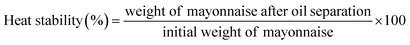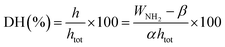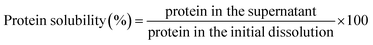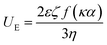Open Access Article
Open Access ArticleMechanistic insights into the improved properties of mayonnaise from the changes in protein structures of enzymatic modification-treated egg yolk†
Wenfei Zhaoa,
Jingnan Zanga,
Mingmin Qinga,
Huiyong Wanga,
Yujie Chi *a and
Yuan Chi*b
*a and
Yuan Chi*b
aCollege of Food Science, Northeast Agricultural University, Harbin, Heilongjiang 150030, China. E-mail: yjchi310@126.com; Tel: +86-13019713698
bCollege of Engineering, Northeast Agricultural University, Harbin, Heilongjiang 150030, China. E-mail: cy207@126.com; Tel: +86-13945678633
First published on 26th September 2022
Abstract
Heat treatment is an important step in mayonnaise production but can affect the quality of mayonnaise because thermal treatment can accelerate oil droplet coalescence. To resolve this issue, in this study, enzymatically modified egg yolks were applied to produce mayonnaise. Egg yolk hydrolyzed with 0.2% neutral protease could effectively produce mayonnaise with superior heat stability, and this effect was attributed to enzymatic modifications that increased the degree of amino acid ionization, the overall hydrophilicity and the ability to adsorb proteins. Moreover, electrophoresis and FT-IR results showed that the enzymatically modified egg yolk proteins had a smaller molecular weight and more flexible structure, which could also favor the improved properties. The study elucidated why mayonnaise prepared by enzymatic modification-treated egg yolk has better thermal stability.
1 Introduction
Mayonnaise is a viscous oil-in-water emulsion that is widely applied in sauces worldwide.1,2 One of the necessary processes for the production of mayonnaise is sterilization, which inactivates pathogenic microorganisms.3 However, under heat treatments, the appearance of mayonnaise becomes undesirable, and its ability to emulsify decreases due to changes in the hydrophilicity and interfacial tension of yolk proteins, which could present an obstacle to the industrial development of mayonnaise; therefore, developing a method to prepare mayonnaise that is stable under high heat would be advantageous.Enzymatic modification is an effective method for enhancing the heat stability of egg yolk,4 and improvements in the heat stability of other proteins have also been found,5,6 which could contribute to the formation of proteins or peptide bonds with smaller molecular weights.7–9 Therefore, we deduced that enzymatically modified egg yolk has great potential for application in the production of emulsified food. Heat stability is very important in the industrial production of food because the temperature increase caused by machine operation is involved in many processes.10 However, to our knowledge, previous studies have mainly investigated the influence of enzymatic modification on emulsification activity. Bao11 showed that improved emulsification activity could be obtained by altering the molecular size. Fu12 found that hydrolyzed egg yolk exhibits excellent emulsification activity due to its improved solubility. Little information is available concerning the heat stability of mayonnaise with enzymatically modified egg yolks. In addition, few studies have focused on the mechanism underlying the improvements from the perspective of protein structures. To some extent, this lack of knowledge will hinder the application of protease-modified egg yolk in mayonnaise.
Therefore, the objective of this study was to prepare protease-modified egg yolks that are suitable for the production of mayonnaise with improved heat stability. Moreover, the physicochemical properties and protein structure of enzymatically modified egg yolks were evaluated to elucidate the mechanism of the improvement. In summary, this work is expected to lay the foundation for the application of enzymatically modified egg yolk in the preparation of mayonnaise, which would ultimately benefit the practical production of mayonnaise.
2 Materials and methods
2.1 Materials
Fresh grade A brown shell eggs, sugar, salt and vinegar were obtained from the Zhongjun supermarket, and soybean oil was obtained from Jiusan Oils & Grains Industries Group Co., Ltd. Food-grade Neutrase was obtained from Shanghai Yuanye Biotechnology Co., Ltd., and ortho-phthalaldehyde (OPA), sodium tetraborate, dithiothreitol (DTT), sodium dodecyl sulfate (SDS), glycine, and 1-anilino-8-naphthalene sulfonic acid were purchased from Sigma-Aldrich Chemicals, Inc. (St. Louis, MO, USA). Other reagents were purchased from Solaibao Technology Co., Ltd. (Beijing, China).2.2 Preparation of liquid egg yolk and separation of plasma and granules
The shells of fresh eggs were broken, and the egg yolks were separated from the egg whites by a separator. The residual albumen was then removed by rolling on filter paper. The vitelline membrane was punctured, and pure egg yolk was collected in a beaker and then slowly stirred to homogenize the sample for subsequent applications. Plasma and granules were prepared according to the method described by Chalamaiah, M. with slight modification.13 The egg yolk was diluted with an equal weight of distilled water (DW), and the sample diluent was then divided into two parts by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C for 40 min. The supernatant and sediment were the egg yolk plasma and granules, respectively.
000 rpm and 4 °C for 40 min. The supernatant and sediment were the egg yolk plasma and granules, respectively.
2.3 Preparation of enzymatically modified egg yolk and mayonnaise
Commercial Neutrase (food grade, 0.05–0.5%) was added to the liquid egg yolk (LEY) and incubated at a constant temperature of 50 °C for 1 h in a thermostatic water bath reactor. Then, enzymatically modified egg yolk was pasteurized at 60 °C for 4 min. The processes used for the preparation of mayonnaise are as follows. Salt, sugar, water, and enzymatically modified egg yolks were mixed at a high speed for 5 min in the corresponding carrier, and oil was added dropwise during the blending process. Subsequently, vinegar was added, and the resulting mixture was stirred for 2 min.2.4 Preparation of emulsion
Egg yolk samples were dispersed into deionized water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 dilution) and stirred magnetically. Then, emulsions were prepared by mixing the oil phase and diluting egg yolk samples (1
20 dilution) and stirred magnetically. Then, emulsions were prepared by mixing the oil phase and diluting egg yolk samples (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) using a high-shear mixer at 12
4) using a high-shear mixer at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 1 min.
000 × g for 1 min.
2.5 Optical microscopy of mayonnaise products
A drop of mayonnaise sample was placed on a slide. The samples were covered with a cover glass and observed when the objective lens was adjusted to 10×.2.6 Heat stability of the mayonnaise
Heat stability was measured according to the method described by Primacella, M.14 Eight milliliters of mayonnaise was poured into a 10 mL centrifuge tube, and a thermal stability test was performed using mayonnaise heated at 70 °C for 30 min. The samples were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 25 min. The separated oil was carefully removed and discarded. The heat stability was calculated as follows:
000 rpm for 25 min. The separated oil was carefully removed and discarded. The heat stability was calculated as follows:2.7 Droplet diameters and particle size distribution
The droplet diameters and particle size distribution (PSD) were estimated using a size tester (Malvern Instruments Co., LTD). The emulsion was diluted 1000-fold with distilled water.2.8 Degree of hydrolysis
The ortho-phthalaldehyde (OPA) method was utilized to measure the degree of hydrolysis (DH) of the egg yolk according to the method described by Nielsen, P. M.15 In brief, the sample was diluted with distilled water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200). The OPA method involves mixing the OPA reagent with the sample diluent (6
200). The OPA method involves mixing the OPA reagent with the sample diluent (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and allowing the components to react. The absorbance of the mixed solution was measured at 337 nm using an ultraviolet spectrophotometer. The DH was calculated using the following formula:
1) and allowing the components to react. The absorbance of the mixed solution was measured at 337 nm using an ultraviolet spectrophotometer. The DH was calculated using the following formula:where h, htot and WNH2 represent the amount of broken peptide bonds per unit weight (mmol g−1), the total amount of peptide bonds per unit weight (8 mmol g−1), and the amount of NH2 per unit weight, respectively. In addition, α = 1.0 and β = 0.4.
2.9 Protein solubility
The protein solubility of the yolk samples that were prepared by different enzyme dosages was determined using the method described by Denmat, M. L.16 with slight modification. The sample was dissolved and stirred in DW for 1 h, and the solution was then centrifuged at 8000 rpm for 20 min. One milliliter of sample without centrifugation and supernatant was removed and allowed to react with 4 mL of biuret reagent for 30 min. The absorbance was then measured at 540 nm, and the protein solubility was calculated as follows:2.10 Zeta potential
The zeta potential (ζ-potential) of the samples was determined according to the method described by Avramenko, N. A.17 with a nanoparticle size tester. The samples were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 using DW and equilibrated for 60 s for further measurement. The electrophoretic mobility (UE) of the protein was examined to obtain the ζ-potential by using the Henry equation as follows:
1000 using DW and equilibrated for 60 s for further measurement. The electrophoretic mobility (UE) of the protein was examined to obtain the ζ-potential by using the Henry equation as follows:where ε, f(κα) and η represent the permittivity, the function related to the ratio of the particle radius to the Debye length, and the dispersion viscosity, respectively.
2.11 Mechanical properties
The mechanical properties (firmness, consistency, cohesiveness, and work of cohesion) were evaluated using a back extrusion rheology approach. Thirty-five milliliters of the enzymatically modified egg yolk was poured into a 50 mL beaker, and the samples were forced upward by lowering a plunger equipped with probe A-BE (ϕ 35 mm). The pretest speed, measuring speed, and posttest speed were 2 mm s−1, 2 mm s−1, and 1 mm s−1, respectively. The test distance was 15 mm, and the trigger force was 5.0 g.2.12 Surface hydrophobicity
The surface hydrophobicity (H0) was measured by applying ANS as a hydrophobic fluorescence probe according to the method described by Xu, X.18 Four milliliters of the sample solution (0.05–1 mg mL−1) was mixed with 20 μL of 8 mmol L−1 ANS and allowed to react in the dark for 15 min. The fluorescence intensity (FI) was then measured at an excitation wavelength of 390 nm and an emission wavelength of 470 nm. The emission and excitation slit widths were both 5 nm, and the slope of the curve at the initial stage was the surface hydrophobicity of the protein molecule.2.13 Intrinsic fluorescence spectra
Fluorescence spectroscopy of the sample was performed following the method of Khan, J. M.19 with a slight modification using a fluorescence spectrophotometer (F-7100 FL spectrophotometer, 3017-008). The protein concentrations were adjusted to 1 mg mL−1 with 10 mmol L−1 phosphate buffer (pH 7.0). The parameter settings were as follows: an excitation wavelength of 280 nm, an emission wavelength of 300–390 nm, a scan speed of 60 nm min−1, and emission and excitation slit widths of 5 nm.2.14 SDS-PAGE
The protein patterns of the egg yolks were analyzed following the method of Gao Y.4 The sample was diluted in 0.5 M NaCl, and the diluent was then mixed with SDS-PAGE sample buffer. SDS-PAGE was conducted, and the gels were stained. A solution of 10% methanol and 10% acetic acid was used for destaining. An image of the gel was captured using a Gel Doc 2000 system (Bio-5000 Microtek, Hsinchu, Taiwan).2.15 Fourier transform infrared spectroscopy (FT-IR)
FT-IR measurements were conducted following the method described by Wang, R.20 After the different treatments, the egg yolk samples were placed on an attenuated total reflection scanning attachment, and the structure of proteins was then determined and recorded at 4000–400 cm−1 by applying FT-IR (Nicolet iS10, Thermo Fisher Scientific Co., Ltd., Waltham, MA, USA).2.16 Statistical analysis
SPSS software was used for the data analyses. The significance of the differences was analyzed by one-way analysis of variance (ANOVA), and p < 0.05 was considered to indicate significance.3 Results and discussion
3.1 Optical microscopy and heat stability analysis
The microscopic diagram can directly reflect the sizes of oil droplets and uniformity of mayonnaise prepared by different hydrolysis degrees of egg yolk. As shown in Fig. 1(A), the droplet size of mayonnaise prepared with fresh egg yolk was large and nonuniform, and the droplet size gradually decreased as the enzyme dosage increased. The change was primarily because egg yolk granules depolymerized and protein solubility increased with increasing enzyme addition.21 In addition, the phenomenon may be closely related to the coalescence of oil droplets; more specifically, under the same heat treatment conditions, only a small amount of oil droplets coalesced in mayonnaise prepared with enzymatic modification-treated egg yolk compared with mayonnaise prepared with fresh egg yolk.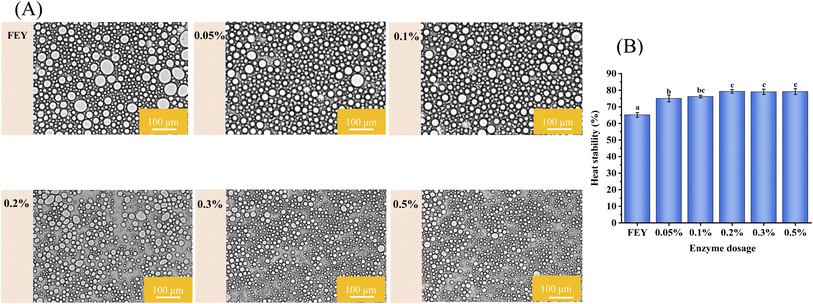 | ||
| Fig. 1 Optical microscopic images (A) and heat stability (B) of mayonnaise prepared by egg yolk modified with different enzyme dosages. | ||
The heat resistance of mayonnaise has a very important effect on the quality of the product. The O/W emulsion is a thermodynamically unstable system, and the motion and collision frequency of the oil droplets increased during thermal operation, leading to coalescence of oil droplets and affecting the properties of the mayonnaise. As shown in Fig. 1(B), the heat stability of mayonnaise first increased and then exhibited a relatively constant final value after the addition of up to 0.2%. This phenomenon might be caused by the presence of peptides. As reported by Primacella, the peptides produced by the hydrolysis of LEY could stabilize the network and reduce the speed of fat coalescence.14 The improvement might also be attributed to the enhanced steric stabilization system, which could enhance the ability of oil droplets to prevent coalescence.22 In addition, enzymatic hydrolysis may increase the negative charge due to the liberation of carboxylic groups, which could increase the electrostatic repulsion between egg yolk proteins or peptides and thus inhibit the interaction between adsorbed and unabsorbed proteins in the emulsion system. Heat stability could not continue to increase with the addition of more than 0.2% enzyme, which may be closely related to the droplet diameter in the emulsions.
3.2 Emulsifying properties
The mean oil droplet diameter in the emulsions could reflect the emulsifying activity of the sample. As shown in Fig. 2(A), the droplet diameters decreased by nearly 60% from 3.9 μm to 1.6 μm. Specifically, a moderate enzyme dosage (0.2%) could efficiently decrease the size of droplets, and a continued increase in the enzyme dosage above 0.2% could not continue to decrease the droplet size. The droplet diameter of the emulsion prepared with enzymatically modified egg yolk was significantly smaller than that of the emulsion prepared with the untreated sample. The decrease in droplet size may have occurred because the enzymatic modification had a significant effect on the minimum droplet size (p < 0.05).23 However, the oil droplet size did not decrease further with the addition of 0.5% enzyme, possibly because a higher DH had little influence on the reduction in interfacial tension and the improvement in emulsifying activity because the peptides could not unfold and reorient at the interface.11 The emulsifying properties could not increase further, which may also be due to an imbalance between hydrophilic and hydrophobic groups.24 Therefore, a suitable addition could be sufficient to improve the emulsifying activity.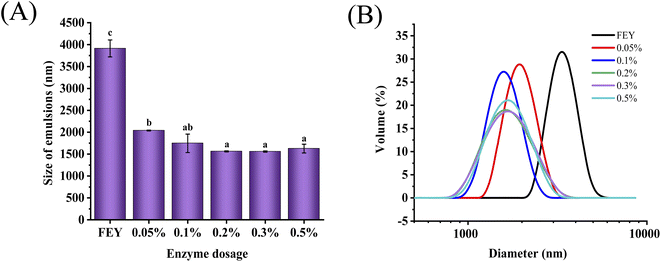 | ||
| Fig. 2 Size of emulsion (A), and particle size distribution of emulsion (B) prepared by egg yolk modified with different enzyme dosages. Different letters indicate significant differences (p < 0.05). | ||
The PSD was also an important value that could reflect the emulsifying activity. As shown in Fig. 2(B), the PSD of the emulsion prepared with enzymatically modified egg yolk exhibited a leftward shift compared to that of the mayonnaise prepared with LEY. A smaller particle size was associated with a stronger adsorption capacity. This phenomenon indicated that the large proteins could be transformed into small particles after enzymatic hydrolysis was performed. In addition, granules formed an insoluble complex through the phosphorus–calcium bridge, which could not be effectively absorbed at the interface compared with soluble protein. Granule protein was destroyed upon enzymatic modification, which could lead to an increase in water solubility, and more water-soluble proteins rapidly adsorbed to the interface to form droplets with relatively small particle sizes. The changes in the diameters and PSD of the droplets were consistent with the alterations in heat stability, which indicated that emulsifying properties could influence the rate of oil droplet merging. The reduced particle size and left-shifted PSD influenced by solubility were more beneficial for the stability of emulsion systems.
3.3 Degree of hydrolysis and protein solubility analysis
As shown in Fig. 3(A), the final DH increased from 0 to 8.9% as the amount of added enzyme increased from 0 to 0.5%. To a certain extent, a greater addition of enzyme was associated with a stronger impact on the egg yolk. Solubility is closely related to the DH and is regarded as the most basic property of proteins, and solubility is also closely related to the interaction between hydrophilic/hydrophobic groups on the protein surface and surrounding solvents. As shown in Fig. 3(A), solubility also increased with increases in the enzyme dosage, which showed the same trend as that found for DH. The soluble protein content increased from 44.6% to 62.6% as the enzyme dosage increased, which may have partially been observed because the apolipoproteins in the egg yolk were transformed into smaller proteins or peptides after enzymatic hydrolysis occurred. The existence of phosphorus–calcium bridges causes high-density lipoprotein and phosvitin to form an insoluble complex under natural conditions, and soluble hydrolysates are formed when water-insoluble proteins are destroyed and cleaved after enzymatic hydrolysis, leading to an increase in protein solubility. Improvements in solubility could inhibit coalescence because the adsorption behavior of soluble proteins was found to be an important prerequisite for the formation of films, and improved solubility is thus favorable for improving emulsifying properties. Therefore, these changes improved the quality of mayonnaise.11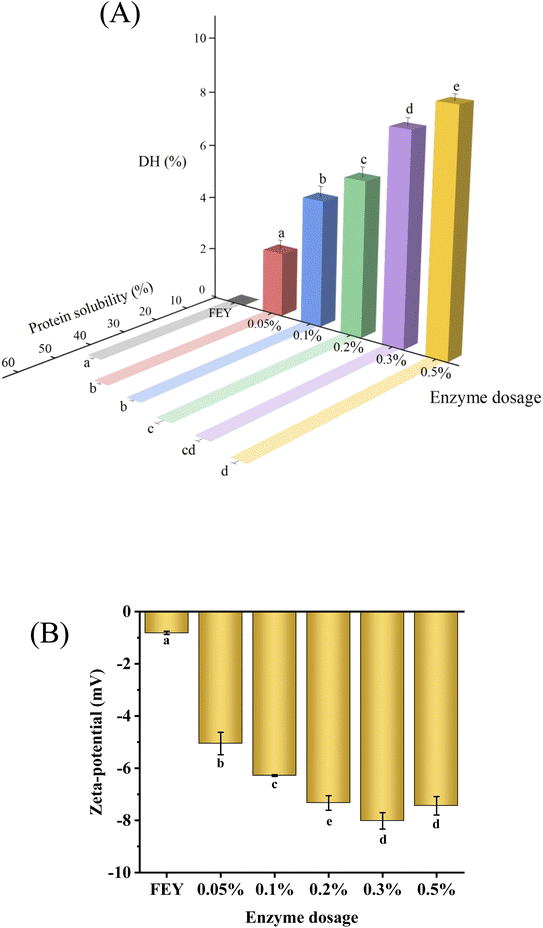 | ||
| Fig. 3 Degree of hydrolysis and protein solubility (A), and zeta potential (B) of egg yolk modified with different enzyme dosages. Different letters indicate significant differences (p < 0.05). | ||
3.4 Zeta potential analysis
The charge of a protein determines its interactions with other charged targets.25 As shown in Fig. 3(B), the absolute value of the surface charge of the sample changed from −0.82 to −8.0 with changes in enzyme addition from 0 to 0.3% and then decreased to −7.44 with the addition of 0.2%. A reasonable explanation for this phenomenon was that hydrolysis increased the negative charge due to the liberation of carboxylic groups,26 and the presence of this repulsion force caused the carboxylic groups to become more resistant to protein–protein interactions. The increase in repulsion between proteins also favored a high repulsive force between emulsions, which helped to increase the steric stabilization forces of oil droplets. In addition, the change in repulsion limited the aggregation behavior of emulsion droplets, which could help enhance the stability of mayonnaise.3.5 Analysis of mechanical properties
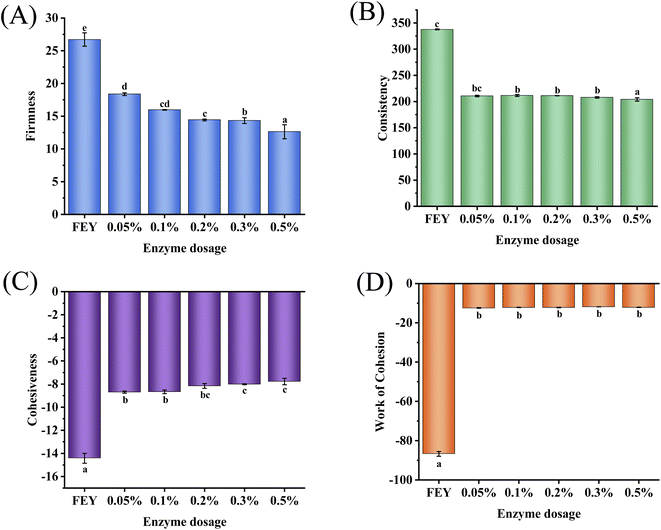
A texture analyzer can accurately detect the position and weight of food samples over time to determine the physical properties of the samples. As shown in Fig. 4, increasing the amount of added enzyme decreased the absolute values of firmness (from 26.715 to 12.625), consistency (from 337.75 to 204.01), cohesiveness (from −14.425 to −7.78), and work of cohesion (from −86.745 to −12.09), which indicated enhanced liquidity. This phenomenon may be caused by changes in interparticle colloidal forces among proteins. The balance of interparticle colloidal forces determines the variation in the consistency of the forces,27 which includes attractive forces (mainly van der Waals and hydrophobic forces) and repulsive forces (mainly electrostatic and steric forces). The mechanical properties decreased with the addition of enzyme, which showed that forces among proteins were decreased because enzymatic hydrolysis degrades proteins from high molecular weight to low molecular weight. Reduced mechanical properties are favorable for mixtures with LEY and other substances; thus, these properties provide a more convenient process of preparing mayonnaise and result in better quality.3.6 Surface hydrophobicity analysis
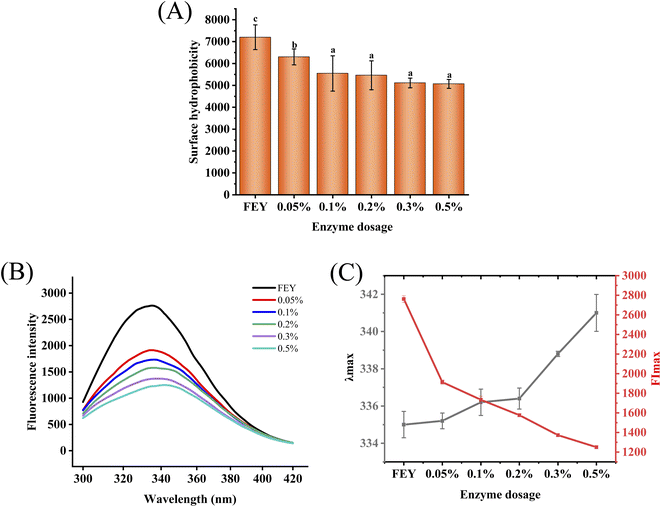
Surface hydrophobicity is a characteristic that represents the conformation and functional properties of proteins.28 LDL and HDL, as important components that affect the heat stability and emulsification of egg yolks, have a particularly high proportion of hydrophobic amino groups;29 thus, the study of surface hydrophobicity is an important task. As shown in Fig. 5(A), the surface hydrophobicity decreased from 7199.88 to 5067.55 as the addition of enzyme increased, and this observed decrease in hydrophobicity could be attributed to the fact that enzymatic hydrolysis breaks down hydrophobic areas. According to Li, Q.,21 this phenomenon may be caused by the exposure and further hydrophobic interactions between hydrophilic and hydrophobic groups after hydrolysis; specifically, further hydrophobic interactions occurred due to the weak steric hindrance between the peptide chains, which in turn decreased the H0.30 Decreases in surface hydrophobicity contribute to increasing the amount of water with which the protein can interact, which could enhance solubility.3.7 Intrinsic fluorescence spectra
The aromatic amino acids in proteins can be applied as fluorescent probes to research protein structure because they can produce fluorescence under excitation.31 Intrinsic fluorescence spectroscopy can reflect the microenvironment of aromatic amino acids, and a decrease in the fluorescence signal may be due to increases in the polarity of the chromophore microenvironment. As shown in Fig. 5(B) and (C), the λmax of the hydrolyzed egg yolk protein shifted from 335.1 nm to 341.0 nm, which suggested that enzymatic hydrolysis changed the polar environment of tryptophan residues. The fluorescence intensity decreased from 2761.5 to 1250.1, and this finding might have been obtained because aromatic groups were destroyed or these hydrolysates had fewer exposed aromatic groups, which would decrease the fluorescence intensity.173.8 SDS-PAGE analysis
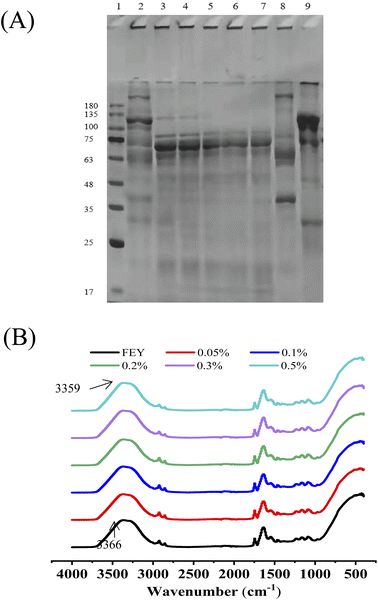
To understand the effect of enzymatic hydrolysis on the change in protein composition, SDS-PAGE profiles were obtained. The composition of proteins in yolk is complex, and based on the difference in density, egg yolk can be separated into plasma and granules through mild centrifugation. Plasma consists mainly of low-density lipoprotein and livetin, and granules consist mainly of high-density lipoprotein and phosvitin. The molecular weights of the two fractions of proteins were observably different. The obtained bands were identified based on the expected molecular weights (MW values). According to the literature, the MW of the egg yolk protein bands were mainly distributed between 20 and 250 kDa. The bands at 15, 17, 55, 68, 85, 93, 122 and 221 kDa represent apo-LDL; those at 31, 47, 78, 33 and 36 kDa represent β-livetin; those at 55 and 73 kDa are α-livetin; those at 203 kDa correspond to γ-livetin; and those at 31, 47, 78 and 110 kDa represent apo-HDL.32 As shown in Fig. 6(A), enzymatic hydrolysis mainly exerted a great influence on polypeptides with large molecular weights (more than 100 kDa); additionally, the band at 135 kDa gradually became shallow and almost disappeared when the addition of enzyme was higher than 0.2%. We also found that small molecular weights (less than 35 kDa) appeared because the high-molecular-weight proteins were degraded to low-molecular-weight protein bands. Another plausible explanation was attributed to new protein groups and the combination of hydrophobic groups and lipoproteins.21 Overall, large molecular weights were converted into smaller proteins or peptides, which were favorable for increasing solubility and emulsifying properties.3.9 FT-IR
FT-IR is a promising approach for obtaining a better understanding of the change in protein structure and functional groups because it provides structural information on the shift of absorption peaks and protein secondary structures. The fundamental frequency vibration of most organic compounds occurs in the mid-infrared region of 4000–400 cm−1. The main peaks at 3100–3600 cm−1 were ascribed to N–H stretching and hydrogen bonding; the peaks at 2800–3000 cm−1 were ascribed to C–H stretching; and the peaks at 1600–1700 cm−1 corresponded to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and hydrogen bonding.33–35 The absorption peaks of egg yolk lipids were found at 1459 cm−1 and 1383 cm−1 and were mainly ascribed to the shear bending vibration of –CH2 and the symmetric bending vibration of –CH3.36,37 As shown in Fig. 6(B), the peak at 3100–3600 cm−1 changed slightly with increases in the enzyme dosage, which indicated that the hydrogen bond force was influenced and led to a change in the functional properties of egg yolk.
O stretching and hydrogen bonding.33–35 The absorption peaks of egg yolk lipids were found at 1459 cm−1 and 1383 cm−1 and were mainly ascribed to the shear bending vibration of –CH2 and the symmetric bending vibration of –CH3.36,37 As shown in Fig. 6(B), the peak at 3100–3600 cm−1 changed slightly with increases in the enzyme dosage, which indicated that the hydrogen bond force was influenced and led to a change in the functional properties of egg yolk.
In addition, the amide I region (1600–1700 cm−1) is the most informative part of the FT-IR spectrum regarding the secondary structure of proteins; thus, a second-derivative procedure was applied using PeakFit software to resolve the overlapping secondary structural elements and thus observe changes in the secondary structure. The α-helix is relatively stable and has a compact and noncavity structure, and the β-turn and β-sheet are relatively stretched ordered structures, whereas the random coil exhibits a disordered structure.38 The percentage of proteins in egg yolks with secondary structures after enzyme hydrolysis is shown in Table 1. With increases in the enzyme dosage, the content of α-helix exhibited a decreasing trend from 33.91% to 32.76%, but the β-turns increased from 16.73% to 18.09%, and the changes in β-sheet and random coil changed only slightly. The phenomena were similar to those reported by Zhang, X.,39 which indicated that the flexibility of protein molecules was enhanced after enzyme hydrolysis, facilitating adsorption at the oil–water interface.40 As reported by Anton,41,42 the flexible and loose protein structure produced by enzyme hydrolysis can rapidly diffuse at the oil–water interface and cover the surface area of the oil droplets. Therefore, the mayonnaise prepared with the modified yolk can be considered to have better quality than that prepared with fresh yolks.
| Samples | β-Sheet (%) | Random coil (%) | α-Helix (%) | β-Turns (%) |
|---|---|---|---|---|
| a Different letter indicates significant difference (p < 0.05). | ||||
| FEY | 14.21 ± 0.03c | 35.15 ± 0.16b | 33.91 ± 0.04e | 16.73 ± 0.18a |
| 0.05% | 14.11 ± 0.03b | 34.67 ± 0.19ab | 33.70 ± 0.05d | 17.52 ± 0.22b |
| 0.1% | 14.04 ± 0.03a | 34.56 ± 0.20a | 33.59 ± 0.06d | 17.81 ± 0.23bc |
| 0.2% | 14.20 ± 0.03c | 34.77 ± 0.18ab | 33.24 ± 0.04c | 17.79 ± 0.19bc |
| 0.3% | 14.09 ± 0.02ab | 35.09 ± 0.19b | 32.93 ± 0.05b | 17.89 ± 0.21bc |
| 0.5% | 14.10 ± 0.03ab | 35.05 ± 0.19b | 32.76 ± 0.05a | 18.09 ± 0.21c |
3.10 Correlation analysis
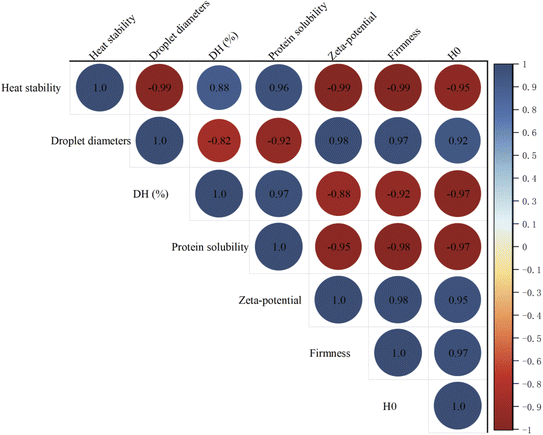
Correlation analysis can be applied to analyze the relationship between mayonnaise heat stability and the characteristics of enzymatically modified egg yolk. As shown in Fig. 7, mayonnaise heat stability was positively related to protein solubility and DH (%), and it was negatively correlated with oil droplet diameters, ξ-potential, and firmness. The results indicated that the improved heat stability of mayonnaise was closely related to the characteristics of enzymatically modified egg yolk, especially its emulsifying properties. The results confirmed that emulsions with smaller droplet sizes could be obtained by using enzymatically modified egg yolk. A smaller droplet size could obviously inhibit oil droplet flocculation induced by heat treatment or other conditions. Moreover, decreased egg yolk firmness was beneficial for mixtures with oil, which improved heat stability. Overall, the heat stability of mayonnaise can be controlled by regulating the emulsifying properties of egg yolk with enzymatic modification.A schematic illustration of the changes in hydrolyzed egg yolk protein and the interfacial stabilization of mayonnaise prepared with protease-modified egg yolk is shown in Fig. 8. Enzymatic modification resulted in the cleavage of plasma and granule proteins, which is represented by the conversion of large molecules to small molecular weight proteins. From the perspective of conformation, enzymatic hydrolysis changed the protein conformation, including changes in hydrophilicity and hydrophobicity, tertiary structure, hydrogen bonding, and secondary structure, which was confirmed by H0, intrinsic fluorescence spectra and FT-IR. Moreover, the adsorption behavior of soluble proteins was found to be an important prerequisite for the formation of films, and improved solubility is thus favorable for improving emulsifying properties. Furthermore, the smaller molecular weight of the particles allowed accumulation in the interfacial layer, enhancing the stability of the emulsion. Emulsions with smaller droplet sizes had improved heat stability. It is also worth noting that hydrolysis increased the negative charge due to the liberation of carboxylic groups, and the presence of this repulsion force caused the carboxylic groups to become more resistant to protein–protein interactions. The increase in repulsion between proteins also favored a high repulsive force between emulsions, which helped to increase the steric stabilization forces of oil droplets. In addition, the change in repulsion limited the aggregation behavior of emulsion droplets, which could help enhance the stability of mayonnaise. From the perspective of back extrusion rheology, the decreased firmness of liquid egg yolk could promote mixing with other materials, which may increase the contact degree and improve the heat stability of mayonnaise.
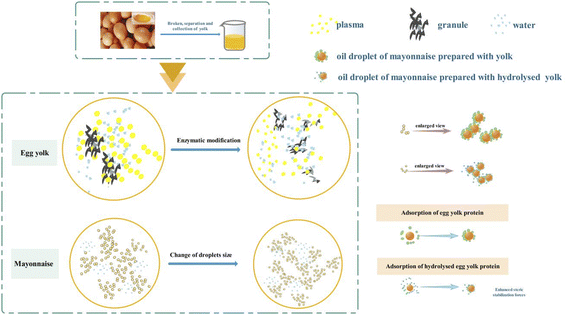 | ||
| Fig. 8 Schematic illustration of the changes of hydrolysed egg yolk protein and interfacial stabilization of mayonnaise prepared with protease-modified egg yolk. | ||
4 Conclusion
Protease-modified egg yolk with 0.2% protease addition has great potential in the preparation of mayonnaise with improved heat stability, and the observed improvements were related to changes in emulsifying properties, surface hydrophobicity and ξ-potential. The mechanism resulting in improved heat stability could be explained by structural characteristics and physicochemical properties. Enzymatic hydrolysis can convert compact structures to relatively stretched ordered structures, destroy aromatic groups, and change the polar environment of tryptophan residues, which makes the protein structure more flexible and thus increases the stability of the emulsion system. Our results suggest that the improvement in the heat stability of mayonnaise can be primarily attributed to the degradation of protein in plasma and granules, which most likely influences the mechanical properties of the yolk and generates smaller emulsion droplets. The study demonstrated the great potential for the application of enzymatically modified egg yolk as a material in the production of mayonnaise and may provide theoretical support for improving the properties of mayonnaise, which could also broaden the industrial application of enzymatically modified egg yolk. Moreover, further study is expected to identify the peptide sequences related to improved heat stability.Author contributions
Wenfei Zhao: investigation, writing-original draft; Jingnan Zang: methodology, software; Mingmin Qing: methodology, data analysis; Huiyong Wang: methodology; Yujie Chi: reviewing and editing, supervision, funding acquisition; Yuan Chi: resources.Conflicts of interest
No conflicts of interest exist regarding the submission of this manuscript, and the manuscript has been approved for publication by all authors. I declare on behalf of my coauthors that the work described here represents original research that has not been published previously and is not under consideration for publication elsewhere, in whole or in part. All the listed authors have approved the enclosed manuscript.Acknowledgements
This work was supported by the China Agriculture Research System of MOF and MARA [grant number CARS-40]; and the Heilongjiang Province Key Research and Development Plan Project “Special liquid egg products processing key technology research and industrialization” [grant number GA21B008].References
- P. Mishra, et al., At-line and inline prediction of droplet size in mayonnaise with near-infrared spectroscopy, Infrared Phys. Technol., 2022, 123, 104155 CrossRef.
- J. H. Li, et al., Hen egg yolk in food industry – a review of emerging functional modifications and applications, Trends Food Sci. Technol., 2021, 115, 12–21 CrossRef CAS.
- F. Guilmineau and U. Kulozik, Influence of a thermal treatment on the functionality of hen's egg yolk in mayonnaise, J. Food Eng., 2007, 78(2), 648–654 CrossRef.
- Y. Gao, et al., Effect of enzymatic hydrolysis on heat stability and emulsifying properties of egg yolk, Food Hydrocolloids, 2019, 97, 105224 CrossRef CAS.
- Y. Zheng, et al., Effects of limited enzymatic hydrolysis, pH, ionic strength and temperature on physicochemical and functional properties of palm (Elaeis guineensis Jacq.) kernel expeller protein, J. Food Sci. Technol., 2015, 52, 6940–6952 CrossRef CAS.
- G. Zhao, et al., Enzymatic hydrolysis and their effects on conformational and functional properties of peanut protein isolate, Food Chem., 2011, 127(4), 1438–1443 CrossRef CAS.
- M. R. Kim, et al., Optimization of the enzymatic modification of egg yolk by phospholipase A2 to improve its functionality for mayonnaise production, LWT–Food Sci. Technol., 2009, 42(1), 250–255 CrossRef CAS.
- D. Gazolu-Rusanova, et al., Role of lysophospholipids on the interfacial and liquid film properties of enzymatically modified egg yolk solutions, Food Hydrocolloids, 2020, 99, 105319 CrossRef CAS.
- K. Daimer and U. Kulozik, Oil-in-water emulsion properties of egg yolk: effect of enzymatic modification by phospholipase A2, Food Hydrocolloids, 2009, 23(5), 1366–1373 CrossRef CAS.
- K. Aganovic, U. Bindrich and V. Heinz, Ultra-high pressure homogenisation process for production of reduced fat mayonnaise with similar rheological characteristics as its full fat counterpart, Innov. Food Sci. Emerg. Technol., 2017, 45, 208–214 CrossRef.
- Z. J. Bao, et al., Effects of degree of hydrolysis (DH) on the functional properties of egg yolk hydrolysate with Alcalase, J. Food Sci. Technol., 2017, 54(3), 1–10 Search PubMed.
- X. Fu, et al., Characterization of enzymatically modified liquid egg yolk: structural, interfacial and emulsifying properties, Food Hydrocolloids, 2020, 105, 105763 CrossRef CAS.
- M. Chalamaiah, et al., Physicochemical and functional properties of leftover egg yolk granules after phosvitin extraction, Food Chem., 2018, 268, 369–377 CrossRef CAS PubMed.
- M. Primacella, T. Wang and N. C. Acevedo, Characterization of mayonnaise properties prepared using frozen-thawed egg yolk treated with hydrolyzed egg yolk proteins as anti-gelator, Food Hydrocolloids, 2019, 96, 529–536 CrossRef CAS.
- P. M. Nielsen, D. Petersen and C. Dambmann, Improved Method for Determining Food Protein Degree of Hydrolysis, J. Food Sci., 2001, 66(5), 642–646 CrossRef CAS.
- M. L. Denmat, M. Anton and V. Beaumal, Characterisation of emulsion properties and of interface composition in O/W emulsions prepared with hen egg yolk, plasma and granules, Food Hydrocolloids, 2000, 14(6), 539–549 CrossRef.
- N. A. Avramenko, N. H. Low and M. T. Nickerson, The effects of limited enzymatic hydrolysis on the physicochemical and emulsifying properties of a lentil protein isolate, Food Res. Int., 2013, 51(1), 162–169 CrossRef CAS.
- X. Xu, et al., Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin, Food Hydrocolloids, 2016, 61, 251–260 CrossRef CAS.
- J. M. Khan, et al., Molecular interaction of Sunset Yellow with whey protein: multi-spectroscopic techniques and computational study, J. Mol. Liq., 2022, 345, 117838 CrossRef CAS.
- R. Wang, et al., Forces involved in freeze-induced egg yolk gelation: effects of various bond dissociation reagents on gel properties and protein structure changes, Food Chem., 2022, 371, 131190 CrossRef CAS PubMed.
- Q. Li, et al., Emulsifying stability of enzymatically hydrolyzed egg yolk granules and structural analysis, Food Hydrocolloids, 2020, 101, 105521 CrossRef CAS.
- Y. Liang, et al., The Heat Stability of Milk Protein-stabilized Oil-in-water Emulsions: A Review, Curr. Opin. Colloid Interface Sci., 2017, 28, 63–73 CrossRef CAS.
- Y. T. Hu, et al., Techniques and methods to study functional characteristics of emulsion systems, J. Food Drug Anal., 2017, 25(1), 16–26 CrossRef CAS PubMed.
- V. D. V. Cornelly, et al., Emulsion properties of casein and whey protein hydrolysates and the relation with other hydrolysate characteristics, J. Agric. Food Chem., 2001, 49(10), 5005–5012 CrossRef.
- R. N. Fernandes, et al., Kinetic stability of the oil-in-water emulsions and dynamic interfacial properties of mixtures of sucrose esters and polysaccharides, Food Chem., 2021, 357, 129693 CrossRef CAS PubMed.
- F. Tamm, et al., Functional properties of pea protein hydrolysates in emulsions and spray-dried microcapsules, Food Hydrocolloids, 2016, 58, 204–214 CrossRef CAS.
- R. Wang, et al., Changes in egg yolk gelation behaviour and mechanisms during freezing, LWT, 2021, 151, 112223 CrossRef CAS.
- J. Gao, et al., Effects of guar gum or xanthan gum addition in conjunction with pasteurization on liquid egg white, Food Chem., 2022, 383, 132378 CrossRef CAS PubMed.
- M. Anton, et al., Filler effects of oil droplets on the rheology of heat-set emulsion gels prepared with egg yolk and egg yolk fractions, Colloids Surf., B, 2001, 21(1–3), 137–147 CrossRef CAS.
- S. B. Zhang and Q. Y. Lu, Characterizing the structural and surface properties of proteins isolated before and after enzymatic demulsification of the aqueous extract emulsion of peanut seeds, Food Hydrocolloids, 2015, 47, 51–60 CrossRef CAS.
- Z. Ma, et al., Inhibiting effect of dry heat on the heat-induced aggregation of egg white protein, Food Chem., 2022, 387, 132850 CrossRef CAS PubMed.
- R. Wang, et al., Changes in gelation, aggregation and intermolecular forces in frozen-thawed egg yolks during freezing, Food Hydrocolloids, 2020, 108, 105947 CrossRef CAS.
- S. Savadkoohi, et al., Structural properties of condensed ovalbumin systems following application of high pressure, Food Hydrocolloids, 2016, 53, 104–114 CrossRef CAS.
- D. Dong and B. Cui, Fabrication, characterization and emulsifying properties of potato starch/soy protein complexes in acidic conditions, Food Hydrocolloids, 2021, 115, 106600 CrossRef CAS.
- W. Xiong, et al., High intensity ultrasound modified ovalbumin: structure, interface and gelation properties, Ultrason. Sonochem., 2016, 31, 302–309 CrossRef CAS.
- Y. Xie, et al., Effects of high-intensity ultrasonic (HIU) treatment on the functional properties and assemblage structure of egg yolk, Ultrason. Sonochem., 2020, 60, 104767 CrossRef CAS PubMed.
- Q. Li, Improved effect of ultrasound-assisted enzymolysis on egg yolk powder: structural properties, hydration properties and stability characteristics, Food Chem., 2022, 382, 32549 Search PubMed.
- X. Du, Characterization of structure, physicochemical properties, and hypoglycemic activity of goat milk whey protein hydrolysate processed with different proteases, LWT, 2022, 159, 113257 CrossRef CAS.
- X. Zhang, et al., Structural characteristics and stability of salmon skin protein hydrolysates obtained with different proteases, LWT, 2022, 153, 112460 CrossRef CAS.
- P. Y. Phoon, M. F. San Martin-Gonzalez and G. Narsimhan, Effect of hydrolysis of soy β-conglycinin on the oxidative stability of O/W emulsions, Food Hydrocolloids, 2014, 35, 429–443 CrossRef CAS.
- M. Anton, V. Beaumal and G. Gandemer, Adsorption at the oil–water interface and emulsifying properties of native granules from egg yolk: effect of aggregated state, Food Hydrocolloids, 2000, 14(4), 327–335 CrossRef CAS.
- M. Anton, et al., Filler effects of oil droplets on the rheology of heat-set emulsion gels prepared with egg yolk and egg yolk fractions, Colloids Surf., B, 2001, 21, 137–147 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04244d |
| This journal is © The Royal Society of Chemistry 2022 |