Open Access Article
Open Access ArticleIn situ fabrication of a novel CdS/ZnIn2S4/g-C3N4 ternary heterojunction with enhanced visible-light photocatalytic performance†
Jingzhe Lia,
Yue Chena,
Liezhen Zhua,
Linfa Liaoa,
Xinmao Wanga,
Xun Xu a,
Lingfang Qiua,
Jiangbo Xi
a,
Lingfang Qiua,
Jiangbo Xi b,
Ping Li
b,
Ping Li *a and
Shuwang Duo
*a and
Shuwang Duo *a
*a
aJiangxi Key Laboratory of Surface Engineering, School of Materials and Mechanical & Electrical Engineering, Jiangxi Science and Technology Normal University, Nanchang, Jiangxi 330013, P. R. China. E-mail: lp1849065552@163.com; dsw@jxstnu.edu.cn
bKey Laboratory of Novel Biomass-Based Environmental and Energy Materials in Petroleum and Chemical Industry, Key Laboratory of Green Chemical Engineering Process of Ministry of Education, Engineering Research Center of Phosphorus Resources Development and Utilization of Ministry of Education, Hubei Key Laboratory of Novel Reactor and Green Chemical Technology, School of Chemistry and Environmental Engineering, Wuhan Institute of Technology, Wuhan, 430073, P. R. China
First published on 11th November 2022
Abstract
In this study, a novel g-C3N4-based ternary heterojunction was rationally designed and constructed by the in situ growth of ZnIn2S4 nanosheets and CdS nanoparticles onto the g-C3N4 nanosheets using a facile two-step oil-bath method. Through optimizing the proportion of ZnIn2S4 and CdS component, g-C3N4 nanosheets coupled with ZnIn2S4 nanosheets and CdS nanoparticles (denoted as CdS/ZnIn2S4/g-C3N4) exhibited obviously higher photocatalytic properties for RhB removal than the single-component and dual-component systems. Among the as-obtained ternary photocatalysts, it was found that the ternary CdS/ZnIn2S4/g-C3N4-0.2 photocatalyst displayed the optimum photocatalytic property (96%) within a short time (30 min), which was almost 27.42 and 1.17 times higher than that of pure g-C3N4 and binary ZnIn2S4/g-C3N4-0.7 composite. The excellent activity of the ternary CdS/ZnIn2S4/g-C3N4 heterostructure is assigned to the synergetic effects of CdS nanoparticles, ZnIn2S4 nanosheets and g-C3N4 nanosheets, which not only broaden the visible-light absorption range, but also improve the charge mobility and separation rate, thus boosting the visible-light-driven photocatalytic property of g-C3N4.
1. Introduction
With the rapid development of modern industrialization, environmental pollution, especially water pollution, has become a deadly threat to human health and environmental security due to its long-term harm to human beings and aquatic organisms.1–3 Traditional techniques such as physical,4 chemical5 and biological methods6 for eliminating residual organic contaminants from water suffer from inefficiency and secondary pollution. Nowadays, photocatalysis, as a green technology, has been widely accepted as a potential alternative method for energy conversion and environmental governance due to its high efficiency and environmentally friendliness.7–9 Unfortunately, insufficient utilization of visible light restricts the practical application of conventional photocatalysts such as TiO2 and ZnO.10,11 Thus, the development of efficient visible-light-responsive photocatalysts is still greatly desired.In recent years, the use of two-dimensional (2D) metal-free graphite carbon nitride (g-C3N4) in the field of visible-light photocatalysis for hydrogen production,12,13 CO2 reduction14,15 and organic pollutants degradation16,17 has attracted extensive attention owing to its excellent stability and environment friendly preparation process. Nonetheless, the pristine g-C3N4 still suffers from poor separation of the photo-induced charge carriers and insufficient absorption of visible light, which severely restrict its actual application.18,19 Various strategies, such as construction of heterojunctions,20,21 elements doping,22,23 and morphologies regulation,24,25 have been proposed to control and modify g-C3N4. Of which, construction of heterojunctions with other narrow-band-gap semiconductors is designated as an effective strategy to overcome the above-mentioned shortcomings of unitary g-C3N4.26,27 Indium zinc sulfide (ZnIn2S4), as a ternary chalcogenide, has been widely used in the field of photocatalysis owing to wide visible-light response range, excellent photoelectric properties, and remarkable chemical stability.28,29 More importantly, ZnIn2S4 possesses an appropriate bandgap structure, which can match well with g-C3N4.30 Actually, the construction of ZnIn2S4/g-C3N4 heterojunction has been proved to be an effective measure to achieve high separation and migration efficiency of photogenerated carriers.31 Additionally, cadmium sulphide (CdS) is also an attractive candidate to couple with g-C3N4 for constructing the heterojunction photocatalyst owing to its excellent electronic and structural properties.32,33 Recently, CdS is widely used as a promoter decorating the main catalyst to further enhance the photocatalytic properties of the ternary heterostructures such as CdS/ZnIn2S4/TiO2,34 CdS-g-C3N4-GA35 and CdS/Bi20TiO32/Bi4Ti3O12.36 Thus, we expect the introduction of CdS nanoparticles to the binary ZnIn2S4/g-C3N4 heterojunction will further enhance the photocatalytic property of g-C3N4.
In this work, we designed and prepared a novel ternary-component CdS/ZnIn2S4/g-C3N4 heterojunction via thermal polymerization followed by a two-step in situ deposition method. The photocatalytic property of the as-obtained ternary samples was investigated via degrading contaminant Rhodamine B (RhB) under visible light irradiation. Owing to the rational construction of ternary composite, CdS/ZnIn2S4/g-C3N4 revealed a faster transfer and separation rate of photo-induced charge carriers as well as a wider visible-light response range compared to that of g-C3N4 and ZnIn2S4/g-C3N4-0.7, and thus obtaining obvious higher photocatalytic activity. Finally, the underlying mechanism of CdS/ZnIn2S4/g-C3N4 is elucidated in detail to explain the improvement of the photocatalytic property.
2. Materials and methods
2.1. Materials
All the chemicals were of analytical grade and used without pretreatment. Ethylenediamine tetraacetic acid disodium (EDTA-2Na) and zinc chloride (ZnCl2) were commercially available from Xilong Science Co., Ltd (Guangdong, China). RhB was bought from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). p-Benzoquinone (BQ) and tertiary butyl alcohol (t-BuOH) were purchased from Shanghai Zhanyun Chemical Co., Ltd (Shanghai, China). Absolute ethanol (EtOH) and hydrochloric acid (HCl) were acquired from Kermel Chemical Reagent Co., Ltd (Tianjin, China). Cadmium chloride hemi (pentahydrate) (CdCl2·2.5H2O) and indium chloride (InCl3) were supplied by Macklin Biochemical Co., Ltd (Shanghai, China). Dicyandiamine (DICY), ammonium chloride (NH4Cl), and thioacetamide (TAA) were originated from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Deionized water was used during the experiments.2.2. Preparation of g-C3N4 nanosheets
The g-C3N4 nanosheets were obtained via a polymerization process according to the literature.37 In detail, 3 g of DICY and 15 g of NH4Cl were transferred into a 100 mL round flask containing 50 mL of deionized water, and then the mixture was heated at 80 °C until completely evaporated. The obtained white powder underwent calcination at 550 °C for 4 h in a muffle furnace under air. Finally, light yellow powder of g-C3N4 nanosheets was obtained.2.3. Synthesis of ZnIn2S4/g-C3N4 binary heterojunctions
The ZnIn2S4/g-C3N4 composites were in situ constructed via oil-bath heating. Typically, 0.2 g of g-C3N4 was dispersed in 50 mL of deionized water (pH = 2.5) by ultrasonic. Then calculated quantity of ZnCl2, InCl3, and TAA were dissolved in the above solution via magnetic stirring. After that, the mixture was heated at 80 °C for 2 h. After washed by water and ethanol three times as well as dried at 60 °C overnight, the target product was obtained and labelled as ZnIn2S4/g-C3N4-X (X presents the weight percent of ZnIn2S4 and g-C3N4). For reference, ZnIn2S4 was also prepared by same procedure but in the absence of g-C3N4.2.4. Synthesis of CdS/ZnIn2S4/g-C3N4 ternary heterojunctions
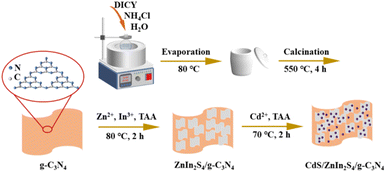
The synthesis of ternary CdS/ZnIn2S4/g-C3N4 heterojunctions was performed by another oil-bath heating process. Briefly, 0.015 g of as-obtained ZnIn2S4/g-C3N4-0.7 was first dispersed in 50 mL of deionized water by magnetic stirring, while 0.05 g CdCl2·2.5H2O and 0.02 g of TAA were dispersed into the above solution. Afterward, the mixture was heated at 70 °C for 2 h. After centrifugation, washing and drying, the target product was collected and labeled as CdS/ZnIn2S4/g-C3N4-Y (Y presents the weight percent of CdS and ZnIn2S4/g-C3N4). For comparison, CdS, CdS/ZnIn2S4-0.2, and CdS/g-C3N4-0.2 were also prepared by same procedure in the absence of ZnIn2S4/g-C3N4-0.7 and using ZnIn2S4 or g-C3N4 instead of ZnIn2S4/g-C3N4-0.7, respectively. The detailed process utilized to prepare the CdS/ZnIn2S4/g-C3N4 composites is depicted in Scheme 1.2.5. Characterization
The microstructure and morphologies were investigated by X-ray diffraction (XRD, XRD-6100), X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi), scanning electron microscopy (SEM, Zeiss Sigma) and transmission electron microscope (TEM, Tecnai G220 S-TWIN). Light absorption property was evaluated by UV-vis diffuse reflectance spectroscopy (UV-vis DRS, UV-2550). Photoluminescence (PL) spectra (excitation at 320 nm) were implemented on a spectrophotometer (ZolixLSP-X500A). The surface specific areas of the samples were measured by N2 isothermal adsorption–desorption spectroscopy (Autosorb-Iq2, Quantachrome). Photoelectrochemical tests were performed on an electrochemical station (CHI660E) three-electrode cell system using 500 W Xe lamp as light source. The total organic carbon (TOC) measures were performed on a TOC analyser (TOC-2000, Metash). Electron spin resonance (ESR) spectra were acquired using an EMXnano spectrometer (Bruker).2.6. Evaluation of the photocatalytic activity
The photodegradation properties were evaluated by using RhB as simulated organic pollutants under the visible light irradiation provided by a 500 W Xe lamp with a cutoff filter (λ ≥ 420 nm). Shortly, photocatalysts (20 mg) were dispersed in fresh RhB aqueous solution (50 mL, 50 mg L−1) and stirred for 60 min in a dark environment to realize the saturation adsorption prior to the light irradiation. After given intervals, aliquots (∼3 mL) were taken out, filtered by 0.22 μm filter membrane, and traced absorbance change at 554 nm on a UV-vis spectrophotometer (Lambda 35, PerkinElmer). Furthermore, the reusability of CdS/ZnIn2S4/g-C3N4-0.2 was also investigated by recycled photocatalytic experiments.3. Results and discussion
The purity and crystalline nature of the specimen series were firstly analyzed by XRD. As displayed in Fig. 1 and S1,† the characteristic pattern of the pure g-C3N4 are assigned to the graphite-like hexagonal phase, which ascribes well to JCPDS card no. 87-1526.38 The observed diffraction peaks of pristine ZnIn2S4 were attributed to hexagonal crystal structure (JCPDS no. 72-0773).39 The diffraction peaks of bare CdS matched well with reported value by JCPDS no. 77-2306.40 For the binary ZnIn2S4/g-C3N4, CdS/ZnIn2S4 or CdS/g-C3N4 photocatalysts, the XRD spectra can well indexed to g-C3N4 and ZnIn2S4 or CdS, which reflects the coexistence of two phases. After CdS was further incorporated into ZnIn2S4/g-C3N4-0.7 composite, several typical peaks of CdS appear, revealing that CdS was successfully presented in CdS/ZnIn2S4/g-C3N4 composite. With the increase of ZnIn2S4 content in the binary ZnIn2S4/g-C3N4 composites (Fig. S1†) and CdS content in the ternary CdS/ZnIn2S4/g-C3N4 composites (Fig. 1), the peak intensity of ZnIn2S4 and CdS gradually increased, respectively. Simultaneously, it can be seen that the diffraction peak of g-C3N4 (002) is appearing at almost same position of the diffraction peaks of ZnIn2S4 (102), CdS (002), and CdS (101), so as to the peak belonging to g-C3N4 is possibly overlapping of the ZnIn2S4 (102), CdS (002), and CdS (101) peaks, and thus possessing a distinguishable diffraction widening. Besides, no other peaks could be detected in the XRD spectra of single-component, binary-component, and ternary-component photocatalysts, indicating that there are no impurities produced during the synthesis.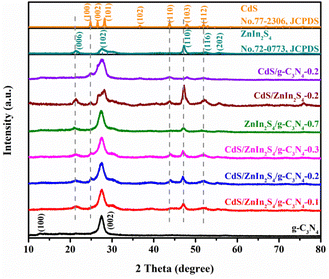 | ||
| Fig. 1 The XRD patterns of g-C3N4, ZnIn2S4, CdS, CdS/g-C3N4-0.2, CdS/ZnIn2S4-0.2, ZnIn2S4/g-C3N4-0.7 and CdS/ZnIn2S4/g-C3N4-Y (Y = 0.1, 0.2 and 0.3). | ||
The surface chemical composition and valence state of CdS/ZnIn2S4/g-C3N4-0.2 were further evaluated by XPS. As depicted in Fig. S2,† the survey XPS spectrum declared the co-occurrence of Cd, S, Zn, In, S, C and N elements in the ternary sample. In the C 1s spectrum (Fig. 2a), the peaks centered at 288.1 and 284.8 eV could be ascribed to graphitic carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and sp2-hybridized carbon (N–C
C) and sp2-hybridized carbon (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), respectively.41 For N 1s (Fig. 2b), the binding energies of peaks at 398.4, 399.5, and 400.7 eV are derived from sp2-hybridized nitrogen (C–N
N), respectively.41 For N 1s (Fig. 2b), the binding energies of peaks at 398.4, 399.5, and 400.7 eV are derived from sp2-hybridized nitrogen (C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), tertiary nitrogen N–(C)3 groups and amino functions carrying hydrogen (C–N–H), separately.42 The S 2p XPS regions could be deconvoluted into two peaks at 161.6 eV (S 2p3/2) and 162.9 eV (S 2p1/2) seen in Fig. 2c, which implies the existence of S2− ions in the ternary composite.43 The high-resolution XPS spectrum of Zn 2p (Fig. 2d) displayed two significant peaks located at 1044.7 eV (Zn 2p1/2) and 1021.6 eV (Zn 2p3/2), which corresponded to the Zn2+ state.44 The In 3d XPS spectrum shows two characteristic peaks at 444.7 and 452.3 eV, corresponding to In 3d5/2 and In 3d3/2 of In3+ in ZnIn2S4 (Fig. 2e).45 The Cd 3d XPS spectrum reveals two strong peaks at 406.0 (Cd 3d5/2) and 412.5 eV (Cd 3d3/2), which are ascribed to the state of Cd2+ (Fig. 2f).46
C), tertiary nitrogen N–(C)3 groups and amino functions carrying hydrogen (C–N–H), separately.42 The S 2p XPS regions could be deconvoluted into two peaks at 161.6 eV (S 2p3/2) and 162.9 eV (S 2p1/2) seen in Fig. 2c, which implies the existence of S2− ions in the ternary composite.43 The high-resolution XPS spectrum of Zn 2p (Fig. 2d) displayed two significant peaks located at 1044.7 eV (Zn 2p1/2) and 1021.6 eV (Zn 2p3/2), which corresponded to the Zn2+ state.44 The In 3d XPS spectrum shows two characteristic peaks at 444.7 and 452.3 eV, corresponding to In 3d5/2 and In 3d3/2 of In3+ in ZnIn2S4 (Fig. 2e).45 The Cd 3d XPS spectrum reveals two strong peaks at 406.0 (Cd 3d5/2) and 412.5 eV (Cd 3d3/2), which are ascribed to the state of Cd2+ (Fig. 2f).46
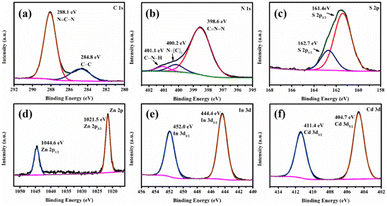 | ||
| Fig. 2 The XPS spectra of CdS/ZnIn2S4/g-C3N4-0.2: C 1s (a), N 1s (b), S 2p (c), Zn 2p (d), In 3d (e), and Cd 3d (f). | ||
The morphology and microstructures of as-obtained photocatalysts were systematically characterized via SEM and TEM, and the results are depicted in Fig. 3. Fig. 3a shows the morphology of as-constructed g-C3N4 with irregular nanosheets. In Fig. 3b, the pure ZnIn2S4 with hierarchical flowerlike architecture consisted of thin nanosheets is also successfully constructed via a simple oil-bath method. As for ZnIn2S4/g-C3N4-0.7 (Fig. 3c), ZnIn2S4 nanosheets uniformly disperse on the surface of g-C3N4, meaning that a typical 2D/2D heterojunction forms between ZnIn2S4 and g-C3N4. The SEM image (Fig. 3d) of CdS/ZnIn2S4/g-C3N4-0.2 clearly shows a laminated structure consisting of ZnIn2S4 nanosheets and g-C3N4 nanosheets. Nevertheless, CdS nanoparticles cannot be clearly distinguished, which may be ascribed to the small particle size of CdS, the corresponding energy-dispersive system (EDS) element mappings (Fig. S3†) indicate the simultaneous existence and even distribution of g-C3N4, ZnIn2S4, and CdS. Fig. 3e shows the TEM image of CdS/ZnIn2S4/g-C3N4-0.2 heterojunction, which consists of g-C3N4 nanosheets, ZnIn2S4 nanosheets, and CdS nanoparticles. High resolution TEM (HR-TEM) image (Fig. 3f) displays the lattice fringes space of 0.32 and 0.31 nm corresponded to the (102) plane of ZnIn2S4 and (101) plane of CdS, separately.47,48 These results further confirm the successful construction of target product.
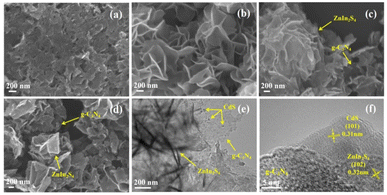 | ||
| Fig. 3 SEM images of g-C3N4 (a), ZnIn2S4 (b), ZnIn2S4/g-C3N4-0.7 (c), and CdS/ZnIn2S4/g-C3N4-0.2 (d); TEM (e) and HR-TEM (f) images of CdS/ZnIn2S4/g-C3N4-0.2. | ||
Commonly, large specific surface areas may provide abundant surface active sites for the absorption of reactant molecules, and thus boosting the photocatalytic property.49 Thereby, the specific surface area and pore volume distribution of g-C3N4, ZnIn2S4/g-C3N4-0.7, and CdS/ZnIn2S4/g-C3N4-0.2 were investigated by N2 adsorption method. As displayed in Fig. 4a, all three samples show the classical type IV isotherms with the type H3 hysteresis loops, indicating the existence of mesoporous structure.50 The specific surface area (Fig. 4a, inset) of pristine g-C3N4 is 25.186 m2 g−1. After successively coupling with ZnIn2S4 and CdS, the specific surface area of g-C3N4 increases from 25.186 m2 g−1 to 40.944 and 49.175 m2 g−1, which suggests that ZnIn2S4 and CdS can obviously improve the BET surface area of g-C3N4 providing more active sites for photocatalysis. Moreover, the pore size distributions are displayed in Fig. 4b, the results show that the pore sizes (Fig. 4b, inset) of all three samples distribute at 2–10 nm, which further confirm the presence of mesopores, the mesoporous characteristics are beneficial for producing high surface areas.
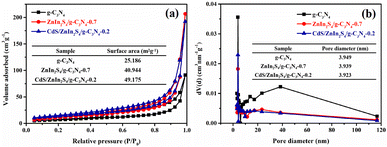 | ||
| Fig. 4 N2 adsorption–desorption isotherms (a) and the corresponding pore size distribution plots (b) of g-C3N4, ZnIn2S4/g-C3N4-0.7 and CdS/ZnIn2S4/g-C3N4-0.2. | ||
The light absorption capacity of the g-C3N4, ZnIn2S4, CdS, ZnIn2S4/g-C3N4-0.7 and CdS/ZnIn2S4/g-C3N4-0.2 photocatalysts was investigated by UV-vis DRS, and the related results are depicted in Fig. 5. Based on the results displayed in Fig. 5a, the light absorption thresholds of g-C3N4, ZnIn2S4, and CdS are approximately 446 and 500, 562 nm, separately, which are basically in accord with the previous reports.51,52 After in situ growing ZnIn2S4 and CdS on g-C3N4, the light absorption threshold of the ZnIn2S4/g-C3N4-0.7 and CdS/ZnIn2S4/g-C3N4-0.2 heterojunctions have significant red shifts, indicating that the combination of ZnIn2S4 and CdS can effectively widen the light absorption range of g-C3N4. The wider the light absorption range, the more conducive it is to utilize visible light, which is more beneficial to enhance the photocatalytic performance. Fig. 5b shows the bandgap energies (Eg) of g-C3N4, ZnIn2S4, and CdS estimated by Tauc plot method,53 which are 2.67, 2.40 and 2.36 eV, separately. To further obtain the band edges of g-C3N4, ZnIn2S4, and CdS, their valence band XPS spectra (VB-XPS) were further measured. According to the VB-XPS results depicted in Fig. 5c, the valence band (VB) potentials of g-C3N4, ZnIn2S4, and CdS are 1.24, 1.51, and 0.83 eV, separately. Eg and edge potentials of VB and conduction band (CB) possess the relationship: Eg = EVB − ECB. As a result, the CB potentials of g-C3N4, ZnIn2S4, and CdS can be determined as −1.43, −0.89, and −1.53 eV, separately. Accordingly, the overall band structure positions of g-C3N4, ZnIn2S4, and CdS can be obtained and the results are shown in Fig. 5d.
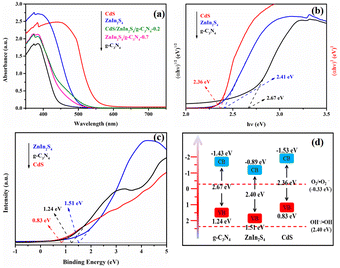 | ||
| Fig. 5 UV-vis DRS of g-C3N4, ZnIn2S4, CdS, ZnIn2S4/g-C3N4-0.7 and CdS/ZnIn2S4/g-C3N4-0.2 (a); the band gap (b), VB-XPS (c), and energy band structure (d) of g-C3N4, ZnIn2S4, CdS. | ||
The internal electron transfer behaviors of photo-induced charge carrier were firstly analyzed by PL technique. As we all know, a lower PL signal represents a higher separation efficiency of photo-generated charge carrier.54 As illustrated in Fig. 6a, intense emission peak near 450 nm can be observed for the pristine g-C3N4, indicating an inherent nature of a low separation rate. In contrast, the PL intensity of the ZnIn2S4/g-C3N4-0.7 and heterojunction obviously quenched. After further coupling with CdS, the obtained CdS/ZnIn2S4/g-C3N4-0.2 ternary heterojunction displays a lower PL signal, indicating that the charge carriers' separation efficiency of g-C3N4 can be accelerated by forming ternary heterojunction with ZnIn2S4 and CdS. To verify this result, photelectrochemical measurements were performed to support the improved separation and transfer efficiency. Generally, higher photocurrent intensity or smaller semicircle radius means higher separation and transfer efficiency of photo-generated charge carriers.55 As displayed in Fig. 6b, the photocurrent intensity of CdS/ZnIn2S4/g-C3N4-0.2 composite is obviously higher that of ZnIn2S4/g-C3N4-0.7 and g-C3N4. The results of electrochemical impedance spectroscopy (EIS) (Fig. 6c) show CdS/ZnIn2S4/g-C3N4-0.2 possesses the lowest charge transfer impedance due to its smaller semicircle radius than the two other samples. The photoelectrochemical results forcefully verify that the constructing of CdS/ZnIn2S4/g-C3N4-0.2 ternary composite significantly promotes the charge carrier transfer and separation, and thus delivering boosting photocatalytic property.
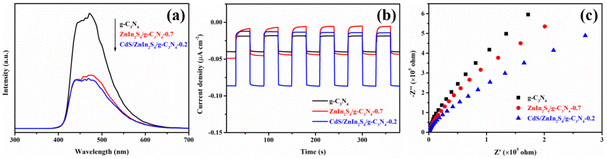 | ||
| Fig. 6 PL spectra (a), transient photocurrent responses (b), and Nyquist plots (c) of g-C3N4, ZnIn2S4/g-C3N4-0.7 and CdS/ZnIn2S4/g-C3N4-0.2. | ||
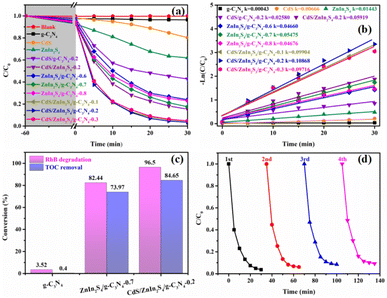
The visible-light photocatalytic activities of the pristine g-C3N4, ZnIn2S4, CdS, CdS/g-C3N4-0.2, CdS/ZnIn2S4-0.2, and a series of ZnIn2S4/g-C3N4 and CdS/ZnIn2S4/g-C3N4 composites were evaluated by the degradation of RhB. As depicted in Fig. 7a, the degradation efficiency of ZnIn2S4 and CdS is obviously higher than that of g-C3N4 due to their wider visible-light response range. The photocatalytic properties of g-C3N4 and ZnIn2S4 are obviously improved after coupling with ZnIn2S4 or CdS. Among of the ZnIn2S4/g-C3N4 composites, ZnIn2S4/g-C3N4-0.2 exhibited the highest degradation efficiency of 82% within 30 min. When ZnIn2S4/g-C3N4-0.2 further coupled with CdS, the photocatalytic activities could be obviously improved. Among the ternary photocatalysts, CdS/ZnIn2S4/g-C3N4-0.7 exhibited the highest degradation efficiency, in which 96% of RhB could be degradated within 30 min. Moreover, the photocatalytic kinetics of RhB degradation were evaluated using the psedo-first-order model (Fig. 7b). The CdS/ZnIn2S4/g-C3N4-0.2 showed the highest apparent rate constant, approximately 0.10868 min−1, which is far greater than that of the pristine g-C3N4 (0.00043 min−1), ZnIn2S4/g-C3N4-0.7 (0.05475 min−1) and CdS/g-C3N4-0.2 (0.02580 min−1) under the same conditions, separately. Furthermore, the TOC measurements were also carried out to investigate the mineralization abilities of photocatalysts under 30 min of visible light irradiation. As illustrated in Fig. 7c, the TOC removal efficiency of CdS/ZnIn2S4/g-C3N4-0.2 (84.65%) is obviously higher than that of g-C3N4 (0.40%) and ZnIn2S4/g-C3N4-0.7 (73.97%), which is coincident with the trend of photodegradation performance. This result fully confirms the high mineralization ability of CdS/ZnIn2S4/g-C3N4-0.2 for RhB. The stability is a crucial factor affecting the actual application of the photocatalyst,56 and thus sequential cycling experiments were performed to detect the stability of the CdS/ZnIn2S4/g-C3N4-0.2 ternary photocatalyst. As displayed in Fig. 7d, the photocatalytic efficiency had no obvious change after four reused tests. Besides, the structure and morphology can also be preserved after undergoing four runs of photodegradation reaction as no noticeable change can be observed in the XRD pattern (Fig. S4†) and SEM image (Fig. S5†). The results prove that the as-prepared ternary catalysts can meet the practical application requirements.
To expound the photocatalytic mechanism of the photocatalytic activity improvement of CdS/ZnIn2S4/g-C3N4 photocatalysts, the predominant active species formed during the photocatalytic process were distinguished via a series of trapping experiments over CdS/ZnIn2S4/g-C3N4-0.2. In the experiments, EDTA-2Na, BQ, and t-BuOH were chosen as the scavengers of hydroxyl radicals (˙OH), radical superoxide (˙O2−), and hole (h+), separately. Fig. 8a exhibits the addition of BQ has a significant effect on the degradation activity, which mean that ˙O2− was the primary active substance during the photocatalytic process. However, after adding EDTA-2Na and t-BuOH, the removal efficiency of RhB decreased from 96% to 90% and 94%, separately, which revealed that h+ had a weak effect on the photocatalytic reaction, while the role of ˙OH could be ignored owing to the negligible effect of EDTA-2Na on the RhB removal efficiency. Consequently, it could be inferred that the active species played a different role during the photocatalytic reaction process with a sequence of ˙O2− > h+ > ˙OH. In addition, the ESR results (Fig. 8b) provided a persuasive proof for the existence of ˙O2− in the photodegradation reaction. No ESR signals were observed under dark, while the signals of the DMPO-˙O2− adducts (intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) could be easily identified after irradiating by visible light, which is fully in accord with the results of radical trapping experiments.
1) could be easily identified after irradiating by visible light, which is fully in accord with the results of radical trapping experiments.
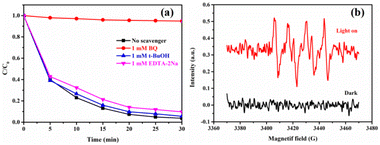 | ||
| Fig. 8 Photodegradation of RhB over the CdS/ZnIn2S4/g-C3N4-0.2 photocatalyst in the presence of different radical scavengers (a); ESR detection of ˙O2− over CdS/ZnIn2S4/g-C3N4-0.2 (b). | ||
Based on the conclusions above, a plausible photocatalytic charge-transfer mechanism of the CdS/ZnIn2S4/g-C3N4 composite for photocatalytic degradation of RhB is put forward and displayed in Scheme 2. Under the visible light irradiation, g-C3N4, ZnIn2S4 and CdS can adsorb photons and generate a great deal of photo-induced electrons (e−) and h+ pairs, where the e− and h+ gather at CB and VB, separately. According to the calculated band structure and close contact interface, e− in the CB of CdS can readily migrate to the CB of ZnIn2S4 by a one-step process or two-step process. Meanwhile, h+ in the VB of ZnIn2S4 transfers to the VB of CdS under the driving force of potential difference. Then, the accumulated e− in ZnIn2S4 will react with dissolved O2 to produce ˙O2− due to its more negative potential than O2/˙O2− (−0.33 eV versus NHE),57 while the VB potential of CdS is too low for h+ to convert –OH into ˙OH (+2.4 eV versus NHE).58 Finally, the ˙O2− and h+ behave as oxidation active site for the degradation of RhB due to their high oxidative capacity.
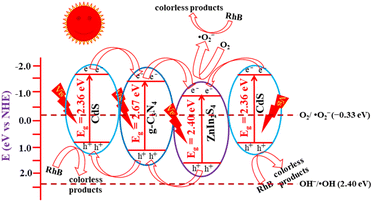 | ||
| Scheme 2 Proposed mechanism for the visible-light photodegradation of RhB over CdS/ZnIn2S4/g-C3N4 ternary composite. | ||
4 Conclusions
In general, the CdS/ZnIn2S4/g-C3N4 ternary photocatalyst was prepared by a facile pyrolysis route coupling with simple two-step oil-bath procedure and the photoactivity was evaluated by degrading RhB under visible light irradiation. As expected, CdS/ZnIn2S4/g-C3N4 ternary composites possess boosted photocatalytic performance, which is not only higher than bare g-C3N4, ZnIn2S4 and CdS, but also even superior to ZnIn2S4/g-C3N4 and CdS/g-C3N4 binary photocatalysts. Among them, the CdS/ZnIn2S4/g-C3N4-0.2 photocatalyst achieves the best photocatalytic activity with a degradation rate of 96%. Additionally, the proposed CdS/ZnIn2S4/g-C3N4-0.2 composite reveals excellent photostability and reusability after four reused tests. The reasonable construction of ternary heterojunction not only facilitates the photogenerated charge carrier separation and migration as well as widen the visible-light response range, but also enhances the specific surface area, all of which are favorable for photocatalytic process. The design and fabrication of the CdS/ZnIn2S4/g-C3N4 ternary heterojunction photocatalysts represent a promising approach to fabricate high efficient g-C3N4-based photocatalysts for environmental remediation.Author contributions
Jingzhe Li: data curation, investigation, writing – original draft. Yue Chen and Liezhen Zhu: investigation, validation, data curation. Linfa Liao and Xinmao Wang: investigation, validation. Xun Xu and Lingfang Qiu: conceptualization, funding acquisition, supervision. Jiangbo Xi: investigation, methodology. Ping Li: conceptualization, methodology, funding acquisition, writing – review & editing, supervision. Shuwang Duo: conceptualization, funding acquisition, project administration, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 41763020), the Natural Science Foundation of Jiangxi Province (No. 20212BAB204020, 20202BABL214040, and 20202BABL213014), and the Postgraduate Innovation Special Fund Project of Jiangxi Provincial Education Department (YC2022-s779).Notes and references
- J. B. Xi, Q. J. Wang, X. M. Duan, N. Zhang, J. X. Yu, H. Y. Sun and S. Wang, Chem. Eng. Sci., 2021, 231, 116303 CrossRef CAS.
- B. S. Li, C. Lai, L. Qin, C. C. Chu, M. M. Zhang, S. Y. Liu, X. G. Liu, H. Yi, J. F. He, L. Li, M. F. Li and L. Chen, J. Colloid Interface Sci., 2020, 560, 701–713 CrossRef CAS PubMed.
- J. Wang, G. Wang, B. Cheng, J. Yu and J. Fan, Chin. J. Catal., 2021, 42, 56–68 CrossRef CAS.
- M. T. Islam, R. Saenz-Arana, C. Hernandez, T. Guinto, M. A. Ahsan, D. T. Bragg, H. Y. Wang, B. Alvarado-Tenorio and J. C. Noveron, J. Environ. Chem. Eng., 2018, 6, 3070–3082 CrossRef CAS.
- T. Luo, H. P. Feng, L. Tang, Y. Lu, W. W. Tang, S. Chen, J. F. Yu, Q. Q. Xie, X. Ouyang and Z. M. Chen, Chem. Eng. J., 2020, 382, 122970 CrossRef CAS.
- X. Yang, G. R. Xu, H. R. Yu and Z. Zhang, Bioresour. Technol., 2016, 211, 566–573 CrossRef CAS PubMed.
- X. Gong, X. G. Ma, F. D. Wan, W. Y. Duan, X. L. Yang and J. R. Zhu, Acta Chim. Sin., 2022, 80, 510–516 CrossRef.
- Z. Y. Zhang, X. L. Xue and X. Y. Chen, Dalton Trans., 2022, 51, 8015–8027 RSC.
- T. Y. Zhao, Z. P. Xing, Z. Y. Xiu, Z. Z. Li, S. L. Yang, Q. Zhu and W. Zhou, Int. J. Hydrogen Energy, 2019, 44, 1586–1596 CrossRef CAS.
- H. Liu, J. Z. Li, P. Li, G. Z. Zhang, X. Xu, H. Zhang, L. F. Qiu, H. Qi and S. W. Duo, Acta Chim. Sin., 2021, 79, 1293–1301 CrossRef.
- P. Molaei and F. R. Moghadam, Mater. Today Commun., 2022, 31, 103677 CrossRef CAS.
- J. W. Fu, Q. L. Xu, J. X. Low, C. J. Jiang and J. G. Yu, Appl. Catal., B, 2019, 243, 556–565 CrossRef CAS.
- H. C. Yang, R. Y. Cao, P. X. Sun, J. M. Yin, S. W. Zhang and X. J. Xu, Appl. Catal., B, 2019, 256, 117862 CrossRef CAS.
- Y. Huo, J. F. Zhang, K. Dai and C. H. Liang, ACS Appl. Energy Mater., 2021, 4, 956–968 CrossRef CAS.
- C. Yang, Q. Y. Tan, Q. Li, J. Zhou, J. J. Fan, B. Li, J. Sun and K. L. Lv, Appl. Catal., B, 2020, 268, 118738 CrossRef CAS.
- Q. H. Shen, C. Y. Wu, Z. Y. You, F. L. Huang, J. S. Sheng, F. Zhang, D. Cheng and H. Yang, J. Mater. Res., 2020, 35, 2148–2157 CrossRef CAS.
- Y. C. Wang, J. Zhou, X. Q. Hao, Y. Wang and Z. G. Zou, Appl. Surf. Sci., 2018, 456, 861–870 CrossRef CAS.
- Y. C. Deng, J. Liu, Y. B. Huang, M. M. Ma, K. Liu, X. M. Dou, Z. J. Wang, S. C. Qu and Z. G. Wang, Adv. Funct. Mater., 2020, 30, 2002353 CrossRef CAS.
- C. Zhang, D. Y. Qin, Y. Zhou, F. Z. Qin, H. Wang, W. J. Wang, Y. Yang and G. M. Zeng, Appl. Catal., B, 2022, 303, 120904 CrossRef CAS.
- X. Zhu, Y. T. Wang, Y. Guo, J. Z. Wan, Y. Yan, Y. X. Zhou and C. Sun, Appl. Surf. Sci., 2021, 544, 148872 CrossRef CAS.
- B. Zhang, X. Y. Hu, E. Z. Liu and J. Fan, Chin. J. Catal., 2021, 42, 1519–1529 CrossRef CAS.
- S. H. Wang, J. W. Zhan, K. Chen, A. Ali, L. H. Zeng, H. Zhao, W. L. Hu, L. X. Zhu and X. L. Xu, ACS Sustainable Chem. Eng., 2020, 8, 8214–8222 CrossRef CAS.
- G. M. Liu, G. H. Dong, Y. B. Zeng and C. Y. Wang, Chin. J. Catal., 2020, 41, 1564–1572 CrossRef CAS.
- F. He, Z. X. Wang, Y. X. Li, S. Q. Peng and B. Liu, Appl. Catal., B, 2020, 269, 118828 CrossRef CAS.
- X. Y. Zhang, X. Y. Wang, J. Q. Meng, Y. Q. Liu, M. Ren, Y. H. Guo and Y. X. Yang, Sep. Purif. Technol., 2021, 255, 117693 CrossRef CAS.
- B. Zhang, H. X. Shi, Y. J. Yan, C. Q. Liu, X. Y. Hu, E. Z. Liu and J. Fan, Colloids Surfaces, A, 2021, 608, 125598 CrossRef CAS.
- W. L. Shi, M. Y. Li, X. L. Huang, H. J. Ren, C. Yan and F. Guo, Chem. Eng. J., 2020, 382, 122960 CrossRef CAS.
- L. Ye, Z. H. Wen, Z. H. Li and H. T. Huang, Solar RRL, 2020, 4, 2000027 CrossRef CAS.
- F. H. Mu, Q. Cai, H. Hu, J. Wang, Y. Wang, S. J. Zhou and Y. Kong, Chem. Eng. J., 2020, 384, 123352 CrossRef CAS.
- L. L. Li, D. K. Ma, Q. L. Xu and S. M. Huang, Chem. Eng. J., 2022, 437, 135153 CrossRef CAS.
- X. M. Liu, S. Q. Wang, F. Yang, Y. C. Zhang, L. S. Yan, K. X. Li, H. Q. Guo, J. J. Yan and J. Lin, Int. J. Hydrogen Energy, 2022, 47, 2900–2913 CrossRef CAS.
- D. D. Ren, W. N. Zhang, Y. N. Ding, R. C. Shen, Z. M. Jiang, X. Y. Lu and X. Li, Solar RRL, 2019, 4, 1900423 CrossRef.
- L. Chen, Y. M. Xu and B. L. Chen, Appl. Catal., B, 2019, 256, 117848 CrossRef CAS.
- H. Liu, J. Z. Li, Y. Chen, X. T. Sun, X. Xu, L. F. Qiu, S. W. Duo and P. Li, New J. Chem., 2022, 46, 7195–7201 RSC.
- J. H. Liu, X. N. Wei, W. Q. Sun, X. X. Guan, X. C. Zheng and J. Li, Environ. Res., 2021, 197, 111136 CrossRef CAS PubMed.
- K. Das, R. Bariki, D. Majhi, A. Mishra, K. K. Das, R. Dhiman and B. G. Mishra, Appl. Catal., B, 2022, 303, 120902 CrossRef CAS.
- M. M. Zhang, C. Lai, B. S. Li, D. L. Huang, G. M. Zeng, P. Xu, L. Qin, S. Y. Liu, X. G. Liu, H. Yi, M. F. Li, C. C. Chu and Z. Chen, J. Catal., 2019, 369, 469–481 CrossRef CAS.
- H. G. Yu, H. Q. Ma, X. H. Wu, X. F. Wang, J. J. Fan and J. G. Yu, Solar RRL, 2020, 5, 2000372 CrossRef.
- F. Min, Z. Q. Wei, Z. Yu, Y. T. Xiao, S. E. Guo, R. J. Song and J. H. Li, Dalton Trans., 2022, 51, 2323–2330 RSC.
- S. W. Sun, H. Cheng, K. Cao, A. Y. Song, C. Q. Xu, J. X. Ba, H. W. Lin, W. C. Qiu, Z. H. Li, D. H. Fan, J. Huang and S. Y. Jin, Energy Fuels, 2022, 36, 2034–2043 CrossRef CAS.
- J. F. Zheng, Z. Xu, S. T. Xin, B. C. Zhu and L. H. Nie, Dalton Trans., 2022, 51, 12883–12894 RSC.
- X. Chang, Y. Wang, X. J. Zhou, Y. Song and M. Y. Zhang, Dalton Trans., 2021, 50, 17618–17624 RSC.
- J. Chen, K. Li, X. Y. Cai, Y. L. Zhao, X. Q. Gu and L. Mao, Mater. Sci. Semicond. Process., 2022, 143, 106547 CrossRef CAS.
- H. Q. Wang, H. P. Jiang, H. J. Wang, Q. Liu and P. W. Huo, Energy Technol., 2022, 10, 2101158 CrossRef CAS.
- Y. Gao, K. Qian, B. T. Xu, F. Ding, V. Dragutan, I. Dragutan, Y. G. Sun and Z. H. Xu, RSC Adv., 2020, 10, 32652–32661 RSC.
- F. Zhang, H. Q. Zhuang, W. M. Zhang, J. Yin, F. H. Cao and Y. X. Pan, Catal. Today, 2019, 330, 203–208 CrossRef CAS.
- W. Chen, R. Q. Yan, G. H. Chen, M. Y. Chen, G. B. Huang and X. H. Liu, Ceram. Int., 2019, 45, 1803–1811 CrossRef CAS.
- Q. L. Yu, G. Z. Wu, T. Zhang, X. D. Zhao, Z. Zhou, L. Liu, W. Chen and P. J. J. Alvarez, Chem. Commun., 2020, 56, 7613–7616 RSC.
- R. H. Zha, T. Shi, L. He, X. Y. Sun, Y. F. Jia and M. Zhang, Dyes Pigm., 2020, 180, 108439 CrossRef CAS.
- L. F. Cui, Y. F. Liu, X. Y. Fang, C. C. Yin, S. S. Li, D. Sun and S. F. Kang, Green Chem., 2018, 20, 1354–1361 RSC.
- X. Deng, D. D. Wang, H. J. Li, W. Jiang, T. Y. Zhou, Y. Wen, B. Yu, G. B. Che and L. Wang, J. Alloys Compd., 2022, 894, 162209 CrossRef CAS.
- G. M. Li, B. Wang, J. Zhang, R. Wang and H. L. Liu, Appl. Surf. Sci., 2019, 478, 1056–1064 CrossRef CAS.
- J. F. Jing, J. Yang, W. L. Li, Z. H. Wu and Y. F. Zhu, Adv. Mater., 2022, 34, 2106807 CrossRef CAS PubMed.
- W. K. Wei, Q. F. Tian, H. S. Sun, P. Liu, Y. Zheng, M. Z. Fan and J. D. Zhuang, Appl. Catal., B, 2020, 260, 118153 CrossRef CAS.
- R. Arcas, E. Peris, E. Mas-Marzá and F. Fabregat-Santiago, Sustainable Energy Fuels, 2021, 5, 956–962 RSC.
- H. Y. Zhang, B. G. Xu, X. Zhang and P. Yang, Environ. Sci.: Nano, 2022, 9, 3397–3406 RSC.
- J. W. Ren, Y. Meng, X. Zhang, Y. Gao, L. X. Liu, X. X. Zhou, Z. G. Zhang, L. B. Zeng and J. Ke, Sep. Purif. Technol., 2022, 296, 121423 CrossRef CAS.
- J. L. Wang, X. Liu, C. S. Li, M. Yuan, B. J. Zhang, J. H. Zhu and Y. Q. Ma, J. Photochem. Photobiol., A, 2020, 401, 112795 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The XRD patterns of ZnIn2S4/g-C3N4 composites, XPS survey spectrum and EDS element mappings of CdS/ZnIn2S4/g-C3N4-0.2, XRD spectra and SEM image of CdS/ZnIn2S4/g-C3N4-0.2 during a four-cycle experiment. See DOI: https://doi.org/10.1039/d2ra06328j |
| This journal is © The Royal Society of Chemistry 2022 |