Open Access Article
Open Access ArticleHydrothermal synthesis of carbon quantum dots with size tunability via heterogeneous nucleation†
Nant Nammahachak a,
Kamonwan Khamphumee Aup-Ngoen*b,
Piyapong Asanithic,
Mati Horpratumd,
Surawut Chuangchote
a,
Kamonwan Khamphumee Aup-Ngoen*b,
Piyapong Asanithic,
Mati Horpratumd,
Surawut Chuangchote ae,
Sutatch Ratanaphan*afg and
Werasak Surareungchaif
ae,
Sutatch Ratanaphan*afg and
Werasak Surareungchaif
aDepartment of Tool and Materials Engineering, King Mongkut's University of Technology Thonburi, 126 Prachauthit Road, Bangmod, Bangkok 10140, Thailand. E-mail: sutatch.ratanaphan@mail.kmutt.ac.th
bMaterials and Nondestructive Testing Laboratory, King Mongkut's University of Technology Thonburi (KMUTT, Ratchaburi), 126 Pracha Uthit Rd, Thung Khru, Bangkok 10140, Thailand. E-mail: kamonwan.aup@kmutt.ac.th
cDepartment of Physics, Faculty of Science, King Mongkut's University of Technology Thonburi, 126 Prachauthit Road, Bangmod, Bangkok 10140, Thailand
dOpto-Electrochemical Sensing Research Team (OEC), National Electronic and Computer Technology Center, 112 Thailand Science Park, Pahonyothin Rd, Khlong Nueng, Khlong Luang, Pathum Thani 12120, Thailand
eResearch Center of Advanced Materials for Energy and Environmental Technology (MEET), King Mongkut's University of Technology Thonburi, 126 Prachauthit Road, Bangmod, Bangkok 10140, Thailand
fNanoscience and Nanotechnology Graduate Program, King Mongkut's University of Technology Thonburi, 126 Prachauthit Road, Bangmod, Bangkok 10140, Thailand
gCenter of Excellence in Theoretical and Computational Science Center (TaCS-CoE), King Mongkut's University of Technology Thonburi, 126 Pracha Uthit Rd, Thung Khru, Bangkok 10140, Thailand
First published on 8th November 2022
Abstract
Hydrothermal synthesis has been extensively utilized for fabricating carbon quantum dots (CQDs). Generally, the average sizes of the CQDs are controlled by using specific precursor concentrations, processing temperatures, and reaction times. In our study, the average size of CQDs can simply be controlled by using a different filling volume of sucrose solution in the hydrothermal reactor while keeping the other experimental parameters constant. If homogeneous nucleation plays a major role in the hydrothermal synthesis, the CQDs synthesized by using different filling volumes should have relatively the same size. Nonetheless, we found that the average size of CQDs is inversely correlated with the filling volumes. Particularly, for the hydrothermal syntheses with the filling volumes of 20%, 50%, and 80%, the average size of the CQDs is 15, 13, and 4 nm, respectively. Therefore, the hydrothermal synthesis of CQDs with size-tunability can be achieved by the heterogeneous process associated with the total surface areas between the precursor and reactor.
Introduction
Carbon quantum dots (CQDs) have drawn considerable attention owing to their excellent biocompatibility, chemical inertness, water solubility, and low toxicity.1–6 There are many synthesis approaches to fabricate CQDs (for example, reduction method,2 pyrolysis,7 acidic oxidation,8 and electrochemical synthesis9), but these methods are rather complicated or expensive compared with a hydrothermal synthesis.1–6 Although carbonization and polymerization play a major role on the average size of CQDs, it is not possible to observe these activities in the black box/hydrothermal reactor.10–12 In other words, our knowledge on the CQD size distributions was only empirically determined from the experimental inputs of hydrothermal process (i.e. concentration of organic precursors, processing temperature, and reaction time).10–12 As a result, nucleation and growth mechanisms of the CQDs are not well described due to limited configurations of the experimental inputs.It was reported that the sizes of CQDs derived from the hydrothermal treatment of sucrose precursor were strongly influenced by the decomposition and polymerization.12 Particularly during the decomposition, sucrose in aqueous solution is hydrolyzed into fructose and glucose, and subsequently decomposed to smaller organic compounds (i.e. furfurals and weak acids).11,12 These organic compounds are then polymerized into larger molecules, which finally lead to the formation of the CQDs in the hydrothermal reactor.12–14 While homogeneous nucleation is well investigated for metals, ceramics, and organic compounds, its activation free energy is generally larger than a heterogeneous nucleation.15–18 Considering that the surface of the hydrothermal reactor could provide favorably kinetic pathways for the nucleation of CQDs, the heterogeneous nucleation is expected to be the key mechanism for the nucleation of the CQDs in the hydrothermal reactor. In other words, the classical nucleation theory19 can be used to evaluate the free energy barriers associated with the formations of critical nuclei for the heterogeneous nucleation. It is known that existences of foreign substances (i.e. reactor wall, impurities, and foreign particle) can significantly reduce the energy barriers for nucleation.15–17 As a result, nuclei nucleated heterogeneously on the reactor wall is more frequently occurred comparing with the one nucleated inside the liquid precursor and the number of heterogeneous nuclei are linearly proportional to the total surface area of the reactor.15,20 In order to study the heterogeneous nucleation in the hydrothermal CQDs, different filling volumes of sucrose solution in the hydrothermal reactor are used while keeping the other experimental parameters constant (such as, reactor geometry, concentration of the precursor, processing temperature, and reaction time). Analyses of the sizes of CQDs and activation free energies for homogeneous and heterogeneous nucleation are also discussed by using the classical nucleation theory.
Experimental section
Sucrose [purity ≥99.5%, Sigma Aldrich] dissolved in deionized water (DI) with 2 wt% has been used for all hydrothermal syntheses of the CQDs. To investigate the influence of precursor filling volumes on the size distributions of CQDs, the precursor was placed into a Teflon lined stainless steel autoclave (diameter = 50 mm, height = 100 mm, and volume = 200 ml) with a different filling volume (20%, 50%, and 80% of the total capacity). The hydrothermal reactor was then annealed in a hot air furnace (Binder) at 180 °C for 2 hours and gradually cooled to a room temperature. Morphology and particle size of CQDs were determined using a high-resolution transmission electron microscope (HRTEM; FEI Tecnai G2 20). Chemical bonding and surface functional groups of the CQDs were characterized using Fourier transform infrared spectroscopy (FTIR, Thermo scientific) with wavenumbers ranging from 800 cm−1 to 3600 cm−1. Raman (Renishaw) spectroscopy with an excitation wavelength of 532 nm was used to characterize the graphitic level of the CQDs. Fluorescence properties of the CQDs were obtained by using a Tecan Infinite M200 spectrophotometer and the quantum yields (QYs) of CQDs were determined by using quinine sulfate as a reference.Results and discussion
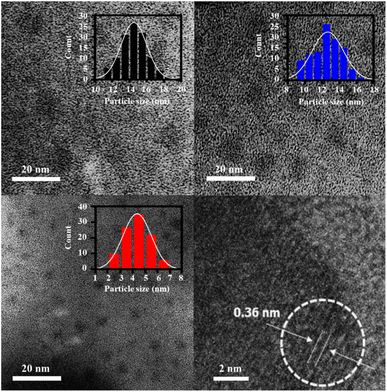
The hydrothermal syntheses of the CQDs with different filling volumes lead to distinct size distributions as shown in TEM images (Fig. 1). Specifically, the average sizes of CQDs increased from 4 ± 1 nm to 13 ± 1.6 nm and 15 ± 1.4 nm when the filling volumes of 80% to 50% and 20% are used, respectively. All experiments are repeated three times with comparable size distributions (Fig. S1, ESI†). While average sizes of the CQDs are inversely correlated with the filling volumes of the sucrose precursor solutions in the hydrothermal reactor (Table S1, ESI†), the size distributions of these CQDs are well described by a normal distribution (Fig. 1a–c). Therefore, growth mechanism of the CQDs, which are composed of well-ordered graphite (002) plane with an interplanar spacing of 0.36 nm10,12 as shown in a HRTEM image (Fig. 1d), are comparable regardless of the filling volumes. The chemical analyses of the CQDs using FTIR and Raman spectroscopies in Fig. 2 clearly demonstrated that the surface functional group of these CQDs are mainly composed of oxygen containing groups (i.e. hydroxyl, carboxylic, and carbonyl), consistent with the previous studies that used glucose as precursors for the hydrothermal synthesis of CQDs.10,12 While the surface functional groups of the CQDs are relatively comparable regardless of their sizes, the intensity ratios between the D and G bands (ID/IG) in Table S1, ESI† indicates that the disorder structures are increased during the growth of the CQDs.11,12,14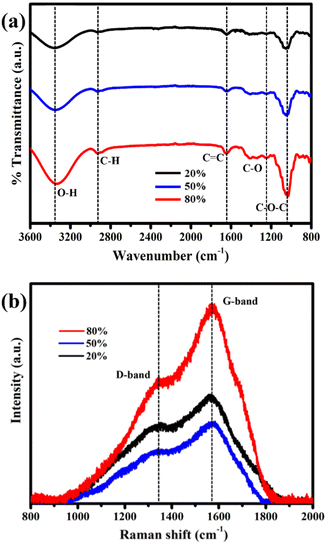 | ||
| Fig. 2 FTIR (a) and Raman spectra (b) of the CQDs synthesized by using the filling volumes of 20% (black), 50% (blue), and 80% (red). | ||
A change in the color from dark to light brown is observed in the hydrothermal solutions synthesized with the filling volumes from 20% to 80% under the ambient light (Fig. S2a, ESI†), however, these solutions containing the CQDs show only green fluorescence under ultraviolet lamp (415 nm). The fluorescent emission spectra of these CQDs show excitation-dependent emission, consistent with the previous studies.11,12,21 Particularly, the peak intensities centered on around 450 nm corresponded to an excitation wavelength of 360 nm (90 nm Stokes shift) are observed in the CQDs specimens regardless of their sizes (Fig. S2b–d, ESI†)). If the QYs is only correlated with the ID/IG, the CQDs with average sizes of 4 nm should have the largest QYs. Nonetheless, the QYs of the CQDs with average sizes of 4 nm (ID/IG = 0.63) and 13 nm (ID/IG = 0.67) are relatively comparable (Table S1, ESI†). Therefore, the fluorescent emission intensities of the CQDs were contributed from the interaction between the sp2 graphite structure and their surrounding media,11,22 resulting in the differences in the QYs of the CQDs with equivalent chemical basis (Table S1, ESI†).
While classical nucleation theory has been used to describe the formation of nanostructures in various materials (metals, ceramics, organic, and inorganic compounds),15–17,23 it was never applied to describe the heterogeneous nucleation in the hydrothermal CQDs. Because the transformations of one-dimensional carbon chains to graphene, two-dimensional graphene, and finally to three-dimensional CQDs are rather complicated and could be strongly influenced by the derivative organic compounds of sucrose.12,24 Ones would doubt the applicability of classical nucleation theory to quantify the nucleation mechanisms in the hydrothermal reactor. However, previous studies demonstrated that homogeneous and heterogeneous nucleation of organic compounds (such as, glycine, amyloid beta, and isonicotinamide) can be well described by the classical nucleation theory.15,25,26 If homogeneous nucleation plays a major role on the nucleation and growth in the hydrothermal synthesis, the CQDs synthesized by using different filling volumes should have comparable size distributions. In addition, if the rates of heterogeneous nucleation are continuous during hydrothermal synthesis, the size distribution of CQDs is expected to be a broad distribution. Nonetheless, the size distributions of CQDs prepared with the different filling volumes are not only different but also have narrow distributions as shown in Fig. 1, suggesting that heterogeneous nucleation is a primary mechanism for the formation of the CQDs. According to the classical nucleation theory,19 the total free energy of homogeneous nucleation of the CQDs with spherical nucleus (ΔGhomo) as a function of its radius (r) is given by
| ΔGhomo = 4/3πr3ΔGυ + 4πr2γgw | (1) |
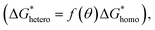 is lower than for the homogeneous nucleation (4.12 × 10−20 J), the overall rate of the CQD nucleation is contributed from the heterogeneous nucleation as shown in Fig. 3a. Note that for the CQDs, the contact angle (θ = 128.7°) is determined from the Young equation.17
is lower than for the homogeneous nucleation (4.12 × 10−20 J), the overall rate of the CQD nucleation is contributed from the heterogeneous nucleation as shown in Fig. 3a. Note that for the CQDs, the contact angle (θ = 128.7°) is determined from the Young equation.17
γtw = γgt + γgwcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ + cos
θ + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ3) = 0.91, it is expected that the smaller organic compounds obtained from a dehydration of sucrose during the heating12,30 are preferably polymerized into larger molecules and finally carbonized into the CQDs on the reactor wall during the cooling period (an inset in Fig. 3a). Assuming that the precursor solutions and the reactor wall are in thermal equilibrium after 2 hours annealing at 180 °C in a hot air furnace, the temperature gradient is expected to promote large amount of the heterogeneous nucleation during the cooling period, resulting in the narrow distributions of CQDs. The fluid flows induced from the temperature gradients20 during the cooling might also enhance the mixing of the heterogeneous nuclei with the derivative precursors in the hydrothermal reactor. Fig. 3b shows the relationships between the filling volumes and the average sizes of the CQDs, graphene hydroxyapatite (GHA), zinc ferrite (ZnFe2O4), and titanium dioxide (TiO2). Interestingly, the average size of the CQDs, GHA, and ZnFe2O4 are all inversely correlated with the precursor filling volumes.31,32 Nonetheless, the sizes of TiO2 are not depended on the filling volumes.33 Although the activation energy for heterogeneous nucleation is also lower than the homogeneous nucleation for TiO2, it should be noted that the energy difference in TiO2 (1.55 × 10−21 J) calculated from TiO2–Teflon (γTiO2t = 57 mJ m−2) and TiO2–DI water (γTiO2w = 72 mJ m−2)34 is significantly lower than the one in the CQD (4.08 × 10−21 J). Considering that the magnitude of energy difference in TiO2 is comparable with the thermal energy (kBT = 6.25 × 10−21 J), it is expected that the rates of homogeneous nucleation and heterogeneous nucleation are negligible different, resulting in the average sizes that are not inversely correlated with the filling volumes.
θ3) = 0.91, it is expected that the smaller organic compounds obtained from a dehydration of sucrose during the heating12,30 are preferably polymerized into larger molecules and finally carbonized into the CQDs on the reactor wall during the cooling period (an inset in Fig. 3a). Assuming that the precursor solutions and the reactor wall are in thermal equilibrium after 2 hours annealing at 180 °C in a hot air furnace, the temperature gradient is expected to promote large amount of the heterogeneous nucleation during the cooling period, resulting in the narrow distributions of CQDs. The fluid flows induced from the temperature gradients20 during the cooling might also enhance the mixing of the heterogeneous nuclei with the derivative precursors in the hydrothermal reactor. Fig. 3b shows the relationships between the filling volumes and the average sizes of the CQDs, graphene hydroxyapatite (GHA), zinc ferrite (ZnFe2O4), and titanium dioxide (TiO2). Interestingly, the average size of the CQDs, GHA, and ZnFe2O4 are all inversely correlated with the precursor filling volumes.31,32 Nonetheless, the sizes of TiO2 are not depended on the filling volumes.33 Although the activation energy for heterogeneous nucleation is also lower than the homogeneous nucleation for TiO2, it should be noted that the energy difference in TiO2 (1.55 × 10−21 J) calculated from TiO2–Teflon (γTiO2t = 57 mJ m−2) and TiO2–DI water (γTiO2w = 72 mJ m−2)34 is significantly lower than the one in the CQD (4.08 × 10−21 J). Considering that the magnitude of energy difference in TiO2 is comparable with the thermal energy (kBT = 6.25 × 10−21 J), it is expected that the rates of homogeneous nucleation and heterogeneous nucleation are negligible different, resulting in the average sizes that are not inversely correlated with the filling volumes.
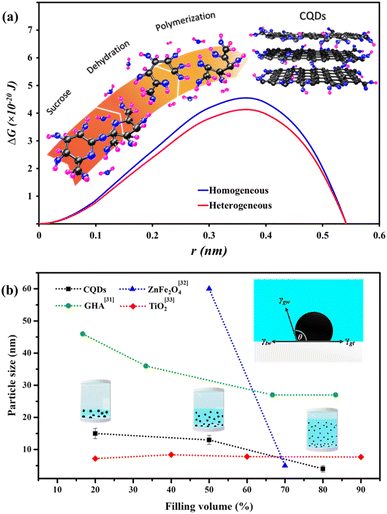 | ||
| Fig. 3 Comparison between activation energies for homogeneous and heterogeneous nucleation of the CQDs (a). The inset in (a) shows formation mechanism of the CQDs composed of two major steps, dehydration of sucrose and polymerization of smaller compounds. Relationship between the filling volumes of hydrothermal precursors and the average sizes of CQDs (b). The heterogeneous nucleation of a CQD on the Teflon surface shows as an inset in (b). The previous results for graphene hydroxyapatite (GHA), zinc ferrite (ZnFe2O4), and titanium dioxide (TiO2)31–33 are also included in the plot. Surface energies, Teflon–DI water (γtw), graphene–Teflon (γgt), and graphene–DI water (γgw) are obtained from ref. 28 and 29. | ||
Conclusions
In summary, hydrothermal carbon quantum dots (CQDs) with average sizes ranging from 4 to 15 nm have been simply obtained by hydrothermal syntheses with different filling volumes of sucrose solution in the hydrothermal reactor. Because the CQDs are synthesized with the same sucrose concentration, hydrothermal autoclave, and temperature profile, the differences in the CQD sizes are strongly influenced by the filling volumes or the heterogeneous surface between the precursor and reactor. In this respect, experimental parameters for a large-scale fabrication of the CQDs should not only be considered the precursor concentrations, processing temperatures, and reaction times, but also included the heterogeneous interfaces.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial supports from the Petchra Pra Jom Klao PhD Research Scholarship from King Mongkut’s University of Technology Thonburi, the Thailand Science Research and Innovation (TSRI) under Fundamental Fund (Project: Advanced Materials and Manufacturing for Applications in New S-curve Industries) and the Development and Promotion of Science and Technology Talents Project (DPST).Notes and references
- C. Wang, Z. Xu, H. Cheng, H. Lin, M. G. Humphrey and C. Zhang, Carbon, 2015, 82, 87–95 CrossRef CAS.
- K. Linehan and H. Doyle, J. Mater. Chem. C, 2014, 2, 6025–6031 RSC.
- Y. Guo, Z. Wang, H. Shao and X. Jiang, Carbon, 2013, 52, 583–589 CrossRef CAS.
- Z. X. Liu, Z. L. Wu, M. X. Gao, H. Liu and C. Z. Huang, Chem. Commun., 2016, 52, 2063–2066 RSC.
- S. Liu, Y. He, Y. Liu, S. Wang, Y. Jian, B. Li and C. Xu, Chem. Commun., 2021, 57, 3680–3683 RSC.
- W. He, W. Weng, X. Sun, Y. Pan, X. Chen, B. Liu and J. Shen, ACS Appl. Nano Mater., 2020, 3, 7420–7427 CrossRef CAS.
- M. C. Ortega-Liebana, N. X. Chung, R. Limpens, L. Gomez, J. L. Hueso, J. Santamaria and T. Gregorkiewicz, Carbon, 2017, 117, 437–446 CrossRef CAS.
- X. Wang, L. Cao, F. Lu, M. J. Meziani, H. Li, G. Qi, B. Zhou, B. A. Harruff, F. Kermarrec and Y.-P. Sun, Chem. Commun., 2009, 3774–3776 RSC.
- Q.-L. Zhao, Z.-L. Zhang, B.-H. Huang, J. Peng, M. Zhang and D.-W. Pang, Chem. Commun., 2008, 5116–5118 RSC.
- Z.-C. Yang, M. Wang, A. M. Yong, S. Y. Wong, X.-H. Zhang, H. Tan, A. Y. Chang, X. Li and J. Wang, Chem. Commun., 2011, 47, 11615–11617 RSC.
- L. Tang, R. Ji, X. Li, K. S. Teng and S. P. Lau, Part. Part. Syst. Charact., 2013, 30, 523–531 CrossRef CAS.
- H. K. Sadhanala, J. Khatei and K. K. Nanda, RSC Adv., 2014, 4, 11481–11485 RSC.
- M. L. Liu, B. B. Chen, C. M. Li and C. Z. Huang, Green Chem., 2019, 21, 449–471 RSC.
- G. Liu, S. Li, M. Cheng, L. Zhao, B. Zhang, Y. Gao, Y. Xu, F. Liu and G. Lu, New J. Chem., 2018, 42, 13147–13156 RSC.
- Y. Kamano, K. Kadota, A. Shimosaka, Y. Shirakawa and J. Hidaka, J. Mol. Liq., 2014, 200, 474–479 CrossRef CAS.
- L. M. Hamm, A. J. Giuffre, N. Han, J. Tao, D. Wang, J. J. D. Yoreo and P. M. Dove, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 1304–1309 CrossRef CAS.
- J. Zhao, M. Wang, H. M. S. Lababidi, H. Al-Adwani and K. K. Gleason, Desalination, 2018, 442, 75–88 CrossRef CAS.
- A. Yoko, S. Okabe, G. Seong, T. Tomai and T. Adschiri, J. Supercrit. Fluids, 2020, 159, 104749 CrossRef CAS.
- K. Kelton and A. L. Greer, Nucleation in Condensed Matter: Applications in Materials and Biology, Elsevier, 2010 Search PubMed.
- Z.-Y. Ma, Z.-L. Yu, Z.-L. Xu, L.-F. Bu, H.-R. Liu, Y.-B. Zhu, B. Qin, T. Ma, H.-J. Zhan, L. Xu, H.-A. Wu, H. Ding and S.-H. Yu, Matter, 2020, 2, 1270–1282 CrossRef.
- P. Suvarnaphaet, C. S. Tiwary, J. Wetcharungsri, S. Porntheeraphat, R. Hoonsawat, P. M. Ajayan, I.-M. Tang and P. Asanithi, Mater. Sci. Eng., C, 2016, 69, 914–921 CrossRef CAS PubMed.
- Z.-H. Wen and X.-B. Yin, RSC Adv., 2016, 6, 27829–27835 RSC.
- D. Gebauer, M. Kellermeier, J. D. Gale, L. Bergström and H. Cölfen, Chem. Soc. Rev., 2014, 43, 2348–2371 RSC.
- J. Gao, J. Yip, J. Zhao, B. I. Yakobson and F. Ding, J. Am. Chem. Soc., 2011, 133, 5009–5015 CrossRef CAS PubMed.
- A. K. Srivastava, J. M. Pittman, J. Zerweck, B. S. Venkata, P. C. Moore, J. R. Sachleben and S. C. Meredith, Protein Sci., 2019, 28, 1567–1581 CAS.
- S. A. Kulkarni, S. S. Kadam, H. Meekes, A. I. Stankiewicz and J. H. ter Horst, Cryst. Growth Des., 2013, 13, 2435–2440 CrossRef CAS.
- J. Yang, X. Li, S. Han, R. Yang, P. Min and Z.-Z. Yu, J. Mater. Chem. A, 2018, 6, 5880–5886 RSC.
- C. D. van Engers, N. E. A. Cousens, V. Babenko, J. Britton, B. Zappone, N. Grobert and S. Perkin, Nano Lett., 2017, 17, 3815–3821 CrossRef CAS PubMed.
- D. Mańko, A. Zdziennicka and B. Jańczuk, Appl. Surf. Sci., 2017, 392, 117–125 CrossRef.
- M. O. Dekaliuk, O. Viagin, Y. V. Malyukin and A. P. Demchenko, Phys. Chem. Chem. Phys., 2014, 16, 16075–16084 RSC.
- H. Nosrati, R. S. Mamoory, F. Dabir, D. Q. Svend Le, C. E. Bünger, M. C. Perez and M. A. Rodriguez, Ceram. Int., 2019, 45, 1761–1769 CrossRef CAS.
- P. S. Yoo, B. W. Lee and C. Liu, IEEE Trans. Magn., 2015, 51, 1–4 Search PubMed.
- A. H. Mamaghani, F. Haghighat and C.-S. Lee, J. Photochem. Photobiol., A, 2019, 378, 156–170 CrossRef CAS.
- A. Saptoro, Y. Kanazawa, M. Asada, Y. Asakuma and C. Phan, Exp. Therm. Fluid Sci., 2016, 72, 228–234 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra05989d |
| This journal is © The Royal Society of Chemistry 2022 |