Open Access Article
Open Access ArticleEffect of electric field on coalescence of an oil-in-water emulsion stabilized by surfactant: a molecular dynamics study
Yudou Wang *,
Shiyan Li,
Yuanwu Zhang,
Zhenlei Zhang,
Shundong Yuan and
Diansheng Wang
*,
Shiyan Li,
Yuanwu Zhang,
Zhenlei Zhang,
Shundong Yuan and
Diansheng Wang
College of Science, China University of Petroleum (East China), Qingdao 266580, China. E-mail: wangyd@upc.edu.cn
First published on 26th October 2022
Abstract
The microscopic understanding of electrocoalescence of oil-in-water (O/W) emulsions stabilized by surfactant is very important to improve the efficiency of electrical demulsification. The behaviors of the coalescence of O/W emulsion stabilized by surfactant in the presence of a direct electric field and a pulsed electric field were explored by nonequilibrium molecular dynamics simulations. According to the simulated results, an electrical method is feasible to demulsify an O/W emulsion stabilized by a surfactant. The configuration and movement of the sodium dodecyl sulfate (SDS) were determined by interactions between SDS molecules themselves and between SDS and oil/water molecules along with the force exerted by the applied electrical field. Two droplets will coalesce into one when the strength of the electric field exceeds 0.4 V nm−1. The SDS group can be broken up by an electric field larger than 0.6 V nm−1. The point when interaction energy between the hexadecane molecules of the two droplets begins to decrease from zero is consistent with the time when the two oil droplets came in contact. The coalescence process can be completed if the two droplets have begun to coalesce, even after the electric field was removed. Otherwise, the coalescence process cannot be completed. To enhance the efficiency of the electrocoalescence of O/W emulsions, strength, frequency and duty ratio of the electric field have to be optimized according to the properties of the emulsion. This research will help us to figure out how electric fields promote the efficiency of electrocoalescence of O/W emulsions with surfactant.
1. Introduction
Fluid emulsification is a common phenomenon in the petroleum industry, whether it is generated artificially or naturally. Water-in-oil (W/O) emulsions and oil-in-water (O/W) emulsions are the two main types of emulsions that are often encountered in the petroleum industry.1,2 Demulsification is needed to separate oil and water before the petroleum can proceed further. Electrical technology is considered to be an efficient method to enhance the demulsification of emulsions and is attracting more and more attention from environmental protection and energy consumption3–5 points of view. Generally, surfactants, which are intentionally added or naturally exist in oil–water systems, would be adsorbed onto the oil–water interface to enhance the stability of the emulsions.6–8 Understanding the electrocoalescence performance of emulsions stabilized by surfactant is very important to improve the efficiency of electrical demulsification.The mechanisms of the electrocoalescence for W/O and O/W emulsions are different. In general, the dispersed water droplets in W/O emulsions are easily polarized and attract each other to coalesce into one larger droplet to achieve demulsification if an electric field is applied to the system. And if the dispersed water droplets are the charged, droplets travel in the electric field could collide with each other and coalesce. Electrical demulsification of W/O emulsions has been studied by many researchers. The effectiveness and feasibility of electric demulsification of W/O emulsion have been proven relevant by researchers.9–11 The effects of electrical field parameters and the properties of the emulsions on the performance of electrocoalescence of W/O emulsions were investigated by experiments and numerical simulations.9,10,12–18 Because the continuous phase is conductive, it is difficult to conduct the method of O/W emulsion system because of the large current density and water electrolysis. In addition, O/W emulsion is generally supposed to be difficult to demulsify by an electrical method because of the nonpolarity of the dispersed oil droplets. However, results of experiments proved that electrical method is effective to demulsify O/W emulsions. Ichikawa et al.4 found that dense O/W emulsion stabilized with ionic surfactant can coalesce in low-voltage constant electric field space. But the O/W emulsions of the same oil stabilized with nonionic polymer surfactant cannot be demulsified by a low electric field. The type of surfactants is a key factor to determine the efficiency of the electric demulsification of O/W emulsion. Ha and Yang9 found that tip-streaming occurs and drops were broken when the O/W emulsion drops are non-uniform. Ren and Kang19 reported that rotational flow and coalescence of O/W emulsion can be stimulated by a bidirectional pulsed electric field. Hu et al.20 studied the influence of electric field intensity, frequency and water content of emulsions on the diameter distribution of the emulsions using demulsification experiments. All these experiments show that the O/W emulsion with net charges can be demulsified by electric fields. Therefore, the electrical demulsification efficiency of the O/W emulsions stabilized by nonionic surfactant is not good because of the nonpolarity of the dispersed oil droplets.4
The experimental evidence show that the electrical demulsification is feasible for both W/O and O/W emulsions. But the mechanisms of electrical demulsification for these two kinds of emulsion are different. Molecular dynamics (MD) simulation is a tool to understand the electrocoalescence procedure from the micro perspective and has been widely used to reveal the behaviors and microscopic mechanisms of electrical demulsification. Up to now, the MD simulation method focuses on the behaviors of coalescence and breakup of water or conducting droplets in the electric field. Wang et al.21,22 found that field strength determines the coalescence of two conducting droplets in electric fields using MD simulation method. He et al.23,24 found that the effects of electrical field strength and the contact time on coalescence behaviors are related by comparing the dynamic behaviors of two conducting droplets in various kinds of electric field. The ions in dispersed aqueous droplets enhanced the elongation and coalescence of salty water droplets and its influence is related to the strength of electric field.25–29 Li et al.30 found the drop–interface electrocoalescence is affected by the hydration effect and the interactions between the particles and water molecules. Results of Li et al.31 show that the coulombic attraction of the droplets is relatively stronger compared to the van der Waals interaction in an electric field. In the above mentioned researches, nitrogen gas was used to replace the continuous water phase to save computational time, which may introduce a different effect on electric demulsification. Li et al.32,33 and Wang et al.34 found that the dispersion attraction of water–oil is very pronounced compared to the interactions between water and nitrogen by studying the droplet electrocoalescence in the oil phase and the gas phase. It should be pointed out that all the above researches of molecular simulation are aimed at the electrical coalescence of W/O emulsion. To the best of the authors' knowledge, only two paper reported the research on electrocoalescence of O/W emulsion by using MD method. Liu et al. explored the distribution of charges and the mechanisms of electrocoalescence of oil droplets.35 Generally, emulsion is a relatively stable system due to the adsorption of surfactants on the oil–water interface in the petroleum industry. The existence of surfactants will greatly affect the behavior and efficiency of electrical demulsification. Liu et al.36 discussed the effects of concentration of surfactant on the behavior of O/W emulsion electrocoalescence under a bidirectional pulsed electric field. But the effects of electric field intensity and the behaviors of droplets after electric field was removed were not discussed.
In this work, we used MD method to study the coalescence behaviors of O/W emulsion stabilized by surfactant with the presence of direct electric fields. The O/W emulsion droplets were generated by molecular dynamics method based on a system where water, surfactant and oil are uniformly distributed initially. The deformation and coalescence of two O/W emulsion droplets were analyzed when uniform electric fields with different strengths were applied. In order to thoroughly understand the microscopic mechanism of electrical demulsification of O/W emulsion, the coalescence behaviors of droplets, the electric dipole moment of surfactant and the interaction between the components of emulsions were studied. To study the electrocoalescence of O/W emulsion droplets in a pulsed electric field, the behaviors of the droplets after the electric field was removed at different times in the process of electrocoalescence were further discussed. This work could help to understand the micro mechanisms of electrocoalescence and enhance the efficiency of separation of O/W emulsions by electric method.
2. Simulation model and method
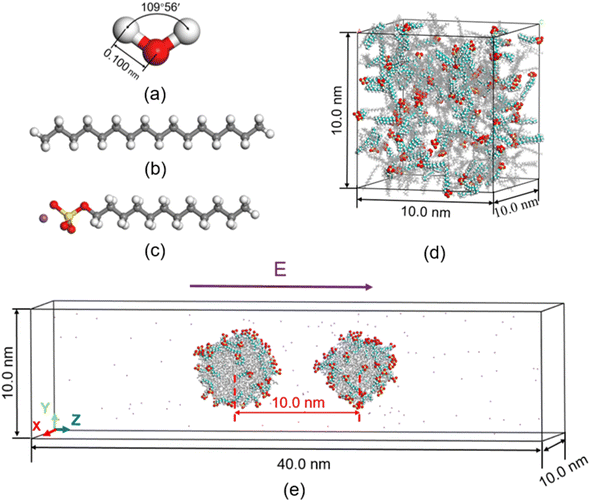
LAMMPS37 (Largescale Atomic/Molecular Massively Parallel Simulator) code.37 are used to perform the simulations. In the most previous study on molecular dynamics simulation of electrocoalescence of water in oil emulsion, the continuous phase was usually replaced by nitrogen gas. Though electrocoalescence of dispersed droplets can be reproduced in nitrogen gas, the interaction of water–oil is very much stronger than that of water–nitrogen. In addition, surfactant plays a very important role in the demulsification of O/W emulsions. To simulate the electrocoalescence of O/W emulsions more accurately, the oil/water/surfactant system was adopted in the present MD simulations. The SPC/E water molecule model is used in the simulations, as shown in Fig. 1a. The composition of oil is very complicated.38 To simplify the simulation, the oil is represented by hexadecane molecules,39 as shown in Fig. 1b. To generate the stable emulsions, different kinds of surfactants, such as cationic, anionic, gemini, etc., were added to the system.40–42. Anionic surfactant, sodium dodecyl sulfate (SDS), was widely used in petroleum industry7,43,44 and was added to the oil/water system, as shown in Fig. 1c.The van der Waals interaction and electrostatic interaction were considered in the non-bond interactions among all atoms in the system. The classical L–J potential model is used to describe the short-range van der Waals interactions, and the Coulomb's law is used to describe the electrostatic interactions:
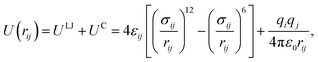 | (1) |
 | (2) |
 | (3) |
The consistent valence force field (CVFF) is used to describe hexadecane and SDS molecules. All the L–J parameters and atomic charges for water, oil, and SDS45–47 are given in Table 1. The cutoff radius of L–J pair potential is 1.2 nm. The electrostatic interaction is calculated by particle–particle particle–mesh (PPPM) with convergence value of 10−4.
| Atom | ε (kcal mol−1) | σ (Å) | q (e) |
|---|---|---|---|
| Water | |||
| O | 0.1553 | 3.1660 | −0.8476 |
| H | 0 | 0 | 0.4238 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Oil | |||
| C/CH3 | 0.0660 | 3.5000 | −0.180 |
| C/CH2 | 0.0660 | 3.5000 | −0.120 |
| H | 0.0300 | 2.5000 | 0.060 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| SDS | |||
| S | 0.25006 | 3.5640 | 1.284 |
| O/O− | 0.17005 | 3.0004 | −0.654 |
| O/S–O–C | 0.17005 | 3.0004 | −0.459 |
| C/O–C | 0.0660 | 3.5000 | 0.017 |
| C/CH3 | 0.0660 | 3.5000 | −0.180 |
| C/CH2 | 0.0660 | 3.5000 | −0.120 |
| H | 0.0300 | 2.5000 | 0.060 |
| Na | 0.0005 | 4.0700 | 1.000 |
Before electrocoalescence of O/W emulsions stabilized by surfactant is simulated, the O/W emulsion droplets have to be generated by molecular dynamics simulations. According to previous study, it is possible to form stable W/O emulsions for a certain amount of oil and water even with the addition of different amounts of surfactants, and different surfactant contents in O/W emulsion may result in the different oil/water interfacial tension and coalescing speed of the droplets.48 In the work by Liu et al.,39 the droplets of O/W emulsion were formed by 100 gas switchable surfactants along with 300 hexadecane molecules. 100 SDS surfactants molecules and 300 hexadecane molecules were also used in the simulation. To form the O/W emulsions, these molecules and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 water molecules were randomly placed in the cubic box with 10 nm length to maintain the system pressure of 1 atm at temperature 298 K, as shown in Fig. 1d. The large water oil ratio ensures that O/W emulsion is formed in this system. The steepest descent method is used to minimize the system and MD simulation is performed for 40 ns with a time step of 2 fs to produce the emulsions. The NPT ensemble with 1 atm and 298 K is used to generate emulsions.
050 water molecules were randomly placed in the cubic box with 10 nm length to maintain the system pressure of 1 atm at temperature 298 K, as shown in Fig. 1d. The large water oil ratio ensures that O/W emulsion is formed in this system. The steepest descent method is used to minimize the system and MD simulation is performed for 40 ns with a time step of 2 fs to produce the emulsions. The NPT ensemble with 1 atm and 298 K is used to generate emulsions.
The MD simulation of electrocoalescence of O/W emulsion requires a larger system. After the O/W emulsion droplets are produced, the two droplets, including hexadecane and SDS molecules, were put in another empty rectangular box (10 × 10 × 40 nm3). The distance of the center of mass of the two droplets is 10 nm. And 133![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 water molecules were added to the box. Opposed-conformation is removed by performing energy minimization. Then, an electric field is applied to the system and 3 ns NVT ensemble is used to simulate the process of electrocoalescence of O/W emulsions. The temperature of the system is kept constant using the Nose–Hoover thermostat. Periodic boundary conditions are used for all simulations.
700 water molecules were added to the box. Opposed-conformation is removed by performing energy minimization. Then, an electric field is applied to the system and 3 ns NVT ensemble is used to simulate the process of electrocoalescence of O/W emulsions. The temperature of the system is kept constant using the Nose–Hoover thermostat. Periodic boundary conditions are used for all simulations.
3. Results and discussion
3.1 Structure of emulsion stabilized by surfactant
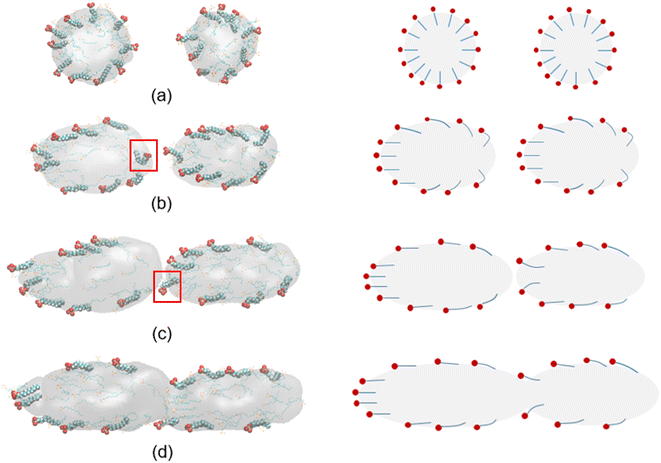
Before discussion the effect of electric field on coalescence of O/W emulsion stabilized by surfactant, emulsification and structure of the droplets have to be studied. Fig. 2 shows the formation process of O/W droplets stabilized by surfactant. Under the interaction of hexadecane molecules, the adjacent molecules aggregate spontaneously into small droplets at the beginning of simulation, and the SDS molecules adsorb on the surface of these oil droplets, as shown in Fig. 2a. As the process goes on, these small droplets collide with each other and fuse into larger ones. We can see that four droplets are generated at 6.0 ns, as shown in Fig. 2b. And finally, two O/W droplets were formed in the box at about 22.0 ns of the simulation, as shown in Fig. 2c. In fact, there are 51 SDS molecules and 170 water molecules in droplet at the lower left of Fig. 2c. And there are 49 SDS molecules and 130 water molecules in droplet at the upper right of Fig. 2c. To show the O/W emulsion system reaches a relative stable status, the system is continuously simulated for 40 ns. The positions and distance between the two droplets remain almost unchanged from 22.0 ns to 40.0 ns simulation time. And the researches on electrocoalescence of O/W emulsion can be conducted using this system.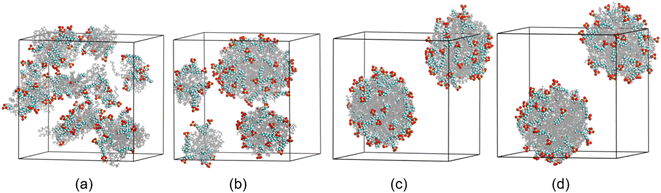 | ||
| Fig. 2 Configurations at different times during the generation of O/W emulsion: (a) 0.5 ns; (b) 6.0 ns; (c) 22.0 ns; (d) 40.0 ns. Water molecules were omitted. | ||
The distributions of some atoms of water, SDS and hexadecane molecules with respect to the center of mass of the droplet are shown in Fig. 3a. Here, the distribution of oil can be represented by the distribution of C atoms of hexadecane molecules. And the distribution of water can be represented by O atoms of water molecules. The hexadecane molecules are mainly distributed in the core of the droplets due to the aggregation behavior of them, and the water molecules are mainly distributed around the droplets. Because the droplet is not an ideal sphere, the oil and water distribution curves overlap to some extent. And the position of the intersection point can be defined as the average radius of the emulsion droplet. The radii of these two emulsion droplets are 2.85 nm and 2.60 nm, respectively, which is comparable to the droplet sizes in other similar MD simulations. In the experimental studies, the radii of droplets were generally in the range of 0.3–0.6 mm.15,49,50 That is, the droplet sizes used in MD simulations are about five orders of magnitude smaller than those used in the experiments. This is because current computing power is not yet capable of performing MD calculations for the enormous number of water molecules in actual droplets. The SDS molecules has a long chain structure, as shown in Fig. 1. The head of chain is negatively charged and shows strong polarity due to the ionization of sodium ions. And tail of the chain with 12 carbon atoms shows strong hydrophobicity. The SDS molecules are adsorbed on the interface of droplets because of the amphiphilic properties of its structure. The negative charged heads of SDS molecules have strong interactions with water and the tails of SDS molecules enter the hexadecane group. Therefore, the density distribution of C atoms of tail chains of droplet 1 has a peak at 2.60 nm, which is in oil phase of droplet. And the density distribution of O atoms of head of chains of droplet 1 has a peak at 3.10 nm, which is in continuous phase of the system. And the droplet 2 has a structure similar to droplet 1. The final negatively charged and hydrophilic shell helped to prevent the coalescence of two droplets and stabilize O/W emulsions.
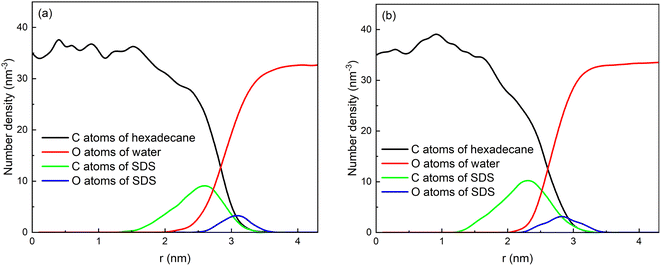 | ||
| Fig. 3 Number density distribution of components with respect to center of mass of the droplets: (a) droplet 1 and (b) droplet 2. | ||
3.2 Effect of direct current electric field on coalescence of oil-in-water emulsion stabilized by surfactant
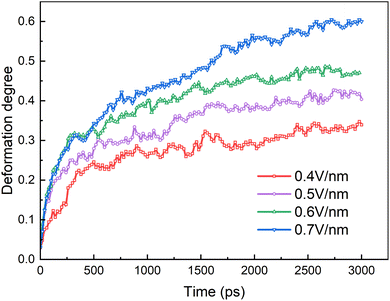
We will first discuss the dynamic evolutions of electrocoalescence of two O/W emulsion droplets at different times when direct current electric fields are 0.4 V nm−1, 0.5 V nm−1, 0.6 V nm−1 and 0.7 V nm−1, respectively. The direction of the electric fields is along the z axis. When the electric field is applied to the system, the charged SDS molecules are subjected to a force opposite to the electric field's direction, which drives the surfactants to move or to have a movement trend to the left end of the emulsion droplets thus deforms and elongates the droplets along the z axis. At the same time, the SDS molecules have to align with the direction of the field under the action of the electric field force. The facing ends of the two droplets attract each other because of the polarization of the droplets. The two droplets do not contact and coalesce during the simulation time of 3000 ps due to the slight deformation of them when electric field is at 0.4 V nm−1, as shown in Fig. 4a. When electric fields are at 0.5 V nm−1, 0.6 V nm−1 and 0.7 V nm−1, the distance between the facing ends of the two emulsion droplets becomes closer and begin to coalesce into one droplet because of the forces mentioned above and the interaction between the hexadecane molecules and the tails of the surfactants. The higher the electric field intensity, the earlier the two droplets begin to contact. With the extension of time, the coalesced droplet became more and more slender. SDS molecules move faster on the surface of droplet under the larger forces exerted by a higher electric field. Therefore, the droplet becomes slender with the increase of electric field strength. The surfactant molecules move on the surface of the droplets along the opposite direction of the electric field when the electric field is applied. Therefore, the number of surfactant molecules on the left end of the droplet increases, while the molecules number of right end decreases as time goes on. The surfactant molecules of the right droplet continue to move to the left through the surface of the left droplet when the two droplets coalesce together. A daughter group of SDS molecules will be generated from the left end of the coalesced droplet at 3000 ps when the electric field is at 0.6 V nm−1. When the electric field increases to 0.7 V nm−1 however, a daughter group of SDS molecules was generated at 3000 ps. The effects of electric field on electrocoalescence are also related to the size of the droplet along with the concentration and distribution of SDS. The critical electric field has to be evaluated for a specific system.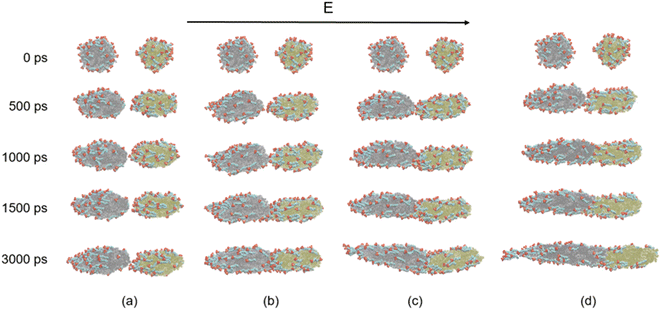 | ||
| Fig. 4 Dynamic evolutions of the electrocoalescence: (a) 0.4 V nm−1; (b) 0.5 V nm−1; (c) 0.6 V nm−1; (d) 0.7 V nm−1. | ||
The coalescence of the droplets is mainly determined by their deformation. The degree of deformation is calculated by:51
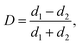 | (4) |
The changes of electric dipole moments of surfactants and hexadecane molecules with time are shown in Fig. 6. The average dipole moments of a molecule along the electric field direction are calculated by
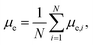 | (5) |
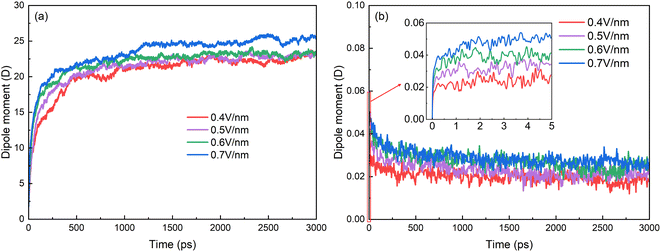 | ||
| Fig. 6 Time evolutions of average of z-component of electric dipole moment of (a) SDS molecules and (b) hexadecane molecules. | ||
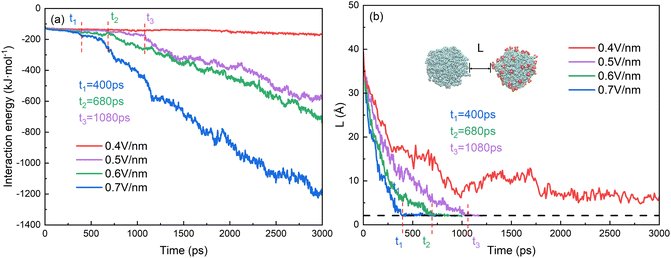
The behaviors of droplets electrocoalescence of O/W emulsion can be explained by the interactions of the two droplets which include the electrostatic energy of the surfactant molecules on the surface of the two droplets, the interaction energy of oil molecules between the two droplets and the interaction energy between SDS molecules of one droplet and the oil molecules of another droplet. Fig. 7a shows the interaction energy between the oil groups of two droplets in the electrocoalescence process with different electric field strengths. The hexadecane molecule is electrically neutral, therefore, the interaction between oil groups of two droplets can be regarded as a short-range interaction though it has a very small electric dipole moment induced by electric field. The interaction energy is almost zero before the two oil groups begin to contact. The interaction energy decreases as soon as the hexadecane molecules of the two droplets begin to make contact with each other. And the interaction energy value continues to decrease as the electrocoalescence process went on. The higher the electric field intensity, the better the droplets coalesce and the lower the interaction energy. The interaction energy value is always equal to zero in the case of 0.4 V nm−1 because of the noncoalescence of the two droplets. The time when the interaction energy decreases from zero corresponds to the time that the two droplets begin to collide. Fig. 7b shows the time evolutions of distance of the two oil droplets. The two droplets quickly approach each other when the electric field just is applied to the system. As time goes on, the rate at which the droplets approaches slows down. The distance of the two oil droplets remains relatively stable after 900 ps in the case of 0.4 V nm−1, which is consistent with the results that the two droplets do not coalesce, as Fig. 4a shows. The times when the distance of the two oil droplets is zero for the case of 0.5 V nm−1, 0.6 V nm−1, and 0.7 V nm−1 are at 1150 ps, 740 ps and 610 ps, respectively. They are consistent with the time that the interaction energy decreases from zero, as Fig. 7a shows.
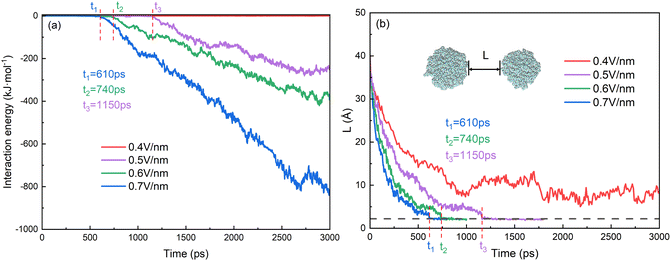 | ||
| Fig. 7 Time evolutions of (a) interaction energy between oil groups of two droplets and (b) distance of two oil droplets. | ||
Fig. 8a shows the interaction energy between surfactant molecules of the right droplet and the oil molecules of the left droplet in the electrocoalescence process with different electric fields. The interaction between SDS and hexadecane molecules gradually becomes stronger as the two droplets slowly approach each other because of the long-range interaction of these two kinds of polarized molecules. When the SDS molecules contact with oil molecules, the weak interaction begins to work and the interaction energy begins to decrease rapidly. The interaction energy continues to decrease as the molecules make better contact. This is consistent with the time evolutions of distance of SDS molecules of the right droplet and the oil molecules of the left droplet, as shown in Fig. 8b. It is clear that surfactant molecule of one droplet comes into contact with the oil of another droplet earlier than the oil molecule. The two droplets aggregate more and interact stronger as electric field increases. The interaction energy value is almost constant in the case of 0.4 V nm−1 because of the noncoalescence of the two droplets.
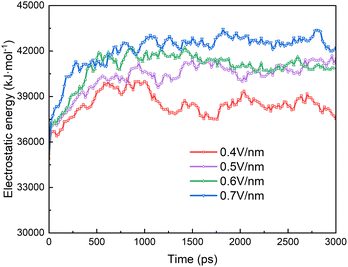
Fig. 9 shows the electrostatic energy of the surfactant molecules on the surface of the two droplets during the electrocoalescence process. The interaction of SDS molecules between the two droplets is a long range force because the molecule is negatively charged. It is determined by the shape and distance of the two droplets and the distributions of the SDS on the surface of the droplets. At the beginning, the electrostatic energy increases rapidly due to the rapid approach of two droplets. The electrostatic effect remains almost unchanged as time going on. The stronger the electric field, the stronger the electrostatic effect.
Ichikawa et al.5 regarded charged O/W emulsion droplets as metal particles with approximately infinite number of charges and explained the behavior of O/W emulsion electrocoalescence theoretically. In fact, the charged surfactant molecule not only moves on the interface of the droplet but also deforms under the actions of the electric field. Fig. 10 shows the instantaneous configurations of SDS molecules at the edge of the droplets when electrical field is 0.5 V nm−1. At the initial state, all surfactant molecules adsorb on the surface of hexadecane droplets. The chain of the surfactant molecules is almost perpendicular to the surface of the droplet and the total electric dipole moment is zero without the presence of a electric field, as shown in Fig. 10a. When the electric field is applied, all SDS molecules have a tendency to align with the external electric field. But the configuration and movement of the SDS are determined by interactions between surfactant molecules along with interactions between surfactant molecules and oil/water molecules and the force exerted by the applied electrical field. The negatively charged surfactant headgroup of the SDS is subject to a force opposite to the direction of the electric field, while the tail remains in the oil phase. Therefore, this molecule is bent and begin to move to the left end of the droplet, as shown by marked molecule in Fig. 10b. At this moment, the electric dipole moment of this molecule begin to decrease. When the two droplets are close enough to each other to make contact, the headgroup on the facing end of the right droplet is bent because of the repulsion from the oil phase of another droplet, though the headgroup is still subject to a force exerted by the electric field. It means the headgroup of surfactants on the facing end is pushed aside by the repulsion from the oil phase of another droplet, as shown by marked molecule in Fig. 10c. The main interaction that linked the two droplets together is the interaction between the hexadecane molecule and the tail of the SDS. Finally, the two droplets coalesce together and the surfactants redistribute gradually on the surface of it, as shown in Fig. 10d.
3.3 Behaviors of oil-in-water emulsion droplets stabilized by surfactant after electric field is removed during electro-coalescence process
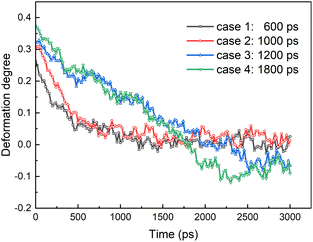
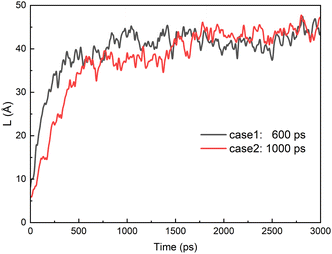
From the results above, we know that the merged droplets will further elongate in the presence of an electric field. If the electric field is strong enough, the droplets will break up and become small droplets, which could reduce the coalescence efficiency. Pulsed electric field is an effective alternative for overcoming this phenomenon. Experiments have shown that a pulsed electric field could drive oil droplets to form oil-droplet chains and coalescence.5,19,20 But the microscopic mechanisms of the demulsification of O/W systems is not clear when the electric field is removed or changed. To study the effects of pulsed electric field on coalescence of O/W emulsion, the behaviors of the droplets are studied in some cases which the electric field is removed at different times during the electro-coalescence process in the 0.5 V nm−1 electric field. Electric field is removed at 600 ps in case 1 when the two droplets obviously do not contact each other. Case 2 is that the electric field is removed at 1000 ps when the oil groups of two droplets will contact immediately. Case 3 is that the electric field is removed at 1200 ps that the oil groups just touch each other at that time. And the case 4 is that the electric field is removed at 1800 ps when the oil groups are fully coalesced. Fig. 11 shows the dynamic evolution of two O/W emulsion droplets after the electric field is removed in the four cases. The time marked on the left is calculated from the time when the electric field is removed. After the electric field is shut down, the droplets in case 1 do not continue to deform and approach each other. And its shape gradually returns to a sphere under the interactions among SDS, hexadecane and water molecules. The droplets become almost spherical at 500 ps after the electric field is removed, as shown in Fig. 11a. The surfactant molecules gradually become evenly distributed over the surface again. Fig. 11b shows the simulation snapshots of the two O/W emulsion droplets in case 2. The behaviors of the two droplets are similar to that of Fig. 11a. The interaction energy of oil molecules between the two droplets and the interaction energy between SDS molecules of one droplet and oil group of another droplet are both zero still, though they likely made contact, as shown in Fig. 7a and 8a. The droplets do not continue to deform and approach each other. The two droplets gradually return to a sphere after the electric field is shut down. Fig. 11c shows the simulation snapshots of the two O/W emulsion droplets in case 3 where the two droplets just coalesce. The corresponding interaction energy of oil molecules between the two droplets and the interaction energy between SDS molecules of one droplet and oil group of another droplet are both less than zero. Because of the interactions between hexadecane molecules of the two oil groups and interactions between SDS and hexadecane molecules, the two droplets continue to merge even after the electric field has been shut down. And merged droplet eventually becomes a sphere 3000 ps after the electric field has been removed, as Fig. 11c shows. This phenomenon is helpful to avoid the oil chain formation in direct current electric fields. Fig. 11d shows the results of the two O/W emulsion droplets in case 4 where the electric field is removed at 1800 ps when the oil groups were fully coalesced. There is no doubt that the merged droplet gradually becomes a sphere after the electric field is removed. Therefore, these droplets can grow to a certain size to be separated by gravity after one or several cycles of applying and removing the electric field.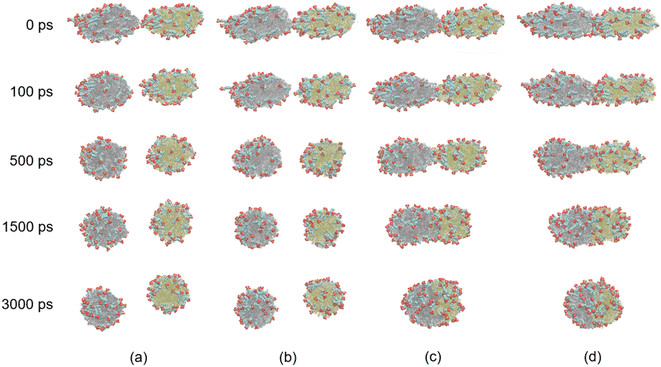 | ||
| Fig. 11 Dynamic evolution of two O/W emulsion droplets after the electric field is removed at different time: (a) case 1: 600 ps; (b) case 2: 1000 ps; (c) case 3: 1200 ps; (d) case 4: 1800 ps. | ||
From the above results, it can be concluded that the coalescence process can continue even after the electric field is removed if the two droplets have already begun to coalesce. Otherwise, the coalescence process cannot be completed. To enhance the efficiency of electrocoalescence of O/W emulsion, strength, frequency and duty ratio of electric field have to be optimized together according to the properties of emulsion.
Fig. 12 shows the degree of deformation of the droplet in the above four cases. The droplets slowly change from an ellipsoid to a sphere under the interaction of hexadecane, surfactant and water molecules whatever they are coalesced or not when electric field is shut down. Therefore, the degree of deformation of the droplet of case 1 and 2 decreases with time and is equal to zero at about 1300 ps after the electric field is removed. But for case 3 and 4, because the coalesced droplet slowly becomes a sphere, the isolated droplets before coalescence become hemispherical. Therefore, the degree of deformation of the droplet of case 3 and 4 eventually becomes negative.
Fig. 13 shows distance of two oil droplets in case 1 and 2 when electric field is removed. The case 3 and 4 are omitted because the droplet is always coalesced during the simulation time. Because the droplets slowly change from ellipsoid to sphere when electric field is removed, the distance of two oil droplets increases with the time for case 1 and 2. The distance becomes relatively stable at about 1000 ps after the electric field is removed. And the final distances of the two oil droplets are almost same for case 1 and 2.
With the removal of electric field, the SDS molecules begin to redistribute on the oil/water interface under the interactions between SDS and oil–water system whatever they are coalesced or not. The chain of the molecule is almost perpendicular to the surface of the droplet with the negative charged head of SDS molecule in the water phase. Therefore, the dipole moments of SDS decrease to zero gradually, as shown in Fig. 14a. The displacement polarization of hexadecane molecules disappeared and the dipole moments of hexadecane molecules decrease to zero rapidly with the removal of electric field, as shown in Fig. 14b. As the results of Section 3.1, the structure of droplet with a negative charge and hydrophilic shell helps to prevent the coalescence of two droplets and stabilize O/W emulsions. Electric field has to be applied to the system for further demulsification. Optimizations of strength, frequency and duty ratio of electric field have to be done to efficiently demulsify the emulsions.
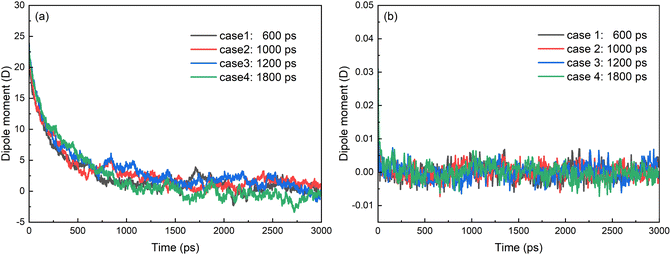 | ||
| Fig. 14 Time evolutions of z-component of electric dipole moment of (a) SDS molecules and (b) hexadecane molecules after electric field is removed. | ||
4. Conclusions
To explore the demulsification probability of O/W emulsions stabilized by surfactant in the presence of electrical fields by using MD simulation method, the deformation and coalescence of two O/W emulsion droplets are analyzed when a uniform electric field is applied and removed. And the electric dipole moment of surfactant, the interaction between surfactant and oil molecules and the deformation of surfactant are studied thoroughly to understand the microscopic mechanism of electrical demulsification of O/W emulsion. The main conclusions are as follows.(1) The method of electrical demulsification is feasible for O/W emulsions stabilized by ionic surfactants.
(2) The O/W emulsion droplets deform and elongate along the direction of electric field because of the forces acted on the surfactant molecules by the external electric field. For the system discussed above, two droplets will coalesce into one within 3 ns when the strength of electric field exceeds 0.4 V nm−1. A daughter group of SDS molecules will be generated from the coalesced droplet when electric field is larger than 0.6 V nm−1.
(3) SDS molecules have a tendency to align with the external electric field. The configuration and movement of the SDS are determined by interaction between SDS molecules, interaction between SDS and oil/water molecules and the force exerted by the applied electrical field. The point that interaction energy between the hexadecane molecules of the two droplets begin to decrease from zero is consistent with the time when the two oil droplets begin to make contact. The interaction energy between the hexadecane molecules of one droplet and SDS of another droplet is similar to this.
(4) The coalescence process can continue after electric field is removed if the two droplets have begun to coalesce. Otherwise, the coalescence process cannot be completed. To enhance the efficiency of electrocoalescence of O/W emulsion, strength, frequency and duty ratio of electric field have to be optimized together according to the properties of emulsion.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This study is financially supported by the National Key Research and Development Program of China (No. 2018YFA0702400), Shandong Province Natural Science Foundation of China (ZR2021ME003), the Fundamental Research Funds for the Central Universities (20CX05014A).References
- R. Zolfaghari, A. Fakhru'l-Razi, L. C. Abdullah, S. S. E. H. Elnashaie and A. Pendashteh, Sep. Purif. Technol., 2016, 170, 377–407 CrossRef CAS.
- A. M. Sousa, M. J. Pereira and H. A. Matos, J. Pet. Sci. Eng., 2022, 210, 110041 CrossRef CAS.
- J. S. Eow, M. Ghadiri, A. O. Sharif and T. J. Williams, Chem. Eng. J., 2001, 84, 173–192 CrossRef CAS.
- T. Ichikawa, K. Itoh, S. Yamamoto and M. Sumita, Colloids Surf., A, 2004, 242, 21–26 CrossRef CAS.
- T. Ichikawa, Colloids Surf., A, 2007, 302, 581–586 CrossRef CAS.
- Y. Yang, Z. Ma, F. Xia and X. Li, J. Water Process Eng., 2020, 36, 101292 CrossRef.
- M. Ahmadi and Z. Chen, Energy Fuels, 2020, 34, 13536–13551 CrossRef CAS.
- O. A. Okunade, N. Yekeen, E. Padmanabhan, A. Al-Yaseri, A. K. Idris and J. A. Khan, J. Pet. Sci. Eng., 2021, 207, 109139 CrossRef CAS.
- J.-W. Ha and S.-M. Yang, J. Colloid Interface Sci., 1999, 213, 92–100 CrossRef CAS PubMed.
- W. Kang, M. Li, H. Yang, X. Kang, F. Wang, H. Jiang, M. Zhang, T. Zhu and B. Sarsenbekuly, J. Ind. Eng. Chem., 2021, 93, 415–422 CrossRef CAS.
- C. Nie, L. Xu, D. Gu, G. Cao, R. Yuan and B. Wang, Energy Fuels, 2016, 30, 9686–9692 CrossRef CAS.
- L. He, D. Yang, R. Gong, T. Ye, Y. Lu and X. Luo, Pet. Sci., 2013, 10, 548–561 CrossRef.
- V. Vivacqua, S. Mhatre, M. Ghadiri, A. M. Abdullah, A. Hassanpour, M. J. Al-Marri, B. Azzopardi, B. Hewakandamby and B. Kermani, Chem. Eng. Res. Des., 2015, 104, 658–668 CrossRef CAS.
- E. Mohammadian, T. S. Taju Ariffin, A. Azdarpour, H. Hamidi, S. Yusof, M. Sabet and E. Yahya, Ind. Eng. Chem. Res., 2018, 57, 13247–13256 CrossRef CAS.
- X. Luo, H. Yin, H. Yan, X. Huang, D. Yang and L. He, Chem. Eng. Sci., 2018, 191, 350–357 CrossRef CAS.
- B. Li, Z. Wang, V. Vivacqua, M. Ghadiri, J. Wang, W. Zhang, D. Wang, H. Liu, Z. Sun and Z. Wang, Chem. Eng. Sci., 2020, 213, 115360 CrossRef CAS.
- H. Yin, X. Luo, K. Xu, H. Yan and L. He, Chem. Eng. Sci., 2020, 224, 115788 CrossRef CAS.
- B. Li, X. Dou, K. Yu, W. Zhang, H. Xu, Z. Sun, Z. Wang and J. Wang, Chem. Eng. Sci., 2022, 248, 117158 CrossRef CAS.
- B. Ren and Y. Kang, Langmuir, 2018, 34, 8923–8931 CrossRef CAS PubMed.
- J. Hu, J. Chen, X. Zhang, J. Xiao, S. An, Z. Luan, F. Liu and B. Zhang, Sep. Purif. Technol., 2021, 254, 117631 CrossRef CAS.
- B.-B. Wang, X.-D. Wang, W.-M. Yan and T.-H. Wang, Langmuir, 2015, 31, 7457–7462 CrossRef CAS PubMed.
- B.-B. Wang, X.-D. Wang, T.-H. Wang, G. Lu and W.-M. Yan, Int. J. Heat Mass Transf., 2016, 98, 10–16 CrossRef.
- X. He, S.-L. Wang, Y.-R. Yang, X.-D. Wang and J.-Q. Chen, J. Mol. Liq., 2020, 312, 113429 CrossRef CAS.
- X. He, B.-X. Zhang, S.-L. Wang, Y.-F. Wang, Y.-R. Yang, X.-D. Wang and D.-J. Lee, J. Mol. Liq., 2021, 341, 117417 CrossRef CAS.
- Q. Cao, L. Li, F. Huang and C. Zuo, Langmuir, 2017, 33, 428–437 CrossRef CAS PubMed.
- Q. Chen, J. Ma, H. Xu and Y. Zhang, J. Mol. Liq., 2019, 290, 111214 CrossRef CAS.
- L. Li, Q. Cao, H. Liu and X. Qiao, J. Mol. Liq., 2021, 332, 115895 CrossRef CAS.
- F. Song, H. Niu, J. Fan, Q. Chen, G. Wang and L. Liu, J. Mol. Liq., 2020, 312, 113195 CrossRef CAS.
- H. Dong, Y. Liu, Y. Zhou, T. Liu, M. Li and Z. Yang, Colloids Surf., A, 2019, 570, 55–62 CrossRef CAS.
- B. Li, X. Dou, K. Yu, N. Li, W. Zhang, H. Xu, Z. Sun, Z. Wang and J. Wang, J. Mol. Liq., 2021, 344, 117875 CrossRef CAS.
- N. Li, Z. Sun, Y. Fan, W. Liu, Y. Guo, B. Li and Z. Wang, J. Mol. Liq., 2021, 321, 114475 CrossRef CAS.
- N. Li, Z. Sun, W. Liu, L. Wei, B. Li, Z. Qi and Z. Wang, J. Mol. Liq., 2021, 333, 115995 CrossRef CAS.
- N. Li, Z. Sun, J. Sun, W. Liu, L. Wei, T. Li, B. Li and Z. Wang, Colloids Surf., A, 2022, 632, 127746 CrossRef CAS.
- Z. Wang, N. Li, Z. Sun, X. Wang, Q. Chen, W. Liu, Z. Qi, L. Wei and B. Li, Sep. Purif. Technol., 2021, 278, 119622 CrossRef.
- S. Liu, H. Zhang, S. Yuan and C. Liu, Chem. J. Chin. Univ., 2021, 42, 2170–2177 Search PubMed , in Chinese..
- S. Liu, S. Yuan and H. Zhang, Molecules, 2022, 27, 2559 CrossRef CAS PubMed.
- S. Plimpton, J. Comput. Phys., 1995, 117, 1–19 CrossRef CAS.
- M. Kunieda, K. Nakaoka, Y. Liang, C. R. Miranda, A. Ueda, S. Takahashi, H. Okabe and T. Matsuoka, J. Am. Chem. Soc., 2010, 132, 18281–18286 CrossRef CAS PubMed.
- X. Liu, Y. Li, S. Tian and H. Yan, J. Phys. Chem. C, 2019, 123, 25246–25254 CrossRef CAS.
- H. Jia, P. Lian, X. Leng, Y. Han, Q. Wang, K. Jia, X. Niu, M. Guo, H. Yan and K. Lv, Fuel, 2019, 258, 116156 CrossRef CAS.
- S. Kiani, D. R. Jones, S. Alexander and A. R. Barron, J. Colloid Interface Sci., 2020, 571, 307–317 CrossRef CAS PubMed.
- P. Lian, H. Jia, X. Wei, Y. Han, Q. Wang, J. Dai, D. Wang, S. Wang, Z. Tian and H. Yan, Fuel, 2021, 283, 119252 CrossRef CAS.
- S. Song, H. Zhang, L. Sun, J. Shi, X. Cao and S. Yuan, Energy Fuels, 2018, 32, 12383–12393 CrossRef CAS.
- M. Ahmadi and Z. Chen, Fuel, 2021, 122718 Search PubMed.
- W. Damm, A. Frontera, J. Tirado-Rives and W. L. Jorgensen, J. Comput. Chem., 1997, 18, 1955–1970 CrossRef CAS.
- J. Shelley, K. Watanabe and M. L. Klein, Int. J. Quantum Chem., 1990, 38, 103–117 CrossRef.
- Y. Yao, Q. Li, M. Lai, Q. Wu, Y. Mo, Q. Li, B. Liu and H. Luo, J. Appl. Phys., 2021, 130, 154701 CrossRef CAS.
- N. Li, Z. Sun, Y. Pang, Z. Qi, W. Liu, W. Li, M. Sun, B. Li and Z. Wang, Sep. Purif. Technol., 2022, 289, 120756 CrossRef CAS.
- M. Mousavichoubeh, M. Shariaty-Niassar and M. Ghadiri, Chem. Eng. Sci., 2011, 66, 5330–5337 CrossRef CAS.
- D. Yang, Y. Sun, L. He, X. Luo, Y. Lü and Q. Gao, Chem. Eng. Res. Des., 2019, 142, 214–224 CrossRef CAS.
- J. S. Eow, M. Ghadiri and A. Sharif, J. Electrost., 2001, 51–52, 463–469 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |