Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Base-promoted highly efficient synthesis of nitrile-substituted cyclopropanes via Michael-initiated ring closure†
Min Ye*,
Fan Xu,
Yun Bai,
Fanglian Zhang,
Wenjia Wang,
Yiping Qian and
Zhengwang Chen *
*
Key Laboratory of Organo-pharmaceutical Chemistry of Jiangxi Province, Gannan Normal University, 341000, China. E-mail: yemin@gnnu.edu.cn; chenzwang2021@163.com; Fax: +86 797-8793670; Tel: +86 797-8793670
First published on 6th October 2022
Abstract
A convenient and efficient annulation reaction has been developed for the general synthesis of dinitrile-substituted cyclopropanes in moderate to excellent yields. A variety of 2-arylacetonitriles and α-bromoennitriles were compatible under the standard conditions. The reaction was achieved through tandem Michael-type addition followed by intramolecular cyclization. The preliminary application of this method was confirmed by the synthesis of the 2,4-dioxo-3-azabicyclo[3.1.0]hexane scaffold.
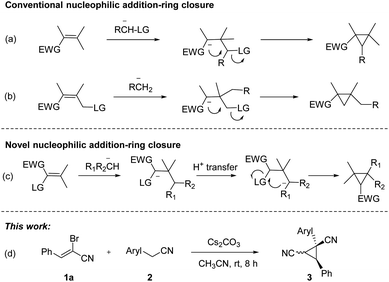
Substituted cyclopropanes, as attractive structural units, are commonly found in a variety of natural products and biologically active compounds.1 The strained structure, interesting bonding characteristics, and value as an internal mechanistic probe of the cyclopropane subunit have attracted the attention of the physical organic community.2 As a consequence, considerable efforts have been made to develop new and effective approaches toward cyclopropane derivatives.3 Classical approaches to cyclopropane synthesis are the Simmons–Smith cyclopropanation.4 Transition-metal-catalyzed cyclopropanation of alkenes with diazo compounds represents a direct protocol for their preparation.5 Furthermore, the new types of cyclopropanation reactions based on nucleophilic addition-ring closure sequence were well documented (Scheme 1a and b).6 Nitrile-substituted cyclopropanes are of great interest as they are versatile templates for the rapid formation of biologically active and synthetically useful functionalized cyclopropane derivatives.7 Recently, nitrile-substituted cyclopropanes were synthesized via transition-metal-catalyzed olefin functionalization with diazoacetonitriles.8 Despite the significant advancement, the development of complementary strategy toward functionalized cyclopropanes by using readily available substrates and cheap agents with high efficiency would be highly desirable.
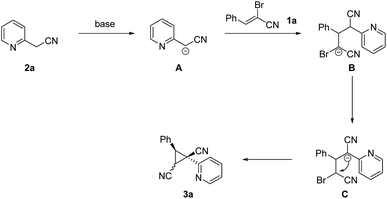
α-Bromoennitrile is a class of readily available intermediate in organic synthesis.9 However, this intermediate is rarely used in organic synthesis compared to its analog α-bromoenal.10 Recently, our group has reported a series of functionalization of 2-arylacetonitriles and their derivatives.11 We hypothesized five-membered nitrogen containing heterocycles could be formed from 2-pyridylacetonitrile and α-bromoennitriles via [3 + 2] annulation. However, dinitrile-substituted cyclopropanes were afforded through a novel Michael-initiated ring closure procedure (Scheme 1c). Herein, we present a base-promoted synthesis of dinitrile-substituted cyclopropanes from 2-arylacetonitriles and α-bromoennitriles under mild conditions via Michael-initiated ring closure (Scheme 1d).
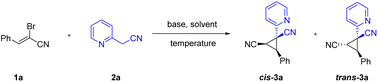
Initially, (Z)-2-bromo-3-phenylacrylonitrile 1a and 2-pyridylacetonitrile 2a were selected as the model substrates for the condition optimization. As illustrated in Table 1, a variety of commonly used organic and inorganic bases were screened. Among the tested organic bases, only DBU was found to afford the desired product in 38% yield (Table 1, entries 1–4). Then various inorganic bases were examined. All of the inorganic bases had some effect on the reaction. We were pleased to find Cs2CO3 was the most suitable base and furnished the corresponding product in 95% yield (Table 1, entries 5–9). The control experiment revealed that the base was indispensable for the cyclization reaction, no product was produced without the addition of the base (Table 1, entry 10). Having this promising result, we subsequently evaluated the effects of several solvents. Solvent screening indicated that besides MeCN, DMF also promoted this reaction, whereas the use of DMSO, H2O, DCE, THF and dioxane resulted in significantly lower yields (Table 1, entries 11–16). Finally, the reaction temperatures were investigated, and decreasing or increasing the temperatures led to diminishing yields (Table 1, entries 17–18). The cis/trans isomers ratio of the product was the same for 0° and room temperature. Therefore, base, solvent and reaction temperature are all essential for this transformation.
| Entry | Base | Solvent | Temp. | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.2 mmol) and base (1.5 equiv.) in solvent (1.0 mL) for 12 h.b Yields of isolated cis-3a and trans-3a are given. Cis refers the two nitriles positioned on the same face of the cyclopropane; trans refers the two nitriles positioned on the opposite face of the cyclopropane. | ||||
| 1 | DABCO | MeCN | rt | Trace |
| 2 | DBU | MeCN | rt | 38 |
| 3 | TEA | MeCN | rt | np |
| 4 | DMAP | MeCN | rt | np |
| 5 | Cs2CO3 | MeCN | rt | 95 |
| 6 | K2CO3 | MeCN | rt | 67 |
| 7 | NaOAc | MeCN | rt | 42 |
| 8 | K3PO4 | MeCN | rt | 89 |
| 9 | KOtBu | MeCN | rt | 84 |
| 10 | — | MeCN | rt | np |
| 11 | Cs2CO3 | DMF | rt | 87 |
| 12 | Cs2CO3 | DMSO | rt | 36 |
| 13 | Cs2CO3 | H2O | rt | np |
| 14 | Cs2CO3 | DCE | rt | 67 |
| 15 | Cs2CO3 | THF | rt | np |
| 16 | Cs2CO3 | Dioxane | rt | 53 |
| 17 | Cs2CO3 | MeCN | 0 | 62 |
| 18 | Cs2CO3 | MeCN | 50 | np |
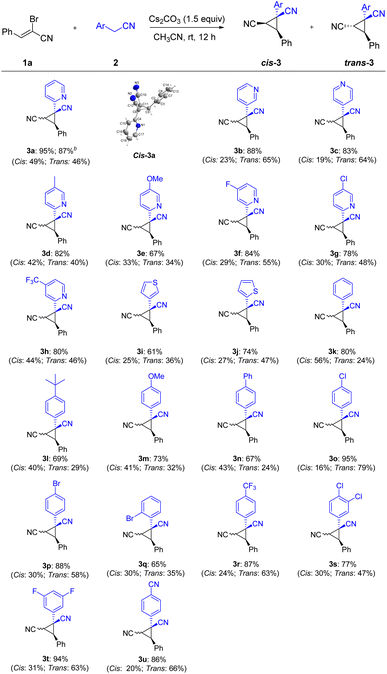
Having the developed optimal conditions for the Michael-initiated ring closure reaction, the substrate scope was investigated. As illustrated in Scheme 2, a wide range of 2-arylacetonitriles were tolerated with (Z)-2-bromo-3-phenylacrylonitrile 1a to render dinitrile-substituted cyclopropanes in moderate to excellent yields (3a–3u). Except for 2-pyridylacetonitrile, 3-pyridyl and 4-pyridyl derivatives also reacted smoothly to generate the products in good yields (3a–3c). The annulation with 2-pyridylacetonitrils bearing electron-donating groups and withdrawing groups in the pyridine ring worked well to deliver the products in satisfactory yields (3d–3h). Thienyl derivatives were reactive to afford the corresponding products, but exhibited lower reactivity compared with pyridyl (3i–3j). In addition to heteroaryl-substituted substrates, various 2-arylacetonitriles were further tested. The reaction conditions were compatible with an array of substituents, such as alkyl, methoxy, phenyl, chloro, bromo, trifluoromethyl, fluoro, and cyano groups (3k–3u). In particular, the aryl bromide could be further functionalized in metal-catalyzed cross-coupling reactions and hold the enormous potential application in pharmaceutical and materials science (3p–3q). To our delight, nitrile-containing substrate could provide the product 3u in 86% yield. Significantly, the annulation reaction could be carried out on large-scale synthesis and formed the product 3a in 87% yield. The structure of cis-3a was verified by X-ray crystal analysis (CCDC: 2141258†).
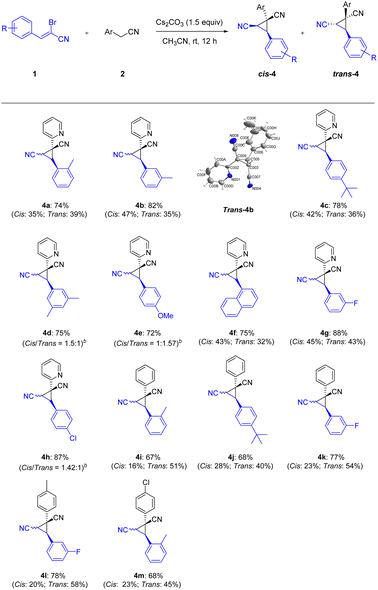
Continuing to examine the generality and scope of the annulation reaction, we explored various α-bromoennitriles under the standard conditions (Scheme 3). α-Bromoennitriles bearing electron-rich or electron-deficient groups on the benzene ring reacted successfully with 2-pyridylacetonitrile to achieve the desired products in good yields (4a–4h). The substrates bearing a methyl at the ortho- and meta-positions of the benzene ring were suitable substrates for the transformation, thus indicating the steric hindrance is negligible (4a–4b). The bulky tert-butyl group was accommodated in this transformation (4c and 4j). The disubstituted α-bromoennitrile proved to be good substrate under the same reaction conditions (4d). The reaction of the fused ring system also yielded the products in satisfactory yields (4f). Finally, phenylacetonitriles also worked well with α-bromoennitriles to obtain the corresponding product in good yields (4i–4m). The structure of trans-4b was further confirmed by X-ray crystal diffraction measurements (CCDC: 2142244†). It implied that this Michael-initiated ring closure reaction can be effective for the construction of dinitrile-substituted cyclopropane library.
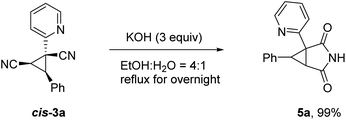
To illustrate the applicability of this reaction, further transformation of product cis-3a was carried out as depicted in Scheme 4. 2,4-Dioxo-3-azabicyclo[3.1.0]hexane scaffold is known to be an important pharmacology agent and synthons for synthesis of functionally substituted cyclopropanes and various spirocompounds.12 The target compound 5a can be readily accessible in excellent yield via a simple hydrolysis reaction. It is worth noting that similar result was obtained for trans-3a.
A tentative mechanism for cyclopropane formation was proposed and outlined in Scheme 5 on the basis of aforementioned results as well as our experimental observations. Initially, carbanion intermediate B was produced via the sequential extraction of hydrogen proton and Michael-type addition process. Then the intermediate B was converted into intermediate C through 1,3-hydride transfer. Finally, the dinitrile-substituted cyclopropane 3a was formed through intramolecular nucleophilic substitution. The diastereomer 3a is the favored product due to steric effects, in which the two aryl groups are located on the opposite face of the plane of the cyclopropane moiety.
In summary, we have explored a convenient and highly efficient annulation reaction of 2-arylacetonitriles and α-bromoennitriles. A wide range of dinitrile-substituted cyclopropanes were obtained in moderate to excellent yields through a novel Michael-initiated ring closure procedure. The advantages of this transformation include readily accessible substrates, transition-metal-free conditions, good functional group tolerance, simple operation, etc. In addition, nitrile-substituted products have potential applications in synthetic and pharmaceutical chemistry. Further synthetic utilization and asymmetric transformations are currently ongoing in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the NSFC (21762003, 21967002), the NSF of Jiangxi Provincial (20212ACB213002, 20212BAB213011) and the NSF of Jiangxi Provincial Education Department (GJJ201422). We acknowledge the Analytical & Testing Center of Beijing Normal University for the high-resolution mass spectrometry analyses.Notes and references
- (a) L. A. Wessjohann, W. Brandt and T. Thiemann, Chem. Rev., 2003, 103, 1625 CrossRef CAS PubMed; (b) C. A. Carson and M. A. Kerr, Chem. Soc. Rev., 2009, 38, 3051 RSC; (c) D. Y. K. Chen, R. H. Pouwer and J.-A. Richard, Chem. Soc. Rev., 2012, 41, 4631 RSC; (d) T. T. Talele, J. Med. Chem., 2016, 59, 8712 CrossRef CAS.
- (a) Z. Goldschmidt and B. Crammer, Chem. Soc. Rev., 1988, 17, 229 RSC; (b) H. N. C. Wong, M. Y. Hon, C. W. Tse, Y. C. Yip, J. Tanko and T. Hudlicky, Chem. Rev., 1989, 89, 165 CrossRef CAS; (c) D. C. Nonhebel, Chem. Soc. Rev., 1993, 22, 347 RSC; (d) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117 CrossRef CAS.
- (a) F. Gnad and O. Reiser, Chem. Rev., 2003, 103, 1603 CrossRef CAS; (b) H. Lebel, J.-F. Marcoux, C. Molinaro and A. B. Charette, Chem. Rev., 2003, 103, 977 CrossRef CAS; (c) H. Pellissier, Tetrahedron, 2008, 64, 7041 CrossRef CAS; (d) M. J. Campbell, J. S. Johnson, A. T. Parsons, P. D. Pohlhaus and S. D. Sanders, J. Org. Chem., 2010, 75, 6317 CrossRef CAS; (e) G. Bartoli, G. Bencivenni and R. Dalpozzo, Synthesis, 2014, 46, 979 CrossRef.
- (a) H. E. Simmons and R. D. Smith, J. Am. Chem. Soc., 1958, 80, 5323 CrossRef CAS; (b) H. E. Simmons and R. D. Smith, J. Am. Chem. Soc., 1959, 81, 4256 CrossRef CAS.
- (a) R. P. Wurz and A. B. Charette, Org. Lett., 2002, 4, 4531 CrossRef CAS; (b) W. Lin and A. B. Charette, Adv. Synth. Catal., 2005, 347, 1547 CrossRef CAS; (c) B. Morandi and E. M. Carreira, Angew. Chem., Int. Ed., 2010, 49, 938 CrossRef CAS; (d) B. Morandi, B. Mariampillai and E. M. Carreira, Angew. Chem., Int. Ed., 2011, 50, 1101 CrossRef CAS.
- (a) C. D. Papageorgiou, S. V. Ley and M. J. Gaunt, Angew. Chem., Int. Ed., 2003, 42, 828 CrossRef CAS; (b) N. Bremeyer, S. C. Smith, S. V. Ley and M. J. Gaunt, Angew. Chem., Int. Ed., 2004, 43, 2681 CrossRef CAS; (c) C. D. Papageorgiou, M. A. Cubillo de Dios, S. V. Ley and M. J. Gaunt, Angew. Chem., Int. Ed., 2004, 43, 4641 CrossRef CAS; (d) R. K. Kunz and D. W. C. MacMillan, J. Am. Chem. Soc., 2005, 127, 3240 CrossRef CAS PubMed; (e) H. M. Hansen, D. A. Longbottom and S. V. Ley, Chem. Commun., 2006, 46, 4838 RSC; (f) C. C. C. Johansson, N. Bremeyer, S. V. Ley, D. R. Owen, S. C. Smith and M. J. Gaunt, Angew. Chem., Int. Ed., 2006, 45, 6024 CrossRef CAS; (g) S. H. McCooey, T. McCabe and S. J. Connon, J. Org. Chem., 2006, 71, 7494 CrossRef CAS; (h) R. Rios, H. Sundén, J. Vesely, G.-L. Zhao, P. Dziedzic and A. Córdova, Adv. Synth. Catal., 2007, 349, 1028 CrossRef CAS; (i) H. Xie, L. Zu, H. Li, J. Wang and W. Wang, J. Am. Chem. Soc., 2007, 129, 10886 CrossRef CAS PubMed.
- (a) D. L. Boger and C. E. Brotherton, J. Am. Chem. Soc., 1986, 108, 6695 CrossRef CAS; (b) X.-Q. Zhu, H.-Y. Wang, J.-S. Wang and Y.-C. Liu, J. Org. Chem., 2001, 66, 344 CrossRef CAS PubMed; (c) S. K. Bagal, M. de Greef and S. Z. Zard, Org. Lett., 2006, 8, 147 CrossRef CAS PubMed; (d) F. Brackmann and A. de Meijere, Chem. Rev., 2007, 107, 4538 CrossRef CAS PubMed; (e) F. Couty, O. David, B. Larmanjat and J. Marrot, J. Org. Chem., 2007, 72, 1058 CrossRef CAS PubMed.
- (a) J. R. Denton, K. Cheng and H. M. L. Davies, Chem. Commun., 2008, 10, 1238 RSC; (b) D. Marcoux, S. Azzi and A. B. Charette, J. Am. Chem. Soc., 2009, 131, 6970 CrossRef CAS; (c) S. Zhu, X. Xu, J. A. Perman and X. P. Zhang, J. Am. Chem. Soc., 2010, 132, 12796 CrossRef CAS PubMed; (d) K. J. Hock, R. Spitzner and R. M. Koenigs, Green Chem., 2017, 19, 2118 RSC; (e) A. L. Chandgude and R. Fasan, Angew. Chem., Int. Ed., 2018, 57, 15852 CrossRef CAS PubMed.
- T. P. M. Goumans, K. van Alem and G. Lodder, Eur. J. Org. Chem., 2008, 2008, 435 CrossRef.
- (a) F. Xia, X.-Y. Chen and S. Ye, J. Org. Chem., 2018, 83, 15178 CrossRef CAS; (b) K.-Q. Chen, Z.-H. Gao and S. Ye, Angew. Chem., Int. Ed., 2019, 58, 1183 CrossRef CAS; (c) S. Jin, S. Fang, R. Ma, Z. Liang, Y. Xu, T. Lu and D. Du, Org. Chem. Front., 2019, 6, 3392 RSC; (d) C. Liu, S. Wu, J. Xu, L. Chen, P. Zheng and Y. R. Chi, Org. Lett., 2019, 21, 9493 CrossRef CAS; (e) S. Sun, M. Lang and J. Wang, Adv. Synth. Catal., 2019, 361, 5704 CrossRef CAS; (f) Z. Chen, W. Jin, Y. Xia, Y. Zhang, M. Xie, S. Ma and C. Liu, Org. Lett., 2020, 22, 8261 CrossRef CAS PubMed; (g) Y. Xie, L. Li, S. Sun, Z. Wu, M. Lang, D. Jiang and J. Wang, Org. Lett., 2020, 22, 391 CrossRef CAS; (h) P. Jiang, J. Guo, M. Gong, X. Zhou, W. Cao, Z. Fu and W. Huang, Org. Biomol. Chem., 2021, 19, 4882 RSC; (i) B. Mondal, R. Maiti, X. Yang, J. Xu, W. Tian, J.-L. Yan, X. Li and Y. R. Chi, Chem. Sci., 2021, 12, 8778 RSC.
- (a) C. Lu, M. Ye, L. Long, Y. Zheng, J. Liu, Y. Zhang and Z. Chen, J. Org. Chem., 2022, 87, 1545 CrossRef CAS PubMed; (b) C. Lu, M. Ye, M. Li, Z. Zhang, Y. He, L. Long and Z. Chen, Chin. Chem. Lett., 2021, 32, 3967 CrossRef CAS; (c) J. Zheng, L. Chen, X. Liu, W. Xu, Y. Wang, Q. He, H. Liu, M. Ye, G. Luo and Z. Chen, ChemistrySelect, 2020, 5, 13198 CrossRef CAS; (d) Z. Chen, P. Liang, F. Xu, Z. Deng, L. Long, G. Luo and M. Ye, J. Org. Chem., 2019, 84, 12639 CrossRef CAS.
- (a) J. W. Epstein, H. J. Brabander, W. J. Fanshawe, C. M. Hofmann, T. C. McKenzie, S. R. Safir, A. C. Osterberg, D. B. Cosulich and F. M. Lovell, J. Med. Chem., 1981, 24, 481 CrossRef CAS; (b) M. G. Rowlands, M. A. Bunnett, A. B. Foster, M. Jarman, J. Stanek and E. Schweizer, J. Med. Chem., 1988, 31, 971 CrossRef CAS PubMed; (c) T. Polonski, M. J. Milewska and A. Katrusiak, J. Org. Chem., 1993, 58, 3411 CrossRef CAS; (d) F. Micheli, P. Cavanni, R. Arban, R. Benedetti, B. Bertani, M. Bettati, L. Bettelini, G. Bonanomi, S. Braggio, A. Checchia, S. Davalli, R. Di Fabio, E. Fazzolari, S. Fontana, C. Marchioro, D. Minick, M. Negri, B. Oliosi, K. D. Read, I. Sartori, G. Tedesco, L. Tarsi, S. Terreni, F. Visentini, A. Zocchi and L. Zonzini, J. Med. Chem., 2010, 53, 2534 CrossRef CAS PubMed; (e) H. Wang and R. Fan, J. Org. Chem., 2010, 75, 6994 CrossRef CAS PubMed; (f) A. N. Vereshchagin, M. N. Elinson, E. O. Dorofeeva, D. V. Demchuk, I. S. Bushmarinov, A. S. Goloveshkin and G. I. Nikishin, Tetrahedron, 2013, 69, 5234 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, characterization of all compounds, copies of 1H and 13C NMR spectra for selected compounds. CCDC 2141258 and 2142244. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ra05393d |
| This journal is © The Royal Society of Chemistry 2022 |