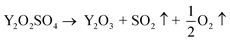Open Access Article
Open Access ArticleDual-mode vehicles with simultaneous thermometry and drug release properties based on hollow Y2O3:Er,Yb and Y2O2SO4:Er,Yb spheres†
Sonali Mohanty ab,
Simona Premcheska
ab,
Simona Premcheska ac,
Joost Verduijn
ac,
Joost Verduijn c,
Hannes Rijckaert
c,
Hannes Rijckaert d,
Andre G. Skirtach
d,
Andre G. Skirtach c,
Kristof Van Hecke
c,
Kristof Van Hecke *b and
Anna M. Kaczmarek
*b and
Anna M. Kaczmarek *a
*a
aNanoSensing Group, Department of Chemistry, Ghent University, Krijgslaan 281-S3, 9000 Ghent, Belgium. E-mail: Kristof.VanHecke@UGent.be; Anna.Kaczmarek@UGent.be
bXStruct, Department of Chemistry, Ghent University, Krijgslaan 281-S3, 9000 Ghent, Belgium
cNanoBiotechnology Group, Department of Biotechnology, Faculty of Bioscience Engineering, Ghent University, Proeftuinstraat 86, 9000 Ghent, Belgium
dSCRiPTS, Department of Chemistry, Ghent University, Krijgslaan 281-S3, 9000 Ghent, Belgium
First published on 21st November 2022
Abstract
Employing luminescence thermometry in the biomedical field is undeniably appealing as many health conditions are accompanied by temperature changes. In this work, we show our ongoing efforts and results at designing novel vehicles for dual-mode thermometry and pH-dependent drug release based on hollow spheres. Hereby for that purpose, we exploit the hollow Y2O3 and Y2O2SO4 host materials. These two inorganic hollow phosphors were investigated and showed to have excellent upconversion Er3+–Yb3+ luminescence properties and could be effectively used as optical temperature sensors in the physiological temperature range when induced by near-infrared CW light (975 nm). Further, doxorubicin was exploited as a model anti-cancer drug to monitor the pH-dependent drug release of these materials showing that they can be used for simultaneous thermometry and drug delivery applications.
Introduction
Health conditions such as cancer, inflammations, heart attacks, and brain seizures lead to hyperlocal temperature changes in the human body.1,2 Based on this knowledge, we know that temperature measurements are of immense importance for diagnostic purposes, starting from localization, mapping, tracing, and ultimately treatment.3,4 For applications in biomedicine the temperature range of 20–50 °C is crucial, and is often referred to as the physiological range. For future real-life application in biomedicine, it is important that the measurements are done in a straightforward and non-invasive way to avoid any unnecessary pain and side effects to the patient.1 To date, techniques such as infrared light (IR) thermography or Raman spectroscopy-based thermometry have been proposed to precisely detect temperature locally and non-invasively.5 Yet, as a downside, IR cameras provide temperature distributions only of the surface, with estimated thermal emissivity from a short distance. Additional disadvantages of this technique are a large temperature uncertainty and a low spatial resolution. On the one hand, although Raman thermometry can effectively probe the temperature of a system <1 μm in both solids and liquids, it struggles with low signal levels and crosstalk with fluorescent molecules. On the other hand, thermometers based on luminescence detection promise obtaining a high thermal sensitivity (Sr > 1% per °C), good thermal resolution (δ < 0.1 °C), thermal imaging through the object surface, in many cases high brightness in the infrared biological windows (650–1700 nm) and measuring in the desired physiologically relevant temperature region.4,6 The development of such luminescence thermometers, and their use in biological systems, could be a solution to important persisting challenges, such as early tumor detection, real-time intra-tumoral in vitro or in vivo thermal sensing, in vivo localized inflammation detection, etc.7,8 In the development of luminescence thermometers, it is also necessary to ensure that the designed materials do not inflict acute toxicity on viable tissue and show good clearance from the body.3Thus far, numerous luminescent materials have already been proposed as luminescent thermometers for potential use in biomedicine e.g., quantum dots (QDs), fluorescent dyes, polymers, lanthanide (Ln)-doped metal–organic frameworks (MOFs), and lanthanide-doped nanoparticles.9–11 Among them, lanthanide-based materials are proving to be excellent materials for luminescence thermometry, as they have a large number of closely-lying emissive excited energy states originating from the 4fn configuration. This allows luminescence detection in a wide range – from the ultraviolet (UV) over the visible to near-infrared (NIR).12 Upconversion (UC) materials have been studied extensively as they have promising applications in three-dimensional displays, optical, optoelectronic, and chemical sensors, solar cells, biological fluorescence labels and probes, anti-counterfeiting, and other applications.13,14 An exciting line of research in the nanotheranostic field now exploits UC luminescence which enhances the drug release of certain model drugs e.g., doxorubicin (DOX) from the transporting vessels.15
Nonetheless, developing efficiently emitting nanothermometers that fulfill all of these prerequisites for in vivo applications is beyond a trivial task. Many reported Ln-based luminescent materials have already shown very good potential for certain biomedical applications, but there are still some significant challenges that need to be resolved, such as obtaining sufficiently high brightness and relative sensitivity, obtaining real-time high spatio-temporal resolution, 3D, reliable, real-time in vivo monitoring at a very small temperature step, and the design of active nanothermometers (combined nanocooler/nanoheater-thermometer systems).16–18 In order to make translational application of these materials, it is important to move towards (nano)theranostics, where the nanothermometers need to show potential to be coupled with a treatment method for a certain targeted disease (e.g., drug delivery, photodynamic therapy, or photothermal therapy).19,20 And simultaneously these materials need to show low-to-no toxicity toward healthy cells and tissue in the human body.
Inorganic hollow materials with controllable composition and shape are very attractive for various applications due to their superior properties which include low density, large specific surface area, and good permeability.21–24 Such materials have great potential for applications in drug delivery, but also photodynamic therapy, sensing, cell labeling, waste removal, or catalysis. One of the most intriguing aspects is that hollow materials also allow the development of multifunctional materials, which can combine several functions in one material, e.g., sensing and drug delivery, which is the aim of this work. For the preparation of inorganic hollow materials both a template, as well as a template-free process can be used.25 As a template often silica, carbon spheres, or polystyrene beads are employed.26,27 Sacrificial templates, such as rare-earth hydroxyl carbonates can also be used.25
In our work, two different inorganic hollow materials were prepared with the notion of combining thermometry (as a diagnostic tool) with drug release in one material. Our synthesis was based on a modified previously reported double-shelled hollow spheres composed of an inner Y2O3 shell doped with Yb3+ and Er3+, and an outer, mesoporous silica shell.28 Also, as a second material – yttrium oxysulfate hollow spheres doped with Yb3+ and Er3+ was prepared, using L-cysteine as the sulfur source, in a hydrothermal synthesis involving the Ostwald ripening process. This synthesis was also based on a previously reported route.29
Ln3+-doped Y2O3 has widely been studied as an upconversion material due to its high thermal stability, chemical durability, relatively low phonon energy, and low thermal expansion of yttrium oxide. In Y2O3:Er,Yb the ratio of the green-to-red emission can easily be tuned by changing the Yb3+ sensitizer concentration, as has been reported previously in literature.30
Rare-earth oxysulfate (RE2O2SO4) materials are important matrix compounds for luminescent materials and have attracted quite some attention in recent years, due to their specific magnetic properties, large oxygen storage volume, and unique luminescent properties.31,32 The RE2O2SO4 material has already shown to be an excellent host for upconversion applications, which is why it was selected for this study alongside the Y2O3 material.33,34 This material has ideal chemical and thermal stability as well as a high size and shape tunability. Many types of synthetic approaches can be used including single precursor decomposition, urea-based aqueous synthesis, molten salt synthesis, combustion synthesis, and others.34 RE2O2SO4 shows very good structural stability when replacing Y3+ ions with other rare-earth metal ions, which is very important for designing materials with a high doping percentage of emissive lanthanide ions. Two different rare-earth ions can easily be co-doped into the crystal structure of the material in optimal ratios without modifying the host structure or significantly changing the lattice constant. This particular oxysulfate has also not been explored much for thermometry or drug release; therefore, it was of interest for our investigation.
In this work, we investigated both Y2O3:Er,Yb and Y2O2SO4:Er,Yb hollow spheres for potential applications such as luminescence thermometers and drug carriers, employing DOX as a model anticancer drug.
Results and discussion
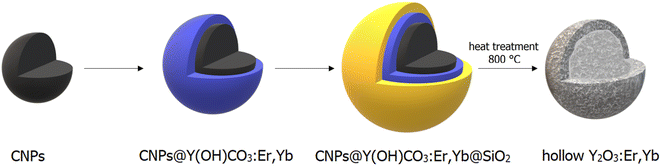
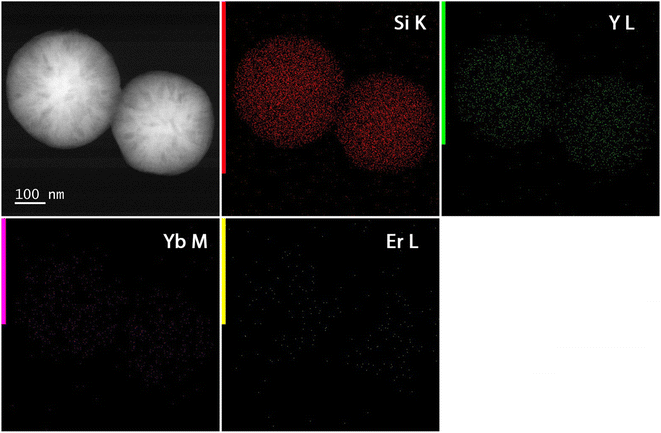
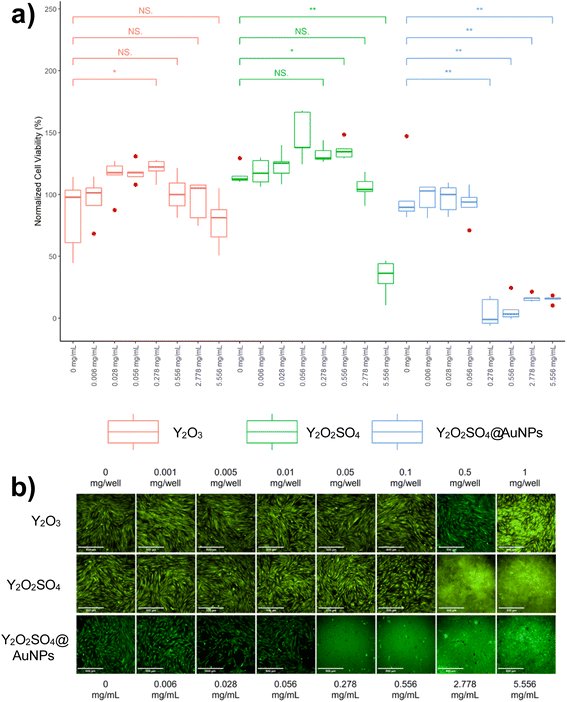
A schematic overview of the development of Y2O3:Er,Yb hollow spheres is presented in Scheme 1. The synthesis of double-shelled Y2O3:Er,Yb is complex and had to be carried out in several steps based on a previously reported synthesis route.28 The detailed synthesis route of the material is presented in the Experimental section. Briefly, carbon nanoparticles (CNPs) of 200–400 nm in size were prepared via a hydrothermal synthesis route. Next, a shell of Y(OH)CO3:Er,Yb was grown around the CNPs template. The CNPs@Y(OH)CO3:Er,Yb particles were then coated with a layer of SiO2 and after drying the CNPs@Y(OH)CO3:Er,Yb@SiO2 product was heat-treated at 800 °C to obtain hollow Y2O3:Er,Yb spheres. On the contrary, the preparation of Y2O2SO4:Er,Yb hollow spheres was simpler undergoing only two synthetic steps, and was also based on a previous report.29 The precursor material was obtained through a hydrothermal synthesis where L-cysteine and PVP (polyvinylpyrrolidone) were used.29 To obtain Y2O2SO4:Er,Yb hollow spheres the precursor material was heat-treated at 850 °C. All developed materials, obtained at different synthetic stages, were characterized by scanning transmission electron microscopy (STEM) with bright field (BF-STEM) and high-angle annular dark-field detector (HAADF-STEM), and energy dispersive X-ray (EDX) mapping, as well as by scanning electron microscopy (SEM) to monitor the morphology of the obtained materials. The Y2O3:Er,Yb, and Y2O2SO4:Er,Yb materials (for Y2O2SO4 both the Y2O2SO4:Er,Yb precursor and Y2O2SO4:Er,Yb heat treated (HT) materials were investigated) were characterized by powder XRD and nitrogen sorption as well as TGA. In Fig. 1 the BF-STEM and SEM images of CNPs (Fig. 1a and b), as well as CNPs@Y(OH)CO3:Er,Yb (Fig. 1c–f) are presented. The CNPs are around 200–400 nm in size, with uniform size distribution. After growing Y(OH)CO3:Er,Yb on the CNPs the size of the spherical particles slightly increases, and rough edges of the Y(OH)CO3:Er,Yb layer are observed. It is clear from the BF-STEM images that the Y(OH)CO3:Er,Yb layer is distributed on all CNPs. SEM images confirm uniform shapes and sizes of the CNPs@Y(OH)CO3:Er,Yb material. In Fig. 2, BF-STEM images of the particles after SiO2 coating and heat-treatment are shown. Hollow Y2O3:Er,Yb spheres of 200–400 nm in size are formed. EDX maps confirm the presence of the SiO2 shell, as well as even distribution of the Er3+ and Yb3+ dopants in the Y2O3 matrix (Fig. 3).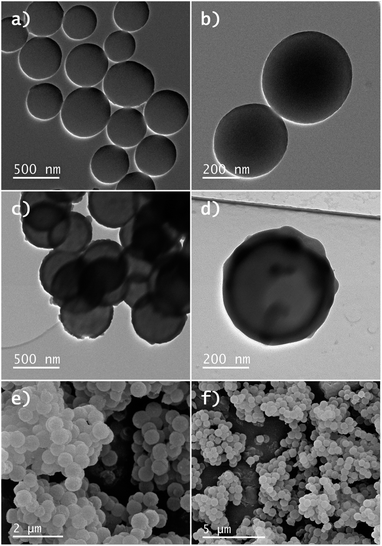 | ||
| Fig. 1 BF-STEM images of (a) and (b) CNPs obtained in the hydrothermal synthesis and (c) and (d) CNPs@Y(OH)CO3:Er,Yb spheres and (e) and (f) SEM images of CNPs@Y(OH)CO3:Er,Yb spheres. | ||
 | ||
| Fig. 2 (a) and (b) BF-STEM images of Y2O3:Er,Yb hollow spheres obtained after heat-treatment in air. | ||
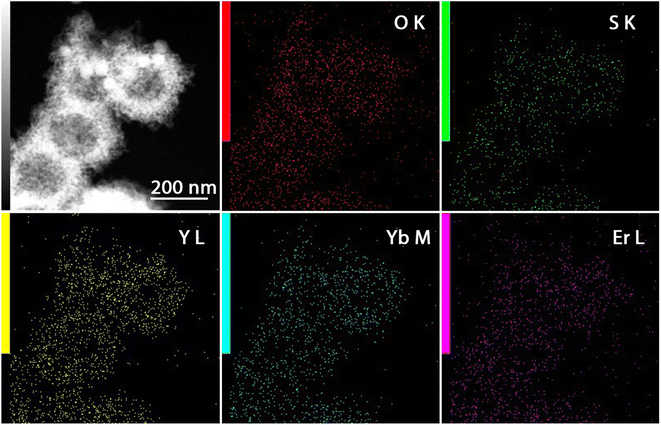
In Fig. 4 the BF-STEM images of the Y2O2SO4:Er,Yb precursor and Y2O2SO4:Er,Yb HT (after heat-treatment) are presented. For both the precursor (Fig. 4a and b) as well as the Y2O2SO4:Er,Yb HT material spherical, urchin-like morphology is obtained. The obtained particles are around 150–200 nm in size, with uniform size distribution. The spheres are hollow even before heat-treatment, without any sufficient changes in morphology (Fig. 4c). Also, for this material, EDX maps confirm an even distribution of the Er3+ and Yb3+ dopant ions in the Y2O2SO4 host matrix (Fig. 5).
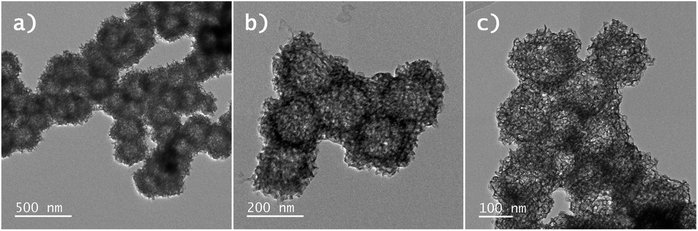 | ||
| Fig. 4 BF-STEM images of (a) and (b) Y2O2SO4:Er,Yb precursor and (c) Y2O2SO4:Er,Yb hollow spheres obtained after heat-treatment in air. | ||
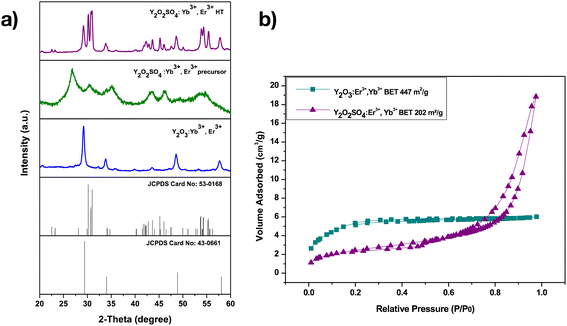
The powder XRD patterns (Fig. 6a) of the Y2O3:Er,Yb, Y2O2SO4:Er,Yb precursor and Y2O2SO4:Er,Yb HT materials are presented in Fig. 6a. For Y2O3:Er,Yb, characteristic peaks, which can be assigned to the cubic-phase Y2O3 (JCPDS card No. 43-0661), are clearly observed. The width of the diffraction peaks for the Y2O2SO4:Er,Yb precursor, in the range from about 20° to 60°, suggests that the precursor has some crystallinity, however, the obtained diffraction pattern is inconsistent with any reported data in the JCPDS card database. After being heat treated at 850 °C for 3 hours in air, it is apparent that the crystallinity of the final product has significantly enhanced. XRD analysis shows that this calcined product is mainly composed of Y2O2SO4 monoclinic phase (JCPDS 53-0168) with traces of the Y2O3 phase as impurity.30 N2 sorption measurements were performed on the Y2O3:Er,Yb, and Y2O2SO4:Er,Yb precursor (Fig. 6b). The BET surface area values of the materials were calculated to be 447 m2 g−1 and 202 m2 g−1, for the Y2O3:Er,Yb and Y2O2SO4:Er,Yb precursor, respectively. We have focused here only on the Y2O2SO4:Er,Yb precursor because we have shown that the upconversion properties of this material are more appealing for thermometry applications compared to those of the heat-treated form.
The porous nature of the spheres with hollow cavities makes them ideal for further drug delivery investigations.34,35
Thermogravimetric (TG) and Differential Thermal (DTA) analyses were done to determine the thermal stability of the materials. TG-DTA curves for both the Y2O3:Er,Yb material and Y2O2SO4:Er,Yb precursor were conducted from room temperature to 1000 °C in air, and the obtained TG-DTA curves by a thermal analyzer are shown in Fig. S1 and S2 in the ESI.† For both materials, the initial weight loss before 100 °C is assigned to the removal of the absorbed H2O. For Y2O3 (Fig. S1†), the two rapid weight loss steps can be assigned to the dehydration and subsequent burning of any remainings of the CNP templates, respectively. The maximum mass loss occurs at about 400 °C, which may be attributed to the conversion from the amorphous Y(OH)CO3 precursor to the crystalline Y2O3. Furthermore, the DTA curve analysis reveals that all reactions are endothermic. As seen in Fig. S2,† the precursor decomposes completely up to around 550 °C. The exothermic peak on the DTA curve centered at 500 °C and 580 °C indicates that the above process is an exothermic reaction caused by the crystallization of Y2O2SO4. However, the weight loss starting at 950 °C is attributed to the decomposition of the SO42− groups in Y2O2SO4, corresponding to the following reaction equation:
The luminescence properties of the developed Y2O3:Er,Yb, Y2O2SO4:Er,Yb precursor and Y2O2SO4:Er,Yb HT hollow spheres were studied at room temperature as well as at varying temperatures upon excitation with a 975 nm continuous wave (CW) laser in powder and in aqueous suspensions. The temperature-dependent emission spectra (288.15–328.15 K; 15–55 °C) of Y2O3:Er,Yb are presented in Fig. 7. The RT emission of solid Y2O3:Er,Yb particles is presented in Fig. S3.† Upon 975 nm laser excitation, strong green upconversion emission due to the 2H11/2 → 4I15/2 (522 nm) and 4S3/2 → 4I15/2 (563 nm) transitions of Er3+ is observed.36 And even stronger red emission, due to the 4F9/2 → 4I15/2 (661 nm) transition can also be observed. The green-to-red emission in the Y2O3 host matrix can be tuned by changing the Er3+ and Yb3+ doping amounts as has previously been described in literature.30 As NIR luminescence thermometers based on the 2H11/2, 4S3/2 → 4I13/2 transitions between 790 and 850 nm have previously been reported, we have also investigated the NIR region, however, this material did not show the 2H11/2, 4S3/2 → 4I13/2 transitions.36 After dispersing the hollow spheres in distilled water, the thermometric properties of the Y2O3:Er,Yb were determined (Fig. 7). Only the green emission region was monitored since we find the peaks relevant for thermometry (as they are thermally coupled levels that can be used for temperature sensing).
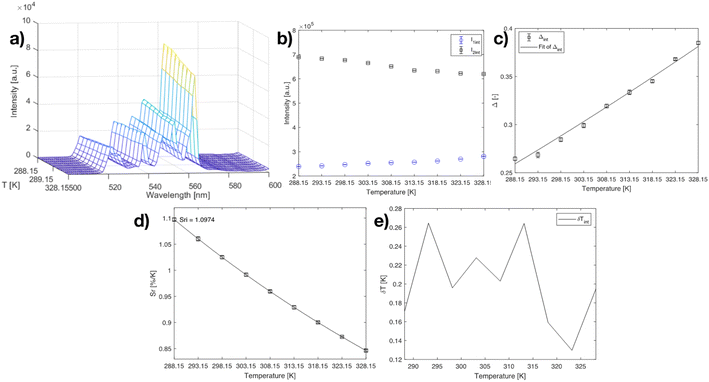 | ||
| Fig. 7 (a) Emission map of Y2O3:Er,Yb at varying temperatures (288.15–328.15 K; step 5 K), (b) intensities of the 522 nm and 563 nm peaks at varying temperatures, (c) calibration curve plot for the Y2O3:Er,Yb material using eqn (2) when the 522 nm and 563 nm peaks are considered. The points are experimental Δ parameters, the solid line shows the least-squares fit to the experimental points. (d) relative sensitivity Sr plot at varying temperatures (288.15–328.15 K) for I522/I563 ratio, the solid lines are a guide for the eyes. (e) temperature uncertainty graph over the monitored temperature range. | ||
The experimental Δ parameter (eqn (1)) was calculated based on the integrated areas under the peaks and was well-fitted using eqn (2).37,38
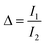 | (1) |
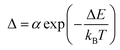 | (2) |
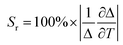 | (3) |
The maximum Sr for the Y2O3:Er,Yb material was calculated to be 1.097 % K−1 at 288.15 K (Fig. 7). The temperature uncertainty (δT) is however the most important parameter to assess the performance of a thermometer (eqn (4)) since it not only includes the relative sensitivity but also the measurement error of the intensity ratio (δΔ).
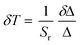 | (4) |
Next, the temperature-dependent emission spectra (288.15–328.15 K; 15–55 °C) of the Y2O2SO4:Er,Yb precursor are presented in Fig. 8. The precursor was selected for thermometric measurements and not the Y2O2SO4:Er,Yb heat-treated material as would be expected, on the account that after heat treatment the green emission necessary for thermometry applications becomes weak compared to the red emission of the material. The RT emission of solid Y2O2SO4:Er,Yb particles after heat treatment is presented in Fig. S4.† Upon dispersing in distilled water, the green emission even further decreases (Fig. S5†) and makes it not suitable for a thermometer material.
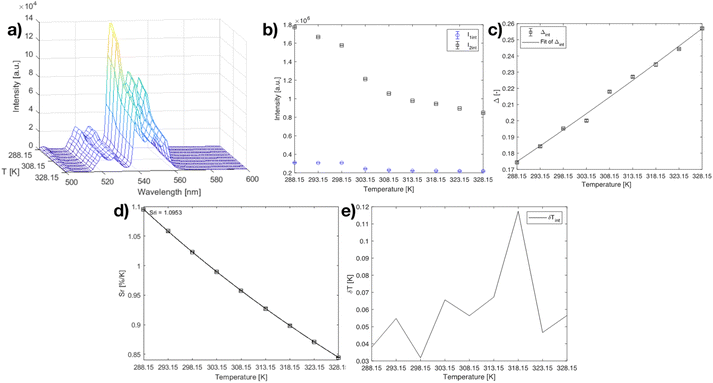 | ||
| Fig. 8 a) Emission map of Y2O2SO4:Er,Yb precursor at varying temperatures (288.15–328.15 K; step 5 K), (b) intensities of the 520 nm and 540 nm peaks at varying temperatures, (c) calibration curve plot for the Y2O2SO4:Er,Yb precursor material using eqn (2) when the 520 nm and 540 nm peaks are considered. The points are experimental Δ parameters, and the solid line shows the least-squares fit to the experimental points. (d) relative sensitivity Sr plot at varying temperatures (288.15–328.15 K) for I520/I540 ratio, the solid lines are a guide for the eyes. (e) temperature uncertainty graph over the monitored temperature range. | ||
Here, the NIR 2H11/2, 4S3/2 → 4I13/2 transitions were visible, however, did not show significant temperature change in the physiological range. For the Y2O2SO4:Er,Yb precursor the experimentally determined intensity ratios between the luminescence emissions from the 2H11/2 and 4S3/2 levels, respectively, were fitted to the model of eqn (1) to obtain ΔE = (631 ± 27) cm−1 (R2 = 0.994), which is close to the values found in literature for fluoride host materials. The maximum Sr for the Y2O2SO4:Er,Yb precursor material was calculated to be 1.095 % K−1 at 288.15 K (Fig. 8), which is very similar to that of the Y2O3:Er,Yb material. The δT < 0.12 K throughout the whole temperature range, showing even better results than for the Y2O3:Er,Yb material.
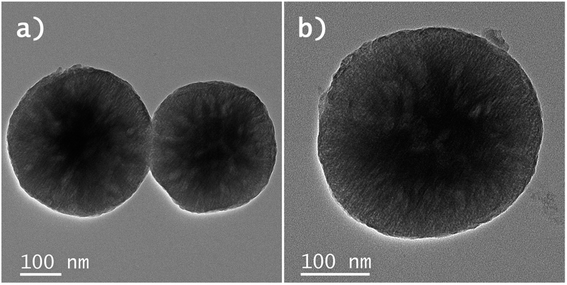
Based on these results it can be concluded that both the Y2O3:Er,Yb and Y2O2SO4:Er,Yb precursor could work well as a physiological thermometer in an aqueous environment. As a proof of principle, on the surface of the Y2O2SO4 precursor additionally gold nanoparticles (AuNPs) were grown in situ (Fig. 9), to potentially use the AuNPs as nano-heaters for the purposes of developing localized hyperthermia or enhancing the rate of drug release.40 We have used such a method previously for other materials like hybrid NaYF4:Er,Yb nano-MOF composites and it can be well translated to purely inorganic materials such as the Y2O2SO4 shown here.41 The applications of these materials were not further explored in the work, the objective was to just show the possibilities from a materials point of view.
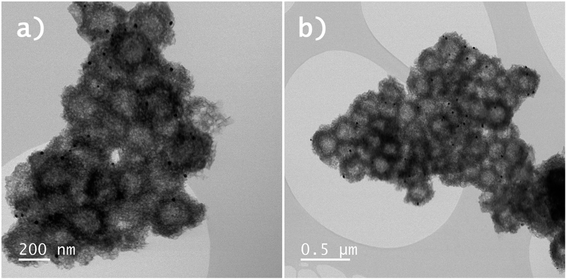 | ||
| Fig. 9 (a) and (b) BF-STEM images Y2O2SO4:Er,Yb@AuNPs precursors hollow spheres. The AuNPs can be observed as small black dots on the surface of the Y2O2SO4:Er,Yb@AuNPs hollow spheres. | ||
To further assess their potential use in real biological systems, the cytotoxicity of these materials was tested employing a PrestoBlue™ cell viability assay on in vitro cultured human cells via fluorescence spectroscopy.41 Simultaneously, widefield microscopy was exploited to visualize the sample-treated, dye-marked cells applying a Green Fluorescent Protein (GFP) long-pass filter. Here, Normal Human Dermal Fibroblasts (NHDF) were seeded at early passage stages when their growth reached 80–90% confluency. The toxicity of the three materials (Y2O3:Er,Yb, Y2O2SO4:Er,Yb precursor, as well as the Y2O2SO4:Er,Yb@AuNPs) was tested in a concentration-dependent manner on five technical replicates per tested concentration per sample, and the results are presented in Fig. 10. The cell viability tests indicate that the aforementioned materials are relatively non-toxic in a wide concentration range. The Y2O3:Er,Yb compound exhibits a relatively non-toxic trend over the entire investigated concentration range and is therefore deemed generally compatible with the NHDFs. For the Y2O2SO4:Er,Yb precursor material, significant toxicity towards fibroblastic cells occurred at a concentration of 1 mg per well or 5.556 mg mL−1, which is a quite high concentration of this compound compared to other similar studies.42,43 However, when AuNPs are additionally incorporated within the precursor matrix Y2O2SO4:Er,Yb, the compound manifests a notable cytotoxic effect, i.e., the cell viability is drastically decreased and varies in a range from 0–25% already at a tested concentration of 0.05 mg per well or 0.278 mg mL−1 of the compound. This result is also strengthened by the widefield microscopy imaging as the spindle-shaped cells are no longer discernible in the range of 2.778–5.556 mg mL−1 for Y2O2SO4:Er,Yb and 0.278–5.556 mg mL−1 for Y2O2SO4:Er,Yb@AuNPs, respectively. However, as with the pure precursor material, such high concentrations would be futile for biomedical applications, so the current finding does not pose a major concern for further investigations of these materials in the appropriate, optimal dose.44 What is noteworthy to highlight is how well the cytotoxicity data and the widefield microscopy images complement each other within different technical replicates of the same sample concentrations. This is a preliminary observation of the potentially low replicate variability and good technical reproducibility of the sample measurements.
Due to the hollow nature of the materials, large surface area, non-toxicity to the chosen in vitro cultured healthy human cells, and good thermometric properties in water, these materials were further considered to be employed as vehicles for dual-mode activity: combined thermometry and drug release. The materials (Y2O2SO4 precursor and Y2O3) were in a subsequent step loaded with doxorubicin – a well-explored model drug used in clinical chemotherapy. First, the drug uptake and then the pH-dependent drug release was studied.
For determination of the drug loading efficiency and the drug release behavior of the rare-earth hollow spheres – Y2O3:Er,Yb and Y2O2SO4:Er,Yb, doxorubicin hydrochloride was chosen as a fluorescent anti-cancer model drug. Because of their large specific surface area and good permeability, both Y2O3 and Y2O2SO4 can store a relatively large amount of DOX molecules. To quantitatively analyze the uptake of DOX during the experiment, a calibration curve of DOX was drawn using a series of solutions with different concentrations of DOX, versus their absorbance at 480 nm. After the sample was loaded with DOX, we evaluated both the drug Loading Capacity (LC%) (eqn S(1) in the ESI†) and the Encapsulation Efficiency (EE%) (eqn S(2) in the ESI†) using both the calibration curve and the measured absorbance value. The LC% of DOX into Y2O3:Er,Yb and Y2O2SO4:Er,Yb hollow spheres was determined to be 37.0% and 38.3%, respectively. The EE% was found to be 58.6% for Y2O3:Er,Yb and 62.0% for Y2O2SO4:Er,Yb.
Drug release depending on the pH change of the environment has been extensively studied, as the pH values of cytoplasm, lysosomes, and endosomes vary from 7.2–7.4, 4.5–6.5, and 5.5–6.6, respectively.44,45 Furthermore, it is known that cancer cells in cancerous tissue have a more acidic pH than in healthy tissue. pH-controlled drug delivery, based on this feature, can be an alternative approach for cancer therapy.
It has previously been reported that DOX drug release from hollow nanoparticles can be enhanced in a more acidic environment.45 Hence it was suggested that DOX molecules might be released faster in mildly acidic tumor areas than in physiological blood environment in vascular compartments.
Therefore, to explore the pH-responsive behavior of both Y2O3:Er,Yb and Y2O2SO4:Er,Yb, we have determined the absorbance at 480 nm as a function of time at pH 7.4, pH 5.5 and pH 4 in PBS buffer at 37 °C (corresponding calibration curves for PBS buffer at pH 7.4, pH 5.5 and pH 4 can be found in Fig. S8–S10 in the ESI†). A schematic overview of the DOX loading and release process is presented in Scheme 2. In our experiment, the drug release for both materials was studied for a period of 72 h. The concentration of DOX molecules in the solutions upon release was determined using UV-Vis measurements performed at different time intervals.
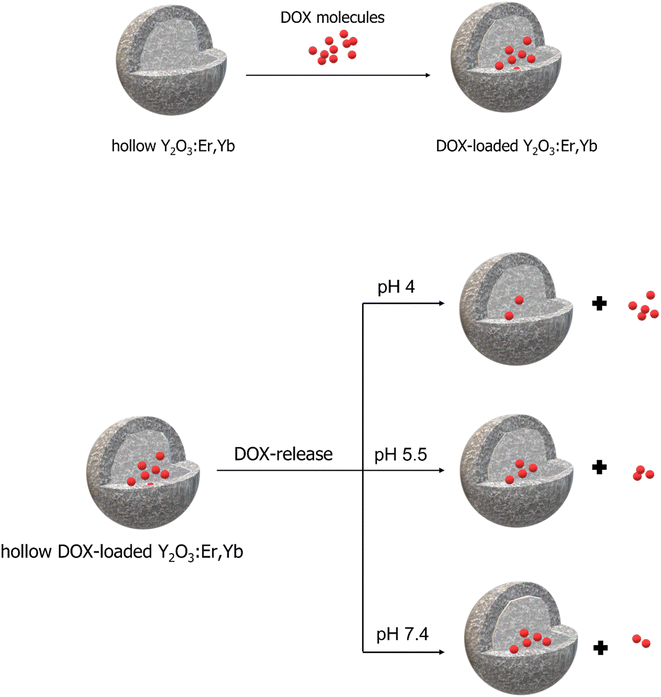 | ||
| Scheme 2 Schematic overview of the DOX loading (top) and release process at different pH values (bottom) using Y2O3:Er,Yb as example. | ||
For Y2O3:Er,Yb, about 9% of DOX was released in the neutral condition at pH 7.4, while ∼16% of DOX was released in the acidic PBS solution at pH 5.5 and around 22% DOX was released at pH 4, within 72 h. Whereas, in a neutral pH 7.4 PBS solution, Y2O2SO4:Er,Yb released approximately 6% of DOX, around 12% in an acidic pH 5 PBS solution, and nearly 26% in a pH 4 PBS solution within 72 h (Fig. 11). Table S1 in the ESI† summarizes the DOX loading and release results for both materials. It is clear from these results that our materials take up DOX very well and release it gradually with clear pH dependency.
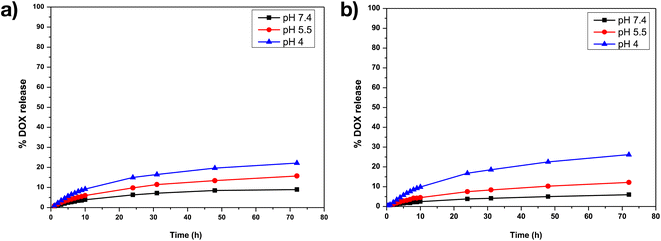 | ||
| Fig. 11 DOX drug release profiles for (a) Y2O3:Er,Yb-DOX, (b) Y2O2SO4:Er,Yb-DOX at pH 7.4, at pH 5.5 and at pH 4 in PBS buffer at 37 °C. | ||
To date, not much work has been done on combining thermometry with drug delivery. However, there are quite some reports on (hollow) inorganic particles for DOX release that can be used as a reference for comparison with our drug loading and release results. For example, Yi et al. reported GdPO4:Ce,Tb hollow spheres for the loading and release of doxorubicin. They showed that when the pH was 5, 47% of the loaded DOX was released in just 10 hours, whereas at pH 7.4, only 30% of the drug was released showing pH-dependent release of this inorganic host. However, drug loading efficiency was only 17%, which is much lower than that of our materials.46 Moreover, Huang et al. reported that the DOX loading efficiency of the (PAH/PSS)2@Gd2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb3+, Er3+@MSNs was only 10.3%. with 9.4% of the DOX being released at pH 7.4 after 72 hours and a faster release profile of roughly 64% over the same period of time at pH 5.2. The fast release of this material can be attributed to the diffusion of DOX loaded in the polymer shell and the outer surface of the silica nanoparticles.47 Ge et al. studied the release pattern of DOX from the hollow Y2O3:Yb3+,Er3+ sphere in PBS and acetate buffer solution (ABS) and found that the release of DOX is essentially nonexistent in a neutral environment (PBS, pH 7.4). In addition, this system exhibits a notable first burst release within 6 hours and almost completely releases after 48 hours in an acidic environment (ABS, pH 5.0).48 This behavior can be explained by the possibility of DOX molecules being present on the outer surface of the particles rather than in their inner hollow cavity or core.
Yb3+, Er3+@MSNs was only 10.3%. with 9.4% of the DOX being released at pH 7.4 after 72 hours and a faster release profile of roughly 64% over the same period of time at pH 5.2. The fast release of this material can be attributed to the diffusion of DOX loaded in the polymer shell and the outer surface of the silica nanoparticles.47 Ge et al. studied the release pattern of DOX from the hollow Y2O3:Yb3+,Er3+ sphere in PBS and acetate buffer solution (ABS) and found that the release of DOX is essentially nonexistent in a neutral environment (PBS, pH 7.4). In addition, this system exhibits a notable first burst release within 6 hours and almost completely releases after 48 hours in an acidic environment (ABS, pH 5.0).48 This behavior can be explained by the possibility of DOX molecules being present on the outer surface of the particles rather than in their inner hollow cavity or core.
It is undoubtedly clear that the type of host material has a huge effect on the loading and release profiles. However, it is not just the material, but also how it is prepared that has an effect on the size, shape, BET surface area, and other factors that clearly subsequently affect the drug uptake and release too. The materials which we have prepared in our work have higher BET surface area values (446 m2 g−1 for Y2O3:Er,Yb and 202 m2 g−1 for Y2O2SO4:Er,Yb precursor, respectively) compared to the above-discussed materials. They show very good DOX loading efficiencies (36.95% and 38.3%, respectively). Nevertheless, the slower DOX release relative to other materials can perhaps be explained by the fact that the loaded DOX diffuses from the pores of materials rather than its surface.49
This work therefore once again highlights possibilities of employing various hybrid and inorganic (nano)materials for biomedical thermometry purposes as well as the potential of simultaneously coupling thermometry with another modality for theranostic purposes as has already been shown for other similar materials in literature.50–53
Conclusions
In this work, we demonstrate how hollow Y2O3:Er,Yb and Y2O2SO4:Er,Yb particles can be used as very sensitive thermometers in the physiological temperature range in an aqueous environment. We show that these materials can additionally be successfully loaded with the model anti-cancer drug DOX and the release can be controlled through pH change, which is appealing as cancer cells have lower pH values than healthy cells and tissue. We have also carried out preliminary cytotoxicity tests, indicating that these materials are mostly non-toxic to in vitro cultivated healthy human cells when used at moderate concentrations without further functionalization. To the best of our knowledge, these are the first inorganic hollow materials proposed for such dual-mode thermometry-drug release application.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work is part of a project that has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant agreement No. 945945). A. M. K. additionally thanks Ghent University for BOF funding (BOF/STG/202002/004). A.G.S. acknowledges FWO (G043322N, I002620N) for support.References
- M. Dramicanin, Luminescence thermometry: methods, materials, and applications, Woodhead Publishing, 2018 Search PubMed.
- E. C. Ximendes, U. Rocha, T. O. Sales, N. Fernández, F. Sanz-Rodríguez, I. R. Martín, C. Jacinto and D. Jaque, Adv. Funct. Mater., 2017, 27, 1702249 CrossRef.
- J. Zhou, B. Del Rosal, D. Jaque, S. Uchiyama and D. Jin, Nat. Methods, 2020, 17, 967–980 CrossRef PubMed.
- A. Bednarkiewicz, L. Marciniak, L. D. Carlos and D. Jaque, Nanoscale, 2020, 12, 14405–14421 RSC.
- X.-d. Wang, O. S. Wolfbeis and R. J. Meier, Chem. Soc. Rev., 2013, 42, 7834–7869 RSC.
- D. Jaque and F. Vetrone, Nanoscale, 2012, 4, 4301–4326 RSC.
- D. Rosenblum, N. Joshi, W. Tao, J. M. Karp and D. Peer, Nat. Commun., 2018, 9, 1–12 CrossRef CAS PubMed.
- N. Degrauwe, A. Hocquelet, A. Digklia, N. Schaefer, A. Denys and R. Duran, Front. Pharmacol., 2019, 10, 450 CrossRef PubMed.
- H. Suo, X. Zhao, Z. Zhang, Y. Wang, J. Sun, M. Jin and C. Guo, Laser Photonics Rev., 2021, 15, 2000319 Search PubMed.
- C. D. Brites, S. Balabhadra and L. D. Carlos, Adv. Opt. Mater., 2019, 7, 1801239 CrossRef.
- M. Peng, A. M. Kaczmarek and K. Van Hecke, ACS Appl. Mater. Interfaces, 2022, 14, 14367–14379 CrossRef CAS PubMed.
- J.-C. G. Bünzli and S. V. Eliseeva, Chem. Sci., 2013, 4, 1939–1949 RSC.
- J. Liu, A. M. Kaczmarek and R. Van Deun, Chem. Soc. Rev., 2018, 47, 7225–7238 RSC.
- C. Duan, L. Liang, L. Li, R. Zhang and Z. P. Xu, J. Mater. Chem. B, 2018, 6, 192–209 RSC.
- A. Bagheri, H. Arandiyan, C. Boyer and M. Lim, Adv. Sci., 2016, 3, 1500437 CrossRef PubMed.
- K. Zhao, J. Sun, F. Wang, A. Song, K. Liu and H. Zhang, ACS Appl. Bio Mater., 2020, 3, 3975–3986 CrossRef PubMed.
- E. Hemmer, P. Acosta-Mora, J. Mendez-Ramos and S. Fischer, J. Mater. Chem. B, 2017, 5, 4365–4392 RSC.
- X. Qin, J. Wang and Q. Yuan, Front. Chem., 2020, 8, 608578 CrossRef PubMed.
- H. Chen, W. Zhang, G. Zhu, J. Xie and X. Chen, Nat. Rev. Mater., 2017, 2, 1–18 Search PubMed.
- X. Y. Wong, A. Sena-Torralba, R. Alvarez-Diduk, K. Muthoosamy and A. Merkoçi, ACS Nano, 2020, 14, 2585–2627 CrossRef CAS PubMed.
- P. Zhao, Y. Zhu, X. Yang, K. Fan, J. Shen and C. Li, RSC Adv., 2012, 2, 10592–10597 RSC.
- H. Terraschke, M. Franzreb and C. Wickleder, Chem. - Eur. J., 2020, 26, 6833–6838 CrossRef CAS PubMed.
- Y. Gao, Y. Qiu, X. Wang, Y. Bi, G. Zhao, F. Ding, Y. Sun and Z. Xu, RSC Adv., 2018, 8, 21857–21862 RSC.
- F. Zhang, G. B. Braun, A. Pallaoro, Y. Zhang, Y. Shi, D. Cui, M. Moskovits, D. Zhao and G. D. Stucky, Nano Lett., 2012, 12, 61–67 CrossRef CAS PubMed.
- Y. Jia, T.-Y. Sun, J.-H. Wang, H. Huang, P. Li, X.-F. Yu and P. K. Chu, CrystEngComm, 2014, 16, 6141–6148 RSC.
- L. Zong, P. Xu, Y. Ding, K. Zhao, Z. Wang, X. Yan, R. Yu, J. Chen and X. Xing, Small, 2015, 11, 2768–2773 CrossRef CAS PubMed.
- Y. Gao, H. Yu, C. Shi, G. Zhao, Y. Bi, B. Xu, F. Ding, Y. Sun and Z. Xu, R. Soc. Open Sci., 2017, 4, 171451 CrossRef PubMed.
- D. Yang, G. Yang, X. Wang, R. Lv, S. Gai, F. He, A. Gulzar and P. Yang, Nanoscale, 2015, 7, 12180–12191 RSC.
- G. Chen, F. Chen, X. Liu, W. Ma, H. Luo, J. Li, R. Ma and G. Qiu, Nano Res., 2014, 7, 1093–1102 CrossRef CAS.
- V. M. Lojpur, P. S. Ahrenkiel and M. D. Dramićanin, Nanoscale Res. Lett., 2013, 8, 1–6 CrossRef PubMed.
- F. Zhao, M. Yuan, W. Zhang and S. Gao, J. Am. Chem. Soc., 2006, 128, 11758–11759 CrossRef PubMed.
- S.-L. Lin, Z.-R. Chen and C. A. Chang, Nanotheranostics, 2018, 2, 243 CrossRef PubMed.
- C. Larquet and S. Carenco, Front. Chem., 2020, 8, 179 CrossRef PubMed.
- J. Zhu, L. Liao, X. Bian, J. Kong, P. Yang and B. Liu, Small, 2012, 8, 2715–2720 CrossRef CAS PubMed.
- Y. Huang, E. Hemmer, F. Rosei and F. Vetrone, J. Phys. Chem. B, 2016, 120, 4992–5001 CrossRef CAS PubMed.
- A. M. Kaczmarek, M. Suta, H. Rijckaert, A. Abalymov, I. Van Driessche, A. G. Skirtach, A. Meijerink and P. Van Der Voort, Adv. Funct. Mater., 2020, 30, 2003101 CrossRef CAS.
- A. M. Kaczmarek, Y. Y. Liu, M. K. Kaczmarek, H. Liu, F. Artizzu, L. D. Carlos and P. Van Der Voort, Angew. Chem., Int. Ed., 2020, 59, 1932–1940 CrossRef CAS PubMed.
- M. Suta and A. Meijerink, Adv. Theory Simul., 2020, 3, 2000176 CrossRef CAS.
- C. D. Brites, P. P. Lima, N. J. Silva, A. Millan, V. S. Amaral, F. Palacio and L. D. Carlos, Nanoscale, 2012, 4, 4799–4829 RSC.
- L. Jauffred, A. Samadi, H. Klingberg, P. M. Bendix and L. B. Oddershede, Chem. Rev., 2019, 119, 8087–8130 CrossRef CAS PubMed.
- H. Rijckaert, S. Premcheska, S. Mohanty, J. Verduijn, A. Skirtach and A. M. Kaczmarek, Phys. B, 2022, 626, 413453 CrossRef CAS.
- M. Asadi, M. Ghahari, S. A. Hassanzadeh-Tabrizi, A. M. Arabi and R. Nasiri, J. Chin. Chem. Soc., 2019, 67, 720–731 CrossRef.
- Y. Bai, Y. Li, R. Wang and Y. Li, ACS Omega, 2020, 5, 5346–5355 CrossRef CAS PubMed.
- ISO 10993-5:2009, Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity, Canadian Standards Association, 2007, pp. 1–11 Search PubMed.
- K. M. Rao, S. Parambadath, A. Kumar, C.-S. Ha and S. S. Han, ACS Biomater. Sci. Eng., 2018, 4, 175–183 CrossRef CAS PubMed.
- Z. Yi, W. Lu, C. Qian, T. Zeng, L. Yin, H. Wang, L. Rao, H. Liu and S. Zeng, Biomater. Sci., 2014, 2, 1404–1411 RSC.
- S. Huang, Z. Cheng, Y. Chen, B. Liu, X. Deng, P. a. Ma and J. Lin, RSC Adv., 2015, 5, 41985–41993 RSC.
- K. Ge, C. Zhang, W. Sun, H. Liu, Y. Jin, Z. Li, X. J. Liang, G. Jia and J. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 25078–25086 CrossRef PubMed.
- W. Feng, X. Zhou, C. He, K. Qiu, W. Nie, L. Chen, H. Wang, X. Mo and Y. Zhang, J. Mater. Chem. B, 2013, 1, 5886–5898 RSC.
- J. You, G. Zhang and C. Li, ACS Nano, 2010, 4(2), 1033–1041 CrossRef PubMed.
- Z. Xu, P. Ma, C. Li, Z. Hou, X. Zhai, S. Huang and J. Lin, Biomaterials, 2011, 32, 4161–4173 CrossRef PubMed.
- S. Premcheska, M. Lederer and A. M. Kaczmarek, Chem. Commun., 2022, 58, 4288–4307 RSC.
- H. Rijckaert, S. Mohanty, J. Verduijn, M. Lederer, B. Laforce, L. Vincze, A. Skirtach, K. Van Hecke and A. M. Kaczmarek, J. Mater. Chem. C, 2022, 10, 10574–10585 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthesis procedure, TG-DTA graphs, additional HAADF-STEM with its EDX maps and additional luminescence spectra. See DOI: https://doi.org/10.1039/d2ra06162g |
| This journal is © The Royal Society of Chemistry 2022 |