Open Access Article
Open Access ArticleDevelopment of a low-melting-point eutectic salt and evaluation of its discharge performance for light weight thermal batteries†
Tae-Young Ahn *,
Hae-Won Cheong,
Seung-Ho Kang,
Jae-In Lee,
Minu Kim and
Yusong Choi
*,
Hae-Won Cheong,
Seung-Ho Kang,
Jae-In Lee,
Minu Kim and
Yusong Choi *
*
Agency for Defense Development, P.O. Box 35, Yuseong, Daejeon 34186, Republic of Korea. E-mail: tyahn84@gmail.com; richpine87@gmail.com
First published on 9th August 2022
Abstract
This paper proposes low-melting-point eutectic salts containing RbCl as electrolytes for light weight thermal batteries. The handleability of the eutectic salts was remarkably improved for commercialisation. Their performance as thermal battery molten-salt electrolytes was verified using tests on a single cell and a 12-cell stacked battery.
Thermal batteries are reserve batteries that are maintained in an inactivated state at room temperature and can be activated within a few seconds by melting a solid electrolyte using a heat source.1–7 Owing to zero self-discharge during storage, thermal batteries can be stored for more than 10 years without any degradation in their performance. In addition, their structural stability and reliability enable them to withstand vibration, shock, and temperature variations; thus, they can be used as power sources for guided weapons and space-launched vehicles.1–7 Thermal batteries operate at a high temperature owing to the heat source. Due to the high operating temperature, thermal batteries have a high ionic conductivity (1–5 S cm−1) compared with lithium-ion batteries (∼10−2 S cm−1). Thus, they have excellent high-output discharge characteristics.8,9 In the case of a lithium secondary battery, it has been reported that the performance decreases due to the low ionic conductivity of the electrolyte below 0 °C. Owing to the high ionic conductivity of thermal batteries, they can be used in a wide temperature range (−55 to +75 °C), including low temperatures, without performance degradation.1
A eutectic salt comprising lithium fluoride (LiF)–lithium chloride (LiCl)–lithium bromide (LiBr) or lithium chloride (LiCl)–potassium chloride (KCl) has been commercialised and used as an electrolyte for thermal batteries.4 An operational temperature of 500 °C or higher must be maintained to melt the solid electrolyte. Thus, the weight of the high-density heat pellet increases owing to the large amount of heat produced, which negatively affects the specific capacity of the thermal battery. Additionally, as the electrode to be used in a thermal battery must possess high thermal stability to sustain its high operational temperature, the electrodes are restricted to materials such as Li–Si alloys for anodes and intermetallic sulphides for cathodes.4,5
The open-circuit voltage of a thermal battery is relatively low compared with that of a Li-ion battery. To apply high-voltage cathode materials in thermal batteries, a eutectic salt with a low melting point must be used as the electrode material because of the thermal instability of the materials used in other batteries, such as Li-ion batteries. Masset and Guidotti8 reported that the melting point of eutectic salts could be reduced by using an electrolyte containing iodine; however, they also found that lithium iodide cannot be applied to commercial thermal batteries owing to the difficulty of handling it in dry room conditions. Therefore, lowering the melting point of the electrolyte, which can be handled in dry room conditions, plays a key role in improving the specific capacity of thermal batteries.
In this paper, we propose a eutectic salt composed of rubidium chloride (RbCl) with improved handleability, a low melting point, and an enhanced specific capacity. We also evaluated the performance of this eutectic salt as an electrolyte in a single cell and a 12-cell stacked thermal battery.
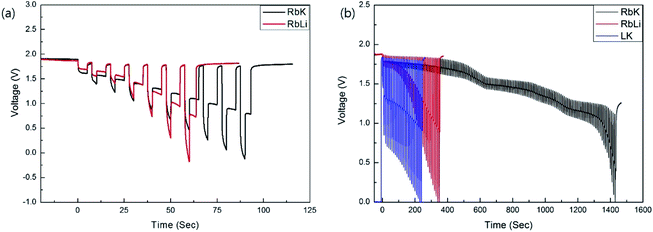
The composition of the RbCl-containing eutectic salt was predicted using the FactSage thermochemical software. To fabricate the predicted eutectic salts, LiBr, KBr, RbCl, and LiCl were purchased from Sigma-Aldrich (St. Louis, MO, USA). The chemical composition of the eutectic electrolytes was 0.54LiBr–0.23KBr–0.23RbCl (wt%, RbK) and 0.17LiCl–0.16LiBr–0.67RbCl (wt%, RbLi). The melted electrolytes were immediately quenched to room temperature (25 °C) in a square-shaped tray, which was fabricated using Inconel alloy to inhibit corrosion by a highly reactive Li-containing electrolyte. The eutectic electrolyte consisted of a 55 wt% eutectic salt and 45 wt% MgO, where the latter serves as a binder to prevent the molten salt from flowing down under the discharge conditions of the thermal battery, operating at high temperature. After manufacturing the eutectic salt with a low melting point, their microstructures were observed using a scanning electron microscope (SEM; FEI-Helios). The melting point and heat of fusion of the fabricated eutectic salts were measured using differential scanning calorimetry (DSC). To carry out the battery discharge process, pellets with a diameter of 26 mm for the electrolyte and electrode were fabricated via cold pressing. The Li–Si alloy and FeS2 were used as the negative and positive electrodes, respectively. The single cells were discharged at 325 and 375 °C while applying a pulse current profile of 0.5 A (0.1 A cm−2) for 2.5 s, 1 A (0.2 A cm−2) for 2.5 s, and 0 A for 1 s (0.1 Aavg cm−2) under a DC electric load (SL-600, Unicorn) at a force of 100 kgf. Before the single-cell discharge was performed, the fabricated single cell was rested for 1 min to allow the electrolyte to melt in the temperature range of 325–375 °C, and the discharge test was terminated when the voltage dropped to 0 V. Twelve battery cells were stacked with minimum thermal insulation around the cells to fabricate the thermal battery. Subsequently, the battery was discharged under a high constant current (0.6 A cm−2).
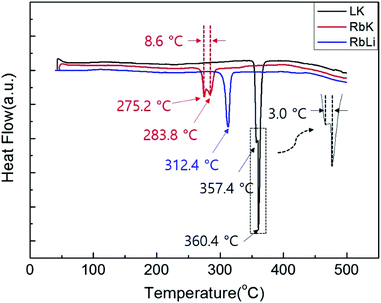
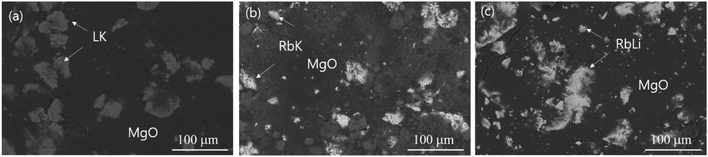
The DSC curves were plotted to measure the melting point and heat of fusion of the fabricated eutectic salts. Fig. 1 shows the DSC curves for the eutectic electrolyte compositions comprising RbK, RbLi, and LiCl–KCl (LK). As shown in Fig. 1, the melting point of the RbK eutectic salts was measured as 275.2 and 283.8 °C. We believe that the two peaks observed in the melting temperature are due to non-uniform mixing; however, commercial LK eutectic salts also show the same pattern. Fig. S1† reveals a marginal difference in the melting points measured via DSC, but the results are consistent with the melting point (238 °C) of eutectic salts calculated using the FactSage thermochemical software.10 Similar to the results obtained for RbK, the melting point of RbLi was measured as 315.2 °C, consistent with the eutectic point temperature of 275 °C calculated by the FactSage thermochemical software. In addition to RbK and RbLi, the melting point of the LK eutectic salt as a comparison group was measured via DSC. Table 1 shows the measured heat of fusion from the DSC results. The heat of fusion of LK, RbK, and RbLi was measured as 195.06, 25.69, and 109.71 J g−1, respectively. RbK was confirmed to exhibit the lowest heat of fusion value (i.e. 25.69 J g−1), which is approximately 7 times lower than that of LK, a commercial electrolyte salt used for thermal batteries. Thus, because the heat of fusion was confirmed to be remarkably low for RbK, it is expected that the heat required to operate the thermal battery will be drastically reduced, thereby reducing the weight of the thermal battery. Fig. 2 shows the SEM images of the LK, RbK, and RbLi eutectic salts after mixing them with MgO; the white contrast in the figure corresponds to the eutectic salts. We found that the RbK and RbLi eutectic salts contained fine particles compared with the LK eutectic salt. The fine particles observed in the SEM images are consistent with the results obtained from filtering out a large number of fine particles when the molten eutectic salt is quenched and pulverised for particle size classification to manufacture an electrolyte for thermal batteries. According to Lai et al.,11 the heat of fusion decreases as the particle size decreases. Thus, one of the reasons for the low melting point and heat of fusion of molten salt containing RbCl is the fine particle size (<∼100 nm) compared with that of the conventional molten salt (LK). Performance testing via single-cell battery discharge is essential for manufacturing thermal batteries. The discharge performance of the battery containing an electrolyte composed of a eutectic salt with a low melting point was evaluated in the temperature range of 325–375 °C. Fig. S2† shows a single cell before and after assembly. The internal resistance of the battery was measured by applying a pulse current, and its value was obtained using the following equation:12
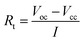 | (1) |
| Li22Si5→ Li13Si4 → Li7Si3 → Li12Si7 | (2) |
| Eutectic salt composition | LK | RbK | RbLi |
|---|---|---|---|
| Heat of fusion (J g−1) | 195.06 | 25.69 | 109.71 |
Li22Si5 is known to possess a high reactivity to moisture; therefore, it is difficult to handle under dry room conditions, i.e. with < 3% relative humidity. Thus, several thermal battery research groups and this study used the Li13Si4 transition. Because the anode is not in excess with respect to the cathode, the plateaus are related to the phase transition Li13Si4 → Li7Si3 and Li7Si3 → Li12Si714,15
It has been previously reported that the sensitivity of alkali halides to moisture decreases from Li to Na and K.8 Therefore, the moisture sensitivity and handling of eutectic salts in dry room conditions designed in this study could be improved by including Rb in the eutectic salt composition. Fig. S3† reveals the discharge profile at 375 °C after manufacturing the electrolyte and storing it in a dry room (RH < 1.5%) for 1 month. Considering a discharge current of 0.25 Aavg, which was 0.5 Aavg in the plotted graph, excellent discharge performance was confirmed even after storage for 1 month in a dry room.
It is generally accepted that the addition of eutectic salt to the cathode and anode contributes to the enhancement of the ionic conductivity of thermal batteries; therefore, eutectic salts comprising LK have been adopted in conventional thermal battery systems. By adding a eutectic salt containing RbCl to the Li–Si alloy (anode) and FeS2 (cathode), the moisture vulnerability is improved, and the discharge performance of the thermal battery can be improved when it operates at temperatures lower than 500 °C, which is the operating temperature of the conventional thermal battery. Fig. 4 shows the discharge results and corresponding internal resistance when a low-melting-point eutectic salt, RbK, and a commercialised salt, LK, are added to the cathode and anode. The discharge time can be observed to be significantly improved in the case of RbK in comparison to LK. Therefore, in terms of internal resistance, it is reasonable to assume that applying RbK to both negative and positive electrodes will lead to an optimal thermal battery performance, especially at low discharge temperatures. Although the quantity of salt added to the anode and cathode is comparably less than the electrolyte, the salt does not adequately melt when discharging at a relatively low temperature when using the LK. This can be attributed to an increase in the internal resistance. If RbK is used as a component of the eutectic salts added to the anode and cathode, it is reasonable to assume that an optimised discharge performance can be achieved in a thermal battery that requires discharge at a relatively low temperature, along with weight reduction, or in a long-life thermal battery. Fig. S4† shows no change in the appearance after the discharge of a single cell. Thus, it can be inferred that the proposed eutectic salt exhibits excellent wettability with the MgO binder. In this study, 45 wt% of MgO was used as the binder; the resultant eutectic salt was tested for comparison with the commercially available eutectic salts. MgO is a ceramic material that increases the internal resistance of the thermal cell.8 When the thermal battery operates at low temperatures, the amount of MgO can be reduced to reduce the internal resistance of the thermal battery. A thermal battery with an enhanced performance can be built based on the optimal composition and microstructures of MgO for this eutectic salt. Fig. S5† shows the results of testing a thermal battery stacked with 12 cells using the proposed low-melting-point eutectic salt. It was confirmed that the battery exhibited an optimal performance even under a high current discharge condition of 0.6 A cm−2. For a thermal battery to be used as a general power source, its specific capacity must be improved. The proposed low-melting-point eutectic salt reduces the amount of high-density heat sources, introducing the possibility of decreasing the weight of the thermal battery. In addition, because the standard of thermal stability required for the electrode of a thermal battery is relaxed, the overall performance, including the specific capacity, can be improved by using a high-voltage cathode material and fine electrode powder with excellent high-power characteristics. Thus, it is expected that the improved thermal battery with excellent discharge performance at low temperatures can be used as a drone power source, especially in low-temperature environments.
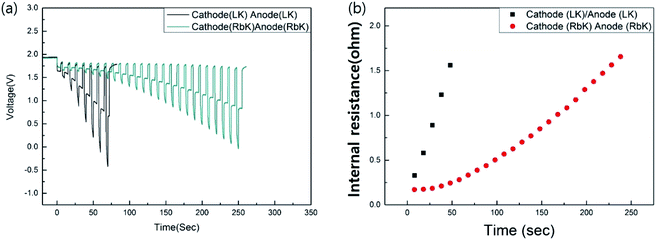 | ||
| Fig. 4 (a) Effect of salt addition on discharge performance and (b) corresponding internal resistance. | ||
In this study, a eutectic salt composition containing RbCl was predicted and fabricated using thermodynamic simulations. The DSC results indicate that the melting points of the fabricated eutectic salts are 275 (RbK) and 312 (RbLi) °C. We confirmed that the thermal cell comprising the RbK eutectic salt could be successfully used for cell operation in a temperature range of 325–375 °C via single-cell discharge. Furthermore, the RbK eutectic salt has excellent storage and handling properties in a dry room (RH < 1.5%).
Conflicts of interest
There are no conflicts to declare.Notes and references
- R. A. Guidotti and P. M. Masset, J. Power Sources, 2006, 161, 1443–1449 CrossRef CAS.
- R. A. Guidotti and P. J. M. Masset, J. Power Sources, 2008, 183, 388–398 CrossRef CAS.
- P. J. Masset and R. A. Guidotti, J. Power Sources, 2008, 177, 595–609 CrossRef CAS.
- P. J. Masset and R. A. Guidotti, J. Power Sources, 2008, 178, 456–466 CrossRef CAS.
- P. Masset, J. Poinso and J. Poignet, J. Power Sources, 2004, 137, 140–144 CrossRef CAS.
- Y. Choi, H. R. Yu, H. Cheong, S. Cho and Y.-S. Lee, Appl. Chem. Eng., 2014, 25, 161–166 CrossRef CAS.
- Y. Choi, H. R. Yu, H. Cheong, S. Cho and Y.-S. Lee, Appl. Chem. Eng., 2014, 25, 72–77 CrossRef CAS.
- P. Masset and R. A. Guidotti, J. Power Sources, 2007, 164, 397–414 CrossRef CAS.
- J. Jaguemont, L. Boulon and Y. Dube, Appl. Energy, 2016, 164, 99–114 CrossRef CAS.
- L. I. Hanxu, Y. Ninomiya, D. Zhongbing and Z. Minxu, Chin. J. Chem. Eng., 2006, 784–789 Search PubMed.
- S. L. Lai, J. Y. Guo, V. Petrova, G. Ramanath and L. H. Allen, Phys. Rev. Lett., 1996, 77, 99–102 CrossRef CAS PubMed.
- S. Fujiwara, M. Inaba and A. Tasaka, J. Power Sources, 2011, 196, 4012–4018 CrossRef CAS.
- C. J. Wen and R. A. Huggins, J. Solid State Chem., 1981, 37, 271–278 CrossRef CAS.
- L. Redey and R. A. Guidotti, Proceedings of the 37th Power Sources Conference, vol. 255, 1996 Search PubMed.
- J. D. P. Payne, K. Giagloglou, C. J. Crouch, G. M. Carins, R. I. Smith, R. Comrie, R. K. B. Gover and J. T. S. Irvine, ChemElectroChem, 2017, 4, 1–9 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03436k |
| This journal is © The Royal Society of Chemistry 2022 |