Open Access Article
Open Access ArticleDABCO-promoted highly diastereo- and regioselective construction of C-3 functionalized spirooxindoles via [3 + 2] cycloaddition of 2-aryl/heteroarylidene-1H-indene-1,3(2H)-diones with N-2,2,2-trifluoroethylisatin ketimines at ambient conditions†
Madavi S. Prasad *a,
Sankar Bharania,
Syed Mastan Shariefa,
Mudavath Ravi
*a,
Sankar Bharania,
Syed Mastan Shariefa,
Mudavath Ravi a,
Murugesan Sivaprakasha,
Biplob Borah
a,
Murugesan Sivaprakasha,
Biplob Borah b and
L. Raju Chowhan
b and
L. Raju Chowhan *b
*b
aAsymmetric Synthesis and Catalysis Laboratory, Department of Chemistry, Central University of Tamil Nadu (CUTN), Tiruvarur-610 005, India
bSchool of Applied Material Sciences, Centre for Applied Chemistry, Central University of Gujarat, Sector-30, Gandhinagar-382030, Gujarat, India. E-mail: rchowhan@cug.ac.in
First published on 6th December 2022
Abstract
The application of 2-aryl/heteroarylidene-1H-indene-1,3(2H)-dione as an activated olefin source in the DABCO-catalyzed [3 + 2] cycloaddition with N-2,2,2-trifluoroethylisatin ketimines has been disclosed. This highly efficient 1,3-dipolar cycloaddition reaction offered a variety of trifluoro methyl group bearing spiro-pyrrolidine linked oxindoles with four consecutive stereocentres in good to excellent yield and excellent diastereoselectivity. The synthetic practicality of the protocol was established by demonstrating the enantioselective construction of spiro-pyrrolidine-oxindoles with two vicinal spiro-quaternary chiral centres in good yield excellent enantioselectivity (>90% ee) by using ultralow loading of quinine as the catalyst at room temperature.
Introduction
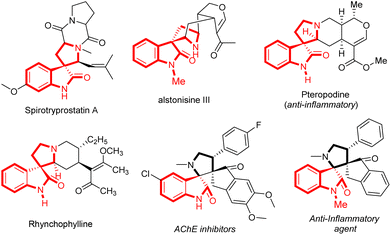
Spirocyclic compounds, especially spirooxindoles, which are categorized by a tetrahedral sp3-hybridized carbon atom at the C-3 position of an orthogonally structured bicyclic structure, have recently drawn tremendous interest in the research and development area of pharmaceuticals, organic, and medicinal chemistry.1 These structural scaffolds are well-featured in a wide variety of natural alkaloids, and synthetic drug candidates.2 Because of the occurrences of their sp3-hybridized quaternary stereocenters at the 3-position, spirooxindoles have often offered excellent lipophilicity, stereochemical geometry, and increased binding potential with many receptors as compared to planar aromatic rings.3 Representative examples of some potential pharmacologically active natural alkaloids and synthetic drug candidates bearing spiro-pyrrolidine oxindoles as the core structure are represented in Fig. 1.4On the other hand, the incorporation of fluorine either as a single molecule or as a substituted molecule into organic compounds either alters or enhances the properties of the parent material, and the resulting compounds may generally display promising metabolic stability, binding potential, stereochemical integrity, or even unprecedented properties.5 Particularly, the installation of the CF3 group adjacent to the nitrogen atom located at the α-position affects the binding potential of the drug receptor by reducing the alkalinity of the amide group.6–10
Recognizing the broad chemical landscape, the significant potential of spiro-pyrrolidine oxindoles, the immense importance of fluorine-containing molecules, and the development of an elegant synthetic strategy for the expeditious stereodivergent construction of spirooxindoles embedded with the fluorine-containing group as the key moiety is highly desired.
For the construction of carbon–carbon, carbon–heteroatom bonds, as well as spirocyclic compounds, the 1,3-dipolar cycloaddition reaction has been demonstrated as one of the most fundamental approaches in organic chemistry.11 Intriguingly, many 1,3-dipoles, including azomethine ylides,12 nitrones,13 carbonyl ylides,14 and others, have been extensively discovered and explored in cycloaddition reactions throughout the past few decades. Among them, recently, N-2,2,2-trifluoroethylisatin ketimines which are easily accessible and highly reactive azomethine ylide precursors15 have been successfully employed in many 1,3-dipolar cycloadditions with various activated olefins and has been demonstrated as one of the efficient approaches for the stereoselective construction of spiro-pyrrolidine oxindoles. In 2015, Wang et al., introduced N-2,2,2-trifluoroethylisatin ketimines as a new type of 1,3-dipoles which was demonstrated for the assembly of spiro[pyrrolidin-3, 2′-oxindole] by a secondary amine catalyzed enantioselective [3 + 2] cycloaddition with enals (Scheme 1a).15 In 2020, Zhou et al., disclosed the utilization of 4-oxo-4H-chromene-3-carboxylic acid as the dipolarophiles in the 1,3-dipolar cycloaddition with N-2,2,2-trifluoroethylisatin ketimines for the assembly of various chromone fused spiro-pyrrolidine oxindoles (Scheme 1b).16 The exploitation of chalcone types of compounds for the [3 + 2] cycloaddition with N-2,2,2-trifluoroethylisatin ketimines was developed by Wang et al., which also leads to the formation of spirooxindoles in good yields with excellent diastereoselectivity (Scheme 1c).17 However, the expeditious construction of spiro-pyrrolidine oxindoles from 2-aryl/heteroarylidene-1H-indene-1,3(2H)-dione as the dipolarophiles was not reported so far. Here, we have demonstrated the DABCO-catalyzed highly efficient and diastereoselective [3 + 2] cycloaddition of N-2,2,2-trifluoroethylisatin ketimines with 2-arylidene-1H-indene-1,3(2H)-dione as the dipolarophiles for the facile construction of diverse spiro-pyrrolidine oxindoles fused with indeno moiety for the first time (Scheme 1d).
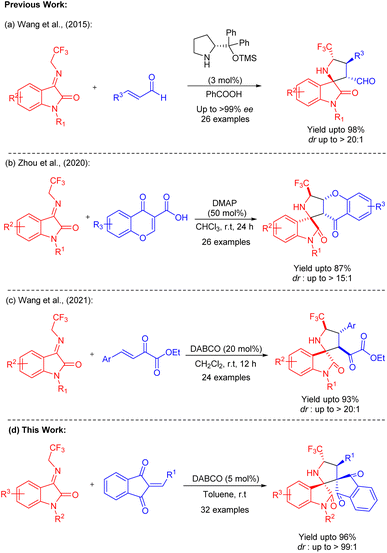 | ||
| Scheme 1 Previous strategy for the construction of spirooxindoles via 1,3-dipolar cycloaddition reactions and the current approach. | ||
Results and discussion
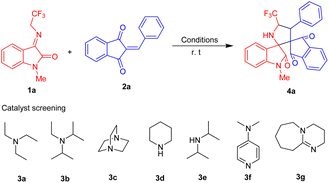
Our initial investigation for optimizing the reaction conditions starts with the execution of the reaction of N-2,2,2-trifluoroethylisatin ketimines 1a and 2-benzylidene-1H-indene-1,3(2H)-dione 2a as the model substrate and the reaction was carried out in presence of different catalytic systems as well as solvent systems at room temperature (Table 1). Using 5 mol% of triethylamine 3a as the basic catalyst, the reaction afforded the corresponding spiro-pyrrolidine oxindole 4a in 37% yield with 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr after 1 hour (Table 1, entry 1). Then we examined the efficiency of various amine catalysts such as N-ethyl-N-isopropylpropan-2-amine 3b (Table 1, entry 2), DABCO 3c (Table 1, entry 3), piperidine 3d (Table 1, entry 4), diisopropylamine 3e (Table 1, entry 5), N,N-dimethylpyridin-4-amine 3f (Table 1, entry 6), and catalyst 3g (Table 1, entry 7) in chloroform as the solvent system for the model reaction at room temperature. A quick survey of the results disclosed in the Table 1 revealed that the reaction conducted in presence of catalysts 3b, 3d, 3e, 3f, and 3g leads to a lower yield of product 4a and necessitates longer reaction time. However, surprisingly it was noticed that using DABCO as the catalyst which also required 38 hours to complete conversion of the product 4a, excellent diastereoselectivity (>20
1 dr after 1 hour (Table 1, entry 1). Then we examined the efficiency of various amine catalysts such as N-ethyl-N-isopropylpropan-2-amine 3b (Table 1, entry 2), DABCO 3c (Table 1, entry 3), piperidine 3d (Table 1, entry 4), diisopropylamine 3e (Table 1, entry 5), N,N-dimethylpyridin-4-amine 3f (Table 1, entry 6), and catalyst 3g (Table 1, entry 7) in chloroform as the solvent system for the model reaction at room temperature. A quick survey of the results disclosed in the Table 1 revealed that the reaction conducted in presence of catalysts 3b, 3d, 3e, 3f, and 3g leads to a lower yield of product 4a and necessitates longer reaction time. However, surprisingly it was noticed that using DABCO as the catalyst which also required 38 hours to complete conversion of the product 4a, excellent diastereoselectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) was achieved (Table 1, entry 3). Inspired by this result, then we carried out the reaction by changing the solvent system from chloroform to dioxane, hexane, acetonitrile, and toluene for optimizing the appropriate solvent for the model reaction. With 5 mol% of DABCO as the catalyst and dioxane as the solvent, an increase in the diastereoselectivity was observed albeit with a low yield of the product even after 43 hours (Table 1, entry 9). The screening of hexane and acetonitrile also furnished the product with a very low yield (Table 1, entry 10–11). To our delight, the reaction in presence of toluene pleasingly afforded the desired product 4a in 91% yield with >99
1 dr) was achieved (Table 1, entry 3). Inspired by this result, then we carried out the reaction by changing the solvent system from chloroform to dioxane, hexane, acetonitrile, and toluene for optimizing the appropriate solvent for the model reaction. With 5 mol% of DABCO as the catalyst and dioxane as the solvent, an increase in the diastereoselectivity was observed albeit with a low yield of the product even after 43 hours (Table 1, entry 9). The screening of hexane and acetonitrile also furnished the product with a very low yield (Table 1, entry 10–11). To our delight, the reaction in presence of toluene pleasingly afforded the desired product 4a in 91% yield with >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr after 5 hours (Table 1, entry 12). On the other hand, increasing or decreasing the catalyst loading had no auspicious effects on the rate of the reaction. Consequently, the utilization of 5 mol% of DABCO as the catalyst and toluene as the solvent was recognized as the best optimum condition for this cycloaddition addition reaction.
1 dr after 5 hours (Table 1, entry 12). On the other hand, increasing or decreasing the catalyst loading had no auspicious effects on the rate of the reaction. Consequently, the utilization of 5 mol% of DABCO as the catalyst and toluene as the solvent was recognized as the best optimum condition for this cycloaddition addition reaction.
| Entry | Catalyst | Solvent | Time (h) | Yieldb (%) | drc |
|---|---|---|---|---|---|
| a Unless otherwise mentioned, all the reactions were performed using 1a (0.11 mmol), 2a (0.1 mmol), and catalyst 3a–g (0.005 mmol), in 0.2 M solvent at room temperature.b Yield refers to the column purified product.c dr was determined for crude product 4a by 1H NMR analysis.d Yield refers to the centrifuge-purified product.e dr was determined for purified product 4a by 1H NMR analysis. | |||||
| 1 | 3a | Chloroform | 1 | 37 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 3b | Chloroform | 2 | 23 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 3c | Chloroform | 38 | 60 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 3d | Chloroform | 6 | 52 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 3e | Chloroform | 4 | 45 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 3f | Chloroform | 10 | 10 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 3g | Chloroform | 3 | 36 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 3c | Toluene | 5 | 64 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 3c | Dioxane | 43 | 51 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 3c | Hexane | 13 | 56 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 3c | Acetonitrile | 26 | 15 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12d,e | 3c | Toluene | 5 | 91 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1 |
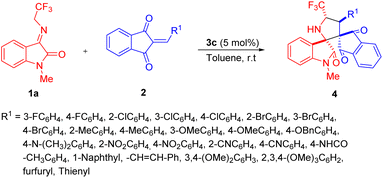
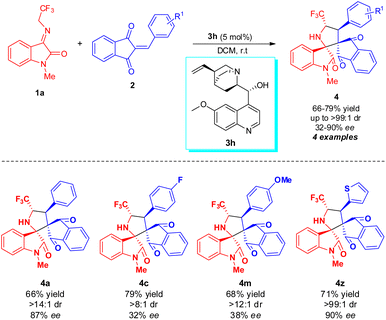
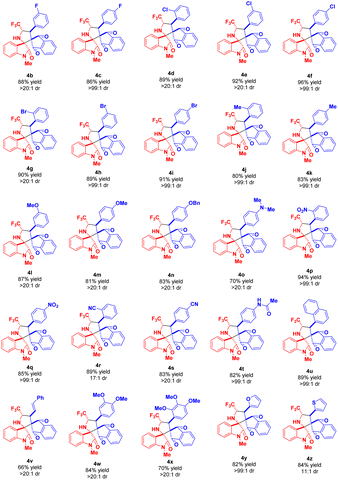
After ascertaining the standard reaction condition, the feasibility of the protocol was investigated by executing the reaction with different N-2,2,2-trifluoroethylisatin ketimines and 2-aryl/heteroarylidene-1H-indene-1,3(2H)-dione. At first, the efficiency of various 1H-indene-1,3(2H)-dione 2b–2z for this DABCO-catalyzed 1,3-dipolar cycloaddition with N-methyl substituted N-2,2,2-trifluoroethylisatin ketimine 1a (R2 = Me, R3 = H) was examined (Scheme 2). From the results summarized in Table 2, it was found that the 1H-indene-1,3(2H)-dione ring bearing diverse electron-withdrawing group at the different positions of R1 were well tolerated under the standard reaction condition and deliver the products 4b–4i in good to excellent yield with excellent diastereoselectivity up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 2, entry 4b–4i). The best yield of the product was obtained for 4-chloro substituted 1H-indene-1,3(2H)-diones (Table 2, entry 4f). Similarly, the reaction was also amenable with broad electron-donating substituents in the different positions of the reaction of 1H-indene-1,3(2H)-dione (Table 2, entry 4j–4o). On the other hand, strong electron-withdrawing groups such as nitro- and cyano substituted 1H-indene-1,3(2H)-dione successfully proceeded for this reaction and delivered the products in almost quantitative yield with good to excellent diastereoselectivity (Table 2, entry 4q–4t). Not only with aryl substitution but heteroaryl substituted 1H-indene-1,3(2H)-dione have also been well documented for the present reaction (Table 2, entry 4y and 4z). With furyl substituted 1H-indene-1,3(2H)-dione, up to >99
1 (Table 2, entry 4b–4i). The best yield of the product was obtained for 4-chloro substituted 1H-indene-1,3(2H)-diones (Table 2, entry 4f). Similarly, the reaction was also amenable with broad electron-donating substituents in the different positions of the reaction of 1H-indene-1,3(2H)-dione (Table 2, entry 4j–4o). On the other hand, strong electron-withdrawing groups such as nitro- and cyano substituted 1H-indene-1,3(2H)-dione successfully proceeded for this reaction and delivered the products in almost quantitative yield with good to excellent diastereoselectivity (Table 2, entry 4q–4t). Not only with aryl substitution but heteroaryl substituted 1H-indene-1,3(2H)-dione have also been well documented for the present reaction (Table 2, entry 4y and 4z). With furyl substituted 1H-indene-1,3(2H)-dione, up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr was observed, while thienyl substitution afforded a moderate dr up to 11
1 dr was observed, while thienyl substitution afforded a moderate dr up to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
| a Unless otherwise mentioned, all the reactions were performed using 1 (0.11 mmol), 2 (0.1 mmol), and catalyst 3c (0.005 mmol), in 0.2 M of toluene at room temperature.b Yield refers to the purified product.c dr was determined for purified product 4 by 1H NMR analysis.d Yield refers to the column purified product (4o, 4t). |
|---|
 |
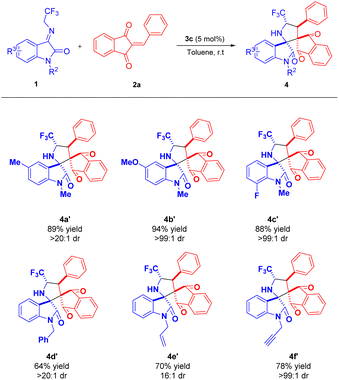
Enlightened by these successful achievements, and to further broaden the scope of this reaction, we carried out the reaction of various N-substituted N-2,2,2-trifluoroethylisatin ketimines with 2-benzylidene-1H-indene-1,3(2H)-dione 2a in presence of 5 mol% of DABCO (3c) in toluene at room temperature. N-Substituted ketimines 1b and 1c having methyl and methoxy group at the C-5 position of the aryl ring efficiently participated in the reaction to provide the respective products 4a′ and 4b′ in 89% and 94% yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and >99
1 and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr respectively, Table 3. The presence of a fluoro group on the C-7 position of the aryl ring of N-methyl substituted ketimines, is also very suitable for this reaction, delivering the product 4c′ in 88% yield with >99
1 dr respectively, Table 3. The presence of a fluoro group on the C-7 position of the aryl ring of N-methyl substituted ketimines, is also very suitable for this reaction, delivering the product 4c′ in 88% yield with >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. On the other hand, changing the methyl substitution on the nitrogen atom of ketimines by benzyl, allyl, and propargyl had no detrimental effect on the yield of the products. While N-benzyl and N-propargyl substituted ketimines 1e and 1g afforded the products 4d′ and 4f′ in 64% and 78% yields with >20
1 dr. On the other hand, changing the methyl substitution on the nitrogen atom of ketimines by benzyl, allyl, and propargyl had no detrimental effect on the yield of the products. While N-benzyl and N-propargyl substituted ketimines 1e and 1g afforded the products 4d′ and 4f′ in 64% and 78% yields with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and >99:1 dr respectively, the N-allyl substituted ketimines leads to a good yield of the product 4e′ but 16
1 and >99:1 dr respectively, the N-allyl substituted ketimines leads to a good yield of the product 4e′ but 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
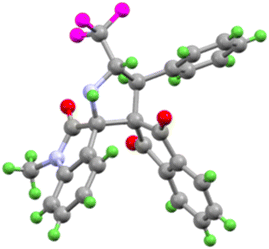
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio. All the synthesized compounds are new and characterized by using 1H NMR, 13C NMR, 19F NMR, HRMS, and FT-TR spectroscopic analysis (see ESI†). The structure of compound 4a is further confirmed by single-crystal X-ray analysis, Fig. 2.
1 diastereomeric ratio. All the synthesized compounds are new and characterized by using 1H NMR, 13C NMR, 19F NMR, HRMS, and FT-TR spectroscopic analysis (see ESI†). The structure of compound 4a is further confirmed by single-crystal X-ray analysis, Fig. 2.
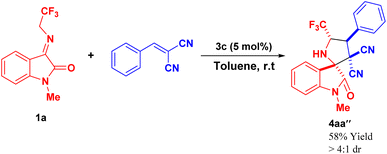
To further established the synthetic potentiality of the present protocol, an enantioselective [3 + 2] cycloaddition of N-2,2,2-trifluoroethylisatin ketimine 1a with 2-benzylidene-1H-indene-1,3(2H)-dione 2a was performed. With the help of 5 mol% of quinine 3h as the organocatalyst, the reaction provided the corresponding spiro-pyrrolidine oxindole product 4a bearing four contiguous stereogenic centres with two vicinal spiro-all carbon quaternary stereogenic centres in good yield (66% yield), excellent enantioselectivity (87% ee). Inspired by this result, we then synthesized a total of four examples of an asymmetric version of the spirooxindole products. The final product 4 was isolated by using flash chromatography (Scheme 3). Furthermore, to validate the synthetic potentiality of the present strategy, we conducted a reaction between arylidene malononitrile and 1a under identical conditions. To our delight, the corresponding product 4aa′′ was accomplished in 58% yield (Scheme 4).
Conclusions
In conclusion, we have demonstrated the exploitation of 2-aryl/heteroarylidene-1H-indene-1,3(2H)-dione as the new olefin source for the expeditious construction of trifluoro methyl containing spiro-pyrrolidine oxindoles fused with indeno moiety via a DABCO-catalyzed [3 + 2] cycloaddition with readily accessible N-2,2,2-trifluoroethylisatin ketimines at ambient temperature. The mild reaction conditions, ultra-low loading of catalyst, energy efficiency, eco-compatible, and diverse functionality are some of the key features of this approach. The synthetic applicability of the present approach was highlighted by the enantioenriched construction of spiro-pyrrolidine oxindole with four stereogenic centres out of which two vicinal spiro-quaternary centers were formed. The developments of further synthetic strategies for improving the enantioselectivity of the synthesized products, indeno fused spiro-pyrrolidine oxindoles may would find the future scopes of applications.Author contributions
MSP: conceptualization-equal, data curation-equal, formal analysis-equal, investigation-equal, writing – original draft-equal, supervision-equal. SB, SMS, MR and MS: investigation and original draft. BB: writing – investigation and original draft. RC: supervision-equal.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was made possible by a grant from the Department of Science and Technology (DST), SERB, New Delhi [grant no. EEQ/2016/000176]. We thank the Central Instrumentation Lab, Department of Chemistry, CUTN, for the NMR (400 MHz) and HRMS facilities. We thank the MSC project students for their help in manuscript preparation. BB thanks UGC-India for the Non-NET fellowship. Author thanks the Central University of Tamil Nadu and the Central University of Gujarat for the infrastructure to carry out the work.Notes and references
- (a) A. J. Boddy and J. A. Bull, Org. Chem. Front., 2021, 8, 1026–1084 RSC; (b) B. Borah, J. Bora, P. Ramesh and L. R Chowhan, RSC Adv., 2022, 12, 12843–12857 RSC; (c) M. Ganesh and S. Suraj, Org. Biomol. Chem., 2022, 20, 5651–5693 RSC; (d) W. H. Zhang, S. Chen, X. L. Liu, X. W. Liu and Y. Zhou, Bioorg. Med. Chem. Lett., 2020, 30, 127410 CrossRef CAS PubMed; (e) M. Zhang, J. X. Wang, S. Q. Chang, X. L. Liu, X. Zuo and Y. Zhou, Chin. Chem. Lett., 2020, 31, 381–385 CrossRef CAS.
- (a) B. Borah and L. R. Chowhan, Tetrahedron Lett., 2022, 104, 154014 CrossRef CAS; (b) V. A. D’yakonov, O. G. A. Trapeznikova, A. de Meijere and U. M. Dzhemilev, Chem. Rev., 2014, 114, 5775–5814 CrossRef PubMed; (c) L. M. Zhou, R. Y. Qu and G. F. Yang, Expert Opin. Drug Discovery, 2020, 15, 603–625 CrossRef CAS PubMed; (d) K. Zhang, H. Han, L. Wang, Z. Zhang, Q. Wang, W. Zhang and Z. Bu, Chem. Commun., 2019, 55, 13681–13684 RSC; (e) Y. Zhu, J. Zhou, S. Jin, H. Dong, J. Guo, X. Bai and Z. Bu, Chem. Commun., 2017, 53, 11201–11204 RSC.
- (a) M. Aldeghi, S. Malhotra, D. L. Selwood and A. W. E. Chan, Chem. Biol. Drug Des., 2014, 83, 450–461 CrossRef CAS PubMed; (b) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS; (c) J. Guo, X. Bai, Q. Wang and Z. Bu, J. Org. Chem., 2018, 83, 3679–3687 CrossRef CAS PubMed; (d) M. Li, W. L. Yang, L. R. Wen and F. Q. Li, Eur. J. Chem., 2008, 16, 2751–2758 CrossRef; (e) N. V. Lakshmi, P. Thirumurugan and P. T. Perumal, Tetrahedron Lett., 2010, 51, 1064–1068 CrossRef CAS; (f) M. Rajeswari, J. Sindhu, H. Singh and J. M. Khurana, RSC Adv., 2015, 5, 39686–39691 RSC.
- (a) T. Peng, T. Liu, J. Zhao, J. Dong, Y. Zhao, Y. Yang, X. Yan, W. Xu and X. Shen, J. Org. Chem., 2022 DOI:10.1021/acs.joc.2c02391; (b) R. C. Elderfield and R. E. Gilman, Phytochem, 1972, 11, 339–343 CrossRef CAS; (c) K. C. Chan, F. Morsingh and G. B. Yeoh, J. Chem. Soc. C, 1966, 2245–2249 RSC; (d) R. G. Geetha and S. Ramachandran, Pharmaceutics, 2021, 13, 1170 CrossRef CAS PubMed; (e) M. Asad, M. N. Arshad, A. M. Asiri, S. A. Khan, M. Rehan and M. Oves, Polycyclic Aromat. Compd., 2022, 42, 5385–5397 CrossRef CAS; (f) A. S. Girgis, Eur. J. Med. Chem., 2009, 44, 91–100 CrossRef CAS PubMed.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC.
- Q. Sun, X. Li, J. Su, L. Zhao, M. Ma, Y. Zhu and R. Wang, Adv. Synth. Catal., 2015, 357, 3187–3196 CrossRef CAS.
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. Del Pozo, A. E. Sorochinsky, S. Fustero and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed.
- W. R. Dolbier Jr, J. Fluorine Chem., 2005, 126, 157–163 CrossRef.
- K. Muller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed.
- T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470–477 CrossRef CAS PubMed.
- (a) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366–5412 CrossRef CAS PubMed; (b) M. Kissane and A. R. Maguire, Chem. Soc. Rev., 2010, 39, 845–883 RSC; (c) M. S. Reddy, N. S. Kumar and L. R. Chowhan, RSC Adv., 2018, 8, 35587–35593 RSC; (d) M. S. Reddy, L. R. Chowhan, N. S. Kumar, P. Ramesh and S. B. Mukkamala, Tetrahedron Lett., 2018, 59, 1366–1371 CrossRef CAS; (e) V. Singh, S. R. Lakshmi and L. R. Chowhan, Front. Chem., 2022, 1095 Search PubMed; (f) P. Roy, S. R. Anjum and D. B. Ramachary, Org. Lett., 2020, 22, 2897–2901 CrossRef CAS PubMed.
- (a) I. Coldham and R. Hufton, Chem. Rev., 2005, 105, 2765–2810 CrossRef CAS PubMed; (b) G. Pandey, P. Banerjee and S. R. Gadre, Chem. Rev., 2006, 106, 4484–4517 CrossRef CAS PubMed; (c) G. Surendra Reddy, A. Suresh Kumar and D. B. Ramachary, Org. Biomol. Chem., 2020, 18, 4470–4478 RSC.
- W. S. Jen, J. J. Wiener and D. W. MacMillan, J. Am. Chem. Soc., 2000, 122, 9874–9875 CrossRef CAS.
- H. Suga, D. Ishimoto, S. Higuchi, M. Ohtsuka, T. Arikawa, T. Tsuchida and T. Baba, Org. Lett., 2007, 9, 4359–4362 CrossRef CAS PubMed.
- M. Ma, Y. Zhu, Q. Sun, X. Li, J. Su, L. Zhao and R. Wang, Chem. Commun., 2015, 51, 8789–8792 RSC.
- X. W. Liu, J. Yue, Z. Li, D. Wu, M. Y. Tian, Q. L. Wang and Y. Zhou, Tetrahedron, 2020, 76, 131678 CrossRef CAS.
- Y. Xiong, X.-X. Han, Y. Lu, H.-J. Wang, M. Zhang and X.-W. Liu, Tetrahedron, 2021, 87, 132112 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1880719. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ra07141j |
| This journal is © The Royal Society of Chemistry 2022 |