Open Access Article
Open Access ArticleTailoring the phosphorus release from biochar-based fertilizers: role of magnesium or calcium addition during co-pyrolysis†
Kaewta Jetsrisuparbab,
Thanawan Jeejailaa,
Chanon Saengthipa,
Pornnapa Kasemsiriab,
Yuvarat Ngernyena,
Prinya Chindaprasirtbc and
Jesper T. N. Knijnenburg *bd
*bd
aDepartment of Chemical Engineering, Khon Kaen University, Khon Kaen 40002, Thailand
bSustainable Infrastructure Research and Development Center, Khon Kaen University, Khon Kaen 40002, Thailand. E-mail: jespth@kku.ac.th
cDepartment of Civil Engineering, Khon Kaen University, Khon Kaen 40002, Thailand
dInternational College, Khon Kaen University, Khon Kaen 40002, Thailand
First published on 26th October 2022
Abstract
The presence of magnesium (Mg) and calcium (Ca) in biochar-based fertilizers is linked to the slow release of phosphorus (P), but these alkali metals have not been systematically compared under identical conditions. In this study, sugarcane filter cake was treated with H3PO4 and MgO or CaO followed by pyrolysis at 600 °C to produce a Mg/P-rich biochar (MgPA-BC) and a Ca/P-rich biochar (CaPA-BC), respectively. The P-loaded biochars were studied by extraction and kinetic release in water over 240 hours to assess the potential P availability. X-ray diffraction and Fourier-transform infrared (FTIR) spectroscopy were used to characterize the pristine and post-kinetics biochars to identify the responsible phases for phosphate release. Additionally, the dissolved P concentrations in the kinetic release experiment were compared to thermodynamic solubility calculations of common Mg and Ca phosphates. Both MgPA-BC and CaPA-BC had P loadings of 73–74 g kg−1 but showed distinctly different release behaviors. Phosphate dissolution from MgPA-BC was gradual and reached 10 g P per kg biochar after 240 hours, with rate-determining phases being Mg2P2O7 (Mg pyrophosphate), MgNH4PO4·6H2O (struvite), and Mg3(PO4)2·22H2O (cattiite). In contrast, CaPA-BC only released 1.2 g P per kg biochar. Phosphate release from CaPA-BC was limited by the low solubility of Ca2P2O7 (Ca pyrophosphate) and (Ca,Mg)3(PO4)2 (whitlockite). Co-pyrolysis with MgO retained P in a more soluble and available form than CaO, making MgO a preferential additive over CaO to immobilize phytoavailable P in biochar-based fertilizers with higher fertilizer effectiveness.
1. Introduction
Phosphorus (P) is an element that is indispensable for living organisms. Globally, many soils have low available P concentrations, and water-soluble P sources have been excessively applied to meet crop requirements. However, only a fraction of the applied P is taken up, and most of it is lost to surface waters through runoff or bound to soil in unavailable forms.1 Ideally, the P release from fertilizers should be synchronized with crop demands in order to improve fertilizer use efficiency and minimize negative environmental impacts, which can be successfully realized in the form of slow release fertilizers.2Thailand is the world's 4th largest producer of sugarcane with a production rate of over 100 million tons of sugarcane per year.3 The main solid waste product in the sugar production process is sugarcane filter cake, a nutrient-rich residue that is left behind when cane juice is filtered. Each ton of crushed sugarcane produces 30–40 kg filter cake.4 Due to its high nutrient content (1–2% of each nitrogen (N) and P per dry weight), the filter cake is often directly used as fertilizer,4 but this may result in eutrophication due to losses of water-soluble nutrients like N and P to surface waters.5
Biochar is a porous carbon-rich material that is produced by thermal treatment of biomass in an oxygen-limited environment. Soil application of biochars can provide (long-term) financial gains to farmers,6,7 and the conversion of sugarcane filter cake into a stable biochar presents an attractive solution to convert this waste into a valuable nutrient-rich soil amendment.8–10 Compared to the raw biomass, biochars have a higher P content in a less water-soluble form with reduced leaching.11,12 Especially the presence of calcium (Ca) and magnesium (Mg) aid to immobilize the P into forms that do not rapidly dissolve in water but are still available to crops.13,14 The conversion of a waste biomass such as sugarcane filter cake into a biochar may thus present an attractive slow release P fertilizer.
To further increase the P loading, biochars are frequently treated with a P source either before or after pyrolysis, and especially pre-pyrolysis treatment of the biomass with P in combination with Ca and/or Mg can produce slow release fertilizers.15 Sugarcane leaves treated with MgO and a P source followed by pyrolysis at 600 °C produced biochar-based fertilizers that presented slow P release in water. Without MgO, the acidic P was rapidly released, highlighting the essential presence of Mg.16 Similarly, the presence of Ca and Mg greatly reduced the water solubility of P when poultry litter was co-pyrolyzed with various P sources, with and without the addition of MgO.17 Biochar-based fertilizers synthesized by co-pyrolysis of cotton straw with bentonite and K3PO4 under microwave irradiation had slow release of P and K in soil and were effective in increasing the growth of pepper seedlings in a pot trial.18 Co-pyrolysis of sewage sludge (a P-rich waste) with CaO produced a biochar that could promote the growth of rice seedlings in hydroponic experiments.19 Sewage sludge biochars modified with MgCl2, CaO and MgO (but not CaCl2) were effective at adsorbing P from solution. The Mg-modified biochars had a higher P release rate than the CaO-modified biochar. Adsorbed P was suggested to be rapidly released, whereas the inherent P in the biochars was released more slowly.20 Also the co-pyrolysis of sawdust and switchgrass with triple superphosphate or bone meal at 500 °C produced P-loaded biochars with slow P release kinetics in water.21
In these previous studies, the gradual P release from biochar-based fertilizers has been linked to the presence of poorly soluble Mg and/or Ca phosphate phases. However, the differences in conditions used (e.g., pyrolysis temperature, raw material, and contents of Mg, Ca and P) make it difficult to directly compare the roles of Mg and Ca. Moreover, few studies have investigated the changes in the biochar during phosphate dissolution process,17 and dissolved P concentrations have been rarely compared with thermodynamic solubility values of the phosphates.22 In this work, sugarcane filter cake was pre-treated with H3PO4 and either MgO or CaO, and subsequently pyrolyzed at 600 °C. The P-loaded biochars were studied for their extractable P concentrations in 2% formic acid and deionized (DI) water, and the phosphate release kinetics were evaluated in DI water over 240 hours. To identify the phosphate release mechanism, the pristine and post-kinetics biochars were characterized by X-ray diffraction and FTIR spectroscopy. The proposed formation and dissolution mechanism of the formed Ca/Mg phosphate phases in the biochars supported by thermodynamic solubility calculations is presented.
2. Materials and methods
2.1 Materials
Dried sugarcane filter cake was obtained from Mitr Phol sugar factory (Chaiyaphum province, Thailand). The as-received filter cake was milled by ball mill to obtain a homogeneous powder. Phosphoric acid (H3PO4, ≥85%, RCI Labscan, Thailand), magnesium oxide (MgO, ≥97%, RCI Labscan, Thailand), calcium oxide (CaO, ≥90%, Kemaus, Australia), formic acid (99%, APS Chemicals, New Zealand), and ammonium persulfate (98%, Loba Chemie, India) were used as received. All solutions were prepared with DI water (18.2 MΩ).2.2 Preparation of biochars
To prepare the modified biochars, the dry filter cake powder was first manually mixed with a given amount of MgO or CaO until a homogeneous mixture was obtained. Each solid mixture was then immersed in a solution of 1 M H3PO4. Caution was taken when adding the H3PO4 solution because the reaction of H3PO4 with MgO and especially with CaO was highly exothermic.23 The pre-treatment with H3PO4 in combination with MgO or CaO was carried out at a P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) biomass ratio of 1
biomass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (w/w) and the atomic ratios of respectively P
12 (w/w) and the atomic ratios of respectively P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg or P
Mg or P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca were 1
Ca were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.16,17 After drying, the pre-treated biomasses (200–300 g per batch) were packed into enclosed clay pots and pyrolysis was done in a muffle furnace at 600 °C for 3 h (heating rate of 6 °C min−1) based on our previous study.16 The biochar yield was calculated by dividing the mass of the produced biochar by the mass of the treated filter cake prior to pyrolysis. An unmodified biochar was prepared under the same pyrolysis conditions.
1.16,17 After drying, the pre-treated biomasses (200–300 g per batch) were packed into enclosed clay pots and pyrolysis was done in a muffle furnace at 600 °C for 3 h (heating rate of 6 °C min−1) based on our previous study.16 The biochar yield was calculated by dividing the mass of the produced biochar by the mass of the treated filter cake prior to pyrolysis. An unmodified biochar was prepared under the same pyrolysis conditions.
Three biochars were produced: (i) unmodified filter cake biochar (BC), (ii) filter cake biochar pre-treated with H3PO4 and MgO (MgPA-BC), and (iii) filter cake biochar pre-treated with H3PO4 and CaO (CaPA-BC).
2.3 Characterization of biochars
The ash contents of the biochar were determined in triplicate by ashing 0.2 g biochar at 500 °C for 8 h. Nitrogen adsorption experiments were carried out at 77 K on a Micromeritics ASAP 2460 (Micromeritics, USA) by measuring a 41-point adsorption–desorption isotherm for each sample. The samples were degassed overnight under vacuum at 200 °C prior to analysis. The BET specific surface area (SBET) was determined at a relative pressure (P/P0) range of 0.05–0.25, the total pore volume (VT) was determined at P/P0 ≥ 0.95, the micropore volume (Vmicro) was obtained from the t-plot, and the average pore diameter (Dp) was calculated with the BJH method using desorption data.X-ray diffraction (XRD) measurements were carried out on a PANalytical EMPYREAN diffractometer (PANalytical B.V., the Netherlands) with Cu Kα radiation operating at 40 kV and 45 mA. Patterns were collected over 2θ = 5–70° with step size 0.02 s−1. The crystalline phases were identified using X'Pert HighScore Plus software (PANalytical B.V., the Netherlands). Fourier transform infrared (FTIR) spectra were collected on a Bruker Alpha II ATR-FTIR (Bruker, Germany) at 4000–600 cm−1 (32 scans, resolution 2 cm−1). Scanning electron microscopy (SEM) analysis was done on a Hitachi SU3800 (Hitachi, Japan) after sputter-coating the samples with gold.
The total metal (Ca, Mg, Fe, and K) and P contents of the biochars were determined in triplicate after digesting the biochars by modified dry ashing.24 After filtration, the Ca, Mg, Fe, and K concentrations were measured by flame atomic absorption spectroscopy on a PinAAcle 900F (Perkin Elmer, Singapore) after dilution in 1% HNO3. For Ca and Mg measurements, each standard and sample solution contained 1000 ppm Sr to eliminate interferences. The P concentrations were measured by UV-vis spectroscopy (Agilent 8453, Agilent Technologies, USA) via the molybdenum blue method at 880 nm.25
The extractable P of the biochars was evaluated in DI water and 2% formic acid according to the modified method of Wang et al.26 The suspensions (0.3 g biochar in 30 mL extractant) were ultrasonicated for 10 min and placed on an orbital shaker (Gallenkamp, UK) at 120 rpm. After 30 min, the suspensions were filtered through a 0.22 μm nylon filter and the extracted P concentrations were measured by UV-vis spectroscopy via the molybdenum blue method. The pH values of the extracts in DI water were recorded with a digital pH meter (OHAUS Starter 3100, OHAUS Corporation, USA), and the average value for each sample was reported as the biochar pH.16
2.4 Phosphorus release from biochar-based fertilizers
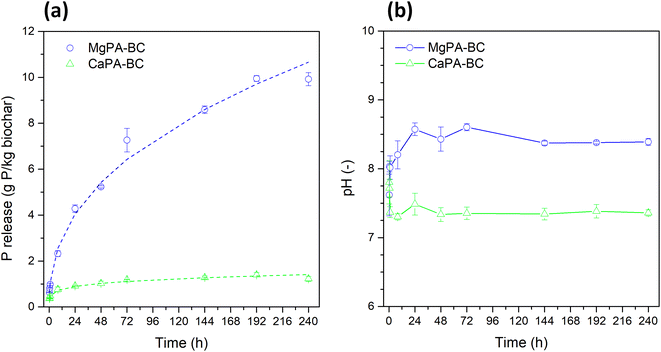
The slow release behavior of phosphate from MgPA-BC and CaPA-BC was studied in triplicate.16,27 The suspensions (1.0 g biochar in 200 mL DI water) were gently mixed (100 rpm) on an orbital shaker (Gallenkamp, UK) in a closed Erlenmeyer flask for 240 hours at room temperature. Periodically, a 2 mL sample was filtered (nylon filter, 0.22 μm) and the dissolved phosphate concentrations were determined with UV-vis spectroscopy via the molybdenum blue method. The solution pH was measured at each collection point after sample withdrawal.The following models were used to describe the phosphate dissolution kinetics: zero order, pseudo-first order, pseudo-second order, Elovich, parabolic diffusion, and power function (Table S1 in ESI†). The suitability of each model to describe the release behavior was assessed by the coefficient of determination (R2) and the standard error of the estimate (SE).16
At the end of the kinetic release experiment (after 240 hours) the biochars were collected by filtration (Whatman no. 42, GE Healthcare Life Sciences, UK), rinsed with DI water, dried for 24 hours at 40 °C, and subsequently analyzed by XRD and FTIR spectroscopy as described previously. These post-kinetics biochars are referred to as MgPA-BC-post and CaPA-BC-post. The Ca and Mg contents in the filtrate after 240 hours were measured by AAS as described previously. Additionally, the solution after 240 hours was digested with ammonium persulfate according to the procedure of Huang and Zhang28 with slight modifications. Each solution was mixed with 6 mL DI water and 1.2 mL of a 4.2% ammonium persulfate solution in a 10 mL screw-cap vial. The vials were tightly closed and digestion was carried out in an oven (Biobase BOV-V45F, Biobase Bioindustry Co. Ltd, China) for 16 hours at 90 °C. The P concentration of each sample after persulfate digestion was determined by UV-vis spectroscopy via the molybdenum blue method.
2.5 Thermodynamic solubility calculations
The solubility of various Mg and Ca phosphates as function of pH was calculated with ChemEQL software (version 3.2)29 assuming ideal conditions. The solubility products of the used phases are given in Table S2 (ESI).†3. Results and discussion
3.1 General properties of the biochars
Selected properties of the produced sugarcane filter cake biochars are summarized in Table 1. The yield of the unmodified sugarcane filter cake biochar (BC) was 64.3%, which is higher than other biochars produced under comparable conditions.30 The high yield may be due to the high ash content (77.5%) that indicated a substantial content of inorganic material. Previous studies on sugarcane filter cake biochars reported a yield of 42–68% and ash contents of 52–58% but at lower pyrolysis temperatures (350–380 °C).10,31 Modification of the biochar with MgO and H3PO4 decreased the yield to 59.9% for MgPA-BC, as also observed previously for sugarcane leaf biochar.16 In contrast, modification with CaO and H3PO4 increased the yield of CaPA-BC to 68.4%, which may be due to the higher atomic mass of Ca compared to Mg. The ash contents in MgPA-BC and CaPA-BC increased to 80.9–82.6% due to the introduction of Mg/Ca and P into the powders. The pH of the biochars ranged from 7.0 to 8.1, in agreement with previous studies.10,32 Compared to BC, the lower pH of MgPA-BC and CaPA-BC may have been due to the presence of free acid groups from the pretreatment of the filter cake with H3PO4.| Sample | Yield (%) | pH | Ash (wt%) | Ca (g kg−1) | Mg (g kg−1) | Fe (g kg−1) | K (g kg−1) | P (g kg−1) | P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg Mg |
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca Ca |
|---|---|---|---|---|---|---|---|---|---|---|
| BC | 64.3 | 8.1 | 77.5 ± 2.6 | 15.8 ± 1.2 | 2.6 ± 0.2 | 10.9 ± 1.1 | 3.1 ± 0.3 | 12.0 ± 1.1 | 3.7 | 1.0 |
| MgPA-BC | 59.9 | 7.7 | 80.9 ± 0.9 | 0.9 ± 0.1 | 40.9 ± 2.7 | 6.0 ± 0.8 | 2.0 ± 0.2 | 74.1 ± 7.3 | 1.4 | 110.5 |
| CaPA-BC | 68.4 | 7.0 | 82.6 ± 1.1 | 92.1 ± 4.6 | 2.4 ± 0.1 | 6.9 ± 0.7 | 2.3 ± 0.4 | 73.4 ± 2.6 | 24.2 | 1.0 |
Sample BC contained small amounts of Ca (15.8 g kg−1), Fe (10.9 g kg−1), K (3.1 g kg−1), and Mg (2.6 g kg−1), which were similar to previous studies on sugarcane filter cake biochars.10,32 In the treated biochars, the concentrations of Fe and K decreased to 6.0–6.9 g kg−1 and 2.0–2.3 g kg−1, respectively, likely due to leaching during pretreatment and dilution due to additive addition. As expected, the Mg content in MgPA-BC increased greatly to 40.9 g kg−1. This Mg content was higher than that of sugarcane leaf biochars16 modified with Mg and P but lower than other Mg-modified biochars.17 The Ca content in MgPA-BC conversely decreased to 0.9 g kg−1, which could have been caused by the leaching of Ca in the H3PO4 pre-treatment solution. Upon pre-treatment with CaO and H3PO4, the Ca content in CaPA-BC greatly increased to 92.1 g kg−1, similar to previous works.17,19
The P content of BC was 12.0 g kg−1 which is typical for sugarcane filter cake biochars,10,32 and increased to 73.4–74.1 g kg−1 for the modified biochars. Such P loadings are comparable to previous works.16,17,21,33 The modified biochars had an approximately equimolar ratio of P to additive cation: the P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg ratio in MgPA-BC was 1.4, and the P
Mg ratio in MgPA-BC was 1.4, and the P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca ratio in CaPA-BC was 1.0.
Ca ratio in CaPA-BC was 1.0.
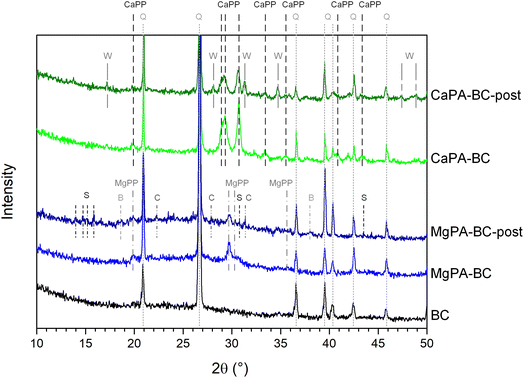
Biochar morphology was studied by scanning electron microscopy (Fig. 1). The unmodified filter cake biochar (BC) was heterogeneous with particles originating from plant fibers in the sugarcane and angular particles that were likely inorganic compounds.5 Compared to BC, the particle size of MgPA-BC was smaller which may have been a result of the pre-treatment with H3PO4. The MgPA-BC consisted of agglomerates of crystalline particles, which were likely a Mg phosphate such as Mg2P2O7.16 In contrast to MgPA-BC, CaPA-BC was more agglomerated and showed the presence of needle-like or tabular particles, possibly Ca2P2O7.34
 | ||
| Fig. 1 Scanning electron microscopy (SEM) images of (a) BC, (b) MgPA-BC, and (c) CaPA-BC at 1000× magnification. | ||
3.2 Specific surface area and porosity
The unmodified sugarcane filter cake biochar (BC) had a specific surface area (SBET) of 32.9 m2 g−1 (Table 2). This SBET was lower than plant-derived biochars produced under similar conditions but comparable to biochars derived from biosolids30 and sugarcane filter cake biochars produced under similar conditions.8,9 Both pre-treatments slightly increased the SBET and VT, and slightly decreased the average pore diameter (Dp), suggesting a small promoting effect of the additives on biochar pore formation. The N2 adsorption experiment demonstrated that the pores consisted of only 14–18% of micropores. Generally, biochars with high ash contents (as is the case here) have low porosity because pore development largely depends on the release of volatile matter during pyrolysis.35| Material | SBET (m2 g−1) | Vmicro (cm3 g−1) | VT (cm3 g−1) | Dp (nm) |
|---|---|---|---|---|
| BC | 32.9 | 0.010 (18%) | 0.054 | 11.7 |
| MgPA-BC | 43.6 | 0.011 (14%) | 0.079 | 7.8 |
| CaPA-BC | 42.6 | 0.011 (16%) | 0.071 | 9.9 |
3.3 Extractable P
The slow P release potential of the biochars was assessed by measuring the extractable P in 2% formic acid (FA) and DI water (DIW) (Fig. 2). Here, FA was used as extraction solution because it provides a good indication of P phytoavailability from high-ash biochars.26 All biochars contained a very small amount of DIW-extractable P (<1 g kg−1) that accounted for only 1–2% of the total P present in those materials, comparable to previous studies.12,19 Since the P release from such biochars is proton-promoted, the alkaline nature of the biochars (pH 7.0–8.1, Table 1) likely limited the P release in water.12 In BC, the FA-extractable P was 8.4 g kg−1, which accounted for 70% of the total P (12 g kg−1, Table 1), indicating that the majority of the P in BC was available to crops. The total P loading, however, was low.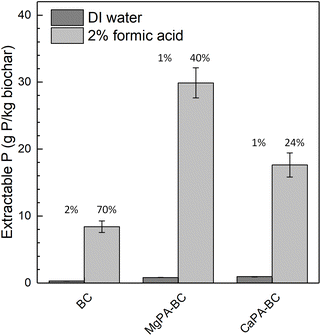 | ||
| Fig. 2 Extractable P from the biochars in DI water (DIW) and 2% formic acid (FA). The values above the bars indicate the percentage of the total P that is extracted with each extraction solution. | ||
Modification of the biochars greatly increased the FA-extractable P, ranging from 18 g kg−1 (CaPA-BC) to 30 g kg−1 (MgPA-BC). These values accounted for 24–40% of the total P in those materials, in agreement with previous studies.16,26 Compared to Ca, particularly the modification with Mg-containing pre-treatments enhanced the P extractability; the percentage of total P in MgPA-BC that was FA-extractable (40%) was almost twice as large as that of CaPA-BC (24%). This may suggest that the P in MgPA-BC is more available to crops than that in CaPA-BC.
All biochars had a much higher FA-extractable P compared to the DIW-extractable P, which indicated the potential for slow release. Based on these results, both MgPA-BC and CaPA-BC were considered promising materials for slow P release fertilizers and were subsequently tested in a 240 hour release study in water.
3.4 Phosphate slow release behavior
The slow release behavior of MgPA-BC and CaPA-BC was evaluated in DI water over 240 hours. The phosphate release (Fig. 3a) and pH of the solutions at each time point (Fig. 3b) showed clear differences between the two materials. The phosphate release from MgPA-BC was initially small (only 1 g P per kg biochar after 1 hour), but this gradually increased to 4 g kg−1 after 24 hours, and to 10 g kg−1 after 240 hours. During dissolution, the pH was slightly alkaline and reached a value of 8.4 after 240 hours. In contrast, the phosphate release from CaPA-BC was much smaller; after 24 hours, only 0.9 g kg−1 P was released, which only slightly increased to 1.2 g kg−1 for 144 hours and then remained constant. The pH of the solution was lower as well and stabilized around 7.4. The higher phosphate release from MgPA-BC compared to CaPA-BC was in agreement with Fang et al.20Six models were used to assess the phosphate release from the modified biochars (Table S1 in ESI†). Based on the R2 and SE values, the phosphate release from both MgPA-BC and CaPA-BC was best described by the power function (Table 3). In previous studies, phosphate release from other biochars also followed the power function,21,22 but also parabolic diffusion,16,21 Elovich,17 and even zero order equation12 have been used. The constants for the power function were a = 1.065 g kg−1 h−0.42 and b = 0.42 (MgPA-BC), and a = 0.478 g kg−1 h−0.20 and b = 0.20 (CaPA-BC). For both biochars, the values of b were less than 1, implying that the release rates decreased with time. For MgPA-BC, b was close to 0.5, in which case the power function approaches the parabolic diffusion model; indeed, the parabolic diffusion model provided the second best fit (Table 3). The a value represents the initial release rate, which indicated that MgPA-BC had an initial phosphate release rate that was more than twice as large as that of CaPA-BC. To identify the dissolution mechanism, the biochars were characterized by X-ray diffraction and FTIR spectroscopy before and after the kinetics dissolution experiments, and the results are presented in the next sections.
| Model | MgPA-BC | CaPA-BC |
|---|---|---|
| R2/SE | R2/SE | |
| Zero order | 0.87/1.46 | 0.67/0.23 |
| Pseudo-first order | 0.97/0.55 | 0.75/0.19 |
| Pseudo-second order | 0.97/0.97 | 0.99/0.19 |
| Elovich | 0.90/1.25 | 0.97/0.07 |
| Parabolic diffusion | 0.98/0.55 | 0.87/0.15 |
| Power function | 1.00/0.43 | 0.99/0.08 |
3.5 X-ray diffraction patterns of pristine and post-kinetics biochars
Fig. 4 shows the XRD patterns of the modified biochars before and after the kinetic release experiment. The pattern of the unmodified biochar (BC) displayed strong peaks at 2θ = 20.9°, 26.6°, 36.5°, 39.5°, 40.3°, 42.4°, and 45.8° that were ascribed to quartz (SiO2). No other crystalline phases were found in BC. In addition to quartz, the Mg-containing pre-treated biochar (MgPA-BC) presented peaks of crystalline Mg pyrophosphate (MgPP, α-Mg2P2O7), in agreement with previous studies.16,17 After the kinetic study, the Mg2P2O7 peaks in MgPA-BC-post have reduced in intensity, suggesting their dissolution or transformation. New peaks in MgPA-BC-post at 2θ = 13–16° and 30.6° indicated the presence of small amounts of struvite (MgNH4PO4·6H2O) or struvite-K (MgKPO4·6H2O), and peaks at 2θ = 18.6° and 38.0° were ascribed to brucite (Mg(OH)2). Small peaks at 2θ = 22.3°, 27.9°, and 31.2° indicated that cattiite (Mg3(PO4)2·22H2O) was present after the kinetic experiment in MgPA-BC-post.The pristine Ca-containing pre-treated biochar (CaPA-BC) contained crystalline Ca pyrophosphate (γ-Ca2P2O7), as also seen in previous studies where P-loaded biochars were formed by pre-treatment with Ca and P.17,21,33 After the kinetic study, peaks for Ca2P2O7 were still present in CaPA-BC-post but reduced in intensity, and new peaks corresponding to (Ca,Mg)3(PO4)2, a Mg-stabilized β-Ca3(PO4)2 phase sometimes referred to as whitlockite, were found at 2θ = 17.0°, 28.2°, 31.5°, 34.9°, 47.4° and 48.9°.
3.6 FTIR spectroscopy of pristine and post-kinetics biochars
Fig. 5 presents the FTIR spectra at 1800–600 cm−1 of the pristine biochars and after the kinetic release experiment. Most vibrations for phosphate groups appear in this region,36,37 and no apparent peaks were observed at higher wavenumbers (Fig. S1 in ESI†). In the FTIR spectrum of BC, the peaks at 690, 777, 797, 1040–1100, and 1168 cm−1 were ascribed to various Si–O vibrations in quartz.38 The peaks at 1040–1100 cm−1 may possibly indicate the presence of C–O stretching of alcohol and ether groups.39 Peaks at 655 and 741 cm−1 were ascribed to Si–O or Al–O vibrations in silicate minerals,38 and the peak at 620 cm−1 may be due to C–C stretching vibrations.39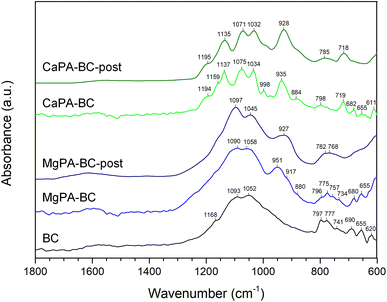 | ||
| Fig. 5 FTIR spectra (1800–600 cm−1) of pristine BC and MgPA-BC and CaPA-BC, and the modified biochars after the 240 hour kinetic release experiment (MgPA-BC-post and CaPA-BC-post, respectively). Approximate peak positions are indicated above each peak. The FTIR spectra over the full range (4000–600 cm−1) can be found as Fig. S1 in the ESI.† | ||
Upon pre-treatment with MgO and H3PO4, the FTIR spectrum of MgPA-BC showed a relative increase in peak intensity around 1090 cm−1 compared to BC, possibly because of incorporation of a (pyro)phosphate,36,40 likely Mg2P2O7 as observed in the XRD patterns (Fig. 4). Possibly, the shoulder peak at 917 cm−1 can also be ascribed to Mg2P2O7.41 The increased intensity of a peak around 951 cm−1 indicated the presence of symmetric stretching mode of P–O bonds of orthophosphate groups,42 and the shoulder at 880 cm−1 was ascribed to HPO4 group vibrations.43 However, more information about the presence of specific phosphate phases in MgPA-BC was difficult to obtain due to the overlap of the phosphate vibrations with the Si–O peaks in the region 800–1300 cm−1.
After the kinetic experiment, the peaks at 1045, 951, and 880 cm−1 decreased in intensity, possibly suggesting the dissolution of an orthophosphate phase. As a result, the broader underlying peak with maximum at 927 cm−1 was found in MgPA-BC-post (this peak was present in MgPA-BC but masked by the peak at 951 cm−1 and only found as a shoulder at 917 cm−1), which was ascribed to Mg2P2O7.41 At lower wavenumbers (600–800 cm−1) peaks have become broader, possibly due to the formation of struvite36 that has an absorbance peak at 770 cm−1 that overlapped with the vibrational peaks of quartz. The cattiite (Mg3(PO4)2·22H2O) that was identified by XRD has its main absorbance peak43 at 980 cm−1 but this peak could not be confirmed with FTIR spectroscopy due to the low quantity and overlap with other quartz/phosphate peaks.
In the FTIR spectrum of CaPA-BC, the peaks at 611, 719, 935, 998, 1034, 1075, 1137, 1159, and 1194 cm−1 were assigned to γ-Ca2P2O7.44 The peak at 884 cm−1 indicated the presence of HPO4 groups from amorphous CaHPO4.45 After the kinetic experiment, there was only little change in the FTIR spectrum of CaPA-BC-post compared to CaPA-BC. Various peaks have broadened, which may suggest a reduction in crystallinity and potentially a transformation of phosphate phases. Moreover, the HPO4 peak at 884 cm−1 reduced in intensity and broadened to become a shoulder peak, possibly indicating its dissolution. The (Ca,Mg)3(PO4)2 as identified in the XRD pattern (Fig. 4) has its main FTIR absorbance peaks in the region 970–1120 cm−1,46 but the presence of these peaks could not be confirmed in CaPA-BC-post due to the overlap with the Ca2P2O7 and SiO2 vibrations.
3.7 Formation and dissolution mechanism of MgPA-BC
Based on the X-ray diffraction analysis (Fig. 4), MgPA-BC contained crystalline P in the form of α-Mg2P2O7. This was further confirmed by the characteristic peaks of α-Mg2P2O7 in the FTIR spectrum of pristine MgPA-BC (Fig. 5) and the presence of micronsized crystals in the SEM image (Fig. 1b). In previous studies, Mg2P2O7 was formed under similar pyrolysis conditions.16,17 The formation of crystalline α-Mg2P2O7 during biochar synthesis is proposed to occur according to the following reactions:| MgO + H3PO4 + 2H2O → MgHPO4·3H2O | (1) |
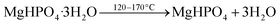 | (2) |
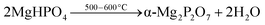 | (3) |
Before pyrolysis, the reaction between MgO and H3PO4 in the pre-treatment process resulted in the formation of MgHPO4·3H2O.47 Upon thermal treatment, dehydration of MgHPO4·3H2O took place around 120–170 °C and resulted in an amorphous MgHPO4 phase.48,49 Further heating converted this amorphous phase into crystalline α-Mg2P2O7 that is formed around 500–600 °C depending on the particle size and heating conditions.49,50 The conversion of amorphous MgHPO4 into crystalline α-Mg2P2O7 follows a complicated mechanism of various polyphosphate chain lengthening and shortening steps.50
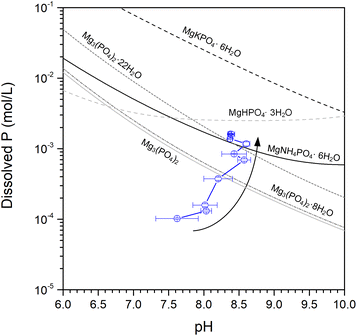
However, Mg2P2O7 was likely not the only P form in MgPA-BC. Aramendía et al.51 estimated with solid-state 31P MAS NMR measurements that when MgHPO4·3H2O was heated to 650 °C the material consisted of a mixture of 15% crystalline Mg3(PO4)2 and 85% crystalline α-Mg2P2O7. At a lower temperature of 500 °C, the Mg3(PO4)2 was amorphous and its proportion was larger than at 650 °C (i.e., >15%). These data suggested that MgPA-BC, which was produced at 600 °C, likely contained a substantial fraction of amorphous Mg3(PO4)2 in addition to the crystalline α-Mg2P2O7. This was also inferred from the presence of an orthophosphate peak at 951 cm−1 in the FTIR spectrum of MgPA-BC (Fig. 5). In addition, other (amorphous) orthophosphate phases may have been present as well. Possibly, some struvite (MgNH4PO4·6H2O) and/or struvite-K (MgKPO4·6H2O) may have been formed by the presence of respectively NH4+ and/or K+ in the biomass.47 Even though struvite is unstable at high temperatures (the loss of water and NH3 from struvite takes place41 below 300 °C), Bekiaris et al.36 found indications that an amorphous struvite phase was present in biochar produced from a solid fraction of digestate at 600 °C. The presence of struvite in biochars was also suggested by Bruun et al.11 from the co-occurrence of Mg and P in SEM-EDX analysis with high spatial correlation, and also Wang et al.12 suggested that a Mg phosphate mineral (possibly struvite) was the main phase responsible for P release from poultry litter biochar. Similarly, struvite-K was previously identified in various biochars.17,52 However, the presence or absence of struvite or struvite-K in MgPA-BC could neither be confirmed nor be ruled out. Possibly, some of these phases may have been present in MgPA-BC, but were masked by other peaks in the XRD pattern and FTIR spectrum. The largely amorphous nature of MgPA-BC and the presence of SiO2 made it difficult to confirm the specific orthophosphates.
Based on the identified phases in XRD and FTIR analysis, the phosphate release from MgPA-BC could be separated into two processes, namely: (1) dissolution of crystalline Mg2P2O7, and (2) dissolution of an amorphous orthophosphate (such as struvite, struvite-K, and/or Mg3(PO4)2). In the XRD pattern of MgPA-BC, α-Mg2P2O7 was the sole crystalline phosphate phase, and the intensity decreased during the dissolution process that suggested its dissolution. We could not find any reliable Ksp values for Mg2P2O7, but it can be inferred that Mg2P2O7 is more soluble than Ca2P2O7 since the presence of Mg2+ ions was reported to increase the Ca2P2O7 solubility.34,53 The dissolution of Mg2P2O7 likely took place via the release of pyrophosphate (P2O74−) ions, which likely remained in solution as pyrophosphate ions, since the conversion of pyrophosphate to orthophosphate takes place only very slowly at room temperature and neutral pH.34 To confirm the release of condensed phosphates (such as pyrophosphates but also longer chains) from MgPA-BC, the solution after the 240 hour kinetic release experiment was digested with a persulfate solution (Table S3 in ESI†). After persulfate digestion, the dissolved P concentration after 240 hours was 68.4 ± 3.3 mg L−1, which is higher than the P concentration before persulfate digestion (49.6 ± 1.4 mg L−1), indicating that indeed some Mg2P2O7 dissolved. It should be noted that the P dissolution from Mg2P2O7 cannot explain the phosphate release kinetics because the dissolved P concentrations in Fig. 3a consist primarily of orthophosphates.54
Thus, in addition to Mg2P2O7 (the only crystalline phosphate phase), an (amorphous) orthophosphate also dissolved from MgPA-BC. To identify the determining phase for phosphate dissolution, the dissolved P concentrations from MgPA-BC were compared with calculated solubility values of various common Mg phosphates (Fig. 6). After 240 hours, struvite and Mg3(PO4)2·22H2O appear to be the rate-limiting phases for long-term phosphate dissolution; the dissolved P concentration agreed very well with the calculated solubility of struvite and Mg3(PO4)2·22H2O (see Fig. 6). Indeed, struvite/struvite-K and Mg3(PO4)·22H2O were the phases that appeared in the XRD pattern of MgPA-BC-post (Fig. 4). In previous experiments, struvite was in equilibrium with Mg3(PO4)2·22H2O under similar alkaline conditions.55 The dissolved Mg concentration after 240 hours (34.3 ± 1.5 mg L−1) was similar to the dissolved P concentrations (Table S3 in ESI†), which further confirmed that an Mg-containing phosphate dissolved from the biochar.
Amorphous Mg3(PO4)2 was possibly also present in MgPA-BC as suggested by the FTIR spectrum (Fig. 5) and previous work.51 It is likely that the amorphous Mg3(PO4)2 converted to crystalline Mg3(PO4)2·22H2O during the kinetic release experiment.
3.8 Formation and dissolution mechanism of CaPA-BC
Similar to MgPA-BC, the XRD pattern of CaPA-BC (Fig. 4) demonstrated the presence of crystalline γ-Ca2P2O7 as the sole crystalline phosphate phase. The characteristic peaks of γ-Ca2P2O7 were also prominent in the FTIR spectrum of CaPA-BC (Fig. 5), as well as the presence of needle-like or tabular crystals in the SEM image (Fig. 1c). In previous studies, crystalline Ca2P2O7 was formed under similar pyrolysis conditions.17,21,33 The formation pathway can be proposed as follows:| CaO + H3PO4 + H2O → CaHPO4·2H2O | (4) |
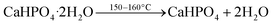 | (5) |
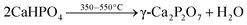 | (6) |
The reaction of CaO with H3PO4 resulted in the formation of CaHPO4·2H2O.23 Upon heating, the CaHPO4·2H2O was dehydrated to form CaHPO4 at around 150–160 °C, which further converted into crystalline γ-Ca2P2O7 at around 350–550 °C.50,56
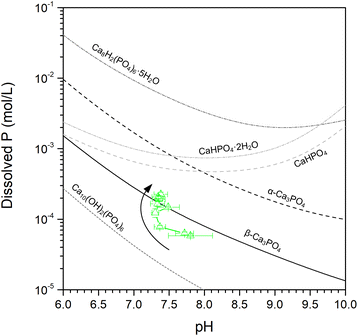
Apart from Ca2P2O7, other Ca phases may have been present as well, either as amorphous or small amounts of crystalline phases. Examples of phases that were previously observed in biochars prepared under similar conditions were CaHPO4,11,17,36 Ca3(PO4)2, Ca8H2(PO4)6·5H2O,22 and hydroxyapatite (Ca10(OH)2(PO4)6).17,22 When comparing the solubility of these phases with the dissolved P concentrations from CaPA-BC (Fig. 7), it appears that β-Ca3(PO4)2 (i.e., (Ca,Mg)3(PO4)2) was the rate controlling phase, in agreement with the XRD pattern of CaPA-BC-post (Fig. 4). In addition, a more soluble phase such as (amorphous) CaHPO4 was likely also present in CaPA-BC, as was suggested by the HPO4 peak at 884 cm−1 in the FTIR spectrum of CaPA-BC (Fig. 5) and since this phase was identified in various Ca- and P-rich biochars.11,17,36
Based on the identified phases, the phosphate dissolution from CaPA-BC was separated into two processes, namely (1) dissolution of crystalline Ca2P2O7, and (2) dissolution of an amorphous orthophosphate (such as (Ca,Mg)3(PO4)2 and/or CaHPO4). The solubility of Ca2P2O7 is very low, with reported Ksp values ranging from 3 × 10−18 to 1.8 × 10−13.53,57 Little to no dissolution of Ca2P2O7 was also confirmed by the fact that the dissolved P concentrations before and after persulfate digestion were not different (Table S3 in ESI†). Lustosa Filho et al.17 also found that crystalline Ca2P2O7 was still present after their 240 hour kinetic release experiment of poultry litter biochars.
Similar to MgPA-BC, the phosphate dissolution from CaPA-BC cannot be solely ascribed to the formation of Ca2P2O7, and an orthophosphate phase must have been responsible for the P release. The dissolved P concentrations did not change any further after 144 hours (Fig. 3a) and equilibrium was reached. When comparing the dissolved P concentrations with the calculated solubilities of various Ca phosphates, the equilibrium P concentration was in good agreement with the calculated solubility of β-Ca3(PO4)2 (Fig. 6b). This was further corroborated by the presence of (Ca,Mg)3(PO4)2 in the XRD pattern of CaPA-BC-post (Fig. 4). In previous studies, the slow phosphate release and poor solubility of P and Ca from various biochars was also ascribed to crystalline (Ca,Mg)3(PO4)2.22,58 It should be noted that the actual solubility of (Ca,Mg)3(PO4)2 may be lower than that of the calculated Mg-free β-Ca3(PO4)2, depending on the Mg content.46
After 240 hours, close to 2% of the P present in CaPA-BC dissolved, and only 0.5% of the total Ca has dissolved (Table S3 in ESI†). This very low amount of Ca dissolved suggested that an additional phase different than CaHPO4 or (Ca,Mg)3(PO4)2 may have been responsible for P dissolution and/or the Ca may have reprecipitated on the biochar surface in the form of (Ca,Mg)3(PO4)2.
4. Conclusions
In this study, sugarcane filter cake was pyrolyzed after pre-treatment with H3PO4 in the presence of MgO (MgPA-BC) or CaO (CaPA-BC) and the potential P availability of the biochars to crops was studied by extraction (DI water and 2% formic acid) and kinetic release in DI water. When prepared under identical conditions, co-pyrolysis of biomass with MgO and CaO produced biochars with identical P loadings of 73–74 g kg−1 but with distinctly different P release profiles. The phosphate dissolution from MgPA-BC was gradual and reached 10 g P per kg biochar after 240 hours, and the rate-determining phases were identified to be Mg pyrophosphate (Mg2P2O7), struvite (MgNH4PO4·6H2O), and cattiite (Mg3(PO4)2·22H2O). In contrast, CaPA-BC only released 1.2 g P per kg biochar and equilibrium was reached after 144 hours. Further P dissolution from CaPA-BC was limited by the low solubility of Ca pyrophosphate (Ca2P2O7) and whitlockite ((Ca,Mg)3(PO4)2). These results demonstrate that while both Mg and Ca aid to immobilize P in biochars, co-pyrolysis with MgO retains the P in a more soluble and available form than CaO. Therefore, for slow release fertilizer applications, addition of MgO (and not CaO) is recommended during co-pyrolysis. The phytoavailability of the biochar-based fertilizers should be confirmed in a plant study, which will be carried out in our future work.Author contributions
Kaewta Jetsrisuparb: conceptualization, methodology, resources, writing – review & editing, supervision, project administration, funding acquisition. Thanawan Jeejaila: investigation. Chanon Saengthip: investigation. Pornnapa Kasemsiri: writing – review & editing, supervision. Yuvarat Ngernyen: writing – review & editing, funding acquisition. Prinya Chindaprasirt: writing – review & editing. Jesper T. N. Knijnenburg: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, visualization, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was conducted under the research on valorisation of industrial food wastes for the development of sustainable products towards a bio-based circular economy by Faculty of Engineering, Khon Kaen University, which has received funding support from Fundamental Fund 2022 (the National Science, Research and Innovation Fund (NSRF), Thailand). The authors would like to thank Mitr Phol for supplying the sugarcane filter cake for this study. The assistance of Wasu Jantapa (biochar synthesis), Kaung Set Linn (slow release experiments) and Siraprapa Suwanree (SEM analysis) is kindly acknowledged.References
- M. R. Hart, B. F. Quin and M. L. Nguyen, J. Environ. Qual., 2004, 33, 1954–1972 CrossRef CAS PubMed.
- J. J. Weeks and G. M. Hettiarachchi, J. Environ. Qual., 2019, 48, 1300–1313 CrossRef CAS PubMed.
- W. Pornprakun, S. Sungnul, C. Kiataramkul and E. J. Moore, Adv. Differ. Equations, 2019, 2019, 257 CrossRef.
- R. D. M. Prado, G. Caione and C. N. S. Campos, Appl. Environ. Soil Sci., 2013, 2013, 581984 Search PubMed.
- P. A. O. George, J. J. C. Eras, A. S. Gutierrez, L. Hens and C. Vandecasteele, Waste Biomass Valorization, 2010, 1, 407–413 CrossRef.
- J. Zheng, J. Han, Z. Liu, W. Xia, X. Zhang, L. Li, X. Liu, R. Bian, K. Cheng, J. Zheng and G. Pan, Agric., Ecosyst. Environ., 2017, 241, 70–78 CrossRef CAS.
- N. R. Pandit, J. Mulder, S. E. Hale, A. R. Zimmerman, B. H. Pandit and G. Cornelissen, Sci. Total Environ., 2018, 637–638, 1333–1341 CrossRef CAS.
- A. J. Eykelbosh, M. S. Johnson, E. Santos De Queiroz, H. J. Dalmagro and E. Guimarães Couto, PLoS One, 2014, 9, e98523 CrossRef.
- A. B. Speratti, M. S. Johnson, H. M. Sousa, G. N. Torres and E. G. Couto, Agronomy, 2017, 7, 49 CrossRef.
- T. K. Choudhary, K. S. Khan, Q. Hussain, M. Ahmad and M. Ashfaq, Arabian J. Geosci., 2019, 12, 617 CrossRef CAS.
- S. Bruun, S. L. Harmer, G. Bekiaris, W. Christel, L. Zuin, Y. Hu, L. S. Jensen and E. Lombi, Chemosphere, 2017, 169, 377–386 CrossRef CAS.
- Y. Wang, Y. Lin, P. C. Chiu, P. T. Imhoff and M. Guo, Sci. Total Environ., 2015, 512–513, 454–463 CrossRef CAS.
- R. Li, W. Teng, Y. Li, W. Wang, R. Cui and T. Yang, J. Cleaner Prod., 2017, 140, 964–970 CrossRef CAS.
- C. Vogel and C. Adam, Environ. Sci. Technol., 2011, 45, 7445–7450 CrossRef CAS PubMed.
- M. Marcińczyk and P. Oleszczuk, J. Cleaner Prod., 2022, 339, 130685 CrossRef.
- S. Suwanree, J. T. N. Knijnenburg, P. Kasemsiri, W. Kraithong, P. Chindaprasirt and K. Jetsrisuparb, Biomass Bioenergy, 2022, 156, 106304 CrossRef CAS.
- J. F. Lustosa Filho, E. S. Penido, P. P. Castro, C. A. Silva and L. C. A. Melo, ACS Sustainable Chem. Eng., 2017, 5, 9043–9052 CrossRef CAS.
- X. An, Z. Wu, J. Yu, G. Cravotto, X. Liu, Q. Li and B. Yu, ACS Sustainable Chem. Eng., 2020, 8, 3181–3190 CrossRef CAS.
- Q. Liu, Z. Fang, Y. Liu, Y. Liu, Y. Xu, X. Ruan, X. Zhang and W. Cao, Waste Manage., 2019, 87, 71–77 CrossRef CAS.
- L. Fang, F. Yan, J. Chen, X. Shen and Z. Zhang, ACS Sustainable Chem. Eng., 2020, 8, 6611–6621 CrossRef CAS.
- L. Zhao, X. Cao, W. Zheng, J. W. Scott, B. K. Sharma and X. Chen, ACS Sustainable Chem. Eng., 2016, 4, 1630–1636 CrossRef CAS.
- K. Sun, M. Qiu, L. Han, J. Jin, Z. Wang, Z. Pan and B. Xing, Sci. Total Environ., 2018, 634, 1300–1307 CrossRef CAS.
- K. L. Elmore and T. D. Farr, Ind. Eng. Chem., 1940, 32, 580–586 CrossRef CAS.
- A. Enders and J. Lehmann, Commun. Soil Sci. Plant Anal., 2012, 43, 1042–1052 CrossRef CAS.
- N. Abeysinghe, K. Jetsrisuparb, K. H. T. Karunarathna, E. P. S. Chandana, S. Suwanree, P. Kasemsiri, P. Chindaprasirt and J. T. N. Knijnenburg, J. Met., Mater. Miner., 2022, 32, 124–133 CrossRef CAS.
- T. Wang, M. Camps-Arbestain, M. Hedley and P. Bishop, Plant Soil, 2012, 357, 173–187 CrossRef CAS.
- Y. Liang, X. Cao, L. Zhao, X. Xu and W. Harris, J. Environ. Qual., 2014, 43, 1504–1509 CrossRef PubMed.
- X. L. Huang and J. Z. Zhang, Talanta, 2009, 78, 1129–1135 CrossRef CAS PubMed.
- B. Müller, Swiss Federal Institute for Environmental Science and Technology (EAWAG), Kastanienbaum, Switzerland, 2015 Search PubMed.
- S. Li, S. Harris, A. Anandhi and G. Chen, J. Cleaner Prod., 2019, 215, 890–902 CrossRef CAS.
- J. O. Fernandes, C. A. R. Bernardino, C. F. Mahler, R. E. Santelli, B. F. Braz, R. C. Borges, M. C. da Cunha Veloso, G. A. Romeiro and F. H. Cincotto, Water, Air, Soil Pollut., 2021, 232, 67 CrossRef CAS.
- A. B. Speratti, M. S. Johnson, H. M. Sousa, H. J. Dalmagro and E. G. Couto, GCB Bioenergy, 2018, 10, 272–286 CrossRef CAS.
- R. Gao, Q. Wang, Y. Liu, J. Zhu, Y. Deng, Q. Fu and H. Hu, Energy Fuels, 2019, 33, 413–419 CrossRef CAS.
- K. P. Pritzker, in Calcium Phosphates in Biological and Industrial Systems, Springer, 1998, pp. 277–301 Search PubMed.
- L. Leng, Q. Xiong, L. Yang, H. Li, Y. Zhou, W. Zhang, S. Jiang, H. Li and H. Huang, Sci. Total Environ., 2021, 763, 144204 CrossRef CAS PubMed.
- G. Bekiaris, C. Peltre, L. S. Jensen and S. Bruun, Spectrochim. Acta, Part A, 2016, 168, 29–36 CrossRef CAS PubMed.
- W. Jastrzebski, M. Sitarz, M. Rokita and K. Bułat, Spectrochim. Acta, Part A, 2011, 79, 722–727 CrossRef CAS PubMed.
- B. Udvardi, I. J. Kovács, P. Kónya, M. Földvári, J. Füri, F. Budai, G. Falus, T. Fancsik, C. Szabó, Z. Szalai and J. Mihály, Sediment. Geol., 2014, 313, 1–14 CrossRef CAS.
- H. Yang, R. Yan, H. Chen, D. H. Lee and C. Zheng, Fuel, 2007, 86, 1781–1788 CrossRef CAS.
- B. C. Cornilsen and R. A. Condrate Sr, J. Phys. Chem. Solids, 1977, 38, 1327–1332 CrossRef CAS.
- M. V. Ramlogan and A. A. Rouff, J. Therm. Anal. Calorim., 2016, 123, 145–152 CrossRef CAS.
- O. Kaygili, C. Tatar and F. Yakuphanoglu, Ceram. Int., 2012, 38, 5713–5722 CrossRef CAS.
- B. Lothenbach, B. Xu and F. Winnefeld, Appl. Geochem., 2019, 111, 104450 CrossRef CAS.
- B. Cornilsen and R. Condrate Sr, J. Inorg. Nucl. Chem., 1979, 41, 602–605 CrossRef CAS.
- Z. Zyman, M. Epple, A. Goncharenko, D. Rokhmistrov, O. Prymak and K. Loza, J. Mater. Sci.: Mater. Med., 2017, 28, 52 CrossRef PubMed.
- X. Li, A. Ito, Y. Sogo, X. Wang and R. Z. LeGeros, Acta Biomater., 2009, 5, 508–517 CrossRef CAS PubMed.
- S. A. Walling and J. L. Provis, Chem. Rev., 2016, 116, 4170–4204 CrossRef CAS.
- N. Petranović, U. Mioč and D. Minić, Thermochim. Acta, 1987, 116, 137–143 CrossRef.
- M. A. Aramendía, V. Borau, C. Jiménez, J. M. Marinas and F. J. Romero, J. Colloid Interface Sci., 1999, 217, 288–298 CrossRef PubMed.
- B. C. Sales, B. C. Chakoumakos, L. A. Boatner and J. O. Ramey, J. Non-Cryst. Solids, 1993, 159, 121–139 CrossRef CAS.
- M. A. Aramendía, V. Borau, C. Jiménez, J. M. Marinas, F. J. Romero and J. R. Ruiz, J. Colloid Interface Sci., 1998, 202, 456–461 CrossRef.
- N. Prakongkep, R. J. Gilkes and W. Wiriyakitnateekul, J. Plant Nutr. Soil Sci., 2015, 178, 732–740 CrossRef CAS.
- W. E. Brown and T. M. Gregory, Arthritis Rheum., 1976, 19, 446–462 CrossRef CAS PubMed.
- E. A. Nagul, I. D. McKelvie, P. Worsfold and S. D. Kolev, Anal. Chim. Acta, 2015, 890, 60–82 CrossRef CAS PubMed.
- A. W. Taylor, A. W. Frazier and E. L. Gurney, Trans. Faraday Soc., 1963, 59, 1580–1584 RSC.
- M. El Hazzat, A. El Hamidi, M. Halim and S. Arsalane, Materialia, 2021, 16, 101055 CrossRef CAS.
- B. H. Wiers, Inorg. Chem., 1971, 10, 2581–2584 CrossRef CAS.
- L. Zhao, X. Cao, Q. Wang, F. Yang and S. Xu, J. Environ. Qual., 2013, 42, 545–552 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Equations for kinetics release, solubility products (Ksp), and dissolved concentrations (P before and after persulfate digestion, Mg, Ca) after 240 hours. See DOI: https://doi.org/10.1039/d2ra05848k |
| This journal is © The Royal Society of Chemistry 2022 |