Open Access Article
Open Access ArticleElectrochemical performance of CoSe2 with mixed phases decorated with N-doped rGO in potassium-ion batteries†
Hui Zheng‡
b,
Han-Shu Xu‡ *ab,
Jiaping Hub,
Huimin Liub,
Lianwei Weib,
Shusheng Wub,
Jin Lib,
Yuhu Huangb and
Kaibin Tang
*ab,
Jiaping Hub,
Huimin Liub,
Lianwei Weib,
Shusheng Wub,
Jin Lib,
Yuhu Huangb and
Kaibin Tang *ab
*ab
aHefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026, People's Republic of China. E-mail: xhs@ustc.edu.cn; kbtang@ustc.edu.cn
bDepartment of Chemistry, University of Science and Technology of China, Hefei, 230026, People's Republic of China
First published on 2nd August 2022
Abstract
Potassium-ion batteries (PIBs) have received much attention as next-generation energy storage systems because of their abundance, low cost, and slightly lower standard redox potential than lithium-ion batteries (LIBs). Nevertheless, they still face great challenges in the design of the best electrode materials for applications. Herein, we have successfully synthesized nano-sized CoSe2 encapsulated by N-doped reduced graphene oxide (denoted as CoSe2@N-rGO) by a direct one-step hydrothermal method, including both orthorhombic and cubic CoSe2 phases. The CoSe2@N-rGO anodes exhibit a high reversible capacity of 599.3 mA h g−1 at 0.05 A g−1 in the initial cycle, and in particular, they also exhibit a cycling stability of 421 mA h g−1 after 100 cycles at 0.2 A g−1. Density functional theory (DFT) calculations show that CoSe2 with N-doped carbon can greatly accelerate electron transfer and enhance the rate performance. In addition, the intrinsic causes of the higher electrochemical performance of orthorhombic CoSe2 than that of cubic CoSe2 are also discussed.
1. Introduction
LIBs are widely used in the field of energy storage due to their high energy density and excellent power density.1–3 Meanwhile, the limited and unevenly distributed lithium resources not only make LIBs expensive, but also severely limits the large-scale application of LIBs.4 Consequently, it is urgent to explore new and low-cost energy storage devices. The working principles of PIBs and LIBs are similar, and the reserves of potassium resources are relatively rich, the cost is low, and the redox potential of PIBs is very close to that of lithium (K+/K: −2.93 V; Li+/Li: −3.04 V).5,6 Therefore, PIBs are one of the ideal substitutes for LIBs, especially in large-scale energy storage applications. Although theoretically feasible, there are still some difficulties in the application of PIBs.7–9 On the one hand, the diffusion process of K+ in the bulk phase of traditional electrode materials is difficult due to its large ion radius, and on the other hand, the large volume change associated with the K+ insertion/extraction of the electrode materials significantly reduces the capacity of the battery.10–12 In order to solve these technical problems, it is necessary to carry out reasonable structural and morphological design of key electrode materials for PIBs.Carbon-based materials usually show the better chemical stability, and can act as an elastic buffer carrier to increase the stability of electrode materials during circulation.13,14 The advantages of rich pore structure and high conductivity make it have excellent performance as protective load material and electrode material, so it is an important anode material of PIBs. Among them, hard carbon and N-doped carbon has more active sites and larger discharge capacity, which has attracted the attention of researchers.13,15,16 However, the current carbon electrode has a lower theoretical capacity of 372 mA h g−1, and the lower working potential will accelerate the formation of dendritic Li/Na/K at a high rate, which will greatly weaken the energy density of the battery and reduce the safety of use.13 Therefore, the development of PIBs anode composites with large capacity and excellent cycling performance is the key to overcome these shortcomings and meet the needs of large-scale energy storage equipment.
Compared with transition metal sulfides, transition metal selenium compounds not only have higher electrical conductivity, but also weaker ionic bonds and larger spacing between metal ions and selenium ions can significantly improve ionic diffusion kinetics and electrochemical performance.17,18 The disadvantage of transition metal selenides is that large particle agglomeration and severe volume expansion often occur in alloying or conversion reactions, resulting in rapid capacity loss and reduced cycle life.19 After a long period of exploration, it has been found that combination of carbon materials and transition metal selenides is an effective method to eliminate the disadvantages when they exist alone through technical means such as nano crystallization, nanostructure engineering and carbon coating.6,20 The volume changes buffered by the construction of metal-carbon and selenium-carbon chemical bonds, improving the electrochemical performance.6,21
After the development in recent years, transition metal selenides with different nano-morphologies, such as CoSe2, Ni3Se4, MoSe2, etc., are often used as anode materials for PIBs.22,23 These transition metal selenides coated with different morphologies and types of carbon show good cycle stability and large residual capacitance in PIBs. For example, Xu et al. prepared CoSe2–MoSe2/rGO composite electrode materials, and the formation of chemical bonds between the two significantly improved the charge transfer in the electrochemical cycle.23 Among these transition metal selenides for PIBs and sodium-ion batteries (SIBs), CoSe2 is special, because according to the current reports, no matter how carbon is used for modification, the electrochemical cycle performance of CoSe2 belonging to the orthorhombic system is always better than that of the cubic system.24–30 In addition, the actual reaction processes from CoSe2 with orthorhombic structure to Co and K2Se have been theoretically given, which is significantly different from LIBs and SIBs.19,20,24,31–33 To further understand the behavior of CoSe2 with different crystal systems in PIBs, more experiments and theoretical calculations are necessary.
In this study, nano-sized CoSe2 was decorated by N-doped reduced graphene oxide (denoted as CoSe2@N-rGO) through a direct one-step hydrothermal synthesis, which contains both orthorhombic and cubic phase. The electrochemical capability of the CoSe2@N-rGO compound was evaluated as anode materials for PIBs. Particularly, the CoSe2@N-rGO nanoparticles delivered a high reversible capacity of 599.3 mA h g−1 at 0.05 A g−1 in the first circle, and exhibited a reversible capacity of 421 mA h g−1 at 0.2 A g−1 after 100 cycles. The density of states (DOS) of different situations and the effect of carbon coating on the DOS was obtained by the DFT calculations, and the intrinsic physics of the excellent electrochemical performance of the orthorhombic CoSe2@N-rGO was further discussed, which is helpful to deepen understanding of composites in the electrochemical reaction.
2. Results
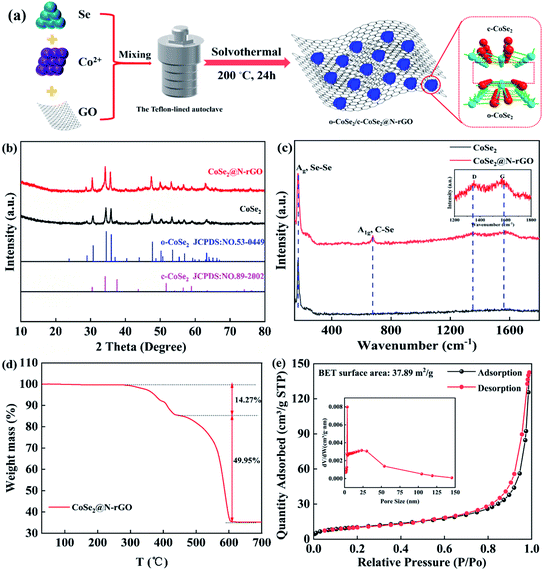
The synthesis strategy for CoSe2 encapsulated by N-doped carbon with a sandwich-like structure in a whole is schematically described in Fig. 1(a). The XRD patterns confirm that CoSe2 contains two phases (Fig. 1(b)), the main phase has an orthorhombic structure (JCPDS no. 53-0449), and the other small amount of phase belongs to cubic phase (JCPDS no. 89-2002). Importantly, this further indicates that we have successfully synthesized CoSe2 containing a small amount of cubic phase, which could form a heterojunction with orthorhombic CoSe2. Moreover, there was no carbon peak observed in XRD patterns, which implies that the low amorphous carbon content and great crystallinity of CoSe2. To gain a better understanding of amorphous carbon, samples CoSe2 and CoSe2@N-rGO were evaluated by Raman spectroscopy (Fig. 1(c)). Compared with CoSe2, there are double distinct peaks at 1337.73 and 1598.55 cm−1 for CoSe2@N-rGO, which correspond to the sp3-hybridization vibration of disordered or defective bands (D-band) and sp2-stretching of graphitic bands (G-band) of amorphous carbon, respectively. From the inset in Fig. 1(c), it is clearly visible from the intensity of D-band on CoSe2@N-rGO is comparable to that of the G-band (the ratio ID/IG ≈ 1.15), revealing that the amorphous coated carbon has more defects and disorder.13,34,35 Meanwhile, the existence of these defects and disorder can also form a strong interaction with CoSe2@N-rGO during electrochemical cycling reactions. In addition, the peak at 173.7 cm−1 (Ag) is assigned to the Se–Se in CoSe2@N-rGO, while a tiny peak associated with C–Se bond appears at 677.2 cm−1 (A1g).36–38 The presence of C–Se bond between CoSe2 and N-rGO is not simple physical contact but it is a kind of chemical connection through C–Se bond.To further understand the thermal stability of CoSe2@N-rGO and confirm the relative contents of CoSe2 and carbon of CoSe2@N-rGO composites, we performed a TG analysis of the as-synthesized samples CoSe2@N-rGO. Fig. 1(d) shows the representative TG curve on heating up from room temperature to 700 °C in hot air at a rate of 10 °C min−1. Firstly, the weight of CoSe2@N-rGO is almost unchanged below 300 °C, which indicates that it has good thermal stability. When the temperature surpasses 300 °C, the weight reduction is attributed to the oxidative decomposition of the N-rGO to CO2 and NO2. However, when the temperature above 410 °C, the rapid weight decrease should be caused by the oxidation of CoSe2 to Co3O4.38,39 Corresponding to the TG curve, the content of organic coating in CoSe2@N-rGO compounds accounts for about 14.27%. Fig. 1(e) and S1† show the isothermal nitrogen adsorption/desorption and nanopore size distribution curves of CoSe2@N-rGO and CoSe2, respectively. After comparison, it is found that the CoSe2 coated with N-doped carbon exhibits higher nitrogen adsorption/desorption value in a whole, and the curve has a smaller hysteresis behavior. The insets in Fig. 1(e) and S1† illustrate that the peaks of pore size before and after N-doped carbon coating are 18 nm and 25 nm, respectively. The introduction of N-rGO can accelerate ion diffusion, which is beneficial to the electrode reaction.40,41 Furthermore, a sharp peak at around 3.5 nm can also be clearly seen in the inset of Fig. 1(e), which corresponds to the averaged nanopore size of N-doped carbon.
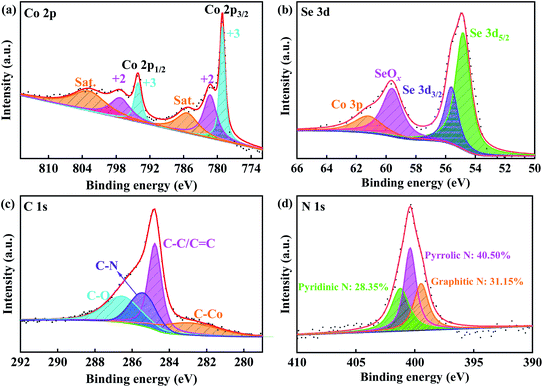
Chemical composition and chemical bonding situation of CoSe2@N-rGO composites can be determined by XPS analysis, as seen in Fig. 2(a)–(d). High-resolution spectra confirmed the existence of Co, Se, C and N elements in the CoSe2@N-rGO (Fig. S2†). Fig. 2(a) shows that there are six peaks on the Co 2p spectrum, which can be well fitted by Co3+, Co2+ and two other satellite peaks. Two broad peaks located at 797.2 eV and 781.3 eV correspond to Co2+ 2p1/2 and Co2+ 2p3/2, respectively, while the peaks at 785.4 eV and 802.8 eV stem from the corresponding satellite peaks of Co2+. The Co–O bond of Co2O3 (Co3+) due to the surface oxidization of CoSe2, resulting in peaks at 779.1 eV and 794.1 eV.37,42,43 The two peaks at 54.8 eV and 55.6 eV in Fig. 2(b) can be assigned to Se 3d5/2 and Se 3d3/2, respectively. The peaks located at 59.6 eV is corresponding to Se–O bond formed by the inevitable surface oxidation of the transition metal selenides during the test.22,23,42 The broad peak at around 61.2 eV is assigned to the Co 3p orbital.41,44
Fig. 2(c) shows the spectrum of C 1s, three peaks located at 284.7 eV, 285.1 eV and 286.3 eV should belong to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, and C–O bonds, respectively.45 Importantly, the C–Co bond can also be observed at 282.4 eV, which indicates that there is not a simple physical contact but a strong interaction between the CoSe2 and the carbon layer, thus ensuring the stability and robustness of the CoSe2@N-rGO composites.46 Furthermore, the carbon is nitrogen-doped, and the N 1s XPS spectrum of nitrogen in the carbon layer is shown in Fig. 2(d). The three peaks at 399.5 eV, 400.4 eV and 401.2 eV are corresponding to graphitic N, pyrrolic N and pyridinic N, respectively. By calculation, the content of each type of nitrogen (graphitic, pyrrolic, and pyridinic) are 31.15%, 40.50% and 28.35%, respectively. The abundant N-doping provides more exogenous defects and active centers to improve electron/ion transport in electrochemical reactions, thereby further improving conductivity and increasing reversible capacity.38,47,48
C, C–N, and C–O bonds, respectively.45 Importantly, the C–Co bond can also be observed at 282.4 eV, which indicates that there is not a simple physical contact but a strong interaction between the CoSe2 and the carbon layer, thus ensuring the stability and robustness of the CoSe2@N-rGO composites.46 Furthermore, the carbon is nitrogen-doped, and the N 1s XPS spectrum of nitrogen in the carbon layer is shown in Fig. 2(d). The three peaks at 399.5 eV, 400.4 eV and 401.2 eV are corresponding to graphitic N, pyrrolic N and pyridinic N, respectively. By calculation, the content of each type of nitrogen (graphitic, pyrrolic, and pyridinic) are 31.15%, 40.50% and 28.35%, respectively. The abundant N-doping provides more exogenous defects and active centers to improve electron/ion transport in electrochemical reactions, thereby further improving conductivity and increasing reversible capacity.38,47,48
In order to directly observe the coating of N-doped reduced graphene oxide on CoSe2 nanoparticles, SEM was used for characterization. Fig. S3(a)† shows that the overall shape of uncoated CoSe2 is not uniform, some are spherical, some are flake, and some are rod-like structures, which also reflects CoSe2 synthesized by one-step method is a mixed phase with multiple structures. The nano-sized CoSe2 results of TEM in Fig. S3(b)† also further verify the results of SEM. It is shown that the CoSe2 nanoparticles are evenly distributed in CoSe2@N-rGO, the SEM images with a large-scale range in Fig. 3(a), signifying that one-step synthesis method we used can also effectively avoid the aggregation of CoSe2 nanoparticles. From Fig. 3(b), the N-doped carbon tightly wraps CoSe2, which enables the chemical structure stability of CoSe2 to be effectively improved during electrochemical cycling. In addition, the size of CoSe2@N-rGO nanoparticles is about 150–200 nm. The TEM and HRTEM images in Fig. 3(c) and S3(d)† further elucidated that the CoSe2@N-rGO with uniform size are homogenously well-encapsulated in N-doped carbon frame network rather than exposed to the surface. Fig. 3(d) shows the HRTEM image of CoSe2@N-rGO, the d = 0.262 nm is assigned to the (210) crystal planes of CoSe2 with cubic crystal structure, whereas the (111) plane of CoSe2 with orthorhombic crystal structure comes down to the interplanar distance of 0.259 nm. In fact, these two different crystals of CoSe2 can be effectively constructed into heterointerface, which can improve the electrochemical reaction kinetics by promoting the transport of alkali metal ions. Additionally, Fig. 3(e) exhibit the evenly distribution of Co, Se, C and N elements in the chosen CoSe2@N-rGO composites. Moreover, from the C, N, Co, and Se peak intensity line traces in the EDX spectra of CoSe2@N-rGO composites (shown in Fig. S3(e)†), it is concluded that C, N, Co, and Se have been almost homogeneously distributed throughout the samples. From the EDS mapping in Fig. S3(c),† it also can be seen that the pristine CoSe2 contains a very small amount of N and C, which is mainly due to the inevitable introduction of C and N from ethylenediamine during the reaction. The prominent Cu element peaks in EDX originate from the copper-containing sample holder.
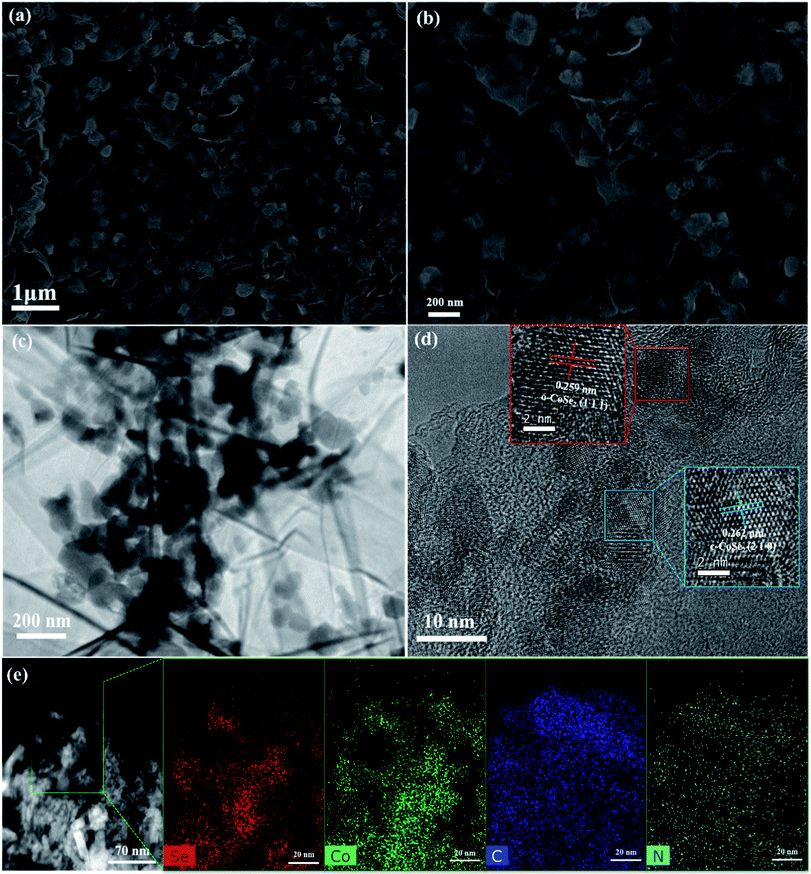 | ||
| Fig. 3 Morphology and microstructure characterizations of the CoSe2@N-rGO: (a and b) SEM, (c) TEM, (d) HRTEM, and (e) EDS mappings of the Se, Co, C and N elements. | ||
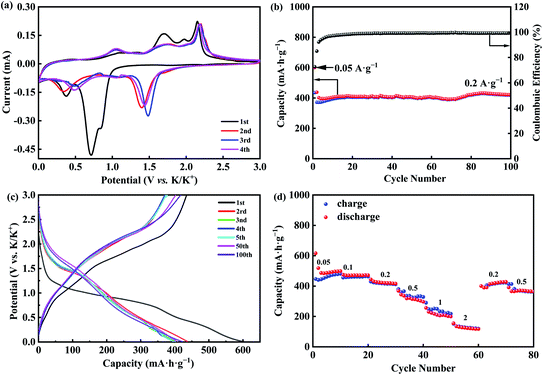
To further understand the mechanism of potassium storage, CR2032 coin-type cells were assembled at room temperature to systematically study the electrochemical performance of the CoSe2@N-rGO. The CV curves of Fig. 4(a) shows that during the first discharge (cathodic) scan, a strong reduction peak locate at 0.71 V and at 0.82 V has a small kink, which corresponds to the formation of Co3KSe4 by inserting K+ into the CoSe2 layer, accompanied by the formation of solid electrolyte interface (SEI) layer on the electrode and electrolyte interface. Meanwhile, due to the reduction of Co3KSe4 to Co and K2Se by K+, another small reduction peak can be observed at 0.35 V. In contrast, the oxidation peaks in 1.12 V and 1.67 V are consistent with the sequential reverse conversion reaction from K2Se and Co to Co3KSe4 in the first charging (anodic) cycle.49 A stronger oxidation peak appears at 2.26 V and a minor peak lies in 2.1 V, which are attributed to the de-intercalation of K+ from Co3KSe4 to CoSe2. In the following CV scans, the peak positions of the reduction peaks shift to higher voltages, while the oxidation peaks are almost unchanged, indicating that the polarization of CoSe2@N-rGO electrode is small.43,50 In fact, the apparent difference between the first scan and the subsequent scan is mainly due to the formation of SEI.51,52
It is well known that the repeated insertion/extraction of large K+ often leads to structural failure, so that cycle performance is vital to rechargeable PIBs. From Fig. 4(b), the CoSe2@N-rGO anode material shows the capacity of 599.3 mA h g−1 at 0.05 A g−1 during the initial circle, and keeps 421 mA h g−1 at 0.2 A g−1 after 100 cycles, which prove CoSe2@N-rGO present better cycling stability. In order to highlight the significant function of N-doped carbon coating, we also tried to conduct electrochemical experiments with uncoated CoSe2, and the results show that the battery capacity decays rapidly with the increase of the number of cycles (shown in Fig. S4†). The charge/discharge profiles in Fig. 4(c) are consistent with the CV profiles. The CoSe2@N-rGO anode provides a large capacity of 599.3 mA h g−1 with a low initial coulombic efficiency of 72.6% during the initial cycle. In the following cycles, the reversible discharge curves have good coincidence, denoting that the electrochemistry maintains the cycle stability. In should be noted that the capacity decreases and coulombic efficiency increases during first three cycles due to formation of stable SEI film.51 Moreover, the discharge curve exhibits a slightly sloping step at around 1.5 V and about 2.0 V on the charge curve, which agree well with the 1.48 V reduction peak and the 2.1 V oxidation peak on the CV curve, respectively. Interestingly, with the increase of the number of cycles, the possible reason for the increase of capacity is the electrochemical reconstruction or electrochemical activation process during the reaction, which can provide plentiful sites for K+-storage.53
Fig. 4(d) demonstrates the rate performance of CoSe2@N-rGO composites at several current densities. The CoSe2@N-rGO reversible capacities obtained are 498.5, 470.9, 415.8, 296.8 and 199.3 mA h g−1 at the different current density of 0.05 A g−1, 0.1, 0.2, 0.5 and 1 A g−1. Interestingly, CoSe2@N-rGO has an excellent reversible capacity of 116.7 mA h g−1 when the current density is even up to 2 A g−1. Importantly, due to the rearrangement of structure in the process of reaction activation and the production of more active sites when K+ insertion/extraction during the reaction, the capacity could recover to close to the previous values of 427.1 and 364.1 mA h g−1 after 60 cycles whether the current density returns to 0.2 or 0.5 A g−1, indicating fine reliability and rate performance.54 For the long-term cycling performance by the extended charge and discharge experiments, the initial discharge capacity shown in Fig. S5(a)† is about 624.9 mA h g−1 at 0.05 A g−1 with initial coulombic efficiency of 72.2%, which is relatively close to the low current 0.05 A g−1 as in Fig. 4(d). As shown in Fig. S5(a) and (b),† the capacity begins to decay after about 200 cycles, while the reversible capacity of CoSe2@N-rGO is only 174.7 mA h g−1 beyond 400 cycles at 1 A g−1. Therefore, it can be speculated that the structural distortion of CoSe2 leads to the decreased long-term cycling performance during the long-term cycling of potassiation/de-potassiation.
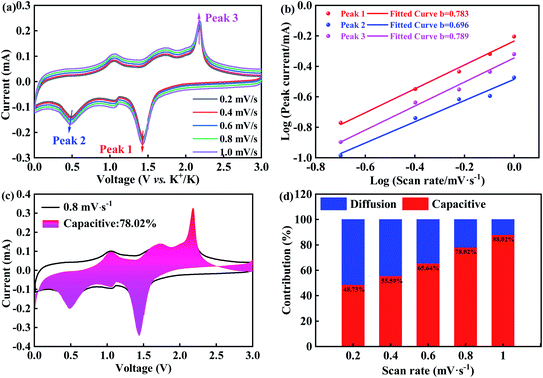
Fig. 5(a) plots the CV curves at various scan rates ranging from 0.2 to 1.0 mV s−1. Overall, the CV loops exhibit similar shapes at different scanning rates, indicating that there is the identical electrochemical process at the scanning rate. Based on the Bruce Dunn's theory,55,56 by the following equations, the relationship between the peak current (i) and scan rate (v) can be expressed:
| i = avb | (1) |
| log(i) = log(a) + blog(v) | (2) |
To investigate their contributions, the following eqn (3) is used,
| i = k1v + k2v1/2 | (3) |
3. Discussion
In this work, we synthesized CoSe2 with two different crystal structures by one-step method, in which the orthorhombic one is the dominant phase. In CoSe2 with mixed phase, the electrochemical cycling process consists of three independent processes, one for orthorhombic system, one for cubic system, and the last is for heterojunction formed by orthorhombic and cubic system, among which the first is the main one. Table S1 and S2† show that as the anode materials of PIBs and SIBs, CoSe2 with orthogonal phase often exhibits better cycling performance than that with cubic phase, although both have experienced different forms of coating. Although some previous experimental works have given the reaction equations during electrochemical cycling, it is inaccurate to simply replace Li/Na with K in the equation of LIBs and SIBs due to the larger radius of K+. The process of K+ intercalation into CoSe2 with orthogonal phase has been carefully studied by the first-principles calculations, and the reaction equation is as follows:58| CoSe2 + 4/3K+ + 4/3e− → 1/3Co3Se4 + 2/3K2Se | (4) |
| Co3Se4 + K+ + e− → Co3KSe4 | (5) |
| Co3KSe4 + K+ + e− → 3CoSe + K2Se | (6) |
| CoSe + 2K+ + 2e− → Co + K2Se | (7) |
In charging process, the reaction equation can be summarized into the following steps:
| 4K2Se + 3Co → Co3KSe4 + 7K+ + 7e− | (8) |
| Co3KSe4 + 2K2Se → 3CoSe2 + 5K+ + 5e− | (9) |
As shown in Fig. 4(a), our CV results are basically consistent with the experimental results of Yu et al., eqn (4)–(7) can also well describe our discharge processes.58 In addition, compared with Yu's results, there is no additional peak on our CV curve, so it can be inferred that the above reaction eqn (4)–(9) are also followed for the CoSe2 with cubic phase during electrochemical cycling.58
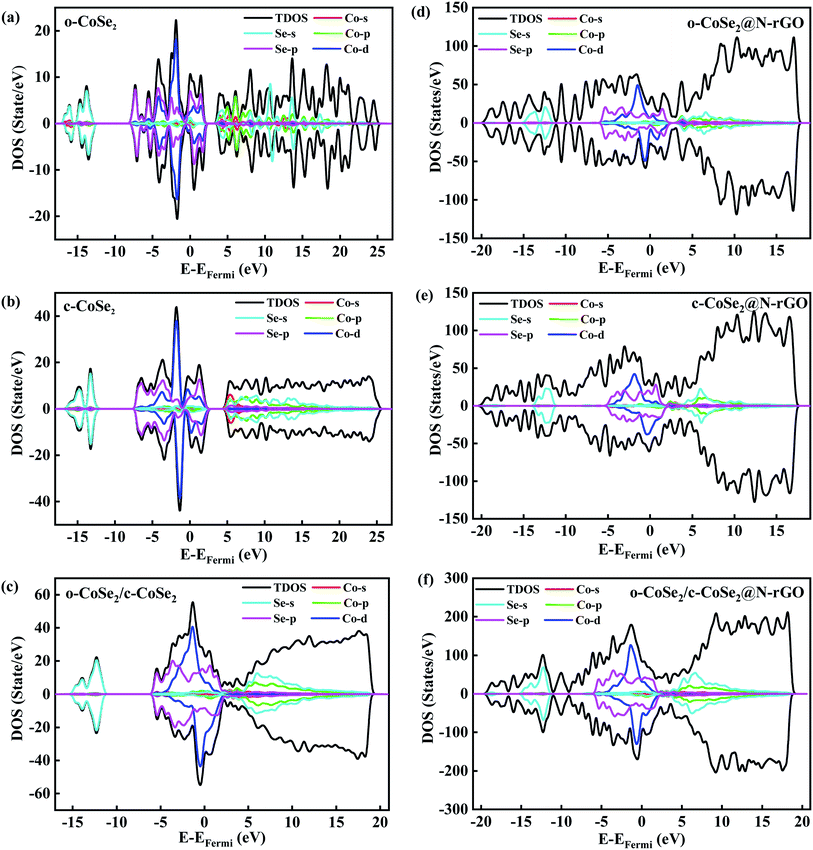
Now we focus on the influence of carbon coating for the electrochemical performance of CoSe2@N-rGO anode material. By means of the DFT calculations, we obtained the DOS profiles of CoSe2 with orthorhombic phases, cubic phases, and the heterojunction constructed by these two structures, respectively. The detailed lattice model used in the DFT calculations is constructed in Fig. S7.† Fig. 6(a) exhibit that the DFT results reveal the metallic behavior of the orthorhombic CoSe2, in which the DOS resides across the Fermi level, giving promising signs for the high conductivity of nanoparticles. Importantly, the electronic configuration of orthorhombic CoSe2 is t2g6eg1, which means that the effective magnetic moment of its ground state is not zero, and therefore, the DOS is asymmetric.58 Compared with CoSe2, Fig. 6(d) shows that the DOS of CoSe2@N-rGO changes greatly. Significantly, the overall DOS of CoSe2@N-rGO increases several times, and the forbidden band between 3–4 V and −8 to −12 V disappears, which fully indicates the conductivity will be batter after coating. Furthermore, the electrochemical impedance spectroscopy (EIS) of Fig. S6† also verifies the significant decrease of the overall resistivity after carbon coating. After comparing Fig. 4(b) and S4,† it can be found the cycle stability of CoSe2@N-rGO is much higher than that of CoSe2. However, the capacity of the first 30 cycles is not as good as CoSe2, indicating that the carbon coating mainly plays a part in maintaining the structure of CoSe2. For CoSe2 with cubic structure, the DOS is shown in Fig. 6(b). The profiles show that the p-orbital of Se and the d-orbital of Co through the Fermi level, indicating that the cubic CoSe2 is metallic. Moreover, its positive (spin up) and negative (spin down) total DOS is symmetrical, which also implies that the Co ions exhibit paramagnetism. Our DFT results are consistent with the previous measurements of transport and paramagnetic behavior.59 The DOS of cubic CoSe2 after carbon coating is shown in Fig. 6(e), which shows a similar result to the Fig. 6(d), namely, the disappearance of the bandgap in a larger energy range.
Theoretically, the capacitance of CoSe2 is certain and independent of the structure. In some transition metal compounds, such as Fe3O4, CoO, NiO, FeF2 and Fe2N, the extra capacitance is mainly derived from the spin-polarized capacitance on the surface of the transition metal nanoparticle, and the high DOS of 3d electrons at the Fermi surface play key role.60 In Fe3O4 LIBs, there exists a space charge zone formed by spin-polarized electrons at the interface between magnetic nanosized Fe particles and Li2O, which is the surface capacitance for extra lithium storage.60 The actual capacitance of CoSe2 with orthogonal structure is higher than that of cubic system may be derived from the surface capacitance. If the magnetic nanoparticles of Co form a surface capacitance with K2Se, then the PIBs capacity should be the same whether it is orthogonal or cubic CoSe2. But in fact, the electrochemical performance of orthogonal CoSe2 is better, no matter PIBs in Table S1† and SIBs in Table S2.† Therefore, for CoSe2@N-rGO PIBs is different from that of Fe3O4 LIBs. It can be speculated that during the electrochemical cycling of CoSe2@N-rGO PIBs, there may be minor remaining orthorhombic CoSe2 and the product K2Se to form surface capacitance of spin-polarized electrons.
In PIBs and SIBs, the heterojunction can be formed between two different phases, such as sodium/potassium-ion storage anode Ni3Se4/CoSe2. For CoSe2 with different phases, it is also possible to form heterojunctions in principle. The DOS profiles of orthorhombic CoSe2/cubic CoSe2 are shown in Fig. 6(c). On one hand, the elimination of the bandgap between 3–5 V and the overall higher total DOS, indicating that the heterointerface of orthorhombic CoSe2/cubic CoSe2 enhances electrical conductivity compared with single phase of CoSe2. Fig. 6(f) shows that the DOS of carbon coated heterojunction is not much different from Fig. 6(d) and (e). In our experiments, since the content of cubic CoSe2 is small, the probability of forming heterojunction is lower. Therefore, in the subsequent experiments, it is necessary to construct a phase that can form a relatively perfect heterojunction between orthorhombic CoSe2 and cubic CoSe2, which should be further improve the specific capacity of PIBs.
4. Conclusion
In summary, we successfully synthesized N-doped rGO tightly encapsulated CoSe2 with mixed phases by one-step hydrothermal method. The experimental and DFT results show that the capacity of CoSe2@N-rGO is 559.3 mA h g−1 during the first charge/discharge at a current density of 0.05 A g−1, and importantly, the capacity remains stable at 421 mA h g−1 after 100 cycles at a high current density of 0.2 A g−1. The excellent rate performance and long cycle life of the CoSe2@N-rGO electrode provide beneficial help for the development of novel PIBs anode materials.Author contributions
Hui Zheng, Han-Shu Xu, Huimin Liu, Lianwei Wei, Shusheng Wu, Jin Li, and Yuhu Huang made the samples, performed the measurements and analyzed the data with help from Han-Shu Xu and Kaibin Tang. Jiaping Hu performed the DFT calculations. Hui Zheng, Han-Shu Xu, and Kaibin Tang wrote the paper with input from all other co-authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We sincerely appreciate the fund support of the National Key R&D Program of China (2021YFB4001401) and the National Natural Science Foundation of China (21671182).References
- J. Zhao, Y. Zhang, Y. Wang, H. Li and Y. Peng, The application of nanostructured transition metal sulfides as anodes for lithium ion batteries, J. Energy Chem., 2018, 27, 1536–1554 CrossRef.
- F. Arshad, J. Lin, N. Manurkar, E. Fan, A. Ahmad, M.-u.-N. Tariq, F. Wu, R. Chen and L. Li, Life Cycle Assessment of Lithium-ion Batteries: A Critical Review, Resour., Conserv. Recycl., 2022, 180, 106104 CrossRef.
- J. Xie and Y. C. Lu, A retrospective on lithium-ion batteries, Nat. Commun., 2020, 11, 2499 CrossRef CAS PubMed.
- S. Xiao, X. Li, T. Li, Y. Xiang and J. S. Chen, Practical strategies for enhanced performance of anode materials in Na+/K+-ion batteries, J. Mater. Chem. A, 2021, 9, 7317–7335 RSC.
- T. L. Kulova, V. N. Fateev, E. A. Seregina and A. S. Grigoriev, A Brief Review of Post-Lithium-Ion Batteries, Int. J. Electrochem. Sci., 2020, 15, 7242–7259 CrossRef.
- Y. Lu, A. P. V. K. Saroja, R. Wei and Y. Xu, Engineering metal selenides for sodium-and potassium-ion batteries, Cell Rep. Phys. Sci., 2021, 2, 100555 CrossRef CAS.
- X. Min, J. Xiao, M. Fang, W. Wang, Y. Zhao, Y. Liu, A. M. Abdelkader, K. Xi, R. V. Kumar and Z. Huang, Potassium-ion batteries: outlook on present and future technologies, Energy Environ. Sci., 2021, 14, 2186–2243 RSC.
- P. Li, H. Kim, K. H. Kim, J. Kim, H. G. Jung and Y. K. Sun, State-of-the-art anodes of potassium-ion batteries: synthesis, chemistry, and applications, Chem. Sci., 2021, 12, 7623–7655 RSC.
- W. Zhang, J. Yin, W. Wang, Z. Bayhan and H. N. Alshareef, Status of rechargeable potassium batteries, Nano Energy, 2021, 83, 105792 CrossRef CAS.
- Y. Dong, C. Yan, H. Zhao and Y. Lei, Recent Advances in 2D Heterostructures as Advanced Electrode Materials for Potassium-Ion Batteries, Small Struct., 2022, 3, 2100221 CrossRef CAS.
- P. Du, L. Cao, B. Zhang, C. Wang, Z. Xiao, J. Zhang, D. Wang and X. Ou, Recent progress on heterostructure materials for next-generation sodium/potassium ion batteries, Renewable Sustainable Energy Rev., 2021, 151, 111640 CrossRef CAS.
- M. M. Rahman, I. Sultana, Y. Fan, B. Yu, T. Tao, C. Hou and Y. Chen, Strategies, design and synthesis of advanced nanostructured electrodes for rechargeable batteries, Mater. Chem. Front., 2021, 5, 5897–5931 RSC.
- Z. Jian, W. Luo and X. Ji, Carbon electrodes for K-ion batteries, J. Am. Chem. Soc., 2015, 137, 11566–11569 CrossRef CAS PubMed.
- P. Xiong, J. Zhu, L. Zhang and X. Wang, Recent advances in graphene-based hybrid nanostructures for electrochemical energy storage, Nanoscale Horiz., 2016, 1, 340–374 RSC.
- Y. An, H. Fei, G. Zeng, L. Ci, B. Xi, S. Xiong and J. Feng, Commercial expanded graphite as a low-cost, long-cycling life anode for potassium-ion batteries with conventional carbonate electrolyte, J. Power Sources, 2018, 378, 66–72 CrossRef CAS.
- J. Bai, H. Wu, S. Wang, G. Zhang, C. Feng and H. Liu, Synthesis of CoSe2-SnSe2 nanocube-coated nitrogen-doped carbon (NC) as anode for lithium and sodium ion batteries, Appl. Surf. Sci., 2019, 488, 512–521 CrossRef CAS.
- I. Hussain, S. Sahoo, C. Lamiel, T. T. Nguyen, M. Ahmed, C. Xi, S. Iqbal, A. Ali, N. Abbas, M. S. Javed and K. Zhang, Research progress and future aspects: Metal selenides as effective electrodes, Energy Storage Mater., 2022, 47, 13–43 CrossRef.
- A. Sobhani and M. Salavati-Niasari, Transition metal selenides and diselenides: Hydrothermal fabrication, investigation of morphology, particle size and and their applications in photocatalyst, Adv. Colloid Interface Sci., 2021, 287, 102321 CrossRef CAS PubMed.
- T. Lu, S. Dong, C. Zhang, L. Zhang and G. Cui, Fabrication of transition metal selenides and their applications in energy storage, Coord. Chem. Rev., 2017, 332, 75–99 CrossRef CAS.
- J. Zhou, Y. Liu, S. Zhang, T. Zhou and Z. Guo, Metal chalcogenides for potassium storage, InfoMat, 2020, 2, 437–465 CrossRef CAS.
- G. D. Park, J. S. Park, J. K. Kim and Y. C. Kang, Recent Advances in Heterostructured Anode Materials with Multiple Anions for Advanced Alkali-Ion Batteries, Adv. Energy Mater., 2021, 11, 2003058 CrossRef CAS.
- Y. Zhang, S. Wei, Z. Zhao, X. Pei, W. Zhao, J. Wang, X. Du and D. Li, Carbon-Encapsulated Ni3Se4/CoSe2 Heterostructured Nanospheres: Sodium/Potassium-Ion Storage Anode with Prominent Electrochemical Properties, Small, 2022, 2107258 CrossRef CAS PubMed.
- Y. Xu, X. Liu, H. Su, S. Jiang, J. Zhang and D. Li, Hierarchical Bimetallic Selenides CoSe2-MoSe2/rGO for Sodium/Potassium-Ion Batteries Anode: Insights into the Intercalation and Conversion Mechanism, Energy Environ. Mater., 2022, 5, 627–636 CrossRef CAS.
- X. Ou, X. Liang, F. Zheng, P. Wu, Q. Pan, X. Xiong, C. Yang and M. Liu, In situ X-ray diffraction investigation of CoSe2 anode for Na-ion storage: Effect of cut-off voltage on cycling stability, Electrochim. Acta, 2017, 258, 1387–1396 CrossRef CAS.
- H. Guo, G. Liu, M. Wang, Y. Zhang, W. Li, K. Chen, Y. Liu, M. Yue and Y. Wang, In Situ Fabrication of Bone-Like CoSe2 Nano-Thorn Loaded on Porous Carbon Cloth as a Flexible Electrode for Na-Ion Storage, Chem.–Asian J., 2020, 15, 1493–1499 CrossRef CAS PubMed.
- N. Yu, L. Zou, C. Li and K. Guo, In situ growth of binder-free hierarchical carbon coated CoSe2 as a high performance lithium ion battery anode, Appl. Surf. Sci., 2019, 483, 85–90 CrossRef CAS.
- J. Geng, S. Zhang, E. H. Ang, J. Guo, Z. Jin, X. Li, Y. Cheng, H. Dong and H. Geng, Modulating the kinetics of CoSe2 yolk-shell spheres via nitrogen doping with high pseudocapacitance toward ultra-high-rate capability and high-energy density sodium-ion half/full batteries, Mater. Chem. Front., 2021, 5, 6873–6882 RSC.
- S. H. Yang, S. K. Park and Y. C. Kang, MOF-Derived CoSe2@N-Doped Carbon Matrix Confined in Hollow Mesoporous Carbon Nanospheres as High-Performance Anodes for Potassium-Ion Batteries, Nano-Micro Lett., 2021, 13, 1–15 CrossRef CAS PubMed.
- F. Kong, J. Wang, J. Chen, S. Tao, B. Qian and H. Luo, MOF-derived ultrasmall CoSe2 nanoparticles encapsulated by an N-doped carbon matrix and their superior lithium/sodium storage properties, Chem. Commun., 2020, 56, 9218–9221 RSC.
- H. Zhou, X. Li, Y. Li, M. Zheng and H. Pang, Applications of MxSey (M = Fe, Co, Ni) and Their Composites in Electrochemical Energy Storage and Conversion, Nano-Micro Lett., 2019, 11, 1–33 CrossRef PubMed.
- Y. Zhao, Y. Yan and J. M. Lee, Recent progress on transition metal diselenides from formation and modification to applications, Nanoscale, 2022, 14, 1075–1095 RSC.
- H. Zhao, Y. Qi, K. Liang, J. Li, L. Zhou, J. Chen, X. Huang and Y. Ren, Interface-Driven Pseudocapacitance Endowing Sandwiched CoSe2/N-Doped Carbon/TiO2 Microcubes with Ultra-Stable Sodium Storage and Long-Term Cycling Stability, ACS Appl. Mater. Interfaces, 2021, 13, 61555–61564 CrossRef CAS PubMed.
- Y. Liu, Z. Chen, H. Jia, H. Xu, M. Liu and R. Wu, Iron-Doping-Induced Phase Transformation in Dual-Carbon-Confined Cobalt Diselenide Enabling Superior Lithium Storage, ACS Nano, 2019, 13, 6113–6124 CrossRef CAS PubMed.
- J. Xie, Y. Zhu, N. Zhuang, H. Lei, W. Zhu, Y. Fu, M. S. Javed, J. Li and W. Mai, Rational design of metal organic framework-derived FeS2 hollow nanocages@reduced graphene oxide for K-ion storage, Nanoscale, 2018, 10, 17092–17098 RSC.
- Z. Zhao, C. Gao, J. Fan, P. Shi, Q. Xu and Y. Min, Dual Confinement of CoSe2 Nanorods with Polyphosphazene-Derived Heteroatom-Doped Carbon and Reduced Graphene Oxide for Potassium-Ion Batteries, ACS Omega, 2021, 6, 17113–17125 CrossRef CAS PubMed.
- Z. Li, L. Y. Zhang, L. Zhang, J. Huang and H. Liu, ZIF-67-derived CoSe/NC composites as anode materials for lithium-ion batteries, Nanoscale Res. Lett., 2019, 14, 1–11 CrossRef PubMed.
- E. Xu, P. Li, J. Quan, H. Zhu, L. Wang, Y. Chang, Z. Sun, L. Chen, D. Yu and Y. Jiang, Dimensional Gradient Structure of CoSe2@CNTs-MXene Anode Assisted by Ether for High-Capacity, Stable Sodium Storage, Nano-Micro Lett., 2021, 13, 1–14 CrossRef CAS PubMed.
- J. Yang, H. Gao, S. Men, Z. Shi, Z. Lin, X. Kang and S. Chen, CoSe2 nanoparticles encapsulated by N-doped carbon framework intertwined with carbon nanotubes: high-performance dual-role anode materials for both Li- and Na-ion batteries, Adv. Sci., 2018, 5, 1800763 CrossRef PubMed.
- Z. Li, H. Xue, J. Wang, Y. Tang, C.-S. Lee and S. Yang, Reduced Graphene Oxide/Marcasite-Type Cobalt Selenide Nanocrystals as an Anode for Lithium-Ion Batteries with Excellent Cyclic Performance, ChemElectroChem, 2015, 2, 1682–1686 CrossRef CAS.
- Y. Xiao, P. Sun and M. Cao, Core-Shell Bimetallic Carbide Nanoparticles Confined in a Three-Dimensional N-Doped Carbon Conductive Network for Efficient Lithium Storage, ACS Nano, 2014, 8, 7846–7857 CrossRef CAS PubMed.
- H. Liu, D. Su, R. Zhou, B. Sun, G. Wang and S. Z. Qiao, Highly Ordered Mesoporous MoS2 with Expanded Spacing of the (002) Crystal Plane for Ultrafast Lithium Ion Storage, Adv. Energy Mater., 2012, 2, 970–975 CrossRef CAS.
- Z. Zhang, B. Zhang, J. Xu, M. Zhang, L. Duan, J. Shen and X. Zhou, Anchoring Carbon-Coated CoSe Nanoparticles on Hollow Carbon Nanocapsules for Efficient Potassium Storage, ACS Appl. Energy Mater., 2021, 4, 6356–6363 CrossRef CAS.
- Y. Wang, Y. Wu, L. Liu, T. Yan, G. Zeng, L. Li, J. Duan and Z. Chen, Bimetallic MOF-derived CoSe2 embedded within N-doped carbon with enhanced lithium storage properties, Solid State Ionics, 2021, 370, 115747 CrossRef CAS.
- N. Hussain, M. Li, B. Tian and H. Wang, Co3Se4 Quantum Dots as an Ultrastable Host Material for Potassium-Ion Intercalation, Adv. Mater., 2021, 33, 2102164 CrossRef CAS PubMed.
- C. Wang, B. Zhang, X. Ou, H. Xia, L. Cao, L. Ming and J. Zhang, Co0.85Se@N-doped reduced graphene oxide hybrid polyhedron-in-polyhedron structure assembled from metal-organic framework with enhanced performance for Li-ion storage, J. Colloid Interface Sci., 2020, 573, 223–231 CrossRef CAS PubMed.
- Y. Xiao, J. Zhang, D. Su, A. Zhang, Q. Jin, L. Zhou, S. Wu, X. Wang, W. Chen and S. Fang, In situ growth of V-shaped CoSe2 nanorods on graphene with C-Co bonding for high-rate and long-life sodium-ion batteries, J. Alloys Compd., 2020, 819, 153359 CrossRef CAS.
- C. Cui, Z. Wei, G. Zhou, W. Wei, J. Ma, L. Chen and C. Li, Quasi-reversible conversion reaction of CoSe2/nitrogen-doped carbon nanofibers towards long-lifetime anode materials for sodium-ion batteries, J. Mater. Chem. A, 2018, 6, 7088–7098 RSC.
- J. Hu, B. Wang, Q. Yu, D. Zhang, Y. Zhang, Y. Li and W. A. Wang, CoSe2/N-doped carbon porous nanoframe as an anode material for potassium-ion storage, Nanotechnology, 2020, 31, 395403 CrossRef CAS PubMed.
- Q. Yu, B. Jiang, J. Hu, C. Y. Lao, Y. Gao, P. Li, Z. Liu, G. Suo, D. He, W. A. Wang and G. Yin, Metallic Octahedral CoSe2 Threaded by N-Doped Carbon Nanotubes: A Flexible Framework for High-Performance Potassium-Ion Batteries, Adv. Sci., 2018, 5, 1800782 CrossRef PubMed.
- H. Hu, J. Zhang, B. Guan and X. W. Lou, Unusual formation of CoSe@carbon nanoboxes, which have an inhomogeneous shell, for efficient lithium storage, Angew. Chem., Int. Ed., 2016, 55, 9514–9518 CrossRef CAS PubMed.
- Z. Liu, K. Han, P. Li, W. Wang, D. He, Q. Tan, L. Wang, Y. Li, M. Qin and X. Qu, Tuning Metallic Co0.85Se Quantum Dots/Carbon Hollow Polyhedrons with Tertiary Hierarchical Structure for High-Performance Potassium Ion Batteries, Nano-Micro Lett., 2019, 11, 1–14 CrossRef.
- W. Wang, J. Zhou, Z. Wang, L. Zhao, P. Li, Y. Yang, C. Yang, H. Huang and S. Guo, Short-Range Order in Mesoporous Carbon Boosts Potassium-Ion Battery Performance, Adv. Energy Mater., 2018, 8, 1701648 CrossRef.
- H. Huang, R. Xu, Y. Feng, S. Zeng, Y. Jiang, H. Wang, W. Luo and Y. Yu, Sodium/potassium-ion batteries: boosting the rate capability and cycle life by combining morphology, defect and structure engineering, Adv. Mater., 2020, 32, 1904320 CrossRef CAS PubMed.
- Y. Liu, Q. Deng, Y. Li, Y. Li, W. Zhong, J. Hu, X. Ji, C. Yang, Z. Lin and K. Huang, CoSe@ N-doped carbon nanotubes as a potassium-ion battery anode with high initial coulombic efficiency and superior capacity retention, ACS Nano, 2021, 15, 1121–1132 CrossRef CAS PubMed.
- V. Augustyn, P. Simon and B. Dunn, Pseudocapacitive oxide materials for high-rate electrochemical energy storage, Energy Environ. Sci., 2014, 7, 1597–1614 RSC.
- T. Brezesinski, J. Wang, J. Polleux, B. Dunn and S. H. Tolbert, Templated nanocrystal-based porous TiO2 films for next-generation electrochemical capacitors, J. Am. Chem. Soc., 2009, 131, 1802–1809 CrossRef CAS PubMed.
- P. Lou, Z. Cui, Z. Jia, J. Sun, Y. Tan and X. Guo, Monodispersed carbon-coated cubic NiP2 nanoparticles anchored on carbon nanotubes as ultra-long-life anodes for reversible lithium storage, ACS Nano, 2017, 11, 3705–3715 CrossRef CAS PubMed.
- Q. Yu, B. Jiang, J. Hu, C. Y. Lao, Y. Gao, P. Li, Z. Liu, G. Suo, D. He and W. Wang, Metallic octahedral CoSe2 threaded by N-doped carbon nanotubes: a flexible framework for high-performance potassium-ion batteries, Adv. Sci., 2018, 5, 1800782 CrossRef PubMed.
- A. Teruya, F. Suzuki, D. Aoki, F. Honda, A. Nakamura, M. Nakashima, Y. Amako, H. Harima, K. Uchima, and M. Hedo, in Fermi Surface and Magnetic Properties in Ferromagnet CoS2 and Paramagnet CoSe2 with the Pyrite-type Cubic Structure, Journal of Physics: Conference Series, IOP Publishing, 2017; p. 012001 Search PubMed.
- Q. Li, H. Li, Q. Xia, Z. Hu, Y. Zhu, S. Yan, C. Ge, Q. Zhang, X. Wang and X. Shang, Extra storage capacity in transition metal oxide lithium-ion batteries revealed by in situ magnetometry, Nat. Mater., 2021, 20, 76–83 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03608h |
| ‡ Hui Zheng and Han-Shu Xu make the same contribution to this work. |
| This journal is © The Royal Society of Chemistry 2022 |