Open Access Article
Open Access ArticleThermodynamics analysis and experiments on Ti-bearing blast furnace slag leaching enhanced by sulfuric acid roasting
Lvshan Zhou *b,
Tongjiang Peng*a,
Hongjuan Suna and
Sanyuan Wangb
*b,
Tongjiang Peng*a,
Hongjuan Suna and
Sanyuan Wangb
aKey Laboratory of Ministry of Education for Solid Waste Treatment and Resource Recycle, Institute of Mineral Materials & Application, Sichuan Engineering Lab of Nonmetallic Mineral Powder Modification & High-quality Utilization, Center of Forecasting and Analysis, School of Environment and Resource, Southwest University of Science and Technology, Mianyang 621010, Sichuan, China. E-mail: tjpeng@swust.edu.cn; kcs103201@163.com
bSchool of Chemistry and Chemical Engineering, Sichuan University of Arts and Science, Dazhou 635000, Sichuan, China
First published on 8th December 2022
Abstract
The potential-pH diagrams of the main components of Ti-bearing blast furnace slag (air-cooled slag) at 298.15 K (25 °C) and an ion activity of 1.00 were drawn by thermodynamic calculation. Thermodynamic analysis showed that the main metal components, when the Ti-bearing blast furnace slag is roasted with concentrated sulfuric acid, could be converted to sulfate. From these analyses, it can be seen that under strong acid conditions, the major metal components could react to form sulfate, and the effective separation of Ti, Mg, and Al can be achieved from both Ca and Si. Further experiments were performed with a 5.0% dilute sulfuric acid solution used to leach a Ti-bearing blast furnace slag sample that had been calcined with concentrated sulfuric acid, at a liquid–solid ratio of 10, a reaction time of 60 min, and a reaction temperature of 338.15 K (65 °C). This led to a leaching ratio of Ti above 85.0%, leaching ratios of Mg and Al higher than 95.0%, and leaching ratios of Fe and Ca of 45.7% and 24.7%, respectively. All these values were higher than the leaching ratios of Ti-bearing blast furnace slag.
1 Introduction
The western Panxi region in China is a well-known place of origin for vanadium-bearing titanomagnetite. After beneficiation, approximately 50.0% of the titanium resources are able to enter the iron concentrate. When the blast furnace method is used to smelt the iron concentrate, the titanium resources enter the blast furnace slag, forming Ti-bearing blast furnace slag. This can afterwards be divided into water-quenched slag and air-cooled slag, according to a cooling method.1 The applications of Ti-bearing blast furnace slag mainly include producing cement, concrete, non-fired and non-steamed bricks, functional materials, and glass-ceramics,2,3 and also the selective extraction of valuable components such as titanium and magnesium.4 The latter process can be mainly divided into two categories: pyrometallurgy and hydrometallurgy.5 Besides pyrometallurgy and hydrometallurgy, there are many reports on the extraction of metals from Ti-bearing blast furnace slag with an electrochemical method, such as molten slag electrolysis, or deoxidation of a solid slag cathode.6,7 The wet extraction process is mainly based on the sulfuric acid method,8 hydrochloric acid method,9 or sodium hydroxide method,10 among other examples. Some research achievements from these works are listed in Table 1. To obtain an air-cooled slag with good crystallinity, the valuable component leaching ratio needs to be low, by using direct acid leaching. To overcome the leaching bottleneck, a salt or acid roasting leaching process represented by ammonium sulfate11,12 and activated roasting-alkali leaching processes13 were reported. In other solid waste applications, the acid roasting leaching process14 has also been effectively developed, providing a reference for the use of Ti-bearing blast furnace slag. Although the new technology has solved the problem of a low leaching ratio, there is still a lack of studies regarding leaching thermodynamic analysis, as well as theoretical studies about the transfer and reaction of the target substance. Without this, it is not possible to achieve an effective foundation for innovation and development of leaching technology.| No. | Leaching agent | Target component | Operating conditions | Result | Reference |
|---|---|---|---|---|---|
| 1 | 50.0 wt% H2SO4 | Ti | Stirring speed 400 rpm, liquid–solid weight ratio 10, reaction temperature 100 °C, reaction time 1 h | 72.3% (water quenched slag) 45.0% (air-cooled slag) | 8 |
| 2 | 20.0 wt% HCl | Ti | Stirring, liquid–solid ratio 100 mL g−1, reaction temperature 100 °C, reaction time 8 h | 44.0% (air-cooled slag) | 9 |
| 3 | 2.0 mol L−1 NaOH | V | Ti-bearing blast furnace slag was modified with titanium slag, SiO2 and O2 at 1450 °C, stirring speed 500 rpm, liquid–solid ratio 3, leaching temperature 95 °C, and leaching time 90 min | 91.3% | 10 |
| 4 | 2.0 mol L−1 HCl | Ti | Ti-bearing blast furnace slag was modified with titanium slag, SiO2 and O2 at 1450 °C, stirring speed 500 rpm, liquid–solid ratio 3, leaching temperature 65 °C, and leaching time 60 min | 97.8% | 10 |
| 5 | 10.0 wt% H2SO4 | Ti | Ti-bearing blast furnace slag was roasted with ammonium sulfate, stirring speed 450 rpm, liquid–solid ratio 5 mL g−1, leaching temperature 90 °C, leaching time 3 h | 94.5% | 11 |
| 6 | 2.5 wt% H2SO4 | Ti | Sulfuric acid curing, stirring speed 300 rpm, leaching temperature 25 °C, leaching time 3 h | 85.96% | 12 |
| 7 | H2O | Al | Ti-bearing blast furnace slag was roasted with NaOH, stirring, liquid–solid ratio 10 mL g−1, leaching temperature 90 °C, leaching time 3 h | 83.0% | 13 |
The potential-pH (E-pH) diagram (Pourbaix diagram) is mainly based on thermodynamic data, and it can reflect the stable existence and transformation trend of substances in different pH systems. It can also intuitively describe several aspects that occur in the solution, like the chemical reaction, equilibrium conditions, progress direction, reaction limits, and others.15 Weathering has an important effect on the extraction of copper, silver, and gold from copper porphyry ore. The results of this study indicated that the mineral near-surface weathering oxidation section corresponds to the oxidation area of the E-pH diagram, while the non-redox enrichment area corresponds to the reducing region. The predicted product is consistent with the actual mineral composition,15 and metal surface passivation is an important means to slow or even prevent metal corrosion. Chandra-ambhorn et al.16 used the E-pH diagram to speculate on the corrosion and passivation of 316 L stainless steel in a mixed solution of sodium chloride and sodium sulfate, verifying the results with lab experiments. These results showed that the E-pH diagram can better predict the corrosion of 316 L stainless steel, as well as provide better theoretical support for its corrosion resistance. Metal recovery in solid waste is an important way to realize solid waste resource utilization. Mu et al.17 reported the pressure conditions and product distribution of leaching vanadium-containing titanium slag, according to the E-pH diagram. Ruwaida et al.18 reported the possibility of monazite acid leaching to recover rare earth metals, based on the E-pH diagram.
Whether looking at hydrometallurgy, metal corrosion, geochemistry, or analytical chemistry, research on E-pH diagrams mostly focuses on pure metal–water systems, and there are still only a small number of studies that cover complex mineral-water systems.19 Although the E-pH diagram is a research result under ideal conditions, it has a powerful role in the development of new industrial production processes, or even the design and preparation of new materials, while it can also provide theoretical support for practical experiments and industrial applications.
The main purpose of hydrometallurgy for Ti-bearing blast furnace slag is the separation of valuable metals.20 Therefore, in hydrometallurgy, the key is for the target components to dissolve as much as possible in the solution. For air-cooled slag, the high crystallization degree makes it difficult for most metals to dissolve, and theoretical research on the leaching process is relatively poor. To achieve a high-efficiency leaching, in this work, a Ti-bearing blast furnace slag was roasted using concentrated sulfuric acid, and the main focus is the chemical reaction and thermodynamics of the leaching process. Based on chemical reaction thermodynamics, E-pH diagram theory, and HSC chemistry 6.0, thermodynamic calculations were carried out, and E-pH diagrams were drawn for the sulfuric acid leaching process on Ti-bearing blast furnace slag. The main objective was to obtain a theoretical scheme for extracting valuable metals from Ti-bearing blast furnace slag, as well as provide theoretical support for the technological design of hydrometallurgy for Ti-bearing blast furnace slag.
2 Materials and methods
2.1 Materials
Titanium-bearing blast furnace slag is an air-cooled slag produced in the Panzhihua Iron and Steel Group in China. After crushing and ball milling, powder with a particle size of less than 0.150 mm is obtained. The sulfuric acid used in the experiment was analytically pure (95.0–98.0 wt%) and purchased from Chengdu Kelong Chemical Reagent Factory. Pure water produced by an ultrapure water machine (Chengdu Ultra Pure Technology Co., Ltd., UPT-11-10T) was used in all experiments.2.2 Experimental
According to previous work from the authors,21 a 100 mL porcelain crucible with a lid was used, containing 10.0 g of Ti-bearing blast furnace slag sample and 15.8 g of concentrated sulfuric acid (which is 1.5 times the stoichiometric ratio of Ti, Mg, Al, and Fe in the sample). The sample was mixed well using a glass rod, and placed for 120 minutes in a 403.15 K (130 °C) tubular reactor for calcination. During the calcination process, air at 5 L min−1 was used to purge the generated gas, while lye and acid were used at the outlets for absorption. Roasted Ti-bearing blast furnace slag (5.0 g) was added to a 100 mL beaker to leach at different values of sulfuric acid concentrations, liquid–solid ratios, reaction times and reaction temperatures, in a constant temperature water bath (Zhengzhou Yingyu Lingke Instrument Equipment Co., Ltd., DF-101S). After the reaction, a filter was applied to obtain the filtrate and residue. The filtrate was analyzed for Ti, Mg, Al, Fe, and Ca content, the residue was washed with ultrapure water to achieve neutrality, and dried in a constant temperature drying oven at 378.15 K (105 °C), and samples were taken for chemical composition analysis. A blank group without sulfuric acid roasting was obtained during the experiment. Three parallel experiments were performed for all samples.2.3 Analysis and characterization
The contents of Ca and Mg in the leaching solution of Ti-bearing blast furnace slag were analyzed using titration,22 and the contents of Ti, Al, and Fe were analyzed using spectrophotometry.23,24 The used instrument was an Evolution™ 300 UV-vis spectrophotometer, produced by Thermo Company in the United States.Phase composition analysis was performed using an X'pert Pro X-ray diffractometer, produced by the Dutch PANalytical Company, for Ti-bearing blast furnace slag raw samples, samples after sulfuric acid roasting, and leaching residues. The test conditions were as follows: Cu target, tube voltage: 40 kV, tube current: 40 mA, transmitting slit (DS): (1/2)°, anti-scatter slit (SS): 0.04 rad, receiving slit (AAS): 5.5 mm, scanning step length: 0.02°, scan range: 3°–80°, continuous scanning.
An Axios X-ray fluorescence spectrometer with a ceramic light tube was used to analyze the chemical composition of the Ti-bearing blast furnace slag sample, with the maximum power set as 2.4 kW. Sample preparation was carried out using the fuse method.
Loss on ignition analysis was performed in a porcelain crucible with a lid that had been calcined to a constant weight. 1.00 g of Ti-bearing blast furnace slag sample (m) was added and the crucible was placed in a 1223.15 K (950 °C) muffle furnace to heat for 15–20 min. Then, the crucible was removed and placed in a desiccator to cool at room temperature, having also been weighed and calcined repeatedly until the crucible weight did not change (m1). The loss on ignition of Ti-bearing blast furnace slag was calculated using XLOI (%) = 100(m − m1)/m.
2.4 E-pH diagram mapping principles
The essence of the acid leaching process is the reaction between H+ ionized by the acid and the leached object, which can be expressed by the general eqn (1).15| aA + xH+ + nE− = bB + yH2O | (1) |
Under isothermal and isopiestic pressure conditions, and ignoring the influence of generated water, the Gibbs free energy change of the reaction can be obtained by eqn (2)–(4).
ΔrG = ΔrGθ + RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln[αBb/(αAa·αH+x)] ln[αBb/(αAa·αH+x)]
| (2) |
| ΔrGθ = ∑viΔfGθ (product) − ∑viΔfGθ (reagent) | (3) |
| ΔrGθ(T) = ΔrHθ(298.15 K) − TΔrSθ(298.15 K) | (4) |
According to the relationship between the Gibbs free energy and electric potential (ΔrG = −nFE), eqn (2) can be expressed as eqn (5).
nFE = −ΔrGθ−2.303RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg(αBb/αAa) − 2.303RTxpH lg(αBb/αAa) − 2.303RTxpH
| (5) |
When n = 0, the pH expression is as shown in eqn (6).
| pH = −ΔrGθ/(2.303RTx) − lg(αBb/αAa)/x | (6) |
When x = 0, the electric potential expression is as shown in eqn (7).
E = −ΔrGθ/nF − 2.303RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg(αBb/αAa)/nF lg(αBb/αAa)/nF
| (7) |
When oxidation–reduction and hydrolysis–neutralization reactions coexist, the electric potential expression is as shown in eqn (8).
E = −ΔrGθ/nF − 2.303RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg(αBb/αAa)/nF − 2.303RTx/nFpH lg(αBb/αAa)/nF − 2.303RTx/nFpH
| (8) |
3 Results and discussion
3.1 Phase and chemical composition analysis of Ti-bearing blast furnace slag (air-cooled slag)
The chemical composition analysis results of Ti-bearing blast furnace slag using the XRF analysis method are displayed in Table 2. The results indicate that the Ti-bearing blast furnace slag mainly includes Ca, Si, Ti, Al, Mg, and Fe, which account for more than 96% of the total chemical content, so the Ti-bearing blast furnace slag has a high comprehensive recycling value. The loss on ignition of the Ti-bearing blast furnace slag was only 4.75%, indicating that its structure is stable and it contains fewer components that are volatile and easy to decompose or gasify.| Composition | Content/% | Composition | Content/% | Composition | Content/% |
|---|---|---|---|---|---|
| a The content of main elements in high-titanium blast furnace slag is expressed in the form of oxides. | |||||
| CaO | 27.37 | SiO2 | 26.51 | TiO2 | 17.92 |
| Al2O3 | 14.33 | MgO | 8.05 | Fe2O3 | 2.59 |
| SO3 | 1.10 | Na2O | 0.77 | MnO | 0.62 |
| K2O | 0.58 | BaO | 0.07 | SrO | 0.04 |
| ZrO2 | 0.02 | Cl | 0.02 | Y2O3 | 0.01 |
| Loss on ignition | 4.75 | ||||
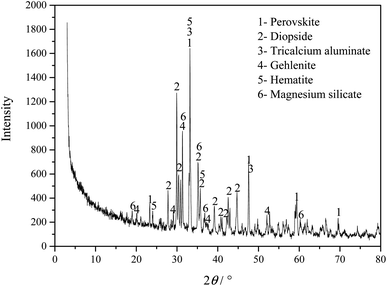
Fig. 1 shows the phase composition analysis of the Ti-bearing blast furnace slag (air-cooled slag), which shows that because the air-cooled slag was naturally cooled, the formed crystal phases have sharp diffraction peaks and high crystallinity. The Ti-bearing blast furnace slag mainly comprises perovskite (CaTiO3, PDF#22-0153), diopside (Ca(Mg, Al))(Si, Al)2O6, (PDF#41-1370), tricalcium aluminate (Ca3Al2O6, PDF#38-1429), gehlenite (Ca2Al2SiO7, PDF#35-0755), hematite (Fe2O3, PDF# 33-0664), and magnesium silicate (MgSiO3, PDF#39-0048).
3.2 Thermodynamic analysis of Ti-bearing blast furnace slag roasted with concentrated sulfuric acid
The possible reactions in the roasting process are shown in eqn (9)–(16), which take into account the roasting of Ti-bearing blast furnace slag with concentrated sulfuric acid, and the phase analysis results.| CaTiO3 + 2H2SO4 = CaSO4 + TiOSO4 + 2H2O | (9) |
| CaMgSi2O6 + 2H2SO4 = CaSO4 + MgSO4 + 2SiO2 + 2H2O | (10) |
| Ca3Al2O6 + 6H2SO4 = 3CaSO4 + Al2(SO4)3 + 6H2O | (11) |
| Ca2Al2SiO7 + 5H2SO4 = 2CaSO4 + Al2(SO4)3 + SiO2 + 5H2O | (12) |
| MgSiO3 + H2SO4 = MgSO4 + SiO2 + H2O | (13) |
| Fe2O3 + 3H2SO4 = Fe2(SO4)3 + 3H2O | (14) |
| 2Fe2O3 + 4H2SO4 = 4FeSO4 + 4H2O + O2 | (15) |
| CaTiO3 + H2SO4 = CaSO4 + TiO2 + H2O | (16) |
Fig. 2 shows graphs plotting the Gibbs free energy change ΔG, and equilibrium constant K for different reactions, when both Ti-bearing blast furnace slag and concentrated sulfuric acid are roasted at different temperatures. The results in Fig. 2 show that the main components in Ti-bearing blast furnace slag can spontaneously react with concentrated sulfuric acid during the roasting process. The equilibrium constants of reactions eqn (11) and (12) are very high, which indicates that the reaction proceeded very thoroughly, and the aluminum in the Ti-bearing blast furnace slag can be easily converted into soluble aluminum sulfate, which is beneficial to leaching. The Gibbs free energies of reactions eqn (13)–(15) are high, but the equilibrium constant is smaller, which means that these reactions can occur in the range of 273.15 K (0 °C) –473.15 K (200 °C), but the degree of positive reaction may not be high. Regarding reaction (16), it is possible that calcium titanate can be transformed into titanium dioxide, but titanium dioxide will decompose in hot concentrated sulfuric acid, and later transform into titanyl sulfate.13
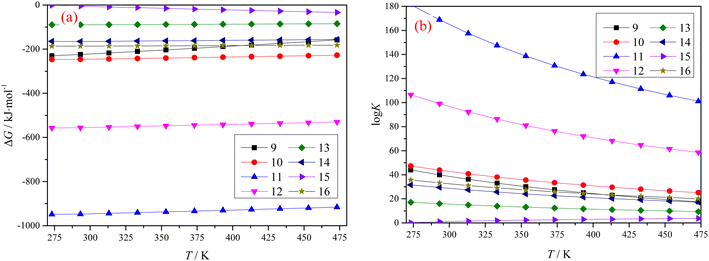 | ||
| Fig. 2 Gibbs free energy change ΔG (a) and equilibrium constant K (b) of different reactions with temperature. | ||
3.3 E-pH diagram of the main components in Ti-bearing blast furnace slag
According to the phase and chemical composition analysis, Ti-bearing blast furnace slag mainly contains perovskite and silicate. The main components were first selected, and the drawing was carried out in light of E-pH diagram principles. Table 3 gives the standard Gibbs free energy that is required for each substance in the E-pH diagram drawing process. When drawing the E-pH diagram of the main component in the Ti-bearing blast furnace slag with a sulfuric acid solution system, the ion concentration is used instead of the ion activity. It is supposed that each ion concentration in the system is 1 mol L−1, and at 298.15 K (25 °C), both the main reaction equilibrium equation and the calculation formula of E-pH are listed in Table 4.| Substance | ΔfGθ/kJ mol−1 | Substance | ΔfGθ/kJ mol−1 | Substance | ΔfGθ/kJ mol−1 |
|---|---|---|---|---|---|
| a c-Crystal, l-liquid, g-gas, aq-aqueous solution, *data were checked by HSC Chemistry 6.0 software. | |||||
| H2(g) | 0 | H+(aq) | 0 | H2O(l) | −237.1 |
| OH−(aq) | −157.3 | O2(g) | 0 | SO42−(aq) | −744.5 |
| CaSO4·2H2O(c) | −1797.5 | CaSO4·0.5H2O(c) | −1436.8 | CaSO4(c) | −1309.1 |
| Ca(OH)2(c) | −897.5 | CaTiO3(c)* | −1688.7 | CaMgSi2O6(c)* | −3248.8 |
| Ca2Al2SiO7(c)* | −4048.5 | Ca3Al2O6(c)* | −3649.2 | Ca2+(aq) | −553.5 |
| H4SiO4(c) | −1333.0 | H2SiO3(c) | −1092.4 | HSiO3−(aq) | −1152.1 |
| SiO2(c) | −856.4 | Mg(c) | 0 | Mg2+(aq) | −454.8 |
| MgO(c) | −569.3 | Mg(OH)2(c) | −833.7 | MgSiO3(c) | −1462.0 |
| Ti(OH)3(c) | −1049.8 | TiO(OH)2(c)* | −1086.7 | Ti3+(aq) | −349.78 |
| TiO2+(aq) | −633.1 | Ti2O3(c) | −1434.2 | TiOSO4(c)* | −1549.9 |
| Fe(c) | 0 | Fe(OH)3(c) | −705 | Fe(OH)2(c) | −490.0 |
| Fe2O3(c) | −742.2 | Fe3O4(c) | −1015.4 | FeOOH(c) | −578.0 |
| Fe3+(aq) | −4.7 | Fe2+(aq) | −78.9 | Al3+(aq) | −485.3 |
| AlO2−(aq) | −830.9 | Al(OH)3(c) | −1306.0 | ||
| No. | Reaction | E-pH equation |
|---|---|---|
| a The reaction Gibbs free energy was obtained using HSC Chemistry 6.0. | ||
| a | O2 + 4H+ + 4e− = 2H2O | E = 1.228 − 0.0592pH |
| b | 2H+ + 2e− = H2 | E = −0.0592pH |
| 1 | CaTiO3 + 4H+ = Ca2+ + TiO2+ + 2H2O | pH = −1.222 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Ca2+][TiO2+]) lg([Ca2+][TiO2+]) |
| 2 | CaTiO3 + 4H+ + SO42− = CaSO4·2H2O + TiO2+ | pH = −0.114 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([TiO2+]/[SO42−]) lg([TiO2+]/[SO42−]) |
| 3 | CaTiO3 + 3H+ + e− + SO42− + 2H2O = CaSO4·2H2O + Ti(OH)3 | E = −0.623 − 0.177pH − 0.0592![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg(1/[SO42−]) lg(1/[SO42−]) |
| 4 | 2CaTiO3 + 6H+ + 2SO42− + 2e− = 2CaSO4·0.5H2O + Ti2O3 + 2H2O | E = −0.437 − 0.178pH − 0.0296![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg(1/[SO42−]2) lg(1/[SO42−]2) |
| 5 | CaTiO3 + 4H+ + SO42− = Ca2+ + TiOSO4 + 2H2O | pH = 6.324 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Ca2+]/[SO42−]) lg([Ca2+]/[SO42−]) |
| 6a | CaTiO3 + 4H+ + 2SO42− = CaSO4·2H2O + TiOSO4 | pH = 4.873 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg(1/[SO42−]2) lg(1/[SO42−]2) |
| 7 | CaTiO3 + SO42− + 6H+ + e− = CaSO4·2H2O + Ti3+ + H2O | E = −0.506 − 0.355pH − 0.0592![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Ti3+]/[SO42−]) lg([Ti3+]/[SO42−]) |
| 8a | CaMgSi2O6 + 4H+ + 2H2O = Ca2+ + Mg2+ + 2H4SiO4 | pH = 5.919 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Ca2+][Mg2+]) lg([Ca2+][Mg2+]) |
| 9a | CaMgSi2O6 + 4H+ = Ca2+ + Mg2+ + 2SiO2 + 2H2O | pH = 7.155 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Ca2+][Mg2+]) lg([Ca2+][Mg2+]) |
| 10a | CaMgSi2O6 + 4H+ = Ca2+ + Mg2+ + 2H2SiO3 | pH = 7.040 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Ca2+][Mg2+]) lg([Ca2+][Mg2+]) |
| 11a | CaMgSi2O6 + 4H+ + SO42− + 4H2O = CaSO4·2H2O + Mg2+ + 2H4SiO4 | pH = 7.043 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Mg2+]/[SO42−]) lg([Mg2+]/[SO42−]) |
| 12a | CaMgSi2O6 + 2H+ + SO42− + 4H2O = CaSO4·2H2O + Mg(OH)2 + 2H2SiO3 | pH = 7.918 − 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg(1/[SO42−]) lg(1/[SO42−]) |
| 13a | CaMgSi2O6 + 2H+ + SO42− + 2H2O = CaSO4·2H2O + Mg(OH)2 + 2SiO2 | pH = 8.148 − 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg(1/[SO42−]) lg(1/[SO42−]) |
| 14 | Ca3Al2O6 + 12H+ = 3Ca2+ + 2Al3+ + 6H2O | pH = 5.905 − 0.0833![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Ca2+]3[Al3+]2) lg([Ca2+]3[Al3+]2) |
| 15a | Ca3Al2O6 + 6H+ = 3Ca(OH)2 + 2Al3+ | pH = 7.790 − 0.167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Al3+]2 lg[Al3+]2 |
| 16a | Ca3Al2O6 + 12H+ + 3SO42− = 3CaSO4·2H2O + 2Al3+ | pH = 10.662 − 0.0833![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Al3+]2/[SO42−]3) lg([Al3+]2/[SO42−]3) |
| 17a | Ca2Al2SiO7 + 4H+ + 3H2O = 2Ca2+ + 2Al(OH)3 + H4SiO4 | pH = 8.804 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Ca2+]2 lg[Ca2+]2 |
| 18a | Ca2Al2SiO7 + 4H+ + 2H2O = 2Ca2+ + 2Al(OH)3 + H2SiO3 | pH = 8.788 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Ca2+]2 lg[Ca2+]2 |
| 19 | Ca2Al2SiO7 + 10H+ = 2Ca2+ + 2Al3+ + 4H2O + H2SiO3 | pH = 1.224 − 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Ca2+]2[Al3+]2) lg([Ca2+]2[Al3+]2) |
| 20 | Ca2Al2SiO7 + 10H+ + 2SO42− + H2O = 2CaSO4·2H2O + 2Al3+ + H4SiO4 | pH = 2.172 − 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Al3+]2/[SO42−]2) lg([Al3+]2/[SO42−]2) |
| 21a | Ca2Al2SiO7 + 4H+ + 2SO42− + 7H2O = 2CaSO4·2H2O + 2Al(OH)3 + H4SiO4 | pH = 11.052 − 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg(1/[SO42−]2) lg(1/[SO42−]2) |
| 22 | Ca2Al2SiO7 + 10H+ + 2SO42− = 2CaSO4·2H2O + 2Al3+ + H2SiO3 | pH = 2.111 − 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg([Al3+]2/[SO42−]2) lg([Al3+]2/[SO42−]2) |
| 23 | Fe2+ + 2e− = Fe | E = −0.409 − 0.0296![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Fe2+] lg[Fe2+] |
| 24 | Fe3+ + e− = Fe2+ | E = 0.769 − 0.0592![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Fe2+]/[Fe3+] lg[Fe2+]/[Fe3+] |
| 25 | Fe(OH)3 + 3H+ = Fe3+ + 3H2O | pH = 1.14 − 0.333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Fe3+] lg[Fe3+] |
| 26 | Fe(OH)3+3H+ + e− = Fe2+ + 3H2O | E = 0.971− 0.177pH − 0.0592![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Fe2+] lg[Fe2+] |
| 27 | Fe(OH)2 + 2H+ = Fe2+ + 2H2O | pH = 6.47 − 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Fe2+] lg[Fe2+] |
| 28 | Fe(OH)3 + H+ + e− = Fe(OH)2 + H2O | E = 0.208 − 0.059pH |
| 29 | Fe(OH)2 + 2H+ + 2e− = Fe + 2H2O | E = −0.026 − 0.059pH |
| 30 | Fe2O3 + 6H+ = 2Fe3+ + 3H2O | pH = −0.628 − 0.167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Fe3+]2 lg[Fe3+]2 |
| 31 | Fe2O3 + 6H+ + 2e− = 2Fe2+ + 3H2O | E = 0.658 − 0.178pH − 0.0296![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Fe2+] lg[Fe2+] |
| 32a | Fe3O4 + 8H+ + 2e− = 3Fe2+ + 4H2O | E = 1.092 − 0.237pH − 0.0296![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Fe2+] lg[Fe2+] |
| 33a | FeOOH + 3H+ = Fe3+ + 2H2O | pH = 0.131 − 0.333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Fe3+] lg[Fe3+] |
| 34a | FeOOH + 3H+ + e− = Fe2+ + 2H2O | E = 0.794 − 0.178pH − 0.0592![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[Fe2+] lg[Fe2+] |
| 35a | FeOOH + H+ + e− = Fe(OH)2 | E = 0.0282 − 0.0592pH |
| 36a | Fe2O3 + 2H+ + 2e− + H2O = 2Fe(OH)2 | E = 0.0296 − 0.0592pH |
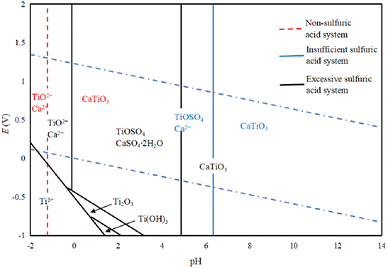
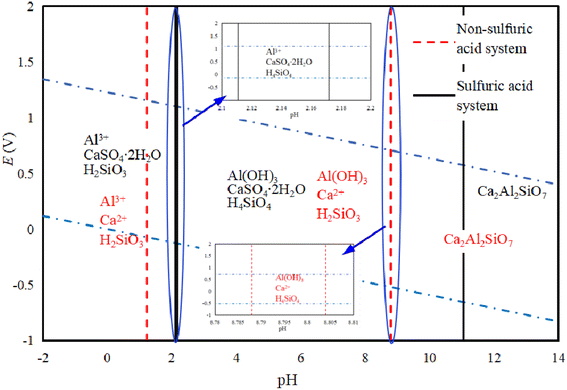
Fig. 3 shows the E-pH diagram of perovskite in a sulfuric acid solution system, which leads to the conclusion that CaTiO3 is relatively stable in a generally aqueous solution system, while TiO2+ will only be generated when the pH is lower than −1.20. In a sulfuric acid solution system, CaTiO3 can react with H2SO4 to generate a more soluble TiOSO4, where the pH of TiO2+ is higher than the one without sulfuric acid. When the sulfuric acid amount is insufficient, calcium is present as Ca2+, while in the case when the amount is sufficient, CaSO4·2H2O will be generated. In the low potential area, the Ti in CaTiO3 can be converted to Ti3+, but it is not stable and will produce Ti(OH)3, Ti2O3, and other products.
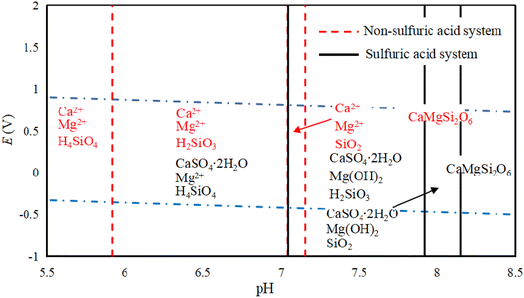
Fig. 4 show the E-pH diagram of diopside in a sulfuric acid solution system. In the solution system, CaMgSi2O6 (diopside) can be decomposed by acid, and Si mainly exists in the form of orthosilicic or metasilicic acid. The existing forms of Mg and Ca are related to the acidic medium, and in a non-sulfuric acid system, they mainly exist in the form of Mg2+ and Ca2+. The main reason for this is that the pH of calcium and magnesium ions that are beginning to precipitate at 298.5 K is 11.4 and 9.43, respectively. Ca2+ and Mg2+ in acidic systems can exist stably without being hydrolyzed to Ca(OH)2 or Mg(OH)2, while in a sulfuric acid medium, Ca mainly forms CaSO4·2H2O, while Mg is hydrolyzed in alkaline solution to produce Mg(OH)2. According to a published work, pyroxene can be decomposed under acidic conditions.26 Regarding diopside, its acid solubility is weak, but its structure can be destroyed by ball milling, thereby increasing its decomposition rate.27
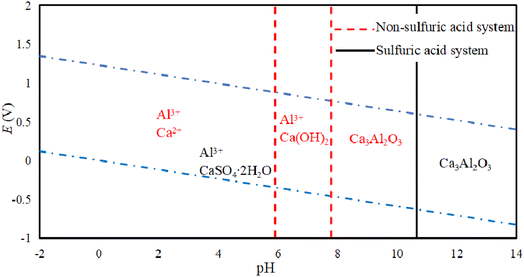
Fig. 5 shows an E-pH diagram of tricalcium aluminate in a sulfuric acid solution system. In an acidic system, Ca3Al2O3 can react with acid to form soluble salts. In the case of Al3+, it can be widely present in systems with a pH lower than 7.80. Because of the presence of SO42− in the system, its existence area tends to expand for Al3+, while Ca2+ released from other ions in the system will quickly generate calcium-containing substances with low solubility, which is consistent with conclusions of Zhao's work.28 It is known that only when pH > 15.3 will Al(OH)3 be generated, while the presence of SO42− will increase the environmental pH requirement for Al(OH)3 generation. Lapeyre et al.29 found that Ca3Al2O3 can react to form Al(OH)4− and CaAl-OH-LDH (layered double hydroxides, LDH), and when SO42− exists, the anions in CaAl-OH-LDH can be exchanged to form CaAl–SO4-LDH. This compound is then hydrated to form Ca3Al2O6·6H2O, although the reaction conditions are preferably alkaline.
Fig. 6 shows the E-pH diagram of yellow feldspar in the sulfuric acid solution system, illustrating that Ca2Al2SiO7 can be converted into soluble calcium and aluminum salts under acidic conditions, where Si exists both as H2SiO3 or H4SiO4. Under alkaline conditions, Al is converted to Al(OH)3, while Ca still exists mainly as ions in the solution. When there is SO42− in the system, Ca is converted to CaSO4·2H2O, while in a system without SO42−, Al3+ can exist under strong acidic conditions, where the increase in pH will promote its hydrolysis to produce Al(OH)3. The experiment also showed that Ca2Al2SiO7 can be decomposed and Al3+ and Ca2+ are released when the pH is lower than 1,30,31 and in contrast to Ca3Al2O3, this compound is not prone to hydration reactions.32 There is a salt of a weak acid base containing Al and Si in the system, where the double hydrolysis reaction can promote the hydrolysis of Al3+, but the conditions are not reached for Ca(OH)2 precipitation in an aqueous solution system with a pH lower than 8.8.33 In the SO42− containing system, the conversion of Ca into CaSO4·2H2O promotes the decomposition of Ca2Al2SiO7 and increases the decomposition pH.
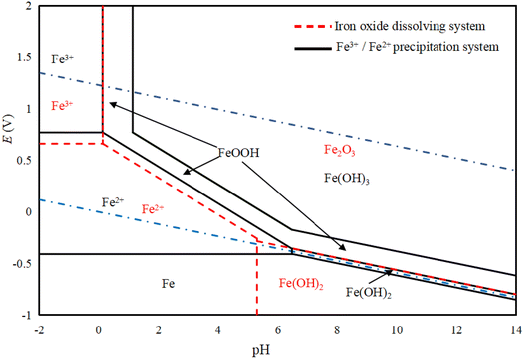
Based on Fig. 7, FeOOH, Fe(OH)3, and Fe2O3 can stably exist in both the water stable region and oxygen stable region. For Fe2+, its stable region is larger than that of Fe3+ in the water stable region, and it is possible for it to be oxidized in the aqueous solution system. When Fe2O3 reacts with an acid, it directly transforms to Fe2+ or Fe3+, without undergoing the formation of FeOOH. In the case of the hydrolysis of Fe2+ and Fe3+, FeOOH is generated first, and then oxidized to Fe(OH)3, which is consistent with the actual experimental results. FeOOH can be prepared in the pH range, and it can be converted into Fe(OH)3 by changing the operating conditions.34
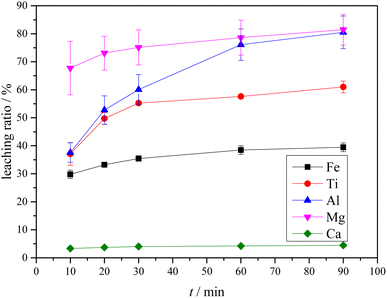
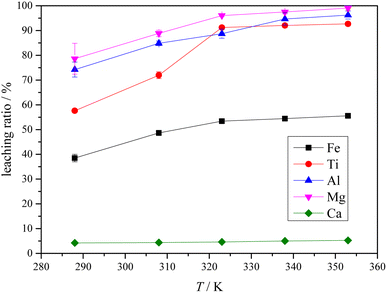
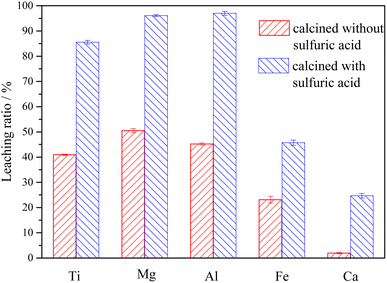
3.4 Leaching of Ti-bearing blast furnace slag activated by sulfuric acid roasting
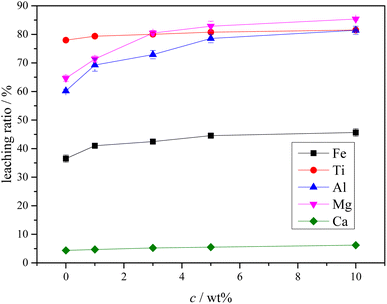
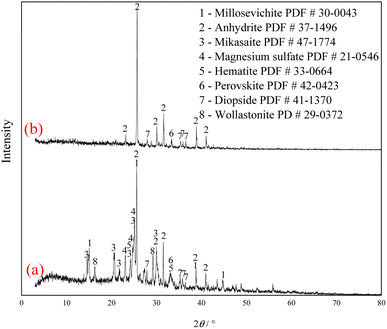
The XRD analysis results of the Ti-bearing blast furnace slag roasted with sulfuric acid and residue of dilute sulfuric acid solution leaching are shown in Fig. 13. In Fig. 13(a), it is clear that the perovskite, pyroxene, maghemite, and tricalcium aluminate in the Ti-bearing blast furnace slag can be converted into sulfate, which is easily soluble in water, when activated by concentrated sulfuric acid roasting. Ti, Mg, Al, and Fe are converted to sulfate and dissolved in the solution, while Ca and Si are enriched in the solid phase. The experimental results show that there is still a certain amount of Fe2O3 (hematite) in the roasting slag (shown in Fig. 13(a)). The main reason for this is that concentrated sulfuric acid has difficulty ionizing H+ in a non-water environment, which makes the acidolysis reaction of Fe2O3 difficult. This achieved result is consistent with the conclusion that the equilibrium constant is small by thermodynamic analysis. There is a small amount of unreacted CaTiO3 (perovskite) in the remaining slag after leaching (shown in Fig. 13(b)), and other Ti is transformed into amorphous TiOSO4, which is dissolved in the sulfuric acid solution during leaching.11 These experiments showed that the leaching solution was green, and when a small amount of potassium hexacyanoferrate(III) (K3[Fe(CN)6]) was dropped into it, a blue precipitate was generated in the solution. This phenomenon shows that there are Fe2+ ions in the leaching solution.
4 Conclusions
Based on the theory of chemical reaction thermodynamics and the potential-pH diagram, the reaction thermodynamics of Ti-bearing blast furnace slag activated by concentrated sulfuric acid was studied. This analysis included the design of the sulfuric acid leaching potential-pH diagram of the main components, as well as a leaching experiment of valuable components in Ti-bearing blast furnace slag roasted with sulfuric acid. The main conclusions are the following:(1) The crystallinity degree of Ti-bearing blast furnace slag was good when it was naturally cooled in the air. The formed perovskite, diopside, tricalcium aluminate, maghemite, hematite, and magnesium silicate could spontaneously react to form sulfate in a concentrated sulfuric acid environment. In addition to the formation reaction of ferrous sulfate, the forward reaction of other chemical reactions can be promoted under heating.
(2) Based on the E-pH diagrams of CaTiO3, CaMgSi2O6, Ca3Al2O3, Ca2Al2SiO7, and Fe2O3, several elements (Ti, Mg, Al, and Fe) could be converted into easily soluble sulfates, under the strong acid system, leading to the achievement of the purpose of their effective separation from Ca and Si.
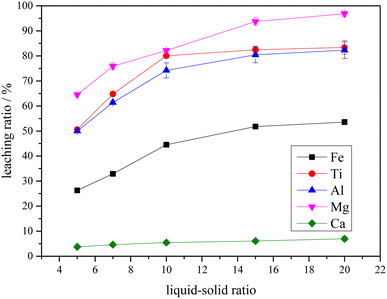
(3) The Ti-bearing blast furnace slag roasted with concentrated sulfuric acid at 403.15 K (130 °C) was used for leaching. When the leaching agent was a 5% dilute sulfuric acid solution, the reaction operating parameters were: a liquid–solid ratio of 10, a reaction time of 60 min, and a reaction temperature of 338.15 K (65 °C). This led to a leaching ratio of Ti over 85.0%, leaching ratios of Mg and Al higher than 95.0%, and leaching ratios of Fe and Ca of 45.7% and 24.7%, respectively, which are higher than the leaching ratios of Ti-bearing blast furnace slag without roasting activation under the same conditions.
(4) Compared with reports in the literature,8 the leaching ratio of titanium increases by approximately 40%, the sulfuric acid amount is reduced by approximately 45%, and the operating conditions are milder.
Author contributions
L. Zhou contributed to the literature search, study design, experimental study, data analysis, and writing—review and editing; T. Peng contributed to the design of the work and data analysis; H. Sun and S. Wang contributed to the work of writing—review and editing.Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.Acknowledgements
This work was financially supported by the Programs of the National Natural Science Foundation of China (No. 41972042), the Sichuan Science and Technology Program (No. 2022YFG0277), and the Opening Project of Key Laboratory of Green Chemistry of Sichuan Institutes of Higher Education (No. LZJ2202).References
- L. Liu and S. L. Yang, Present State and Perspectives of Complex Utilization on Panzhihua BF Slag, Light Metals, 2007, pp. 48–50, DOI:10.13662/j.cnki.qjs.2007.07.013.
- L. X. He, Application of High Ti-Bearing Blast Furnace Slag in Field of Building Materials, Adv. Mater. Res., 2014, 1052, 392–395, DOI:10.4028/www.scientific.net/AMR.1052.392.
- K. Chen, Y. Li, X. Meng, L. Meng and Z. Guo, New integrated method to recover the TiO2 component and prepare glass-ceramics from molten titanium-bearing blast furnace slag, Ceram. Int., 2019, 45, 24236–24243, DOI:10.1016/j.ceramint.2019.08.134.
- W. Zhang, L. Zhang, Y. Li and X. Li, An environmental procedure to extract titanium components and metallic iron from Ti-bearing blast furnace slag, Green Process. Synth., 2015, 4, 307–316, DOI:10.1515/gps-2015-0031.
- Y. Yang, J. L. Liang, H. Li, D. X. Huo and S. S. Xie, Resource Recycling progress of Panzhihua Titanium Bearing Blast Furnace Slag, Multipurpose Utilization of Mineral Resources, 2018, pp. 12–15, DOI:10.3969/j.issn.1000-6532.2018.02.003.
- Q. Zhao, N. Ye, L. Li and F. Yan, Oxalate Coprecipitation Process Synthesis of 5 V Cathode Material LiNi0.5Mn1.5O4 and Its Performance, Xiyou Jinshu Cailiao Yu Gongcheng, 2010, 39, 1715–1718, DOI:10.1016/S1875-5372(10)60130-0.
- X. Zou, X. Lu, W. Xiao, Z. Zhou, Q. Zhong and W. Din, Direct electrochemical extraction of Ti5Si3from Ti/Si-containing metal oxide compounds in molten CaCl2, Shanghai Jiaotong Daxue Xuebao, 2013, 18, 111–117, DOI:10.1007/s12204-013-1373-6.
- T. Jiang, H. G. Dong, Y. F. Guo, G. H. Li and Y. B. Yang, Study on leaching Ti from Ti bearing blast furnace slag by sulphuric acid, Miner. Process. Extr. Metall., 2010, 119, 33–38, DOI:10.1179/037195509x12585446038807.
- F. Jia, R. Liu and M. Yang, Leaching of High-Ti-Bearing Blast-Furnace Slag Directly in Hydrochloric Acid, Nanjing Shida Xuebao, Ziran Kexueban, 2012, 12, 43–49 CAS.
- Han, J. Zhang, J. Zhang, X. Chen and L. Zhang, Extraction of vanadium and enrichment of titanium from modified Ti-bearing blast furnace slag, Hydrometallurgy, 2021, 201, 105577 CrossRef CAS.
- Z. Bian, Y. L. Feng and H. R. Li, Extraction of valuable metals from Ti-bearing blast furnace slag using ammonium sulfate pressurized pyrolysis-acid leaching processes, Trans. Nonferrous Met. Soc. China, 2020, 30, 2836–2847, DOI:10.1016/s1003-6326(20)65425-5.
- L. Wang, L. Chen, W. Liu, G. Zhang, S. Tang, H. Yue, B. Liang and D. Luo, Recovery of titanium, aluminum, magnesium and separating silicon from titanium-bearing blast furnace slag by sulfuric acid curing–leaching, Int. J. Miner., Metall. Mater., 2022, 29, 1705–1714, DOI:10.1007/s12613-021-2293-3.
- S. He, T. Peng and H. Sun, Titanium Recovery from Ti-Bearing Blast Furnace Slag by Alkali Calcination and Acidolysis, JOM, 2019, 71, 3196–3201, DOI:10.1007/s11837-019-03575-9.
- J. Kim and G. Azimi, Recovery of scandium and neodymium from blast furnace slag using acid baking-water leaching, RSC Adv., 2020, 10, 31936–31946, 10.1039/d0ra05797e.
- H. H. Huang, The Eh-pH Diagram and Its Advances, Metals, 2016, 6, 23, DOI:10.3390/met6010023.
- S. Chandra-ambhorn, W. Wachirasiri and G. Lothongkum, E-pH Diagrams for 316 L Stainless Steel in Chloride Solutions Containing SO42-, Anti-Corros. Methods Mater., 2016, 63, 431–436, DOI:10.1108/ACMM-10-2014-1454.
- W. Mu, T. Zhang, Z. Dou, G. LÜ and Y. Liu, φ-pH diagram of V-Ti-H2O system during pressure acid leaching of converter slag containing vanadium and titanium, Trans. Nonferrous Met. Soc. China, 2011, 21, 2078–2086, DOI:10.1016/s1003-6326(11)60976-x.
- N. Y. Fatin, N. M. S. Liyana, H. W. I. Wan and A. R. Ruwaida, Thermodynamic Evaluation of the Aqueous Stability of Rare Earth Elements in Sulfuric Acid Leaching of Monazite through Pourbaix Diagram, Anti-Corros. Methods Mater., 2019, 19, 1647–1656, DOI:10.1016/j.matpr.2019.11.193.
- Z. Cao, B. Ma, C. Wang, Y. Chen, B. Liu, P. Xing and W. Zhang, E-pH diagrams for the metal-water system at 150 °C: thermodynamic analysis and application for extraction and separation of target metals from saprolitic laterite, Miner. Eng., 2020, 152, 106365, DOI:10.1016/j.mineng.2020.106365.
- Y. Cai, N. Song, Y. Yang, L. Sun, P. Hu and J. Wang, Recent progress of efficient utilization of titanium-bearing blast furnace slag, Int. J. Miner., Metall. Mater., 2022, 29, 22–31, DOI:10.1007/s12613-021-2323-1.
- S. He, Study on Principle and Chemical Kinetics of Valuable Components Extraction and Separation From Panzhihua High Titanium-Blast Furnace Slag, Doctor dissertation, Southwest University of Science and Technology, China, 2020.
- Z. Zhang, X. H. Fan and L. H. Xue, Continuous determination of calcium and magnesium in iron ore by EDTA complexometric titration, Yejin Fenxi, 2011, 31, 74–78, DOI:10.13228/j.issn.1000.
- GB/T 5124.4-1985, Methods for chemical analysis of cemented carbide-the peroxide photometric method for the determination of titanium content, China Standard Press. Beijing, 1985 Search PubMed.
- GB/T 5750.6-2006, Standard Inspection Method for Drinking Water-Metal Index. China Standard Press, Beijing, 2006 Search PubMed.
- J. G. Speight, Lange's Handbook of Chemistry, 16th edn, 2005 Search PubMed.
- P. Semsari Parapari, M. Irannajad and A. Mehdilo, Effect of acid surface dissolution pretreatment on the selective flotation of ilmenite from olivine and pyroxene, Int. J. Miner. Process., 2017, 167, 49–60, DOI:10.1016/j.minpro.2017.07.017.
- Y. Xiong, C. Li, B. Liang and J. Xie, Leaching behavior of air cooled Ti-bearing blast-furnace slag in hydrochloric acid, Chin. J. Nonferrous Met., 2008, 18, 557–563, DOI:10.19476/j.ysxb.1004.0609.2008.03.030.
- M. Zhao, Removal and Mechanism of Inorganic Anions from Aqueous Solution by Tricalcium Aluminate, Master degree thesis, Nanchang University, 2014.
- J. Lapeyre, H. Ma, M. Okoronkwo, G. Sant and A. Kumar, Influence of water activity on hydration of tricalcium aluminate-calcium sulfate systems, J. Am. Ceram. Soc., 2020, 103, 3851–3870, DOI:10.1111/jace.17046.
- K. K. Takaaki Wajima, H. Ishimoto, O. Tamada and T. Nishiyama, The synthesis of zeolite-P, Linde Type A, and hydroxysodalite zeolites from paper sludge ash at low temperature (80 °C): optimal ash-leaching condition for zeolite synthesis, Am. Mineral., 2015, 89, 1694–1700, DOI:10.2138/am-2004-11-1215.
- T. Wajima, Effects of Step-Wise Acid Leaching with HCl on Synthesis of Zeolitic Materials from Paper Sludge Ash, Minerals, 2020, 10, 402, DOI:10.3390/min10050402.
- G. Li, P. B. Bai, Y. M. Tian and X. C. Kong, Preparation and Properties of Gehlenite with Magnesium Slag as a raw Material, Journal of Taiyuan University of Science and Technology, 2013, 34, 435–439, DOI:10.3969/j.issn.1673-2057.2013.06.008.
- J. Qin, C. Yang, C. Cui, J. Huang, A. Hussain and H. Ma, Ca2+ and OH− release of ceramistes containing anorthite and gehlenite prepared from waste lime mud, J. Environ. Sci., 2016, 47, 91–99, DOI:10.1016/j.jes.2016.03.013.
- X. Xiong and L. X. Zhou, synthesis of iron oxyhydroxides of different crystal forms and their roles in adsorption and removal of Cr(VI)from aqueous solutions, Acta Petrol. Mineral., 2008, 27, 559–566 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |