Open Access Article
Open Access ArticleExtraction of alumina from high-alumina fly ash by ammonium sulfate: roasting kinetics and mechanism
Xiaoying Li a,
Bo Hubc,
Nengsheng Liu
a,
Bo Hubc,
Nengsheng Liu *a,
Xueqing Liubc,
Chengwei Liuc,
Xintao Hec and
Sufang Hebc
*a,
Xueqing Liubc,
Chengwei Liuc,
Xintao Hec and
Sufang Hebc
aFaculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China. E-mail: liunengshengms@163.com
bResearch Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming 650093, China
cFaculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming 650093, China
First published on 21st November 2022
Abstract
The recycling of aluminum is commonly an important step to achieve the high value-added utilization of fly ash, which is a kind of solid waste generated from coal-fired power plants. In this study, high-alumina fly ash was efficiently activated by ammonium sulfate method and the alumina was efficiently extracted. The effects of roasting temperature, roasting time, and ammonium sulfate/high-alumina fly ash mass ratio on the leaching rate of alumina were fully analyzed, and the roasting kinetics and reaction mechanism in the roasting process were discussed. The experimental results showed that the leaching rate of alumina in the roasted material achieved 93.57% with the roasting temperature of 673 K, the roasting time of 60 min, and the mass ratio of ammonium sulfate to high-alumina fly ash of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The roasting kinetics showed that the reaction between high-alumina fly ash and ammonium sulfate was controlled by internal diffusion, the apparent activation energy was 37.40 kJ mol−1, which accorded with the reaction kinetic equation 1 − 2x/3 − (1 − x)2/3 = 2.9546
1. The roasting kinetics showed that the reaction between high-alumina fly ash and ammonium sulfate was controlled by internal diffusion, the apparent activation energy was 37.40 kJ mol−1, which accorded with the reaction kinetic equation 1 − 2x/3 − (1 − x)2/3 = 2.9546![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[−37
exp[−37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400/(RT)]t. The reaction mechanism showed that the aluminum and structural damage mullite in the high-alumina fly ash reacted with molten ammonium sulfate to form (NH4)3Al(SO4)3 and NH4Al(SO4)2. Finally, (NH4)3Al(SO4)3 was transformed into NH4Al(SO4)2 with the increase of temperature.
400/(RT)]t. The reaction mechanism showed that the aluminum and structural damage mullite in the high-alumina fly ash reacted with molten ammonium sulfate to form (NH4)3Al(SO4)3 and NH4Al(SO4)2. Finally, (NH4)3Al(SO4)3 was transformed into NH4Al(SO4)2 with the increase of temperature.
1. Introduction
Fly ash, generated from coal-fired power plants,1,2 is the largest kind of industrial solid waste in China.3–5 At present, the annual emissions of fly ash in China is 600–800 million tons,6–8 which is expected to reach 925 million tons in 2024.9 However, the utilization rate of fly ash is only 25%,10 resulting in the accumulation of more than 3 billion tons of fly ash.11 The long-term and large-scale accumulation of fly ash not only causes environmental pollution,12–14 but also has great harm to crop and human body, so the various treatments have been explored to improve this problem, involving architecture, agriculture, materials, metallurgy, environment and other fields. In order to utilize fly ash and reduce its damage, it is necessary to convert it into high value-added products,15 which has attracted widespread attention in soil remediation, ceramic material preparation, synthetic catalysts, zeolite preparation, valuable metal recovery and other fields.16–18It is worth mentioning in particular that there is a special kind of fly ash, which comes from Shanxi and the middle and west of Inner Mongolia with the abundant aluminum content up to 40–60%,19 called high-alumina fly ash.20,21 Due to the aluminum content in high-alumina fly ash is comparable to a medium grade bauxite, so it can be used as a potential raw material to replace bauxite and recover alumina, alleviate the shortage of bauxite resources in China, and reduce the environmental pollution caused by fly ash accumulation.19 However, aluminum in high-alumina fly ash mainly exists in the form of stable mullite, leading to the difficulty for the direct extraction of aluminum.22 Therefore, the key to the utilization of high-alumina fly ash is to destroy the structure of mullite through activation.
There are many methods about fly ash activation, such as acid method, alkali method, thermal activation by calcination, sodium hydrogen sulfate roasting method,23 thermal activation by potassium bisulfate,24 potassium pyrosulfate calcination activation method,19 etc. The traditional and effective activation method of high-alumina fly ash is alkali fusion method, which means that high-alumina fly ash is mixed with solid alkali such as NaOH, KOH, NaCO3, Na2SO4 or K2SiO3 (ref. 5) evenly and roasted at high temperature to achieve the purpose of activation.25,26 However, the process of alkali fusion is usually high cost, high temperature, high energy consumption and low activation efficiency.27 Therefore, in terms of environment and economy, it is necessary to find a new activator to further reduce energy consumption.
Ammonium sulfate method is a newly developed activation technology in recent years,28,29 which can produce alumina products with high purity and has high commercial value. Especially, the activation process of ammonium sulfate, with low cost and recyclable tail gas, does not involve strong acid, strong alkali or strong corrosive agent and is less corrosive to the equipment, so there is a broad industrial application prospect with its unique advantages.30,31 Briefly speaking, the ammonium sulfate method involves the reaction of ammonium sulfate with high alumina fly ash in the temperature range of 300–400 °C to produce soluble aluminum sulfate, which converts mullite, an inert component of high alumina fly ash, into silica and soluble aluminum salts, thus facilitating the extraction of aluminum and silica elements and the preparation of silica materials.31 For instance, Li et al.31 conducted three times recrystallization of ammonium aluminum sulfate solutions obtained by this method to obtain high-purity alumina with a purity of more than 99.9%. Park32 extracted high concentration alumina from fly ash by ammonium sulfate low-temperature sintering method, the extraction rate of aluminum can reach 94.36%. Li33 studied the process of extracting alumina from fly ash by ammonium sulfate, and the recovery rate was 95.6%. Frederic J. Doucet et al.34 studied the feasibility of recovering aluminum from ammonium sulfate and found that 95% of aluminum could be recovered at 1 h, 500 °C, with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 weight ratio of fly ash to ammonium sulfate. Their results indicated that ammonium sulfate method was a good activation method and the study of it was very necessary.
6 weight ratio of fly ash to ammonium sulfate. Their results indicated that ammonium sulfate method was a good activation method and the study of it was very necessary.
At present, most researches on ammonium sulfate activation of high-alumina fly ash were mainly focused on the optimization of experimental process parameters to efficiently extract alumina, the in-depth studies on roasting kinetics and reaction mechanism in the roasting process, which is important to the efficient activation and comprehensive utilization of high-alumina fly ash, have been less addressed. Therefore, in order to promote further development of alumina extraction from high-alumina fly ash via the ammonium sulfate method, this method was first used to treat the raw material high-alumina fly ash from a power plant in Inner Mongolia, China. On the basis of studying the effect of process parameters on the extraction rate of alumina from high-alumina fly ash, the thermodynamics and kinetics, as well as associated factors in roasting process were examined, and the reaction mechanism was deduced. This study aims to reveal the reaction mechanism of ammonium sulfate activation high-alumina fly ash, optimize the roasting parameters and kinetic parameters, and provide certain theoretical guidance for the efficient activation and comprehensive utilization of high-alumina fly ash.
2. Experimental
2.1. Raw materials and reagents
High-alumina fly ash came from a thermal power plant in Inner Mongolia, China; the ammonium sulfate ((NH4)2SO4) and the sulfuric acid (H2SO4) used in the experiment were analytically pure and obtained from Tianjin Ruijinte Chemicals Co., Ltd, China; and the water used in the experiment was distilled water.2.2. Experimental method
First, the high-alumina fly ash and ammonium sulfate were mixed evenly with a certain mass ratio in the crucible, and then heated in a muffle furnace at a heating rate of 10 °C min−1. When the set temperature was reached, the timing device was started immediately.35 After sintering, the sample was cooled, grinded, weighed and characterized. Subsequently, this sample was transferred to the beaker and leached by sulfuric acid of 10% (with the liquid–solid ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 90 °C for 1 h, followed by vacuum filtration, washing, and drying. Finally, the Al3+ content in the filtrate was determined by Inductively coupled plasma-optical emission spectrometer, and afterwards the aluminum leaching rate (η), which can be used as the evaluation basis for the activation effect of high-alumina fly ash, was calculated using η = (VcCx)/(MWx) × 100%.36 Where M (g) is the quantity of raw high-alumina fly ash material, Wx (wt%) is the mass fraction of aluminum in the raw high-alumina fly ash material, Cx (g L−1) is the mass concentration of Al3+ ions in the acid leaching solution, and Vc (L) is the volume of the prepared acid leaching solution.4
1) at 90 °C for 1 h, followed by vacuum filtration, washing, and drying. Finally, the Al3+ content in the filtrate was determined by Inductively coupled plasma-optical emission spectrometer, and afterwards the aluminum leaching rate (η), which can be used as the evaluation basis for the activation effect of high-alumina fly ash, was calculated using η = (VcCx)/(MWx) × 100%.36 Where M (g) is the quantity of raw high-alumina fly ash material, Wx (wt%) is the mass fraction of aluminum in the raw high-alumina fly ash material, Cx (g L−1) is the mass concentration of Al3+ ions in the acid leaching solution, and Vc (L) is the volume of the prepared acid leaching solution.4
2.3. Sample characterization
The elemental analysis of raw material was carried out by X-ray fluorescence (XRF, XRF-1800, Shimadzu, Kyoto, Japan, instrument working voltage 40 kV, working current 95 mA, prt 8 mL min−1). The crystalline phases and surface morphology of raw material and important solid roasted material were examined and scanned by X-ray diffraction (XRD, TTRAX III, Japan, Cu-Kα radiation, tube current 700 mA, tube voltage 40 kV, step sizes 0.2°, scanning range 2θ = 5°–90°, scanning rate 10° min−1), scanning electron microscopy (SEM, Nova NanoSEM 450, FEI, USA, acceleration voltage: 50 V to 30 kV, magnification 20–1000k). The aluminum content in aluminum liquid was determined by an inductively coupled plasma-optical emission spectrometer (ICP-OES, ICAP7400 Radial, Thermo Scientific, USA). The carbon contents analysis was performed via an infrared carbon and sulfur analyzer (CS 2000, ELTRA, Germany). The particle size distribution was measured using Rise-2002 laser particle size analyzer (Jinan Runzhi Technology Co., Ltd.).3. Results and discussion
3.1. Characterization of raw high-alumina fly ash
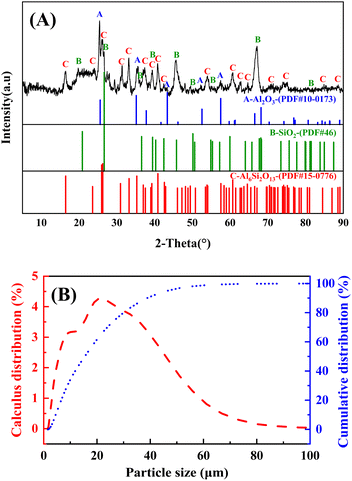
In order to obtain the phase transformation and chemical reactions of high-alumina fly ash and ammonium sulfate in the roasting process, the raw material of high-alumina fly ash was first characterized by XRF, XRD and SEM to understand its chemical compositions, phase compositions and appearance morphology.The chemical compositions (mass fraction) of raw fly ash were measured by XRF and infrared carbon and sulfur analyzer, and the results were presented in Table 1. The main components were SiO2 and Al2O3, with the contents were 37.40% and 46.22%, respectively. Obviously, it is a kind of high-alumina fly ash. As well, there were a little amount of Fe2O3 (3.05%), CaO (5.17%), TiO2 (3.51%) and C (2.54%) in this sample.
| Component | SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | C |
|---|---|---|---|---|---|---|
| Content/wt% | 37.40 | 46.22 | 3.05 | 5.17 | 3.51 | 2.54 |
In addition, the phase compositions of raw high-alumina fly ash were studied by XRD and the results were shown in Fig. 1(A). According to PDF#10-0173, PDF#46, PDF#15-0076 and previous literature,30,37,38 the diffraction peaks at 16.44°, 23.99°, 25.99°, 31.40°, 33.16°, 37.37°, 39.39°, 40.80°, 42.43°, 54.01°, 60.74°, 62.55°, 64.70°, 70.95°, 74.94°, 84.87° and 88.65° were assigned to mullite (Al6Si2O13); while the peaks at 19.72°, 26.60°, 36.08°, 39.39°, 45.66°, 50.10°, 54.96°, 67.12° and 81.85° were corresponded to amorphous SiO2; in addition, Al2O3 phase could be also found at peaks of 25.42°, 35.53°, 43.33°, 52.23°, and 57.48°. The particle size distribution of high alumina fly ash in Fig. 1(B) showed that the maximum particle size was 30 μm, and more than 98% of the material diameter was distributed in the range of 0–60 μm, and the material size was relatively fine. The surface morphology of high-alumina fly ash was characterized by SEM, as shown in Fig. 2. Fig. 2(A) was obtained at 8k magnification, it can be seen that high-alumina fly ash was composed of a large number of spherical, spherical particles, and rod-like particles.30 Fig. 2(B) was shot at the magnification of 150k, which further showed that the spherical particles in Fig. 2(A) were smooth and dense. It had a stable structure with a particle size of about 0.9 μm.
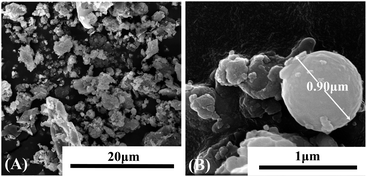 | ||
| Fig. 2 SEM image of raw high-alumina fly ash. (A) Low magnification image and (B) high magnification image. | ||
3.2. Optimization of roasting parameters
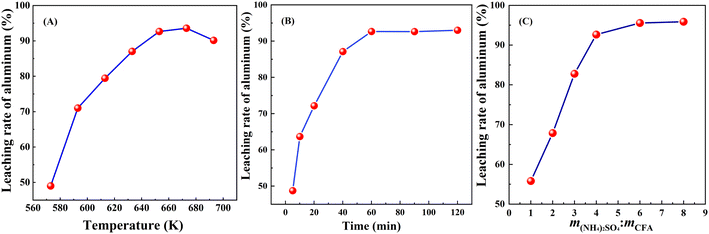
For the roasting procedure, the alumina extraction efficiency was significantly influenced by several operational parameters such as the roasting temperature, roasting time and mass ratio of ammonium sulfate to high-alumina fly ash. In order to balance the roasting efficiency and cost, optimize the roasting parameters and discover the roasting kinetics of ammonium sulfate and high-alumina fly ash, the optimization of the roasting conditions was necessary. Therefore, the law of change of aluminum extraction rate under different roasting conditions was investigated, as shown in Fig. 3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As evident from Fig. 3(B), the leaching rate of aluminum increased rapidly when the roasting time was extended from 5 min to 60 min before reaching a plateau. With a roasting time of 5 min, the leaching rate of aluminum was only 48.73% because the time employed was too short to facilitate the reaction between high-alumina fly ash and ammonium sulfate.3 It was found that the leaching rate of aluminum was over 93% when the roasting time was 60 min. However, with a further extension of the roasting time, there was little improvement in the leaching rate of aluminum, which indicates that 60 min is adequate for activating the high-alumina fly ash. Any longer not only increased the amount of energy consumed, but also increased the process time involved and affected process efficiency.3 Therefore, 60 min was set as the optimal roasting time.
1. As evident from Fig. 3(B), the leaching rate of aluminum increased rapidly when the roasting time was extended from 5 min to 60 min before reaching a plateau. With a roasting time of 5 min, the leaching rate of aluminum was only 48.73% because the time employed was too short to facilitate the reaction between high-alumina fly ash and ammonium sulfate.3 It was found that the leaching rate of aluminum was over 93% when the roasting time was 60 min. However, with a further extension of the roasting time, there was little improvement in the leaching rate of aluminum, which indicates that 60 min is adequate for activating the high-alumina fly ash. Any longer not only increased the amount of energy consumed, but also increased the process time involved and affected process efficiency.3 Therefore, 60 min was set as the optimal roasting time.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6
1, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on the leaching rate of aluminum, experiments were performed with roasting temperature 673 K and roasting time 60 min, and the results were shown in Fig. 3(C). When the mass ratio of ammonium sulfate to high-alumina fly ash was 1
1) on the leaching rate of aluminum, experiments were performed with roasting temperature 673 K and roasting time 60 min, and the results were shown in Fig. 3(C). When the mass ratio of ammonium sulfate to high-alumina fly ash was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the leaching rate of aluminum was 55.83%, which indicated that there was insufficient ammonium sulfate to react all the mullite in the high-alumina fly ash.3 The leaching rate of aluminum increased with an increase in the mass ratio of ammonium sulfate to high-alumina fly ash: when the mass ratio of ammonium sulfate to high-alumina fly ash was 6
1, the leaching rate of aluminum was 55.83%, which indicated that there was insufficient ammonium sulfate to react all the mullite in the high-alumina fly ash.3 The leaching rate of aluminum increased with an increase in the mass ratio of ammonium sulfate to high-alumina fly ash: when the mass ratio of ammonium sulfate to high-alumina fly ash was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the leaching rate of aluminum reached 93.57%, which showed that high-alumina fly ash can be fully activated by ammonium sulfate under this condition.3 However, with a further increase in the amount of ammonium sulfate added, there was little improvement in the leaching rate of aluminum, and the higher mass ratio was found to have little effect on high-alumina fly ash activation. For example, when the mass ratio of ammonium sulfate to high-alumina fly ash was 8
1, the leaching rate of aluminum reached 93.57%, which showed that high-alumina fly ash can be fully activated by ammonium sulfate under this condition.3 However, with a further increase in the amount of ammonium sulfate added, there was little improvement in the leaching rate of aluminum, and the higher mass ratio was found to have little effect on high-alumina fly ash activation. For example, when the mass ratio of ammonium sulfate to high-alumina fly ash was 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the leaching rate of aluminum reached 93.88%, which was only 0.31% higher than that when the mass ratio was 6
1, the leaching rate of aluminum reached 93.88%, which was only 0.31% higher than that when the mass ratio was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, leading to an increase in acid consumption and energy consumption in the subsequent acid leaching process. The results indicated that the maximum economic benefit was provided when the ammonium sulfate/high alumina fly ash mass ratio was 6
1, leading to an increase in acid consumption and energy consumption in the subsequent acid leaching process. The results indicated that the maximum economic benefit was provided when the ammonium sulfate/high alumina fly ash mass ratio was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.As mentioned previously, the optimum roasting conditions for using ammonium sulfate to roast and activate high-alumina fly ash were as follows: the roasting temperature was 673 K, the roasting time was 60 min, and mass ratio of ammonium sulfate to high-alumina fly ash was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Under these conditions, the leaching rate of aluminum reached 93.57%. According to previous literature (Table 2),30,40,41 the leaching rate of aluminum in this study was higher than most of them, which evidenced that this study was essential. It can be seen from the Fig. 3 that the roasting temperature had a great influence on the extraction rate of aluminum. Next, the roasting kinetics was discussed.
1. Under these conditions, the leaching rate of aluminum reached 93.57%. According to previous literature (Table 2),30,40,41 the leaching rate of aluminum in this study was higher than most of them, which evidenced that this study was essential. It can be seen from the Fig. 3 that the roasting temperature had a great influence on the extraction rate of aluminum. Next, the roasting kinetics was discussed.
3.3. Roasting kinetics analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
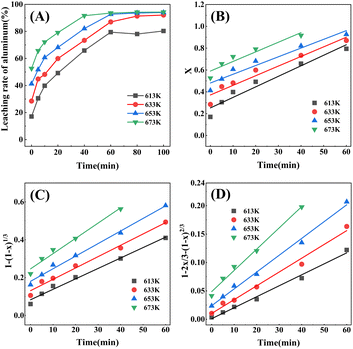
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the leaching conditions were the same, the effects of roasting temperature and roasting time on roasting process were studied at 613 K, 633 K, 653 K, and 673 K roasting for 0 min, 5 min, 10 min, 20 min, 40 min, 60 min, 80 min, 100 min respectively, and the results were shown in Fig. 4(A). It can be seen that the leaching rate of aluminum increased with the increase of roasting temperature and roasting time, especially the former one affected more significantly. It is worth emphasizing that the extraction rate of aluminum at 0 min was not 0, due to the fact that there was a period of temperature rise before the set temperature was reached, during which the reaction had already occurred, so the extraction rate was not 0.35 In addition, the change of aluminum leaching rate was divided into two stages. In the former stage, with the extension of roasting time, the aluminum leaching rate continued to increase under different temperature conditions; in the latter stage, the aluminum leaching rate of each temperature curve tended to be stable with the extension of time. Considering comprehensively, in the roasting temperature range of 613–673 K, the roasting time range of kinetic fitting was 0–60 min for 613–653 K, and the roasting time range of kinetic fitting was 0–40 min for 673 K.
1 and the leaching conditions were the same, the effects of roasting temperature and roasting time on roasting process were studied at 613 K, 633 K, 653 K, and 673 K roasting for 0 min, 5 min, 10 min, 20 min, 40 min, 60 min, 80 min, 100 min respectively, and the results were shown in Fig. 4(A). It can be seen that the leaching rate of aluminum increased with the increase of roasting temperature and roasting time, especially the former one affected more significantly. It is worth emphasizing that the extraction rate of aluminum at 0 min was not 0, due to the fact that there was a period of temperature rise before the set temperature was reached, during which the reaction had already occurred, so the extraction rate was not 0.35 In addition, the change of aluminum leaching rate was divided into two stages. In the former stage, with the extension of roasting time, the aluminum leaching rate continued to increase under different temperature conditions; in the latter stage, the aluminum leaching rate of each temperature curve tended to be stable with the extension of time. Considering comprehensively, in the roasting temperature range of 613–673 K, the roasting time range of kinetic fitting was 0–60 min for 613–653 K, and the roasting time range of kinetic fitting was 0–40 min for 673 K.
| Equations | Equations: g(x) = k(T)t | Reaction mechanisms |
|---|---|---|
| a Where x is the extraction rate of aluminum; k is the apparent rate constant; t is the reaction time. | ||
| F1(x) | x = k1t | External diffusion |
| F2(x) | 1 − (1 − x)1/3 = k2t | Chemical reaction |
| F3(x) | 1 − 2x/3 − (1 − x)2/3 = k3t | Internal diffusion |
| Time/min−1 | Temperature/K | F1(x) | F2(x) | F3(x) |
|---|---|---|---|---|
| 0–60 | 613 | 0.95048 | 0.98879 | 0.99108 |
| 0–60 | 633 | 0.94293 | 0.98608 | 0.98720 |
| 0–60 | 653 | 0.94477 | 0.99281 | 0.99476 |
| 0–40 | 673 | 0.90794 | 0.98026 | 0.99109 |
The simulated data of the most probably roasting kinetics were shown in Fig. 4(D). The apparent reaction rate constant k was obtained by calculating the slope of the reaction models, the values of R2 were obtained by linear fitting, and the values of k and R2 were shown in Table 5. It can be seen from Table 5 that the apparent reaction rate constant k increased from 0.00193 to 0.00374 when the thermodynamic temperature was increased from 613 K to 673 K, indicating that the thermodynamic temperature had a great influence on apparent reaction rate constant, and the increase of the thermodynamic temperature could accelerate apparent reaction rate constant.
| Temperature (K) | Apparent reaction rate constant k (s−1) | Linear correlation coefficient R2 |
|---|---|---|
| 613 | 0.00193 | 0.99108 |
| 633 | 0.00243 | 0.98720 |
| 653 | 0.00296 | 0.99476 |
| 673 | 0.00374 | 0.99109 |
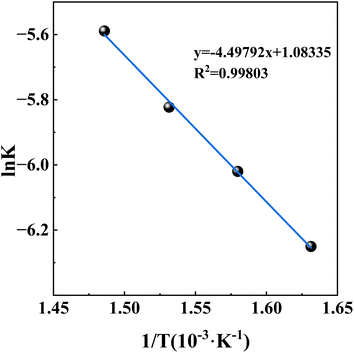
According to the Arrhenius formula ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = ln
k = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − Ea/RT (where k is the rate constant; A is the frequency factor; Ea is the apparent activation energy, J mol−1; R is the mole gas constant (8.314 J mol−1 K−1) and T is the thermodynamic temperature), ln
A − Ea/RT (where k is the rate constant; A is the frequency factor; Ea is the apparent activation energy, J mol−1; R is the mole gas constant (8.314 J mol−1 K−1) and T is the thermodynamic temperature), ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k was used to regress T−1 to explore the relationship between chemical reaction rate constant and temperature, and the results were shown in Fig. 5. It was very obvious that ln
k was used to regress T−1 to explore the relationship between chemical reaction rate constant and temperature, and the results were shown in Fig. 5. It was very obvious that ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k had a better linear relationship with T−1 (the R2 is close to 1 with a value of 0.99803). Combining ln
k had a better linear relationship with T−1 (the R2 is close to 1 with a value of 0.99803). Combining ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = ln
k = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − Ea/RT with y = −4.49792x + 1.08335 in Fig. 5, the values of apparent activation energy Ea and A were obtained with 37.40 kJ mol−1 and 2.9546, respectively. There were some differences compared to the literature,31 which was normal because of different raw materials, different chemical reagents and different chemical reactions. Bringing the experimental data into 1 − 2x/3 − (1 − x)2/3 = kt, the kinetic equation of high-alumina fly ash–ammonium sulfate system in the roasting activation process was obtained: 1 − 2x/3 − (1 − x)2/3 = 2.9546
A − Ea/RT with y = −4.49792x + 1.08335 in Fig. 5, the values of apparent activation energy Ea and A were obtained with 37.40 kJ mol−1 and 2.9546, respectively. There were some differences compared to the literature,31 which was normal because of different raw materials, different chemical reagents and different chemical reactions. Bringing the experimental data into 1 − 2x/3 − (1 − x)2/3 = kt, the kinetic equation of high-alumina fly ash–ammonium sulfate system in the roasting activation process was obtained: 1 − 2x/3 − (1 − x)2/3 = 2.9546![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[−37
exp[−37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400/(RT)]t.
400/(RT)]t.
3.4. Reaction mechanism
In order to further deduce the reaction mechanism of roasting process, the activated materials under different roasting conditions were characterized and analyzed by thermodynamics.The possible chemical reactions in the roasting process are listed below:48,49
| 6(NH4)2SO4 + Al2O3 = 2(NH4)3Al(SO4)3 + 6NH3↑ + 3H2O↑ | (1) |
| 18(NH4)2SO4 + Al6Si2O13 = 6(NH4)3Al(SO4)3 + 18NH3↑ + 9H2O↑ + 2SiO2↑ | (2) |
| (NH4)2SO4 = NH4HSO4 + NH3↑ | (3) |
| Al6Si2O13 + 12(NH4)2SO4 = 6NH4Al(SO4)2 + 2SiO2 + 18NH3↑ + 9H2O↑ | (4) |
| Al2O3 + 4(NH4)2SO4 = 2NH4Al(SO4)2 + 6NH3↑ + 3H2O↑ | (5) |
| Fe2O3 + 3(NH4)2SO4 = Fe2(SO4)3↑ + 6NH3↑ + 3H2O↑ | (6) |
| (NH4)2SO4 = 2NH3↑ + H2O↑ + SO3↑ | (7) |
| 4(NH4)3Al(SO4)3 + Al2O3 = 61NH4Al(SO4)2 + 6NH3↑ + 3H2O↑ | (8) |
| NH4HSO4 = NH3↑ + H2O↑ + SO3↑ | (9) |
By roasting, alumina in high-alumina fly ash can be converted to soluble (NH4)3Al(SO4)3 and NH4Al(SO4)2 by the reaction between molten ammonium sulfate and alumina and damaged mullite.
The high-alumina fly ash–ammonium sulfate mixtures were roasted and activated at different temperatures (573–693 K) with the mass ratio of ammonium sulfate to high-alumina fly ash of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
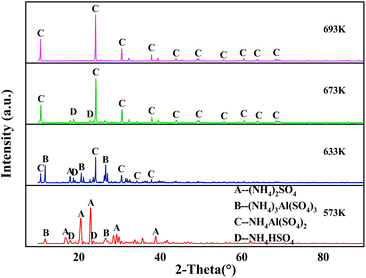
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, roasting time of 60 min. And the phase transformations of the mixtures were investigated by XRD. The XRD comparison charts of the high-alumina fly ash–ammonium sulfate mixtures were shown in Fig. 6, the main XRD peaks for different materials are listed in Table 6.31,38,50 Obviously, the main phases of the sample roasted at 573 K were found to be (NH4)2SO4 and (NH4)3Al(SO4)3, and there was a small amount of NH4HSO4, indicating that the molten ammonium sulfate had reacted with alumina and the damaged mullite to form ammonium aluminum sulfate, so the reactions (1)–(3) were verified.31 At the same time, the extraction rate of aluminum in the roasted material was 49%, which indicated that the reaction degree was limited. After roasted at 633 K, a new phase –NH4Al(SO4)2– were observed in the sample, so the reactions (4) and (5) began to happen under this condition.31,48,50 Simultaneously, the extraction rate of aluminum in the roasted material was 86.82%, indicating that a large number of aluminum-containing materials in high-alumina fly ash had reacted with ammonium sulfate to form soluble ammonium aluminum sulfate. In addition, the main phases were NH4HSO4 and NH4Al(SO4)2 in the sample at 673 K. Compared with 633 K, the peaks of NH4HSO4 and NH4Al(SO4)2 continued to exist and were enhanced, but the characteristic peaks of (NH4)2SO4 and (NH4)3Al(SO4)3 disappeared which due to the reactions (1)–(8) were over, respectively.48,50,51 At this time, the extraction rate of aluminum in the roasted material reached 93.57%, the reaction between high-alumina fly ash and ammonium sulfate was relatively complete, achieving high efficiently activate high-alumina fly ash. As the roasting temperature continued to rise to 693 K, the characteristic peak of NH4HSO4 disappeared, the characteristic peak of NH4Al(SO4)2 continued to exist, and the extraction rate of aluminum in the roasted material under this condition was 90.14%. Compared to 673 K, the extraction rate was decreased, which may be related to the decomposition of ammonium salt in the raw material and the relatively low mass ratio of ammonium sulfate to high-alumina fly ash in the material,3 reaction (9) was finished.48,50,51
1, roasting time of 60 min. And the phase transformations of the mixtures were investigated by XRD. The XRD comparison charts of the high-alumina fly ash–ammonium sulfate mixtures were shown in Fig. 6, the main XRD peaks for different materials are listed in Table 6.31,38,50 Obviously, the main phases of the sample roasted at 573 K were found to be (NH4)2SO4 and (NH4)3Al(SO4)3, and there was a small amount of NH4HSO4, indicating that the molten ammonium sulfate had reacted with alumina and the damaged mullite to form ammonium aluminum sulfate, so the reactions (1)–(3) were verified.31 At the same time, the extraction rate of aluminum in the roasted material was 49%, which indicated that the reaction degree was limited. After roasted at 633 K, a new phase –NH4Al(SO4)2– were observed in the sample, so the reactions (4) and (5) began to happen under this condition.31,48,50 Simultaneously, the extraction rate of aluminum in the roasted material was 86.82%, indicating that a large number of aluminum-containing materials in high-alumina fly ash had reacted with ammonium sulfate to form soluble ammonium aluminum sulfate. In addition, the main phases were NH4HSO4 and NH4Al(SO4)2 in the sample at 673 K. Compared with 633 K, the peaks of NH4HSO4 and NH4Al(SO4)2 continued to exist and were enhanced, but the characteristic peaks of (NH4)2SO4 and (NH4)3Al(SO4)3 disappeared which due to the reactions (1)–(8) were over, respectively.48,50,51 At this time, the extraction rate of aluminum in the roasted material reached 93.57%, the reaction between high-alumina fly ash and ammonium sulfate was relatively complete, achieving high efficiently activate high-alumina fly ash. As the roasting temperature continued to rise to 693 K, the characteristic peak of NH4HSO4 disappeared, the characteristic peak of NH4Al(SO4)2 continued to exist, and the extraction rate of aluminum in the roasted material under this condition was 90.14%. Compared to 673 K, the extraction rate was decreased, which may be related to the decomposition of ammonium salt in the raw material and the relatively low mass ratio of ammonium sulfate to high-alumina fly ash in the material,3 reaction (9) was finished.48,50,51
| Temperature | Main phase | 2theta (°) | ||||
|---|---|---|---|---|---|---|
| 573 K | (NH4)2SO4 | 16.76 | 20.52 | 22.94 | 29.32 | 38.84 |
| (NH4)3Al(SO4)3 | 11.78 | 26.62 | — | — | — | |
| NH4HSO4 | 17.94 | 23.58 | — | — | — | |
| 633 K | (NH4)2SO4 | 17.86 | — | — | — | — |
| (NH4)3Al(SO4)3 | 11.78 | 20.62 | 26.62 | — | — | |
| NH4Al(SO4)2 | 10.64 | 24.14 | 30.52 | 34.28 | 37.88 | |
| NH4HSO4 | 18.68 | — | — | — | — | |
| 673 K | NH4Al(SO4)2 | 10.72 | 24.22 | 30.62 | 37.92 | 43.94 |
| 49.46 | 55.64 | 60.64 | 63.94 | 68.46 | ||
| NH4HSO4 | 18.78 | 22.84 | — | — | — | |
| 693 K | NH4Al(SO4)2 | 10.64 | 24.12 | 30.54 | 37.86 | 43.86 |
| 49.72 | 55.52 | 60.56 | 63.88 | 68.38 | ||
The chemical compositions of the roasted sample and the acid leached residue were measured by XRF and were shown in Table 7. Sample 1 was roasted at a roasting temperature of 673 K, roasting time of 60 min, and a mass ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of ammonium sulfate to high alumina fly ash, and the total amount of elements measured by XRF was 67.5% less than 100%, which was because nitrogen and hydrogen could not be measured. The main elements were S (31.1043%), O (27.0454%), Al (5.0143%), Si (2.3086%), with higher S, O content, which corresponded to the NH4HSO4 and NH4Al(SO4)2 phases in XRD (Fig. 6 – 673 K). Sample 2 was 0.71 g residue obtained from 10 g sample 1 after acid leaching, the total amount of elements measured by XRF was 85.7%, the main elements were Si (33.6134%), O (44.6783%), Al (6.0732%), the content of Si and O was high, which corresponded to the SiO2 phase in XRD (Fig. 7), while Al was the percentage of 0.71 g residue, so the amount of residual Al amount was very small and it was calculated that 91.4% of Al was extracted by XRF. Fig. 7 showed the XRD pattern of the residue after roasting with ammonium sulfate high alumina fly ash with a mass ratio of 6
1 of ammonium sulfate to high alumina fly ash, and the total amount of elements measured by XRF was 67.5% less than 100%, which was because nitrogen and hydrogen could not be measured. The main elements were S (31.1043%), O (27.0454%), Al (5.0143%), Si (2.3086%), with higher S, O content, which corresponded to the NH4HSO4 and NH4Al(SO4)2 phases in XRD (Fig. 6 – 673 K). Sample 2 was 0.71 g residue obtained from 10 g sample 1 after acid leaching, the total amount of elements measured by XRF was 85.7%, the main elements were Si (33.6134%), O (44.6783%), Al (6.0732%), the content of Si and O was high, which corresponded to the SiO2 phase in XRD (Fig. 7), while Al was the percentage of 0.71 g residue, so the amount of residual Al amount was very small and it was calculated that 91.4% of Al was extracted by XRF. Fig. 7 showed the XRD pattern of the residue after roasting with ammonium sulfate high alumina fly ash with a mass ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 673 K for 60 min and leaching with 10% volume fraction of sulfuric acid at 90 °C for 60 min at a liquid to solid ratio of 10
1 at 673 K for 60 min and leaching with 10% volume fraction of sulfuric acid at 90 °C for 60 min at a liquid to solid ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
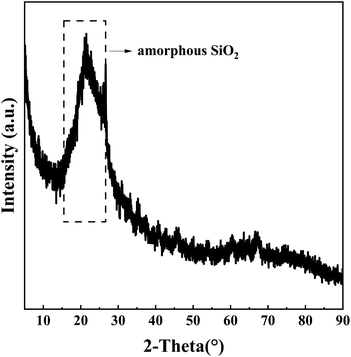
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Only amorphous silica was detected in the residue, and other substances such as mullite and aluminum-containing compounds were not detected, showing that NH4HSO4 and NH4Al(SO4)2 generated during roasting were completely dissolved in sulfuric acid solution, and the aluminum in high-alumina fly ash was leached out by the acid, further confirming that high-alumina fly ash can be activated efficiently by ammonium sulfate.
1. Only amorphous silica was detected in the residue, and other substances such as mullite and aluminum-containing compounds were not detected, showing that NH4HSO4 and NH4Al(SO4)2 generated during roasting were completely dissolved in sulfuric acid solution, and the aluminum in high-alumina fly ash was leached out by the acid, further confirming that high-alumina fly ash can be activated efficiently by ammonium sulfate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of ammonium sulfate to high alumina fly ash; sample 2 was 0.71 g residue obtained from 10 g sample 1 after acid leaching)
1 of ammonium sulfate to high alumina fly ash; sample 2 was 0.71 g residue obtained from 10 g sample 1 after acid leaching)
| Sample | O | Al | Si | P | S | K | Ca | Ti | Fe | Zn | Sr | Y | Zr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27.0454 | 5.0143 | 2.3086 | — | 31.1043 | 0.0668 | 0.7211 | 0.5349 | 0.4616 | 0.1473 | 0.0334 | — | 0.0290 |
| 2 | 44.6783 | 6.0732 | 33.6134 | 0.1143 | 0.2510 | 0.1227 | 0.0887 | 0.4048 | 0.2348 | — | 0.0246 | 0.0046 | 0.0529 |
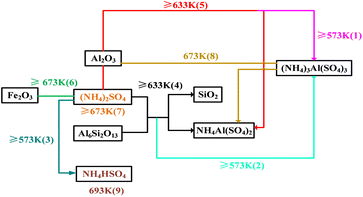
Based on the analysis of XRD patterns, the reaction path of the high-alumina fly ash–ammonium sulfate mixtures under the experimental conditions was obtained, as shown schematically in Fig. 8.
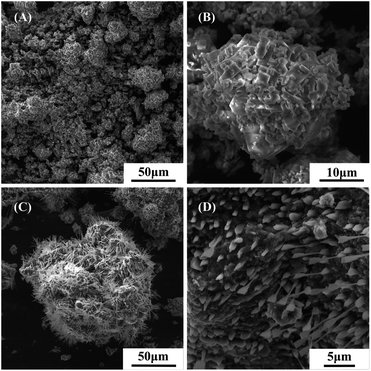
In the condition of the temperatures of 573 K and 633 K, and duration of 60 min, the SEM images of high-alumina fly ash–ammonium sulfate mixtures were shown in Fig. 9. Fig. 9(A) showed that the particle structure of the mixture was similar, and there was agglomeration between particles. Fig. 9(B) showed that the material appearance and morphology further demonstrated this melt aggregation phenomenon, however, the degree of melt aggregation of the material was low. The results confirmed that the mass transfer rate of high-alumina fly ash ammonium sulfate was accelerated and the occurrence of reactions was promoted by melt polymerization at 573 K, the reactions (1)–(3) were confirmed again. Correspondingly, the XRD patterns of the activated materials showed that the main phases of the activated materials were ammonium sulfate and ammonium aluminum sulfate. Fig. 9(C) showed that the outer surface of each particle was similar and whiskers were formed. Fig. 9(D) further confirmed the appearance of crystal morphology. Correspondingly, XRD patterns of activated materials showed that the main phases of activated materials were (NH4)2SO4, (NH4)3Al(SO4)3, NH4Al(SO4)2 and NH4HSO4, indicating that a large number of aluminum-bearing materials in high-alumina fly ash had reacted with ammonium sulfate to form soluble ammonium aluminum sulfate, which confirmed the occurrence of reactions (1)–(5) again.
The above XRD and SEM analysis were complementary and revealed the mechanism of the activation process.
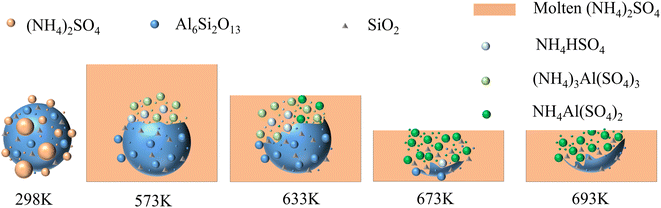
Fig. 10 was the reaction mechanism diagram. It can be seen that at 298 K, the reaction did not occur because the temperature range of roasting activation reaction was not reached. With the increase of temperature, ammonium sulfate reached the melting point and became a molten state. At 573 K, the molten ammonium sulfate reacted with the structurally damaged mullite to form (NH4)3Al(SO4)3, coinciding with reactions (1) and (2), and some ammonium sulfate was decomposed into NH4HSO4, such as reaction (3). At 633 K, the reactions (1)–(3) were enhanced and a new phase NH4Al(SO4)2 was formed, coinciding with reactions (4) and (5). At 673 K, (NH4)2SO4 disappeared, indicating that the reactions (1)–(7) were ended. Moreover, (NH4)3Al(SO4)3 had completely disappeared and all transformed into NH4Al(SO4)2, reaction (8) was completed. At 693 K, it can be found that only NH4Al(SO4)2 was observed, and NH4HSO4 was completely disappeared, reaction (9) was over. At the same time, the further decomposition of ammonium salt in the raw material produced ammonia, sulfur trioxide and other gases, which made the effective mass ratio of ammonium sulfate to high-alumina fly ash in the material decrease, and resulted in the whole reaction degree and the leaching rate of aluminum were decreased.
In brief, the amorphous silicon dioxide contained in high-alumina fly ash was unchanged during the whole reaction process, indicating that the reaction of high-alumina fly ash–ammonium sulfate system was mainly the reaction between molten ammonium sulfate and structurally damaged mullite.
4. Conclusions
On the basis of thermodynamic analysis, ammonium sulfate was used as an activator to roast and activate high-alumina fly ash. The effects of roasting process (roasting temperatures, roasting times, mass radio of ammonium sulfate to high-alumina fly ash) on the aluminum extraction were discussed, and the reaction mechanism was investigated through chemical reactions and phase transformation in the roasting process. A summary and main conclusions are as follows:(1) The main components of high-alumina fly ash were mullite (Al6Si2O13) and amorphous SiO2, as well as impurity oxide Fe2O3. It was found that temperature had a great influence on the effect of high-alumina fly ash activation. The highest aluminum extraction rate of 93.57% was achieved when ammonium sulfate was roasted with high alumina fly ash at a mass ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 673 K for 60 min.
1 at 673 K for 60 min.
(2) The roasting activation process of high alumina fly ash–ammonium sulfate system was a liquid–solid reaction process and controlled by internal diffusion, with an apparent activation energy of 37.40 kJ mol−1. The kinetic equation was: 1 − 2x/3 − (1 − x)2/3 = 2.9546![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[−37
exp[−37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400/(RT)]t.
400/(RT)]t.
(3) The phase transformation and morphology of high-alumina fly ash–ammonium sulfate system roasted at different temperatures were thoroughly investigated by XRD and SEM. The alumina and structurally damaged mullite in high-alumina fly ash can react with the molten ammonium sulfate. At 573 K, the ammonium sulfate salt was (NH4)3Al(SO4)3; at 633 K, the ammonium sulfate salts were mainly (NH4)3Al(SO4)3 and NH4Al(SO4)2; at 673 K and 693 K, the ammonium sulfate salt was NH4Al(SO4)2. In the reaction process, the appearance morphology of the material agglomerated, which enhanced the mass transfer process and promoted the occurrence of reactions.
(4) Based on these investigations and analysis, the reaction mechanism of high-alumina fly ash–ammonium sulfate mixtures was mainly the reaction between molten ammonium sulfate and structurally damaged mullite.
Author contributions
Xiaoying Li performed the experiments and analysis and wrote the main manuscript text. Bo Hu and Xueqing Liu helped Xiaoying Li in analyzing kinetics and mechanism. Xintao He and Chengwei Liu gave some guidance on the means of the analysis of the material. Nengsheng Liu and Sufang He guided the research and revised the draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support provided by the National Natural Science Foundation of China (No. 51704137) and Yunnan Ten Thousand Talents Plan Young & Elite Talents Project (YNWR-QNBJ-2018-067 and YNWR-QNBJ-2020-002).References
- S. S. Alterary and N. H. Marei, J. King Saud Univ., Sci., 2021, 33, 101536 CrossRef.
- P. Laxmidhar and D. Subhakanta, Emerging Mater. Res., 2020, 9, 921–934 CrossRef.
- T. Hu, L. Y. Kou, L. Yang, B. C. Zhou and J. W. Zhou, Powder Technol., 2021, 377, 739–747 CrossRef.
- L. Y. Kou, J. W. Zhou, L. Yang, T. Hu and B. C. Zhou, Mater. Res. Express, 2020, 7, 025515 CrossRef.
- Y. W. Xing, F. Y. Guo, M. D. Xu, X. H. Gui, H. S. Li, G. S. Li, Y. C. Xia and H. S. Han, Powder Technol., 2019, 353, 372–384 CrossRef.
- W. Liu, J. W. Liang, C. Fu, B. B. Zeng, M. P. Huang, G. Yang, X. D. Luo, D. An, S. Wei, Z. P. Xie and G. Z. Xu, Ceram. Int., 2022, 48, 18588–18595 CrossRef.
- J. J. Yang, H. J. Sun, T. J. Peng, L. Zeng and L. Chao, Clean Technol. Environ. Policy, 2022, 24, 1507–1519 CrossRef.
- G. Li, M. Li, X. Zhang, P. Cao, H. Jiang, J. Luo and T. Jiang, Int. J. Min. Sci. Technol., 2022, 32, 563–573 CrossRef.
- H. Yuan, Y. Yong, C. Wei, W. Shu, Z. Dongmei and Y. Ce, Think Tank Era, 2019, 29, p. 274.
- Z. Zimar, D. Robert, A. Zhou, F. Giustozzi, S. Setunge and J. Kodikara, J. Environ. Manage., 2022, 312, 114926 CrossRef PubMed.
- G. Sun, J. Zhang, B. Hao, X. Li, M. Yan and K. Liu, Chemosphere, 2022, 298, 134136 CrossRef.
- Y. Liang, S. Chen, J. X. Zhong, H. Ding, Z. L. Zhu and S. Li, J. Sol-Gel Sci. Technol., 2022, 103, 185–194 CrossRef.
- V. Gadore and M. Ahmaruzzaman, J. Water Proc. Eng., 2021, 41, 101910 CrossRef.
- E. V. Fomenko, N. N. Anshits, L. A. Solovyov, Y. V. Knyazev, S. V. Semenov, O. A. Bayukov and A. G. Anshits, ACS Omega, 2021, 6, 20076–20085 CrossRef.
- L. Y. Sun, K. Luo, J. R. Fan and H. L. Lu, Fuel, 2017, 199, 22–27 CrossRef.
- J. K. Zhang, K. J. Wen and L. Li, Fuel, 2021, 286, 119386 Search PubMed.
- T. Seki, K. Nakamura, Y. Ogawa and C. Inoue, Environ. Monit. Assess., 2021, 193, 225 CrossRef.
- D. Langauer, V. Cablik, S. Hredzak, A. Zubrik, M. Matik and Z. Dankova, Materials, 2021, 14, 1267 CrossRef.
- C. B. Guo, L. Zhao, J. Yang, K. H. Wang and J. J. Zou, J. Cleaner Prod., 2020, 271, 122703 CrossRef.
- J. E. Nyarko-Appiah, W. Yu, P. Wei, W. Jiang, Z. Liang and S. Gong, Fuel, 2022, 310, 122434 CrossRef.
- J. Chen, R. Yang, Z. Zhang and D. Wu, J. Hazard. Mater., 2022, 421, 126817 CrossRef PubMed.
- G. H. Bai, Y. H. Qiao, B. Shen and S. L. Chen, Fuel Process. Technol., 2011, 92, 1213–1219 CrossRef.
- C. B. Guo, J. J. Zou, Y. S. Jiang, T. P. Huang, Y. Cheng and C. D. Wei, J. Mater. Sci., 2014, 49, 4315–4322 CrossRef.
- C. Guo, J. Zou, S. Ma, J. Yang and K. Wang, Minerals, 2019, 9, 585 CrossRef.
- S. Sanchindapong, C. Narattha, M. Piyaworapaiboon, S. Sinthupinyo, P. Chindaprasirt and A. Chaipanich, J. Therm. Anal. Calorim., 2020, 142, 167–174 CrossRef.
- J. Cao, P. Wang and Q. Sun, Z. Anorg. Allg. Chem., 2020, 646, 1666–1670 CrossRef.
- Z. Cai and Y. Zhang, RSC Adv., 2017, 7, 36917–36922 RSC.
- J. Ding, S. Ma, S. Shen, Z. Xie, S. Zheng and Y. Zhang, Waste Manag., 2017, 60, 375–387 CrossRef.
- D. Valeev, P. Bobylev, N. Osokin, I. Zolotova, I. Rodionov, C. Salazar-Concha and K. Verichev, J. Cleaner Prod., 2022, 363, 132360 CrossRef.
- W. Yan, X. Haixia, X. Yujun and L. Jianan, J. Phys.: Conf. Ser., 2019, 1347, 012111 CrossRef.
- P. Wang, L. S. Li and D. Z. Wei, Chin. J. Chem. Eng., 2014, 22, 1027–1032 CrossRef.
- H. C. Park, Y. J. Park and R. Stevens, Mater. Sci. Eng., A, 2004, 367, 166–170 CrossRef.
- L. Li, X. Liao, Y. Wu and Y. Liu, Extracting Alumina from Coal Fly Ash with Ammonium Sulfate Sintering Process, Orlando, FL, 2012 Search PubMed.
- F. J. Doucet, S. Mohamed, N. Neyt, B. A. Castleman and E. M. van der Merwe, Hydrometallurgy, 2016, 166, 174–184 CrossRef.
- L.-L. Sui and Y.-C. Zhai, Trans. Nonferrous Met. Soc. China, 2014, 24, 848–853 CrossRef.
- L. Tao, L. Wang, K. Yang, X. Wang, L. Chen and P. Ning, RSC Adv., 2021, 11, 5741–5752 RSC.
- B. Zhou, J. Zhou, T. Hu, L. Yang, G. Lin and L. Zhang, Mater. Res. Express, 2018, 6, 015502 CrossRef.
- R. C. Wang, Y. C. Zhai and Z. Q. Ning, Int. J. Miner., Metall. Mater., 2014, 21, 144–149 CrossRef CAS.
- N. Li, J. Guo, Z. Chang, H. Dang, X. Zhao, S. Ali, W. Li, H. Zhou and C. Sun, RSC Adv., 2019, 9, 23908–23915 RSC.
- Y. S. Wu, P. Xu, J. Chen, L. S. Li and M. C. Li, Chin. J. Chem. Eng., 2014, 22, 1363–1367 CrossRef.
- L. Y. Sun, K. Luo, J. R. Fan and H. L. Lu, Fuel, 2017, 199, 22–27 CrossRef.
- L. Tian, A. Gong, X. G. Wu, J. H. Li, C. W. Liu, X. Q. Yu and Z. F. Xu, Powder Technol., 2020, 373, 362–368 CrossRef.
- L. Jie, Y. Li, H. Duan, X. Guo and Y. Zhai, Russ. J. Non-Ferrous Metals, 2019, 59, 596–604 CrossRef.
- B. Wang, T. Liu, Y. M. Zhang and J. Huang, Minerals, 2017, 7, 43 CrossRef.
- X. R. Zhu, X. H. Liu and Z. W. Zhao, Hydrometallurgy, 2019, 186, 83–90 CrossRef CAS.
- J. Ma, Y. Zhang, Y. Qin, Z. Wu, T. Wang and C. Wang, Ultrason. Sonochem., 2017, 35, 304–312 CrossRef CAS PubMed.
- W. J. Zhang, C. Y. Wang and B. Z. Ma, Trans. Nonferrous Met. Soc. China, 2019, 29, 859–867 CrossRef CAS.
- G. Q. Zhang, T. Hu, W. J. Liao and X. D. Ma, J. Environ. Chem. Eng., 2021, 9, 105332 CrossRef CAS.
- F. Y. Meng, X. S. Li, L. Shi, Y. J. Li, F. Gao and Y. Z. Wei, Miner. Eng., 2020, 157, 106561 CrossRef CAS.
- G. S. Li, X. L. Xiong, L. P. Wang, L. Che, L. Z. Wei, H. W. Cheng, X. L. Zou, Q. Xu, Z. F. Zhou, S. G. Li and X. G. Lu, Metals, 2019, 9, 1256 CrossRef CAS.
- S. Chaudhury, K. D. S. Mudher and V. Venugopal, J. Nucl. Mater., 2003, 322, 119–125 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |