurbit[n]uril via a hydrogen atoms bond. Unlike other complexes, Fe3+ is not directly coordinated with the cucurbit[n]uril portal. The free water molecules bridge the coordinated water molecule and the carbonyl oxygen atom in the other cucurbit[n]uril portal via hydrogen atoms bonding (shown in the blue curve in Fig. 5c), the specific performance is O1–O2W and O2–O2W, and the distance between them is 2.606 and 2.573 Å respectively. Fig. 5d is the stacking diagram of complex 4 viewed along the a-axis. It can be seen from the figure that there are obvious gaps between the cucurbit[n]urils and the ClO4− counter ions are dispersed in these gaps.
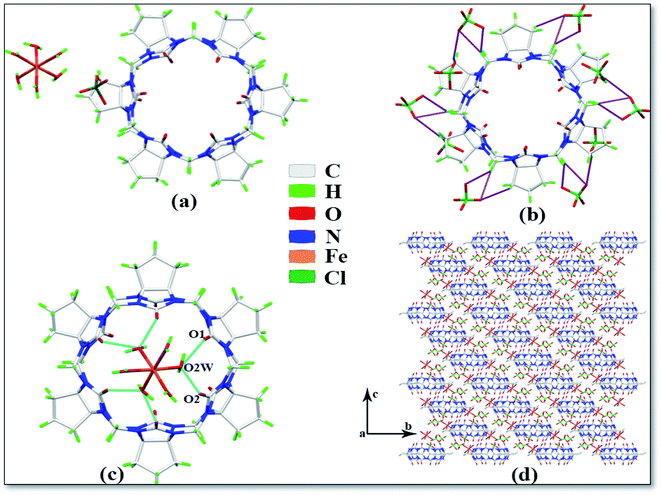 |
| | Fig. 5 Crystal structure of complex 4: (a) Asymmetric unit, (b) ion–dipole interactions, (c) hydrogen atoms bond interactions and (d) stacking diagram viewed along the a-axis. | |
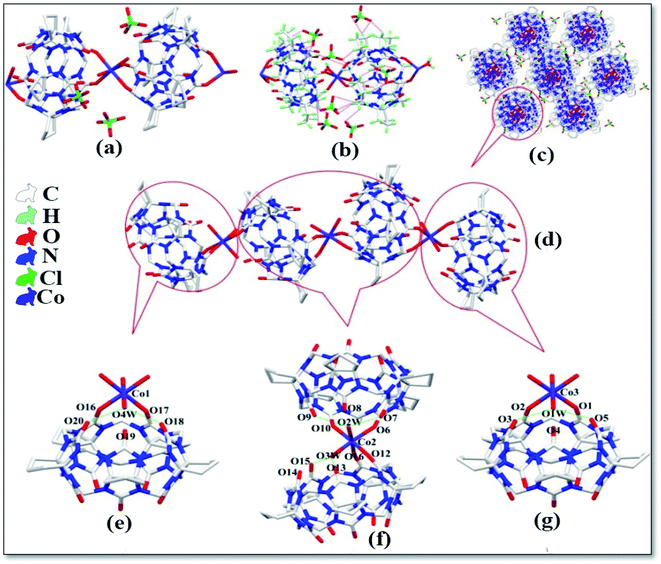
The asymmetric unit of complex 5 is shown in Fig. 6a, which contains one CyP6Q[6], one Co2+ ion, four coordinated water molecules and two free perchlorate ions. The Co2+ ion of the complex has a twisted octahedral coordination configuration. Each Co2+ ion is coordinated with two carbonyl oxygen atoms and four water molecules at one portal of the cucurbit[n]uril and the Co2+ ion is coordinated with the carbonyl oxygen atom of the cucurbit[n]uril. The coordination bond length is 2.081–2.179 Å, the coordination bond length with the coordinated water molecule oxygen is 2.023–2.108 Å and the bond angle around the Co2+ ion ranges from 85.77 to 102.61°. There is a hydrogen atoms bond formed between the water molecule participating in the coordination bond and the carbonyl oxygen atom at the portal of the cucurbit[n]uril, which is connected with the carbonyl oxygen atom at the lower portal of the other CyP6Q[6] via a hydrogen atoms bond. The free water molecule bridges the coordinated water molecule and carbonyl oxygen atom of another cucurbit[n]uril portal via hydrogen atoms bonding (Fig. 6b), which are specifically expressed as O7–O2W and O11–O3W, and the distance between them is 2.699 and 2.788 Å, respectively. Each CyP6Q[6] is surrounded by eight free perchlorate ions, which are connected via ion–dipole interactions, the ion–dipole interactions distances range from 2.332 Å to 2.673 Å. The mode of action is shown in Fig. 6c. Fig. 6d is the stacking diagram of complex 5 viewed along the b-axis.
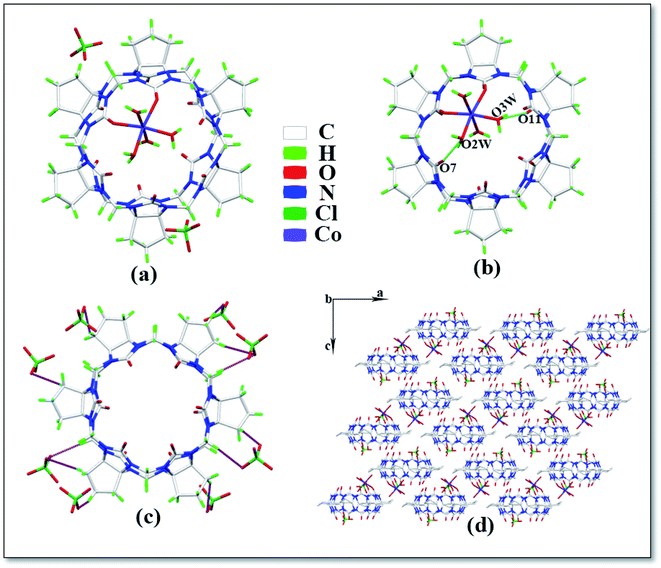 |
| | Fig. 6 Crystal structure of complex 5: (a) asymmetric unit, (b) ion–dipole interactions, (c) hydrogen atoms bond interactions and (d) stacking diagram viewed along the b-axis. | |
The asymmetric unit structure diagram of complex 6 is shown in Fig. 7a, which contains two CyP6Q[6], four Ni2+ ions, twenty coordinated water molecules, two free water molecules and eight free ClO4− ions. Among the four Ni2+ ions in this complex, two Ni2+ ions are directly coordinated with the carbonyl oxygen atom of the CyP6Q[6] portal and the Ni2+ ion of the complex has a distorted octahedral coordination configuration. Each Ni2+ ion is coordinated with two carbonyl oxygen atoms and four water molecules at one portal of the CyP6Q[6]. The coordination bond length between Ni2+ and the carbonyl oxygen atom of CyP6Q[6] is 2.080–2.087 Å, which is coordinated with the coordinated water molecule oxygen atoms. The position bond length is 1.951–2.105 Å and the bond angle around the Ni2+ ion is in the range of 77.88–96.21°. The other two Ni2+ ions are coordinated with six water molecules, but due to the forces between the CyP6Q[6] molecules, these two Ni2+ ions are not in a regular coordination configuration; it is a twisted octahedral configuration like the two Ni2+ ions participating in the coordination bond, the coordination bond length between the Ni2+ ion and coordinated water molecule oxygen atom is 1.951–2.105 Å, and the bond angle around the Ni2+ ion is 77.88–96.21°. There is a ClO4− counter ion contained in the cavity of CyP6Q[6], which is fixed in the cavity of CyP6Q[6] via ion–dipole interactions and hydrogen atoms bonding, and the remaining ClO4− ions are passed through the ion and the dipole effect surrounds the cucurbit[n]uril (Fig. 7b), the ion–dipole interactions distances range from 2.523 Å to 2.707 Å. Fig. 7c shows the cucurbit[n]urils are mainly connected via hydrogen atoms bonding in this complex. The coordinated water molecules on the Ni2+ ion that are not directly coordinated with CyP6Q[6] and the CyP6Q[6] molecules are directly connected. The coordinated water molecule on the coordinated Ni2+ ion, acts as a bridging compound connecting the CyP6Q[6] molecules. There are also hydrogen atoms bonds formed between the free water molecules and ClO4− ions in the cavity and coordinated water molecules and the carbonyl oxygen atom of CyP6Q[6]. Fig. 7d is the stacking diagram of the complex viewed along the a-axis. It can be clearly seen from the figure that there are obvious gaps between the CyP6Q[6] molecules and the ClO4− counter ions are dispersed in these gaps.
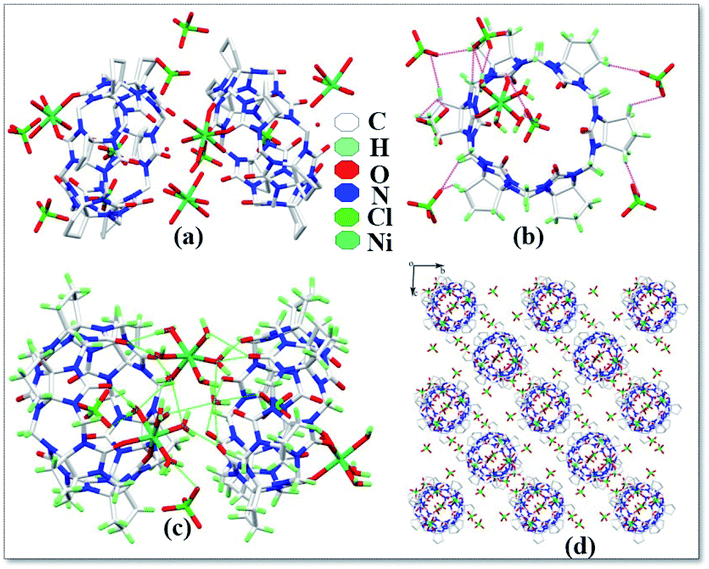 |
| | Fig. 7 Crystal structure of complex 6: (a) asymmetric unit, (b) ion–dipole interactions, (c) hydrogen atoms interactions and (d) stacking diagram viewed along the a-axis. | |
4. Conclusions
In this article, we reported the coordination of cyclopentanocucurbit[5]uril(CyP5Q[5]) and cyclopentanocucurbit[5]uril(CyP6Q[6]) with Fe(ClO4)3, Co(ClO4)2 and Ni(ClO4)2. In complex 1, 2, 3 and 5, the metal ion is directly coordinated with the portal of the cucurbit[n]uril to form a one-dimensional supramolecular chain system or independent system; in complex 4, the metal ion is not directly coordinated with the cucurbit[n]uril portal, but after forming Fe(H2O)6, it interacts with the cucurbit[n]uril port via hydrogen atoms bonding. Complex 6 is quite special. In this system, there are both metal ions directly coordinated with the cucurbit[n]uril portal and metal ions free on the outer surface of the cucurbit[n]uril. This further improved the gaps in the study of the complexes of cyclopentyl-substituted cucurbit[n]urils with metal ions, and has laid a theoretical foundation for the application of cucurbit[n]urils in the adsorption of metal ions.
Funding statement
This work was supported by the National Natural Science Foundation of China (Grant No.22161010).
Ethics statement
No related experiments.
Conflicts of interest
The authors declare on conflict of interest.
Acknowledgements
We thank the experts of the Key Laboratory of Macrocyclic Chemistry and Supramolecular Chemistry of Guizhou University for their technical guidance. We also thank several anonymous reviewers and editors who provided comments that greatly improved the manuscript.
References
- W. A. Freeman, W. L. Mock and N. Y. Shih, Cucurbituril, J. Am. Chem. Soc., 1981, 103, 7367–7368 CrossRef CAS.
- K. Kim, N. Selvapalam and D. H. Oh, Cucurbiturils-A New Family of Host Molecules, J. Inclusion Phenom. Macrocyclic Chem., 2004, 50, 31–36 CrossRef CAS.
- A. Day, A. P. Arnold, R. J. Blanch and B. Sunshall, Controlling factors in the synthesis of cucurbituril and its homologues, J. Org. Chem., 2001, 66, 8094–8100 CrossRef CAS PubMed.
- A. I. Day, R. J. Blanch, A. P. Arnold, S. Lorenzo, G. R. Lewis and I. Dance, A Cucurbituril-Based Gyroscane: A New Supramolecular Form, Angew. Chem., 2002, 41, 275–277 CrossRef CAS.
- S. Liu, P. Y. Zavalij, V. Sindelar and L. Isaacs, Reasons Why Aldehydes Do Not Generally Participate in Cucurbit[n]uril Forming Reactions, J. Am. Chem. Soc., 2005, 127, 16798–16799 CrossRef CAS PubMed.
- X. J. Cheng, L. L. Liang, K. Chen, N. N. Ji, X. Xiao, J. X. Zhang, Y. Q. Zhang, S. F. Xue, Q. J. Zhu, X. L. Ni and Z. Tao, Twisted Cucurbit[14]uril, Angew. Chem., Int. Ed., 2013, 52, 7252–7255 CrossRef CAS PubMed.
- A. Flinn, G. C. Hough, J. F. Stoddart and D. J. Williams, Decamethylcucurbit[5]uril, Angew. Chem., Int. Ed., 1992, 31, 1475–1477 CrossRef.
- J. Z. Zhao, H. J. Kim, J. H. Oh, S. Y. Kim, J. W. Lee, S. Sakamoto, K. Yamaguchi and K. Kim, Cucurbit[n]uril Derivatives Soluble in Water and Organic, Solvents, Angew. Chem., 2001, 113, 4363–4365 CrossRef.
- F. Wu, L. H. Wu, X. Xiao, Y. Q. Zhang, S. F. Xue, Z. Tao and A. I. Day, Locating the cyclopentano cousins of the cucurbit[n]uril family, J. Org. Chem., 2012, 77, 606–611 CrossRef CAS PubMed.
- L. H. Wu, X. L. Ni, F. Wu, Y. Q. Zhang, Q. J. Zhu, S. F. Xue and Z. Tao, Crystal structures of three partially cyclopentano-substituted cucurbit[6]urils, J. Mol. Struct., 2009, 920, 183–188 CrossRef CAS.
- S. Y. Jon, N. Selvapalam, D. H. Oh, J. K. Kang, S. Y. Kim, Y. J. Jeon, J. W. Lee and K. Kim, Facile Synthesis of Cucurbit[n]uril Derivatives via Direct Functionalization: Expanding Utilization of Cucurbit[n]uril, J. Am. Chem. Soc., 2003, 125, 10186–10187 CrossRef CAS PubMed.
- H. Isobe, S. Sato and E. Nakamura, Synthesis of Disubstituted Cucurbit[6]uril and Its Rotaxane Derivative, Org. Lett., 2002, 4, 1287–1289 CrossRef CAS PubMed.
- X. L. Ni, X. Xiao, H. Cong, Q. J. Zhu, S. F. Xue and Z. Tao, Self-Assemblies Based on the “Outer-Surface Interactions” of Cucurbit[n]urils: New Opportunities for Supramolecular Architectures and Materials, Acc. Chem. Res., 2014, 47, 1386–1395 CrossRef CAS PubMed.
- S. Y. Jon, N. Selvapalam, D. H. Oh, J. K. Kang, S. Y. Kim, Y. J. Jeon, J. W. Lee and K. Kim, Facile Synthesis of Cucurbit[n]uril Derivatives via Direct Functionalization: Expanding Utilization of Cucurbit[n]uril, J. Am. Chem. Soc., 2003, 125, 10186–10187 CrossRef CAS PubMed.
- L. B. Lu, D. H. Yu, Y. Q. Zhang, Q. J. Zhu, S. F. Xue and Z. Tao, Supramolecular assemblies based on some new methyl-substituted cucurbit[5]urils through hydrogen atoms bonding, J. Mol. Struct., 2008, 885, 70–75 CrossRef CAS.
- Y. Meng, W. W. Zhao, J. Zheng, D. f. Jiang, J. Gao, Y. M. Jin and P. H. Ma, Host-guest modes and supramolecular frameworks of complexes of tetramethyl cucurbit[6]uril with 4-chloroaniline and 4,4′-diaminostilbene, RSC Adv., 2021, 11, 3470–3475 RSC.
- J. Zheng, W. W. Zhao, Y. Meng, Y. M. Jin, J. Gao and P. H. Ma, Supramolecular Self-Assembly of Cyclopentyl-Substituted Cucurbit[n]uril with Fe3+, Fe2+, and HClO4 Based on Outer Surface Interaction, Cryst. Res. Technol., 2021, 56, 2000183 CrossRef CAS.
- S. Y. Cheng, W. W. Zhao, X. N. Yang, Y. Meng, L. T. Wei, Z. Tao and P. H. Ma, The binding behaviours between cyclopentanocucurbit[6]uril and three amino acids, R. Soc. Open Sci., 2021, 8, 202120 CrossRef CAS PubMed.
- J. Gao, Y. Meng, W. W. Zhao, D. F. Jiang, Y. M. Jin, J. Zheng, X. N. Yang and P. H. Ma, Host-Guest Interactions of Cyclopentanocucurbit[6]uril with Alkyl Imidazolium Hydrochlorides, Chem. Res. Chin. Univ., 2021, 1–5 Search PubMed.
- G. S. Fang, W. Q. Sun, W. X. Zhao, R. L. Lin, Z. Tao and J. X. Liu, Host-guest complexation of di-cyclohexanocucurbit[6]uril and hexa-cyclohexanocucurbit[6]uril with alkyldiammonium ions: a comparative study, Org. Biomol. Chem., 2016, 14, 674–679 RSC.
- C. Zhang, Y. Q. Zhang, S. F. Xue, Q. J. Zhu and T. Zhu, Coordination of lanthanide cations to a symmetrical dicyclohexanocucurbit[6]uril in the presence of tetrachlorozincate facilitates isolation of lighter lanthanides, Inorg. Chim. Acta, 2016, 450, 258–262 CrossRef CAS.
- Y. M. Jin, D. F. Jiang, Y. Meng, J. Gao, J. Zheng and P. H. Ma, Crystal structure of self-assembled inclusion complex of symmetric dicyclohexanocucurbit[6]uril with 1H-benzotriazole, J. Inclusion Phenom. Macrocyclic Chem., 2021, 1–7 Search PubMed.
- Y. Zhao, V. Mandadapu, H. Iranmanesh, J. E. Beves and A. I. Day, The Inheritance Angle: A Determinant for the Number of Members in the Substituted Cucurbit[n]uril Family, Org. Lett., 2017, 19, 4034–4037 CrossRef CAS PubMed.
- L. T. Wei, Y. Q. Zhang, K. Z. Zhou, L. L. Zhan, Y. X. Qu, Z. Tao and P. H. Ma, Coordination of fully substituted cyclopentano cucurbit[5]uril with alkali cation in the presence of tetrachloridezicate anion, Inorg. Chim. Acta, 2016, 445, 1–7 CrossRef CAS.
- L. T. Wei, Y. Q. Zhang, K. Z. Zhou, L. L. Zhan, Y. X. Qu, Z. Tao and P. H. Ma, Coordination of fully substituted cyclopentano cucurbit[5]uril with alkaline earth cations in the presence of tetrachlorozincate anions, Inorg. Chim. Acta, 2016, 453, 277–283 CrossRef CAS.
- Y. X. Qu, Y. Q. Zhang, K. Z. Zhou, L. T. Wei, L. L. Zhan, S. Y. Cheng, Z. Tao and P. H. Ma, Interaction of cyclopentano cucurbit[6]uril with alkaline earth cations and supramolecular assemblies with aid of [ZnCl4]2-, Chemistryselect, 2017, 2(16), 4360–4363 CrossRef CAS.
- Y.-X. Qu, K.-Z. Zhou, K. Chen, Y.-Q. Zhang, X. Xiao, Q. Zhou, Z. Tao, P.-H. Ma and G. Wei, Coordination and supramolecular assemblies of fully substituted cyclopentanocucurbit[6]uril with lanthanide cations in the presence of tetrachlorozincate anions, and their potential applications, Inor. Chem., 2018, 57(12), 7412–7419 CrossRef CAS PubMed.
- W. W. Zhao, L. T. Wei, K. Z. Zhou and P. H. Ma, A study on the coordination of fully substituted cyclopentanocucurbit[5,6]uril with copper and zinc ions, ChemistrySelect, 2019, 4, 11674–11677 CrossRef CAS.
- J. Zheng, Y. Meng, L. Zhang, X. N. Yang and P. H. Ma, Metal-induced different structures of four cyclopentanocucurbit[5]uril-based complexes, Inorg. Chim. Acta, 2022, 529, 120669 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2022 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a
*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 692,1 2
692,1 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 694,2 2
694,2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 707,3 2
707,3 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 712,4 20
712,4 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713 ref. 5 and 2
713 ref. 5 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 724.6 These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/datarequest/cif. The crystal parameters, data acquisition conditions, and parameters of the complexes 1–3 are listed in Table 1 and 4–6 are listed in Table 2.
724.6 These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/datarequest/cif. The crystal parameters, data acquisition conditions, and parameters of the complexes 1–3 are listed in Table 1 and 4–6 are listed in Table 2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 242/1737
242/1737![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 835/1592
835/1592![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 988/937
988/937![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 385(5)
385(5)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022/969
022/969![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 444/2245
444/2245







