Open Access Article
Open Access ArticleOrganic–inorganic hybrid [NH3(CH2)6NH3]ZnBr4 crystal: structural characterization, phase transitions, thermal properties, and structural dynamics†
Ae Ran Lim *ab and
Huiyeong Juc
*ab and
Huiyeong Juc
aGraduate School of Carbon Convergence Engineering, Jeonju University, Jeonju 55069, Korea
bDepartment of Science Education, Jeonju University, Jeonju 55069, Korea. E-mail: aeranlim@hanmail.net; arlim@jj.ac.kr
cKorea Basic Science Institute, Seoul Western Center, Seoul 03759, Korea
First published on 10th October 2022
Abstract
Organic–inorganic hybrid [NH3(CH2)6NH3]ZnBr4 crystals were prepared by slow evaporation; the crystals had a monoclinic structure with space group P21/c and lattice constants a = 7.7833 Å, b = 14.5312 Å, c = 13.2396 Å, β = 90.8650°, and Z = 4. They underwent two phase transitions, at 370 K (TC1) and 430 K (TC2), as confirmed by powder X-ray diffraction patterns at various temperatures; the crystals were stable up to 600 K. The nuclear magnetic resonance spectra, obtained using the magic-angle spinning method, demonstrated changes in the 1H and 13C chemical shifts were observed near TC1, indicating changing structural environments around 1H and 13C. The spin–lattice relaxation time, T1ρ, increased rapidly near TC1 suggesting very large energy transfer, as indicated by a large thermal displacement around the 13C atoms of the cation. However, the environments of 1H, 14N, and C1 located close to NH3 in the [NH3(CH2)6NH3] cation did not influence it significantly, indicating a minor change in the N–H⋯Br hydrogen bond with the coordination geometry of the ZnBr4 anion. We believe that the information on the physiochemical properties and thermal stability of [NH3(CH2)6NH3]ZnBr4, as discussed in this study, would be key to exploring its application in stable, environment friendly solar cells.
1. Introduction
Solar cells based on organic–inorganic hybrid materials have been extensively studied. Typically, CH3NH3PbX3 (X = Cl, Br, and I) thin-film photovoltaic devices have been used as solar cells;1–6 however, these substances readily decompose in humid air and are toxic because of the presence of Pb. Therefore, it is necessary to develop eco-friendly hybrid perovskite solar cells. Recently, a new series of perovskite compounds [(CH3)2NH2]Zn(HCOO)3, consisting of an organic cation and a metal ion bridged by formate ions, has been reported.7–13 They have the potential for application in novel memory storage and manipulation devices. In addition, research on type of organic–inorganic hybrid perovskites [NH3(CH2)nNH3]BX4 (n = 2, 3, 4, …; B = Mn, Co, Cu, Zn, Cd; X = Cl, Br, I), with a focus on the optimization of the perovskite structure and dynamics investigation, is rapidly garnering attention. The physical properties of organic–inorganic hybrids depend on their organic cations, inorganic anion coordination geometry of the metal ions (BX42− or BX62−), and halogen ions.14–24 The organic cations of hybrid perovskites induce structural flexibility and nonlinear optical properties, whereas the inorganic anion is attributable for thermal and mechanical properties.25,26 The crystal structures with B = Mn, Cu, and Cd consist of alternate octahedrons, (BX6)2−, and organic chains, and is two-dimensional structure. The ammonium ion of the organic group is connected by a N–H⋯X hydrogen bond to the halide ion of the inorganic layer, rendering such structures good candidates for proton conduction.16 The isolated tetrahedral structures with B = Co and Zn are formed, where an inorganic layer of (BX4)2− is sandwiched between the organic cation, and zero-dimensional structure.18,27–29 The crystal consists of unassociated tetrahedrally distorted (BX4)2− anions and cations linked by H bonds to X− ions. Structural rearrangements due to conformational changes in the chains are important for long-chain alkylenediammonium materials [NH3(CH2)nNH3]BX4, with n >> 4.30 Among them, the organic–inorganic perovskite type [NH3(CH2)6NH3]ZnBr4 (1, 6-hexanediammonium tetrabromozincate(II)), containing [NH3(CH2)6NH3] cations and layered ZnBr4 anions (with Zn atoms surrounded by four Br atoms to form the ZnBr4 tetrahedron), is an interesting hybrid material.Ishiha and Horiuchi31 measured the 81Br nuclear quadrupole resonance (NQR) frequencies of [NH3(CH2)6NH3]ZnBr4 below room temperature. Four resonance signals were assigned to the four bromine atoms in the tetrahedron. The N–H⋯Br hydrogen bonds between the cations and anions were considered based on these temperature dependences. In addition, the phase transition temperature, measured by differential scanning calorimetry (DSC), was not observed below room temperature, but was observed at 380 K and 424 K above room temperature; the melting point was 475 K.31 The crystal structure and electronic properties of [NH3(CH2)6NH3]ZnCl4 similar to the [NH3(CH6)NH3]ZnBr4 crystal were reported by Mostafa and El-khiyami.16 Although the NQR data and phase transition temperatures have been reported, to the best of our knowledge, investigation of crystal structure, thermodynamic stability and structural dynamics with respect to temperature change has not been widely conducted.
This study is the first to investigate the crystal structures, phase transition temperature (TC), and thermodynamic properties of [NH3(CH2)6NH3]ZnBr4 crystals with zero-dimensional. Secondly, nuclear magnetic resonance (NMR) chemical shifts and spin–lattice relaxation times (T1ρ) for 1H and 13C were also measured to understand the coordination geometry and molecular dynamics of the organic [NH3(CH2)6NH3] cation near TC. In addition, the effect of temperature on the static 14N NMR spectra was investigated to elucidate the atomic configurations of the cation. The change in the coordination geometry in response to temperature change was explained by the changes of N–H⋯Br hydrogen bonds between the cations and tetrahedral ZnBr4 anions. The investigation of the crystal structures and physicochemical properties of the phase transition mechanism conducted herein will expand the application scope of [NH3(CH2)6NH3]ZnBr4 crystals in environmentally friendly solar cells.
2. Experimental
To obtain [NH3(CH2)6NH3]ZnBr4 single crystals, an aqueous solution containing NH2(CH2)6NH2·2HBr (Aldrich, USA) and ZnBr2 (Aldrich, 99.99%, USA) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was slowly evaporated in a thermostat at 300 K. Colorless single crystals (10 × 3 × 2 mm) were grown for approximately five weeks. The single crystal grown here was colorless and transparent, with a long rectangular shape.
1 ratio was slowly evaporated in a thermostat at 300 K. Colorless single crystals (10 × 3 × 2 mm) were grown for approximately five weeks. The single crystal grown here was colorless and transparent, with a long rectangular shape.
Fourier transformation infrared (FT-IR) spectra in the 4000–500 cm−1 range were measured using an FT-IR spectrometer (PerkinElmer, L1600300) with a compressed KBr pellet.
The lattice parameters at various temperatures were determined by single-crystal X-ray diffraction (XRD) at the Seoul Western Center of the Korea Basic Science Institute (KBSI). A colorless crystal block was picked up with paratone oil and mounted on a Bruker D8 Venture PHOTON III M14 diffractometer equipped with a graphite-monochromated Mo-Kα (λ = 0.71073 Å) radiation source and a nitrogen cold stream (−50 °C). Data was collected and integrated using SMART APEX3 (Bruker, 2016) and SAINT (Bruker, 2016). The absorption was corrected by a multi-scan method implemented in SADABS. The structure was solved using direct methods and refined by full-matrix least-squares on F2 using SHELXTL.32 All non-hydrogen atoms were refined anisotropically, and the hydrogen atoms were added to their geometrically ideal positions. Additionally the powder XRD patterns of the [NH3(CH2)6NH3]ZnBr4 crystals were measured at several temperatures using an XRD system with a Mo-Kα radiation source.
The DSC experiments were performed using a DSC 25 apparatus (TA Instruments, USA) at heating and cooling rates of 10 K min−1 from 200 to 570 K in a nitrogen gas atmosphere.
Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) experiments were performed on a thermogravimetric analyzer (TA Instruments, USA) at the heating rate identical to that utilized for DSC at 300–873 K under nitrogen gas.
The NMR chemical shifts of the [NH3(CH2)6NH3]ZnBr4 crystals were measured using a Bruker 400 MHz Avance II+ NMR spectrometer (Bruker, Germany) at KBSI. The Larmor frequency for 1H magic-angle spinning (MAS) NMR was ωo/2π = 400.13 MHz, and that for the 13C MAS NMR experiment was ωo/2π = 100.61 MHz. To minimize spinning sidebands, the MAS speeds for 1H and 13C were measured at 5 kHz. Tetramethylsilane (TMS) was used as a reference material for accurate NMR chemical shift measurements. The spin–lattice relaxation time, T1ρ, values were obtained using a π/2–τ pulse, followed by a spin-lock pulse of duration τ, and the π/2 pulse widths for 1H and 13C were measured by a previously published method.33 Static 14N NMR resonance frequencies were recorded with a Larmor frequency of ωo/2π = 28.90 MHz using the one-pulse method and NH4NO3 was used as the reference material. The temperature during the NMR experiment ranged from 180 to 430 K; owing to the limitations of the instrument, measurements at higher temperatures were not possible.
3. Results and discussion
3.1. FT-IR spectra
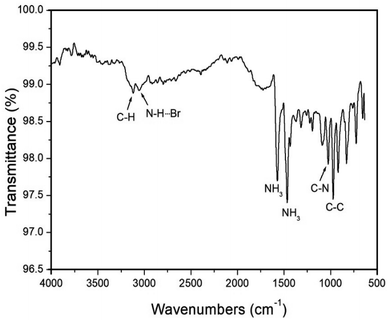
The FT-IR spectrum within the 4000–500 cm−1 range was recorded at room temperature. The result is shown in Fig. 1, and the peak near 3128 cm−1 is assigned to the C–H mode. And, the peak at 3058 cm−1 is due to the N–H⋯Br hydrogen bond, and those at 1577 and 1469 cm−1 correspond to the asymmetric deformation of NH3 and symmetric deformation of NH3, respectively. The peaks near 1025 and 973 cm−1 are defined to the C–N and C–C mode.3.2. Crystal structure and phase transition
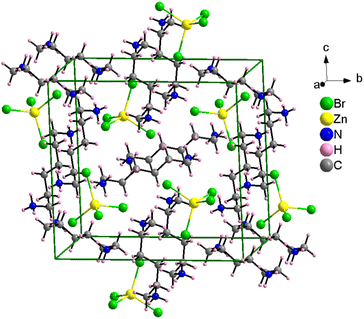
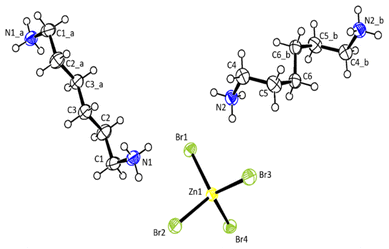
Single-crystal XRD patterns for [NH3(CH2)6NH3]ZnBr4 crystals were obtained at several temperatures. At 300 K, the hybrid was found to have crystallized as a monoclinic system with a P21/c space group and had lattice constants a = 7.7833 (2) Å, b = 14.5312 (4) Å, c = 13.2396 (3) Å, β = 90.8650 (1)°, and Z = 1. Table 1 lists single-crystal XRD and refinement data of the [NH3(CH2)6NH3]ZnBr4 crystal, and Fig. 2 shows its structure. The atomic numbering scheme and thermal ellipsoids for the H atoms are shown in Fig. 3, and their bond lengths and angles are summarized in Table 2. The Zn atom is coordinated by four Br atoms, forming a nearly regular tetrahedron of ZnBr4. The hydrogen atoms of each formula unit are able to form hydrogen bonds N–H⋯Br. The changes in the lattice parameters at 230, 250, 270, 300, and 350 K are shown in Fig. 4, where a, b, and c have different thermal expansion upon with increasing temperature and β increases slightly with increasing temperature.| Chemical formula | C6H18N2ZnBr4 |
| Weight | 503.23 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| T (K) | 300 |
| a (Å) | 7.7833 |
| b (Å) | 14.5312 |
| c (Å) | 13.2396 |
| β (°) | 90.8650(10) |
| Z | 4 |
| V (Å3) | 1497.24 |
| Radiation type | Mo-Kα |
| Wavelength (Å) | 1.71073 |
| Reflections collected | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 490 |
| Independent reflections | 3572 (Rint = 0.0452) |
| Goodness-of-fit on F2 | 1.047 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0300, wR2 = 0.0633 |
| R indices (all data) | R1 = 0.0421, wR2 = 0.0677 |
| Bond-length (Å) and bond-angle (°) | |||
|---|---|---|---|
| Br(1)–Zn(1) | 2.4139 (5) | Br(3)–Zn(1)–Br(1) | 110.07 (2) |
| Br(2)–Zn(1) | 2.4333 (5) | Br(3)–Zn(1)–Br(4) | 111.31 (2) |
| Br(3)–Zn(1) | 2.3822 (6) | Br(1)–Zn(1)–Br(4) | 108.08 (2) |
| Br(4)–Zn(1) | 2.4264 (5) | Br(3)–Zn(1)–Br(2) | 112.00 (2) |
| Br(1)–Zn(1)–Br(2) | 107.482 (19) | ||
| Br(4)–Zn(1)–Br(2) | 107.73 (2) | ||
| N(1)–C(1) | 1.487 (5) | ||
| C(1)–C(2) | 1.523 (5) | ||
| C(2)–C(3) | 1.522 (5) | ||
| C(3)–C(3) | 1.507 (7) | ||
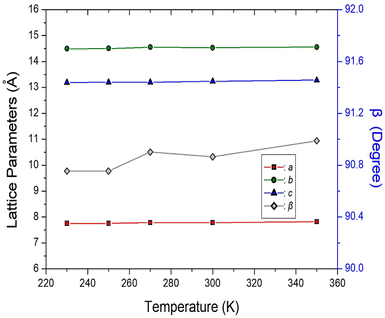 | ||
| Fig. 4 The lattice parameters a, b, c, and β of [NH3(CH2)6NH3]ZnBr4 crystal according to the temperatures. | ||
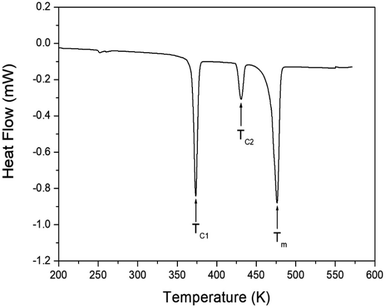
The DSC analysis was performed on the [NH3(CH2)6NH3]ZnBr4 crystals at a heating rate of 10 K min−1. Two strong endothermic peaks at 370 and 476 K and a weak peak at 430 K were observed (Fig. 5). A very small peak was also observed at 250 K. The phase transition temperatures were defined as TC1 = 370 K and TC2 = 430 K; 476 K was defined as the melting temperature, Tm. An analysis of the lattice parameters indicated that the peak at 250 K was independent of the phase transition temperature. According to the result previously reported by Ishiha and Horiuchi,31 [NH3(CH2)6NH3]ZnBr4 undergoes structural phase transitions at 389 and 424 K, whereas no phase transition was observed below room temperature. The Tm was reported to be 475 K. The phase transition temperatures obtained in this study were 370 and 430 K, which were slightly different from the previously reported results,31 however, the Tm was remarkably similar. The slight differences in phase transition temperatures may vary depending on the heating rate in the DSC experiment and may also show some differences depending on the growth conditions of the crystal.
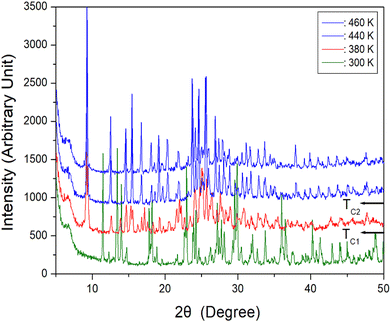
To further confirm the phase transitions, powder XRD patterns of the [NH3(CH2)6NH3]ZnBr4 crystals were obtained after heating; the results in the measuring range (2θ) of 5–50° are shown in Fig. 6.
The XRD pattern at 300 K (olive) was slightly different from that recorded at 380 K (red); this difference is related to TC1. Further, the XRD pattern recorded at 380 K was different from those recorded at 440 K and 460 K exhibited a clear change in structure, which is attributed to TC2. The phase transition temperatures shown in the XRD results are in reasonable agreement with the endothermic peaks in the DSC curves.
3.3. Thermal properties
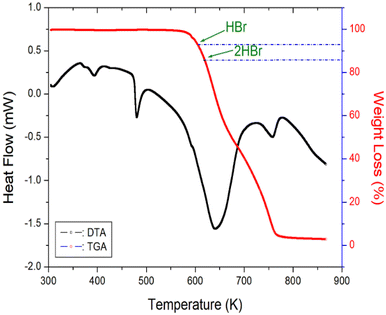
The TGA and DTA results measured at a heating rate of 10 K min−1 are shown in Fig. 7. As the temperature increased, the molecular weight of the [NH3(CH2)6NH3]ZnBr4 crystals decreased. The molecular weight loss began at approximately 600 K, which was defined as the partial thermal decomposition temperature, Td, where at 5% weight loss occurred. From the total molecular weight of 503.23 mg, the residual amounts after the decomposition of HBr and 2HBr were calculated using eqn (1) and (2), respectively:33| {[NH2(CH2)6NH2·HBr]ZnBr3 + HBr (g)}/[NH3(CH2)6NH3]ZnBr4 = 92.75% | (1) |
| {[NH2(CH2)6NH2]ZnBr2 + 2HBr (g)}/[NH3(CH2)6NH3]ZnBr4 = 85.51% | (2) |
Thus, molecular weight losses of 7% and 14% were due to the decomposition of HBr and 2HBr, respectively, and the decomposition temperatures of HBr and 2HBr obtained by TGA were 604 and 620 K, respectively. The 55% weight loss was mainly attributed to organic decomposition. The mass rapidly decreased in the 600–750 K range, with a corresponding mass loss of 95% near 750 K. Further, the endothermic peak at 476 K, obtained from DSC, was confirmed by a polarizing microscope, which showed that the single crystal had started to melt at 470 K; thus, the endothermic peak at 476 K was denoted as the melting temperature.
3.4. 1H NMR chemical shifts and spin–lattice relaxation times
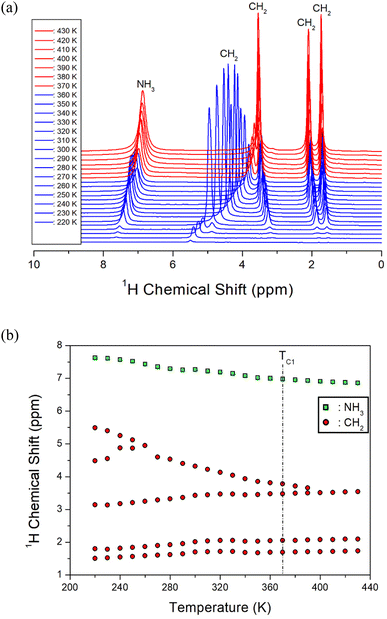
Initially, the 1H NMR chemical shifts of the [NH3(CH2)6NH3]ZnBr4 crystals with spinning speeds of 5 and 10 kHz at 300 K were measured (ESI 1†). However, the chemical shifts measured at the two different spinning rates were observed at the same position; thus, NMR chemical shifts with respect to temperature change were measured at 5 kHz only. The in situ 1H chemical shifts according to temperature change are shown in Fig. 8(a), and the change in chemical shifts is shown in Fig. 8(b) for better understanding. Below 260 K, six resonance lines were observed instead of the two 1H signals expected from NH3 and CH2. NH3 denotes 1H in NH3, and the remaining four or five signals appeared on the right side represent 1H in the six CH2. At 300 K, the 1H chemical shift for NH3 was recorded at 7.27 ppm, and those for the CH2 were obtained at 4.32, 3.42, 2.01, and 1.69 ppm. Two 1H chemical shifts at approximately 4.5–5.5 ppm overlap into one signal at 260 K; in addition, two 1H chemical shifts near 3.5 ppm overlap into one signal at 400 K. Below TC1, one 1H peak was observed for H around C3 at the center of cation as shown in the structure in Fig. 3, and four 1H peaks were observed for H around C2 and C1 with different surrounding environments. However, above TC1, the symmetry around 1H improved such that one 1H peak was observed for each of C1, C2, and C3. The 1H chemical shifts for NH3 are continuous regardless of TC1, as expressed by the different colors in each phase (Fig. 8(a)), while those for CH2 show a slight change near TC1.The 1H MAS NMR spectra were measured by changing the delay time at each temperature, and the plots of spectral intensity as a function of delay times were expressed as mono-exponential curves.
The recovery traces of the magnetization were characterized by the spin–lattice relaxation time, T1ρ, according to eqn (3):29,34–36
| IH(τ) = IH(0)exp(−τ/T1ρ) | (3) |
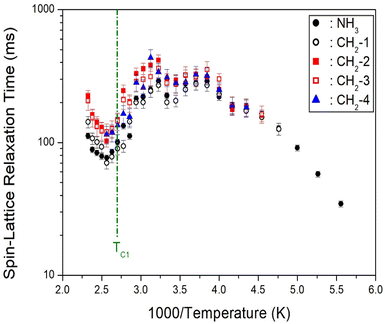 | ||
| Fig. 9 1H NMR spin–lattice relaxation times of NH3 and CH2 in [NH3(CH2)6NH3]ZnBr4 as a function of inverse temperature. | ||
3.5. 13C NMR chemical shifts and spin–lattice relaxation times
The 13C chemical shifts in the MAS NMR spectra with respect to increasing temperature are shown in Fig. 10. The 13C chemical shift for TMS at 300 K was recorded at 38.3 ppm, and this value was set as the standard reference. In the [NH3(CH2)6NH3] cation structure (inset of Fig. 10), CH2 close to NH3 at both ends of the cation was labeled C1, CH2 at the center of the six CH2 chains was labeled C3, and CH2 between C3 and C1 was labeled C2. From 180 to 350 K, the chemical shifts showed a slight change with temperature, while those near TC1 abruptly changed. At 300 K, the 13C chemical shifts were, 41.69 and 39.86, 29.55 and 27.56, and 24.94 and 20.50 ppm for C1, C2, and C3, respectively. The 13C chemical shifts at 360 K were recorded at 42.48 and 41.36 ppm for C1, 27.93 and 27.00 ppm for C2, and 26.01 ppm for C3. All 13C chemical shifts for C1, C2, and C3 in the cation rapidly changed near TC1, indicating that the structural environment around 13C changed near TC1. The 13C peaks corresponding to the chemical shifts for C1, C2, and C3 (Fig. 10) below TC1, including those on the opposite side of C3 at the center of cation, were observed far away from each other. In contrast, the 13C peaks for C1, C2, and C3 above TC1 were found to be close to each other. This result implies that the symmetry of the environment around C1, C2, and C3 above TC1 is improved as indicated by the 1H chemical shifts.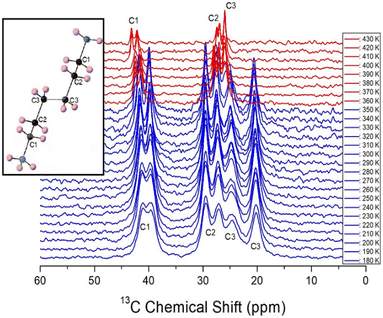 | ||
| Fig. 10 In situ 13C MAS NMR spectra of [NH3(CH2)6NH3]ZnBr4 at several temperatures (inset: structure of cation). | ||
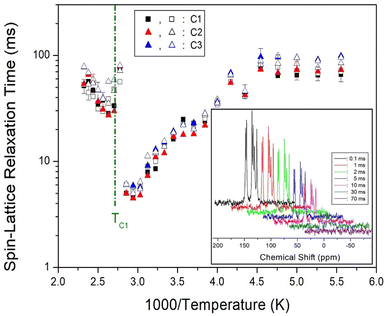
The 13C MAS NMR spectra measured the change in the intensities with increasing delay time at a spinning rate of 5 kHz at each temperature. The recovery traces of the 13C nuclei for the delay times ranging from 0.1 to 70 ms at 300 K are represented in the inset of Fig. 11. All decay curves for C1, C2, and C3 were described by a mono-exponential function, and the 13C T1ρ values from the slopes of their recovery traces were obtained as a function of 1000/T (Fig. 11). The 13C T1ρ values rapidly decreased with increasing temperature, while the values near TC1 increased 10 times in 5 ms, similar to the rapid change in the 13C chemical shifts. Here, the T1ρ values for C1, C2, and C3 were similar within the error range.
3.6. Static 14N resonance frequency
The static NMR spectra for 14N at both ends of the cation in the [NH3(CH2)6NH3]ZnBr4 single crystals are shown inset of Fig. 12 (circled in red). The spectra in the temperature range of 180–400 K were obtained, and the direction of the applied magnetic field was measured with respect to the arbitrary direction of the single crystal. 14N has a spin number of 1, and two resonance signals are expected by the quadrupole interaction.33 The 14N resonance frequency was very low at 28.90 MHz, and it was not easy to obtain a resonance signal by wiggling of the base line. However, because the intensity was small and the line width was relatively broad, it was difficult to distinguish the signals (inset of Fig. 12). The resonance frequencies for the 14N NMR spectra are shown in Fig. 12 at several temperatures. It can be seen that two resonance lines slightly increased with increasing temperature. The same pairs for 14N are indicated by symbols of the same color. However, the 14N signals were difficult to observe at temperatures above 350 K. In the vicinity of TC1, the line width of the 14N signal rapidly widened, making it difficult to detect. The continuous change in the 14N resonance frequency with temperature change indicated a change in the coordination geometry of the environment around N, implying a change in the quadrupole coupling constant, e2qQ/h.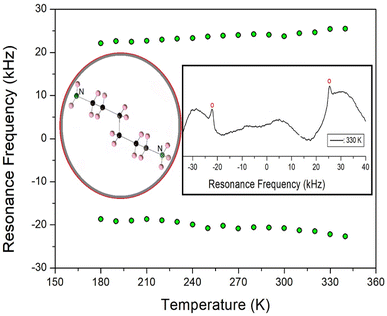 | ||
| Fig. 12 14N resonance frequency of [NH3(CH2)6NH3]ZnBr4 as a function of temperature (inset: structure of cation, the 14N NMR spectrum at 330 K). | ||
4. Conclusions
The crystal structures, phase transitions, structural geometries, thermal stabilities, and molecular dynamics of the [NH3(CH2)6NH3]ZnBr4 crystals were investigated through XRD, DSC, TGA, and NMR experiments. It was discovered that the crystals belong to a monoclinic system with a P21/c space group at 300 K, and the lattice constants are a = 7.7833 Å, b = 14.5312 Å, c = 13.2396 Å, β = 90.8650°, and Z = 4. The crystals undergo two phase transitions, at 370 K (TC1) and 430 K (TC2), as determined by their powder XRD patterns. Our results showed that the thermal property is stable, with a thermal decomposition temperature of approximately 600 K. In the NMR spectra, the changes in the 1H and 13C chemical shifts were observed near TC1, indicating that the structural environment around 1H and 13C changed. This further suggests that the energy transfer above TC1 is very large, indicated by the large thermal displacement around the 13C atoms. The influence of 1H, 14N, and C1 located close to NH3 in the [NH3(CH2)6NH3]ZnBr4 crystals was not significant, indicating a minor change in the N–H⋯Br hydrogen bond related to the coordination geometry of the ZnBr4 anion.This material is lead-free, environmentally friendly, and stable at relatively high temperature; therefore, it is potentially applicable in solar cells.
Author contributions
A. R. Lim performed NMR experiments and wrote the manuscript. H. Ju performed XRD experiment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2018R1D1A1B07041593 and 2016R1A6A1A03012069).References
- O. Knop, R. E. Wasylishen, M. A. White, T. S. Cameron and M. J. M. Van Oort, Can. J. Chem., 1990, 68, 412 CrossRef.
- Q. Chen, N. D. Marco, Y. Yang, T.-B. Song, C.-C. Chen, H. Zhao, Z. Hong, H. Zhou and Y. Yang, Nano Today, 2015, 10, 355 CrossRef.
- I. M. Hermes, S. A. Bretschneider, V. W. Bergmann, D. Klasen, J. Mars, W. Tremel, F. Laquai, H.-J. Butt, M. Mezger, R. Berger, B. J. Rodriguez and S. A. L. Weber, J. Phys. Chem., 2016, 120, 5724 CrossRef PubMed.
- E. Strelcov, Q. Dong, T. Li, J. Chae, Y. Shao, Y. Deng, A. Gruverman, J. Huang and A. Centrone, Sci. Adv., 2017, 3, e1602165 CrossRef.
- S. K. Abdel-Aal, A. S. Abdel-Rahman, G. G. Kocher-Oberlehner, A. Ionov and R. Mozhchil, Acta Crystallogr., Sect. A: Found. Adv., 2017, 70, C1116 Search PubMed.
- Y. Liu, L. Collins, R. Proksch, S. Kim, B. R. Watson, B. Doughty, T. R. Calhoun, M. Ahmadi, A. V. Ievlev, S. Jesse, S. T. Retterer, A. Belianinov, K. Xiao, J. Huang, B. G. Sumpter, S. V. Kalinin, B. Hu and O. S. Ovchinnikova, Nat. Mater., 2018, 17, 1013 CrossRef PubMed.
- T. Asaji, Y. Ito, J. Seliger, V. Zagar, A. Gradisek and T. Apih, J. Phys. Chem., 2012, 116, 12422 CrossRef PubMed.
- T. Asaji and K. Ashitomi, J. Phys. Chem., 2013, 117, 10185 Search PubMed.
- M. Simenas, A. Ciupa, M. Maczka, A. Poppl and J. Banys, J. Phys. Chem., 2015, 119, 24522 Search PubMed.
- N. Abhyankar, J. J. Kweon, M. Orio, S. Bertaina, M. Lee, E. S. Choi, R. Fu and N. S. Dalal, J. Phys. Chem., 2017, 121, 6314 CrossRef PubMed.
- M. Simenas, S. Balciunas, M. Trzebiatowska, M. Ptak, M. Maczka, G. Volkel, A. Poppl and J. Banys, J. Mater. Chem. C, 2017, 5, 4526 RSC.
- M. Simenas, S. Balciunas, A. Ciupa, L. Vilciauskas, D. Jablonskas, M. Kinka, A. Sieradzki, V. Samulionis, M. Maczka and J. Banys, J. Mater. Chem. C, 2019, 7, 6779 RSC.
- M. Simenas, M. Ptak, A. H. Khan, L. Dagys, V. Balevicius, M. Bertmer, G. Volkel, M. Maczka, A. Poppl and J. Banys, J. Phys. Chem., 2018, 122, 10284 CAS.
- A. H. Mahmoudkhani and V. Langer, Acta Crystallogr., Sect. B: Struct. Sci., 2002, E58, m592 Search PubMed.
- Z. Cheng and J. Lin, CrystEngComm, 2010, 12, 2646 RSC.
- M. F. Mostafa and S. S. El-khiyami, J. Solid State Chem., 2014, 209, 82 CrossRef CAS.
- S. Gonzalez-Carrero, R. E. Galian and J. Perez-Prieto, Part. Part. Syst. Charact., 2015, 32, 709 CrossRef.
- S. K. Abdel-Adal, G. Kocher-Oberlehner, A. Ionov and R. N. Mozhchil, Appl. Phys. A, 2017, 123, 531 CrossRef.
- K. Pradeesh, G. S. Yadav, M. Singh and G. Vijaya Prakash, Mater. Chem. Phys., 2010, 124, 44 CrossRef.
- S. Saikumar, J. J. Ahmad, G. Baumberg and G. Vijaya Prakash, Scr. Mater., 2012, 67, 834 CrossRef.
- B. Staskiewicz, O. Czupinski and Z. Czapla, J. Mol. Struct., 2014, 1074, 723 CrossRef.
- S. Ahmad, C. Hanmandlu, P. K. Kanaujia and G. Vijaya Prakash, Opt. Mater. Express, 2014, 4, 1313 CrossRef.
- Z. Czapla, J. Przeslawski, M. Crofton, J. Janczak, O. Czupinski, A. Ingram and M. Kostrzewa, Phase Transitions, 2017, 90, 637 CrossRef.
- H.-Y. Zhang, Z. Wei, P.-F. Li, Y.-Y. tang, W.-Q. Liao, H.-Y. Ye, H. Cai and R.-G. Xiong, Angew. Chem., Int. Ed., 2018, 57, 526 CrossRef CAS PubMed.
- W. Zang and R.-G. Xiong, Chem. Rev., 2012, 112, 1163 CrossRef PubMed.
- A. R. Lim and S. H. Kim, ACS Omega, 2021, 6, 27568 CrossRef PubMed.
- W. Liu, J. Xing, J. Zhao, X. Wen, K. Wang, L. Peixiang and Q. Xiong, Adv. Opt. Mater., 2017, 5, 1601045 CrossRef.
- J.-C. Bissey, N. Filloleau, N.-B. Chanh, R. Berger and S. Flandrois, Solid State Commun., 1998, 106, 385 CrossRef.
- A. R. Lim, S. H. Kim and Y. L. Joo, Sci. Rep., 2021, 11, 8408 CrossRef PubMed.
- R. Kind, S. Plesko, P. Gunter, J. Roos and J. Fousek, Phys. Rev. B, 1981, 23, 5301 CrossRef.
- H. Ishiha and K. Horiuchi, Hyperfine Interact., 2004, 159, 149 CrossRef.
- SHELXTL v 6.10, Bruker AXS, Inc., Madison, Wisconsin, USA, 2000 Search PubMed.
- A. R. Lim, Sci. Rep., 2020, 10, 20853 CrossRef PubMed.
- A. Abragam, The Principles of Nuclear Magnetism, Oxford Univ. press, 1961 Search PubMed.
- R. K. Harris, Nuclear Magnetic Resonance Spectroscopy, Pitman Pub., UK, 1983 Search PubMed.
- J. L. Koenig, Spectroscopy of Polymers, Elsevier, New York, 1999 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: XRD data, bond distances and angles, and hydrogen-bond geometrics of the crystal structure. CCDC 2169730. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ra04834e |
| This journal is © The Royal Society of Chemistry 2022 |