Open Access Article
Open Access ArticleSuperhydrophobicity and conductivity of polyester-conductive fabrics using alkaline hydrolysis
Suhyun Lee *ab
*ab
aDepartment of Fashion Design, Jeonbuk National University, Jeonju, 54896, Republic of Korea. E-mail: suhyun14@jbnu.ac.kr
bResearch Institute of Human Ecology, College of Human Ecology, Jeonbuk National University, Jeonju, 54896, Republic of Korea
First published on 15th August 2022
Abstract
This study attempted to develop a superhydrophobic conductive fabric to solve the problem of functional deterioration due to oxidation of conductive fabrics. To this end, a superhydrophobic surface was achieved by introducing nano-roughness to the surface of the polyester-conductive fabric by using alkaline hydrolysis, and then lowering the surface energy through hydrophobic coating. In order to derive optimal processing conditions with excellent superhydrophobicity while maintaining conductivity and mechanical properties, changes in surface structure, conductivity, superhydrophobicity and durability of conductivity against air and water were evaluated according to alkaline hydrolysis duration. As the alkaline hydrolysis duration increased, the polyester surface was etched and the silver oxide particles formed on the conductive yarns, creating non-uniform nano-roughness. The weight, tensile strength, and strain of the conductive fabric decreased due to alkaline hydrolysis, and the change was noticeable after 30 min of alkaline hydrolysis. In addition, as the alkaline hydrolysis duration increased, the surface resistance of the conductive fabric slightly increased and the surface temperature by electric heating performance decreased, but it still showed excellent conductivity. After 30 min of alkaline hydrolysis, a contact angle of more than 150° and a shedding angle of less than 10° achieved a superhydrophobic surface. This superhydrophobic surface prevented the reaction of silver with air or water in spite of prolonged exposure to air and repeated contact with water to maintain electrical properties, thereby improving the durability of conductivity. This study is significant in that it achieved dual roughness for superhydrophobicity on the surface of conductive fabrics using a relatively easy and simple method by applying alkaline hydrolysis, which is commonly used with polyester fabric. Therefore, it is expected to solve the problem of conductivity loss of the use of conductive fabric in various environments.
1. Introduction
Conductive fabric is promising for wearable electronics, owing to its good flexibility and light weight.1 Generally, conductive fabric is composed of nonconductive fabric and conductive materials such as metal, carbon nanotubes, graphene, and conductive polymers.2,3 Silver has attracted much attention as a conductive material for a conductive fabric because of its distinctive properties, such as good conductivity, chemical stability, and catalytic and antibacterial activities.4 The stability of the electrical conductivity is of critical importance. During application, wearable devices with conductive fabrics may come into contact with corrosive media such as humid air, rainwater, and sweat, resulting in partial corrosion, oxidation, or damage to the conductive pathways.1 Therefore, it is necessary to develop durable conductive fabric with anti-oxidation properties and high electrical conductivity.A superhydrophobic surface is defined as a surface with a water contact angle greater than 150°, and a shedding angle lower than 10°.5–7 As these surfaces have exhibited great potential for their self-cleaning, anti-fouling, and water-repellent properties, functional superhydrophobic materials are desired to further broaden application fields including electric sensors, self-repairing, and smart clothing.8 The combination of superhydrophobicity and conductivity can not only endow the materials with multifunctionality in practical applications but also prolong the lifetime of electronics under wet or corrosive environment.9 In particular, in the case of conductive fabrics, their use is limited due to oxidation degradation caused by air and moisture, and thus conductivity loss may occur. In addition, ensuring the durability of conductivity in the environments of daily use that may cause contamination and promoting reductions in the number of launderings is also essential to increase the lifespan of conductive fabrics.2,10
Lower surface energy and dual surface roughness are two critical factors to obtain superhydrophobicity.7 Methods for introducing nano-roughness for superhydrophobicity on the fabric include bottom-up approaches that include chemical deposition, colloidal assemblies, layer-by-layer deposition, and sol–gel methods, and top-down approaches that include templation, photolithography, or etching of the surface with plasma, UV-laser, and alkaline treatment.11,12
Wang et al.10 developed a conductive cotton fabric with excellent water resistance by electroless plating silver on the surface of fibers after silane modification. Zheng et al.8 fabricated a conductive superhydrophobic cotton fabric via the layer-by-layer assembly of carbon nanotubes and modification of polydimethylsiloxane, and the fabric-based pressure sensor possessed stable and regular responses to detect human motions even under wet and humid conditions. Kang et al.1 reported a method to fabricate a highly conductive and superhydrophobic fabric. They synthesized silver nanoparticles on cotton fiber surfaces via SnCl2 and Tollen's reagent (silver nitrate and ammonium hydroxide) spraying, and then covered the surface with a layer of cerium myristate by electrodeposition. Most of the studies that have implemented conductivity and superhydrophobicity so far have utilized the method of growing or depositing conductive nanoparticles such as silver or copper on general fabrics such as cotton or polyester. However, this approach entails complicated processes such as pre-treatment for functionalization of the fabric surface, and the growth and adhesion of conductive particles on the fabric. In addition, it has various limitations, such as the problem of functional deterioration due to the dropping of conductive particles and the harmfulness of nanoparticles to the human body and environment.10,13,14 Therefore, Cai et al.15 introduced surface roughness by etching the conductive materials. They developed a superhydrophobic conductive material by introducing a mechanical abrasion method to create microscale and nanoscale roughness on the surface of hydrophobic polypropylene with carbon fibers.
Among the approaches for introducing nano-roughness to the fabric surface, alkaline hydrolysis, which is well known for the weight reduction of polyester fabric, is used as a practical method of introducing nano-roughness on the fiber surface by etching through chemical and morphological changes in polyester. Through alkaline hydrolysis, the nucleophilic attack of hydroxide ions on the carbonyl group of the polyester chains leads to breakage of the ester linkages and makes some nano-scale pits and craters on the surface.16–19 In addition, the pits appear to be enlarged due to preferential hydrolysis of the polymer around the softener particles such as titanium dioxide at locations identified as having low crystallinity.17 This irregular surface after hydrolysis could be induced to an appropriate roughness to create a superhydrophobic surface.20 Meanwhile, in alkaline solution, silver on the conductive yarns is oxidized to AgO, and the AgO is finally transformed into Ag+.21 In this process, the smooth surface of the silver-coated conductive yarn may be roughened, which can contribute to superhydrophobicity. However, this reaction can weaken the electrical properties of conductive yarns. Therefore, in order to form a superhydrophobic surface on a conductive fabric, it is important to derive processing conditions that can maintain electrical properties as much as possible while forming physical dual roughness caused by alkaline hydrolysis.
The objective of this study was to develop a superhydrophobic conductive fabric using alkaline hydrolysis and hydrophobic coating. Accordingly, a polyester and silver-coated polyimide blended fabric was produced using a flat knitting machine and treated with an alkaline hydrolysis process to introduce dual roughness on the fabric surface. Subsequently, chemical vapor deposition was conducted with n-dodecyltrimethoxysilane (DTMS) to lower the surface energy. Silane treatment of fabrics is known for increasing hydrophobicity and improving the water resistance of conductive fabric.6 To evaluate the effect of alkaline hydrolysis, the changes in morphology, mechanical, and chemical properties, and superhydrophobicity according to hydrolysis duration were confirmed. Conductive properties were analyzed through surface resistance, electric heating performance, and changes in surface resistance with exposure to air and water.
2. Experimental section
2.1. Materials
In this study, 100% polyester yarn of 150 denier and conductive yarn (Silver Tech 120 Tex 28, AMANN, Germany) were used. This conductive yarn was hybrid with 32 filaments of silver-coated polyamide and 44 filaments of polyester, and their thickness was approximately 250 deniers. The resistance of the yarns was less than 530 Ω m−1. Sodium hydroxide (>97%) from Junsei Chemical (Japan) was used for alkaline hydrolysis, and n-dodecyltrimethoxysilane (DTMS, >93.0%) from Sejin CI (Korea) was utilized for hydrophobic coating. All the chemicals were used without further purification.2.2. Preparation of conductive knitted fabrics
A conductive knitted fabric was fabricated from polyester yarn and conductive yarn using a computerized flat knitting machine (330HP. W, Stoll, Germany). Simultaneously, two-ply of polyester yarns and one-ply of conductive yarn were combined and put into the knitting machine. The structure of the knitted fabric was fixed using an interlock stitch, which are the simplest double-knit fabrics produced by interlocking two 1 × 1 rib structures.2.3. Fabrication of superhydrophobic surface on knitted fabrics
To introduce nano-roughness on the conductive knitted fabric surfaces, the samples were subjected to alkaline hydrolysis. After cutting the knitted fabric into 10 cm × 10 cm squares, the pieces were immersed in a 30% w/w sodium hydroxide solution, etched at 70 °C for 15, 30, 45, and 60 min. It was then rinsed with flowing distilled water until the pH was 7.The alkaline hydrolyzed knitted fabric was hydrophobized using DTMS, which is a low surface energy material. A total of 10 mL of DTMS was placed in a ceramic dish at the bottom of a sieve in which the alkaline hydrolyzed samples were laid. It was later placed in a drying oven at 120 °C for 3 h to vaporize the DTMS and to subsequently vapor-coat the fabrics.
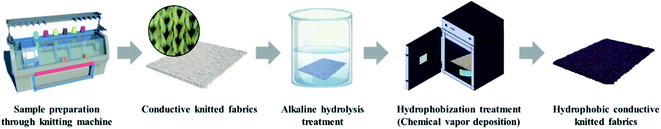
The schematic process of the alkaline hydrolysis and hydrophobization of conductive knitted fabric is shown in Fig. 1.
2.4. Characterization
The weight loss of the fabrics due to alkaline hydrolysis was calculated as in eqn (1):
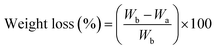 | (1) |
The change in tensile strength of the alkaline hydrolyzed fabrics was measured by the ASTM D 5035 strip method. A sample 2.5 cm width and 10 cm length in the wale direction was fixed in a clamp with a distance of 5.0 cm. Tensile strength was measured utilizing a universal testing machine (Instron-5543, USA) with 1 kN load at an extension rate of 30 cm min−1.
The chemical compositions of the sample surfaces before and after alkaline hydrolysis and hydrophobization were characterized using a high-resolution X-ray diffractometer (HR-XRD, D8 Advance, Bruker, Germany) and an energy-dispersive X-ray microanalysis system (EDX, Aztec, Oxford instrument, UK) coupled to a FE-SEM, respectively. For XRD analysis, the conductive yarns extracted from the fabric samples.
The sample was attached to a slide glass using 3 M tape, and a 3.5 ± 0.3 μl drop of deionized water was placed on the surface under investigation. The contact angle for the water droplet was recorded 1 s after dropping. An average value was calculated by measuring 10 different sites on each sample.
Meanwhile, the shedding angle of the samples was measured according to Zimmermann et al.'s22 method, and was determined as the tilting angle at which a drop of water (12.5 ± 0.3 μl) slid for at least 2 cm. The reported shedding angle values for the samples were the average of five measurements on different point areas in the wale directions.
The electric heating properties were observed at the surface temperature by applying a voltage using a thermal imaging camera (Testo 868, Testo SE&Co. KGaA, Germany) to investigate the heating performance of the conductive knitted fabric caused by the heat resistance. The voltage was limited to 3 V using DC power supply (TS3020A, Toyotech, Japan). Thermal images of the surface were captured 1 min after applying the voltage. The average surface temperature was calculated by extracting the area corresponding to the fabric sample based on the captured image using the IRSofte program (Testo, Germany).
To examine the effect of the superhydrophobic properties on conductivity changes caused by repetitive exposure to water, the AATCC 22 spray test method was used. The sample was placed on top of a substrate inclined by 45°, and 250 mL of distilled water was poured on the sample from 15 cm above it for 25–30 s, falling in a spray form. After the spraying the entire volume, the sample was removed, and dried in oven at 105 °C for 1 h to vaporize the water. Conductivity changes after repetitive moisture exposure were observed by repeating the spray test 20 times and measuring the surface resistance every 5 tests.
3. Results and discussions
3.1. Surface morphologies according to alkaline hydrolysis
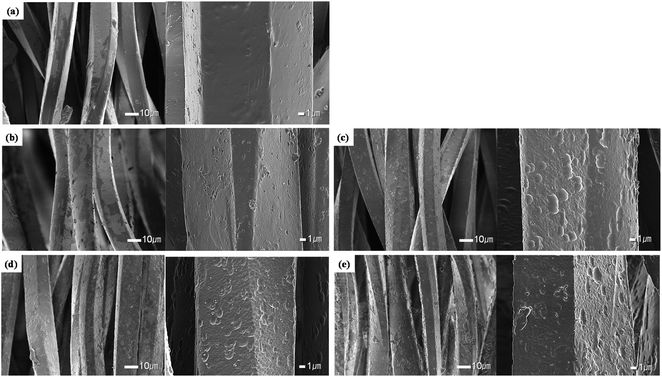
In order to confirm the roughness and changes to the surface of the polyester and conductive yarns as a function of the alkaline hydrolysis, surface images were obtained with a SEM, and the results are shown in Fig. 2 and 3.Fig. 2 shows the appearance of the polyester yarn from the conductive knitted fabrics according to the alkaline hydrolysis duration. Untreated polyester yarns showed a flat and smooth surface (Fig. 2(a)), while the alkaline hydrolyzed polyester yarns showed fine nano-sized pits and cavities on the fiber surface. Alkaline hydrolysis causes a nucleophilic attack of hydroxide ions on the carbonyl groups of polyester chains, thereby inducing chain cleavage at ester bonds and creating some nano-sized pits and craters on the fiber surface.16–19 This nano-roughness of the fiber surface appeared more clearly as the hydrolysis duration increased from 15 min to 60 min (Fig. 2(b)–(e)), and as the fiber surface was etched, it was confirmed that the titanium oxide particles used as matting agents were exposed to the surface. In addition, ethylene glycol and carboxylate/hydroxyl functional groups of polyester formed by alkaline hydrolysis can reduce Ag+ ions separated from the conductive yarn back to Ag nanoparticles,4 or as the Ag+ ions were unstable in the alkaline solution, they were easily formed Ag2O.24 This phenomenon was observed in the samples treated with alkaline hydrolysis duration from after 30 min, which was relatively long so that the reduction reaction of Ag+ ions could occur. As shown in Table 1, the silver component was detected on the surface of the polyester fiber through EDX analysis. However, the amount was very small, probably because it was difficult to accurately detect the silver particles formed on the fiber surface.
| Elements in wt% | ||||||
|---|---|---|---|---|---|---|
| C | O | Na | Ti | Ag | All | |
| Polyester yarn in untreated fabric | 56.58 | 43.42 | — | — | — | 100 |
| Polyester yarn in alkaline treated fabric (60 min) | 64.94 | 32.37 | 0.28 | 1.08 | 1.33 | 100 |
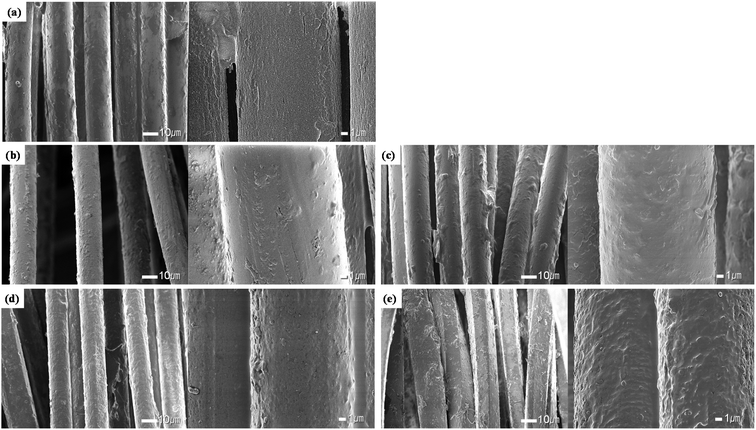
On the other hand, a change different from that of polyester yarns was observed on the surface of the silver coated conductive yarns, as shown in Fig. 3. It was confirmed that a thin layer of silver surrounded the surface of the conductive yarns in the untreated fabric. However, when the alkaline hydrolysis was performed, the smooth surface of the fiber became non-uniform and rough. This trend was clearer as the alkaline hydrolysis duration increased. This observation is consistent with the study by Yan et al.,21 which observed changes from immersing silver nano-coated fabric in alkaline perspiration solution. The silver metal is oxidized in an alkaline solution and an anodic oxide layer was formed on the silver.25,26 The anodic oxidation of Ag metal in alkaline solution proceed via a two-step mechanism. The first step is the oxidation of Ag to Ag2O and formation of a 3D primary Ag(I) oxide overlayer.26,27 The second oxidation step is the oxidation of Ag2O to Ag2O2, which proceeds via nucleation and crystal growth processes.25,27 Therefore, as the alkaline hydrolysis duration increased, the formation of the silver oxide layer is considered to have increased due to oxidation of the silver particles deposited on the surface of the conductive yarn, making the fiber surface rough and uneven. In particular, the most surface changes can be seen in Fig. 3(d) and (e), where the alkaline hydrolysis duration were 45 min and 60 min. According to the study of Aksu et al.,27 when an Ag film was treated in 6 M NaOH solution, a fibrous structure was shown on the surface of the film due to formation of an Ag2O layer. Cheng et al.28 also reported that Ag2O particles were formed of various sizes ranging from tens to hundreds of nanometers by oxidation of Ag along with the anodic sweep.
Through the appearance observation, it was confirmed that the polyester yarn was etched by alkaline hydrolysis, and that the conductive yarn formed a silver oxide layer on the fiber surface due to the oxidation of silver, thereby forming non-uniform nano roughness.
3.2. Physical properties and chemical compositions
Fig. 4 shows the results for the weight changes of conductive knitted fabric with varying alkaline hydrolysis durations. The weight loss following alkaline hydrolysis gradually decreased until 60 min. The rate of weight loss according to hydrolysis duration was 7% at 15 min, 19% at 30 min, 22% at 45 min, and 26% at 60 min.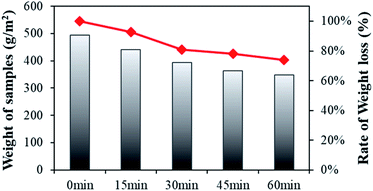 | ||
| Fig. 4 Weight loss of the polyester-conductive knitted fabric according to the alkaline hydrolysis duration. | ||
The reduction in weight after alkaline hydrolysis affected the mechanical properties of the conductive knitted fabrics. Fig. 5 presents the tensile strength and strain curves measured in the wale direction of each sample for varying alkaline hydrolysis durations. The tensile strength and strain at breakpoint were 662 ± 12 N and 122 ± 11% for untreated, 640 ± 2 N and 112 ± 2% for 15 min, 528 ± 26 N and 100 ± 1% for 30 min, 427 ± 18 N and 87 ± 5% for 45 min, and 247 ± 29 N and 91 ± 10% for 60 min. The tensile strength decreased by 96.6% at 15 min, 79.8% at 30 min, 64.5% at 45 min, and 37.3% at 60 min of alkaline hydrolysis, while the tensile strain decreased by 91.9% at 15 min, 81.8% at 30 min, 71.3% at 45 min, and 74.9% at 60 min. The treatment of the polyester fabric with sodium hydroxide could result in lower tensile strength due to the creation of pits on the surface of the fabric possibly acting as nucleation centers for crack formation.17,29–31 In addition, nano craters formed on the polyester fiber surface and the reduction of fiber diameter seemed to affect tensile strength.19 Notably, both tensile strength and strain decreased sharply after 30 min of alkaline hydrolysis. As shown in Fig. 2 and 3, under the conditions of 45 min and 50 min of alkaline hydrolysis, not only the surface change of the polyester fiber but also the surface of the conductive yarn changed very significantly. Therefore, it is considered that the change in the surface structure of the two fibers greatly decreased the mechanical properties of the conductive knitted fabrics.
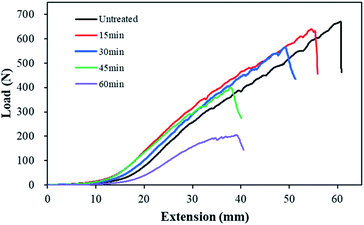 | ||
| Fig. 5 Tensile strength and strain of the polyester-conductive knitted fabric according to the alkaline hydrolysis duration. | ||
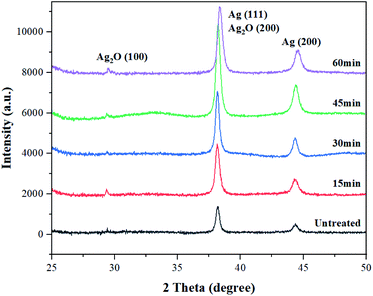
The chemical composition and surface changes of the conductive yarns from the knitted fabrics according to alkaline hydrolysis were examined through XRD analysis. As shown in Fig. 6, the XRD spectra pattern of untreated conductive yarn showed two well-resolved diffraction peaks at 38.1° and 44.2°, which correspond to the face centered cubic crystalline structure of silver (111) and (200) planes respectively.21,32 However, when alkaline hydrolysis was carried out, new diffraction peaks appeared at 29.5° and 38.1°, corresponding to the silver oxide (100) and (200) planes, respectively.33,34 These diffraction peaks became stronger as the alkaline hydrolysis duration increased. Therefore, as the alkaline hydrolysis duration increased, silver particles of the conductive yarns reacted with NaOH to form more silver oxide on the surface of the fiber.
The EDX images of the alkaline hydrolyzed and DTMS-coated conductive knitted fabrics are shown in Fig. 7(a) and (b), respectively. A Si component with hydrophobic properties was detected through EDX analysis of the fabric surface, which confirmed its hydrophobization.
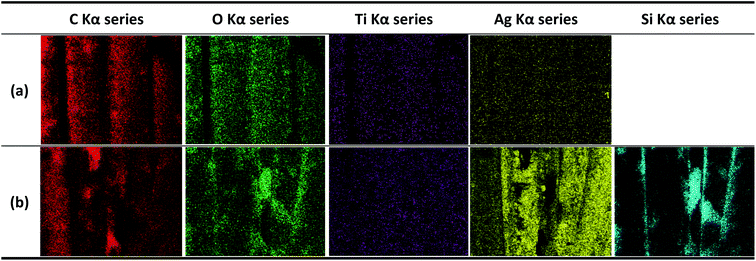 | ||
| Fig. 7 Energy-dispersive X-ray microanalysis system image of (a) alkaline hydrolyzed and (b) hydrophobic coated conductive knitted fabrics (30 min). | ||
3.3. Surface resistivity and electric heating performance
The change of electrical properties according to alkaline hydrolysis and hydrophobic coating were observed by measuring the surface resistance and electrical heating performance.Table 2 summarizes the surface resistance and change of resistance with respect to the untreated sample of each fabric according to the duration of alkaline hydrolysis and hydrophobic coating. The surface resistance of the untreated fabric is 3.6 Ω sqm−1. This is because current flows as electrons move through the silver layer on the surface of the conductive yarns.35 When alkaline hydrolysis was conducted, however, the surface resistance increased as the silver layer was oxidized by hydroxyl groups from the alkaline solution. The Ag2O layer is highly resistive compared to the Ag.36,37 Therefore, as alkaline hydrolysis duration increased, more Ag2O phases were formed, and as a result, the surface resistance of conductive knitted fabrics was increased by up to 34%. Meanwhile, the surface resistance of all samples increased after hydrophobic coating. The electrical property of the fabric is closely related to its water content. In general, the electrical resistance of fabrics decreases gradually as moisture regain increases.37 When DTMS is coated on the conductive knitted fabric by chemical vapor deposition, DTMS molecules penetrated not only the fabric surface but also between the yarns and the fibers to form a hydrophobic layer.2 As a result, the hydrophobic layer created on the silver of the conductive yarns prevented the absorption of moisture and the flow of electron, and increased the surface resistance.
| Untreated | 15 min | 30 min | 45 min | 60 min | |
|---|---|---|---|---|---|
| Alkaline hydrolysis | |||||
| Surface resistance (Ω sqm−1) | 3.6 ± 0.2 | 4.1 ± 0.4 | 4.5 ± 0.1 | 4.7 ± 0.4 | 4.8 ± 0.3 |
| Change (%) | — | 14% | 25% | 30% | 34% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Hydrophobic coating | |||||
| Surface resistance (Ω sqm−1) | 3.7 ± 0.4 | 4.2 ± 0.3 | 4.5 ± 0.4 | 4.51.0 | 4.6 ± 0.9 |
| Change (%) | 2% | 16% | 25% | 25% | 27% |
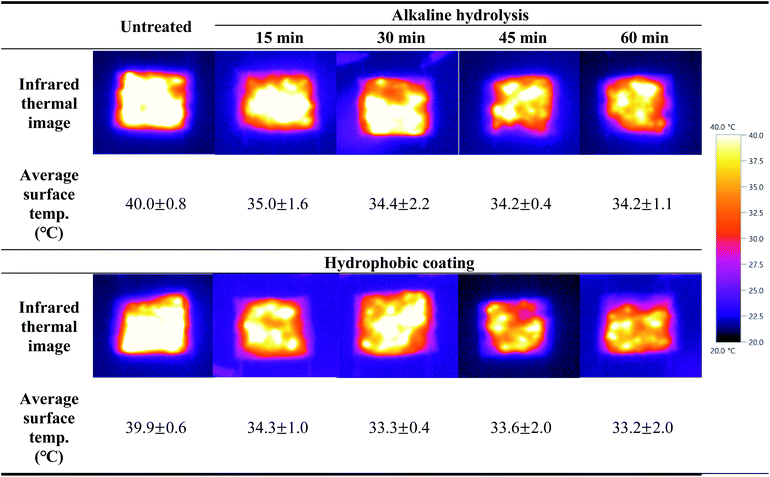
After both sides of the fabric sample were connected with electric wires and voltage of 3 V was applied, the change in surface temperature were observed through infrared thermal images for 1 min to investigate the electrical heating performance of the conductive knitted fabric according to the alkaline hydrolysis and hydrophobic coating.
As shown in Table 3, the electric heating performance was similar to surface resistance results. The average surface temperature of the untreated fabric was 40.0 °C, an increase of 20 °C. This is due to resistance heating. When electrons pass through the silver of the conductive yarns, they collide with molecules, causing friction and consuming energy, and generating chaotic heat motion without direction. Therefore, for the heating mechanism of conductive knitted fabrics, as electricity flows through the silver layer plated on the fiber surface, the fiber is heated by the energy generated from the resistance, and the generated heat is transferred by conduction or convection.2,38 On the other hand, after alkaline hydrolysis, the average surface temperature of the conductive knitted fabric decreased by about 5 °C compared to the untreated surface. This is because the surface resistance increased as silver was oxidized by alkaline solution, and the continuous network of the silver layer was destroyed.28 However, since the difference in surface resistance according to the alkaline hydrolysis duration was less than 1 Ω sqm−1, the average surface temperature of each alkaline hydrolyzed fabrics was almost similar. After the hydrophobic coating process was performed, the average surface temperature of all samples decreased. As described above, an insulating layer was formed on the surface of the conductive knitted fabrics by the hydrophobic coating, which prevented the flow of electricity and reduced thermal conductivity, thereby reducing heat generation according to voltage application.2
Overall, the heating performance was slightly weakened due to alkaline hydrolysis and hydrophobic coating, but nonetheless, the surface temperature of all fabrics increased by about 10 °C or more even when a relatively low voltage of 3 V was applied, showing excellent electric heating performance.
3.4. Superhydrophobicity
The variation in the superhydrophobicity of conductive knitted fabrics by alkaline hydrolysis and hydrophobic coating was evaluated based on water contact and shedding angles. As shown in Table 4, the contact angle of the untreated fabrics was 135.8°, indicating that it was a hydrophobic surface. According to previous studies, the surface energy of polyester was 41 mJ m−2 and the flat film showed a contact angle of 84° with respect to water,39 while the surface energy of silver coated on the polyimide fiber was 1250 mJ m−2 and the contact angle of the smooth silver surface was 79°, indicating hydrophilicity.40,41 Therefore, although the surface energy was slightly increased due to the insertion of the silver coated conductive yarns, the knitted fabric composed of yarns exhibited better hydrophobicity than the film when micro-roughness due to fiber bundles was generated.Conversely, when the fabric was hydrolyzed by alkaline solution, the contact angle of the conductive knitted fabric gradually decreased as the hydrolysis duration increased, so that water droplets were completely absorbed under the 60 min condition. Alkaline hydrolysis is a traditional method for polyester fabrics to improve the hydrophilicity and chemical reactivity. The nucleophilic attack of a base to the carbonyl carbon results in the hydrolysis of the ester bond, and alkaline hydrolyzed polyester shows increased hydrophilic end groups such as carboxyl and hydroxyl.4,20,42 In addition, when silver changes to silver oxide, the affinity toward the liquid changes. As the duration of hydrolysis increases, the oxidation state of the conductive yarn increases, and electrons are clustered in the polar molecules of water around the oxides, changing the electronic properties of the surface.43 As a result, as the hydrolysis duration increases, the hydrophilic end groups on the surface of the polyester fiber are increased and more silver oxide was more generated, becoming the surface of the conductive knitted fabric hydrophilic.
When DTMS deposition was carried out, the contact angle increased to 141.2° for untreated, 147.8° for 15 min, 150.5° for 30 min, 155.3° for 45 min, and 155.9° for 60 min. From 30 min, the samples showed a contact angle of 150° or more, achieving a superhydrophobic surface. Abundant hydrophobic alkyl chains of DTMS could reduce the surface energy of alkaline hydrolyzed knitted fabrics.44 In particular, as the alkaline hydrolysis duration increased, micro-nano roughness for superhydrophobicity was realized due to craters and pits formed on the polyester yarns and the roughened surface of the conductive yarns, and the contact angle was greatly increased.20,30,45
For superhydrophobicity, the effect of introducing dual roughness structures through alkaline hydrolysis can be confirmed by measuring the shedding angle. As shown in Table 5, the water droplet adhered to the surface of untreated sample and did not roll off even at a 90°. However, when the surface energy was lowered by coating with DTMS, the shedding angle was sharply decreased and the water droplet rolled off at 15.4°. When the nano roughness was realized on the surface of the fibers by alkaline hydrolysis processing, the shedding angle was decreased less than 10°, indicating a superhydrophobic surface. The nano craters and pits on the polyester yarns and roughen surface of the conductive yarns by alkaline hydrolysis generated hierarchical structure on the fabric, and the trapped air between fibers and liquid became larger, resulting in close to the Cassie–Baxter state.38 However, even if the alkaline hydrolysis duration was increased to more than 30 min, the shedding angle did not decrease further but showed similar values. In order for water droplets to roll-off on the fabric surface, the contact area between the liquid and the solid should be controlled by optimally combining the micro-roughness of the yarn and the nano-roughness created by the alkaline hydrolysis.45 Therefore, it is considered that there was a limit to uniformly controlling the contact area with water droplets because the directions of the nano-roughness formed on the surface by the etching of the polyester yarns and the oxidation of the conductive yarns through alkaline hydrolysis was different.
| Untreated | Hydrophobic coated | Alkaline hydrolysis + hydrophobic coating | ||||
|---|---|---|---|---|---|---|
| 15 min | 30 min | 45 min | 60 min | |||
| Shedding angle (°) | >90 | 15.4 ± 1.0 | 13.4 ± 1.9 | 9.7 ± 0.9 | 9.5 ± 0.4 | 10.1 ± 0.4 |
The superhydrophobicity of the conductive knitted fabric achieved by the alkaline hydrolysis and hydrophobic coating was maintained even at the moment of heat generation by passing an electric current through the fabric, as shown in Fig. 8.
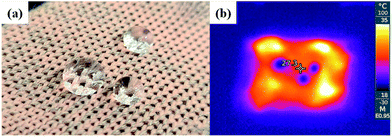 | ||
| Fig. 8 Photo (a) and infrared thermal image (b) of sample with electric heating performance and superhydrophobicity (alkaline hydrolyzed for 30 min and hydrophobic coated fabric). | ||
3.5. Durability of conductivity by air and water
Silver has excellent conductivity; however, when exposed to the atmosphere at room temperature, its resistance increases gradually and its color changes to dark brown.46,47 Therefore, for various applications, the conductive knitted fabric should have good conductivity as well as durability due to air and moisture to which it might be exposed in the use environment. In this study, conductive durability evaluation was performed by prolonged exposure to air and repeated water contact. For this purpose, based on the superhydrophobicity and conductivity results, a 30 min alkaline hydrolysis fabric was selected as the test sample.Untreated, only hydrophobic coated, alkaline hydrolyzed, and alkaline hydrolyzed and hydrophobic coated samples were stored at room temperature for 16 weeks to observe changes in their surface resistance as a function of time. Fig. 9(a) shows the rate of change of the surface resistance value as a function of time with respect to the initial surface resistance value. As a result, the final surface resistance of the untreated fabric left at room temperature for 16 weeks was 3.84 Ω sqm−1, indicating almost no change in resistance due to air, while alkaline hydrolyzed conductive fabric showed 10.12 Ω sqm−1 of the final surface resistance, indicating an increase of 124% compared to the initial value. The silver oxide formed by alkaline solution reacted with O2 to form Ag2O3 more rapidly in air and lost its original conductivity.36 On the other hand, the hydrophobic coated fabrics showed little change in resistance even after 16 weeks. For hydrophobic coated fabric and alkaline hydrolyzed and hydrophobic coated fabric, the final surface resistances were 3.66 Ω sqm−1 and 5.19 Ω sqm−1, respectively, only up to 15% higher than their initial surface resistances. Thus, the rate of increase of the surface resistance of the hydrophobic coated fabrics was lower than that of the non-coated sample when exposed to air. Therefore, it was confirmed that the hydrophobic coating used in this study was effective in blocking the penetration of air by effectively covering the conductive yarns by the introduction of superhydrophobicity into the conductive knitted fabrics.
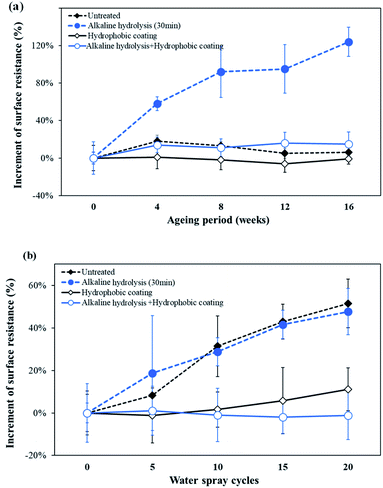 | ||
| Fig. 9 Effect of (a) atmospheric ageing and (b) water on the surface resistance of conductive knitted fabrics. | ||
Electrical properties of the conductive fabrics are affected by the water of the environment.48 Therefore, it is necessary to examine whether the superhydrophobic processing contributes to maintaining conductivity by repeated exposure to water. Accordingly, an experiment was performed using the spray test method and the results are shown in Fig. 9(b).
The final surface resistance of the untreated fabric after being exposed to water 20 times was increased by 52% over to the initial value. For the untreated fabric, an abrupt surface resistance rise was caused by the exposure of the silver metal to water without protection. This tendency was also observed in alkaline hydrolyzed fabric. For the hydrophobic coated sample, the final surface resistance increased by only 11%. The water mostly bounced off the surface due to the water-repellency by hydrophobicity of the coated fabrics, preventing water from contacting the silver-coated yarns. Thus, it was confirmed that conductivity losses caused by water were delayed by effectively blocking contact of the metal with moisture using the hydrophobic coating. In particular, fabrics that achieved superhydrophobic surfaces by alkaline hydrolysis and hydrophobic coating showed little change in surface resistance even after 20 repeated exposures to water. The superhydrophobicity with exceptional water repellency of a fabric not only depends on the chemical composition of its outer surface, but also its texture which controls its roughness and the amount of air trapped in the fabric when in contact with water.11 The superhydrophobic surface reduces the contact area between liquid and solid by forming trapped air pockets on the surface due to the micro-nano dual roughness.5,6,11 Water droplets falling on the superhydrophobic surface do not adhere to the surface, but rebound and roll down.49–51 Therefore, in this study, the conductive fabric with a superhydrophobic surface had a small contact area with water, and even if water droplets collided with the fabric surface, they could not adhere to it and rolled off. As a result, repeated exposure to water did not affect the surface resistance of the superhydrophobic conductive fabric, as almost no water was left on the surface.
Therefore, it was confirmed that the conductivity of the conductive knitted fabric could be improved by preventing the silver from being oxidized by exposure to air and water by introducing a hydrophobic coating. In particular, the superhydrophobic surface implemented on the conductive fabric effectively prevented the loss of conductivity due to water exposure by reducing the contact area between fabric and water.
4. Conclusions
A superhydrophobic surface was introduced to solve the problem that silver-based conductive fabrics lose their conductivity due to oxidation by air and water. A conductive knitted fabric composed by polyester and silver-coated yarns was prepared by weft-knitted machine, and alkaline hydrolysis processing was performed to realize nano-roughness on the fabric surface through etching of the polyester and the phase change of the silver. The treated fabrics were evaluated for conductivity, superhydrophobicity, and durability of conductivity by oxidation according to alkaline hydrolysis duration and hydrophobic coating.Nano-roughness was introduced by etching the surface of the polyester yarn through alkaline hydrolysis, and as the duration increased, the surface of the polyester was etched more, showing clear nano-roughness. Meanwhile, the surface of the silver conductive yarns was oxidized by reaction with NaOH and changed to Ag2O or AgO, which made the surface of the conductive yarns rough and non-uniform. The weight, tensile strength and strain of the conductive knitted fabric decreased due to the alkaline hydrolysis, and the change was noticeable after 30 min of alkaline hydrolysis.
In terms of conductivity, the surface resistance of conductive knitted fabric increased as the alkaline hydrolysis duration increased. This is because the NaOH and silver reacted to form more Ag2O phase with high resistance. In addition, the subsequently hydrophobic coating increased the surface resistance of all conductive fabrics, with and without alkaline hydrolysis, as the hydrophobic layer prevented the absorption of moisture and the flow of electrons. However, despite the increase in surface resistance by alkaline hydrolysis and hydrophobic coating, even when a low voltage of 3 V was applied, the surface temperature of all fabrics increased by more than 10 °C, demonstrating excellent electric heating performance.
After 30 min of alkaline hydrolysis, a contact angle of 150° or more and a shedding angle of less than 10° were achieved a superhydrophobic surface. This was made possible by etching of the polyester and the formation of Ag2O particles of the conductive yarn through alkaline hydrolysis to form the micro-nano dual roughness on the conductive knitted fabric, and lowering the surface energy with a hydrophobic coating.
As a condition to achieve minimal surface resistance change and superhydrophobicity, 30 min alkaline hydrolysis duration was selected as the optimal condition. This superhydrophobic surface improved the durability of the conductivity by preventing the reaction between silver and air or water. In particular, the surface resistance of the untreated conductive fabric after 20 instances of water exposure was increased by 52% from the initial vale, while the superhydrophobic coated fabric increased by only 11%, showing excellent durability against water.
This study is significant in that it achieved dual roughness for superhydrophobicity in a relatively simple way by applying alkaline reduction processing, which is commonly used in polyester, to extend the application of conductive fabrics by preventing oxidation by air and water. It is expected that it will be possible to solve the problem of functional deterioration due to various usage environment of conductive fabrics usage in smart clothing and wearable devices. However, this method has a limitation that it can be applied only to polyester-based blended fabrics. Therefore, continued research is needed on the introduction of superhydrophobic surfaces to secure the durability of conductive fabrics using more diverse materials and fibers.
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.Acknowledgements
This research was supported by “Research Base Construction Fund Support Program” funded by Jeonbuk National University in 2022. And this research also received support from the National Research Foundation (NRF) of Korea funded by the Korean government (MSIP: Grant No. 2021R1F1A1060287).References
- Z. Kang, Y. He, J. Sang, H. Hirahara and D. Chen, Adv. Mater. Interfaces, 2021, 8, 2100651 CrossRef CAS.
- S. Lee and C. H. Park, RSC Adv., 2018, 8, 31008 RSC.
- S. Lee and C. H. Park, Text. Res. J., 2019, 89, 2376–2394 CrossRef CAS.
- V. Allahyarzadeh, M. Montazer, N. H. Nejad and N. Samadi, J. Appl. Polym. Sci., 2013 DOI:10.1002/app.38907.
- W. S. Tung and W. A. Daoud, J. Mater. Chem., 2011, 21, 7858 RSC.
- S. Park, J. Kim and C. H. Park, Text. Res. J., 2017, 87, 193–207 CrossRef CAS.
- S. Wang, K. Liu, X. Yao and L. Jiang, Chem. Rev., 2015, 115, 8230–8293 CrossRef CAS PubMed.
- L. Zheng, X. Su, X. Lai, W. Chen, H. Li and X. Zeng, Mater. Lett., 2019, 253, 230–233 CrossRef CAS.
- X. Su, H. Li, X. Lai, Z. Chen and X. Zeng, ACS Appl. Mater. Interfaces, 2018, 10, 10587–10597 CrossRef CAS.
- L. Wang, D. He, J. Li, B. He and L. Qian, Cellulose, 2021, 28, 5881–5893 CrossRef CAS.
- S. Park, J. Kim and C. H. Park, J. Eng. Fibers Fabr., 2015, 10, 1–18 Search PubMed.
- X. M. Li, D. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 1350–1368 RSC.
- A. Seaton, L. Tran, R. Aitken and K. Donaldson, J. R. Soc., Interface, 2010, 7, S119–S129 CrossRef CAS.
- M. N. Moore, Environ. Int., 2006, 32, 967–976 CrossRef CAS PubMed.
- Z. Cai, L. Shen, X. Wang and Q. Guo, Compos. Sci. Technol., 2018, 164, 238–247 CrossRef CAS.
- S. Ghapanvari, A. Khoddami and H. Hasani, Textiles and Light Industrial Science and Technology, 2015, 4, 27–34 CrossRef.
- H. Kim, J. H. Oh and C. H. Park, Text. Res. J., 2021, 91, 1274–1289 CrossRef CAS.
- S. M. M. Kabir and J. Koh, Color. Technol., 2017, 133, 209–217 Search PubMed.
- M. S. Han, Y. Park and C. H. Park, Fibers Polym., 2016, 17, 241–247 CrossRef CAS.
- Z. Mazrouei-Sebdani and A. Khoddami, Prog. Org. Coat., 2011, 72, 638–646 CrossRef CAS.
- Y. Yan, H. Yang, J. Li, X. Lu and C. Wang, Text. Res. J., 2012, 82, 1422–1429 CrossRef CAS.
- J. Zimmermann, S. Seeger and F. A. Reifler, Text. Res. J., 2009, 79, 1565–1570 CrossRef CAS.
- A. Kaynak, E. Håkansson and A. Amiet, Synth. Met., 2009, 159, 1373–1380 CrossRef CAS.
- Y. Tai, C. Xu and H. Chen, Mater. Lett., 2016, 180, 144–147 CrossRef CAS.
- S. S. Abd El Rehim, H. H. Hassan, M. A. Ibrahim and M. A. Amin, Monatsh. Chem., 1998, 129, 1103–1117 CAS.
- D. Lützenkirchen-Hecht and H. H. Strehblow, Surf. Interface Anal., 2006, 38, 686–690 CrossRef.
- E. Sanli, B. Z. Uysal and M. L. Aksu, Int. J. Hydrogen Energy, 2008, 33, 2097–2104 CrossRef CAS.
- H. Luo, X. Ji and S. Cheng, RSC Adv., 2020, 10, 8453 RSC.
- A. Moazami, M. Montazer and M. K. Dolatabadi, Fibers Polym., 2016, 17, 1359–1370 CrossRef CAS.
- S. Lee and C. H. Park, Text. Res. J., 2018, 88, 777–789 CrossRef CAS.
- A. Ziashahabi, M. Prato, Z. Dang, R. Pousalehi and N. Naseri, Sci. Rep., 2019, 9, 11839 CrossRef PubMed.
- K. T. Sullivan, C. Wu, N. W. Peikiel, K. Gaskell and M. R. Zachariah, Combust. Flame, 2013, 160, 438–446 CrossRef CAS.
- S. Ravichandran, V. Paluri, G. Kumar, K. Loganathan and B. R. K. Venkata, J. Exp. Nanosci., 2016, 11, 445–458 CrossRef CAS.
- S. Lee and J. Eng, J. Eng. Fibers. Fabr., 2022, 17, 1–47 Search PubMed.
- H. R. Hong, J. Kim and C. H. Park, RSC Adv., 2018, 8, 41782 RSC.
- A. H. Hammad, M. S. H. Abdel-Wahab and A. Shahrie, Digest Journal of Nanomaterials and Biostructures, 2016, 11, 1245–1252 Search PubMed.
- U. K. Barik, S. Srinivasan, C. L. Nagendra and A. Subrahmanyam, Thin Solid Films, 2003, 429, 129–134 CrossRef.
- X. Wang, W. Xu, W. Li and W. Cui, Text. Res. J., 2009, 79, 753–760 CrossRef CAS.
- H. Kim and S. Lee, Text. Res. J., 2019, 89, 4114–4130 CrossRef CAS.
- S. Sigurdsson and R. Shishoo, J. Appl. Polym. Sci., 1997, 66, 1591–1601 CrossRef CAS.
- S. Schubert, J. Meiss, L. Müller-Meskamp and K. Leo, Adv. Energy Mater., 2013, 3, 438–443 CrossRef CAS.
- A. Safaee, D. K. Sarkar and M. Farzaneh, Appl. Surf. Sci., 2008, 254, 2493–2498 CrossRef CAS.
- J. H. Oh and C. H. Park, Adv. Mater. Interfaces, 2020, 7, 2000127 CrossRef CAS.
- M. Mirzaeian, A. A. Ogwu, H. F. Jirandehi, S. Aidarova, Z. Ospanova and N. Tsendzughul, Colloids Surf., A, 2017, 519, 223–230 CrossRef CAS.
- H. Yu, M. Wu, G. Duan and X. Gong, Nanoscale, 2022, 14, 1296 RSC.
- X. G. Cao and H. Y. Zhang, Powder Technol., 2012, 226, 53–56 CrossRef CAS.
- B. T. Liu and S. X. Huang, RSC Adv., 2014, 4, 59226 RSC.
- H. Kim and S. Lee, Fibers Polym., 2021, 22, 276–284 CrossRef CAS.
- Q. Kong, Z. Li, X. Ren, H. Gu and W. Ma, Des. Monomers Polym., 2021, 24, 164–174 CrossRef PubMed.
- D. J. Lee, H. M. Kim, Y. S. Song and J. R. Youn, ACS Nano, 2021, 6, 7656–7664 CrossRef PubMed.
- S. LeClear, J. LeClear, Abhijeet, K. C. Park and W. Choi, J. Colloid Interface Sci., 2016, 461, 114–121 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |