Open Access Article
Open Access ArticleDiscovery of 4-nitro-3-phenylisoxazole derivatives as potent antibacterial agents derived from the studies of [3 + 2] cycloaddition†
Yan Zhanga,
Zhiwu Longa,
Longjia Yan ab,
Li Liuab,
Lan Yangab and
Yi Le
ab,
Li Liuab,
Lan Yangab and
Yi Le *ab
*ab
aSchool of Pharmaceutical Sciences, Guizhou University, Guiyang 550025, China. E-mail: yile2021@163.com
bGuizhou Engineering Laboratory for Synthetic Drugs, Guiyang 550025, China
First published on 8th September 2022
Abstract
Polysubstituted phenylisoxazoles were designed and synthesized to discover new antibacterial agents via [3 + 2] cycloaddition. Thirty-five compounds with a phenylisoxazole scaffold were characterized by NMR, HRMS, and X-ray techniques. After being evaluated against Xanthomonas oryzae (Xoo), Pseudomonas syringae (Psa), and Xanthomonas axonopodis (Xac), 4-nitro-3-phenylisoxazole derivatives were found to better antibacterial activities. Further studies have shown that the EC50 values of these compounds were much better than that of the positive control, bismerthiazol.
Introduction
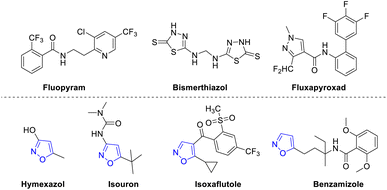
Plant diseases have been one of the main factors restricting food security.1 These diseases not only affect the yield of crops but also hamper the quality of food. The use of antibacterial agents and fungicides has greatly reduced the economic losses caused by plant diseases.2 However, there are also some negative effects along with the social benefits. Due to the unreasonable application of these agents, the resistance of pathogens has become increasingly serious, which also has a tremendous impact on non-target organisms.3 Additionally, these effects bring great pressure to the environment. With the extensive attention to health and increasing environmental awareness, the development of efficient, low toxic, and green antibacterial agents or fungicides has become a hot topic in the field of pesticide research.4 A large number of pesticides have been found in the market.5 For example, fluopyram (Fig. 1) was developed and applied to control the plant pathogens in crops such as rice, citrus, and kiwi fruit.6 Bismerthiazol (Fig. 1) was used to control the effect of rice bacterial blight and bacterial leaf streak.7 In 2012, fluxapyroxad (Fig. 1) was developed as a new pesticide for corresponding plant diseases.8Isoxazole derivatives are important heterocyclic compounds that are widely used in pesticides.9 For instance, 5-methylisoxazol-3-ol (hymexazol in Fig. 1) is considered as a soil disinfectant and plant growth regulator.10 Isouron (Fig. 1), a selective herbicide, is mostly employed to control the weeds such as paspalum and white grass.11 Isoxaflutole (Fig. 1) belongs to early sulfone herbicides, and are mainly applied in corn and sugarcane fields.12 Benzamizole, a herbicide cell division inhibitor (Fig. 1), is usually used in broad-leaved plants including cereal crops, broad beans, peas, trees, and grapes.13 Based on the characteristics and wide application of isoxazole derivatives, the rapid and efficient construction of various isoxazole derivatives has become the focus of researchers.14 Among the synthetic methods of isoxazole compounds, the most efficient strategy is [3 + 2] cycloaddition.15 Therefore, many chemists have used this method to synthesize isoxazole compounds and apply them to develop new pesticides.16
Our group has focused on the development of small molecules with various biological activities for several years.17 Considering the urgent need for novel pesticides containing isoxazole rings and our previous research on heterocyclic compounds,18 we designed and synthesized phenylisoxazole derivatives as a novel pesticide in agriculture. Furthermore, there are only a few reports on phenylisoxazoles as antibacterial agents in literature. In this study, we first developed a new synthetic method for constructing a phenylisoxazole ring with diverse substituent groups. Subsequently, we screened the biological activities of these compounds to obtain novel pesticides.
Results and discussion
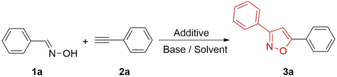
Mohammed et al. reported benzaldehyde oxime (1a) reacted with phenylacetylene (2a) to afford compound 3a in the presence of NCS and DBU in 2015.19 Initially, we repeated the reaction and obtained the isolated yield of 63%. When the additive NCS was increased to two equivalents, the yield still remained at 65% (Table 1, entry 1). Later, we screened different solvents to improve the yield and identified that DMF is still the best solvent (Table 1, entries 1–4). The different additives such as NBS, NIS, PIFA, chloramine-T were also used to accelerate the reaction.20 Unfortunately, these additives were all ineffective in improving the yield (Table 1, entries 5–8). Subsequently, we found that different bases could severely influence the reaction rate (Table 1, entries 9–18). When triethylamine was used as the base, the separated yield reached 85% (Table 1, entry 18), indicating that the weak organic base was ideal for accelerating the yield. Interestingly, the yield decreased slowly with the increase in temperature (Table 1, entries 19–21). This may be due to the substrate side effects accompanied with the increase in temperature, which reduced the reaction yield. Finally, the optimal condition was determined in the presence of TEA and DMF at room temperature (Table 1, entry 18).| Entry | Additive | Base | Solvent | T (°C) | Yield (%) |
|---|---|---|---|---|---|
| a Reagents and conditions: 1a (1 mmol), 2a (1.2 mmol), additive (2 mmol), base (1 mmol), solvent (6 mL), 6 h.b Isolated yields.c Reagents and conditions: 1a (1 mmol), 2a (1.2 mmol), NCS (1.2 mmol), DBU (1 mmol), DMF (6 mL), 6 h. | |||||
| 1 | NCS | DBU | DMF | 25 | 65 (63)c |
| 2 | NCS | DBU | CH2Cl2 | 25 | 38 |
| 3 | NCS | DBU | THF | 25 | 31 |
| 4 | NCS | DBU | Dioxane | 25 | None |
| 5 | NBS | DBU | DMF | 25 | 17 |
| 6 | NIS | DBU | DMF | 25 | 19 |
| 7 | PIFA | DBU | DMF | 25 | 39 |
| 8 | Chloramine | DBU | DMF | 25 | 37 |
| 9 | NCS | Cs2CO3 | DMF | 25 | 43 |
| 10 | NCS | K2CO3 | DMF | 25 | 51 |
| 11 | NCS | NaOtBu | DMF | 25 | 51 |
| 12 | NCS | Na2CO3 | DMF | 25 | 53 |
| 13 | NCS | Pyrrolidine | DMF | 25 | 47 |
| 14 | NCS | NaOH | DMF | 25 | 52 |
| 15 | NCS | DMAP | DMF | 25 | 55 |
| 16 | NCS | DIEA | DMF | 25 | 80 |
| 17 | NCS | DABCO | DMF | 25 | 63 |
| 18 | NCS | TEA | DMF | 25 | 85 |
| 19 | NCS | TEA | DMF | 50 | 84 |
| 20 | NCS | TEA | DMF | 75 | 79 |
| 21 | NCS | TEA | DMF | 100 | 74 |
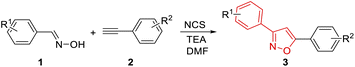
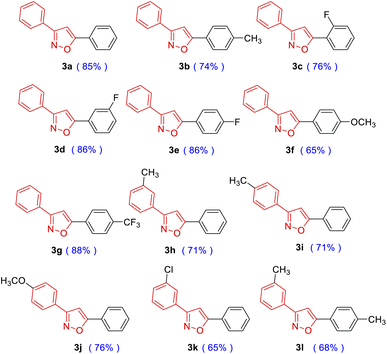
Having the optimal condition in hand (Table 1, entry 18), the scope was explored in different substituted phenyloxime derivatives and phenylacetylene derivatives (Table 2). Phenyloximes with electron-donating groups –CH3 and –OCH3 resulted in lower yields than 2a, while electron-withdrawing groups –F and –CF3 resulted in higher yields than 2a (3a–3g). Furthermore, the steric effect of substituents on the phenyl ring also decreased the yield of this [3 + 2] cycloaddition (3c–3e). In addition, phenyloxime derivatives such as 3-methyl (2h), 4-methyl (2i), 4-methoxy (2j), 3-chloro (2k) benzaldehyde oximes produced the desired products in 65–76% yield (3h–3l).
| a Reagents and conditions: 1 (1 mmol), 2 (1.2 mmol), NCS (2 mmol), TEA (1 mmol), DMF (6 mL), 25 °C, 6 h. |
|---|
 |
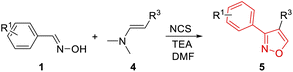
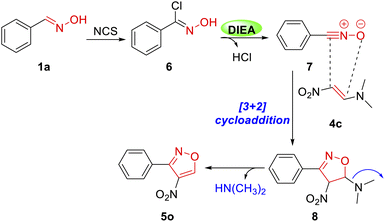
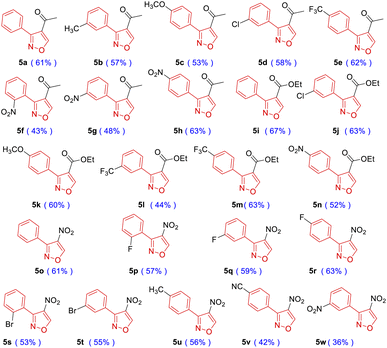
Structural diversity is very crucial for screening new antibacterial agents.21 To ensure molecular diversity, 3,4-disubstituted isoxazoles were synthesized (Table 3), and benzaldehyde oxime (1a) and commercially available 4-dimethylamino-but-3-en-2-one (4a) were chosen as the starting materials. Under the identical conditions as mentioned previously, compound 5a was isolated with 61% yield (Table 3). The structure of 5a was confirmed by NMR and HRMS, which was consistent with that obtained from other cycloadditions using hypervalent iodine reagent as the catalyst.20 Subsequently, a phenyl ring with electron-donating groups –CH3 (5b) and –OCH3 (5c) produced lower yields, while electron-withdrawing groups –CF3 (5e) and –NO2 (5h) produced higher yields than 5a. The steric effect of substituents on the phenyl ring significantly decreased the yield of this cycloaddition (5f, 5g and 5h). Besides, 3-dimethylamino-acrylic acid ethyl ester (4b) was also used in this reaction instead of compound 4a. Fortunately, the final targets 5i–5n were successfully synthesized with 44–67% yields. The effect of substituents on the yield was similar to 5a–5h. Nitroisoxazole derivatives have a wide range of biological activities, such as antitumor, antibacterial, and anti-inflammatory.22 To investigate the antibacterial activities of nitroisoxazoles, 4-nitro-3-phenylisoxazole derivatives were prepared. Dimethyl-(2-nitrovinyl)-amine (4c) reacted with corresponding phenyloximes (1) to give the products 5o–5w. The yield was moderate from 36% to 63%.
| a Reagents and conditions: 1 (1 mmol), 4 (1.2 mmol), NCS (2 mmol), TEA (1 mmol), DMF (6 mL), 25 °C, 6 h. |
|---|
 |
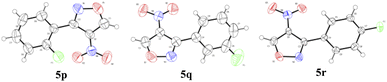
In addition, the NMR spectra of compound 5o was consistent with the data in the literature.23 However, the test result of HRMS was inconsistent with the actual values. This phenomenon was observed in all the 4-nitro-3-phenylisoxazole derivatives. To ensure the structures of this series of products, compounds 5p, 5q, and 5r were characterized by X-ray diffraction analysis. As shown in Fig. 2, the deposition numbers in CCDC (Cambridge Crystallographic Data Centre) were 2130131 (5p), 2130134 (5q), and 2153382 (5r). These results indirectly indicate that the structures of compounds 5o–5w were appropriate.
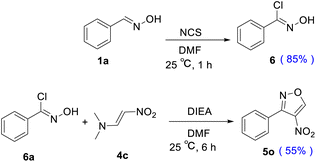
To investigate the mechanism, the benzaldehyde oxime 1a and compound 4c were subjected to undergo [3 + 2] cycloaddition. Based on the observed results and the reports in the literature,24 the regioselectivity of the [3 + 2] cycloaddition reaction between nitrile N-oxides and conjugated nitroalkenes was determined by the nucleophilic attack of oxygen atom from the CNO moiety on the activated electrophilic 2-position of nitroalkene. Considering several mechanisms related to the 32CA reaction,25 we described a plausible mechanism in Scheme 1. Initially, the NCS chlorinated oxime 1a afforded an intermediate 6. Then, compound 6 was dechlorinated under base DIEA to form nitrile oxide 7. Finally, it was reacted with compound 4c to afford the isoxazole compound 5o through [3 + 2] cycloaddition with 55% yield.
In order to explain the pathway of this mechanism, we conducted two complementary experiments. As shown in Scheme 2, under the optimized conditions, the benzaldehyde oxime 1a was converted to compound 6 with 85% yield in the presence of NCS. The NMR spectra of 6 was consistent with the literature.26 At last, compound 6 reacted with (E)-N,N-dimethyl-2-nitroethen-1-amine 4c at 25 °C to afford the required product 5o in 55% yield. These complementary experiments successfully proved our proposed mechanism in Scheme 1.
With the target compounds 3a–3l and 5a–5w in hand, we screened in vitro screening of antibacterial activities against representative plant diseases including Xanthomonas oryzae (Xoo), Xanthomonas axonopodis (Xac), and Pseudomonas syringae (Psa) at 100 μg mL−1 and 50 μg mL−1 according to the previous report.27 The results are listed in Table 4. The activities of compounds 3a–3l and 5a–5n were lower than those of bismerthiazol and fluopyram.28 It was interesting to note that the activities of compounds 5o–5w were much better than the positive controls. Surprisingly, the inhibitions against Xoo and Xac were more than 90% at the concentration of 100 μg mL−1 and 50 μg mL−1. Therefore, compounds 5o–5w were selected for further studies as novel antibacterial agents.
| Entry | Cpd | Xoo (μg mL−1) | Xac (μg mL−1) | Psa (μg mL−1) | |||
|---|---|---|---|---|---|---|---|
| 100 | 50 | 100 | 50 | 100 | 50 | ||
| a The average of three trials. | |||||||
| 1 | 3a | — | — | — | — | 9.6 ± 2.1 | — |
| 2 | 3b | — | — | — | — | 10.7 ± 3.8 | — |
| 3 | 3c | — | — | 38.2 ± 3.7 | 15.0 ± 2.4 | 15.8 ± 1.4 | — |
| 4 | 3d | — | — | 35.5 ± 2.8 | 12.9 ± 1.7 | 12.6 ± 1.1 | — |
| 5 | 3e | — | — | 36.1 ± 3.2 | 14.6 ± 2.5 | 14.9 ± 0.8 | — |
| 6 | 3f | — | — | — | — | 14.5 ± 4.5 | — |
| 7 | 3g | — | — | 43.5 ± 5.9 | 38.1 ± 2.1 | 19.6 ± 1.5 | — |
| 8 | 3h | — | — | 35.1 ± 3.7 | 20.3 ± 5.0 | 18.6 ± 5.8 | — |
| 9 | 3i | — | — | 44.8 ± 3.6 | 21.6 ± 2.5 | 19.3 ± 4.5 | 18.3 ± 7.4 |
| 10 | 3j | — | — | 14.4 ± 2.7 | — | 16.8 ± 4.9 | 9.3 ± 4.1 |
| 11 | 3k | — | — | 46.7 ± 3.6 | 36.1 ± 4.1 | 19.2 ± 4.2 | — |
| 12 | 3l | — | — | 72.8 ± 2.5 | 35.1 ± 1.3 | 16.0 ± 0.9 | 5.8 ± 2.2 |
| 13 | 5a | 29.6 ± 0.9 | 22.4 ± 3.6 | 34.4 ± 3.5 | 25.3 ± 2.6 | 27.4 ± 0.4 | 17.7 ± 0.2 |
| 14 | 5b | 41.1 ± 5.7 | 28.7 ± 3.1 | 45.8 ± 1.7 | 29.5 ± 4.3 | 27.0 ± 5.7 | 14.9 ± 2.8 |
| 15 | 5c | 32.9 ± 4.1 | 31.5 ± 2.2 | 39.0 ± 8.2 | 12.3 ± 6.0 | 27.6 ± 4.9 | 16.5 ± 2.0 |
| 16 | 5d | 42.4 ± 5.2 | 27.6 ± 3.4 | 46.7 ± 1.8 | 28.6 ± 4.1 | 26.8 ± 5.3 | 13.7 ± 2.5 |
| 17 | 5e | 42.2 ± 1.7 | 10.4 ± 5.9 | 64.0 ± 4.6 | 56.8 ± 5.1 | 29.6 ± 2.2 | 16.0 ± 3.0 |
| 18 | 5f | 33.5 ± 1.1 | 24.1 ± 1.6 | 60.6 ± 7.4 | 46.2 ± 4.2 | 26.8 ± 2.7 | 14.3 ± 2.6 |
| 19 | 5g | 37.9 ± 6.9 | 26.8 ± 8.3 | 51.9 ± 8.4 | 29.3 ± 6.6 | 25.3 ± 1.9 | 12.0 ± 1.4 |
| 20 | 5h | 48.4 ± 2.8 | 19.3 ± 4.7 | 51.3 ± 2.5 | 26.0 ± 6.5 | 25.7 ± 1.5 | 13.4 ± 1.6 |
| 21 | 5i | 34.9 ± 1.6 | 26.5 ± 5.1 | 70.4 ± 5.0 | 51.6 ± 3.4 | 26.0 ± 0.7 | 14.8 ± 5.1 |
| 22 | 5j | 37.7 ± 1.4 | 28.9 ± 4.3 | 63.7 ± 4.5 | 46.2 ± 2.8 | 28.2 ± 0.5 | 16.4 ± 5.8 |
| 23 | 5k | 36.8 ± 1.9 | 28.7 ± 6.7 | 72.9 ± 6.1 | 53.7 ± 4.9 | 27.1 ± 0.8 | 15.3 ± 6.6 |
| 24 | 5l | 42.2 ± 1.7 | 10.4 ± 5.9 | 74.0 ± 4.6 | 56.8 ± 7.1 | 26.5 ± 0.7 | 16.6 ± 4.5 |
| 25 | 5m | 33.5 ± 1.1 | 24.1 ± 1.6 | 60.6 ± 2.4 | 46.2 ± 5.2 | 27.1 ± 0.8 | 15.3 ± 3.6 |
| 26 | 5n | 41.2 ± 1.8 | 26.7 ± 1.5 | 62.1 ± 2.1 | 42.0 ± 4.8 | 28.8 ± 1.2 | 16.8 ± 3.4 |
| 27 | 5o | 97.7 ± 0.2 | 93.6 ± 0.3 | 99.5 ± 0.4 | 98.5 ± 0.1 | 60.8 ± 3.2 | 55.6 ± 6.5 |
| 28 | 5p | 97.7 ± 1.0 | 96.8 ± 0.0 | 97.9 ± 1.0 | 96.4 ± 0.6 | 66.3 ± 5.6 | 63.7 ± 5.2 |
| 29 | 5q | 97.7 ± 0.1 | 94.0 ± 0.3 | 97.8 ± 0.7 | 95.6 ± 0.1 | 65.4 ± 4.5 | 57.0 ± 4.6 |
| 30 | 5r | 96.4 ± 0.1 | 93.5 ± 0.1 | 99.2 ± 0.2 | 97.7 ± 0.2 | 58.4 ± 1.4 | 41.7 ± 1.3 |
| 31 | 5s | 97.8 ± 0.1 | 97.6 ± 0.1 | 97.9 ± 0.3 | 97.5 ± 0.0 | 55.0 ± 4.1 | 51.7 ± 5.3 |
| 32 | 5t | 97.9 ± 0.1 | 96.6 ± 0.1 | 99.8 ± 0.4 | 97.6 ± 0.6 | 53.3 ± 0.5 | 48.0 ± 1.9 |
| 33 | 5u | 96.0 ± 0.3 | 69.4 ± 6.5 | 99.6 ± 0.2 | 95.4 ± 0.0 | 42.7 ± 4.5 | 21.7 ± 0.7 |
| 34 | 5v | 97.5 ± 0.1 | 94.1 ± 0.5 | 98.9 ± 0.0 | 98.7 ± 0.1 | 44.2 ± 5.0 | 40.8 ± 1.6 |
| 35 | 5w | 97.5 ± 0.1 | 94.1 ± 0.5 | 98.9 ± 0.0 | 98.7 ± 0.1 | 44.2 ± 5.0 | 40.8 ± 1.6 |
| Bismerthiazol | 73.9 ± 1.1 | 29.3 ± 1.7 | 78.8 ± 6.6 | 46.7 ± 2.3 | 39.0 ± 3.5 | 15.6 ± 4.1 | |
As shown in Table 5, compounds 5o–5w exhibited much better EC50 values against Xoo, Xac, and Psa than bismerthiazol and fluopyram.28 Preliminary structure–activity relationship can also be found in Table 5. The ortho-substituted derivatives (5p, 5s) have better activities than meso-substituted derivatives (5q, 5t) and para-substituted derivatives (5r, 5u, and 5v). Against Xoo, the large-steric substituted compounds 5t (–Br, 9.1 μg mL−1) and 5w (–NO2, 7.9 μg mL−1) have lower EC50 than small-steric substituted compound 5q (–F, 12.6 μg mL−1). A similar phenomenon was observed against Xac. However, against Psa, small-steric substituted compound 5q (12.6 μg mL−1) showed a better effect than large-steric substituted compound 5t (47.0 μg mL−1).
| Compound | Regression equation | Correlation coefficient (r) | EC50 (μg mL−1) |
|---|---|---|---|
| a The average of three trials. | |||
| Xanthomonas oryzae (Xoo) | |||
| Bismerthiazol | y = 0.5792x + 2.3536 | 0.9595 | 82.3 ± 5.1 |
| 5o | y = 3.2815x + 0.658 | 0.9856 | 15.0 ± 0.8 |
| 5p | y = 3.6552x + 7.277 | 0.9683 | 11.7 ± 0.4 |
| 5q | y = 3.7164x + 3.068 | 0.9883 | 12.6 ± 0.5 |
| 5r | y = 3.5643x + 2.158 | 0.9835 | 13.4 ± 0.4 |
| 5s | y = 6.55167x + 0.248 | 0.9530 | 7.6 ± 0.3 |
| 5t | y = 5.3958x + 0.8359 | 0.9838 | 9.1 ± 0.3 |
| 5u | y = 3.1172x + 0.4496 | 0.9667 | 15.9 ± 0.8 |
| 5v | y = 1.6916x + 0.0594 | 0.9870 | 29.5 ± 1.4 |
| 5w | y = 6.0038x + 2.4873 | 0.9861 | 7.9 ± 0.4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Xanthomonas axonopodis (Xac) | |||
| Bismerthiazol | y = 0.8141x + 0.3426 | 0.9599 | 61.0 ± 4.4 |
| 5o | y = 19.477x + 1.2731 | 0.9394 | 2.5 ± 0.1 |
| 5p | y = 15.73x + 6.1445 | 0.9082 | 2.8 ± 0.2 |
| 5q | y = 9.3762x + 5.5612 | 0.9371 | 4.7 ± 0.2 |
| 5r | y = 6.8912x + 5.5944 | 0.9804 | 6.4 ± 0.3 |
| 5s | y = 10.836x + 18.611 | 0.9176 | 2.9 ± 0.2 |
| 5t | y = 13.73x + 10.154 | 0.9309 | 2.9 ± 0.2 |
| 5u | y = 7.3972x + 5.8451 | 0.9466 | 6.0 ± 0.3 |
| 5v | y = 6.922x + 5.6802 | 0.9762 | 6.4 ± 0.4 |
| 5w | y = 12.884x + 14.465 | 0.9782 | 2.8 ± 0.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Pseudomonas syringae (Psa) | |||
| Bismerthiazol | >100 | ||
| 5o | y = 0.6055x + 23.34 | 0.9104 | 44.0 ± 3.8 |
| 5p | y = 1.1501x + 19.741 | 0.9581 | 26.3 ± 2.2 |
| 5q | y = 0.9974x + 13.765 | 0.9199 | 36.3 ± 2.7 |
| 5r | y = 0.6203x + 14.995 | 0.9779 | 56.4 ± 5.1 |
| 5s | y = 0.699x + 6.2785 | 0.9784 | 62.6 ± 5.3 |
| 5t | y = 0.9171x + 6.8663 | 0.9650 | 47.0 ± 3.7 |
| 5u | y = 0.6372x + 14.585 | 0.9821 | 55.6 ± 4.6 |
| 5v | >100 | ||
| 5w | >100 | ||
Conclusions
In summary, an efficient method was developed to prepare polysubstituted phenylisoxazoles via [3 + 2] cycloaddition. This series of phenylisoxazole derivatives were characterized and evaluated at 100 μg mL−1 and 50 μg mL−1 against Xoo, Xac, and Psa. The results suggested that 4-nitro-3-phenylisoxazole derivatives performed excellent antibacterial activities. Further studies on the EC50 values have shown that these compounds were much better than bismerthiazol. However, the antibacterial mechanism of these 4-nitro-3-phenylisoxazole derivatives is ongoing in our lab.Author contributions
Yan Zhang and Zhiwu Long synthesized the compounds and performed the spectral studies; Longjia Yan and Li Liu contributed to the design and implementation of the research; Lan Yang and Yi Le tested bioactive activities, and wrote the manuscript with input from all authors.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was financially supported by Guizhou University Found for Newly Enrolled Talent ([2019]15), Guizhou University Found for Cultivation ([2019]65), and the Guizhou Provincial Science and Technology Foundation ([2020]1Y09).Notes and references
- J. L. Dangl, D. M. Horvath and B. J. Staskawicz, Science, 2013, 341, 746–751 CrossRef CAS PubMed.
- (a) M. Liu, Z. Shi, X. Zhang, M. Wang, L. Zhang, K. Zheng, J. Liu, X. Hu, C. Di, Q. Qian, Z. He and D. L. Yang, Nat. Plants, 2019, 5, 389–400 CrossRef CAS PubMed; (b) Y. Sun, Y. X. Zhu, P. J. Balint-Kurti and G. F. Wang, Trends Plant Sci., 2020, 25, 695–713 CrossRef CAS PubMed; (c) S. Chakraborty and A. C. Newton, Plant Pathol., 2011, 60, 2–14 CrossRef.
- (a) F. Gao, T. Wang, J. Xiao and G. Huang, Eur. J. Med. Chem., 2019, 173, 274–281 CrossRef CAS PubMed; (b) P. E. Busby, K. G. Peay and G. Newcombe, New Phytol., 2016, 209, 1681–1692 CrossRef CAS PubMed; (c) T. Zhou, R. Hu, L. Wang, Y. Qiu, G. Zhang, Q. Deng, H. Zhang, P. Yin, B. Situ, C. Zhan, A. Qin and B. Z. Tang, Angew. Chem., Int. Ed. Engl., 2020, 59, 9952–9956 CrossRef CAS.
- (a) S. Lehmann, M. Serrano, F. L'Haridon, S. E. Tjamos and J. P. Metraux, Phytochemistry, 2015, 112, 54–62 CrossRef CAS PubMed; (b) J. G. Schaart, C. C. M. van de Wiel, L. A. P. Lotz and M. J. M. Smulders, Trends Plant Sci., 2016, 21, 438–449 CrossRef CAS; (c) H. Derksen, C. Rampitsch and F. Daayf, Plant Sci., 2013, 207, 79–87 CrossRef CAS.
- (a) D. Aboushady, S. S. Rasheed, J. Herrmann, A. Maher, E. M. El-Hossary, E. S. Ibrahim, A. H. Abadi, M. Engel, R. Müller, M. Abdel-Halim and M. M. Hamed, Bioorg. Chem., 2021, 117, 105422 CrossRef CAS; (b) J. Wang, P. L. Zhang, M. F. Ansari, S. Li and C. H. Zhou, Bioorg. Chem., 2021, 113, 105039 CrossRef CAS PubMed.
- A. Harsanyi, A. Lückener, H. Pasztor, Z. Yilmaz, L. Tam, D. S. Yufit and G. Sandford, Eur. J. Org. Chem., 2020, 3872–3878 CrossRef CAS.
- C. Shen, W. Lu, Y. Huang, J. Wu and H. Zhang, Chem. Eng. J., 2015, 260, 411–418 CrossRef CAS.
- J. V. Mercader, A. Abad-Somovilla, C. Agulló and A. Abad-Fuentes, J. Agric. Food Chem., 2017, 65, 9333–9341 CrossRef CAS PubMed.
- (a) A. Sysak and B. Obmińska-Mrukowicz, Eur. J. Med. Chem., 2017, 137, 292–309 CrossRef CAS; (b) Y. Y. Zhang, S. Gao, Y. X. Liu, C. Wang, W. Jiang, L. X. Zhao, Y. Fu and F. Ye, J. Agric. Food Chem., 2020, 68, 3403–3414 CrossRef CAS PubMed; (c) H. Z. Zhang, Z. L. Zhao and C. H. Zhou, Eur. J. Med. Chem., 2018, 144, 444–492 CrossRef CAS PubMed.
- X. Ma, J. Chen and X.-H. Du, Org. Process Res. Dev., 2019, 23, 1152–1158 CrossRef CAS.
- S. C. Cheng, R. H. Lee, J. Y. Jeng, C. W. Lee and J. Shiea, Anal. Chim. Acta, 2020, 1102, 63–71 CrossRef CAS PubMed.
- F. Li, Z. Ye, Z. Huang, X. Chen, W. Sun, W. Gao, S. Zhang, F. Cao, J. Wang, Z. Hu and Y. Zhang, Bioorg. Chem., 2021, 117, 105452 CrossRef CAS PubMed.
- R. López-Ruiz, R. Romero-González, S. Martín-Torres, A. M. Jimenez-Carvelo and L. Cuadros-Rodríguez, J. Chromatogr. A, 2022, 1664, 462791 CrossRef.
- (a) O. B. Bondarenko and N. V. Zyk, Chem. Heterocycl. Compd., 2020, 56, 694–707 CrossRef CAS; (b) A. Sysak and B. Obmińska-Mrukowicz, Eur. J. Med. Chem., 2017, 137, 292–309 CrossRef CAS; (c) G. N. Pairas, F. Perperopoulou, P. G. Tsoungas and G. Varvounis, ChemMedChem, 2017, 12, 408–419 CrossRef CAS PubMed; (d) J. Zhu, J. Mo, H.-z. Lin, Y. Chen and H.-p. Sun, Bioorg. Med. Chem., 2018, 26, 3065–3075 CrossRef CAS PubMed.
- (a) C. Rajasekhar, S. Durgamma and A. Padmaja, J. Heterocycl. Chem., 2014, 51, 1727–1734 CrossRef CAS; (b) T. V. Hansen, P. Wu and V. V. Fokin, J. Org. Chem., 2005, 70, 7761–7764 CrossRef CAS; (c) J. E. Grob, J. Nunez, M. A. Dechantsreiter and L. G. Hamann, J. Org. Chem., 2011, 76, 10241–10248 CrossRef CAS; (d) S. Kankala, R. Vadde and C. S. Vasam, Org. Biomol. Chem., 2011, 9, 7869–7876 RSC; (e) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- (a) S. Guo, J. Wang, X. Zhang, S. Cojean, P. M. Loiseau and X. Fan, Bioorg. Med. Chem. Lett., 2015, 25, 2617–2620 CrossRef CAS; (b) D. C. B. da Silva-Alves, J. V. dos Anjos, N. N. M. Cavalcante, G. K. N. Santos, D. M. d. A. F. Navarro and R. M. Srivastava, Bioorg. Med. Chem., 2013, 21, 940–947 CrossRef CAS PubMed.
- (a) Y. Zhang, Q. Wang, L. Li, Y. Le, L. Liu, J. Yang, Y. Li, G. Bao and L. Yan, J. Enzyme Inhib. Med. Chem., 2021, 36, 1205–1216 CrossRef CAS; (b) L. Yan, Q. Wang, L. Liu and Y. Le, J. Enzyme Inhib. Med. Chem., 2022, 37, 832–843 CrossRef CAS.
- Y. Le, Y. Zhang, Q. Wang, N. Rao, D. Li, L. Liu, G. Ouyang and L. Yan, Tetrahedron Lett., 2021, 68, 152903 CrossRef CAS.
- S. Mohammed, R. A. Vishwakarma and S. B. Bharate, RSC Adv., 2015, 5, 3470–3473 RSC.
- A. Yoshimura, M. E. Jarvi, M. T. Shea, C. L. Makitalo, G. T. Rohde, M. S. Yusubov, A. Saito and V. V. Zhdankin, Eur. J. Org. Chem., 2019, 6682–6689 CrossRef CAS.
- (a) M. Xu, P. Wu, F. Shen, J. Ji and K. P. Rakesh, Bioorg. Chem., 2019, 91, 103133 CrossRef CAS PubMed; (b) M. Nazir, M. Saleem, M. I. Tousif, M. A. Anwar, F. Surup, I. Ali, D. Wang, N. Z. Mamadalieva, E. Alshammari, M. L. Ashour, A. M. Ashour, I. Ahmed, Elizbit, I. R. Green and H. Hussain, Biomolecules, 2021, 11, 957 CrossRef CAS PubMed; (c) T. Wood, K. Bertheussen and N. Martin, Org. Biomol. Chem., 2019, 18, 514–517 RSC.
- (a) S. J. Liu, Q. Zhao, C. Peng, Q. Mao, F. Wu, F. H. Zhang, Q. S. Feng, G. He and B. Han, Eur. J. Med. Chem., 2021, 217, 113359 CrossRef CAS PubMed; (b) N. Muthineni, N. S. Kumar, L. C. Rao, V. D. Kumar, S. Misra, L. R. Chowhan and H. M. Meshram, ChemistrySelect, 2016, 1, 4197–4202 CrossRef CAS; (c) V. Dočekal, S. Petrželová, I. Císařová and J. Veselý, Adv. Synth. Catal., 2020, 362, 2597–2603 CrossRef; (d) X. M. Hu, H. Dong, Y. D. Li, P. Huang, Z. Tian and P.-A. Wang, RSC Adv., 2019, 9, 27883–27887 RSC.
- H. Hopf, A. F. Mourad and P. G. Jones, Beilstein J. Org. Chem., 2010, 6, 68–71 CrossRef PubMed.
- (a) K. Zawadzińska, M. Ríos-Gutiérrez, K. Kula, P. Woliński, B. Mirosław, T. Krawczyk and R. Jasiński, Molecules, 2021, 26, 6774–6792 CrossRef; (b) R. Jasiński, E. Jasińska and E. Dresler, J. Mol. Model., 2016, 23, 13–21 CrossRef PubMed; (c) K. Kula and K. Zawadzińska, Curr. Chem. Lett., 2021, 10, 6–19 Search PubMed; (d) P. Wolinski, A. Kacka-Zych, O. M. Demchuk, A. Lapczuk-Krygier, B. Miroslaw and R. Jasinski, J. Clean. Prod., 2020, 275, 122086–122099 CrossRef CAS.
- (a) G. Mloston, K. Urbaniak, A. Linden and H. Heimgartner, Helv. Chim. Acta, 2015, 98, 453–461 CrossRef CAS; (b) A. Fryzlewicz, A. Kacka-Zych, O. M. Demchuk, B. Miroslaw, P. Wolinski and R. Jasinski, J. Clean. Prod., 2021, 292, 126079–126094 CrossRef CAS; (c) R. Jasinski, Comput. Theor. Chem., 2018, 1125, 77–85 CrossRef CAS; (d) R. Jasinski, RSC Adv., 2015, 5, 101045–101048 RSC; (e) R. Jasinski, Tetrahedron Lett., 2015, 56, 532–535 CrossRef CAS; (f) R. Jasinski, Monatsh. Chem., 2015, 146, 591–599 CrossRef CAS PubMed.
- T. Chau, H. Dhondt, M. Flipo, B. Déprez and N. Willand, Tetrahedron Lett., 2015, 56, 4119–4123 CrossRef.
- J. Shi, M. Ding, N. Luo, S. Wan, P. Li, J. Li and X. Bao, J. Agric. Food Chem., 2020, 68, 9613–9623 CrossRef CAS PubMed.
- J. Chen, C. Yi, S. Wang, S. Wu, S. Li, D. Hu and B. Song, Bioorg. Med. Chem. Lett., 2019, 29, 1203–1210 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2130131, 2130134 and 2153382. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2ra05009a |
| This journal is © The Royal Society of Chemistry 2022 |